Abstract
Training techniques that prepare laboratory animals to participate in testing via cooperation are useful tools that have the potential to benefit animal wellbeing. Understanding how animals systematically vary in their cooperative training trajectories will help trainers to design effective and efficient training programs. In the present report we document an updated method for training rhesus monkeys to cooperatively participate in restraint in a ‘primate chair.’ We trained 14 adult male macaques to raise their head above a yoke and accept yoke closure in an average of 6.36 training days in sessions that lasted an average of 10.52 min. Behavioral observations at 2 time points prior to training (approximately 3 y and 1.3 y prior) were used to quantify behavioral reactivity directed toward humans and toward other macaques. Individual differences in submissive–affiliative reactivity to humans but not reactivity toward other monkeys were related to learning outcomes. Macaques that were more reactive to humans were less willing to participate in training, were less attentive to the trainer, were more reactive during training sessions, and required longer training sessions, longer time to yoke, and more instances of negative reinforcement. These results suggest that rhesus macaques can be trained to cooperate with restraint rapidly and that individual difference data can be used to structure training programs to accommodate variation in animal temperament.
Abbreviation: PRT, positive reinforcement training
Training techniques that prepare laboratory animals to voluntarily participate in husbandry, medical, and experimental procedures are useful tools that can be implemented easily in diverse settings. Typically, such training is accomplished by using positive reinforcement blended with negative reinforcement and desensitization. This type of training, which is known as ‘cooperative’ training,4 can be used to elicit a whole host of behaviors, including presenting limbs for venipuncture or injection,10,28,33 sitting still in one location for medical checks,20,27 and being secured into a primate chair.4,19,23,29 While new methods for training are being developed to promote welfare, interest in understanding how individuals differ in response to stressors, environmental interventions, and health outcomes5,6,17,20 and on how these individual differences influence training-related activities has surged.9,27 These studies are guided by the idea that understanding how stable attributes of individual animals lead to individual differences in welfare outcomes (for example, variation in self-stimulating and self-injurious behavior,17,25 diarrhea;26 for a review and discussion8) can be used to develop effective training and management plans. In the present report, we build on our previous work4 by documenting a training technique that can be used to train rhesus macaques to participate in restraint in a primate chair. Second, we evaluate the relationship between individual differences in human-related submissive–affiliative reactivity as they relate to variation in learning.
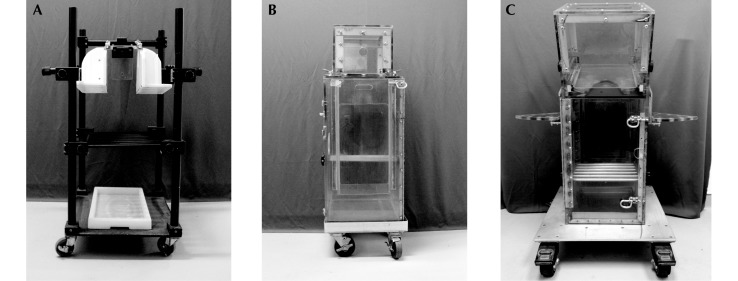
Chair restraint is one of the most common forms of restraint used in the NHP laboratory (for an extensive review, see reference 4). Primate chairs typically secure the animal into a ‘frame’ (Figure 1 A) at a tethering point (typically at the neck). This form of restraint usually has been accomplished by placing a collar on the animal and training it to allow a pole to be affixed to the collar.2,19,23,29 Once the animal is on the pole, it is led to a frame onto which the collar clips (that is, the open chair). In contrast to open chairs, closed chairs are acrylic boxes with internal seats and a wide variety of door configurations (Figure 1 B and C). These chairs typically secure the animal at the neck by a yoke that is slid into place and prevents the animal from pulling its head down into the body of the chair; external shelves (Figure 1 C) can provide the opportunity for additional restraint at the arm or wrist when access to the limbs is necessary. Because animals can move from their cages into the closed chairs, such chairs do not require collars or attachment to a pole. Our group previously demonstrated that chair training for an acrylic box chair could be accomplished by using cooperative techniques in approximately 14 d of 30-min daily training sessions, with animals that were essentially training-naïve.4 In that study, 14 macaques were trained to enter an acrylic box chair (Figure 1 B), raise their heads through an opening in the top of the chair, and have their heads secured by use of a sliding ‘yoke.’
Figure 1.
Standard primate restraint chairs. (A) Pole and collar chair. Closed box chairs: (B) the training chair and (C) the slant-topped testing chair.
A new project in our group necessitated training 14 chair-naïve macaques to participate in chairing so that we could record eye-tracking and psychophysiologic data noninvasively. Unlike our previous training cohort,4 animals in the present group were raised outside in large social groups (60 to 120 macaques; 0.2 ha chain-link enclosures) at the California National Primate Research Center and were relocated indoors as adults as part of a study on the neurobiology of socioaffective behavior. On relocation, all animals were housed with a macaque that they knew from their large social group and who was also a study subject. During this period, we collected behavioral observations of each animal with his pair-mate, allowing us to quantify affective reactivity (for example, the number of affective behaviors produced by a monkey in a given period of time) directed toward other animals as well as behaviors directed toward humans. In addition, we began training these animals using positive reinforcement methods to encourage positive macaque–human interactions (for example, gently touching humans rather than grabbing) and curtail disruptive behavior (for example, being aggressive toward husbandry staff). In the time between the initial behavioral observations and chair training, macaques continued to be trained to participate in experiments by using positive reinforcement, and additional data were collected on their reactivity to humans as part of the widely used,11,12,14,16,17,21,31 standardized human intruder test. As a result, when chair training of the present cohort began, there were documented individual differences in human-directed submissive–affiliative reactivity collected at 2 time points for a cohort of animals that had moderate experience with positive reinforcement training (PRT).
