Abstract
Urethane anesthesia preserves many reflex functions and is often the preferred anesthetic for urodynamic studies in rats. Because of the toxicity profile of urethane, its use as an anesthetic typically is limited to acute and terminal investigations. Alternative anesthetic options are needed for longitudinal studies of micturition reflexes in rats. In this study, we evaluated propofol anesthesia administered at constant rate infusion at different planes of anesthesia in rats for combined cystometrography and external urethral sphincter (EUS) EMG in rats. No reflex micturition was noted after rats received 100%, 80%, or 60% of a previously reported anesthetic dose of propofol. At 40% of the standard propofol dose, a subset of rats showed reflex voiding, with bladder contractions and associated EUS EMG activity. In contrast, urethane anesthesia at a surgical plane allowed for reflex voiding with bladder contractions and EUS activation. Latency to leaking or voiding was longer in rats under propofol anesthesia than in those under urethane anesthesia. In a subset of rats with reflex voiding under propofol anesthesia, voiding efficiency was decreased compared with that of rats anesthetized with urethane. We conclude that propofol anesthesia suppresses micturition reflexes in rats more efficiently than did urethane. Propofol is a suitable anesthetic for longitudinal studies in rats, but its use for urodynamic evaluations is limited in these animals due to its marked suppression of both bladder contractions and EUS EMG activation.
Abbreviation: EUS, external urethral sphincter
Urodynamic studies typically are performed in rats to evaluate micturition reflexes after spinal cord injury and repair and to evaluate the effects of experimental drugs on lower urinary tract function.5,15,26 These urodynamic investigations usually are performed under general anesthesia and may include both cystometrography and EMG of the external urethral sphincter (EUS).6,22 However, evoked autonomic and motor responses are suppressed by anesthetic agents, an aspect that presents an important technical limitation to consider when designing experimental protocols for lower urinary tract studies in anesthetized subjects.3,7,24 Urethane is often the preferred anesthetic agent for acute urodynamic studies in rats because of its relatively modest suppression of cystometrography and EUS EMG,7-9,21 but its use typically is limited to terminal experimental studies because of its toxicity profile.12,19 Inhalant anesthetics, such as isoflurane, often are preferred for survival surgeries and nonterminal experimental procedures in rats,10,15 but they exhibited significantly greater suppression of micturition reflexes than did urethane in direct comparisons between the 2 agents in combined cystometrography and EUS EMG in rats.7 It is therefore of interest to investigate the possible use of alternative anesthetic agents for physiologic studies in rats to improve longitudinal experimental designs.
Although propofol, an injectable anesthetic agent, is often used for surgical procedures in humans and described as suitable for intraoperative monitoring of somatosensory evoked potentials,13,20 studies using propofol for physiologic studies in rats are sparse. Therefore, we evaluated the potential utility of propofol as an anesthetic agent for urodynamic studies in rats. An additional goal was to refine urodynamic protocols for longitudinal studies and consequently decrease the overall number of rats needed. We developed a protocol to perform a combination of cystometrography and EUS EMG in rats under different planes of propofol anesthesia, which was administered by using constant-rate intravenous infusion to maintain a consistent depth of anesthesia for stable urodynamic recording. For control purposes, a separate set of rats underwent urodynamic studies under urethane anesthesia.
Materials and Methods
A total of 19 female naïve and pathogen-free Sprague–Dawley rats (weight, 210 to 260 g; Charles River Labs, Wilmington, MA) were included in the study. Female rats were used to facilitate comparison with urodynamic data collected from our ongoing urodynamic studies in neurologically intact rats and in rats after cauda equina injury and repair.6-8,15 The rats were divided into 2 groups: those that underwent urethane anesthesia (n = 10) and those that received propofol anesthesia (n = 10). The animal procedures, including rat surgeries, postoperative care, and termination of experiments, were performed at an AAALAC-accredited institution and according to the standards established by the Guide for the Care and Use of Laboratory Animals.16 The experimental protocols were approved by the IACUC at the University of California–Irvine. The rats were housed in pairs in cages and had free access to food and water. All rats were housed in a room with a 12:12-h light:dark cycle. All efforts were made to minimize any animal suffering and the number of rats used for the study.
Surgical preparations.
