Abstract
Information regarding effective anesthetic regimens for neonatal rat pups is limited. Here we investigated whether isoflurane or sevoflurane anesthesia maintains physiologic parameters more consistently than does hypothermia anesthesia in neonatal rat pups. Rat pups (age, 4 d) were randomly assigned to receive isoflurane, sevoflurane, or hypothermia. Physiologic parameters monitored at 1, 5, 10, and 15 min included heart rate (HR), respiratory rate (RR), and oxygen saturation (%SpO2). Other parameters evaluated were loss and return of righting reflex, paw withdrawal reflex, and maternal acceptance. Corticosterone and glucose were sampled at 20 min and 24 h after anesthesia induction. Once a surgical plane of anesthesia was achieved, a skin incision was made on the right lateral thigh. After the procedure, all pups were accepted and cared for by their dam. Isoflurane- and sevoflurane-treated pups maintained higher HR, RR, %SpO2, and glucose levels than did hypothermia-treated pups. For both the isoflurane and sevoflurane groups, HR and RR were significantly lower at 10 and 15 min after anesthesia than at 1 min. Compared with hypothermia, isoflurane and sevoflurane anesthesia provided shorter times to loss of and return of the righting reflex. Although corticosterone did not differ among the groups, glucose levels were higher at 20 min after anesthesia induction than at 24 h in all anesthetic groups. We conclude that both isoflurane and sevoflurane anesthesia maintain physiologic parameters (HR, RR, %SpO2) more consistently than does hypothermia anesthesia in 4-d-old rat pups.
Abbreviations: HR, heart rate; RR, respiratory rate; SpO2, oxygen saturation
Neonatal rodent anesthesia is technically challenging, and only limited information on anesthetic techniques has been published. Neonatal rodents are often used in survival surgeries including stereotaxic,2,3,6,8 spinal,20 cardiovascular,28,29 thoracic,13,21 laparoscopic,41 reproductive,17 and dermal1 procedures. Anesthesia of neonates is challenging because of the narrow therapeutic windows for commonly used anesthetics.38 Physiology and pharmacology differ significantly between neonates and adults and affect an anesthetic agent's pharmacodynamics effects. The immature organ structure and function in neonates often results in compromised respiratory and cardiac function in response to anesthetics.38 Compared with adults, neonates are more prone to anesthetic complications, including hypothermia and hypoglycemia, and their drug-metabolism mechanisms are less efficient.4 Additional physiologic differences affecting anesthetic technique include the greater permeability of the blood–brain barrier, higher body-water content, less mature hepatic microsomal enzyme systems, and lower albumin concentrations in neonates than adults.12 Many of the anesthetics that are used frequently in adult rodents, such as ketamine and pentobarbital, are not ideal options for neonates because they often lead to inadequate anesthetic depth, prolonged recovery, or death.7
The most recent comparative evaluation of anesthesia techniques for neonatal rodents was published in 1997,7 prior to the widespread use of the inhalant anesthetics isoflurane and sevoflurane. The anesthetics evaluated in that publication included hypothermia, ketamine, pentobarbital, fentanyl–droperidol, and methoxyflurane.7 In contrast, current scientific protocols for the anesthesia of neonatal rodent commonly use isoflurane, sevoflurane, or hypothermia.6,14,24,25,31 Isoflurane and sevoflurane are commonly used inhalant anesthetics. The advantages of inhalant agents include their low blood gas solubility (which leads to both rapid induction of and recovery from anesthesia), precise control of anesthetic depth, and minimal hepatic metabolism. Disadvantages of inhalant anesthetics include the complexity and expense of delivery systems, waste gas in the environment, and the potential for exposure of personnel. In addition, isoflurane is pungent, causing breath holding in humans,5 and whether this mode of induction is noxious for rodents is unknown. Compared with isoflurane, sevoflurane is less irritating to the respiratory mucosa in humans,11 but whether rodents experience a similar benefit remains unknown. Furthermore, volatile anesthetics have been implicated to cause neuronal degeneration in neonatal pups.23,24
In contrast to inhalant anesthesia, hypothermic anesthesia involves immersing a neonatal rodent in an ice bath after protecting the pup in a latex sleeve to reduce the pain associated with rapid chilling.7 Previous studies indicate that neonatal rodents exposed to severe hypothermia, with subsequent recovery from respiratory and cardiac arrest, do not exhibit subsequent learning deficits.18,36 However, hypothermia in human neonates has been linked to adverse complications including impaired platelet function and coagulation, increased wound infection, cardiac arrhythmia, and prolonged recovery from anesthesia.35
Currently data comparing the effects of isoflurane, sevoflurane, and hypothermic anesthesia in neonatal rodents are unavailable. In the present study, we investigated various physiologic parameters under 3 anesthetic methods. We hypothesized that physiologic parameters in neonatal rats after isoflurane and sevoflurane anesthesia would be more consistent than those after hypothermic anesthesia.
Materials and Methods
Animals.
