Abstract
Background:
Considering that establishment of reference value of nuchal translucency (NT)-related to the crown rump length (CRL) during the first trimester will be helpful for determining an appropriate cutoff level for screening of increased NT thickness-related abnormalities, we determined the NT thickness and investigated its relation with different chromosomal and nonchromosomal abnormalities among a large sample size of pregnant Iranian women.
Materials and Methods:
In this analytic cross-sectional study, pregnant women who were in their first trimester were enrolled at their antenatal visit. Using an abdominal ultrasonography, the fetal NT thickness of the studied population was measured. Those with increased NT thickness were determined. The reference value of NT thickness (5th, 25th, 50th, 75th, and 95th percentiles) within each 5-mm range of CRL and during the 11th, 12th, and 13th gestational weeks were determined. The presences of the different chromosomal and nonchromosomal abnormalities were compared in women with different percentiles of NT thickness who underwent amniocentesis and those who did not.
Results:
1,614 pregnant women were evaluated. The mean NT thickness was 1.30 ± 0.54 mm. Increased NT thickness >2 mm and >95th percentile according to their gestational age (GA) was detected in 89 (5.5%) and 58 (3.6%) pregnant women. The reference 95th percentile value range for NT was 1.8-2.35 and increased NT thickness according to our obtained values was associated significantly with chromosomal abnormalities.
Conclusion:
The obtained reference range in our studied population was different from that reported for other ethnic groups and it is suggested that using this values are more favorable for screening of chromosomal abnormalities during the first trimester of pregnancy than the recommended single cutoff value.
Keywords: Crown rump length (CRL), nuchal translucency (NL), reference values
INTRODUCTION
Nuchal translucency (NT) is the normal fluid-filled subcutaneous space between the back of the fetal skin and the overlying skin.[1] NT is visible and can be measured by ultrasonographic imaging between 11 weeks and 14 weeks gestation.[2] Increased NT is associated with different fetal chromosomal and nonchromosomal abnormalities. There is growing evidence that increased NT thickness during the first trimester of pregnancy in a chromosomally normal fetus is associated with numerous fetal structural abnormalities, genetic syndromes, heart defects, and poor perinatal outcomes such as miscarriage and intrauterine death.[3,4,5]
The first definition for increased NT was a measure >95th percentile for a given crown rump length (CRL) and a NT value of 2.5-3 mm, which was reported as a normal range for the marker. Recently, some studies indicated that NT >99th percentile or NT value that exceeds of 3.5 mm are associated with the most common adverse outcomes.[6,7]
The utility of NT as a sensitive and noninvasive ultrasonographic marker for screening and detection of aneuploidies and major structural anomalies in modern obstetrical practice has been demonstrated recently. Its use as a new screening method for the mentioned purposes has been developed in many developed countries.[8,9,10]
Since the introduction of NT thickness, several studies worldwide have determined the normal range of NT in different populations. The results were different regarding the normative value of NT. One of the explanations for the reported great variety of NT thickness range is ethnic variation.[11,12,13,14,15] However, there are still controversies regarding the role of ethnicity on the value of NT. Some reported a significant role of ethnicity in this regard, whereas others did not support the association.[16,17] However, recently the establishment of reference value for NT in different populations was performed. It is suggested that ethnic and region-specific reference value of NT could have a significant impact on its screening efficacy and using a single cutoff for fetal NT could not be an appropriate tool in this field.[18]
So considering that establishment of reference value of NT related to the CRL during the first trimester will be helpful for determining an appropriate cutoff level for screening of increased NT thickness-related abnormalities and the presence of few reports in this field among the Iranian population, in this study we determined the reference values of NT thickness among Isfahani pregnant women to evaluate the role of ethnicity on the normative value of NT as well as the association of increased NT thickness with chromosomal and nonchromosomal abnormalities during the first trimester.
