Abstract
Background:
Colorectal cancer (CRC) is the second cause of cancer-related deaths worldwide. There have been several studies reporting the proximal tumor shift, especially in Western countries. In the present study, we investigated the clinicopathologic and anatomical distributions of colorectal tumors in Iranian CRC patients.
Materials and Methods:
In this retrospective cohort study, 258 patients with CRC from 2008 to 2013 were evaluated. Comparison of variables was performed using Pearson's chi-square test and Fisher's exact test depending on the nature of the data.
Results:
A total of 258 patients including 124 (48.1%) females and 134 (51.9%) males enrolled in this study. The majority of cancers were detected in the rectosigmoid, i.e., 98 (38%) followed by the left colon, i.e., 84 (32.6%) and the right colon, i.e., 76 (29.5%). In the present study, we observed the significant association between metastases, adjuvant therapy, family history, and history of inflammatory bowel disease (IBD) with tumor, node, and metastasis (TNM) staging (P < 0.001). In univariate analysis, there was a strong association between overall survival (OS) and stage II CRC (P = 0.03). However, the predictive value was lost in multivariate analysis (P = 0.145).
Conclusion:
Unlike the majority of previous studies on Iranian CRC patients, we observed a considerably higher occurrence of right-sided colon cancers (84 versus 76). Although this phenomenon did not reach the statistical significance rate, based on recent studies on Iranian population including the present one, the pattern of anatomical distribution of colorectal tumors has been changed toward the proximal colon. This requires an urgent need to provide other strategies and complementary detecting approaches in order to identify proximal tumors in Iranian CRC patients.
Keywords: Colorectal cancer (CRC), Iran, proximal shift, survival
INTRODUCTION
Colorectal cancer (CRC) is the third most common cancer in the USA and the second cause leading to cancer deaths although intense screening programs have declined the incidence rate.[1,2] The majority of cases (90%) were above 50 years of age in both the genders.[3] It has been demonstrated that approximately 1.23 million cases are detected each year worldwide.[4] Apart from age having a first-degree relative diagnosed with CRC, physical inactivity and overweight are the other main risk factors.[5,6] Adenomatous polyps are considered to be precursors of the majority of CRCs both in the hereditary and sporadic types.[7] Diagnosis of CRC in the early stages is fundamental for further management and if the case is presented with metastasis, the survival will be lesser than 10%.[8] Colonoscopy is considered as a standard goal to identify adenomatous polyps or other suspicious legions in CRC.[9,10] However, the specificity and sensitivity of this technique for the detection of right-sided colon tumors (proximal region) is low and controversial. This requires the application of other modalities apart from colonoscopy in order to identify premalignant tumors located at the right side of the colon. Based on the National Cancer Institute report, between the early 1970s and late 1990s there was a 6% increase in the rate of proximal colon cancers than left tumors.[2] The “left-to-right shift” model in CRC primary was reported in epidemiological studies in the late 1970s.[11] Several studies thereafter have indicated a proximal shift of tumors in different ethnic groups.[2,12,13] Several studies in the USA indicated that approximately 50% of proximal tumors belong to the elderly population.[14,15,16] Therefore, proximal tumors might also imply an aging population. In addition, it has been demonstrated that tumors in the proximal region have distinct pathologic, molecular features and different treatment outcome compared to distal lesions. So an understanding of the pattern of anatomical distribution of the tumors in every ethnic group would help in proceeding with the appropriate medical approach for each patient. In this study, we investigated the clinicopathologic features and anatomical distribution of CRC tumors in Iranian CRC patients.
MATERIALS AND METHODS
In this retrospective cohort study, 275 patients with pathologically documented CRC who referred to the Gastroenterology and Liver Diseases Research Center, Shahid Beheshti University of Medical Sciences from 2008 to 2013 enrolled in this study. Patients with hereditary nonpolyposis colorectal cancer (HNPCC) and polyposis syndromes including familial adenomatous polyposis were excluded from the study. Demographic data including the age at diagnosis, gender, tumor location, pathological type of tumor (grade and stage of tumor), chemotherapy history, anemia, history of inflammatory bowel diseases (IBD), family history of CRC and diabetes, metastasis status in the regional lymph node, and location of CRC metastasis in case of presentation were recorded. The TNM staging system was applied to determine the severity of disease and the local or distant extent of disease spread. The TNM staging system of the American Joint Committee on Cancer (AJCC) is the preferred and standard staging system for CRC. Written informed consent was taken from patients and the local ethics committee approved the study protocol, which was in accordance with the principles of the Helsinki Declaration. All subjects were Iranian and genetically unrelated. Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) 15.0 statistical package (Chicago, IL, USA). Comparison of variables was performed using Pearson's chi-square test, Fisher's exact test, or the Mann-Whitney U test, depending on the nature of the data. Relationships among the clinicopathologic factors were analyzed using the chi-square test. For survival analyses, the following variables were evaluated: Age, tumor location, sex, tumor-node-metastasis stage, and grade of differentiation (well/moderate versus poor), history of chemotherapy, diagnosis age, family history, and microsatellite instability (MSI). Overall survival (OS) analyses were done through a Cox proportional hazard function for both univariate and multivariate analyses, and Kaplan-Meier (log-rank test) curves were plotted. Significance for all statistics were recorded if P < 0.05. OS was defined as the time from histopathological diagnosis to death from any cause. Patients were followed up until September 2013. Patients who died due to reasons unrelated to CRC were censored at the time of death and were excluded from the analysis.
