Abstract
Background:
The association of metabolic syndrome (MS) with left ventricular (LV) hypertrophy is controversial. The objective of our study was to investigate the influence of MS on LV mass and geometry in community-based hypertensive patients among Han Chinese.
Materials and Methods:
This study included 1733 metabolic syndrome patients according to the International Diabetes Federation (IDF) definition and 2373 non-MS hypertension patients. LV hypertrophy was diagnosed by the criteria of LV mass ≥49.2 g/m2.7 for men and 46.7 g/m2.7 for women. LV geometric patterns (normal, concentric remodeling, concentric or eccentric hypertrophy) were calculated according to LV hypertrophy and relative wall thickness. Logistic regression analysis was used to determine odds ratio (OR) and 95% confidence interval (CI) of MS for LV hypertrophy and LV geometry abnormality.
Results:
The LV mass and LV mass index were higher in the MS group than in the non-MS group. In multiple adjusted models. LV mass index, LV mass, interventricular septum, and post wall were raised with the increased number of MS disorders. MS was associated with increased LV hypertrophy risk (unadjusted OR 1.38; 95% CI 1.21-1.57); age, sex, and blood pressure (BP; adjusted OR 1.39; 95% CI 1.22-1.59). MS was also associated with increased risk of eccentric hypertrophy in male and female patients. MS was only associated with increased risk of concentric hypertrophy in female patients; and MS was not associated with concentric remodeling.
Conclusion:
LV mass and LV mass index were associated with the increased number of MS disorders in the Chinese community-based hypertensive population. MS was not only associated with increased LV hypertrophy risk, but also associated with concentric and eccentric LV geometry abnormality, especially in females.
Keywords: Hypertension, left ventricular (LV) geometry abnormality, LV hypertrophy, metabolic syndrome (MS)
INTRODUCTION
Metabolic syndrome (MS) is associated with a twofold increase in the risk of coronary heart disease (CHD) and stroke events.[1] Echocardiographic hypertrophy is an independent risk factor of cardiac morbidity and mortality and all cause of mortality.[2,3]
The association of MS with LV hypertrophy is controversial. A study in black women and men indicated that the degree of MS was strongly related to LV mass and its wall thickness components.[4] Another study conducetd in Native American concluded that in different components of the MS, only high blood pressure (BP) was associated with increased LV mass and prevalence of LV hypertrophy.[5] Recent studies indicated that the impact of MS on LV remodeling is significantly influenced by gender, and the metabolic sequence of MS is more important for LV remodeling in women.[6,7] The number of study subjects in previously was no more than 1572 in the Atherosclerosis Risk in Communities (ARIC) Study.[4] Because MS is a disease with at least three components of cardiovascular risk factors, the scale of the study samples could directly affect the results. This community-based study included 4106 subjects with or without MS.
Our purpose was to investigate the influence of MS on LV mass and geometry in community-based hypertensive patients among the Han Chinese.
MATERIALS AND METHODS
Study population
Details of our study protocol have been described previously.[8] Briefly, this community-based cross-sectional study was conducted in XinYang county, located in the central China, from 2004 to 2005. We used a multistage cluster sampling method to select a representative sample of rural community residents aged 40-75 years. The total 13,444 subjects (5270 men and 8174 women) underwent the survey, and yielded a response rate of 84.9%. Of them, 5421 hypertension patients were identified and thoroughly examined. Hypertension was defined as diastolic blood pressure (DBP) of ≥90 mmHg, systolic blood pressure (SBP) of ≥140 mmHg, physician diagnosis, or current medication for hypertension [as defined by the World Health Organization (WHO) 1999]. Laboratory tests were performed in the central laboratory included determinations of levels of serum sodium, potassium, creatinine, uric acid, blood urea nitrogen (BUN), total cholesterol, triglyceride, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, and glucose.
The study protocol was reviewed and approved by the ethical committees of the FuWai Hospital and local hospitals. All participants gave their informed consent before they were recruited, and reported themselves to be of Han nationality. All investigators were trained at the Cardiovascular Institute, Chinese Academy of Medical Science (Beijing, China) and to be eligible by test.