MATERIALS AND METHODS
In this analytic cross-sectional study, pregnant women referred to a private radiology center for ultrsonographic assessment during the antenatal visit in their first trimester were enrolled. The study was performed from January 2013 to December 2013 in Isfahan, Isfahan Province, Iran.
The protocol of the study was approved by the Regional Ethics Committee of Isfahan University of Medical Sciences.
Pregnant women with gestational age (GA) of 11-13 weeks and 6 days and/or CRL 45-84 mm were included.
The pregnant women were selected by the consecutive method. Those who did not agree to have the ultrasonography performed, with multiple pregnancies, fetal malformation, and those with inappropriate cooperation were excluded. Written informed consent was obtained from all the selected participants. The selected pregnant women underwent abdominal ultrasonography. The sonography was performed by an expert radiologist. The fetal NT thickness of the studied population was measured.
Those with NT thickness of 2 mm were considered as women with increased NT thickness.[19]
The mean of CRL and GA were compared in women with and without increased NT thickness.
The reference value of NT thickness (5th, 25th, 50th, 75th, and 95th percentiles) within each 5-mm range of CRL and during the 11th, 12th, and 13th gestational weeks were determined.
Women with NT thickness of >95th percentile were determined. The women were followed up and fetal outcomes were evaluated by the neonatologists at birth. The presence of different chromosomal abnormalities as well as nonchromosomal abnormalities including cardiac malformation, genitourinary or renal abnormalities, diaphragmatic hernia, spontaneous miscarriage, and intrauterine fetal death (IUFD) were compared in women with different percentiles of NT thickness who underwent amniocentesis and those who did not.
Ultrasonographic measurements
The ultrasonographic measurements were performed in pregnant women in a supine position.
Fetal CRL and NT thickness measurements were performed by transabdominal ultrsonography using a multi fz: 3.5 MHz tranduser (GE Volusun 730). The measurement was performed based on the criteria recommended by the Fetal Medicine Foundation (FMF).[20] According to the criteria, the fetus should be in a neutral position, with the head aligned with the spine in a way that fetus occupied at least 75% of the image. NT was defined as the black area between the inner skin outlines echo and the outer border of the soft tissue overlying the cervical spine.
The maximal thickness of the black area was measured with caliper placed on the inner borders of the NT space, perpendicular to the long axis of the fetus when a sagittal section with a neutral position of the fetus was obtained. The measurements were recorded to the nearest 0.1-mm interval. At least three NK measurements were taken and the largest was recorded.
CRL was measured at the same time and recorded.
Statistical analysis
Data were analyzed using Statistical Package for the Social Sciences (SPSS) version 21 (SPSS Inc., Chicago, IL, USA). Using regression equation, the expected 5th, 25th, 50th, 75th, and 5th percentile values of NT thickness according to the CRL categories of CRL (5-mm interval) and GA (11th, 12th, and 13th weeks) were obtained. Quantitive and qualitative values were compared using the t-test and chi-square test, respectively. P value of <0.05 was considered to be statistically significant.
RESULTS
During this study, 1,614 pregnant women were evaluated. Among the studied pregnant women 382 (23.7%), 871 (54.0%), and 361 (22.4) were in the 11th, 12th, and 13th gestational week. The mean of GA, CRL, and NT thickness in the studied population were 12.46 ± 0.62 weeks, 59.35 ± 8.35 mm, and 1.30 ± 0.54 mm, respectively. Pearson correlation test indicated that there was a significant positive correlation between NT and CRL (r = 0.238, P < 0.001), NT and GA (r = 0.24, P < 0.001 and GA) and CRL (r = 0.8, P < 0.001).
Increased NT thickness (NT >2 mm) was detected in 89 (5.5%) pregnant women. The mean of CRL and GA in pregnant women with normal and increased NT thickness are presented in Table 1.
Table 1.