RESULTS
A total of 258 CRC patients including 124 (48.1%) females and 134 (51.9%) males were enrolled in this retrospective cohort study. The characteristics of the patients enrolled in this study are present in Table 1. The mean age of the participants was 56.4 ± 16 years. As it is shown in Table 1, the majority of cancers were detected in the rectosigmoid, i.e., 98 (38%) followed by the left colon, i.e., 84 (32.6%) and the right colon, i.e., 76 (29.5%). In the present study, the anatomical distribution of tumors was similar in left- and right-sided colons. However, we did not observe any significant difference between the right- and the left-sided cancers with respect to gender, age at diagnosis, stage, and grade of tumor. Among 124 women with colon cancer, the majority of the cases, i.e., 35 (28.2%) were right-sided, whereas in 134 males left-sided colon cancer was dominant in 53 (39.6) cases. In the present study, most of the cases were aged above 50 years, i.e., 180 (69.7%) and only 30.3% cases were aged under 50 years. The majority of the patients 126 (48.8%) in this study were in stage II. However, only 20 (7.7%) cases were diagnosed in stage IV. In terms of differentiation most of the tumors, i.e., 111 (43.0%) were well-differentiated and 51 (19.8%) were poorly differentiated. The most common sites of CRC metastasis were the liver, i.e., 31 (62%) followed by the ovary, i.e., 11 (22%). In eight cases, other metastatic organs were involved. According to our findings, family history of CRC was detected in 73 (28.3%) cases. The clinicopathologic features of the study population according to differentiation are presented in Table 2. Based on our findings, there was a significant association between TNM stage and the differentiation status (P < 0.001). In 126 stage II cases, 64 (50.8%) were well-differentiated and only 17 (13.5%) were poorly differentiated, which are present in Table 2. We also observed a significant association between the tumor location and differentiation status (P < 0.001). Among 98 cases in the rectosigmoid, 38 (38.8) were moderately differentiated, 18 (18.4) were poorly differentiated, and 37 (37.8) were well-differentiated [Table 3]. The clinicopathologic features of the study population according to TNM staging are presented in Table 4. The significant association was detected among the variables including metastases, adjuvant therapy, family history, and history of IBD with TNM staging P < 0.001. In Table 5, the clinicopathologic features of the study population according to location status are presented and in Table 6 the clinicopathologic features of the study population according to chemotherapy status are presented. According to Table 6, we found a significant association between TNM staging and chemotherapy status and among 85 patients in stage III, 77 (90.6%) received chemotherapy (P < 0.001). In this study, we evaluated the 5-year OS based on the clinical outcome available. According to our findings, pathologic tumor stages did not have association with survival (P = 0.05); similar to TNM staging; chemotherapy, family history, tumor location, and differentiation also showed no significant relationship with survival (P > 0.05). Based on survival curves, patients with stages II and III had poorer survival [Figure 1]. However, we observed rather a similar survival for patients with stages II and III CRC (P = 0.05). Kaplan-Meier curves for OS of patients according to differentiation status revealed that poorly differentiated tumors had a poorer survival rate compared with well-differentiated and moderately differentiated tumors; however, the result was did not reach a significant rate (log rank P = 0.06, Figure 2). We also observed that patients older than 44 years of age had a poorer OS rate than younger patients (P = 0.02). In patients with a younger age at diagnosis of CRC (<44), there was a better OS than older patients; however, the difference did not reach statistical significance (P = 0.16). All results for univariate and multivariate analyses are shown in Table 7. Multivariate analysis was performed to identify factors with independent prognostic significance and to calculate hazard ratios (HRs). The analysis included tumor location, TNM stage, differentiation, and mucinous characteristics. In univariate analysis, there was a strong association between OS and stage II CRC (P = 0.03). However, the predictive value was lost in multivariate analysis.