The definition of metabolic syndrome
The definition of MS was defined with reference to the International Diabetes Federation (IDF) definition.[9] Asian central obesity: Waist circumference ≥90 cm for men, ≥80 cm for women, together with at least two of the following criteria:
Triglycerides level: ≥1.7 mmol/L or treatment for this abnormality;
HDL cholesterol (HDL-C): < 1.29 mmol/L for women or <1.03 mmol/L for men or treatment for this abnormality;
Hypertension: Arterial BP ≥130/85 mmHg or on antihypertensive medication;
Fasting plasma glucose: ≥5.6 mmol/L or previously diagnosed type 2 diabetes.
Echocardiographic methods
Transthoracic echocardiography was performed according to the standard protocol[10] that included M-mode, two-dimensional (2D), and color Doppler recordings from the parasternal long-axis and short-axis windows, as well as 2D and color Doppler evaluations from the apical window to yield 2-, 3-, and 4-chamber images with an HP 5500 (Phillips Medical System, Boston, Massachusetts, USA) or an HDI 3000 (ATL, Bothell, Washington, USA). The transducer frequency was 2.5-3.5 MHz. Optigo echocardiographic recorders (Agilent, Boston, Massachusetts, USA) were used occasionally to screen subjects who could not reach the local study center. The echocardiographic examination was supervised by two physician-echocardiographers with at least 2 years of experience. Two technicians from each center performed all the echocardiographic studies. Before the study, they were trained in the echocardiographic protocol at the Cardiovascular Institute, Chinese Academy of Medical Science.
Echocardiographic measurements
Correct orientation of planes for 2D and Doppler imaging was confirmed using standard procedures.[10] LV internal dimension and septal and posterior wall thicknesses were measured on up to three cardiac cycles at end-diastole and end-systole according to the American Society of Echocardiography recommendations.[10] Once optimal orientation of the LV views could not be obtained, as is common in subjects who are overweight or over age 60, correctly oriented 2D linear dimension measurements were made by the leading-edge convention of the American Society of Echocardiography.[10]
Calculation of derived variables
LV mass was calculated using the equation: 0.8 × 1.04 [(IVS + LVEDD + PW)3-LVEDD3] + 0.6, which yields values closely related (R = 0.90) to necropsy LV weight.[11] LV mass was divided by height2.7 to obtain LV mass index (LVMI). RWTm (relative wall thickness)[12] was calculated by (IVS + PW)/LVEDD, RWTp[10] was calculated by 2 × PW/LVEDD, where IVS is interventricular septum, PW is posterior wall, and LVEDD is LV end-diastolic diameter. BSA (body surface area) was calculated by using the Du Bois formula:[13] 0.0071843 × [weight (kg)]0.4253 × [height (cm)]0.725 LVH was diagnosed by using the criteria of LVMI more than 49.2 g/m2.7 for men and 46.7 g/m2.7 for women.[14] A partition value of 0.43[14] was used for RWTp and 0.45[15] for RWTm, respectively.
Normal geometry was present when LVMI and RWT were normal, whereas normal LVMI and increased RWT identified concentric remodeling. Increased LVMI but normal RWT identified eccentric LV hypertrophy, and increases of both variables identified concentric LV hypertrophy.[16]
Statistic analysis
SPSS software version 13.0 (SPSS, Inc. Chicago, Illinois, USA) was used for data management and statistical analysis. Data are reported as mean ± standard deviation (SD) for continuous variables and as frequency (percentage) for qualitative variables. Differences between proportions were assessed by x2 test or Fisher's exact test. For continuous variables, differences between two groups were assessed by independent sample t-test; differences between multiple groups and analysis of covariance (ANCOVA) were performed by analysis of variance (ANOVA). A binary logistic regression model was used to determine the odds ratio (OR) of LVH and LV geometry for MS. Adjusted OR and 95% confidence intervals (CIs) were calculated. A two-tailed value of P < 0.05 was considered significant.
