Abstract
Objective
Transsphenoidal surgery (TS) for sellar lesions is an established and safe procedure, but complications can occur, particularly involving the neuroendocrine system. We hypothesized that postoperative care of TS patients would be optimized when performed by a coordinated team including a pituitary neurosurgeon, endocrinologists, and a specialty nurse.
Methods
We implemented a formalized, multidisciplinary team approach and standardized postoperative protocols for the care of adult patients undergoing TS by a single surgeon (J.N.B.) at our institution beginning in July 2009. We retrospectively compared the outcomes of 214 consecutive TS-treated cases: 113 cases prior to and 101 following the initiation of the team approach and protocol implementation. Outcomes assessed included the incidence of neurosurgical and endocrine complications, length of stay (LOS), and rates of hospital readmission and unscheduled clinical visits.
Results
The median LOS decreased from 3 days preteam to 2 days postteam (P<.01). Discharge occurred on postoperative day 2 in 46% of the preteam group patients compared to 69% of the postteam group (P<.01). Rates of early postoperative diabetes insipidus (DI) and readmissions within 30 days for syndrome of inappropriate antidiuretic hormone (SIADH) or other complications did not differ between groups.
Conclusion
Implementation of a multidisciplinary team approach was associated with a reduction of LOS. Despite earlier discharge, postoperative outcomes were not compromised. The endocrinologist is central to the success of this team approach, which could be successfully applied to care of patients undergoing TS, as well as other types of endocrine surgery at other centers.
INTRODUCTION
Transsphenoidal surgery (TS) is a commonly performed neurosurgical procedure, with approximately 20% of primary brain tumors surgically removed by this approach annually in the United States (1). Resection via the TS approach is the mainstay of therapy for the majority of sellar and parasellar tumors (2). In the hands of a skilled neurosurgeon, TS is an established and relatively safe procedure. When it is performed at a high-volume hospital, adverse outcomes including mortality, morbidity, and operative complications are reduced (3). While most patients undergo the procedure without complications, they are at risk for hormone and electrolyte imbalances, cerebrospinal fluid (CSF) leaks, neurological deficits, and infection during the early postoperative period (4–7).
Care of TS patients is primarily under the direction of the neurosurgical team, but input from endocrinologists is often provided in the perioperative and early postoperative periods. In particular, endocrinologists typically monitor for pituitary-adrenal dysfunction and fluid or electrolyte changes (8–10). A variety of algorithms for managing these issues have been proposed, but no single approach has proven to be superior, often leading to care that is individualized for each hospital. Regardless of the perioperative management algorithm undertaken, the preference for a coordinated, multidisciplinary team approach that includes the pituitary neurosurgeon, endocrinologist, and other relevant health professionals has been proposed (8,9,11). The implementation of a multidisciplinary team approach and the standardization of clinical pathways for postoperative management of other specific diseases has improved outcomes and provided more cost-effective delivery of care (12,13). The impact of implementing standardized clinical protocols and a multidisciplinary team approach to the outcome of TS has not been tested. Beginning in July 2009, our center instituted a change to our program of clinical care of pituitary tumors, instituting a formalized and structured multidisciplinary team approach with the adoption of standard protocols. This clinical program was implemented to improve patient care, facilitate earlier discharge, and reduce hospital readmissions for endocrine complications. In this study, we set out to compare outcomes in patients who underwent TS prior to and after the adoption of our structured team approach with respect to perioperative and early postoperative outcomes.
