Abstract
The traditionally cited recommendations for the preoperative restriction of food (including bedding) and water in pigs do not appear to be evidence-based. As a preliminary step in elucidating a rationale for and standardizing preoperative food and water restriction (PFWR), this structured review recorded recent reported practices in PFWR in laboratory pigs and its consequences. Medline, Google Scholar and Web of Science databases were searched for recently published (2012 – 2014) recovery surgery procedures in pigs. Information pertaining to PFWR practices, as delineated in the ARRIVE guidelines, was extracted from the 233 articles retrieved. Food withdrawal was described in 73 of the 233 (31%) papers evaluated, bedding withdrawal in 5 articles (2%), and water withholding in 13 publications (6%) papers. Food, bedding, and water withdrawal regimens had a median (range) duration of 12 (4 to 48), 48 (48 to 72), and 12 (2 to 12) h, respectively. Compared with other types of procedures, articles describing gastrointestinal or abdominal surgery were more likely to report fasting regimes. Liquid diets were described in 11 of the 233 (5%) publications evaluated. Adverse effects of PFWR effects were not reported. These data reveal considerable variation in PFWR practices. The stress of fasting coupled with the absence of evidence for current recommendations makes the rationale and standards for PFWR in pigs worthy of further study.
Abbreviation: PFWR, preoperative food and water restriction
Despite the common recommendation of preoperative food and water restriction (PFWR), evidence regarding the need for (and efficacy of) this practice (including the removal of edible bedding) before surgery in pigs is limited. Although intestinal transit times in pigs are rapid, with stomach emptying requiring only a few hours,46 minimal recommended periods of food restriction begin at 6 h.11,18 Authorities justify PFWR on the grounds that it prevents emesis and reduces the accumulation of gastrointestinal gas.16,17. Reduced gas accumulation is desirable because it optimizes the functional residual capacity of the lung and pulmonary ventilation17,18,49 and facilitates gastrointestinal tissue handling and surgery.17,49 Furthermore, one authority stated that “gastrointestinal distension interferes with venous return and by causing capillary stasis, edema of the gut wall and blood pooling in the mesenteric veins predisposes to acute cardiovascular failure. Handling of gas-distended bowel and ‘packing off’ from the operative field increases the risk of apnea and shock due to tension on the mesenteric root.”17 However, the incidence of these problems is unknown and controversial: one study18 states that vomiting at induction of or recovery from anesthesia is rare in pigs. This opinion contrasts with the assertion that “swine readily vomit under anesthesia if food is present in the stomach.”17
The UK Animals (Scientific Procedures) Act (1986)22 categorizes the withholding of food or water as a category A procedure because excessive periods are likely to cause suffering, distress and/or lasting harm for which there is considerable evidence. The Guide for the Care and Use of Agricultural Animals in Research and Teaching14 states that “animals must be provided with feed and water on a regular schedule …Where exceptions are required by an experimental or instructional protocol, these must be justified in the protocol and may require approval by the Institutional Animal Care and Use Committee.” Food and water restriction in pigs causes multiple adverse effects, including behavioral,6,10,33,48 biochemical,2,4,15,32,36 endocrine,2,6-8,15,32,39,42 hematologic,8 microbiologic,20,31 temperature2,25 and gastric ulcerative1,40 changes. Fasting in pigs also leads to signs of increased hunger when food subsequently is offered.38 This characteristic is important because different periods of preoperative starvation may result in varying degrees of postoperative hunger and consequently appetite, which is a recognized indicator of postoperative wellbeing in pigs 19, 30, 46. Consequently, indiscriminate periods of preoperative starvation may obfuscate the assessment of convalescence. In this respect, breed may be important, given that the hormonal control of appetite differs between commercial and minipigs.29
The Guide for the Care and Use of Laboratory Animals26 states that “animals should have access to potable, uncontaminated drinking water according to their particular requirements. Other authors18 advocate preoperative water restriction “to ensure an empty stomach.” However, water restriction alone in pigs has been shown to lead to decreased circulating blood volume and to decreased packed cell volume when food was withdrawn as well.23 Severe water restriction can lead to hypovolemia, which will aggravate the adverse cardiovascular effects of anesthetics and surgery.
The adverse effects of resource restriction are probably age-dependent because the food and water requirements of pigs change with age.41 In children, the metabolic response to food restriction is different and more severe than is the pattern in adults.27 In pigs, the adverse effects of PFWR are likely to be greater in juvenile animals because food and water requirements per unit body mass are greater. Several publications16,18,46 do not discriminate between different ages of animals, whereas others11,49 discuss only fasting in adult animals.