Table 1.
Clinical characteristics of patients with CRC

Table 2.
TNM staging in patients with CRC
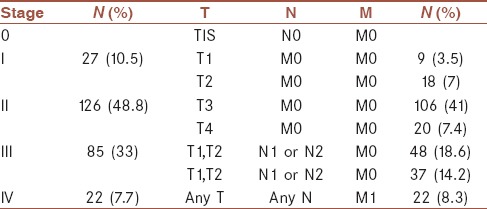
Table 3.
Clinicopathologic features of the study population according to differentiation
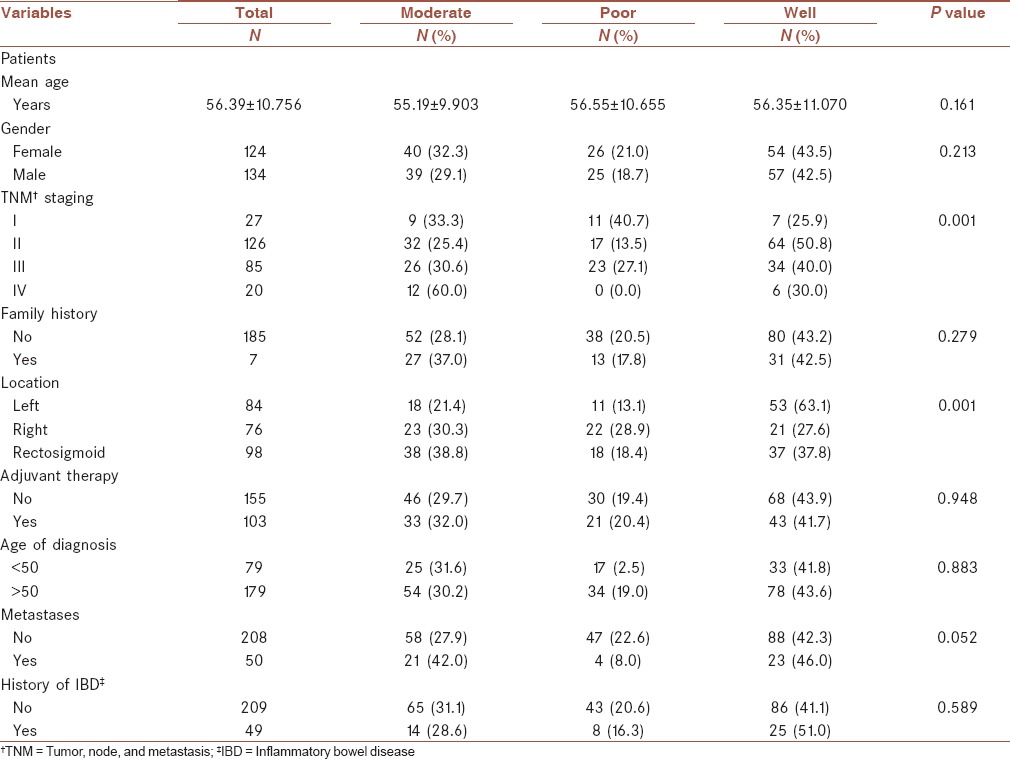
Table 4.
Clinicopathologic features of the study population according to TNM staging

Table 5.