To allow for urodynamic recording, all rats underwent a brief surgical procedure for the placement of a bladder catheter and EUS EMG electrodes. Accordingly, 9 of the 19 rats in the study were anesthetized by using urethane (1.2 g/kg SC; Sigma–Aldrich, St Louis, MO) for placement of the catheter and electrodes as well as subsequent urodynamic recording. The remaining 10 rats anesthetized by using isoflurane (2% to 2.5%, Phoenix Pharmaceuticals, Burlingame, CA) for the placement of the EMG electrodes, bladder catheter, and intravenous catheter for propofol infusion.
A midline abdominal incision was made to expose the urinary bladder. A catheter with a flared tip (PE-50, Instech Laboratories, Plymouth Meeting, PA) was inserted through the top of the bladder dome, and the tip was positioned inside the bladder. The opposite end of the bladder catheter was attached to a 3-way connector connected to both a programmable infusion pump (World Precision Instruments, Sarasota, FL) and a pressure transducer (Biopac Systems, Santa Barbara, CA) to support bladder infusion and cystometrography, respectively. Next, 2 epoxy-coated 50-μm platinum–iridium wire electrodes (A-M Systems, Sequim, WA) were hooked at the tip of a 27-gauge needle and inserted into the EUS bilaterally under direct microscopic visualization. The needle then was withdrawn, leaving the electrodes embedded in the muscle. The electrodes were connected to a data acquisition system (MP150; Biopac Systems, CA), and the abdominal incision was closed. For the subset of rats in the propofol group, the left jugular vein was exposed, and an intravenous catheter (PE-50, Instech Laboratories) was placed and connected to a programmable infusion pump for subsequent drug administration.
The toe-pinch reflex was used to determine the level of anesthesia in all rats and was abolished in all animals during catheter and electrode placement under urethane or isoflurane anesthesia. The surgical preparation took approximately 30 min for each rat. At the completion of the surgical procedures, the isoflurane was discontinued. For the propofol group, an anesthetized state was maintained by using a continuous intravenous infusion of propofol (Abbott Laboratories, Abbott Park, IL) at a rate of 1 mg/kg/min. This rate of infusion was defined for the propofol group as a 100% dosage owing to a previous study, which showed that this dose reliably abolished the rectifying response and allowed the placement of a clip-on pulse oximeter in rats.27 The urethane-anesthetized group remained at a surgical level of anesthesia, confirmed by a negative toe-pinch reflex, during urodynamic recording.
Urodynamic recording.
Cystometrograms and EUS EMG were obtained at a 1-kHz sampling rate during continuous bladder infusion with saline (0.12 mL/min) in rats maintained on either urethane or propofol anesthesia. No filter was used for cystometrography; filters between 100 and 500 Hz were used for the EUS EMG activity. For rats anesthetized with propofol, separate urodynamic recordings were obtained at infusion rates of 100%, 40% (0.4 mg/kg), 60% (0.6 mg/kg), and 80% (0.8 mg/kg) of the maximal dosage (1 mg/kg/min). The duration of propofol infusion was 30 min for each dosage, and the urodynamic data for analysis were obtained during the last 15 min of each dose of propofol. The rationale for this aspect of the experimental design reflects the rapid onset and washout of propofol after intravenous infusion, given that the half-time for brain turnover of propofol in rats is 2.4 min.11 A total of 3 consecutive voiding cycles within the first hour of urodynamic recording were collected and analyzed for urethane-anesthetized rats. Toe-pinch reflex responses were categorized as absent or present in response to the use of nonlocking forceps to pinch the hindlimb toes throughout the urodynamic recording sessions. The withdrawal of the ipsilateral hindlimb in response to the applied toe-pinch was determined as a positive reflex response.
Endpoint.
After urodynamic recording, the propofol-anesthetized rats received the 100% dose for an additional 15 min until the toe-pinch reflex was abolished. In addition, absence of the toe-pinch reflex was confirmed in urethane-anesthetized rats. Thereafter, all rats underwent euthanasia by cardiac incision, according to recommendations provided by the American Veterinary Medical Association.
Outcome measures.
Several functional outcome measures were studied. Latency to first urine leak or void was defined as the time between the start of bladder infusion until the first leakage of urine and was used to calculate bladder capacity as the infusion rate multiplied by time. Voiding efficiency was calculated from the difference between the bladder capacity and residual volume divided by the bladder capacity. Maximal intravesical pressure, intercontraction interval, pressure threshold, resting pressure, expulsion time, and contraction duration were determined according to established criteria.7,23 For EUS EMG recording, the peak amplitudes of EUS tonic and bursting activity during bladder filling and voiding were measured.6,7 The cystometrograms and EUS EMG activity were analyzed by using AcqKnowledge 4.1 (Biopac Systems).