A standardized approach to PFWR is desirable because the different physiologic effects of varying restriction periods may introduce ‘noise’ into physiologic data and consequently reduce study power. Contemporary medical anesthetic practice in the United Kingdom allows the provision of a light meal 6 h before anesthesia and clear fluids until 2 h before surgery in adults and children older than 6 mo.45 There is increasing interest in human medicine in the use of preoperative carbohydrate-dense drinks to provide rapidly available glucose without increasing the risk of emesis.45 Given that pigs are omnivorous and monogastric and are commonly used to model humans, developing a standardized approach similar to that used in contemporary medical practice deserves consideration. Standardizing restriction regimens with the goal of achieving a similar perioperative metabolic state could optimize the translational power of studies.
The ARRIVE (Animal Research: Reporting in Vivo Experiments) guidelines mandate reporting of “husbandry conditions” in articles describing animal experiments, including the type of food available, and the animals’ access to food and water. Prompted by this mandate and the disagreement extant in contemporary textbooks, the proposed review aimed to establish reported practices for PFWR in laboratory pigs and to record reported justifications, advantages, and disadvantages as a preliminary step to establishing the rationale for and possible standardization of PFWR in pigs.
Materials and Methods
Article selection.
A literature review was conducted between 3 January 2014 and 12 March 2014 by using the search terms ‘pig’ OR ‘pigs’ OR ‘minipig’ OR ‘minipigs’ OR ‘swine’ AND ‘surgery’ OR ‘surgical’ and NOT ‘guinea’ in the search engines MEDLINE (1950 to 2014), Web of Knowledge (1956 to 2014), and Google Scholar, to identify peer-reviewed articles written in English and published between 2012 and 2014. Articles that described nonrecovery surgical procedures were not reviewed because we assumed that inappropriate PFWR would be less consequential in nonrecovery compared with recovery experiments. Only journals available in electronic format from the University of Edinburgh library (approximately 25,000) were examined.
The titles and abstracts of all articles obtained were screened to differentiate nonrecovery from recovery experiments; the body text was examined when this differentiation was in doubt. Subsequent analysis was only conducted on articles describing recovery surgery when 4 or more pigs were involved and postoperative recovery was at least 24 h.
Article analysis.
The selected articles (233) were examined for information regarding experimental approval; feed, bedding, and water withdrawal; and the procedure performed. We categorized the experimental approval as present (that is, an explicit statement relating to approval or review of the specific experiment), or adhering to guidelines (a statement that the procedure complied with, for example, NIH guidelines), or not present.
The Adobe ‘Find’ tool (Adobe Systems, San Jose, CA) was used to search selected articles for ‘food’ or ‘water’ or ‘straw’ or ‘withdraw-’ or ‘withh-’ or ‘starv-’ or ‘fast-’—if none of these was found, an author (GB) scanned the Materials and Methods section for mention of food, water, or bedding withdrawal. Details pertaining to liquid diet or bowel preparation were recorded also.
After initial data extraction, operations were categorized as being either ‘intraabdominal or gastrointestinal surgeries or not, and the extent of preoperative resource restriction was compared statistically. For the comparison of intraabdominal or extraabdominal procedures, we excluded endoscopic studies from the dataset (based on screening of the Materials and Methods section). This was done to prevent data skewing, as an empty gut facilitates endoscopic examination.
Information on the pigs’ age and breed were recorded, along with any reported adverse effects that could conceivably be linked with PFWR (specifically emesis, regurgitation, hypoglycemia, postoperative pica, and behavioral anomalies). Data were entered onto am Excel (Microsoft, Redmond, WA) spreadsheet.
No attempt was made to contact the authors of articles that reported no information, because the aim was to examine reported methods rather than those actually used. The subscription of selected articles to the ARRIVE guidelines (2010) was established by consulting the NC3R's website (https://www.nc3rs.org.uk/arrive-animal-research-reporting-vivo-experiments).
Statistics.
The proportions of identified elements in the spreadsheet were calculated and expressed as percentages. A χ2 or Yates χ2 test was used to examine the relationships between the number of articles describing abdominal or gastrointestinal tract surgery and preoperative fasting.
Results
Overall.
The frequency of reporting food, bedding and water restriction varied considerably. Food withdrawal was described in 73 of the 233 (31%) publications, bedding withdrawal in 5 (2%), and water restriction in 13 (6%). The total number of pigs involved in the articles reviewed was 3907. Experimental approval was recorded in 198 of the 233 (85%) papers assessed, and adherence to guidelines on animal welfare was indicated in 14 articles (6%). No mention was made of study approval or compliance with welfare guidelines in 21 papers (9%).