METHODS
Implementation of a Multidisciplinary Team and Standardized Postoperative Protocols
Prior to July 2009, perioperative and early postoperative care of patients undergoing TS for sellar and parasellar lesions at our hospital was under the direction of the inpatient neurosurgical team who consulted the endocrinology service as needed, typically to manage endocrine complications that had occurred. Thus, management varied in a number of regards such as glucocorticoid coverage protocols, early postoperative monitoring, and plans for postdischarge monitoring. Beginning in July 2009, we formed a formal, structured, multidisciplinary team and developed postoperative protocols. Under this approach, all patients admitted for TS are routinely evaluated at the time of surgery by each member of the team consisting of a single neurosurgeon specializing in TS, a neuroendocrinologist (endocrinology attending specializing in the care of pituitary tumor patients), an endocrinology fellow, and a designated trained nurse specializing in pituitary disease (Table 1). Each patient is evaluated preoperatively if possible, on the day of surgery, after surgery, and daily during their hospital stay by a neuroendocrinology attending in conjunction with the endocrinology fellow. Standardized perioperative monitoring and glucocorticoid coverage protocols (Table 1) are carried out by the neuroendocrinology team. Patient education is undertaken by the neuroendocrinology team and pituitary specialty nurse during the hospital stay and prior to discharge. A formalized discharge plan including, if necessary, instructions for close monitoring of fluid intake and output once discharged is established. Patients are contacted by phone within the postop week by the nurse. All patients have laboratory testing on postoperative day (POD) 7, and the results are reviewed by the neuroendocrinologist. The team also arranges appropriate follow-up with an endocrinologist for every patient beyond this first week.
Table 1.
Multidisciplinary Team and Standard Postoperative Protocols
| Multidisciplinary team |
|
| In-hospital DIa surveillance |
|
| Pituitary-adrenal axis assessment |
|
| Discharge planning |
|
| Early postoperative follow-up |
|
Abbreviations: BMP = basic metabolic profile; DI = diabetes insipidus; I/O = fluid intake and output; SIADH = syndrome of inappropriate antidiuretic hormone.
DI diagnostic criteria: Urine volume >250 cc/hour for 2 to 3 hours and urine specific gravity <1.005 (or urine osmolality < 200 mOsm/kg H2O). Indications for desmopressin therapy: Patient unable to maintain adequate oral fluid intake, urine output >> fluid intake or hypernatremia.
Patients with preoperative hypopituitarism discharged on replacement therapy routinely. Glucocorticoids are tapered postoperatively based on am cortisol assessment and results of Cortrosyn® stimulation test as needed (8).
Including education on stress dose steroid use for patients discharged on glucocorticoids.
Hospital and Postoperative Record Review
We retrospectively reviewed the records of 214 consecutive patients who underwent TS by a single neurosurgeon (J.N.B.) at our institution in the 2.5 years immediately before and after implementation of the team approach. We chose this time frame in an attempt to yield at least 100 patients in each group. Data were collected from hospital records and the office records of the neurosurgeon and endocrinologists. Information collected included patient demographic data, medical history, medications, clinical presentation, and preoperative laboratory values. Details of the patients’ surgeries including surgical technique and complications were obtained from the operative report and hospital records. Results of tumor pathologic examinations and imaging studies including magnetic resonance imaging and computed tomography scans were collected. Data collected from the hospital and outpatient records included all available serum cortisol and sodium levels; doses of preoperative, perioperative, and postoperative glucocorticoids administered; and therapy provided for diabetes insipidus (DI). Patients were considered to have DI if this diagnosis was given by the endocrinologist or neurosurgeon in the records, and/or if the patient received desmopressin (DDAVP) during their hospitalization. Patients were considered to have syndrome of inappropriate antidiuretic hormone (SIADH) if the diagnosis was given by the endocrinologist or neurosurgeon in their records, if fluid restriction was administered for hyponatremia, and/or if the patient required readmission for SIADH. The need for pre- and postoperative pituitary hormone replacement was recorded. The total length of hospital stay (LOS) was recorded. LOS was counted from the day of TS to discharge, excluding days of hospitalization prior to the TS date. Ten patients in the preteam group were hospitalized from 2 to 8 days prior to TS, and 10 in the postteam group were hospitalized from 1 to 12 days before TS occurred.
The neurosurgeon’s and endocrinologists’ records and laboratory results were reviewed to verify that early postoperative follow-up was conducted as specified in the plan. Values of sodium and cortisol obtained 1 week postoperatively were recorded. Patient records were reviewed for emergency department visits or hospital readmissions within the early postoperative period and up to 30 days after hospital discharge.
This retrospective study was conducted in accordance with Institutional Review Board (IRB) approval from the Columbia University Medical Center IRB.
