Abstract
Objective:
In the surgery of breast cancer, axillary reverse mapping (ARM) is the identification and preservation of arm draining lymph node (ARM node) during an axillary dissection. The assumption is that the ARM node is different from node draining breast and is unlikely to be involved even in the patients with axillary nodal metastases. If we can identify and preserve ARM node using lymphoscintigraphy; morbidity of lymphedema, as seen with axillary dissection, may be avoided.
Materials and Methods:
Pathologically proven 50 breast cancer patients undergoing initial surgery (cTx-4, cN0-2, and Mx-0) were included in this study. Less than 37 MBq, 0.5 ml in equally divided doses of filtered 99mTc sulfur colloid was injected intradermally into the second and third web spaces. ARM nodes in the axilla were identified with the help of Gamma Probe intraoperatively; however, their location was noted with the reference to specific anatomical landmarks and sent for histopathological examination after excision.
Results:
The ARM node was successfully identified in 47/50 cases (sensitivity - 94%). In 40 out of 47 cases (85%), the location of the ARM node was found to lateral to the subscapular pedicle, above the second intercostobrachial nerve and just below the axillary vein. Of the 47 patients in whom ARM node/s were identified, metastasis was noted in 5 of them (10%). Four out of these 5 patients had the pN3 disease.
Conclusion:
ARM node exists, and it is feasible to identify ARM node using radio isotope technique with an excellent sensitivity. ARM node seems to have a fairly constant location in more than 80% cases. It is involved with metastasis (10% cases) only when there are multiple lymph nodal metastases in the axilla.
Keywords: 99mTc sulfur colloid, axillary reverse mapping, breast cancer, lymphedema, sentinel lymph node scintigraphy
INTRODUCTION
The status of the axillary lymph nodes is the most important predictor of outcome in breast carcinoma patients. Axillary lymph node dissection (ALND) or sentinel lymph node biopsy (SLNB) is the method used for assessing axillary nodal metastases. But, during ALND, arm lymphatics are not distinguished from breast lymphatics and many a times sacrificed unnecessarily. Transection of arm lymphatics during an ALND most likely results in lymphedema and is perhaps the most widely published complication of ALND. Although the sentinel lymph node (SLN) clearly reflects the status of the axillary lymph nodal basin and is less morbid, it has not prevented lymphedema. There is no doubt that lymphedema is minimized with SLN dissection (SLND) in comparison with ALND, as highlighted in eight clinical trials comparing the SLND and ALND methods of axillary staging. Rates of lymphedema with SLND were much lower than those with ALND, in the range of 0–13%, compared with 7–77% for ALND.[1,2,3,4,5,6,7] Although less, there is some risk of lymphedema with SLND as well. Further, with a positive SLNB, ALND is still the standard of care. So, lymphedema is the major concern in this particular group also. It is hypothesized that this higher than expected rate of lymphedema may be secondary to disruption of low-lying arm lymphatics (i.e. arm lymphatics lying in close proximity to breast lymphatics) during an SLND procedure.[8] Identification and ultimately, protection of these low-lying arm lymphatics through axillary reverse mapping (ARM) could be a technique to prevent lymphedema. This new concept is termed as ARM. The goal in ARM is identification and preservation of lymph node draining the ipsilateral arm during standard ALND procedure rather than its removal. This is the reverse of the SLND where first lymph node draining breast is identified and removed for histopathological examination.
ARM involves retrieving all breast-related lymph nodes but leaving the intact of the main lymphatic drainage chain of the upper limb. Thus, it reduces the incidence of lymphedema in breast cancer patients requiring an axillary dissection. The assumption is that lymph node draining the upper limb is different from that draining the breast and it is likely to be uninvolved even in those patients with documented axillary nodal metastases. This is supported by the anatomist's description that the different groups (lateral/brachial group) of axillary nodes may be involved in the lymphatic drainage of the arm.[9,10,11,12,13] ARM concept is also a direct surgical implication of an anatomical description of the lymphatic territories of the upper limb published in 2007 by Suami et al.[14]
Identification of ARM node can be performed by either blue dye or radio tracers or both. Limitations of the dye method include insufficient identification rate of the ARM node (sensitivity <70%), persistent blue stain at the site of injection lasting up to 2 years as well as allergy to blue dye. In radiotracer method, 99mTc sulfur colloid is used in a filtered (0.22 μ Millipore filter) or unfiltered form. There is a worldwide variation in radiopharmaceuticals used for lymphoscintigraphy. 99mTc antimony trisulfide colloid (particle size, 0.015–0.3 μm) and 99mTc nanocolloid (particle size, 0.05–0.8 μm) are used in Australia and Europe. A 99mtc sulfur colloid is used in the United States in a filtered (0.22 μ Millipore filter) or unfiltered form. Radiopharmaceuticals described above are routinely used for lymphoscintigraphy and SLN procedures, and same can also be used for ARM procedure.