Food restriction.
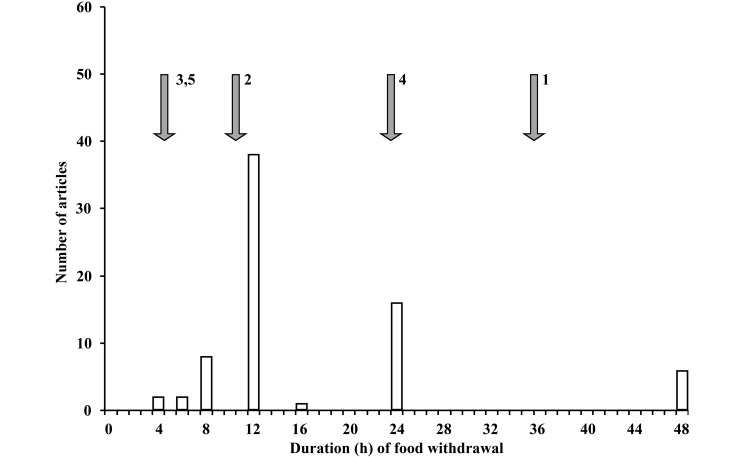
The median (range) duration of food withdrawal was 12 (4 to 48) h (Figure 1). Justification for fasting was provided in only 2 publications. One paper stated, “overnight fasting for 12 hours ...to avoid complications such as vomiting during the introduction of anesthesia and resuscitation and gastric distension.”28 The authors of the other article justified feed restriction “to decrease bowel residue.”5
Figure 1.
Histogram showing duration (h) of food withdrawal. Open arrows show recommendations from leading textbooks. Numbers associated with arrows are the citation numbers for the supporting references.
Bedding withdrawal.
Removal of consumable bedding (including wood chips) for 48 h was described in 2 papers and for 72 h in another 3 publications. The papers that described bedding withdrawal for 3 d involved endoscopic surgical procedures and laparotomy; the others described laparotomy and laparoscopy procedures. None of the authors of these articles provided justification for the withdrawal of bedding.
Water restriction.
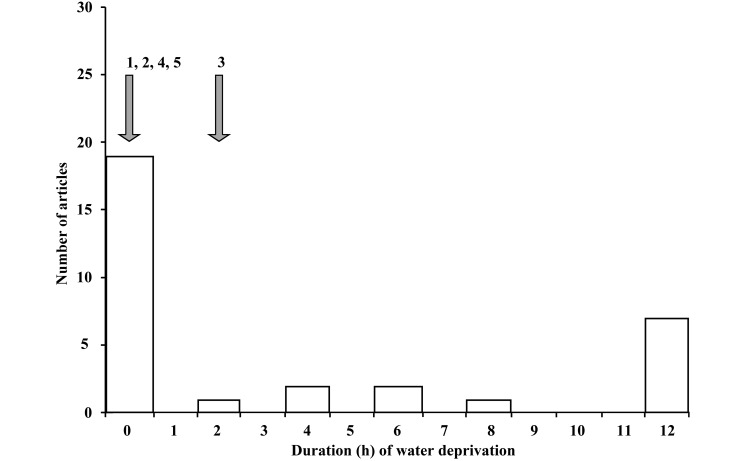
The median (range) duration of water restriction was 12 (2 to 12) h (Figure 2). Water restriction was described in papers reporting endoscopy or abdominal surgery (10 papers), orthopedic surgery (2 publications), and thoracotomy (1 article). In addition, another 24 papers noted the provision of water until the induction of anesthesia. In contrast, 7 articles of the 13 articles described water restriction for 12 h without explicit justification for the practice.
Figure 2.
Histogram showing duration (h) of water restriction. Open arrows show recommendations from leading textbooks. Numbers associated with arrows indicate the citation numbers for the supporting references.
Provision of a liquid diet.
The provision of a liquid diet for 48 h was noted in 5 papers and for 72 h in 4 articles among a total of 11 reports involving gastrointestinal tract surgery. The duration was not reported in the remaining 2 papers, one of which described the use of an ‘electrolyte-rich liquid.’ The other article did not specify the nature of the liquid diet offered.
Procedures and resource restriction.