The present report describes the training methods used to cooperatively train 14 adult male rhesus macaques to participate in chair restraint. During this process, we noticed marked variability in the number of days that were required for training, the speed at which animals did what was asked of them, and the number of times negative reinforcement was used. In particular, 2 animals seemed to have particularly slow learning trajectories and scored poorly on the training outcome measures. Given that temperament-related individual differences have previously been related to training outcomes in NHP (for example, rhesus macaques,9 chimpanzees;29 for a review see reference 8), we were interested to see whether we could predict cooperative chair-training metrics according to individual difference variables collected earlier in the project. As such, a series of a posteriori (or post hoc) individual differences analyses were conducted to evaluate variation in learning as it related to variation in behavioral reactivity 3 y prior to training. As detailed following, submissive–affiliative reactivity directed toward humans (but not toward other monkeys) predicted the speed of learning, willingness to cooperate, and the number of times negative reinforcement was required.
Materials and Methods
Experimental procedures were developed in consultation with the staff at the California National Primate Research Center. All protocols were approved by the University of California–Davis IACUC.
Animals.
Adult male rhesus macaques (n = 14; age [mean ± 1 SD], 7.87 ± 0.55 y) were chair trained by using efficient cooperative restraint training.4 The macaques were part of a study to investigate the effect of anterior cingulate cortex damage on social behavior, physiology, and affect.
Rearing and experimental history.
Macaques were born and reared in the outdoor field cages at the California National Primate Research Center (0.2 ha; 30.5 m × 61.0 m × 2.4 m; approximately 60 to 120 animals per cage). Macaques were 4.78 ± 0.58 y old when they were removed from their large social groups and relocated into standard indoor cages. Cage sizing over the course of the study period was determined by animal weight and was one of 3 sizes (61 cm × 66 cm × 81 cm, 85.5 cm × 68 cm × 82 cm, and 113 cm × 69 cm × 93 cm). After relocation, each macaque was paired with a familiar animal from his large outdoor cage. At 10.0 ± 2.3 mo after moving indoors, macaques underwent surgery at 5.61 ± 0.60 y of age, during which they received either bilateral ibotenic acid lesions to the anterior cingulate cortex or sham operations.
After a recovering from surgery, macaques participated in several experiments to assess their social behavior and affective processing. Animals were chair trained as documented following to prepare them for participation in an upcoming psychophysiology experiment (modeled after that in reference 3). We collected data on the chair-training process to compare the length of time required to train these macaques with that of our previous cohort,4 to evaluate the efficacy of our training procedures. At the time of the current experiment, animals had been living indoors for approximately 3 y. All animals were socially housed in standard indoor cages that varied in size according to each animal's weight. Four subjects were housed continuously (24 h daily, 7 d each week) with another animal; the other 10 animals were housed intermittently (a minimum of 7 h daily for 7 d a week) with another animal because of dietary intake restrictions. Animals were fed monkey chow and oat–rice–pea enrichment twice daily, produce biweekly, and had unlimited access to water 24 h daily. In addition, subjects had continuous access to nylon bones or Kong toys and standard enrichment (for example, videos of NHP weekly). Rooms were maintained on a 12:12-h light:dark cycle at 26 °C.
Chair-training.
Trainers’ and animals’ training histories.
The trainers for this experiment (the authors) had 5 to 10 y of experience with rhesus monkeys and were experienced in using cooperative techniques for chair training.4 Trainers were assisted by other members of the laboratory who also had experience working with the animals on this project.
Prior to chair training, all animals had experience with PRT with the trainers from the present study. Animals were trained basic behaviors, such as touching a target gently and staying still at a certain location in their cage (that is, ‘stationing’). Through this process, animals learned the meaning of our bridging stimulus (first, a clicker, then a verbal cue). A bridging stimulus is an event marker identifying the desired response, bridging the time between the response and delivery of a treat.22 We elected to use a verbal bridge32 for the present training, rather than a clicker13,15,30,32 (perhaps the most ubiquitous bridging stimulus), to allow the trainer to have both hands free during training.
Chair-training procedure.
The training room and box chair used for initial training were identical to those used in our previous report.4 We refer to this chair as the ‘training chair’ throughout. Macaques were moved from the cages in which they lived to a cage in a room in another building, which we refer to as a ‘holding room.’ Animals were moved by using transfer boxes (31 cm × 56 cm × 40 cm), as is the standard for moving animals at our facility (animals walk into the transfer box from their cages, and then are carried by using a cart to a new location). Macaques entered the chair in the holding room (simply by walking from the cage into the box chair) and then were wheeled into a second room for their training sessions. We elected to use this configuration of caging solely because of the other experimental demands on our space.