Mean ± SD of CRL and GA in pregnant women with normal and increased NT thickness

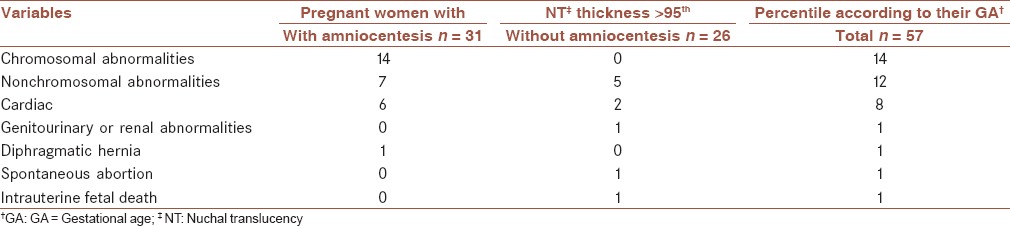
The expected 5th, 25th, 50th, 75th, and 95th percentile values of NT thickness to CRL and GA are listed in Tables 2 and 3. Using the obtained reference value of NT, 58 (3.6%) pregnant women were determined as those with NT thickness >95th percentile according to their GA. During follow-up, 31/58 (53.4%) underwent amniocentesis. Distribution of chromosomal and nonchromosomal abnormalities in pregnant women with NT thickness >95th percentile according to their GA in total and among those with and without amniocentesis are presented in Table 4. Frequency of chromosomal abnormalities were significantly higher in those pregnant women with increased NT thickness who underwent the amniocentesis procedure (P = 0.001). The frequency of different nonchromosomal abnormalities were not significantly different between the two studied groups (P > 0.05).
Table 2.
The expected 5th, 25th, 50th, 75th, and 95th percentile values of NT thickness (mm) to CRL
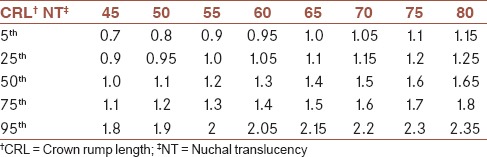
Table 3.
The expected 5th, 25th, 50th, 75th, and 95th percentile values of NT thickness (mm) to gestational age (GA)
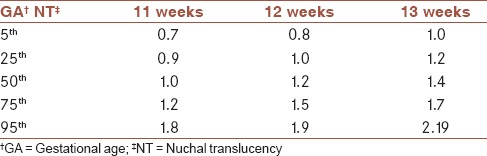
Table 4.
Distribution of chromosomal and nonchromosomal abnormalities in pregnant women with NT thickness >95th percentile according to their GA in total and among those with and without amniocentesis
DISCUSSION
In this study, we determined the reference values of NT thickness among pregnant Isfahani women to evaluate the role of ethnicity on the normative value of NT as well as the association of increased NT thickness with chromosomal and nonchromosomal abnormalities during the first trimester. The results indicated that the reference 95th percentile value range for NT was 1.8-2.35 and increased NT thickness according to our obtained values was associated significantly with chromosomal abnormalities.
Several reports from different parts of the worlds and Iran have demonstrated the utility of NK measurement for screening different chromosomal and nonchromosomal abnormalities.[20,21,22,23]
Most of the studies have used the recommended definition for NT thickness by the FMF (i.e., 2.5-3 mm),[6] whereas recent studies reported that using NT thickness as a continuous variable was more appropriate than using a single cutoff value for the fetal NT and consequently, the outcomes of its increased values and screening programs.[18] So, establishment of reference values of NT have been developed in different regions and ethnic groups worldwide.
Though there were studies in Iran, which investigated the association between increased NT value and Down syndrome[22] and adverse pregnancy outcome including miscarriage, fetal loss, and fetal abnormalities,[23] there was not any study, which reported the normative value of NT thickness for the Iranian population. So, this study was designed to determine the ethnic specific reference value of NT thickness for pregnant Iranian women. Our results indicated that the median NT thicknesses for a CRL between 45 mm and 80 mm ranged from 1.00 to 1.65 mm, and the 95th percentiles ranged from 1.8 to 2.35 mm. The median NT thickness for GA were 1.0 mm, 1.2 mm, and 1.4 mm for gestational age of 11 weeks, 12 weeks, and 13 weeks, respectively, and the 95th percentiles of NT thickness were 1.8, 1.9, and 2.2 for gestational age of 11 weeks, 12 weeks, and 13 weeks, respectively.