Clinicopathologic features of the study population according to location status
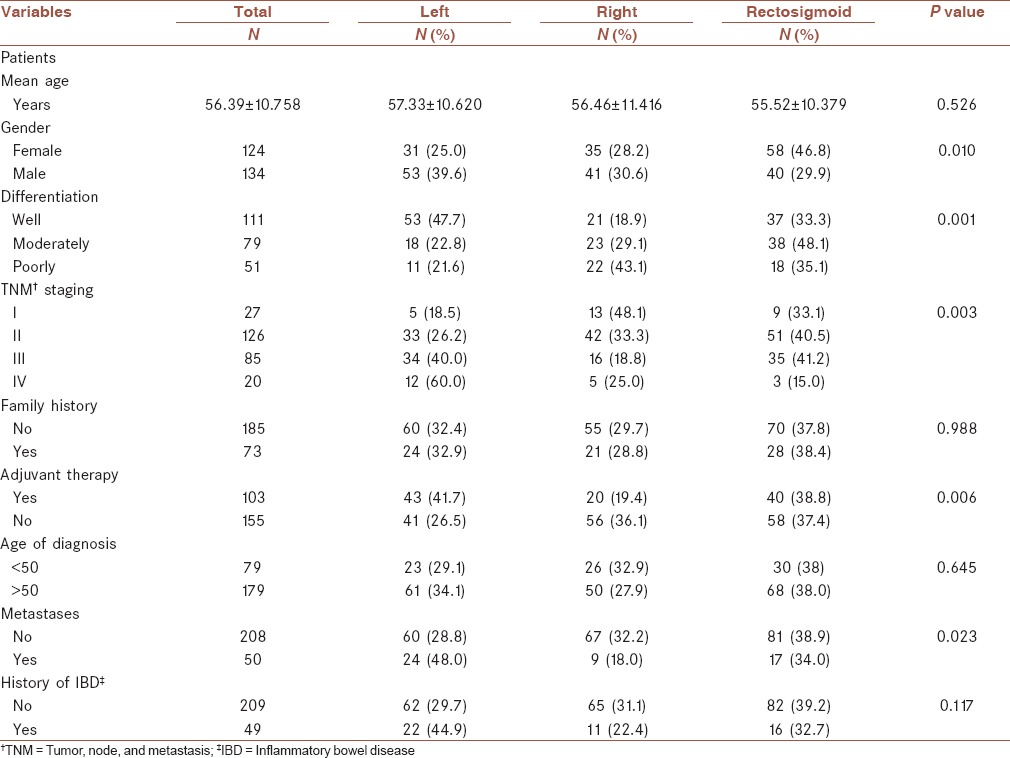
Table 6.
Clinicopathologic features of the study population according to chemotherapy status

Figure 1.

Kaplan-Meier curves of overall survival in colorectal cancer patients according to the stages (n = 258). The survival curves showed that patients with stages II and III had poorer survival. However, we observed rather the similar survival curves for patients with stages II and III CRC. Log-rank P = 0.05
Figure 2.

Kaplan-Meier curves of overall survival of patients according to differentiation status. While the poorly differentiated tumors had a poorer survival rate compared with well-differentiated and moderately differentiated tumors, the result was not reach a significant rate. Log-rank P = 0.06
Table 7.
Univariate and multivariate analysis for clinical variables in this study

DISCUSSION
CRC is considered as the third and the fourth most common cancer in men and women, respectively, in the Iranian population.[17] It has been reported that 5,000 new cases of CRC are diagnosed each year.[17] In this regard, there have been several studies that evaluated the characteristics of CRC patients in Iran.[18,19,20,21] The detection rate of CRC via colonoscopy is approximately 85% in distal, whereas it accounts for 0% to 55% of proximal colon cancers.[22,23,24] It has been demonstrated that distal and proximal colorectal lesions harbor distinct molecular and clinical characteristics.[25,26,27,28] All these differences between the two sites are primarily due to embryonic tissue where they originate and the lifestyle and habits of individuals.[25,29] Previous studies revealed that in comparison to left-sided colon cancers, right-sided tumors mostly occur at an older age and in the female gender, present with advanced stages, and have increased tumor sizes with poorly differentiated features, poorer prognosis, and a larger amount of positive lymph nodes.[15,27,30,31] In the molecular pattern, tumors mostly present with MSI and CpG island methylator phenotype (CIMP).[26] The immunology of the proximal tumors is also different in comparison to the distal colon. It has been noted that intraepithelial T-cells in the proximal colon is higher than the distal colorectum in healthy individuals.[32,33] In this regard, some factors are reported to increase the risk of developing proximal tumors including the intake of high fat,[34] whereas in the distal colon, low consumption of fruits and vegetables and high meat and protein consumptions are the main contributors.[35,36] Anatomical site of tumors has a peculiar epidemiology, pathogenesis, molecular features.[37,38,39] Identification of the dynamic shift of CRC tumors in population would shed light on better screening and management of patients as the proximal colon cancers raise the challenge due to limitations in screening technics.[40] In the present study, the anatomical distribution of tumors was similar in left- and right-sided colons (32.6% versus 29.5%) and we did not observe any significant difference between the right- and the left-sided cancers with respect to gender, age at diagnosis, stage, and grade of tumor in the Iranian population. In consistennce with our study, several other studies in Asia have observed no significant anatomical distribution.[41,42,43] In line with our study in the Iranian population, Bafandeh et al. also revealed no difference in the age of diagnosis and right-sided or left-sided tumors.[44] Several studies on Iranian CRC cases revealed that the majority of tumors are located on the left side of the colon.[42,45,46] Omranipour et al. study on 442 CRC patients including 157 (35.5%) colon cancers and 285 (64.5%) rectal cancers demonstrated that 43.3% of the colon cancers were located on the right side and 56.7% were left-sided. However, Omranipour et al. did not find any statistically significant increase rate in right-sided cancer during the period of 15 years.[47] In contrast, Mahmodlou et al. reported a significant number of tumors located in the right colon, i.e., 192 (35%) followed by the left colon, i.e., 110 (20%).[48] In other ethnic groups Weiss et al. found that within stage II, CRC patients with proximal tumors had lower mortality; however, patients with stage III distal tumors had a higher mortality rate.