RESULTS
Clinical characteristics of the study population
Of 5421 hypertensive patients, 90% (N = 4869) underwent echocardiography, and 89% (N = 4805) were measured for LV mass. The patients with hypertrophic cardiomyopathy (N = 8), valvular heart diseases (N = 108), pulmonary hypertension (N = 7), and 412 patients with CHDs were excluded. Excluding the patients without reliable waist circumferences and lab test data (N = 164), a total of 4106 patients with integrated clinical and echocardiographic data were enrolled in the present study. The clinical characteristics of the study population were described in Table 1. This study comprised 1733 MS patients (1433 female patients and 298 male patients) and 2373 non-MS hypertension patients (1309 female patients and 1064 male patients).
Table 1.
Clinical characteristics of the study population
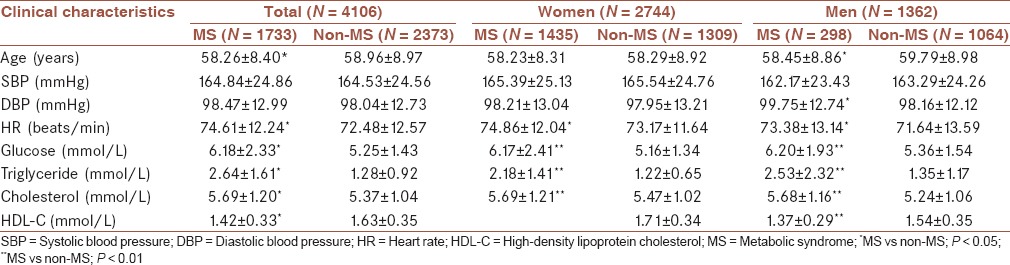
MS and left ventricular mass
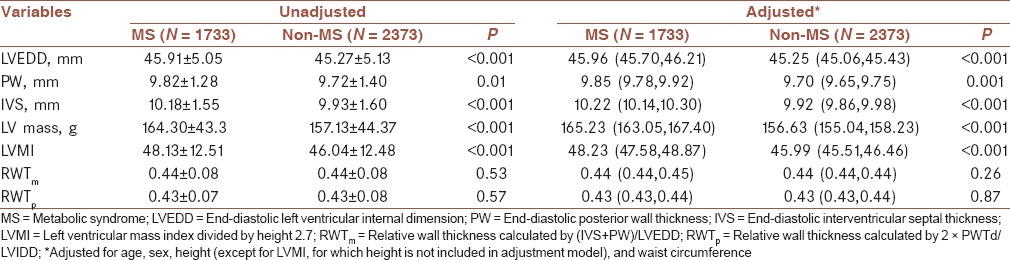
The echocardiographic data of the study population were described in Table 2. The LVEDD, PW, IVS, LV mass, and LVMI were higher in the MS group than in the non-MS group. In the ANCOVA adjusted for age and sex, the differences still remained, like the results in the model adjusted for age, sex, height, and waist circumference. RWTm and RWTp were no differences between the MS and non-MS groups in the unadjusted and adjusted models [Table 2].
Table 2.
The echocardiographic characteristics of patients with MS and without MS
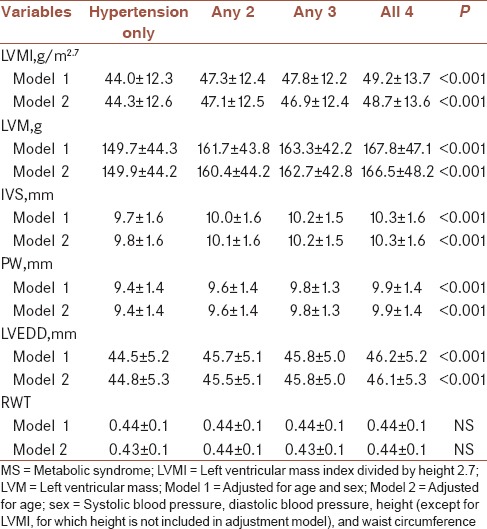
According to the number of MS disorders (hypertension, central obesity, dyslipidemia, and glucose intolerance), the patients were categorized into four groups [Table 3]. LVMI, LVM, IVS, and PW were raised with the increasing number of MS disorders. In the model adjusted for age and sex, and that adjusted for age, sex, SBP, DBP, height, waist circumference, the differences still remained significant [Table 3].