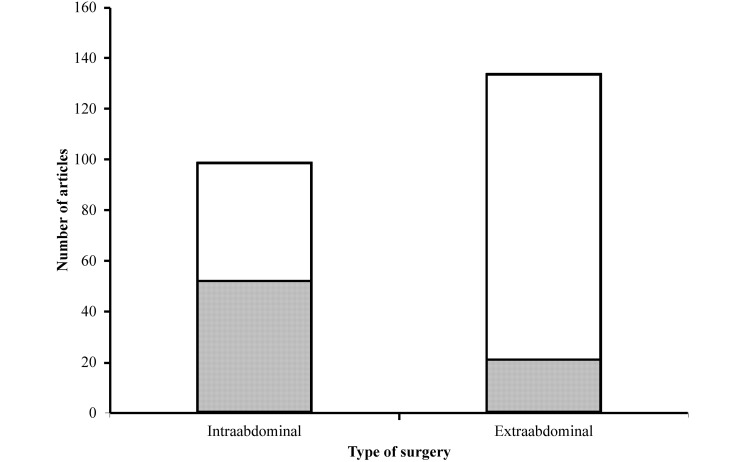
There was a significant (P = 2.03× 10−9, χ2 test) difference in preoperative fasting practices between articles that described surgery involving the abdomen or gastrointestinal tract and those that did not (Figure 3). All 12 articles describing gastrointestinal natural-orifice transluminal endoscopy prescribed preoperative food withdrawal. When these publications were excluded, the result remained statistically significant (P = 8.49× 10−7, χ2 test; Figure 2). Mechanical bowel preparation was described in one paper13: “Starting 48 h prior to surgery, each pig was given a mechanical bowel preparation with 1.5 gal of NuLYTELY (Braintree Laboratories, Braintree, MA) daily.”
Figure 3.
Preoperative fasting descriptions in publications describing intraabdominal surgery or extraabdominal surgery. The gray area depicts the number of papers that recorded that the pigs were fasted, and the white area depicts the number of papers in which the fasting of pigs was not recorded. The difference is statistically significant (P = 2.03 × 10-9, χ2 test).
Emesis.
One study reported vomiting in one pig on the first postoperative day; no other paper reported problems associated with either the provision or restriction of food or water. Assessment of food intake as a marker of postoperative pain or discomfort was described in 12 papers, 9 of which described preoperative food restriction.
Age and breed.
The age of the pigs ranged from 2 d to 6 y; however pigs’ ages were not reported in enough papers or in sufficient detail to allow an association between PFWR practices and age to be determined. None of the articles screened explicitly stated that the animals’ age had influenced the period of resource restriction selected. The use of minipigs was described in 19 publications, and commercial breeds were noted in 54 papers; breed information was explicitly described in 49 papers. Feed restriction times did not differ between minipigs and commercial pigs (H = 1.17 (1,n = 73); P > 0.05, Kruskal–Wallis test).
Discussion
Preoperative restriction of food, bedding, or water in pigs was described in 73, 5, and 11 papers respectively, and all papers that described the restriction of water or bedding also included food deprivation. Consequently, 160 of the 233 (69%) publications evaluated did not describe a fasting practice, indicating that either the resources were not withdrawn or that the resources were withdrawn but the restriction was not reported. The current study identified 12 articles published by ARRIVE-subscribing journals: only 3 of these articles described preoperative fasting.
The variation in reported food restriction practices encountered in this review mirrored the considerable variation encountered in published recommendations. The duration of feed withdrawal ranged from 4 to 48 h. This variation is concerning because various periods might affect biochemical variables differently and differentially influence study results. Food restriction lasting 12 to 18 h in pigs going to slaughter6 causes near-complete depletion of liver glycogen stores; alterations in leptin, ghrelin, and cortisol levels occur after 24 h of restriction in 18-d-old pigs.42 Furthermore, adult pigs demonstrated increased aggression when fasted for 18 h, an outcome that might affect postoperative management.6 By increasing variation in recorded data, varying periods of PFWR might reduce study power and thus require greater numbers of animals achieve adequate statistical power.
Postoperative food consumption was used as a measure of pain in 12 of the selected papers; 9 of these described preoperative food restriction. Preoperative fasting might increase an animal's postoperative hunger, thus potentially masking signs of discomfort. Feed restriction in pigs frequently results in a subsequent compensatory increase in feed intake.38
The one study that reported postoperative emesis involved gastrectomy, and thus emesis was unlikely to be associated with the fasting practice used (12 h of food restriction). Some veterinary anesthesia textbooks2,17 cite emesis as the principle reason for preoperative food restriction in pigs, although this view is not unanimous. For example, another author16 acknowledges that “pigs are usually fasted for 12 h prior to induction of anesthesia, although vomiting on induction is rare.” Of the 233 articles in the current review, 160 did not report food restriction and also did not report postoperative vomiting. This pattern may indicate either that vomiting did not occur or that it was not reported. Although Suidae are able to vomit,47 the incidence of this complication with regard to anesthesia and surgery is unrecorded. One study of food restriction and intestinal distension in pigs found that 2 to 5 h of restriction significantly decreased intestinal distension as compared with that observed without food restriction; stress was also noted to promote intestinal distension.33 No articles screened in the current review reported adverse consequences associated with food, bedding, or water restriction. Although these complications may not have occurred, it also is possible that they did occur but were not noted or that they were attributed to causes other than resource deprivation.