[27] Proximal tumors have gender specific features, which seem to be related to the age of patients. In a study by Yuuki Iida et al., they reported that female CRC patients presented with a higher number of proximal tumors than distal tumors. They revealed that the right-sided shift elevated with increasing age (P < .0001).[49] In the present study, we also evaluated the characteristics of CRC patients and the association of these clinicopathologic variables with the survival status. We observed that most of the cases were in stage II, i.e., 126 (48.8%). This is in contrast to a previous study by Mahmodlou et al. who reported that the most Iranian CRC patients were in stages III and IV (57%).[48] Based on survival curves, patients with stages II and III had poorer survival. However, we observed rather the similar survival curves for patients with stages II and III CRC (P = 0.05). In our study, most of the cases were aged above 50 years, i.e., 180 (69.7%) and only 78 (30.3) cases were younger than 50 years. We observed that patients aged older than 44 years had poorer OS rate than younger patients (P = 0.02). When we evaluated the 5-year OS based on the clinical outcome available, we did not observe a significant association between tumor stages and survival (P = 0.05); in addition, other factors including chemotherapy, family history, tumor location, and differentiation showed no significant relationship with survival as well (P > 0.05). In univariate analysis, there was a strong association between OS and stage II CRC (P = 0.03). However, the predictive value was lost in multivariate analysis. In line with our study, Hermann Brenner in 2012 evaluated the 5-year relative survival for European CRC patients with regard to age, stage at diagnosis, and location. They reported that the survival rate increased in all European regions over time and this rate was more remarkable in younger cases than in older patients, for earlier than for more advanced cancer stages, and also for rectum cancer than for colon cancer.[50] In another study on the Iranian population, Moradi et al. reported that the worst survival rate was detected in young patients (aged less than 20 years) and in patients aged more than 80 years. They revealed that the 5-year OS in Iranian CRC patients was 41% (45% for female and 39% for men).[20] Another valuable paper by Zuli Yang et al. evaluated demographic data and prognosis in a series of CRC patients aged 44 years and below.[51] They found that in comparison to older patients, this group of patients had larger tumors, poorly differentiated, infiltrative growth type, mucinous, and signet-ring cell adenocarcinoma, and they mostly had advanced TNM stages. They reported that histological grade, TNM stage, and recurrence were considered as independent factors related to survival in the younger group.
CONCLUSION
Unlike the majority of previous studies on Iranian CRC patients, which indicated the left-sided predominance of colorectal tumors, in this study we observed the higher occurrence of right-sided colon cancers. However, this phenomenon did not reach the statistical significance rate. According to recent cohort studies on the Iranian population including the present one, the pattern of anatomical distribution of colorectal tumors has been following the Western populations. This might have been due to several environmental and lifestyle factors, which contributed to this anatomical shift. The differences in genetic and molecular pathologic profiles in each side of the colon and the fact that colonoscopy alone fails to identify all the proximal lesions calls for an urgent need to provide other strategies and complementary detecting approaches in order to identify proximal tumors in Iranian CRC patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
AUTHOR'S CONTRIBUTION
All authors contributed in the conception of the work, conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work.
Acknowledgements
We thank all patients and their families who participated in this study. This study was accomplished at the Gastroenterology and Liver Diseases Research Center, Research Institute for Gastroenterology and Liver Diseases, Shahid Beheshti University of Medical Sciences, Tehran, Tehran Province, Iran with research project code number 681.