Statistical Analysis
Descriptive statistics were calculated, frequency for categorical data and mean ± SD for continuous data. Preteam values were compared to those of the postteam group. Comparisons were carried out by Wilcoxon rank sum-test for continuous variables. Fisher exact tests were used to compare categorical data. P<.05 was considered significant. Statistical analyses were performed using Prism 6.0 (GraphPad Software, Inc, San Diego, CA).
RESULTS
Patient Characteristics
The characteristics of the preteam (113 patients) and postteam (101 patients) groups are shown in Table 2. The groups were similar with regard to age, sex distribution, and preoperative maximal tumor diameter. Sixteen and 13 patients in the pre- and postteam groups had undergone a prior TS procedure, respectively (P>.05). Postoperative pathologies were similar in both groups. Most tumors were confirmed to be pituitary adenomas; approximately 9% (19/214) were other types of lesions such as craniopharyngiomas, other cystic lesions, and metastases.
Table 2.
Preoperative Patient Characteristics and Pathologic Diagnoses
| Preteam (n = 113) |
Postteam (n = 101) |
|
|---|---|---|
| Age (years)a | 50.7 ± 1.4 | 52.4 ± 1.4 |
| Sex: # Female (% Female) | 58 (51%) | 49 (49%) |
| Lesion maximal diameter (cm) | 2.1 ± 0.1 | 2.0 ± 0.1 |
| Micro/macro (#) | 15/98 | 13/88 |
| Prior pituitary surgery | 16 | 13 |
| Hypopituitarism (%)b | 37% | 40% |
| Surgical Resection (# GTR/# PR) | 106/7 | 89/12 |
| Pathologic diagnosis | ||
| Pituitary adenoma | ||
| Nonfunctioning | 57 (50%) | 61 (61%) |
| GH | 15 (13%) | 12 (12%) |
| ACTH | 11 (10%) | 11 (11%) |
| Prolactin | 10 (9%) | 9 (9%) |
| TSH | 4 (4%) | 1 (1%) |
| Gonadotropin | 2 (2%) | 0 (0%) |
| Cysts | ||
| Rathke cleft cyst | 7 (7%) | 3 (3%) |
| Arachnoid cyst | 1 (1%) | 1 (1%) |
| Craniopharyngioma | 1 (1%) | 1 (1%) |
| Pituitary cyst | 1 (1%) | 0 (0%) |
| Other lesions | ||
| Chronic inflammatory lesion | 1 (1%) | 0 (1%) |
| Metastatic squamous cell | 0 (0%) | 1 (1%) |
| carcinoma | 1 (1%) | 0 (0%) |
| Lymphoma | 1 (1%) | 0 (0%) |
| Metastatic neuroendocrine tumor | 1(1%) | 0 (0%) |
Abbreviations: ACTH = adrenocorticotrophic hormone; GH = growth hormone; GTR = gross total resection; TS-transsphenoidal surgery, macromicroadenoma; TS = transsphenoidal surgery.
Data are presented as mean ± SD. No categories were significantly different between the groups.
Hypopituitarism was defined as deficiency of 1 or more axis based on these criteria: free thyroxine below the lower limit of normal, morning cortisol <10 µg/dL, and/or peak cortisol <18 µg/dL after Cortrosyn® stimulation testing, total testosterone below the lower limit of normal in men or amenorrhea in premenopausal women, GH deficiency based on standardized criteria (8, 31). Diabetes insipidus was considered separately and not part of the diagnosis of hypopituitarism.
Postoperative Complications
Rates of endocrine complications following surgery were similar in the pre- and postteam groups. The most common endocrine-related complication was DI, occurring in 27% and 25% of the pre- and postteam groups, respectively (P>.05, Table 3). In the majority of cases, DI was transient, requiring DDAVP therapy on POD 1 and 2. In the preteam group, 19 patients received 1 dose of DDAVP therapy, 6 patients received 2 doses, 3 patients 3 doses, and 1 patient 4 doses. In the postteam group, 15 patients received 1 dose of DDAVP, 5 patients received 2 doses, and 4 patients were managed without DDAVP. One additional patient in each group was discharged on standing DDAVP therapy and had persistent DI at the 30-day follow-up. Postoperative SIADH occurred in 11% (n = 12) of patients in the preteam and 9% (n = 9) of the postteam group (P>.05). In the preteam group, SIADH occurred in 3 patients while still hospitalized, 5 patients required readmission after discharge, and 4 patients were managed as outpatients without readmission. In the postteam group, SIADH occurred in 1 patient during postoperative hospitalization, 5 patients required readmission, and 3 patients were managed after discharge without readmission.