Only 5 publications described the removal of consumable bedding. Because food withdrawal alone is a significant stressor in pigs,39 the removal of bedding—under conditions where straw is the only form of environmental enrichment—may be particularly stressful. Bedding removal deprives pigs of the ability to root, which is widely recognized as a feature of positive welfare. The European Commission Directive 2001/93/EC,12 directs that “pigs must have permanent access to sufficient quantity of material, such as straw, to enable proper investigation and manipulation activities.” The Guide for the Care and Use of Agricultural Animals in Research and Teaching14 recognizes that “the risk of tail biting is elevated and activity is depressed if pigs initially reared with straw are subsequently housed without straw.” Despite these statements, 2 foundational texts17,46 emphasize the necessity of withholding bedding to ensure an absence of oral intake. However, the recommended periods of restriction differ widely (24 to 36 h in reference 15 compared with 6 to 8 h in reference 45).
Because only one veterinary textbook (reference 18) recommends preoperative water restriction (2 h before preanesthetic medication), our retrieval of 7 papers describing 12 h of water restriction was unexpected. All 7 papers that reported water restriction recommended concomitant food restriction. Combined restriction of food and water for 12 h or more can cause a significant reduction in blood volume in pigs;23 this reduction in blood volume increases the risk of hypovolemic shock, particularly in operations in which substantial blood loss occurs. Although none of the publications we reviewed reported adverse consequences of water restriction, none justified the practice either.
The use of liquid diets was reported in 11 studies for preoperative periods of as long as 72 h. Whereas 9 articles described withdrawal of this diet before anesthesia, the remaining 2 described its availability until the induction of anesthesia. In human patients, the preoperative provision of carbohydrate-dense drinks (typically 12.5% carbohydrate3) until 2 h before anesthesia is increasing, to decrease anxiety,21 postoperative insulin resistance,37 and associated morbidities35 without an increase in fecal volume. The provision of similar liquids deserves consideration in pigs, which frequently are viewed as one of the closest gastrointestinal models for human conditions from the perspectives of size, diet, and gastrointestinal tract anatomy.
The preoperative fasting practices applied to pigs differed significantly between those facing intraabdominal surgery compared with those undergoing nonabdominal operations. Previously, it was thought that fasting human patients decreased the risk of anastomotic dehiscence,44 minimized the death of devascularised tissue,9 and improved surgical access,34 thus perhaps prompting recommendations for bowel preparation in pigs. For example, one text recommended prior to surgery that “castor oil (2.5 mg/kg) be given orally via gavage tube, but care should be taken to avoid esophageal reflux.50 In another, a soapy enema was advised “where surgery or radiography of the descending colon is to be performed.”17 Despite these recommendations, only one paper in the current review reported the use of mechanical bowel preparation (a mixture of polyethylene glycol, sodium chloride, sodium bicarbonate and potassium chloride) before laparotomy and rectal anastomosis.13 However, evidence from human surgical practice currently supports the notion that “mechanical bowel preparation does not decrease anastomotic leakage, abscessation, or wound sepsis.”43 In addition, a recently conducted randomized controlled trial for gynecologic laparoscopy found a small but statistically significant improvement in surgical visualization in patients who received a liquid diet for 48 h in addition to mechanical bowel preparation preoperatively.51 However, those patients also demonstrated a statistically significant increase in postoperative morbidities, including headache, weakness, tiredness, and overall discomfort. The authors of that study concluded that “the adverse consequences of liquid diet provision and mechanical bowel preparation outweighed the benefits.”51
The ages of pigs studied did not seem to affect the degree of resource restriction imposed; however, the paucity of information on animal age precluded its statistical investigation. Indeed, one article described the use of “10 healthy adult pigs (Large White) with a mean weight of 31.5 ± 2.5 kg (range, 27 to 35 kg),” indicating an unfamiliarity with porcine growth rates that may be widespread in the biomedical research community. Age is an important consideration because a given period of food restriction is likely to have a greater effect on lighter animals. One investigation of pigs at different weights (20, 40, 60, 80 kg) found that the lighter (usually younger) pigs ate more meals.24
Breed was identified in 49 of the 233 papers reviewed, but the number of breeds was not adequate for statistical analysis. However, interbreed variation may affect the response to food restriction substantially. At a body mass of 30 kg—a popular size of experimental pig—the Gottingen minipig's growth rate has plateaued or is close to a plateau phase. This situation contrasts with pigs of commercial breeds, which are still actively growing at this body mass. Consequently, food restriction in 30-kg pigs is more likely to produce severe consequences in animals bred from commercial lines than in minipigs.