Statistical analysis.
Quantitative data are expressed as means ± SE. The 6 experimental groups analyzed were rats receiving urethane, 100% propofol, 80% propofol, 60% propofol, and 40% propofol with or without voiding contractions. For all parameters pertinent to voiding contractions, a total of 3 measurements were averaged to create a mean value for each rate. One-way ANOVA and post t tests (Newman–Keuls multiple-comparison test; Prism 4.0, GraphPad Software, La Jolla, CA) were applied to the data to compare the latency to the first leak or void and the voiding efficiency. The t test allowed for comparisons between the group demonstrating voiding contractions during the 40% propofol dose and rats under urethane anesthesia. We regarded a P value of less than 0.05 to indicate a statistically significant difference between groups.
Results
Urodynamic studies were performed to compare the effects of urethane and propofol anesthesia on lower urinary tract function in rats (Figure 1). For this purpose, cystometrography and EUS EMG were investigated after a standard dose of urethane and after various doses of propofol. Each group initially comprised 10 rats. One subject in the urethane group was lost before the start of recordings, and this subject was not replaced. A surgical plane of anesthesia was obtained by using a standard dose of urethane (1.2 g/kg SC) and maintained during recording in adult female rats (n = 9). The toe-pinch reflex was abolished in all rats after the urethane administration. Subsequent urodynamic recordings were performed, and evoked micturition reflexes were demonstrated in all animals. In response to continuous bladder filling, all rats showed reflex bladder contractions associated with EUS EMG activity, including signs of EUS bursting during voiding.
Figure 1.
Representative examples of cystometrographic and EUS EMG activity in a rat under urethane anesthesia. Two consequent voiding contractions (A, upper tracing) by saline infusion associated with EUS EMG activity (A, lower tracing) are shown. A more rapid time scale indicates the expulsion time (ET) during voiding (B, upper tracing) as well as the tonic and bursting EUS activity (B, lower tracing); RP, resting pressure: PT, pressure threshold; ET, expulsion time; ICI, intercontraction interval; CD, contraction duration; max IVP, maximal intravesical pressure.
We also used a previously reported dose of propofol (1 mg/kg/min continuous intravenous infusion)27 as an anesthetic agent for urodynamic recording in adult female rats (n = 10). However, no micturition reflexes could be evoked in any of the subjects after this dose of propofol. The dose of propofol subsequently was reduced to 80%, 60%, and 40% of the original dose and was administered by continuous intravenous infusion. All rats remained sedated and demonstrated no spontaneous extremity or head movements at the original or any of the reduced doses. Micturition reflexes could be detected only in the subset of rats maintained on 40% of the original propofol dose (Figure 2). Specifically, 6 of the 10 rats exhibited voiding contractions with associated EUS EMG activity at 40% of the standard dose of propofol. Although all rats were immobilized by the administered propofol, all rats receiving 40% of the standard dose of propofol demonstrated ipsilateral limb withdrawal after toe pinching, and subsets of the rats also showed a positive toe-pinch response at the higher doses of propofol (Table 1). Even at 100% of the standard dose of propofol, 4 of 10 rats showed a positive toe-pinch reflex.