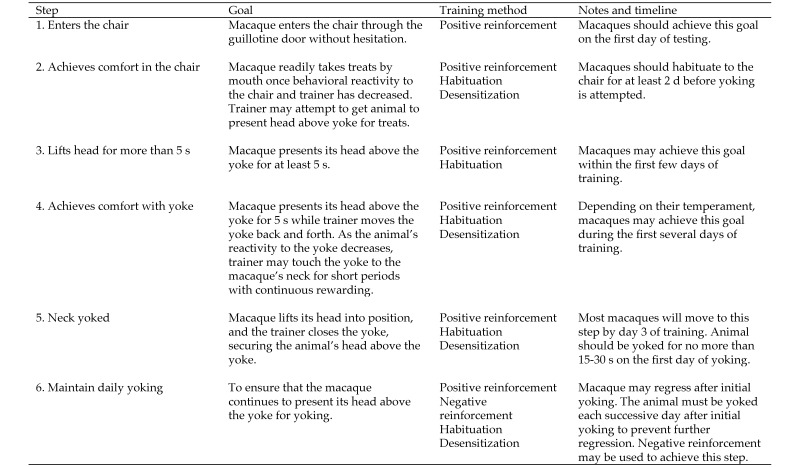
The step-by-step training procedure, including steps, associated goals, and training timelines, is detailed in Figure 2. Initially, only PRT techniques were used to train macaques to enter the training chair and lift their heads above the yoke. Animals were bridged verbally when they lifted their heads above the yoke and then were rewarded with a food treat (small pieces of dried fruit, cereal, and so forth). Once animals willingly lifted their heads above the yoke, the yoke was closed, securing them in place. At this point, negative reinforcement was used when needed to get the animals to consistently present their head above the yoke for yoking. For example, when animals tucked their heads below their bodies and stayed in that position, unresponsive to verbal cues, for more than 2 to 3 min, the chair bottom was slid up toward the yoke, limiting the amount of space they had to move about in the chair. Training procedures followed the step-by-step procedures outlined previously.4
Figure 2.
Training procedure.
Modifications were made to the training procedure that expedited training for this group as compared with those described previously.4 The first, and perhaps most significant, modification was that none of the macaques were collared in preparation for chair training. As such, no animals were ever moved into ‘yoking position’ with a pole. Our previous anecdotal experience (described in reference 4) suggested that using a pole to move animals into position increased the number of training days required. Similarly, we believe that learning was compromised when animals accidently hit their collars on the yokes. For those reasons, we did not use collars for the current group.
A second modification made to the training procedure was that the macaques were desensitized to having the yoke touch their neck (step 4, Figure 2). To accomplish this goal, the trainer touched the yoke against the animal's neck for short periods (a few seconds) and delivered treats to the monkey as the yoke made contact with his neck. We repeated this process several (approximately 8 to 12 times per session). Most animals desensitized to the yoke contact quickly, within one training day, as evidenced by decreased behavioral reactivity each time the yoke touched their necks.
The third and final variation made to the training procedure was that we successively increased the amount of time the animal was yoked each day. Macaques were yoked for only a few seconds on the first day that we closed the yoke. We then built up to longer durations (maximum, 20 min). Macaques were given continuous high-value treats (for example, pieces of dried fruit, grape halves, and so forth) for the entire duration of neck restraint on these first few days they were yoked.
Collection of chair-training data and criteria for training completion.
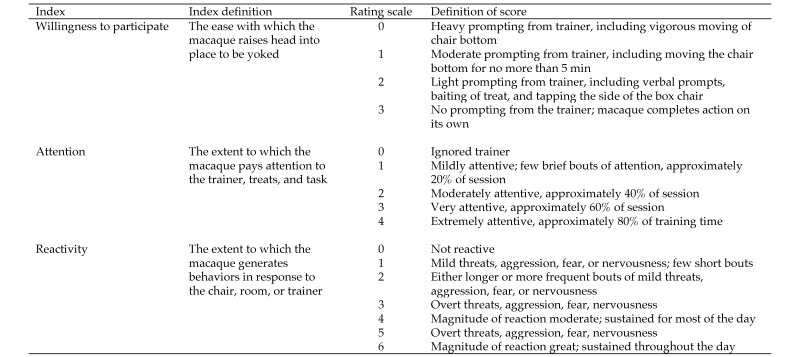
Behavioral indices were collected for each animal on each day of chair training (Figure 3) and were modified from those described previously.4 Each macaque's ‘willingness to participate’ in the session, their attention to the trainer, and their reactivity to the trainer and being in the chair were recorded. The duration (min) of each session and the time needed to yoke each animal (seconds) were recorded also.
Figure 3.
Behavioral indices recorded during training. The frequencies of grimaces and lipsmacks were aggregated to reflect submissive–affiliative reactivity to humans.
We defined an animal as trained according to the definition used previously.4 Macaques were deemed to be trained when they presented their necks for yoking on 2 consecutive days in less than 5 min with scores of 2 or greater on the willingness-to-participate index. Although setting the time criterion to 5 min might be considered very liberal, this duration was chosen: 1) to be consistent with our previous chair training process,4 2) because we knew from previous experience4 that macaques present for chairing more rapidly as the number of experiences in the chair increases during training and testing, and 3) because, whenever possible, the goal was to use only positive reinforcement and therefore to give animals ample time to perform the desired behavior without negative reinforcement. Because we elected to continue to chair macaques in the training chair after they were deemed to be trained according to those criteria, we also recorded instances when training regressions occurred—that is, days during which animals were not willing to present their heads for yoking within 5 min. If a regression event occurred, we continued to chair the animal until he once again met criteria for being trained.
Individual difference measures.
Observations during indoor acclimation.