Table 3.
Postoperative Complicationsa
| Preteam (n = 113) |
Postteam (n = 101) |
|
|---|---|---|
| Endocrine | ||
| DI | 30 (27%) | 25 (25%) |
| SIADH | 12 (11%) | 9 (9%) |
| Adrenal insufficiency | 2 (2%) | 0 (0%) |
| Neurosurgical | ||
| Subarachnoid hemorrhage | 1 (1%) | 1 (1%) |
| Subdural hematoma | 0 (0%) | 1 (1%) |
| Sellar blood clot | 0 (0%) | 1 (1%) |
| CSF leak | 1 (1%) | 3 (3%) |
| Hydrocephalus | 1 (1%) | 0 (0%) |
| Meningitis | 1 (1%) | 1 (1%) |
| Epistaxis | 1 (1%) | 0 (0%) |
| Medical | 5 (4%) | 2 (2%) |
Abbreviations: CSF = cerebrospinal fluid; DI = diabetes insipidus; SIADH = syndrome of inappropriate antidiuretic hormone
There were no significant differences between the 2 groups.
Rates of neurosurgical complications did not differ between the pre- and postteam groups (Table 3). Medical complications were observed in 4% and 2% of the pre- and postteam patients during the course of their hospitalization, respectively (P>.05). These included in the pre-team group, 2 instances of atrial fibrillation and 1 each of urinary tract infection, urinary retention, and ischemic colitis and in the postteam group, 1 instance each of uncontrolled hypertension and acute gout attack. No deaths occurred.
LOS and Hospital Readmissions
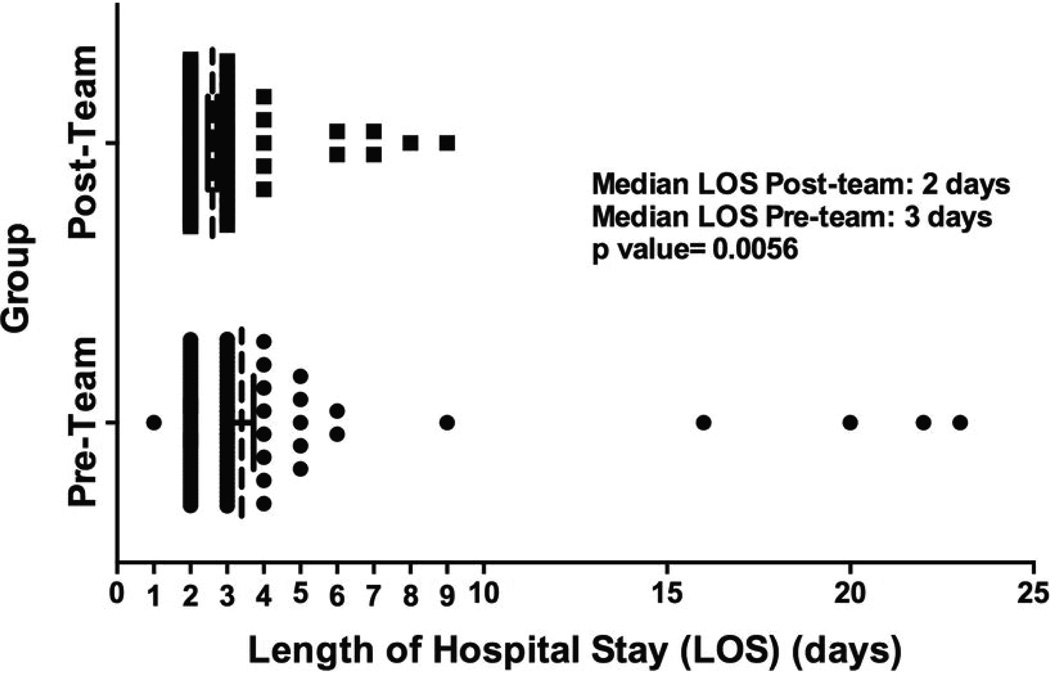
LOS was shorter in the postteam compared to the preteam group. The median LOS was 3 days in the preteam compared to 2 days in the postteam group (P<.01, Fig. 1). Discharge on POD 2 occurred in 46% and 69% of the preand postteam groups, respectively (P<.01) (Table 4). The mean LOS in the preteam group was 3.4 ± 3.5 days versus 2.6 ± 1.3 days in the postteam group (P = .03).