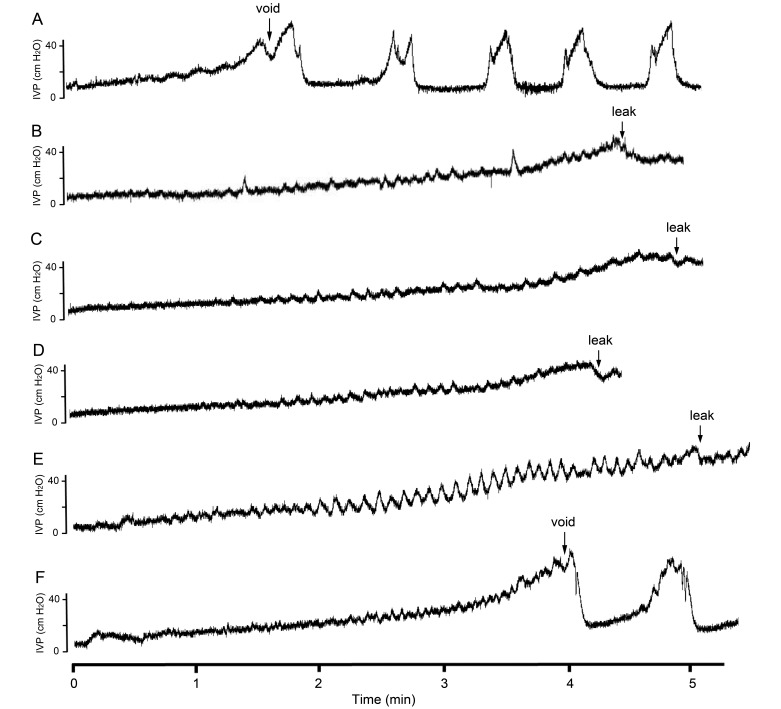
Figure 2.
Representative examples of micturition reflexes in urethane and propofol groups. The graphs show the cystometrograms from 6 experimental conditions during the infusion of saline into the bladder of rats anesthetized by using (A) urethane, (B)100% propofol dose, (C) 80% propofol dose, (D) 60% propofol dose, (E) 40% propofol without voiding contractions, and (F) 40% propofol dose with voiding contractions. Note the onset of reflex voiding in the urethane group (A) and in the subgroup of rats under 40% propofol dose with voiding contractions (F); the other groups lack reflex micturition but instead show a bladder leak response at a later time of onset (B through E).
Table 1.
Toe-pinch reflexes during urodynamic recording
| Propofol (% relative to full dose) |
||||||
| Urethane | 100% | 80%nv | 60%nv | 40%nv | 40%v | |
| No. of rats | 9 | 10 | 10 | 10 | 4 | 6 |
| Toe-pinch reflex | ||||||
| positive | 0 | 4 | 5 | 9 | 4 | 6 |
| negative | 9 | 6 | 5 | 1 | 0 | 0 |
nv, nonvoiding response to bladder filling; v, voiding response to bladder filling
Toe-pinch responses were detected in subsets of rats under propofol anesthesia at 100%, 80%, and 60% doses, whereas all subjects showed positive toe-pinch reflexes at 40% of the full propofol dose. At the 40% propofol dose, a subset of rats (n = 6) showed reflex voiding (v), whereas the rest of the rats (n = 4) had nonvoiding (nv) responses to bladder filling. All rats under urethane anesthesia showed reflex voiding.
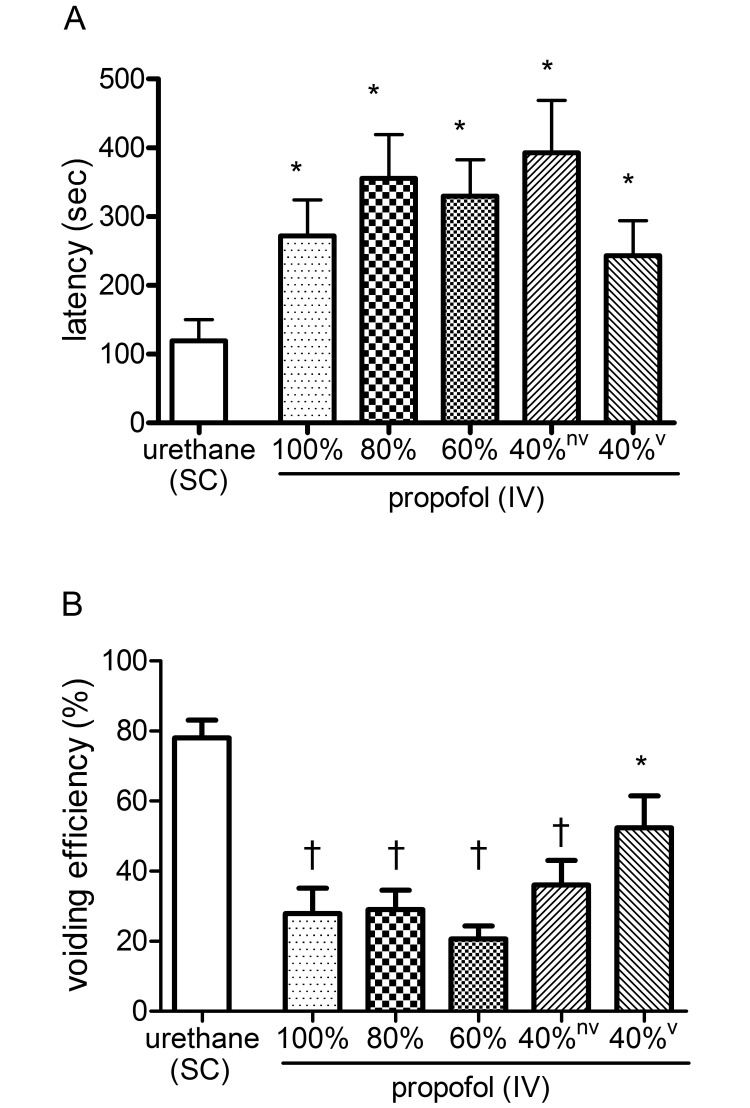
Next, the latency between the start of bladder infusion with saline and sign of the first bladder leak or voiding was determined in rats under either urethane or propofol anesthesia (Figure 3). The urethane group showed a latency to first void of 120 ± 31 s (n = 9). When compared with the urethane group, rats (n = 10) under 100%, 80%, and 60% of the standard dose of propofol showed significantly (P < 0.05) prolonged latency to first urine leak at 272 ± 52, 356 ± 64, and 330 ± 53 s, respectively. At 40% of the standard propofol dose, the latency to first leak was prolonged at 393 ± 76 s (P < 0.05; n = 4), and the latency to first void was prolonged at 243 ± 51 s (P < 0.05; n = 6) compared with the urethane group.
Figure 3.