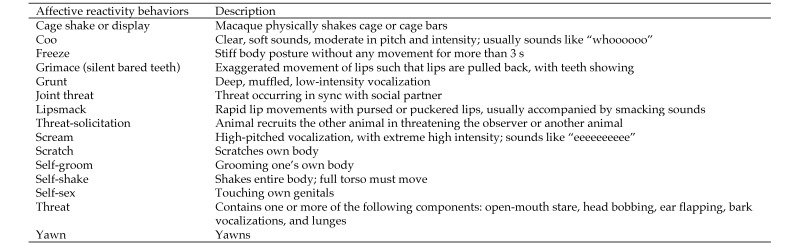
A series of behavioral observations of each animal's social behavior and reactivity to a human observer were collected while the macaques were adjusting to living indoors in pairs. At the time, macaques were paired for 24 h day, 7 d each week. Observations occurred, on average, 70 ± 8 d after moving animals indoors and 3 y prior to chair training. Three familiar observers meeting an interrater reliability of 90% collected 15 focal samples of 5 min each1 from each animal across 3 wk. All observations occurred between 1500 and1730, once husbandry activities were completed for the day. Behaviors of interest (Figure 4) were collected using The Observer 5.0 (Noldus, Wageningen, The Netherlands). Observation order was randomized across day.
Figure 4.
Behavioral ethogram.
Indoor acclimation data were used to compute indices capturing variation in social behavior and affective reactivity that might relate to the efficiency of chair training. Specifically, we were interested in whether affective reactivity in general (for example, with other monkeys, to events occurring in the housing room) or that directed to the human observer specifically might predict efficiency. The hypothesis tested was that systematic variation between reactivity directed toward humans and the chair-training metrics would occur because chair training required close interactions with the human observers. We also hypothesized that a macaque's ‘style’ of interacting with humans might differentially predict learning. For example, macaques that appeared to be submissive or affiliative with humans might have different learning outcomes than those that were aggressive toward humans. To those ends, a variable to reflect affective reactivity directed toward other monkeys (that is, all faces, vocalizations, and so forth that are thought to communicate an internal state; Figure 4) and a variable to reflect submissive–affiliative human reactivity (that is, number of bared teeth and lipsmack displays) were computed. We used the number of threats directed to the observer as an index of aggression-related reactivity to the observer. For each index, the frequency of behaviors was summed across each animal's 15 observations.
Human intruder testing.
Approximately 1.75 y after the animals moved indoors, 1 y after surgery, and 16 mo before chair training at 6.50 ± 0.56 y of age, macaques completed a standardized task to measure reactivity to threat, the human intruder task.12,14,16,17,21,31 During testing, an unfamiliar human presented himself in front of the macaque in a low-threat condition (in profile so that his vision is directed away from the monkey) and a high-threat condition (staring directly at the monkey) in each of 2 positions (distant, approximately 1 m from the monkey; close, approximately 0.3 m from the monkey). Each trial lasted 1 min. Each macaque was tested by using the same protocol on each of 5 d of testing in a standard primate cage (85.5 cm × 68 cm × 82 cm) in an unfamiliar room.
Discrete behaviors were recorded by an observer who met a 90% interrater reliability standard prior to the experiment. Behaviors of interested were collected by using 1–0 sampling16,17 (1 when a behavior was expressed, 0 when it did not occur) in 10-s bins (resulting in 6 data points per condition). Given the results of the previous individual differences analyses, only behaviors related to submission–affiliation (bared teeth and lipsmack) were aggregated to provide a measure of submissive–affiliative reactivity. Data were averaged across 5 test days creating a single index that reflected mean submissive–affiliative reactivity to the human intruder.
Data analysis.
Statistical analyses were completed by using SPSS version 22 (IBM, Armonk, NY). Data were log transformed in cases where they were not normally distributed (as indicated following); in these cases, raw means are presented for ease of interpretation. For the individual difference analyses, individual difference variables were regressed onto the chair training metrics. Individual difference variables were entered into the analyses as raw scores (rather than being centered), because 0 had a clear interpretation (that is, no behavior, no reactivity). We also explored interactions between the individual difference variables and curvilinear relationships. When both the linear and curvilinear models fit the data, we selected the model that accounted for the most variance and appeared to visually fit the data the best. When appropriate, we tested negative binomial and Poisson distribution regression by using models built to accommodate zero-inflated data sets. In cases when model fit was equivalent, we selected the simplest model.
Results
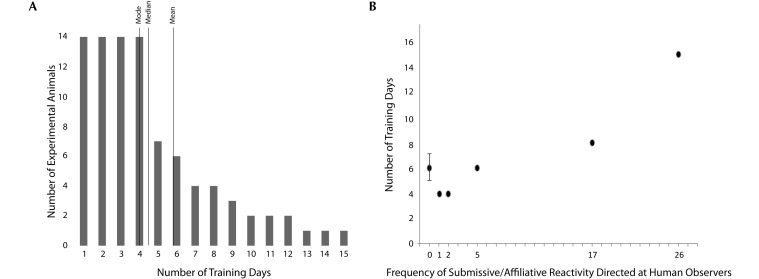
According to the criteria previously used in our group4 regarding successful completion of training, the present group of 14 male rhesus macaques was trained in 6.36 ± 3.48 (mean ± 1 SD) training days (Figure 5 A). There were no differences between control animals and macaques with anterior cingulate cortex damage (t12 = 0.18, P = 0.857; analysis performed on log-transformed data).
Figure 5.
Number of days required to train. (A) Descriptive statistics related to the number of days required to be trained. (B) Relationship between human-directed submissive–affiliative reactivity during indoor acclimation observations and the number of days required for training. The reactivity score (x axis) reflects the total number of behaviors generated during the observations. Error bars indicate the SEM for the 8 macaques that evidenced no human-directed affective reactivity. Two animals each generated 1 submissive–affiliative reaction toward humans but required the same number of days to train, thus there are no error bars on that point.