REFERENCES
- 1.Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220–41. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 2.Cucino C, Buchner AM, Sonnenberg A. Continued rightward shift of colorectal cancer. Dis Colon Rectum. 2002;45:1035–40. doi: 10.1007/s10350-004-6356-0. [DOI] [PubMed] [Google Scholar]
- 3.Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W. SEER Cancer Statistics Review, 1975-2008. Bethesda, MD: National Cancer Institute; 2011. p. 19. [Google Scholar]
- 4.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 5.Brändstedt J, Wangefjord S, Nodin B, Gaber A, Manjer J, Jirström K. Gender, anthropometric factors and risk of colorectal cancer with particular reference to tumour location and TNM stage: A cohort study. Biol Sex Differ. 2012;3:23. doi: 10.1186/2042-6410-3-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyle T, Keegel T, Bull F, Heyworth J, Fritschi L. Physical activity and risks of proximal and distal colon cancers: A systematic review and meta-analysis. J Natl Cancer Inst. 2012;104:1548–61. doi: 10.1093/jnci/djs354. [DOI] [PubMed] [Google Scholar]
- 7.Lindgren G, Liljegren A, Jaramillo E, Rubio C, Lindblom A. Adenoma prevalence and cancer risk in familial non-polyposis colorectal cancer. Gut. 2002;50:228–34. doi: 10.1136/gut.50.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ries LA, Melbert D, Krapcho M, Stinchcomb D, Howlader N, Horner M, et al. SEER Cancer Statistics Review, 1975-2005. Bethesda, MD: National Cancer Institute; 2008. pp. 1975–2005. [Google Scholar]
- 9.Quintero E, Castells A, Bujanda L, Cubiella J, Salas D, Lanas Á, et al. Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N Engl J Med. 2012;366:697–706. doi: 10.1056/NEJMoa1108895. [DOI] [PubMed] [Google Scholar]
- 10.Segnan N, Senore C, Andreoni B, Azzoni A, Bisanti L, Cardelli A, et al. SCORE3 Working Group-Italy. Comparing attendance and detection rate of colonoscopy with sigmoidoscopy and FIT for colorectal cancer screening. Gastroenterology. 2007;132:2304–12. doi: 10.1053/j.gastro.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 11.Beart RW, Melton LJ, 3rd, Maruta M, Dockerty MB, Frydenberg HB, O’Fallon WM. Trends in right and left-sided colon cancer. Dis Colon Rectum. 1983;26:393–8. doi: 10.1007/BF02553382. [DOI] [PubMed] [Google Scholar]
- 12.Larsen IK, Bray F. Trends in colorectal cancer incidence in Norway 1962-2006: An interpretation of the temporal patterns by anatomic subsite. Int J Cancer. 2010;126:721–32. doi: 10.1002/ijc.24839. [DOI] [PubMed] [Google Scholar]
- 13.Singh H, Demers AA, Xue L, Turner D, Bernstein CN. Time trends in colon cancer incidence and distribution and lower gastrointestinal endoscopy utilization in Manitoba. Am J Gastroenterol. 2008;103:1249–56. doi: 10.1111/j.1572-0241.2007.01726.x. [DOI] [PubMed] [Google Scholar]
- 14.Rabeneck L, Davila JA, El-Serag HB. Is there a true “shift” to the right colon in the incidence of colorectal cancer? Am J Gastroenterol. 2003;98:1400–9. doi: 10.1111/j.1572-0241.2003.07453.x. [DOI] [PubMed] [Google Scholar]
- 15.Saltzstein SL, Behling CA. Age and time as factors in the left-to-right shift of the subsite of colorectal adenocarcinoma: A study of 213,383 cases from the California Cancer Registry. J Clin Gastroenterol. 2007;41:173–7. doi: 10.1097/01.mcg.0000225550.26751.6a. [DOI] [PubMed] [Google Scholar]
- 16.Cheng L, Eng C, Nieman LZ, Kapadia AS, Du XL. Trends in colorectal cancer incidence by anatomic site and disease stage in the United States from 1976 to 2005. Am J Clin Oncol. 2011;34:573–80. doi: 10.1097/COC.0b013e3181fe41ed. [DOI] [PubMed] [Google Scholar]
- 17.Fakheri H, Janbabai G, Bari Z, Eshqi F. The epidemiologic and clinical-pathologic characteristics of colorectal cancers from 1999 to 2007 in Sari. JMUMS. 2008;18:58–66. [Google Scholar]
- 18.Iravani S, Kashfi SM, Azimzadeh P, Lashkari MH. Prevalence and characteristics of colorectal polyps in symptomatic and asymptomatic Iranian patients underwent colonoscopy from 2009-2013. Asian Pac J Cancer Prev. 2014;15:9933–7. doi: 10.7314/apjcp.2014.15.22.9933. [DOI] [PubMed] [Google Scholar]