Fig. 1.
LOS (vertical dashed lines) in the pre- and postteam groups. The median LOS was shorter in the postteam group (2 days) than in the preteam group (3 days) (P = .0056). LOS = length of stay.
Table 4.
LOS
| Preteam | Postteam | P value | |
|---|---|---|---|
| (n = 113) | (n = 101) | ||
| Median LOS (days) | 3 | 2 | <.01 |
| Discharged on POD 2 | 46% | 69% | <.001 |
| Median LOS Patients with DI (days) | 3 | 3 | ns |
| DI discharged in POD 2 | 33% | 40% | ns |
| Median LOS uncomplicated DI (days) | 3 | 2 | .09 |
| Uncomplicated DI discharged on POD 2 | 35% | 61% | .1 |
| Total unscheduled clinical visits | 12 (11%) | 9 (9%) | ns |
| Total readmissions | 6 (5%) | 6 (6%) | ns |
| Readmission for SIADH | 5 | 4 | ns |
Abbreviations: DI = diabetes insipidus; LOS = length of stay; ns = not significant; POD = postoperative day; SIADH = syndrome of inappropriate antidiuretic hormone.
Of the patients who developed DI during their hospitalization, LOS did not differ in subjects in the 2 groups, and discharge occurred by POD 2 in 33% and 40% of the pre- and postteam groups, respectively (P = .58, Table 4). In patients with DI and no other medical or neurosurgical complications, the median LOS were 3 days in the preteam patients and 2 days in the postteam patients (P = .09), and the percentage of them discharged on POD 2 was 35% in the preteam compared to 61% in the postteam patients (P = .12). While 100% of the postteam group received an in-hospital endocrine consult, only 40% of the preteam group did. However, among the preteam patients, LOS did not differ between those who received the endocrinology consult and those who did not (P = .28).
Unscheduled Postoperative Visits and Hospital Readmissions
Unscheduled visits (readmissions and emergency department visits) occurred in 11% of the preteam and 9% of the postteam groups (P = .81). Hospital readmissions within 30 days of discharge occurred in 5% of the preteam and 6% of the postteam groups (P = .9). Readmissions in the preteam group were for SIADH (n = 5) and hemorrhage/ clot (n = 1), while in the postteam group they were for SIADH (n = 5) and CSF leak (n = 1). Other emergency department visits in the preteam group were for dizziness (n = 2), polyuria (n = 1), nosebleed (n = 1), and adrenal insufficiency (n = 2); in the postteam group, ED visits were for headache (n = 2) and shortness of breath (n = 1). The rates of readmission or visits for endocrine-related problems did not differ between groups.
New postoperative hypopituitarism that persisted at POD 30 occurred in 12 (6%) patients overall, with 8/113 (7%) and 4/101(4%) patients in the pre- and postteam groups, respectively. Discharge on glucocorticoid replacement occurred in 62/113 (55%) of the preteam group and 43/101 (43%) of the post-team group (P = .08). After excluding patients with preoperative secondary adrenal insufficiency or Cushing disease, a greater percentage of the remainder of the pre-team (43/113, 38%) compared to the postteam (25/101, 25%) were discharged on glucocorticoid replacement therapy (P = .04). Record review showed evidence of a check of 1-week postoperative electrolytes and morning cortisol, and follow-up telephone call in 66/113 (58%) of patients in the preteam and 93/101 (92%) of the postteam group (P<.0001).