The study population (n = 77) comprised 8 litters of 3-d-old male and female Sprague–Dawley rat pups (Crl:CD[SD]IGS, Charles River Laboratories, Hollister, CA), which were housed at our facility as litters of 9 or 10 pups with the dam for 1 d before use in experiments. Rats were free of rat coronavirus, rat Theiler virus, Kilham rat virus, rat parvovirus, Toolan H1 virus, rat minute virus, lymphocytic choriomeningitis virus, murine adenovirus types 1 and 2, reovirus type 3, Sendai virus, pneumonia virus of mice, Mycoplasma pulmonis, mites, lice, and pinworms. Animals were housed in static microisolation cages on a 12:12-h dark:light cycle. Rat dams were provided unrestricted access to a commercial diet (Teklad Global 18% Protein Rodent Diet 2018, Harlan Laboratories, Madison, WI) and water filtered by reverse osmosis. All experiments were approved by the Stanford Administration Panel for Laboratory Animal Care. All rats were treated in accordance with the Guide for the Care and Use of Laboratory Animals22 in an AAALAC-accredited facility. Dams and litters were examined on arrival to our facility, and all pups were determined to be healthy and acceptable as study subjects. All pups within a litter were tested on postnatal day 4.
Anesthesia and surgical incision.
Rat pups were randomly assigned to 3 experimental treatment groups (n = 24 each group) and 2 unmanipulated control groups (5 pups total). Rats in treatment groups were monitored after initiation of anesthesia by isoflurane, sevoflurane, or hypothermia. Pups were left with the dam until time of induction of anesthesia and tested in a randomized alternating manner between anesthetic treatment groups. Each pup was weighed prior to the start of anesthesia. For the isoflurane and sevoflurane groups, anesthesia was induced by using an induction chamber (2 L 100% O2 containing either isoflurane at 5% or sevoflurane at 8%) and maintained by using a nose cone (500 mL of 100% O2 with either isoflurane at 3.5% ± 0.1% [1.5 to 2 minimum alveolar concentration; minimum alveolar concentration, 7.28%] or sevoflurane 5% ± 0.4% [1.5 to 2 minimum alveolar concentration; minimum alveolar concentration, 1.86%]) with pups on a circulating-water heating pad.25 Pups in the hypothermia group were placed on top of a latex sleeve, placed in an ice bath, and held in position until anesthesia was attained. Anesthetic parameters measured included heart rate (HR), respiratory rate (RR), and oxygen saturation (%SpO2). The first measurement was recorded at 1 min after adequate anesthetic depth had been attained (no paw withdrawal reflex), and thereafter measurements were taken at 5, 10, and 15 min after induction. Heart rate and %SpO2 were measured by using the PhysioSuite MouseSTAT (Kent Scientific Corporation, Torrington, CT), and respiratory rate was assessed visually. The times to loss of righting reflex and paw withdrawal were recorded. At 15 min after induction and confirmation of a surgical plane of anesthesia (that is, lack of paw withdrawal), a 0.5-cm skin incision was made over the right lateral thigh. The incision was closed with cyanoacrylate glue (Vetbond, 3M Animal Care Products, St Paul, MN).
After closure of the incision, half of the pups in each experimental group were maintained under anesthesia for an additional 5 min. At the end of this period, a final measurement of physiologic parameters was completed, the pups (including 2 unmanipulated pups as controls) were euthanized by decapitation, and blood was collected in a 0.5-mL microfuge tube for analysis of serum corticosterone and glucose. For the other half of each group, pups (including 3 unmanipulated controls) were numbered with colored permanent ink after the closure of the surgical incision, placed on a circulating-water heating pad, and allowed to recover from anesthesia. Time to recovery (return of righting reflex) was recorded and, once ambulatory, the pups were returned to their dams. Pups were observed closely after their return to the dam to ensure maternal acceptance. At 24 h after the induction of anesthesia, the pups were weighed and euthanized by rapid decapitation. Blood was collected into a 0.5-mL microfuge tube for analysis of corticosterone and glucose.
Corticosterone and glucose analysis.
Blood was allowed to clot in 0.5-mL microfuge tubes, spun in a microcentrifuge, and the serum collected and stored in a –80 °C freezer. Serum was stored until all samples had been collected; all samples then were run in a single batch. Rat serum corticosterone was measured by using a quantitative enzyme immunoassay (Corticosterone HS EIA, Immunodiagnostic Systems, Scottsdale, AZ) according to the manufacturer's recommendations, including 1:20 dilution with the calibrator diluent and heating of the samples. The absorbance at 450 nm of each well was determined by using a microplate reader. Corticosterone concentrations were calculated by using a standard curve derived from assaying calibration samples ranging from 0 to 18.8 ng/mL. The assay has a lower limit of detection of 0.17 ng/mL and less than 10% intraassay variation. The serum glucose concentration was determined by using an automated clinical chemistry system (Dimension Xpand, Siemens, Malvern, PA).
Statistical analysis.