Table 3.
The echocardiographic characteristics of patients according to number of metabolic syndrome disorders
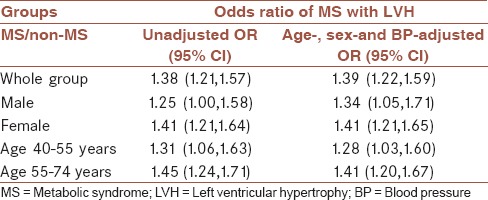
MS was associated with increased LVH risk (unadjusted OR 1.38; 95% CI 1.21-1.57; age, sex-and BP-adjusted OR 1.39; 95% CI 1.22-1.59, in Table 4). This tendency was also observed in males and females. Among age strata, MS was a risk factor of LVH in the age group of 40-55 years as well as the age group of 55-74 years [Table 4].
Table 4.
The association of MS with LVH
MS and LV geometry abnormality
MSs were associated with increasing prevalence of concentric hypertrophy and eccentric hypertrophy [Table 5]. MS was associated with increased risk of concentric hypertrophy (unadjusted OR 1.44; 95% CI 1.21-1.72; multiple-adjusted OR 1.49; 95% CI 1.25-1.78) in the whole group and in the female patients (unadjusted OR 1.58; 95% CI 1.28-1.95; multiple-adjusted OR 1.63; 95% CI 1.31-2.02), but not with the male patients (unadjusted OR 1.11; 95% CI 0.80-1.53; multiple-adjusted OR 1.16; 95% CI 0.84-1.62). This risk remained coincident in the 40-55 years group (unadjusted OR 1.36; 95% CI 1.00-1.84; multiple-adjusted OR 1.36; 95% CI 1.00-1.84) and in the 55-74 years age group (unadjusted OR 1.54; 95% CI 1.24-1.92; multiple-adjusted OR 1.50; 95% CI 1.20-1.87).
Table 5.
The association of metabolic syndrome with left ventricular geometry abnormality
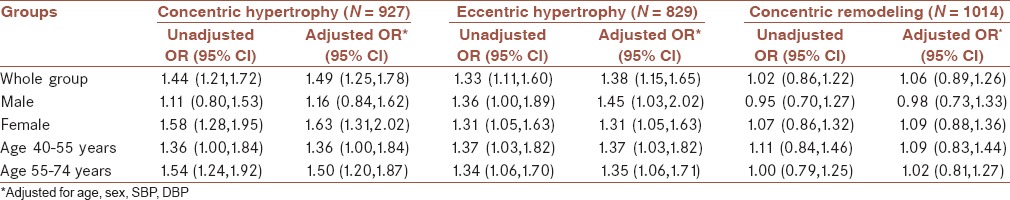
MS was also associated with increased risk of eccentric hypertrophy (unadjusted OR 1.33; 95% CI 1.11-1.60; multiple-adjusted OR 1.38; 95% CI 1.15-1.65) in the whole group and in the female patients (unadjusted OR 1.31; 95% CI 1.05-1.63; multiple-adjusted OR 1.31; 95% CI 1.05-1.63), as well as the male patients (unadjusted OR 1.36; 95% CI 1.00-1.89; multiple-adjusted OR 1.45; 95% CI 1.03-2.02). This risk also remained coincident in the 40-55 years age group (unadjusted OR 1.37; 95% CI 1.03-1.82; multiple-adjusted OR 1.37; 95% CI 1.03-1.82) and in the 55-74 years age group (unadjusted OR 1.34; 95% CI 1.06-1.70; multiple-adjusted OR, 1.35; 95% CI 1.06-1.71).
However, MS was not associated with concentric remodeling regardless in the whole group, in the female and male groups, and in different age groups [Table 5].