DISCUSSION
At our center, the implementation of a structured multidisciplinary team approach and standardized postoperative protocols was associated with a reduction in LOS following surgery. Reduced LOS did not compromise patient safety because unscheduled postoperative visits or readmissions within 30 days after surgery, including those for SIADH, did not increase. After implementation of our team approach, our use of glucocorticoid replacement therapy on discharge was reduced, and no readmissions for adrenal insufficiency occurred. In our experience, a multidisciplinary team approach to the care of patients undergoing TS reduces hospitalization duration without compromising patient safety.
The importance of a multidisciplinary approach to the care of patients with pituitary tumors in general, and specifically after TS, has been emphasized (9,11). Good rationale existed for us to implement this approach, which could improve care and reduce health resource utilization. Collaborative decision making regarding diagnosis, plans for clinical and radiographic investigations, need for surgery, and preoperative medical management are likely to result in the most appropriate use of surgery and preoperative optimization of endocrine function. The collaborative approach should thus improve outcome. The endocrinologist’s input postoperatively is crucial since complications are often endocrinologic in nature. At our center, the endocrinologist is at the center of the team and assumes primary responsibility for follow-up care. Postoperative protocols have been advocated by others for the care of patients undergoing TS (14–16), but these were not formally evaluated. The value of such protocols has been shown for thyroid surgery; use of a standardized algorithm after thyroidectomy reduces complication rates (12) and may contribute to cost reductions (13). The main outcome we observed after adoption of our team approach and standard protocols was a significant reduction in LOS from a median of 3 to 2 days. LOS after TS varies among reports. The experience of the surgeon and clinical center are important in determining LOS. An analysis of over 5,000 surgical procedures from the National Inpatient Sample from 1996 to 2000 showed shorter LOS for higher volume hospitals and surgeons (3). The time frame of a series is relevant since LOS decreased by 4.6% per year over the 4 years of this analysis (3), and LOS decreased over the 9 years of a TS series for Cushing disease (5). Increasing surgical expertise may reduce LOS in longitudinal studies because there is an operative learning curve for TS (17). In our series, however, all procedures were performed by a single experienced, specialized pituitary neurosurgeon over a relatively short period of time at the same high-volume center, thereby reducing the likelihood that surgical or hospital factors could explain the observed reduction in LOS.
Reductions in LOS over time could be related to improved surgical techniques. However, newer techniques may not reduce LOS; differences in LOS after endoscopic and microscopic TS have not been definitively shown. In an endonasal microscopic technique series from 1998 to 2005, the median LOS was 3.0 days (18), and mean LOS after endoscopic procedures performed from 2004 to 2007 was 2.8 days (19). In 2 concurrent series, each comparing procedures performed at the same hospital, LOS was shorter following endoscopic (mean 2.4 days) versus microscopic (mean 3.0 days) procedures in 1 study (20), but LOS was similar between the 2 groups in the second study (microscopic mean 2.4 days, endoscopic 2.5 days) (16). In our series, postteam patients all underwent the microscopic TS approach, and the mean and median LOS were 2.6 and 2 days, respectively, the same as those in a recent endoscopic series (21). Thus, our data suggest no difference in LOS based on approach when the procedure is performed by an experienced pituitary surgeon.
LOS after pituitary surgery also increases as complication rate rises (5,16). Ours was low but is similar to others (2,20–22), and there was no significant difference between the pre- and postteam groups. The complication rate falls as the surgeon’s experience increases (22). When performed by specialized neurosurgeons, the mortality rate of TS is <1% (3,23). Since these factors influencing LOS after TS did not change over time at our center, they are unlikely to have contributed to the observed LOS reduction.