Measurements of anesthesia parameters (HR, RR, %SpO2); body weight; times to loss and return of the righting reflex and of loss of the paw withdrawal reflex; and corticosterone and glucose concentrations underwent analysis for repeated measures with Bonferroni correction for multiple comparisons (SPSS, IBM, Somers, NY). Data are expressed as mean ± SEM. A P value of less than 0.05 was considered as significant.
Results
Maternal behavior toward and weight gain in pups.
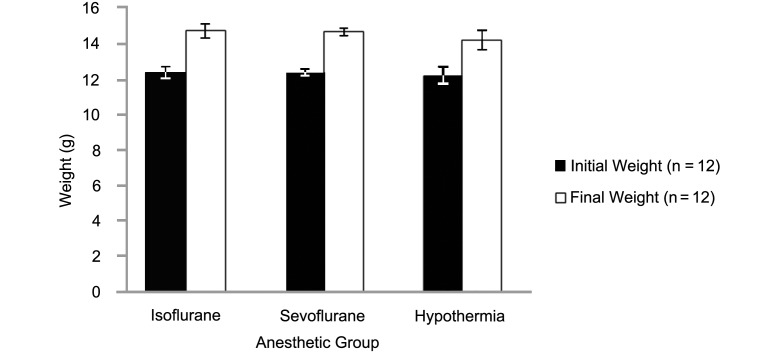
All 8 dams accepted and cared for pups that were returned to them after the surgical procedure. In addition, weight gain at 24 h after anesthesia induction did not differ among groups (Figure 1).
Figure 1.
Initial body weight and at 24 h after the induction of anesthesia in the isoflurane, sevoflurane, and hypothermia groups.
Effects of anesthesia on HR, RR, and %SpO2.
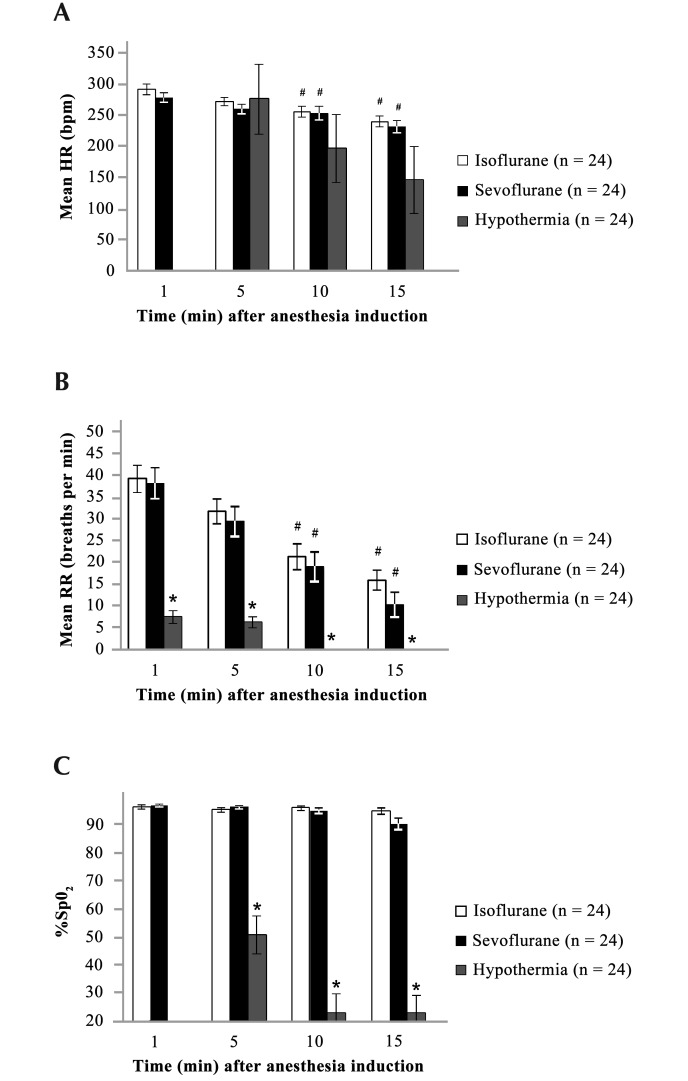
Figure 2 summarizes the mean HR, RR, and %SpO2 recorded at 5-min intervals after the induction of anesthesia in the isoflurane, sevoflurane, and hypothermia groups. In the isoflurane and sevoflurane groups, baseline HR was 290 ± 8.8 and 278 ± 7.8 bpm, respectively, at 1 min after anesthetic induction and 240 ± 8.9 and 231 ± 9.5 bpm at 15 min, respectively. HR gradually declined over time as the anesthetic depth increased and was significantly (P < 0.05) lower at the 10- and 15-min time points than at 1 min. For the hypothermia group, HR was 275 ± 56 bpm at 5 min after anesthetic induction and 146 ± 54 bpm at 15 min (this mean value includes readings that registered as 0). RR for the isoflurane, sevoflurane, and hypothermia groups gradually declined from a baseline of 39 ± 3, 38 ± 4, and 10 ± 1 breaths per minute, respectively, at the 1-min time point to 16 ± 2, 10 ± 3, and 0 breaths per minute at 15 min, respectively. Similar to HR, the RR gradually decreased over time due to increased depth of anesthesia, with a significant (P < 0.05) difference noted between the RR at 10 and 15 min as compared with that at 1 min for both the isoflurane and sevoflurane groups. In addition, the RR for the hypothermia group was significantly lower at all time points compared with those of both the isoflurane and sevoflurane groups. For the isoflurane and sevoflurane anesthetic groups, the mean %SpO2 at 1 min after induction (97% ± 1% and 97% ± 0%, respectively) was maintained at the 15-min time point (95% ± 1% and 91% ± 2%, respectively). In the hypothermia group, the %SpO2 measured at 5 min (51% ± 8%), 10 min (23% ± 7%), and 15 min (23% ± 7%) after induction (means at 10 and 15 min include readings that registered as 0) were significantly (P < 0.05) lower than the corresponding values for the isoflurane and sevoflurane groups.