DISCUSSION
Our study results indicated that in our Chinese community-based hypertensive population, LV mass and LVMI were higher in the MS group than in the non-MS group adjusted by age, sex, height, and waist circumference. Furthermore, LV mass and LVMI were associated with the increasing number of MS disorders. In multiple-adjusted regression models, MS was not only associated with increased LVH risk, but also associated with concentric and eccentric LV geometry abnormality. Female MS patients carried higher risk for concentric and eccentric hypertrophy.
MS is a cluster of interrelated risk factors of metabolic origin that appear to directly promote the development of atherosclerotic cardiovascular disease.[17] The definitions of MS proposed by different organizations attempt to set forth simple diagnostic criteria to be used in clinical practice to identify patients who manifest the multiple components of MS.[18] The widely used definitions of MS in clinical settings including the National Cholesterol Education Program (NCEP) of the USA[19] and the International Diabetes Federation (IDF).[20] In this study, the IDF definition was used to diagnose MS because IDF recommended that the cutoff points for waist circumference be specific to an ethnic group; in addition, the previous study of our group indicated that the IDF-defined MS was more strongly associated with CHD than the NCEP definition.[21]
The formula used to calculate LVH is still a source of controversy. LV mass indexation was performed by a variety of different methods and PVs for men and women to diagnosis LVH. The values of LVMIBSA were 116 g/m2 for men and 104 g/m2 for women,[22] 125 g/m2 for men and 110 g/m2 for women,[23] 131 g/m2 for men and 100 g/m2 for women,[24] or 125 g/m2 for men and women. The values of LVMI were 51 g/m2.7 for men and women or 49.2 g/m2.7 for men and 46.7 g/m2.7 for women.[14] Different PVs lead to marked discrepancies in the prevalence of hypertrophy and the distribution of LV geometric patterns, which may affect the clinical treatment strategy of the patients. Our study indicated that the LV mass indexed by height2.7 is more sensitive to diagnosis of LVH and LV mass/height2.7 (49.2/46.7) was with increasing concomitant cerebrovascular diseases (P < 0.05).[25]
The results of the ARIC study indicated that LV mass and LVMI were associated with number of disorders in both women and men.[4] A previous study on a population of hypertensive patients showed that the relationship between MS and LV hypertrophy was not affected by gender.[25] Recent studies indicated that the metabolic sequence of MS is more important for LV remodeling in women.[6,7] In our study, MS was associated with increased LVH risk in both genders. Female MS patients carried higher risk for concentric and eccentric hypertrophy; male MS patients only had risk for eccentric hypertrophy. Our study supports that MS is more important for LV remodeling in women.
Our study population was a large community-based sample, and to our knowledge, this is the largest number for echocardiographic evaluation of LV mass, at least in Chinese. Another characteristic of our study subjects was that they were a community-based hypertension population enrolled in a rural area, which could avoid selection bias and better represent the Chinese hypertensive population. Furthermore, echocardiographic estimates of LV mass are more sensitive and specific than electrocardiography (ECG).[26,27] Last but not least, the definitions of MS and LVH were both based on our previous studies.
CONCLUSION
LV mass and LVMI were associated with the increasing number of MS disorders in a Chinese community-based hypertensive population. MS was not only associated with increased LVH risk but also associated with concentric and eccentric LV geometry abnormality, especially in females.
Financial support and sponsorship
The study was supported by grants from the National Natural Science Foundation of China (No. 81100160, 81470504), the Ministry of Science and Technology, and Beijing Municipal Commission of Science and Technology (No. 7040001).
Conflicts of interest
There are no conflicts of interest.
AUTHOR'S CONTRIBUTION
RH contributed in the conception of the work, conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work, and agreed for all aspects of the work. JH contributed in the conception of the work, drafting and revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. SW contributed in the conception of the work, conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. KS contributed in the conception of the work, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. XG contributed in the conception of the work, conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. HX contributed in the conception of the work, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. NW contributed in the conception of the work, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. JC contributed in the design of the work, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. YZ contributed in the conception and design of the work, drafting and revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. KS contributed in the conception and design of the work, drafting and revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. HW contributed in the conception and design of the work, drafting and revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work.