We found no increase in adverse endocrine outcomes, unscheduled postoperative visits, or readmissions in our postteam group despite a reduction of LOS to 2 days. In contrast, a recent study that examined the safety and cost-effectiveness of discharge on POD 1 reported that LOS fell on average from 2.2 to 1.3 days, but unscheduled postoperative re-evaluations occurred in roughly 1 in 3 patients in the early discharge group (24). Differing from our protocol, that study routinely discharged patients on 6 weeks of glucocorticoid coverage and checked electrolytes after discharge at the 6-week follow-up visit (24). We routinely contacted our patients in the first week after surgery and checked electrolytes and cortisol levels on POD 7. We cannot determine if our close early postoperative followup helped prevent readmissions or if discharge on POD 2 is optimal since it may allow sufficient time for most early postoperative DI to resolve and for development of a tailored discharge plan.
The most common complications of pituitary surgery are disorders of water balance, specifically DI and SIADH (25), for which close observation and rapid initiation of treatment are essential (11). Postoperative DI can increase LOS. Despite our protocols for DI diagnosis and treatment, we only found a trend for reduced LOS for patients with uncomplicated DI in our postteam group. Our 25% rate of new postoperative DI is similar to another recent series that reported a 28% rate (6). In an endoscopic series, DI occurred in 11%, but this may reflect more stringent diagnostic criteria (21). The overall incidence of delayed hyponatremia after TS varies from 23% (26) to 9% (27), and symptomatic hyponatremia has been reported in 7% (27) and 5% (24) of TS cases. SIADH incidence peaks 7 to 9 days postoperatively (6,27,28). We hypothesized that routine measurement of serum sodium levels on POD 7 would allow for increased detection of SIADH and increase our ability to manage hyponatremia in the outpatient setting. Surprisingly, we found no decrease in readmissions for SIADH in the postteam group. Earlier detection of mild hyponatremia at POD 4 or 5 could have staved off readmission, but patients with severe SIADH may require rehospitalization regardless of early detection. New hypopituitarism was rare, occurring in just 6% of patients, a rate similar to a previously reported 5% rate (29).
Secondary adrenal insufficiency due to manipulation or damage to the stalk or pituitary during surgery or by the tumor is less common but essential to assess for after TS. Centers vary in their glucocorticoid coverage protocols. Patients with preoperative adrenal insufficiency receive stress dose glucocorticoids perioperatively and continued replacement on discharge (14). In those without known adrenal insufficiency, perioperative glucocorticoid supplementation is administered by some but not all centers (8,14). Our standard protocol provides stress doses of dexamethasone perioperatively followed by a rapid taper with evaluation of morning cortisol on POD 2. We continue glucocorticoid replacement on discharge if cortisol is <10 µg/dL on POD 2 (8). After adoption of our follow-up protocols, we significantly reduced our prescription of glucocorticoids on discharge, potentially reducing adverse effects of unnecessary glucocorticoids. We did check 1-week postoperative cortisol levels in a significantly greater percentage of the postteam group, and none developed adrenal insufficiency after discharge. Other centers measure morning cortisol from POD 1 to 3, but the criteria for replacement range from ≤4 to ≤15 µg/dL (10,11,15,30). At one center that holds glucocorticoids postoperatively in patients without preoperative adrenal insufficiency or Cushing disease unless morning cortisol on POD1 or POD2 is ≤4 µg/dL, none developed adrenal crisis (15).
Our study has certain limitations. The retrospective nature of our data collection could have influenced our findings. Data capture for the postteam group may have been greater than for the preteam group as the former was more often than not followed postoperatively by our endocrine team. Ideally, a study randomizing patients to the team approach or usual care would be conducted, but this was not done. Because we added a number of measures to the care of the postteam group, we cannot determine which ones contributed to the LOS reduction. We do not believe that the care administered by the endocrine team differed between the pre- and postteam groups, but in the preteam group, Endocrine consults were requested less often and typically after complications arose, which may have contributed to the longer LOS. Factors other than what we measured could also have contributed to reduce LOS. It should also be acknowledged that the team approach is labor intensive on the part of the endocrinology team.