Training sessions averaged 10.52 ± 7.67 min, and it took an average of 5.33 ± 9.35 min to yoke animals during their training sessions (including the final day that they reached criterion for being trained). In general, macaques were fairly willing to participate (score, 2.34 ± 0.68), attended to the trainer and the task well (3.22 ± 0.86), and were minimally reactive (0.88 ± 1.00). Of the 14 macaques, 9 were trained exclusively with PRT; negative reinforcement (for example, moving the chair bottom up) was used in combination with PRT for the other 5 animals. Of those 5 macaques, 1 required negative reinforcement once, 2 required it 3 times, 1 required it 5 times, and the remaining animal required it 10 times.
Given that 7 of the 14 animals were trained in just 4 d, we elected to continue subsequently chairing them to ensure that they were actually cooperative and understood what was being asked of them. All macaques completed at least 8 training sessions in the chair. During this time, 5 of the 14 animals experienced regression events; 4 macaques had one regression event and the remaining animal had 2 regression events. On average, the animals were yoked on these subsequent sessions in 101.19 ± 97.11 s. During these sessions, 7 of the 14 macaques presented for yoking in less than 1 min, 4 of the animals presented for yoking less than 2 min, and 3 of the animals presented for yoking in less than 4 minutes (omitting the days in which one animal regressed).
Comparison with previous training group.
To determine whether our training had become more efficient since the original cohort,4 we compared the number of days required to train and the average session length between groups. The current cohort was trained in significantly fewer days than was the original cohort (t26 = 6.27, P < 0.000001; current cohort, 6.36 ± 3.48 d; previous cohort, 14.14 ± 3.08 d). Similarly, the present cohort was trained in significantly shorter sessions than was the original cohort (t26 = 6.93, P < 0.000001; current cohort, 10.52 ± 7.67 d; previous cohort, 26.54 ± 4.11 d).
Individual differences in cooperative learning.
Indoor acclimation reactivity.
The relationships between the general affective reactivity variable (reflecting behavior with other macaques) and the learning metrics were evaluated first. Neither general affective reactivity (nor its squared term) was related to willingness to participate, attention, reactivity, session length, time required to yoke, the number of negative reinforcement instances required, or the number of days required to train. Similarly, aggression-related reactivity directed toward the observer did not predict any of the learning metrics.
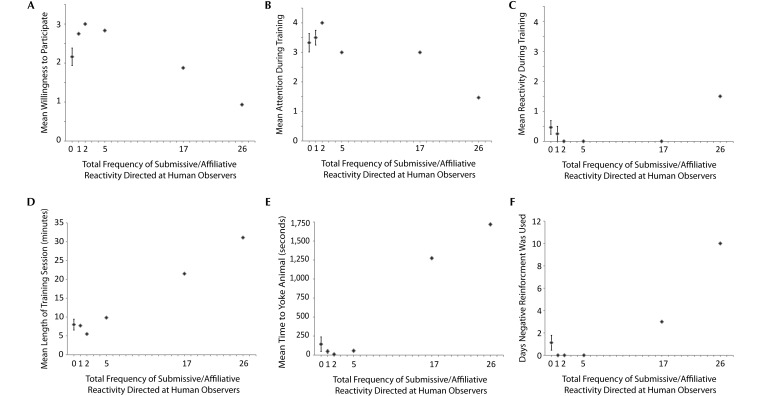
In contrast, submissive–affiliative reactivity directed toward the human observer did predict learning outcomes. Willingness to participate and attention were both predicted by submissive–affiliative reactivity (Figure 6). In both cases, the linear and curvilinear models fit the data, with the curvilinear models accounting for slightly more variance (willingness to participate:2 β = –0.63, F1,13 = 8.08, P = 0.015, R2 = 0.60; attention:2 β = –0.59, F1,13 = 6.35, P = 0.027). In all cases, higher frequencies of submissive–affiliative directed human reactivity predicted worse training performance (that is, less willingness to participate and less attention), although the magnitude of the effect was greater for the highest levels of submissive–affiliative reactivity and essentially constant across the lower levels of submissive–affiliative reactivity. There was a significant linear relationship between reactivity during training and submissive–affiliative reactivity to humans (β = 0.78, F1,13 = 18.64, P = 0.001, R2 = 0.61). Macaques that were more submissive–affiliative toward humans were more reactive during training (Figure 6 C). Similarly, there was a significant linear relationship between submissive–affiliative reactivity to humans and the length of training sessions (β = 0.90, F1,13 = 53.18, P = 0.00001, R2 = 0.82 (Figure 6 D) as well as the average number of seconds required to yoke the macaques (β = 0.92, F1,12 = 56.54, P = 0.00001, R2 = 0.84; Figure 6 E). The squared submissive–affiliative reactivity to humans term predicted the number of instances of negative reinforcement required to train, however the relationship was such that the although the most negative reinforcement was required by the macaques that were most submissive–affiliative, a few animals that were not submissive–affiliative reactive to humans also required negative reinforcement (β = 0.85, F1,13 = 29.96, P = 0.0001, R2 = 0.81; Figure 6 F). Finally, the squared submissive–affiliative reactivity toward humans term significantly predicted the number of days required to train (β = 0.68, F1,13 = 10.30, P = 0.008, R2 = 0.46; Figure 5 B). The number of days required to train was essentially consistent, on average, across the lowest levels of submissive–affiliative reactivity but was much greater for the macaques that were highest in submissive–affiliative reactivity.