Acknowledgments
The study was supported by grants from the National Natural Science Foundation of China (nos. 81100160 and 81470504), the Ministry of Science and Technology, and Beijing Municipal Commission of Science and Technology (No. 7040001).
REFERENCES
- 1.Fan J, Song Y, Chen Y, Hui R, Zhang W. Combined effect of obesity and cardio-metabolic abnormality on the risk of cardiovascular disease: A meta-analysis of prospective cohort studies. Int J Cardiol. 2013;168:4761–8. doi: 10.1016/j.ijcard.2013.07.230. [DOI] [PubMed] [Google Scholar]
- 2.Zile MR, Gottdiener JS, Hetzel SJ, McMurray JJ, Komajda M, McKelvie R, et al. Prevalence and significance of alterations in cardiac structure and function in patients with heart failure and a preserved ejection fraction. Circulation. 2011;124:2491–501. doi: 10.1161/CIRCULATIONAHA.110.011031. [DOI] [PubMed] [Google Scholar]
- 3.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–6. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 4.Burchfiel CM, Skelton TN, Andrew ME, Garrison RJ, Arnett DK, Jones DW, et al. Metabolic syndrome and echocardiographic left ventricular mass in blacks: The atherosclerosis risk in communities (ARIC) study. Circulation. 2005;112:819–27. doi: 10.1161/CIRCULATIONAHA.104.518498. [DOI] [PubMed] [Google Scholar]
- 5.Chinali M, Devereux RB, Howard BV, Roman MJ, Bella JN, Liu JE, et al. Comparison of cardiac structure and function in American Indians with and without the metabolic syndrome (the Strong Heart Study) Am J Cardiol. 2004;93:40–4. doi: 10.1016/j.amjcard.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Nicolini E, Martegani G, Maresca AM, Marchesi C, Dentali F, Lazzarini A, et al. Left ventricular remodeling in patients with metabolic syndrome: Influence of gender. Nutr Metab Cardiovasc Dis. 2013;23:771–5. doi: 10.1016/j.numecd.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Tadic MV, Ivanovic BA, Petrovic M, Celic V, Neskovic A. Gender influence on left ventricular structure and function in metabolic syndrome. Are women at greater risk? J Clin Ultrasound. 2013;41:538–45. doi: 10.1002/jcu.22016. [DOI] [PubMed] [Google Scholar]
- 8.Wang SX, Xue H, Zou YB, Sun K, Fu CY, Wang H, et al. Prevalence and risk factors for left ventricular hypertrophy and left ventricular geometric abnormality in the patients with hypertension among Han Chinese. Chin Med J (Engl) 2012;125:21–6. [PubMed] [Google Scholar]
- 9.Wang W, Kong J, Sun J, Wang CY, Chen HY, Jiang YF, et al. Epidemiological investigation of metabolic syndrome and analysis of relevant factors in north-eastern China. J Int Med Res. 2010;38:150–9. doi: 10.1177/147323001003800117. [DOI] [PubMed] [Google Scholar]
- 10.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Chamber Quantification Writing Group; American Society of Echocardiography's Guidelines and Standards Committee; European Association of Echocardiography. Recommendations for chamber quantification: A report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, et al. Echocardiographic assessment of left ventricular hypertrophy: Comparison to necropsy findings. Am J Cardiol. 1986;57:450–8. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 12.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–67. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 13.Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition. 1989;5:303–13. [PubMed] [Google Scholar]
- 14.Cioffi G, Russo TE, Stefenelli C, Selmi A, Furlanello F, Cramariuc D, et al. Severe obstructive sleep apnea elicits concentric left ventricular geometry. J Hypertens. 2010;28:1074–82. doi: 10.1097/hjh.0b013e328336c90a. [DOI] [PubMed] [Google Scholar]