Figure 2.
(A) Heart rate (HR), (B) respiratory rate (RR), and (C) percentage oxygen saturation (%SpO2) at 1 to 15 min after induction of anesthesia in neonatal rats. Data are given as mean ± SEM. In the hypothermia group, HR and %SpO2 could not be measured at T1, and mean measurements for HR and RR at 10 and 15 min included those measurements recorded as 0. *, Values at the same time point differ significantly (P < 0.05) among anesthesia groups; #, value is significantly (P<0.05 ) different from that at 1 min in the same anesthesia group.
Loss of righting reflex, loss of paw withdrawal, and return of righting reflex.
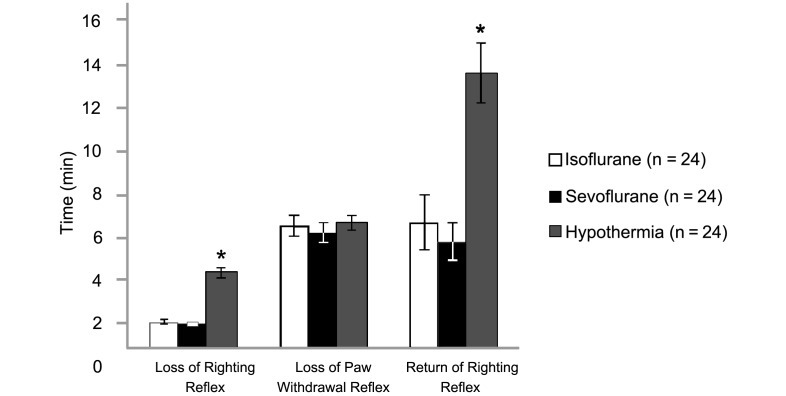
Neonatal rats lost their righting reflex significantly (P < 0.05) faster in both the isoflurane (1 ± 0 min) and sevoflurane (1 ± 0 min) groups than in the hypothermia (3 ± 0 min) group (Figure 3). However, the paw withdrawal reflex was lost at nearly the same time in all 3 groups (isoflurane, 6 ± 1 min; sevoflurane, 5 ± 1 min; hypothermia, 6 ± 0 min). During recovery (that is, after inhalational agents had been discontinued or rewarming in the hypothermia group), the return of righting reflex was significantly more rapid in pups treated with isoflurane (6 ± 1 min) or sevoflurane (5 ± 1 min) than in those in the hypothermia group (13 ± 1 min).
Figure 3.
Times to loss of the righting reflex, loss of the paw withdrawal reflex, and return of the righting reflex. Data are given as mean ± SEM. *, Value is significantly (P < 0.05) different from those for the other groups.
Corticosterone and glucose.