Statistical analysis of the (A) latency to the first void or leak and (B) voiding efficiency (B) for the urethane and propofol groups. Value differs significantly (*, P < 0.05; †, P < 0.001) between groups.
Voiding efficiency was determined as an indicator of overall lower urinary tract function (Figure 3). Under urethane anesthesia, the voiding efficiency was 78 ± 5% (n = 9). When compared with the urethane group, rats (n= 10) under 100%, 80%, and 60% of the standard dose of propofol demonstrated a significantly (P < 0.001) decreased voiding efficiency of 28% ± 7%, 29% ± 6%, and 21% ± 4%, respectively. At 40% of the standard dose of propofol, rats without and with voiding contractions showed a decreased voiding efficiency of 36% ± 7%; (P < 0.001; n = 4) and 52 ± 9% (P < 0.05; n = 6), respectively, when compared with the urethane-anesthetized rats.
For a more detailed assessment of the anesthetic agents on the contractile properties of the bladder and EUS function, we compared cystometrographic and EUS recordings between the urethane group (n = 9) and the propofol group exhibiting reflex bladder contractions at 40% of the standard dose of propofol (n = 6). The urethane and propofol groups did not differ in regard to resting pressure, pressure threshold, maximal intravesical pressure, contraction duration, intercontraction interval, and expulsion time as determined from the cystometrograms. Similarly the 2 groups did not differ in regard to the peak EUS tonic and bursting activity or the duration of EUS bursting (Table 2).
Table 2.
Findings from cystometrography and external urethral sphincter EMG of anesthetized rats
| Urethane n = 9 | Propofol (40% dose) n = 6 | |
| Cystometrography | ||
| Resting pressure (cm H2O) | 5.3 ± 0.9 | 6.9 ± 0.9 |
| Pressure threshold (cm H2O) | 16.4 ± 1.6 | 24.1 ± 4.54 |
| Maximal intravesical pressure (cm H2O) | 33.8 ± 1.8 | 35.9 ± 4.0 |
| Contraction duration (s) | 20.4 ± 2.3 | 19.9 ± 4.2 |
| Intercontraction interval (s) | 104.1 ± 18.2 | 115.4 ± 24.7 |
| Expulsion time (s) | 5.8 ± 0.7 | 7.9 ± 2.6 |
| External urethral sphincter EMG | ||
| Amplitude of tonic activity (mV) | 0.063 ± 0.016 | 0.066 ± 0.020 |
| Amplitude of bursting activity (mV) | 0.130 ± 0.026 | 0.134 ± 0.041 |
| Bursting period (s) | 3.8 ± 0.7 | 4.3 ± 1.0 |
Data are given as mean ± SEM.