- 19.Moghimi-Dehkordi B, Safaee A, Zali MR. Prognostic factors in 1,138 Iranian colorectal cancer patients. Int J Colorectal Dis. 2008;23:683–8. doi: 10.1007/s00384-008-0463-7. [DOI] [PubMed] [Google Scholar]
- 20.Moradi A, Khayamzadeh M, Guya M, Mirzaei HR, Salmanian R, Rakhsha A, et al. Survival of colorectal cancer in Iran. Asian Pac J Cancer Prev. 2009;10:583–6. [PubMed] [Google Scholar]
- 21.Iravani S, Nazemalhosseini-Mojarad E, Kashfi SM, Azimzadeh P. Screening of colorectal diseases among individuals without family history in a private hospital, Tehran, Iran from 2011 to 2013. Translational Gastrointestinal Cancer. 2014;3:165–8. [Google Scholar]
- 22.Singh H, Nugent Z, Mahmud SM, Demers AA, Bernstein CN. Predictors of colorectal cancer after negative colonoscopy: A population-based study. Am J Gastroenterol. 2010;105:663–74. doi: 10.1038/ajg.2009.650. [DOI] [PubMed] [Google Scholar]
- 23.Brenner H, Chang-Claude J, Seiler CM, Rickert A, Hoffmeister M. Protection from colorectal cancer after colonoscopy: A population-based, case-control study. Ann Intern Med. 2011;154:22–30. doi: 10.7326/0003-4819-154-1-201101040-00004. [DOI] [PubMed] [Google Scholar]
- 24.Mulder SA, van Soest EM, Dieleman JP, van Rossum LG, Ouwendijk RJ, van Leerdam ME, et al. Exposure to colorectal examinations before a colorectal cancer diagnosis: A case-control study. Eur J Gastroenterol Hepatol. 2010;22:437–43. doi: 10.1097/MEG.0b013e328333fc6a. [DOI] [PubMed] [Google Scholar]
- 25.Bufill JA. Colorectal cancer: Evidence for distinct genetic categories based on proximal or distal tumor location. Ann Intern Med. 1990;113:779–88. doi: 10.7326/0003-4819-113-10-779. [DOI] [PubMed] [Google Scholar]
- 26.Iacopetta B. Are there two sides to colorectal cancer? Int J Cancer. 2002;101:403–8. doi: 10.1002/ijc.10635. [DOI] [PubMed] [Google Scholar]
- 27.Weiss JM, Pfau PR, O’Connor ES, King J, LoConte N, Kennedy G, et al. Mortality by stage for right- versus left-sided colon cancer: Analysis of surveillance, epidemiology, and end results--Medicare data. J Clin Oncol. 2011;29:4401–9. doi: 10.1200/JCO.2011.36.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siegel RL, Ward EM, Jemal A. Trends in colorectal cancer incidence rates in the United States by tumor location and stage, 1992-2008. Cancer Epidemiol Biomarkers Prev. 2012;21:411–6. doi: 10.1158/1055-9965.EPI-11-1020. [DOI] [PubMed] [Google Scholar]
- 29.Toyomura K, Yamaguchi K, Kawamoto H, Tabata S, Shimizu E, Mineshita M, et al. Relation of cigarette smoking and alcohol use to colorectal adenomas by subsite: The self-defense forces health study. Cancer Sci. 2004;95:72–6. doi: 10.1111/j.1349-7006.2004.tb03173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hansen IO, Jess P. Possible better long-term survival in left versus right-sided colon cancer - a systematic review. Dan Med J. 2012;59:A4444. [PubMed] [Google Scholar]
- 31.Derwinger K, Gustavsson B. Variations in demography and prognosis by colon cancer location. Anticancer Res. 2011;31:2347–50. [PubMed] [Google Scholar]
- 32.Selby WS, Janossy G, Jewell DP. Immunohistological characterisation of intraepithelial lymphocytes of the human gastrointestinal tract. Gut. 1981;22:169–76. doi: 10.1136/gut.22.3.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kirby JA, Bone M, Robertson H, Hudson M, Jones DE. The number of intraepithelial T cells decreases from ascending colon to rectum. J Clin Pathol. 2003;56:158. doi: 10.1136/jcp.56.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.West DW, Slattery ML, Robison LM, Schuman KL, Ford MH, Mahoney AW, et al. Dietary intake and colon cancer: Sex- and anatomic site-specific associations. Am J Epidemiol. 1989;130:883–94. doi: 10.1093/oxfordjournals.aje.a115421. [DOI] [PubMed] [Google Scholar]
- 35.Annema N, Heyworth JS, McNaughton SA, Iacopetta B, Fritschi L. Fruit and vegetable consumption and the risk of proximal colon, distal colon, and rectal cancers in a case-control study in Western Australia. J Am Diet Assoc. 2011;111:1479–90. doi: 10.1016/j.jada.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 36.Oh SW, Kim YH, Choi YS, Chang DK, Son HJ, Rhee PL, et al. The comparison of the risk factors and clinical manifestations of proximal and distal colorectal cancer. Dis Colon Rectum. 2008;51:56–61. doi: 10.1007/s10350-007-9083-5. [DOI] [PubMed] [Google Scholar]