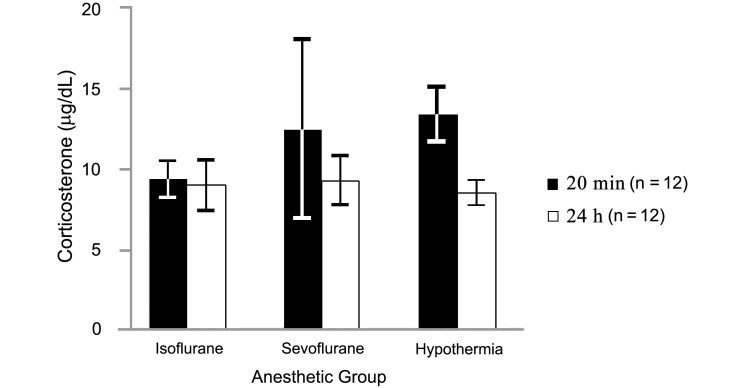
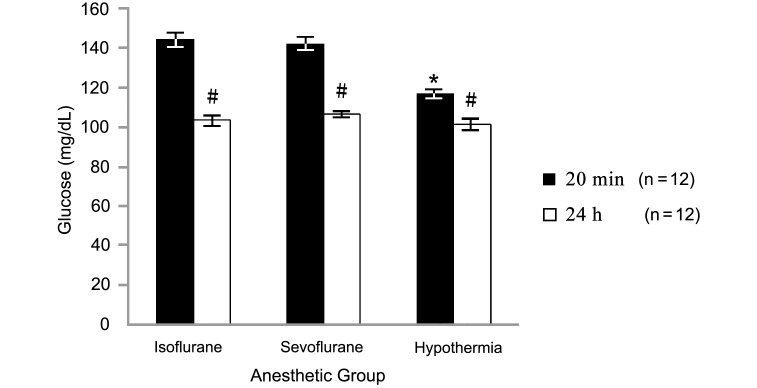
Mean corticosterone levels (Figure 4) in blood samples collected at 20 min after induction were 9.4 ± 1.1 μg/dL in the isoflurane group, 12.6 ± 5.6 μg/dL in the sevoflurane group, and 13.5 ± 1.8 μg/dL in the hypothermia group and did not differ from those in samples collected 24 h after anesthesia induction (9.0 ± 1.6, 9.3 ± 1.6, and 8.5 ± 0.8 μg/dL, respectively). However serum glucose values (Figure 5) were significantly (P < 0.05) higher at the 20-min point (isoflurane, 144.1 ± 3.9 mg/dL; sevoflurane, 142.1 ± 3.3 mg/dL; hypothermia, 116.9 ± 2.2 mg/dL) than at the 24-h point (isoflurane, 103.2 ± 2.5; sevoflurane, 106.4 ± 1.7; hypothermia, 101.4 ± 2.9 mg/dL). In addition, glucose levels at 20 min after induction were higher in the isoflurane and sevoflurane groups than in the hypothermia group.
Figure 4.
Serum corticosterone at 20 min and 24 h after the induction of anesthesia in neonatal rats. Data are given as mean ± SEM. #, Value at 20 min after induction is significantly (P < 0.05) different from that at 24 h after induction within the same group.
Figure 5.
Serum glucose at 20 min and 24 h after the induction of anesthesia in neonatal rats. Data are given as mean ± SEM. *, Value is significantly (P < 0.05) different from those of other groups; #, value at 20 min is significantly (P < 0.05) different from that at 24 h within the same group.
Discussion
This study demonstrates that for short, minor surgical procedures on neonatal rats, all 3 methods of anesthesia (isoflurane, sevoflurane, and hypothermia) are adequate for maintaining a surgical plane. Our results support the hypothesis that, unlike hypothermia anesthesia, isoflurane and sevoflurane anesthesia delivered in 100% O2 by using a precision vaporizer results in measurable physiologic parameters that are within the normal range (HR, RR, and %SpO2).
Neonatal anesthesia and surgery has been reported to result occasionally in postoperative mortality as a result of cannibalism or neglect by the dam. Previous studies have indicated that cannibalism after anesthetic and surgical treatment of pups is low in incidence overall and most severe among dams already in distress or with disturbed pups.10 We noted no complications associated with maternal acceptance of or weight gain by the pups in any of the treatment groups.
Pups in the isoflurane and sevoflurane groups had more stable anesthetic parameter measurements (HR, RR and %SpO2) throughout the procedure than did the hypothermia group. With increasing anesthetic depth, pups in the isoflurane and sevoflurane groups developed a decrease in RR and HR, and pups in the sevoflurane group also exhibited a decrease in %SpO2 under 100% supplemental oxygen.. Hypothermia pups were in apparent respiratory arrest, and the majority of the HR and %SpO2 measurements were detected as 0 because they were below the threshold of our instrument (the PhysioSuite Mouse STAT machine limits measurement to 40 to 900 bpm for and 70% to 100% for %SpO2). Pup survival is aided by the tolerance of the neonatal brain to low tissue temperature and oxygen partial pressure, but this tolerance decreases during the first week postpartum.15,33,36
It is crucial to consider the duration of the intended procedure when choosing an anesthetic method. One study7 reported that 1 of 10 neonatal pups (age, 4 d) kept hypothermic for more than 30 min failed to recover, and another study24 demonstrated that 10-d-old pups required mechanical ventilation to survive a 60-min isoflurane anesthetic procedure. These studies demonstrate that prolonged anesthesia necessitates additional precautions, which were not evaluated in the current study.
Induction time (as determined by loss of the righting reflex) was considerably longer for hypothermia anesthesia than for isoflurane or sevoflurane groups. Neonatal rodents are particularly tolerant of large declines in body temperature leading to a decrease in activity and motor consciousness. However, hypothermic anesthesia remains controversial because the chilling process has the potential to be painful.40 The behavioral responses of pups to noxious stimulation are generally whole-body, wriggling responses, which become more localized and more typical of adult responses as the pups mature.38 The use of latex sleeves is recommended during hypothermia anesthesia, because previous research has indicated that this practice protects pups from contact with ice during the chilling process, resulting in less struggling and vocalization than that in unprotected pups.7 The distinctive odor of isoflurane may be adverse to neonates during anesthesia induction. Given that isoflurane is an irritant anesthetic whereas sevoflurane is nonirritating, sevoflurane may be less aggravating to the respiratory tract during the induction period.37 The amount of discomfort experienced during the induction period in each anesthetic group is unknown.