Discussion
In this study, we established a protocol that enables the evaluation of lower urinary tract function in rats under propofol anesthesia administered by continuous intravenous infusion. Reflex micturition was evaluated by using concurrent cystometrography and EUS EMG. Rats anesthetized with a standard dose of propofol did not show any reflex bladder contractions in response to saline infusion into the bladder. When 80% or 60% of a standard dose of propofol was administered, no reflex bladder contractions were elicited, but all rats showed limb withdrawal to toe pinch, indicative of a decreased plane of anesthesia. At 40% of a standard dose of propofol, all rats remained immobilized with a positive response to toe pinch, and a subset of 6 of 10 subjects demonstrated reflex bladder contractions. When the propofol administered was reduced to less than 40% of a standard dose, sedation could not be maintained, and urodynamic recording was not performed. For control purposes, a separate group of rats was placed under a surgical plane of anesthesia provided by a single subcutaneous dose of urethane, and all subjects in this group showed reflex bladder contractions. We recognize that there are some differences between the urethane and propofol groups with regard to surgical preparation and administration routes for the 2 anesthetic agents, and these differences were not controlled for, because an overall goal was to compare experimentally relevant protocols. We conclude that micturition reflexes are markedly more suppressed by propofol than by urethane under the test conditions.
When investigating the effects of anesthetic agents on physiologic functions, the interpretability of the results depends on the ability to determine and compare levels of anesthesia. For the present control group, an established dose of urethane was used to provide a surgical plane of anesthesia, and all rats under urethane anesthesia lacked detectable withdrawal responses to noxious toe pinch. Continuous infusion of saline into the bladder resulted in evoked bladder contractions detected by cystometrography and EUS tonic and phasic activity, including voiding-associated bursting in rodents, demonstrated by EUS EMG. The urodynamic data obtained in rats anesthetized with urethane were in agreement with previous functional studies under similar conditions.7-9,24 In the experimental group, urodynamic studies were performed in rats anesthetized with a previously reported dose of propofol.27 The selected propofol dose has been shown previously to abolish the rectifying reflex and to allow placement of a clip-on pulse-oximeter.27 In the present series, the standard dose of propofol infusion produced sedation and immobilized all rats, but 40% of the subjects showed limb withdrawal in response to a noxious toe pinch. Neither reflex micturition nor voiding occurred in rats sedated with the standard propofol dose in response to continuous bladder infusion of saline. We therefore conclude that the rats anesthetized with our standard propofol dose were at a lighter plane of anesthesia than were those under urethane anesthesia but their micturition reflexes were suppressed more.
In a prior study on the effect of different anesthetic agents on micturition reflexes, bolus administration (10 mg/kg) followed by intravenous infusion (0.2 mg/kg/min) of propofol resulted in suppression of micturition reflexes in male Sprague–Dawley rats.24 A more recent study reported the ability to obtain reflex micturition in propofol-anesthetized female Wistar rats, in which bladder pressures associated with the resting and micturition periods did not differ from corresponding pressures in unanesthetized rats.25 In the present study, we explored whether a reflex bladder contraction and associated EUS EMG activity could be elicited during constant-rate intravenous infusion of different doses of propofol. Although no reflex micturition could be evoked in rats anesthetized by our standard dose of propofol, both bladder contractions and EUS activity occurred in a subset of subjects when the propofol dose was decreased to 40% of the standard dose, which maintained the sedative and immobilizing effects of the drug. The latency to void was prolonged and the voiding efficiency was reduced in the propofol group when the drug was administered at 40% of the standard dose, but several outcome measures related to cystometrography and EUS EMG did not differ from those obtained in rats under urethane anesthesia. Therefore, even the subset of rats showing micturition reflexes and voiding at the lowest sedating dose of propofol demonstrated increased urine retention, as indicated by the prolonged latency to first void. The impaired detrusor contractility also may have contributed to the associated decreased voiding efficiency.