Figure 6.
Submissive–affiliative reactivity directed toward humans during indoor acclimation observations predicts training outcomes. (A) Average willingness to participate. (B) Average attention. (C) Average reactivity. (D) Average duration of training sessions. (E) Average time to yoke (F) Number of trainings days during which negative reinforcement was required. The reactivity score (x axis) reflects the total number of behaviors generated during the observations. Error bars represent the SEM for the 8 macaques with the reactivity score of 0 and the 2 animals with the reactivity score of 1.
Human intruder reactivity.
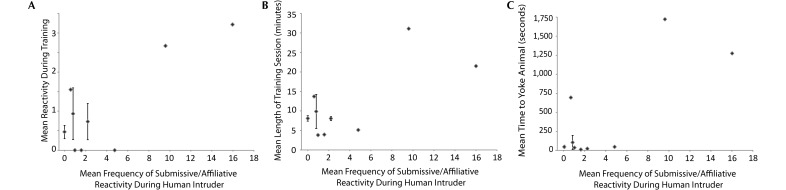
As was the case regarding the indoor acclimation reactivity analyses, submissive–affiliative reactivity directed toward the human observer during human intruder testing predicted learning outcomes. Reactivity during training, the length of the training session, and time required to yoke were all predicted by submissive–affiliative reactivity (Figure 7). There was a significant linear relationship between reactivity during training and submissive–affiliative reactivity to humans (β = 0.76, F1, 13 = 16.73, P = 0.001, R2 = 0.58). Macaques that were more submissive–affiliative toward humans were more reactive during training (Figure 7 A). Similarly, there was a significant linear relationship between submissive–affiliative reactivity to humans and the duration of training sessions (β = 0.70, F1,13 = 11.80, P = 0.005, R2 = 0.50; Figure 7 B) as well as the average number of seconds required to yoke the animals (β = 0.79, F1,12 = 18.78, P = 0.001, R2 = 0.63; Figure 7 C).
Figure 7.
Submissive–affiliative reactivity directed toward humans during the human intruder task predicts learning outcomes. (A) Average reactivity. (B) Average duration of training sessions. (C) Average time to yoke. The reactivity score (x axis) reflects the mean reactivity over the 5 testing days. Error bars represent the SEM in cases in which multiple macaques had the same reactivity score.
Submissive–affiliative reactivity during the indoor acclimation observations was highly correlated with that during the human intruder task (r = 0.81, P = 0.005).
Discussion
The data from the present training study demonstrated that we were able to train PRT-experienced monkeys to cooperate during chair restraint quickly—6.36 d on average, with many monkeys meeting the criteria on training day 4. Furthermore, stable (across time and context) individual differences in the extent to which monkeys were reactive to humans predicted the efficiency of this training. Together, these findings suggest that not only can animals be rapidly trained to participate in restraint by using cooperative techniques but also that trainers can design training programs according to variations in animal temperament.
Given that training proceeded so rapidly, we continued to chair the macaques after they reached our established criteria for successful completion of training. Continuing to chair them allowed us to evaluate whether macaques regressed in their training, and 5 of the 14 animals did regress at least once. Given that our definition of regression was ‘not presenting the head for yoking in less than 5 min,’ it is possible that some of the variation that we saw in regression was simply due to monkeys having an ‘off day.’ This idea is underscored by the fact that, typically, after a single regression event, monkeys returned to chairing and presented their heads within the desired timeframe. Although our criterion for training—allowing macaques as long as 5 min to present their necks to be yoked—might be interpreted as lenient, it is worth noting that 11 of the 14 animals consistently presented within 2 min, with 7 of those macaques presenting consistently in less than 1 min. Perhaps the animals might have been taught to present for yoking more rapidly if negative reinforcement had been used more liberally.
Our success with this cohort represented a significant improvement on our previous chair-training experience. Compared with our previous experience, the number of training sessions required for animals to meet the training criterion was significantly decreased as was the duration of training sessions. Several explanations for this pattern of effects are possible, many of which could and should be explored empirically. The first possibility is that we are simply better trainers, having previously worked with another cohort.4 A second possibility is that having a history of PRT improves future PRT outcomes.18 The macaques in the current study were previously clicker trained, trained with a verbal bridge, and trained to touch a human hand gently, to offer left or right hands, and some to station in a particular location. Beyond knowing how to learn, having a PRT history with particular trainers also reinforces positive learning-oriented relationships with those trainers.24 Perhaps this relationship aided in increasing the efficiency of the training. Our previous cohort did not have such a learning history. Regardless of mechanism, the current findings underscore that our previous average of approximately 14 d to chair train was reproducible.
Perhaps the most interesting finding of the current study is that the extent to which monkeys react submissively to humans predicts their learning outcomes at later time points. Given that submissive–affiliative reactivity predicted behavioral reactivity during chair training, these findings also lend support for the stability of reactivity over time. Individual differences in this reactivity were recorded at 2 different time points—early in the study period (when animals were adjusting to life indoors) and during a threat-relevant experiment (approximately 2 y later). A particular type of reactivity predicted learning metrics: submissive (or affiliative) reactivity directed toward humans; general affective reactivity with other macaques and aggression-related affective reactivity to humans did not predict learning outcomes. It is worth noting that individual difference effects were driven by 2 very reactive animals in both sets of behavioral data. But those 2 animals were not only consistently reactive—they also were consistently poorer learners across metrics used to evaluate our cooperative techniques. Given the small sample size of this study and the limited variance in submissive–affiliative reactivity, it is crucial to replicate these findings in larger, more diverse samples. The finding that 2 of our 14 macaques were particularly challenged by cooperative chair training is consistent with our previous report,4 suggesting that trainers should expect that approximately 15% of their subjects will face such learning challenges.