The aims and objectives of this prospective study were to assess the feasibility of detecting ARM node with filtered 99mTc-sulphur colloid, to assess the sensitivity of this technique and to establish a standard protocol of lymphoscintigraphy. We also aimed to assess the consistency in the location of ARM node and incidence of metastases in the same.
MATERIALS AND METHODS
Patients
Patients with pathologically proven breast cancer with clinically palpable or nonpalpable axillary lymph nodes (Tx-4, N0-2, and Mx-0) undergoing primary breast surgery plus axillary dissection were included in the study. Patients who had previous surgery of the upper limb or axilla and the patients who received neo adjuvant chemotherapy were excluded. A total of 50 patients were enrolled in the study. Only 1 patient among these 50 patients underwent sentinel node mapping also.
Procedure
There is no specific patient preparation for the procedure. Less than 37 MBq of aseptically prepared filtered 99mTc sulfur colloid (filtered with 0.22 μ Millipore filter) in a total volume of 0.5 ml was intradermally injected in equal divided doses into the second and third web spaces of the ipsilateral hand after induction of general anesthesia just before commencing the breast surgery. Usual precautions like gentle shaking of the syringe prior to injection was undertaken to avoid the clumping of colloidal particles together. After injecting, each site was massaged for 1–2 min to facilitate lymphatic flow.
All patients underwent either breast conservation surgery or mastectomy as decided by a surgical oncologist. Routine ALND was performed in all patients irrespective of the finding of ARM procedure. A hand held battery operated collimated Gamma Probe (SinoRx Gamma Finder, USA), was used intraoperatively for the identification of ARM lymph node. Any node with count statistics of at least 10 times the background counts at a location remote from the injection site either in the axilla or along the upper limb drainage area was considered to be an ARM node/s. ARM nodes in the axilla were identified, and their location was noted in relation to specific anatomical landmarks such as a subscapular pedicle, second intercostobrachial (ICB) nerve, and axillary vein [Figure 1]. All the ARM lymph node/s was successfully excised and ex vivo counting of lymph node was also performed to reassess the completeness of surgery. Finally, all the excised nodes were marked and sent for histopathological examination. During each procedure, the time interval at which the first ARM node was localized was noted to calculate the average transit time. For the case who received sentinel node biopsy in addition to ARM, SLNB was performed first, followed by ARM.
Figure 1.
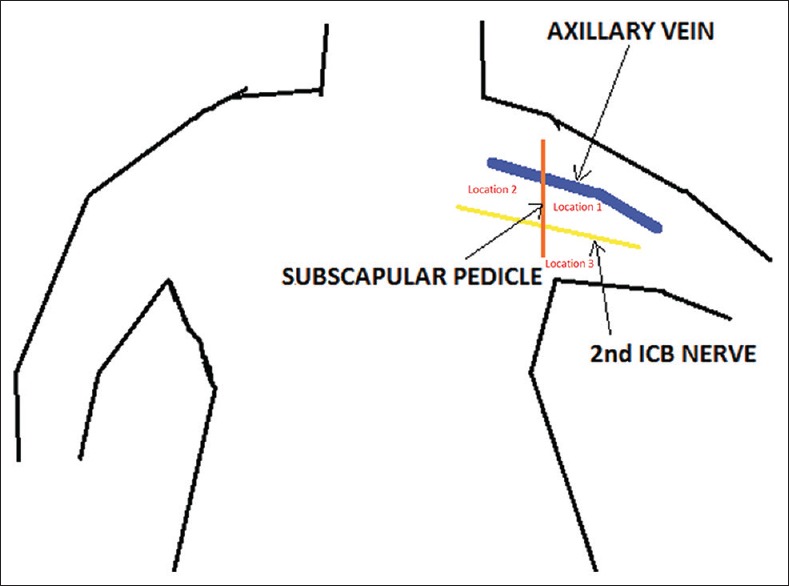
Axillary anatomy. Note the axillary anatomy with described landmarks
Ethical standards
Ethical Committee Clearance was obtained before commencing the study. All persons gave their informed consent prior to their inclusion in the study.