Recovery time was 7 min longer for hypothermia anesthesia than for isoflurane or sevoflurane. A longer recovery time is associated with a delayed return of essential physiologic functions, including respiratory and cardiovascular function, body temperature, and normal behavior. In addition, whether pups experience pain during the recovery period after hypothermia anesthesia is a cause of concern. During the rewarming process, pups emit ultrasonic calls before other spontaneous movements begin,19 and these ultrasonic vocalizations did not became responsive to the inhibitory effect of contact with the dam until their core temperature reached 26 °C.19 As long as external circulating-water heating pads were provided, neonates in the isoflurane and sevoflurane groups maintained more consistent body temperature and recovered faster than did those in the hypothermia group.
The stress-induced release of glucocorticoids by the adrenal cortex is a basic physiologic response in mammals.32 Rats secrete the glucocorticoid corticosterone in response to a variety of stressors. Although mice and rats are similar, data regarding corticosterone secretion in mice cannot be used for direct comparison to rats. We sought to measure potential differences in the corticosterone level among these 3 anesthetic groups.32 Given that rats have significant diurnal variation in corticosterone due to circadian rhythm,23 anesthesia and subsequent blood collection was performed in an alternating order among treatment groups. This study showed no differences in corticosterone levels among anesthesia groups at either 20 min or 24 h after induction. A difference in corticosterone levels may be hard to detect due to the age of the pups, given that the early postnatal period (days 4 through 14) is considered a stress-hyporesponsive period.30 During this time, pups respond weakly to stressors, and the stress response is quantitative rather than qualitative, meaning the magnitude of corticosterone released in response to stress is depressed.32,39 The 20-min and 24-h corticosterone values did not differ in any of the 3 anesthetic treatments. In neonatal rodents, serum corticosterone levels increase significantly within 5 min after a stressor and reach a peak at 15 min, returning to normal 60 min after the stressor.39 Given that our samples were collected at 5 min and 24 h after the surgical procedure, we were unable to quantify a difference in the corticosterone response during the recovery period. This issue would be a useful route of inquiry for future studies, because some studies suggest the rewarming period after hypothermia anesthesia might be a stressor. Although hypothermic pups had a lower glucose level at 20 min after induction than did isoflurane or sevoflurane animals, additional factors should be considered regarding the use of hypothermia as an anesthetic method of choice.
In neonatal pups, hypoglycemia is a relevant procedure-related complication because of its influence on lost nursing time.24 Alternatively, hyperglycemia may be of significance for brief procedures, given the stress associated with anesthesia.16 Glucose significantly increases 3 to 8 min after a stressor, with its highest levels persisting for approximately 20 min; this pattern fits within our selected postprocedural collection times.14 The magnitude of the increase corresponds to the degree of environmental stress.9 The mean reported glucose level of 4-d-old rat pups after anesthesia with halothane is 137 ± 10.4 mg/dL26. This concentration is similar to the values we obtained 20 min after anesthesia with isoflurane and sevoflurane but is higher than the level produced after hypothermia anesthesia. Volatile anesthetics have been shown to impair insulin secretion and glucose utilization, due to a decrease in the glucose-induced inhibition of ATP-sensitive potassium channel activity in pancreatic β cells.34 Interestingly, hypothermia anesthesia has been associated with hyperglycemia, because it simultaneously decreases insulin sensitivity and reduces insulin secretion by pancreatic islet cells.27 The difference in the 20-min glucose levels among the 3 anesthetic groups was not clinically significant. The 24-h glucose values were significantly lower than the 20-min values in the present study and the mean for rat neonates that is reported in the literature.26 We suspect the difference from the reported value can be attributed to differences in collection methods and the associated stress response to anesthesia.
In summary, we found that all 3 methods of anesthesia tested—isoflurane, sevoflurane, and hypothermia—were appropriate techniques for short-term anesthesia of neonatal rats. Isoflurane and sevoflurane offer additional physiologic stability and may be more appropriate choices in older pups for longer anesthetic procedures. Future studies should continue to analyze potential anesthesia-related changes that occur in neonatal rodents, ideally focusing on histology, physiologic parameters (that is blood pressure), and gene expression and genetic markers relevant to neurodevelopment, organ function, behavior, and pain. When choosing an anesthetic technique for a procedure in a neonate, factors for consideration should include the availability of equipment, scavenging of waste gas, familiarity of personnel with the agent, and potential effect of the anesthetic on research outcomes.
Acknowledgment
This study was supported by the NIH Research Education Program for Laboratory Animal Veterinarians, training grant no. 5R25Od01045202-02. We are grateful for the assistance of Ms Janis Atuk-Jones in formatting and reviewing the manuscript.