Findings from the current study are complementary to other studies of NHP that have demonstrated that individual differences influence learning. For example, NHP that were unwilling to interact with a novel object presented at their cage were less likely to learn to touch a target via PRT.9 Similarly, chimpanzees who were rated to be high in ‘openness’—a personality trait reflecting curiosity, creativity, and willingness to engage with the environment—were more likely to participate in blood glucose testing, the steps of which were trained by using PRT.27 Together, findings on temperament and training suggest that we should be able to a priori predict, on future occasions, which animals will require the most experience in order participate in being chaired. This knowledge might be used to balance training groups and set trainer expectations on individual animal training time. What we do not want is to encourage the biased selection of animals for studies based on these parameters. Of note, the indoor acclimation individual difference variables were measured during a critical period of adjustment (to indoor relocation),7 a period during which many researchers elect not to collect experimental data.
There are 2 important caveats associated with the individual difference findings. First, the effects are driven by a small number of highly submissive–affiliative animals. These macaques consistently influenced all of the metrics however, and this consistency in patterning provides further support for our claims regarding individual differences. Second, there was modest variation in the learning outcomes even for animals that evidenced no submissive–affiliative reactivity toward humans. This finding suggests that there may be another source or sources of individual variation in cooperative learning. Identifying these sources could be a fruitful avenue for future research. Additional studies should also investigate whether other temperamental variables or individual differences recorded at a different point of time during animals’ experimental history influence learning of this sort.
Once macaques were successfully trained in the training chair, they transitioned to another chair to use for testing (Figure 1 C; previously used in references 2, 18, and 20). The testing chair had a slanted top and a yoke that closed from the back. Having anticipated that this design might be an issue for training, we elected to first train animals in a flat-top chair used previously in the group.4 The logic for this choice was that we predicted that the macaques might react negatively to having the yoke close from behind (animals faced away from the sliding yoke to sit comfortably in the slant-top design). Animals required several sessions in the new chair to present their necks readily—that is, the transition between chairs was not perfectly smooth. The macaques, however, did get used to this design over time, but we suggest using chairs in which the yoke closes from the front (as in Figure 1 B).
By using cooperative training techniques, such as the chair-training method described in this report, we hope that researchers are able to move toward experimental techniques that allow NHP to actively participate in the research protocols and toward greater reliance on noninvasive methods. Understanding how individual animals will progress along learning trajectories according to features of their temperament and behavior likely will provide trainers with important information that they need to design training schedules and set learning expectations on an animal-by-animal bases. We hope that these new insights about cooperative training help to further refine research protocols and improve the wellbeing of laboratory animals.
Acknowledgment
This research was supported by grant R37MH57502, F32MH087067 (to EBM) and by the base grant of the California National Primate Research Center (RR00169). EBM was supported by grant K99MH10138 during the preparation of this manuscript.
References
- 1.Altmann J. 1974. Observational study of behavior: sampling methods. Behaviour 49:227–266. [DOI] [PubMed] [Google Scholar]
- 2.Anderson JH, Houghton P. 1983. The pole and collar system: a technique for handling and training nonhuman primates. Lab Anim 12:47–49. [Google Scholar]
- 3.Bliss-Moreau E, Machado CJ, Amaral DG. 2013. Macaque cardiac physiology is sensitive to the valence of passively viewed sensory stimuli. PLoS One 8:e71170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bliss-Moreau E, Theil JH, Moadab G. 2013. Efficient cooperative restraint training with rhesus macaques. J Appl Anim Welf Sci 16:98–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boccia ML, Laudenslager ML, Reite ML. 1995. Individual differences in macaques’ responses to stressors based on social and physiological factors: implications for primate welfare and research outcomes. Lab Anim 29:250–257. [DOI] [PubMed] [Google Scholar]
- 6.Capitanio JP. 2010. Individual differences in emotionality: social temperament and health. Am J Primatol 73:507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Capitanio JP, Kyes RC, Fairbanks LA. 2006. Considerations in the selection and conditioning of Old World monkeys for laboratory research: animals from domestic sourcesl. ILAR J 47:294–306. [DOI] [PubMed] [Google Scholar]
- 8.Coleman K. 2011. Indivdiual differences in termperamnet and behavioral management practices for nonhuman primates. Appl Anim Behav Sci 137:106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coleman K, Tully LA, McMillan JL. 2005. Temperament correlates with training success in adult rhesus macaques. Am J Primatol 65:63–71. [DOI] [PubMed] [Google Scholar]