RESULTS
A total of 50 patients with breast cancer underwent ARM lymph node detection procedure from April 2012 to August 2012. The median age was 50 years (range: 29–82 years) [Table 1]. The ARM node was successfully identified in 47/50 cases (sensitivity - 94%). ARM node could not be detected in 3 patients [Tables 2 and 3]. In 2/3 cases, the axillary dissection commenced immediately after the radiotracer injection (i.e., 20 min and 30 min, respectively), and no radioactive lymph node was localized in the axilla. In the third case, the fibro fatty tissue which showed elevated counts with the Gamma Probe did not have any lymph node on histopathological examination. In 16 cases, more than one ARM nodes (range: 2–4, mean nodes - 2.6) in the axilla showed elevated counts intra operatively.
Table 1.
Patient profile
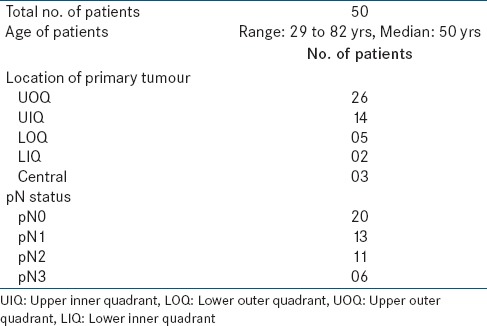
Table 2.
Results - ARM nodes

Table 3.
Results - location of ARM nodes
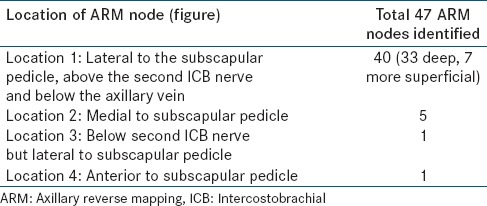
In 40 out of 47 cases (85%), the location of the ARM node was found lateral to the subscapular pedicle, above the second ICB nerve and just below the axillary vein [Figure 1]. In 7 out of these 40 cases (17.5%), it was found to be at a more superficial location, but still lateral to the subscapular pedicle line. In 5/47 (10.6%) cases, the node was more medially located. In 1 case, it was located on the ICB nerve but lateral to the subscapular vessels. In another case, the ARM node was located anterior to the subscapular pedicle [Table 3]. In 9/47 cases, when there was a delay in starting axillary dissection, a second node was identified, usually along the lateral aspect of the subscapular pedicle. In 6/47 cases, three nodes and 1/47 case, four nodes were identified. The average number of ARM nodes with elevated counts, as seen during surgery, was 1.4 nodes per patient.
The median number of lymph nodes removed in axillary dissection was 16 (range: 6–26). Out of total 50 patients, 30 patients had histopathologically proven node positivity (60%) for metastasis (pN1 -13, pN2 -11 and pN3 -6) [Table 1]. Of the 47 patients in whom ARM node/s were identified, metastasis was noted in 5 of them [Table 2]. These 5 patients were analyzed with respect to different variables such as quadrantic location of primary tumor, the location of ARM node, nodal staging, and to identify if there were a correlation. (a) Location of primary tumor - The quadrantic location of the primary tumor in the breast (i.e., upper inner quadrant [UIQ], upper outer quadrant, lower inner quadrant, lower outer quadrant [LOQ], and central) was variable in these 5 patients. LOQ-3 patients, UIQ-1, central-1 (b) Location of ARM node - ARM node was situated: (i) Lateral to latissimus dorsi pedicle, above the ICB nerve and inferior to axillary vein-3 patients. (ii) Medial to subscapular pedicle-2 patients. (c) Nodal stage - Four of these 5 patients had a pathological N3 disease (15/18, 14/15, 10/11, and 10/15 nodes positive/total number of nodes, respectively). In 4/4 patients, nodes in axilla looked clinically enlarged till level III. In 1/4 patient, 3 ARM nodes were detected; while 3 out of 4 patients showed solitary ARM node. One out of 5 patients had pathological N2 disease (with 6/16 nodes being positive for metastasis) and nodes in axilla looked enlarged to level I.
DISCUSSION
Successful identification of the ARM node was possible in 94% of patients; however, in three of them the ARM node could not be identified. A possible explanation for nonlocalization of the same could be inadequate time given for the radiotracer to reach the draining node.