References
- 1.Boon L, Manicourt D, Marbaix E, Vandenabeele M, Vanwijck R. 1992. A comparative analysis of healing of surgical cleft lip corrected in utero and neonates. Plast Reconstr Surg 89:11–17. [PubMed] [Google Scholar]
- 2.Cetin A, Komai S, Eliava M, Seeburg PH, Osten P. 2007. Stereotaxic gene delivery in the rodent brain. Nat Protoc 1:3166–3173. [DOI] [PubMed] [Google Scholar]
- 3.Chambers RA, Moore J, McEvoy JP, Levin ED. 1996. Cognitive effects of neonatal hippocampal lesions in a rat model of schizophrenia. Neuropsychopharmacology 15:587–594. [DOI] [PubMed] [Google Scholar]
- 4.Clowry GJ, Flecknell PA. 2000. The successful use of fentanyl–fluanisone (‘Hypnorm’) as an anaesthetic for intracranial surgery in neonatal rats. Lab Anim 34:260–264. [DOI] [PubMed] [Google Scholar]
- 5.Cregg N, Wall C, Green D, Mannion D, Casey W. 1996. Humidification reduces coughing and breath-holding during inhalation induction with isoflurane in children. Can J Anaesth 43:1090–1094. [DOI] [PubMed] [Google Scholar]
- 6.Cunningham MG, McKay RD. 1993. A hypothermic miniaturized stereotaxic instrument for surgery in newborn rats. J Neurosci Methods 47:105–114. [DOI] [PubMed] [Google Scholar]
- 7.Danneman PJ, Mandrell TD. 1997. Evaluation of 5 agents or methods for anesthesia of neonatal rats. Lab Anim Sci 47:386–395. [PubMed] [Google Scholar]
- 8.Davidson S, Truong H, Nakagawa Y, Giesler GJ., Jr 2010. A microinjection technique for targeting regions of embryonic and neonatal mouse brain in vivo. Brain Res 1307:43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Boer SF, Koopmans SJ, Slangen JL, Van der Gugten J. 1990. Plasma catecholamine, corticosterone, and glucose responses to repeated stress in rats: effect of interstressor interval length. Physiol Behav 47:1117–1124. [DOI] [PubMed] [Google Scholar]
- 10.DeSantis DT, Schmaltz LW. 1984. The mother–litter relationship in developmental rat studies: cannibalism vs caring. Dev Psychobiol 17:255–262. [DOI] [PubMed] [Google Scholar]
- 11.Feiss P. 2000. New halogenated agents: should I change my practice? Minerva Anestesiol 66:264–267. [PubMed] [Google Scholar]
- 12.Flecknell PA, Richardson CA, Popovic A. 2007. Laboratory animals. p 983–988. In: Tranquilli WJ, Thurmon JC, Grimm KA. Lumb and Jones’ veterinary anesthesia. Ames (IA): Blackwell Publishing [Google Scholar]
- 13.Frimpong-Boateng K, Surh C. 2013. Neonatal thymectomy prolongs the permeability of enteric antigens and promotes the strong activation of peripheral CD4 T cells (P3258). J Immunol 190:136.11. [Google Scholar]
- 14.Gotoh H, Matsumoto Y, Imamura K. 2004. General anesthesia of infant mice by isoflurane inhalation for medium-duration surgery. Exp Anim 53:63–65. [DOI] [PubMed] [Google Scholar]
- 15.Greer JJ, Carter JE, Allan DW. 1996. Respiratory rhythm generation in a precocial rodent in vitro preparation. Respir Physiol 103:105–112. [DOI] [PubMed] [Google Scholar]
- 16.Halter JB, Beard JC, Porte D., Jr 1984. Islet function and stress hyperglycemia: plasma glucose and epinephrine interaction. Am J Physiol 247:E47–E52. [DOI] [PubMed] [Google Scholar]
- 17.Hary L, Dupouy JP, Gregoire I. 1986. Effects of castration and testosterone on the pituitary and adrenal responses of the newborn rat to ether inhalation. Neuroendocrinology 42:137–142. [DOI] [PubMed] [Google Scholar]
- 18.Hill RW, Eshuis RK. 1988. Learning in mature mice (Peromyscus leucopus) subjected to deep hypothermia as neonates. J Comp Psychol 102:44–48. [DOI] [PubMed] [Google Scholar]
- 19.Hofer MA, Shair HN. 1992. Ultrasonic vocalization by rat pups during recovery from deep hypothermia. Dev Psychobiol 25:511–528. [DOI] [PubMed] [Google Scholar]
- 20.Hu D, Hu R, Berde CB. 1997. Neurologic evaluation of infant and adult rats before and after sciatic nerve blockade. Anesthesiology 86:957–965. [DOI] [PubMed] [Google Scholar]
- 21.Hu D, Miller SC. 2014. Evidence of a lineage shift between natural (NK) killer cells and T lymphocytes in the spleen and blood of neonatally thymectomized, young adult C3H mice. Curr Pediatr Res 18:43–47. [Google Scholar]
- 22.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th edition. Washington (DC): National Academics Press. [Google Scholar]