In conclusion, the current review reveals wide variation in restriction protocols and absence of supportive evidence, which precludes standardization. We believe that the cost:benefit ratio of PFWR in laboratory pigs needs to be evaluated formally. Until that time, the decision whether to withhold food and water preoperatively in pigs—and for how long—should involve consideration of the animals’ age, growth rate, breed, and pregnancy and disease status and the operation to be performed. The case for particularly aggressive measures may be more defensible in animals facing gastrointestinal surgery. However, in this specific subgroup, the provision of a carbohydrate-dense drink for 12 h or so preoperatively may mitigate the adverse effects of food and water restriction.
References
- 1.Amory J, Mackenzie A, Pearce G. 2006. Factors in the housing environment of finisher pigs associated with the development of gastric ulcers. Vet Rec 158: 260–264. [DOI] [PubMed] [Google Scholar]
- 2.Bertol TM, Ellis M, Ritter MJ, McKeith FK. 2005. Effect of feed withdrawal and handling intensity on longissimus muscle glycolytic potential and blood measurements in slaughter-weight pigs. J Anim Sci 83:1536–1542. [DOI] [PubMed] [Google Scholar]
- 3.Bilku DK, Dennison AR, Hall TC, Metcalfe MS, Garcea G. 2014. Role of preoperative carbohydrate loading: a systematic review. Ann R Coll Surg Engl 96:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boomgaardt J, McDonald BE. 1969. Comparison of fasting plasma amino acid patterns in the pig, rat, and chicken. Can J Physiol Pharmacol 47:392–395. [DOI] [PubMed] [Google Scholar]
- 5.Brigic A, Southgate A, Sibbons P, Clark SK, Fraser C, Kennedy RH. 2013. Full-thickness laparoendoscopic colonic excision in an experimental model. Br J Surg 100:1649–1654. [DOI] [PubMed] [Google Scholar]
- 6.Brown SN, Knowles TG, Edwards JE, Warriss PD. 1999. Relationship between food deprivation before transport and aggression in pigs held in lairage before slaughter. Vet Rec 145:630–634. [DOI] [PubMed] [Google Scholar]
- 7.Buonomo FC, Baile CA. 1991. Influence of nutritional deprivation on insulin-like growth factor I, somatotropin, and metabolic hormones in swine. J Anim Sci 69:755–760. [DOI] [PubMed] [Google Scholar]
- 8.Clemens ET, Schultz BD, Brumm MC, Jesse GW, Mayes HF. 1986. Influence of market stress and protein level on feeder pig hematologic and blood chemical values. Am J Vet Res 47:359–362. [PubMed] [Google Scholar]
- 9.Cohn I, Jr, Rives JD. 1955. Antibiotic protection of colon anastomoses. Ann Surg 141:707–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Day JEL, Kyriazakis I, Lawrence AB. 1995. The effect of food deprivation on the expression of foraging and exploratory behaviour in the growing pig. Appl Anim Behav Sci 42:193–206. [Google Scholar]
- 11.Dugdale A. 2010. Veterinary anesthesia. Ames (IA): John Wiley and Sons. [Google Scholar]
- 12.European Parliament and the Council of the European Union. 2001. Directive 2001/93/EC of 09 November 2001 amending Directive 91/630/EEC laying down minimum standards for the protection of pigs. Off J Eur Communities 316:36. [Google Scholar]
- 13.Fajardo AD, Amador-Ortiz C, Chun J, Stewart D, Fleshman JW. 2011. Evaluation of bioabsorbable Seamguard for staple line reinforcement in stapled rectal anastomoses. Surg Innov 19:288–294. [DOI] [PubMed] [Google Scholar]
- 14.Federation of Animal Science Societies 2010. Guide for the care and use of agricultural animals in research and teaching. Champaign (IL): Federation of Animal Sciences [Google Scholar]
- 15.Fernandez X, Meunier-Salaun MC, Ecolan P, Mormède P. 1995. Interactive effect of food deprivation and agonistic behavior on blood parameters and muscle glycogen in pigs. Physiol Behav 58:337–345. [DOI] [PubMed] [Google Scholar]