This study also shows the feasibility of lymphoscintigraphy for the identification of ARM node. The technique is easy and simple to perform. As the radio pharmaceutical is injected after induction of anesthesia, there is no pain or discomfort to the patient. There is no risk of any anaphylactic reaction or infection. The problem of prolonged staining by the blue dye is avoided. Nos et al. used blue dye with less volume (0.5 cc) to reduce skin discoloration in initial 8 patients in their series, which yielded poor identification rate of ARM nodes. Subsequently, they used radio isotope method, which showed 91% detection rate.[15] Thompson et al. described 61% ARM detection rate with blue dye technique in their series.[16]
Anatomically the location of the ARM node was fairly constant in 85% of the cases in our series. The most common site of this node was within the area bounded by the subscapular vessels medially, the ICB nerve inferiorly and the axillary vein superiorly [Figure 1]. In 15% of the cases, the ARM node was located either medial to the subscapular pedicle or inferior to ICB nerve or anterior to subscapular pedicle [Table 3]. At such location (15% cases), it may not be possible to preserve the ARM node because the central group lymph nodes are closely related to breast lymphatic drainage. This may prove to be a limitation of the surgical technique. The detection of the second tier nodes along the lateral aspect of the subscapular vessels, lower down, seems to indicate that this may be one of the routes for the lymphatics of the arm to traverse. A study by Nos et al. had 9% ARM nodes in breast lymphatic drainage area,[15] which is comparable to our findings. However, other three different studies had 42.7%, 33%, and 37.5% ARM nodes in breast lymphatic drainage area respectively.[8,16,17]
We also attempted to assess the factors which can predict the metastatic involvement of ARM nodes in breast cancer. Our study included patients with pN0 to pN3 nodal staging. This study attempted to confirm whether the ARM nodes were always free of metastatic disease across all pN stages. In our series, we found that 10% of ARM nodes that is, in 5 patients these nodes were actually positive for metastasis out of which four had pN3 disease, which is comparable to 14% metastatic involvement in study by Nos et al. in which the patients with metastatic lymph nodes had pN3 nodal status.[15] Other studies revealed all ARM nodes to be free of metastasis; however, these studies had patients with N0 nodal status.[8,16,17] These findings constitute an important limitation of the technique. There are two explanations that can account for the metastatic involvement of the ARM nodes. The first is when the ARM node is located in the central group. The central nodal group is highly related to the lymphatic drainage of the breast. The second explanation relates to the natural progression of the metastatic disease through the nodes of the axilla. It is striking that the 5 cases with metastatic involvement of the ARM nodes were found in patients who had significant axillary tumor burden, that is, pN3 (10 or more metastatic nodes) in our study. But, it is difficult to predict preoperatively the involvement of the entire axilla with metastatic disease, despite careful clinical examination and all the available modern imaging. However, 80% cases (4/5 cases) showed clinically enlarged lymph nodes till level III intra operatively. Careful clinical examination of lymph nodes intraoperatively may help in predicting metastatic involvement of ARM node. We also evaluated other parameters such as quadrantic location of the primary tumor in breast, location of ARM node, primary tumor histopathology, etc. These factors were noncontributory for predicting the metastasis in ARM nodes. However, a number of cases with metastatic ARM nodes is small (5 cases), making it insufficient to draw any conclusions regarding the prediction of metastatic involvement in them. Overall, the incidence of metastasis in ARM node is low as noted in this series (10% ARM nodes positive for metastasis). The next challenge is how to predict this node positivity preoperatively. Although our current study demonstrated that RN method could successfully identify ARM node, further considerations would be required for the clinical application of this technique because lymphatic channels bound for ARM must be kept intact during axillary node dissection to avoid lymphedema after the dissection. Visualization of these lymphatic channels is not easy by RN methods. The combination of ARM and SLN mapping would be another issue because ARM nodes cannot be differentiated from SLNs by RN methods.