Our experience suggests that the degree of suppression of micturition reflexes is not necessarily related to the route of drug administration but rather to the pharmacologic properties of the individual agents. In the rat, injectable urethane is markedly less suppressive of micturition reflexes compared with a volatile anesthetic, isoflurane.7 However, the injectable administration of propofol in the present study suppressed reflex voiding to a greater extent than did by isoflurane in our prior studies, in that a sedating and immobilizing dose of isoflurane in rats allowed for reflex voiding with bladder contractions and EUS EMG activation in all subjects.7
Propofol has been used in urodynamic studies in dogs. A direct comparison between the volatile anesthetic, sevoflurane, and propofol at a wide range of infusion rates showed that both anesthetics are suitable for urethral pressure recording in female dogs.2 Additional studies have validated that propofol anesthesia is suitable for the evaluation of urethra function during different rates of bladder infusion and for the evaluation of various experimental drug effects on the urethral pressure.4,14
The effects of individual anesthetic agents on lower urinary tract functions can vary between species. Some species may be particularly sensitive to an agent and show depressed function at low doses. The present study showed a high degree of suppression of lower urinary tract reflex function in rats, even at the minimally sedative and nonsurgical planes provided by propofol anesthesia. The specific mechanisms for the observed discrepancies between anesthetic agents are not well understood. However, genomic variations in the GABA receptor, which propofol acts on, may be one possible explanation for the different effects of propofol between species.1,18 In addition, propofol is metabolized by phase II uridine diphosphate glycosyl transferance, and possible enzyme polymorphisms may exist between different species and organ systems.17
In summary, we developed a protocol to evaluate of the effects of propofol on micturition reflexes in rats. We demonstrated that propofol highly suppresses lower urinary-tract function, with impaired bladder contractions and EUS EMG activation, in rats over a broad range of anesthetic dosing. In addition, when reflex micturition was achieved in a subset of rats at a minimally sedating dose of propofol, voiding efficiency was markedly lower than that in rats anesthetized by urethane at a surgical plane. We conclude that propofol offers only limited use for urodynamic studies in rats. Propofol may have some utility in experimental studies in rats, in part because of the possibility to perform longitudinal studies under propofol anesthesia, but its pronounced suppressive effect on reflex micturition must be considered when designing investigations of lower urinary tract function.
Acknowledgments
The studies have been supported by grants from The Dr Miriam and Sheldon G Adelson Medical Research Foundation and NIH NINDS (grant no. NS042719).
References
- 1.Buggy DJ, Nicol B, Rowbotham DJ, Lambert DG. 2000. Effects of intravenous anaesthetic agents on glutamate release: a role for GABAA receptor-mediated inhibition. Anesthesiology 92:1067–1073. [DOI] [PubMed] [Google Scholar]
- 2.Byron JK, March PA, DiBartola SP, Chew DJ, Muir WW., 3rd 2003. Comparison of the effect of propofol and sevoflurane on the urethral pressure profile in healthy female dogs. Am J Vet Res 64:1288–1292. [DOI] [PubMed] [Google Scholar]
- 3.Cannon TW, Damaser MS. 2001. Effects of anesthesia on cystometry and leak-point pressure of the female rat. Life Sci 69:1193–1202. [DOI] [PubMed] [Google Scholar]
- 4.Carofiglio F, Hamaide AJ, Farnir F, Balligand MH, Verstegen JP. 2006. Evaluation of the urodynamic and hemodynamic effects of orally administered phenylpropanolamine and ephedrine in female dogs. Am J Vet Res 67:723–730. [DOI] [PubMed] [Google Scholar]