- 16.Flecknell P. 2009. Laboratory animal anaesthesia. London (UK): Academic Press. [Google Scholar]
- 17.Green CJ. 1979. Animal anaesthesia, Laboratory animal handbooks, no 8. London (UK): Laboratory Animals. [Google Scholar]
- 18.Hall L, Clarke K, Trim C. 2001. Anaesthesia of the pig. p 367–383. In Hall LW, Clarke KW, Trim CM. Veterinary anaesthesia, 10th ed. London (UK): Elsevier. [Google Scholar]
- 19.Harvey-Clark CJ, Gilespie K, Riggs KW. 2000. Transdermal fentanyl compared with parenteral buprenorphine in postsurgical pain in swine: a case study. Lab Anim 34:386–398. [DOI] [PubMed] [Google Scholar]
- 20.Harvey RB, Anderson RC, Young CR, Swindle MM, Genovese KJ, Hume ME, Droleskey RE, Farrington LA, Ziprin RL, Nisbet DJ. 2001. Effects of feed withdrawal and transport on cecal environment and Campylobacter concentrations in a swine surgical model. J Food Prot 64:730–733. [DOI] [PubMed] [Google Scholar]
- 21.Hausel J, Nygren J, Lagerkranser M, Hellström PM, Hammarqvist F, Almström C, Lindh A, Thorell A, Ljungqvist O. 2001. A carbohydrate-rich drink reduces preoperative discomfort in elective surgery patients. Anesth Analg 93:1344–1350. [DOI] [PubMed] [Google Scholar]
- 22.Hollands C. 1986. The Animals (Scientific Procedures) Act 1986. Lancet 328:32–33. [DOI] [PubMed] [Google Scholar]
- 23.Houpt TR, Yang H. 1995. Water deprivation, plasma osmolality, blood volume, and thirst in young pigs. Physiol Behav 57:49–54. [DOI] [PubMed] [Google Scholar]
- 24.Hsia LC, Wood-Gush DGM. 1984. The temporal patterns of food intake and allelomimetic feeding by pigs of different ages. Appl Anim Ethol 11:271–282. [Google Scholar]
- 25.Ingram DL, Mount LE. 1973. The effects of food intake and fasting on 24-hourly variations in body temperature in the young pig. Pflugers Arch 339:299–304. [DOI] [PubMed] [Google Scholar]
- 26.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 27.Koletzko B, Goulet O, Hunt J, Krohn K, Shamir R, Parenteral Nutrition Guideline Working Group; European Society for Clinical Nutrition and Metabolism; European Society of Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN); European Society of Paediatric Research (ESPR) 2005. Guidelines on paediatric parenteral nutrition of the European Society of Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) and the European Society for Clinical Nutrition and Metabolism (ESPEN), supported by the European Society of Paediatric Research (ESPR). J Pediatr Gastroenterol Nutr 41:S1–S87. [DOI] [PubMed] [Google Scholar]
- 28.Krikri A, Alexopoulos V, Zoumakis E, Katsaronis P, Balafas E, Kouraklis G, Karayannacos PE, Chrousos GP, Skalkeas G. 2013. Laparoscopic vs open abdominal surgery in male pigs: marked differences in cortisol and catecholamine responses depending on size of the surgical incision. Hormones 12:283–291. [DOI] [PubMed] [Google Scholar]
- 29.Larsen MO, Rolin B, Wilken M, Carr RD, Svendsen O, Bollen P. 2001. Parameters of glucose and lipid metabolism in the male Göttingen minipig: influence of age, body weight, and breeding family. Comp Med 51:436–442. [PubMed] [Google Scholar]
- 30.Malavasi LM, Nyman G, Augustsson H, Jacobson M, Jensen-Waern M. 2006. Effects of epidural morphine and transdermal fentanyl analgesia on physiology and behaviour after abdominal surgery in pigs. Lab Anim 40:16–27. [DOI] [PubMed] [Google Scholar]
- 31.Morishita Y, Ogata M. 1970. Studies on the alimentary flora of pig. V. Influence of starvation on the microbial flora. Nihon Juigaku Zasshi 32:19–24. [DOI] [PubMed] [Google Scholar]
- 32.Müller MJ, Paschen U, Seitz HJ. 1982. Starvation-induced ketone body production in the conscious unrestrained miniature pig. J Nutr 112:1379–1386. [DOI] [PubMed] [Google Scholar]
- 33.Murray A, Robertson W, Nattress F, Fortin A. 2001. Effect of preslaughter overnight feed withdrawal on pig carcass and muscle quality. Can J Anim Sci 81:89–97. [Google Scholar]