- 23.Kant GJ, Mougey EH, Meyerhoff JL. 1986. Diurnal variation in neuroendocrine response to stress in rats: plasma ACTH, β-endorphin, β-LPH, corticosterone, prolactin, and pituitary cyclic AMP responses. Neuroendocrinology 43:383–390. [DOI] [PubMed] [Google Scholar]
- 24.Loepke AW, McCann JC, Kurth CD, McAuliffe JJ. 2006. The physiologic effects of isoflurane anesthesia in neonatal mice. Anesth Analg 102:75–80. [DOI] [PubMed] [Google Scholar]
- 25.Orliaguet G, Vivien B, Langeron O, Bouhemad B, Coriat P, Riou B. 2001. Minimum alveolar concentration of volatile anesthetics in rats during postnatal maturation. Anesthesiology 95:734–739. [DOI] [PubMed] [Google Scholar]
- 26.Papworth TA, Clubb SK. 1995. Clinical pathology in the neonatal rat. Comp Haematol Intl 5:237–250. [Google Scholar]
- 27.Polderman KH, Herold I. 2009. Therapeutic hypothermia and controlled normothermia in the intensive care unit: practical considerations, side effects, and cooling methods. Crit Care Med 37:1101–1120. [DOI] [PubMed] [Google Scholar]
- 28.Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA. 2011. Transient regenerative potential of the neonatal mouse heart. Science 331:1078–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Porrello ER, Mahmoud AI, Simpson E, Johnson BA, Grinsfelder D, Canseco D, Mammen PP, Rothermel BA, Olson EN, Sadek HA. 2012. Regulation of neonatal and adult mammalian heart regeneration by the miR-15 family. Proc Natl Acad Sci USA 110:187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roskoden T, Linke R, Schwegler H. 2005. Transient early postnatal corticosterone treatment of rats leads to accelerated aquisition of a spatial radial maze task and morphological changes in the septohippocampal region. Behav Brain Res 157:45–53. [DOI] [PubMed] [Google Scholar]
- 31.Sanders RD, Patel N, Hossain M, Ma D, Maze M. 2005. Isoflurane exerts antinociceptive and hypnotic properties at all ages in Fischer rats. Br J Anaesth 95:393–399. [DOI] [PubMed] [Google Scholar]
- 32.Sapolsky RM, Meaney MJ. 1986. Maturation of the adrenocortical stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res 396:64–76. [DOI] [PubMed] [Google Scholar]
- 33.Singer D. 1999. Neonatal tolerance to hypoxia: a comparative–physiological approach. Comp Biochem Physiol A Mol Integr Physiol 123:221–234. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka K, Kawano T, Tomino T, Kawano H, Okada T, Oshita S, Takahashi A, Nakaya Y. 2009. Mechanisms of impaired glucose tolerance and insulin secretion during isoflurane anesthesia. Anesthesiology 111:1044–1051. [DOI] [PubMed] [Google Scholar]
- 35.Tander B, Baris S, Karakaya D, Ariturk E, Rizalar R, Bernay F. 2005. Risk factors influencing inadvertent hypothermia in infants and neonates during anesthesia. Paediatr Anaesth 15:574–579. [DOI] [PubMed] [Google Scholar]
- 36.Tattersall GJ, Milsom WK. 2003. Hypothermia-induced respiratory arrest and recovery in neonatal rats. Respir Physiol Neurobiol 137:29–40. [DOI] [PubMed] [Google Scholar]
- 37.TerRiet MF, DeSouza GJA, Jacobs JS, Young D, Lewis MC, Herrington C, Gold MI. 2000. Which is most pungent: isoflurane, sevoflurane, or desflurane? Br J Anaesth 85:305–307. [DOI] [PubMed] [Google Scholar]
- 38.van Sluyters RC, Obernier A. 2004. Guidelines for the care and use of mammals in neuroscience and behavioral research. Contemp Top Lab Anim Sci 43:48–52. [Google Scholar]
- 39.Walker CD, Scribner KA, Cascio CS, Dallman MF. 1991. The pituitary–adrenocortical system of neonatal rats is responsive to stress throughout development in a time-dependent and stressor-specific fashion. Endocrinology 128:1385–1395. [DOI] [PubMed] [Google Scholar]
- 40.Wolf S, Hardy JD. 1941. Studies on pain. Observations on pain due to local cooling and on factors involved in the ‘cold pressor’ effect. J Clin Invest 20:521–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhai K, Chang Y, Wei B, Liu Q, Leblais V, Fischmeister R, Ji G. 2014. Phosphodiesterase types 3 and 4 regulate the phasic contraction of neonatal rat bladder smooth myocytes via distinct mechanisms. Cell Signal 26:1001–1010. [DOI] [PubMed] [Google Scholar]