- 5.Chang HY, Cheng CL, Chen JJ, de Groat WC. 2006. Serotonergic drugs and spinal cord transections at different segmental levels indicate that different spinal circuits are involved in tonic and bursting external urethral sphincter activity in rats. Am J Physiol Renal Physiol 292:F1044–F1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang HY, Havton LA. 2008. Reestablished micturition reflexes show differential activation patterns after lumbosacral ventral root avulsion injury and repair in rats. Exp Neurol 212:291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang HY, Havton LA. 2008. Differential effects of urethane and isoflurane on external urethral sphincter electromyography and cystometry in rats. Am J Physiol Renal Physiol 295:F1248–F1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang HH, Havton LA. 2013. Serotonergic 5HT(1A) receptor agonist (8-OH-DPAT) ameliorates impaired micturition reflexes in a chronic ventral root avulsion model of incomplete cauda equina–conus medullaris injury. Exp Neurol 239:210–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng CL, de Groat WC. 2004. The role of capsaicin-sensitive afferent fibers in the lower urinary tract dysfunction induced by chronic spinal cord injury in rats. Exp Neurol 187:445–454. [DOI] [PubMed] [Google Scholar]
- 10.Deckardt K, Weber I, Kaspers U, Hellwig J, Tennekes H, van Ravenzwaay B. 2007. The effects of inhalation anaesthetics on common clinical pathology parameters in laboratory rats. Food Chem Toxicol 45:1709–1718. [DOI] [PubMed] [Google Scholar]
- 11.Dutta S, Ebling WF. 1998. Formulation-dependent brain and lung distribution kinetics of propofol in rats. Anesthesiology 89:678–685. [DOI] [PubMed] [Google Scholar]
- 12.Field KJ, Lang CM. 1988. Hazards of urethane (ethyl carbamate): a review of the literature. Lab Anim 22:255–262. [DOI] [PubMed] [Google Scholar]
- 13.Fung NY, Hu Y, Irwin MG, Chow BE, Yuen MY. 2008. Comparison between sevoflurane–remifentanil and propofol–remifentanil anaesthesia in providing conditions for somatosensory evoked potential monitoring during scoliosis corrective surgery. Anaesth Intensive Care 36:779–785. [DOI] [PubMed] [Google Scholar]
- 14.Hamaide AJ, Verstegen JP, Snaps FR, Onclin K, Balligand MH. 2003. Validation and comparison of the use of diuresis cystometry and retrograde filling cystometry at various infusion rates in female Beagle dogs. Am J Vet Res 64:574–579. [DOI] [PubMed] [Google Scholar]
- 15.Hoang TX, Pikov V, Havton LA. 2006. Functional reinnervation of the lower urinary tract after cauda equina injury and repair. J Neurosci 26:8672–8679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Institute for Laboratory animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed Washington (DC): National Academies Press. [Google Scholar]
- 17.Iohom G, Fitzgerald D, Cunningham AJ. 2004. Principles of pharmacogenetics—implications for the anaesthetist. Br J Anaesth 93:440–450. [DOI] [PubMed] [Google Scholar]
- 18.Iwata N, Cowley DS, Radel M, Roy-Byrne PP, Goldman D. 1999. Relationship between a GABAA α6 Pro385Ser substitution and benzodiazepine sensitivity. Am J Psychiatry 156:1447–1449. [DOI] [PubMed] [Google Scholar]
- 19.Koblin D. 2002. Urethane: help or hindrance? Anesth Analg 94:241–242. [DOI] [PubMed] [Google Scholar]
- 20.Liu EH, Wong HK, Chia CP, Lim HJ, Chen ZY, Lee TL. 2005. Effects of isoflurane and propofol on cortical somatosensory evoked potentials during comparable depth of anaesthesia as guided by bispectral index. Br J Anaesth 94:193–197. [DOI] [PubMed] [Google Scholar]
- 21.Maggi CA, Meli A. 1986. Suitability of urethane anesthesia for physiopharmacological investigations. Part 3: other systems and conclusions. Experientia 42:531–537. [DOI] [PubMed] [Google Scholar]
- 22.Maggi CA, Giuliani S, Santicioli P, Meli A. 1986. Analysis of factors involved in determining urinary bladder voiding cycle in urethane-anesthetized rats. Am J Physiol 251:R250–R257. [DOI] [PubMed] [Google Scholar]
- 23.Maggi CA, Santicioli P, Meli A. 1986. The nonstop transvesical cystometrogram in urethane-anesthetized rats: a simple procedure for quantitative studies on the various phases of urinary bladder voiding cycle. J Pharmacol Methods 15:157–167. [DOI] [PubMed] [Google Scholar]
- 24.Matsuura S, Downie JW. 2000. Effect of anesthetics on reflex micturition in the chronic cannula-implanted rat. Neurourol Urodyn 19:87–99. [DOI] [PubMed] [Google Scholar]
- 25.Ozkurkcugil C, Ozkan L. 2010. Effects of anesthetics on cystometric parameters in female rats. Int Urol Nephrol 42:909–913. [DOI] [PubMed] [Google Scholar]
- 26.Pikov V, Wrathall JR. 2001. Coordination of the bladder detrusor and the external urethral sphincter in a rat model of spinal cord injury: effect of injury severity. J Neurosci 21:559–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tung A, Herrera S, Fornal CA, Jacobs BL. 2008. The effect of prolonged anesthesia with isoflurane, propofol, dexmedetomidate, or ketamine on neural cell proliferation in the adult rat. Anesth Analg 106:1772–1777. [DOI] [PubMed] [Google Scholar]