- 34.Muzii L, Angioli R, Zullo MA, Calcagno M, Panici PB. 2003. Bowel preparation for gynecological surgery. Crit Rev Oncol Hematol 48:311–315. [DOI] [PubMed] [Google Scholar]
- 35.Noblett SE, Watson DS, Huong H, Davison B, Hainsworth PJ, Horgan AF. 2006. Preoperative oral carbohydrate loading in colorectal surgery: a randomized controlled trial. Colorectal Dis 8:563–569. [DOI] [PubMed] [Google Scholar]
- 36.Nordstrom JW, Windels HF, Typpo JT, Meade RJ, Stockland WL. 1970. Influence of site of blood withdrawal and stage of fast on concentrations of plasma free amino acids in the growing pig. J Anim Sci 31:874–884. [DOI] [PubMed] [Google Scholar]
- 37.Nygren J. 2006. The metabolic effects of fasting and surgery. Best Pract Res Clin Anaesthesiol 20:429–438. [DOI] [PubMed] [Google Scholar]
- 38.Owen JB, Ridgman WJ, Wyllie D. 2010. The effect of food restriction on subsequent voluntary intake of pigs. Anim Prod 13:537–546. [Google Scholar]
- 39.Parrott RF, Misson BH. 1989. Changes in pig salivary cortisol in response to transport simulation, food and water deprivation, and mixing. Br Vet J 145:501–505. [DOI] [PubMed] [Google Scholar]
- 40.Pocock EF, Bayley HS, Roe CK. 1968. Relationship of pelleted, autoclaved and heat-expanded corn or starvation to gastric ulcers in swine. J Anim Sci 27:1296–1301. [Google Scholar]
- 41.Quiniou N, Dubois S, Noblet J. 2000. Voluntary feed intake and feeding behaviour of group-housed growing pigs are affected by ambient temperature and body weight. Livest Prod Sci 63:245–253. [Google Scholar]
- 42.Salfen BE, Carroll JA, Keisler DH. 2003. Endocrine responses to short-term feed deprivation in weanling pigs. J Endocrinol 178:541–551. [DOI] [PubMed] [Google Scholar]
- 43.Slim K, Vicaut E, Launay-Savary MV, Contant C, Chipponi J. 2009. Updated systematic review and meta-analysis of randomized clinical trials on the role of mechanical bowel preparation before colorectal surgery. Ann Surg 249:203–209. [DOI] [PubMed] [Google Scholar]
- 44.Smith SR, Connolly JC, Gilmore OJ. 1983. The effect of faecal loading on colonic anastomotic healing. Br J Surg 70:49–50. [DOI] [PubMed] [Google Scholar]
- 45.Søreide E, Eriksson LI, Hirlekar G, Eriksson H, Henneberg SW, Sandin R, Raeder J, Task Force on Scandinavian Preoperative Fasting Guidelines, Clinical Practice Committee Scandinavian Society of Anaesthesiology and Intensive Care Medicine) 2005. Preoperative fasting guidelines: an update. Acta Anaesthesiol Scand 49:1041–1047. [DOI] [PubMed] [Google Scholar]
- 46.Swindle MM, Smith AC. 2007. Swine in the laboratory: surgery, anesthesia, imaging, and experimental techniques. Boca Raton (FL): CRC Press. [Google Scholar]
- 47.Szelenyi I, Herold H, Göthert M. 1994. Emesis induced in domestic pigs: a new experimental tool for detection of antiemetic drugs and for evaluation of emetogenic potential of new anticancer agents. J Pharmacol Toxicol Methods 32:109–116. [DOI] [PubMed] [Google Scholar]
- 48.Toscano MJ, Lay DC, Jr, Craig BA, Pajor EA. 2007. Assessing the adaptation of swine to 57 hours of feed deprivation in terms of behavioral and physiological responses. J Anim Sci 85:441–451. [DOI] [PubMed] [Google Scholar]
- 49.Tranquilli WJ, Thurmon JC, Grimm KA. 2013. Lumb and Jones veterinary anesthesia and analgesia. Hoboken (NJ): John Wiley and Sons. [Google Scholar]
- 50.Ward JD. 2008. Preparation for surgery, p 226. In: Schantz JT. A manual for laboratory animal management. vol. 5 Hackensack (NJ):World Scientific. [Google Scholar]
- 51.Won H, Maley P, Salim S, Rao A, Campbell NT, Abbott JA. 2013. Surgical and patient outcomes using mechanical bowel preparation before laparoscopic gynecologic surgery: a randomized controlled trial. Obstet Gynecol 121:538–546. [DOI] [PubMed] [Google Scholar]