- 37.van Engeland M, Derks S, Smits KM, Meijer GA, Herman JG. Colorectal cancer epigenetics: Complex simplicity. J Clin Oncol. 2011;29:1382–91. doi: 10.1200/JCO.2010.28.2319. [DOI] [PubMed] [Google Scholar]
- 38.Ang PW, Loh M, Liem N, Lim PL, Grieu F, Vaithilingam A, et al. Comprehensive profiling of DNA methylation in colorectal cancer reveals subgroups with distinct clinicopathological and molecular features. BMC Cancer. 2010;10:227. doi: 10.1186/1471-2407-10-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Minoo P, Zlobec I, Peterson M, Terracciano L, Lugli A. Characterization of rectal, proximal and distal colon cancers based on clinicopathological, molecular and protein profiles. Int J Oncol. 2010;37:707–18. doi: 10.3892/ijo_00000720. [DOI] [PubMed] [Google Scholar]
- 40.Corleto VD, Pagnini C, Cattaruzza MS, Zykaj E, Di Giulio E, Margagnoni G, et al. Is proliferative colonic disease presentation changing? World J Gastroenterol. 2012;18:6614–9. doi: 10.3748/wjg.v18.i45.6614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gomez D, Dalal Z, Raw E, Roberts C, Lyndon PJ. Anatomical distribution of colorectal cancer over a 10 year period in a district general hospital: Is there a true “rightward shift”? Postgrad Med J. 2004;80:667–9. doi: 10.1136/pgmj.2004.020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fazeli MS, Adel MG, Lebaschi AH. Colorectal carcinoma: A retrospective, descriptive study of age, gender, subsite, stage, and differentiation in Iran from 1995 to 2001 as observed in Tehran University. Dis Colon Rectum. 2007;50:990–5. doi: 10.1007/s10350-007-0248-z. [DOI] [PubMed] [Google Scholar]
- 43.Goh KL, Quek KF, Yeo GT, Hilmi IN, Lee CK, Hasnida N, et al. Colorectal cancer in Asians: A demographic and anatomic survey in Malaysian patients undergoing colonoscopy. Aliment Pharmacol Ther. 2005;22:859–64. doi: 10.1111/j.1365-2036.2005.02646.x. [DOI] [PubMed] [Google Scholar]
- 44.Bafandeh Y, Khoshbaten M, Eftekhar Sadat AT, Farhang S. Clinical predictors of colorectal polyps and carcinoma in a low prevalence region: Results of a colonoscopy based study. World J Gastroenterol. 2008;14:1534–8. doi: 10.3748/wjg.14.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bafandeh Y, Daghestani D, Esmaili H, Aharizad S. Distribution of cancer and adenomatous polyps in the colorectum: Study in an Iranian population. Asian Pac J Cancer Prev. 2006;7:65–8. [PubMed] [Google Scholar]
- 46.Pahlavan PS, Kanthan R. The epidemiology and clinical findings of colorectal cancer in Iran. J Gastrointestin Liver Dis. 2006;15:15–9. [PubMed] [Google Scholar]
- 47.Omranipour R, Doroudian R, Mahmoodzadeh H. Anatomical distribution of colorectal carcinoma in Iran: A retrospective 15-yr study to evaluate rightward shift. Asian Pac J Cancer Prev. 2012;13:279–82. doi: 10.7314/apjcp.2012.13.1.279. [DOI] [PubMed] [Google Scholar]
- 48.Mahmodlou R, Mohammadi P, Sepehrvand N. Colorectal cancer in northwestern Iran. ISRN Gastroenterol 2012. 2012 doi: 10.5402/2012/968560. 968560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iida Y, Kawai K, Tsuno NH, Ishihara S, Yamaguchi H, Sunami E, et al. Proximal shift of colorectal cancer along with aging. Clin Colorectal Cancer. 2014;13:213–8. doi: 10.1016/j.clcc.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 50.Brenner H, Bouvier AM, Foschi R, Hackl M, Larsen IK, Lemmens V, et al. EUROCARE Working Group. Progress in colorectal cancer survival in Europe from the late 1980s to the early 21st century: The EUROCARE study. Int J Cancer. 2012;131:1649–58. doi: 10.1002/ijc.26192. [DOI] [PubMed] [Google Scholar]
- 51.Yang Z, Kang L, Wang L, Xiang J, Cai G, Cui J, et al. Characteristics and long-term survival of colorectal cancer patients aged 44 years and younger. Clin Transl Oncol. 2012;14:896–904. doi: 10.1007/s12094-012-0876-1. [DOI] [PubMed] [Google Scholar]