The distribution of the NT thickness for CRL has been reported in many studies. The median NT thicknesses has been reported to be 1.2-1.9 mm, 1.22-2.10 mm, and 1.19-1.73 mm for a CRL between 45 mm and 80 mm in Japan, Korea, and Brazil, respectively.[11,12,13] Our reported median value was lower than the other reports.
The 95th NT thickness percentiles have been reported to be 2.1-3.2 mm, 2.14-2.3 mm, 1.57-2.10 mm, 1.00-2.90 mm, and 1.84-2.35 mm for a CRL between 45 mm and 80 mm in Japan, Korea, Brazil, Thailand, and China, respectively.[11,12,13,14,15] Our results were similar to the reported reference value range of Brazil.[13] Although there was no report from the Eastern Mediterranean region in this field, the values were not similar to the values reported from the Asian countries.
Reported variations in the index measurements in the different studies might have been due to factors such as radiologist experience, quality of the ultrasound, method of measurement, and an inappropriate fetal and nuchal cord position. In addition, as mentioned by Kor-anantaku et al. in Thailand some investigators have considered the average of two or three measurements of NT thickness, whereas others considered the largest measurement.[14]
There are controversial reports regarding the impact of ethnicity on NT thickness values and its utility for screening. Thilaganathan et al. have investigated the possible role of ethnicity on NT screening and concluded that the reported differences could not have a significant impact in this regard.[24] Many other studies have also showed that ethnic differences in NT measurements are not clinically significant, especially when it used for screening of Down syndrome.[17,24,25] However, it seems that using ethnic-specific reference values of NT thickness could help us in the first trimester screening programs mainly for chromosomal abnormality, especially when they are integrated with other ultrasonographic and biochemical measurements.
In this study using the single cutoff value of 2 mm, 5.5% of the studied pregnant women were considered to have high-risk pregnancy and after using our obtained reference value the rate decreased to 3.6%. Thus, it seems that using normative values of NT thickness is more useful for the first trimester screening and it could optimize the screening results by reducing false positive cases.
In addition, there was significant association between performing the amniocentesis procedure and detection of chromosomal abnormalities among women with increased NT thickness.
The advantage of the current study was a larger sample size of enrolled pregnant women.
The limitation of the current study was that we did not determine the sex-specific reference value of 95th percentiles of NT and its association with both chromosomal and nonchromosomal abnormalities. We followed up only pregnant women with increased NT thickness and did not determine the frequency of the mentioned abnormalities in pregnant women with normal NT. It was due to the reason that follow-up of that large a sample size was not assessable in the framework of the current study. In addition, we enrolled the patients who were referred to a single referral radiologic center, which could not be a representative sample of the whole population. It is suggested that the large sample size of the studied population could partially alleviate the abovementioned limitation.
Further, the planning of further studies that also determine the 99th percentile values of NT thickness is recommended because recent studies demonstrated that chromosomal and nonchromosomal abnormalities are mainly associated with the 99th percentile value of NT thickness.[7]
The results of our study indicated the reference value of NT thickness in a large sample size of Isfahani pregnant women. The obtained reference range in our studied population was different from that reported for other ethnic groups and it is suggested that using this values are more favorable for screening of chromosomal abnormalities during the first trimester of pregnancy than the recommended single cutoff value. The relation between increased NT thicknesses with chromosomal abnormalities also confirms its utility. The results of the current study could be used as baseline information for other follow-up studies and designing first trimester screening programs.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
AUTHOR'S CONTRIBUTION
All authors contributed in the conception of the work, conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work.