- 15.Gebker R, Mirelis JG, Jahnke C, Hucko T, Manka R, Hamdan A, et al. Influence of left ventricular hypertrophy and geometry on diagnostic accuracy of wall motion and perfusion magnetic resonance during dobutamine stress. Circ Cardiovasc Imaging. 2010;3:507–14. doi: 10.1161/CIRCIMAGING.109.923672. [DOI] [PubMed] [Google Scholar]
- 16.Ganau A, Devereux RB, Roman MJ, de Simone G, Pickering TG, Saba PS, et al. Patterns of left ventricular hypertrophy and geometric remodeling in essential hypertension. J Am Coll Cardiol. 1992;19:1550–8. doi: 10.1016/0735-1097(92)90617-v. [DOI] [PubMed] [Google Scholar]
- 17.Noda H, Iso H, Saito I, Konishi M, Inoue M, Tsugane S JPHC Study Group. The impact of the metabolic syndrome and its components on the incidence of ischemic heart disease and stroke: The Japan public health center-based study. Hypertens Res. 2009;32:289–98. doi: 10.1038/hr.2009.14. [DOI] [PubMed] [Google Scholar]
- 18.Cerezo C, Segura J, Praga M, Ruilope LM. Guidelines updates in the treatment of obesity or metabolic syndrome and hypertension. Curr Hypertens Rep. 2013;15:196–203. doi: 10.1007/s11906-013-0337-4. [DOI] [PubMed] [Google Scholar]
- 19.Scordo KA. Managing hyperlipidemia. The updated cholesterol treatment guidelines. Nurse Pract. 2014;39:28–33. doi: 10.1097/01.NPR.0000450386.99732.e9. [DOI] [PubMed] [Google Scholar]
- 20.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome – A new world-wide definition. A consensus statement from the International Diabetes Federation. Diabet Med. 2006;23:469–80. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 21.Li WJ, Xue H, Sun K, Song XD, Wang YB, Zhen YS, et al. Cardiovascular risk and prevalence of metabolic syndrome by differing criteria. Chin Med J (Engl) 2008;121:1532–6. [PubMed] [Google Scholar]
- 22.Devereux RB, Dahlof B, Levy D, Pfeffer MA. Comparison of enalapril versus nifedipine to decrease left ventricular hypertrophy in systemic hypertension (the PRESERVE trial) Am J Cardiol. 1996;78:61–5. doi: 10.1016/s0002-9149(96)00228-7. [DOI] [PubMed] [Google Scholar]
- 23.Cuspidi C, Meani S, Valerio C, Fusi V, Sala C, Zanchetti A. Left ventricular hypertrophy and cardiovascular risk stratification: Impact and cost-effectiveness of echocardiography in recently diagnosed essential hypertensives. J Hypertens. 2006;24:1671–7. doi: 10.1097/01.hjh.0000239305.01496.ca. [DOI] [PubMed] [Google Scholar]
- 24.Kutlay S, Dincer I, Sengül S, Nergizoglu G, Duman N, Ertürk S. The long-term behavior and predictors of left ventricular hypertrophy in hemodialysis patients. Am J Kidney Dis. 2006;47:485–92. doi: 10.1053/j.ajkd.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 25.Wang S, Xue H, Zou Y, Sun K, Fu C, Wang H, Hui R. Left ventricular hypertrophy, abnormal ventricular geometry and relative wall thickness are associated with increased risk of stroke in hypertensive patients among the Han Chinese. Hypertens Res. 2014;37:870–4. doi: 10.1038/hr.2014.88. [DOI] [PubMed] [Google Scholar]
- 26.Mule G, Cusimano P, Nardi E, Cottone S, Geraci C, Palermo A, et al. Relationships between metabolic syndrome and left ventricular mass in hypertensive patients: Does sex matter? J Hum Hypertens. 2008;22:788–95. doi: 10.1038/jhh.2008.69. [DOI] [PubMed] [Google Scholar]
- 27.Devereux RB, Casale PN, Wallerson DC, Kligfield P, Hammond IW, Liebson PR, et al. Cost-effectiveness of echocardiography and electrocardiography for detection of left ventricular hypertrophy in patients with systemic hypertension. Hypertension. 1987;9:II69–76. doi: 10.1161/01.hyp.9.2_pt_2.ii69. [DOI] [PubMed] [Google Scholar]