CONCLUSION
This study revalidates the previously done studies on ARM. ARM node exists, and it is feasible to identify the ARM node using a very simple and easy radio isotope technique. Radioisotope technique provides an excellent sensitivity in detecting ARM node without any significant side effects. The protocol we followed provides good sensitivity and allows early identification of ARM node without much delay post radiotracer injection. ARM node seems to have a fairly constant location in more than 80% cases. It is involved with the metastasis only when there are multiple lymph nodal metastases in the axilla. A long-term follow-up study with the preservation of ARM node during axillary surgery is required to assess the real advantage of lymph edema prevention by ARM technique.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Schrenk P, Rieger R, Shamiyeh A, Wayand W. Morbidity following sentinel lymph node biopsy versus axillary lymph node dissection for patients with breast carcinoma. Cancer. 2000;88:608–14. doi: 10.1002/(sici)1097-0142(20000201)88:3<608::aid-cncr17>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 2.Haid A, Köberle-Wührer R, Knauer M, Burtscher J, Fritzsche H, Peschina W, et al. Morbidity of breast cancer patients following complete axillary dissection or sentinel node biopsy only: A comparative evaluation. Breast Cancer Res Treat. 2002;73:31–6. doi: 10.1023/a:1015234318582. [DOI] [PubMed] [Google Scholar]
- 3.Swenson KK, Nissen MJ, Ceronsky C, Swenson L, Lee MW, Tuttle TM. Comparison of side effects between sentinel lymph node and axillary lymph node dissection for breast cancer. Ann Surg Oncol. 2002;9:745–53. doi: 10.1007/BF02574496. [DOI] [PubMed] [Google Scholar]
- 4.Blanchard DK, Donohue JH, Reynolds C, Grant CS. Relapse and morbidity in patients undergoing sentinel lymph node biopsy alone or with axillary dissection for breast cancer. Arch Surg. 2003;138:482–7. doi: 10.1001/archsurg.138.5.482. [DOI] [PubMed] [Google Scholar]
- 5.Schijven MP, Vingerhoets AJ, Rutten HJ, Nieuwenhuijzen GA, Roumen RM, van Bussel ME, et al. Comparison of morbidity between axillary lymph node dissection and sentinel node biopsy. Eur J Surg Oncol. 2003;29:341–50. doi: 10.1053/ejso.2002.1385. [DOI] [PubMed] [Google Scholar]
- 6.Rönkä R, von Smitten K, Tasmuth T, Leidenius M. One-year morbidity after sentinel node biopsy and breast surgery. Breast. 2005;14:28–36. doi: 10.1016/j.breast.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 7.Leidenius M, Leivonen M, Vironen J, von Smitten K. The consequences of long-time arm morbidity in node-negative breast cancer patients with sentinel node biopsy or axillary clearance. J Surg Oncol. 2005;92:23–31. doi: 10.1002/jso.20373. [DOI] [PubMed] [Google Scholar]
- 8.Boneti C, Korourian S, Bland K, Cox K, Adkins LL, Henry-Tillman RS, et al. Axillary reverse mapping: Mapping and preserving arm lymphatics may be important in preventing lymphedema during sentinel lymph node biopsy. J Am Coll Surg. 2008;206:1038–42. doi: 10.1016/j.jamcollsurg.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 9.Sappey PC. Paris: Adrien Delahaye; 1874. Anatomie, Physiologie, Pathologie Des Vaisseaux Lymphatiques. [Google Scholar]
- 10.Delamere G, Poirier P, Cuneo B. The lymphatics. In: Charpy PP, editor. A trearise of human anatomy. Westminster: Archibald Constable; 1903. [Google Scholar]
- 11.Rouviere H. Paris: Masson et Cie Edit; 1932. Anatomie Des Lymphatiques De L’homme. [Google Scholar]
- 12.Turner-Warwick RT. The lymphatics of the breast. Br J Surg. 1959;46:574–82. doi: 10.1002/bjs.18004620004. [DOI] [PubMed] [Google Scholar]
- 13.Sobotta J. Munchen, Wien, Baltimore, Paris: Urban Schwarzenberg, Maloine S. A. Editeurs; 1977. Atlas d’Anatomie Humaine. [Google Scholar]
- 14.Suami H, Taylor GI, Pan WR. The lymphatic territories of the upper limb: Anatomical study and clinical implications. Plast Reconstr Surg. 2007;119:1813–22. doi: 10.1097/01.prs.0000246516.64780.61. [DOI] [PubMed] [Google Scholar]
- 15.Nos C, Kaufmann G, Clough KB, Collignon MA, Zerbib E, Cusumano P, et al. Combined axillary reverse mapping (ARM) technique for breast cancer patients requiring axillary dissection. Ann Surg Oncol. 2008;15:2550–5. doi: 10.1245/s10434-008-0030-z. [DOI] [PubMed] [Google Scholar]
- 16.Thompson M, Korourian S, Henry-Tillman R, Adkins L, Mumford S, Westbrook KC, et al. Axillary reverse mapping (ARM): A new concept to identify and enhance lymphatic preservation. Ann Surg Oncol. 2007;14:1890–5. doi: 10.1245/s10434-007-9412-x. [DOI] [PubMed] [Google Scholar]
- 17.Casabona F, Bogliolo S, Valenzano Menada M, Sala P, Villa G, Ferrero S. Feasibility of axillary reverse mapping during sentinel lymph node biopsy in breast cancer patients. Ann Surg Oncol. 2009;16:2459–63. doi: 10.1245/s10434-009-0554-x. [DOI] [PubMed] [Google Scholar]