Acknowledgments
The study was supported by Isfahan University of Medical Sciences (research project number; 393486).
REFERENCES
- 1.Nicolaides KH, Azar G, Byrne D, Mansur C, Marks K. Fetal nuchal translucency: Ultrasound screening for chromosomal defects in first trimester of pregnancy. BMJ. 1992;304:867–9. doi: 10.1136/bmj.304.6831.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Szabó J, Gellén J. Nuchal fluid accumulation in trisomy-21 detected by vaginosonography in first trimester. Lancet. 1990;336:1133. doi: 10.1016/0140-6736(90)92614-n. [DOI] [PubMed] [Google Scholar]
- 3.Vogel M, Sharland GK, McElhinney DB, Zidere V, Simpson JM, Miller OI, et al. Prevalence of increased nuchal translucency in fetuses with congenital cardiac disease and a normal karyotype. Cardiol Young. 2009;19:441–5. doi: 10.1017/S1047951109990655. [DOI] [PubMed] [Google Scholar]
- 4.Bilardo CM, Müller MA, Pajkrt E, Clur SA, van Zalen MM, Bijlsma EK. Increased nuchal translucency thickness and normal karyotype: Time for parental reassurance. Ultrasound Obstet Gynecol. 2007;30:11–8. doi: 10.1002/uog.4044. [DOI] [PubMed] [Google Scholar]
- 5.Saldanha FA, Brizot Mde L, Moraes EA, Lopes LM, Zugaib M. Increased fetal nuchal translucency thickness and normal karyotype: Prenatal and postnatal follow-up. Rev Assoc Med Bras. 2009;55:575–80. doi: 10.1590/s0104-42302009000500022. [DOI] [PubMed] [Google Scholar]
- 6.Salman Guraya S. The associations of nuchal translucency and fetal abnormalities; significance and implications. J Clin Diagn Res. 2013;7:936–41. doi: 10.7860/JCDR/2013/5888.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Souka AP, Von Kaisenberg CS, Hyett JA, Sonek JD, Nicolaides KH. Increased nuchal translucency with normal karyotype. Am J Obstet Gynecol. 2005;192:1005–21. doi: 10.1016/j.ajog.2004.12.093. [DOI] [PubMed] [Google Scholar]
- 8.Stefanovic V, Äyräs O, Eronen M, Paavonen1 J, Tikkanen M. Clinical utility of nuchal translucency screening. Res Rep Neonatol. 2014;4:169–76. [Google Scholar]
- 9.Snijders RJ, Noble P, Sebire N, Souka A, Nicolaides KH. UK Multicentre Project on assessment of risk of trisomy 21 by maternal age and fetal nuchal-translucency thickness at 10-14 weeks of gestation. Fetal Medicine Foundation First Trimester Screening Group. Lancet. 1998;352:343–6. doi: 10.1016/s0140-6736(97)11280-6. [DOI] [PubMed] [Google Scholar]
- 10.Miron P, Côté YP, Lambert J. Nuchal translucency thresholds in prenatal screening for down syndrome and trisomy 18. J Obstet Gynaecol Can. 2009;31:227–35. doi: 10.1016/S1701-2163(16)34121-4. [DOI] [PubMed] [Google Scholar]
- 11.Hasegawa J, Nakamura M, Hamada S, Matsuoka R, Ichizuka K, Sekizawa A, et al. Distribution of nuchal translucency thickness in Japanese fetuses. J Obstet Gynaecol Res. 2013;39:766–9. doi: 10.1111/j.1447-0756.2012.02037.x. [DOI] [PubMed] [Google Scholar]