- 10.Coleman K, Pranger L, Maier A, Lambeth SP, Perlman JE, Thiele E, Schapiro SJ. 2008. Training rhesus macaques for venipuncture using positive reinforcement techniques: a comparison with chimpanzees. J Am Assoc Lab Anim Sci 47:37–41. [PMC free article] [PubMed] [Google Scholar]
- 11.Corcoran CA, Pierre PJ, Haddad T, Bice C, Suomi SJ, Grant KA, Friedman DP, Bennett AJ. 2012. Long-term effects of differential early rearing in rhesus macaques: behavioral reactivity in adulthood. Dev Psychobiol 54:546–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davidson RJ, Kalin NH, Shelton SE. 1993. Lateralized response to diazepam predicts temperamental style in rhesus monkeys. Behav Neurosci 107:1106–1110. [DOI] [PubMed] [Google Scholar]
- 13.Fernström AL, Fredlund H, Spångberg M, Westlund K. 2009. Positive reinforcement training in rhesus macaques—training progress as a result of training frequency. Am J Primatol 71:373–379. [DOI] [PubMed] [Google Scholar]
- 14.Fox AS, Shelton SE, Oakes TR, Davidson RJ, Kalin NH. 2008. Trait-like brain activity during adolescence predicts anxious temperament in primates. PLoS One 3:e2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gillis TE, Janes AC, Kaufman MJ. 2012. Positive reinforcement training in squirrel monkeys using clicker training. Am J Primatol 74:712–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gottlieb DH, Capitanio JP. 2012. Latent variables affecting behavioral response to the human intruder test in infant rhesus macaques (Macaca mulatta). Am J Primatol 75:314–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gottlieb DH, Capitanio JP, McCowan B. 2013. Risk factors for stereotypic behavior and self-biting in rhesus macaques (Macaca mulatta): animal's history, current environment, and personality. Am J Primatol 75:995–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harlow HF. 1949. The formation of learning sets. Psychol Rev 56:51–65. [DOI] [PubMed] [Google Scholar]
- 19.Howell LL, Hoffman JM, Votaw JR, Landrum AM, Jordan JF. 2001. An apparatus and behavioral training protocol to conduct positron emission tomography (PET) neuroimaging in conscious rhesus monkeys. J Neurosci Methods 106:161–169. [DOI] [PubMed] [Google Scholar]
- 20.Izzo GN, Bashaw MJ, Campbell JB. 2011. Supplemental material for enrichment and individual differences affect welfare indicators in squirrel monkeys (Saimiri sciureus). J Comp Psychol 125:347–352. [DOI] [PubMed] [Google Scholar]
- 21.Kalin NH, Shelton SE, Davidson RJ, Kelley AE. 2001. The primate amygdala mediates acute fear but not the behavioral and physiological components of anxious temperament. J Neurosci 21:2067–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laule GE, Bloomsmith MA, Schapiro SJ. 2003. The use of positive reinforcement training techniques to enhance the care, management, and welfare of laboratory primates. J Appl Anim Welf Sci 6:163–173. [DOI] [PubMed] [Google Scholar]
- 23.McMillan JL, Perlman JE, Galvan A, Wichmann T, Bloomsmith MA. 2014. Refining the pole-and-collar method of restraint: emphasizing the use of positive training techniques with rhesus macaques (Macaca mulatta). J Am Assoc Lab Anim Sci 53:61–68. [PMC free article] [PubMed] [Google Scholar]
- 24.Minier DE, Tatum L, Gottlieb DH, Cameron A, Snarr J, Elliot R, Cook A, Elliot K, Banta K, Heagerty A, McCowan B. 2011. Human-directed contra-aggression training using positive reinforcement with single and multiple trainers for indoor-housed rhesus macaques. Appl Anim Behav Sci 132:178–186. [Google Scholar]
- 25.Novak MA. 2003. Self-injurious behavior in rhesus monkeys: new insights into its etiology, physiology, and treatment. Am J Primatol 59:3–19. [DOI] [PubMed] [Google Scholar]
- 26.Prongay K, Park B, Murphy SJ. 2013. Risk factor analysis may provide clues to diarrhea prevention in outdoor-housed rhesus macaques (Macaca mulatta). Am J Primatol 75:872–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reamer LA, Haller RL, Thiele EJ, Freeman HD, Lambeth SP, Schapiro SJ. 2014. Factors affective initial training success of blood glucose testing in captive chimpanzees (Pan troglodytes). Zoo Biol 33:212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reinhardt V. 2003. Working with rather than against macaques during blood collection. J Appl Anim Welf Sci 6:189–197. [DOI] [PubMed] [Google Scholar]
- 29.Ruys JD, Mendoza SP, Capitanio JP, Mason WA. 2004. Behavioral and physiological adaptation to repeated chair restraint in rhesus macaques. Physiol Behav 82:205–213. [DOI] [PubMed] [Google Scholar]
- 30.Schapiro SJ, Bloomsmith MA, Laule GE. 2003. Positive reinforcement training as a technique to alter nonhuman primate behavior: quantitative assessments of effectiveness. J Appl Anim Welf Sci 6:175–187. [DOI] [PubMed] [Google Scholar]
- 31.Williamson DE, Coleman K, Bacanu SA, Devlin BJ, Rogers J, Ryan ND, Cameron JL. 2003. Heritability of fearful-anxious endophenotypes in infant rhesus macaques: a preliminary report. Biol Psychiatry 53:284–291. [DOI] [PubMed] [Google Scholar]
- 32.Veeder CL, Bloomsmith M, McMillan J, Perlman J, Martin A. 2009. Positive reinforcement training to enhance the voluntary movement of group-housed sooty mangabeys (Cercocebus atys atys). J Am Assoc Lab Anim Sci 48:192–195. [PMC free article] [PubMed] [Google Scholar]
- 33.Videan EN, Fritz J, Murphy J, Howell S, Heward CB. 2005. Does training chimpanzees to present for injection lead to reduced stress? Lab Primate Newslett 44:1–2. [Google Scholar]