- 12.Chung JH, Yang JH, Song MJ, Cho JY, Lee YH, Park SY, et al. The distribution of fetal nuchal translucency thickness in normal Korean fetuses. J Korean Med Sci. 2004;19:32–6. doi: 10.3346/jkms.2004.19.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Araujo Júnior E, Pires CR, Martins WP, Nardozza LM, Filho SM. Reference values of nuchal translucency thickness in a Brazilian population sample: Experience from a single center. J Perinat Med. 2014;42:255–9. doi: 10.1515/jpm-2013-0141. [DOI] [PubMed] [Google Scholar]
- 14.Kor-Anantakul O, Suntharasaj T, Suwanrath C, Chanprapaph P, Sirichotiyakul S, Ratanasiri T, et al. Distribution of normal nuchal translucency thickness: A multicenter study in Thailand. Gynecol Obstet Invest. 2011;71:124–8. doi: 10.1159/000320754. [DOI] [PubMed] [Google Scholar]
- 15.Sun Q, Xu J, Hu SQ, Chen M, Ma RM, Lau TK, et al. Distribution and normal reference range of fetal nuchal translucency thickness in Kunming pregnant women in the first trimester. Zhonghua Fu Chan Ke Za Zhi. 2012;47:514–7. [PubMed] [Google Scholar]
- 16.Huang T, Wang F, Boucher K, O’Donnell A, Rashid S, Summers AM. Racial differences in first trimester nuchal translucency. Prenat Diagn. 2007;27:1174–6. doi: 10.1002/pd.1866. [DOI] [PubMed] [Google Scholar]
- 17.Chen M, Lam YH, Tang MH, Lee CP, Sin SY, Tang R, et al. The effect of ethnic origin on nuchal translucency at 10-14 weeks of gestation. Prenat Diagn. 2002;22:576–8. doi: 10.1002/pd.363. [DOI] [PubMed] [Google Scholar]
- 18.Taipale P, Hiilesmaa V, Salonen R, Ylöstalo P. Increased nuchal translacency as a marker for fetal chromosomal defects. N Engl J Med. 1997;337:1654–8. doi: 10.1056/NEJM199712043372303. [DOI] [PubMed] [Google Scholar]
- 19.Kim SM, Jun JK. Simplified protocol of nuchal translucency measurement: Is it still effective? Obstet Gynecol Sci. 2013;56:307–11. doi: 10.5468/ogs.2013.56.5.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kagan KO, Avgidou K, Molina FS, Gajewska K, Nicolaides KH. Relation between increased fetal nuchal translucency thickness and chromosomal defects. Obstet Gynecol. 2006;107:6–10. doi: 10.1097/01.AOG.0000191301.63871.c6. [DOI] [PubMed] [Google Scholar]
- 21.Barati M, Zargar M, Masihi S, Taherpour S. Evaluation of nuchal translucency measurement in first trimester pregnancy. Int J Fertil Steril. 2011;5:35–8. [PMC free article] [PubMed] [Google Scholar]
- 22.Elahifar MA, Hasanzadeh M, Dahmardeh H, Elahifar A. The relationship between thickness of nuchal translucency and Down syndrome in the first trimester of pregnancy. Zahedan J Res Med Sci. 2012;14:26–8. [Google Scholar]
- 23.Tahmasebpour A, Rafiee NB, Ghaffari S, Jamal A. Increased nuchal translucency and pregnancy outcome. Iran J Public Health. 2012;41:92–7. [PMC free article] [PubMed] [Google Scholar]
- 24.Thilaganathan B, Khare M, Williams B, Wathen NC. Influence of ethnic origin on nuchal translucency screening for Down's syndrome. Ultrasound Obstet Gynecol. 1998;12:112–4. doi: 10.1046/j.1469-0705.1998.12020112.x. [DOI] [PubMed] [Google Scholar]
- 25.Hsu JJ, Hsieh CC, Chiang CH, Lo LM, Hsieh TT. Preliminary normal reference values of nuchal translucency thickness in Taiwanese fetuses at 11-14 weeks of gestation. Chang Gung Med J. 2003;26:12–9. [PubMed] [Google Scholar]