CONCLUSION
In conclusion, our data suggest that the team approach and use of standardized protocols for postoperative care will reduce LOS and thereby costs associated with this procedure since LOS may be the most influential factor in determining TS-associated health care costs (16). We did not find changes in a number of factors reported by others to relate to LOS, which supports the conclusion that our team approach and protocol were the driving forces behind the LOS reduction. Both improved confidence in the safety of early discharge and the safeguards inherent in the followup procedures may have facilitated discharge on POD 2. A multidisciplinary team approach and standard postoperative protocols for monitoring endocrine function in the immediate postoperative period and on discharge appear to benefit patients undergoing TS.
Acknowledgments
Funded by NIH grants DK070600, DK073040, and DK064720, the National Center for Advancing Translational Sciences, and NIH grant UL1 TR000040. A.S.C. was supported by the Doris Duke foundation.
Dr. Michelle Lee is an emplyee at Sanofi
Abbreviations
- CSF
cerebrospinal fluid
- DDAVP
desmopressin
- DI
diabetes insipidus
- LOS
length of stay
- POD
postoperative day
- SIADH
syndrome of inappropriate antidiuretic hormone
- TS
transsphenoidal surgery
Footnotes
DISCLOSURE
The other authors have no multiplicity of interest to disclose.
REFERENCES
- 1.Jane JA, Jr, Sulton LD, Laws ER., Jr Surgery for primary brain tumors at United States academic training centers: results from the Residency Review Committee for neurological surgery. J Neurosurg. 2005;103:789–793. doi: 10.3171/jns.2005.103.5.0789. [DOI] [PubMed] [Google Scholar]
- 2.Zada G, Kelly DF, Cohan P, Wang C, Swerdloff R. Endonasal transsphenoidal approach for pituitary adenomas and other sellar lesions: an assessment of efficacy, safety, and patient impressions. J Neurosurg. 2003;98:350–358. doi: 10.3171/jns.2003.98.2.0350. [DOI] [PubMed] [Google Scholar]
- 3.Barker FG, 2nd, Klibanski A, Swearingen B. Transsphenoidal surgery for pituitary tumors in the United States, 1996–2000: mortality, morbidity, and the effects of hospital and surgeon volume. J Clin Endocrinol Metab. 2003;88:4709–4719. doi: 10.1210/jc.2003-030461. [DOI] [PubMed] [Google Scholar]
- 4.Sudhakar N, Ray A, Vafidis JA. Complications after trans-sphenoidal surgery: our experience and a review of the literature. Br J Neurosurg. 2004;18:507–512. doi: 10.1080/02688690400012459a. [DOI] [PubMed] [Google Scholar]
- 5.Patil CG, Lad SP, Harsh GR, Laws ER, Jr, Boakye M. National trends, complications, and outcomes following transsphenoidal surgery for Cushing’s disease from 1993 to 2002. Neurosurg Focus. 2007;23:E7. doi: 10.3171/foc.2007.23.3.9. [DOI] [PubMed] [Google Scholar]
- 6.Aulinas A, Colom C, Ybarra J, Muñoz F, Tresserras P, Resmini E, Webb SM. Immediate and delayed postoperative morbidity in functional and non-functioning pituitary adenomas. Pituitary. 2012;15:380–385. doi: 10.1007/s11102-011-0331-2. [DOI] [PubMed] [Google Scholar]
- 7.Berker M, Hazer DB, Yücel T, Gürlek A, Cila A, Aldur M, Onerci M. Complications of endoscopic surgery of the pituitary adenomas: analysis of 570 patients and review of the literature. Pituitary. 2012;15:288–300. doi: 10.1007/s11102-011-0368-2. [DOI] [PubMed] [Google Scholar]
- 8.Ausiello JC, Bruce JN, Freda PU. Postoperative assessment of the patient after transsphenoidal pituitary surgery. Pituitary. 2008;11:391–401. doi: 10.1007/s11102-008-0086-6. [DOI] [PubMed] [Google Scholar]
- 9.Inder WJ, Alford FP. Pituitary masses: the importance of a multidisciplinary approach. Med J Aust. 2007;187:522–523. doi: 10.5694/j.1326-5377.2007.tb01395.x. [DOI] [PubMed] [Google Scholar]
- 10.Dumont AS, Nemergut EC, 2nd, Jane JA, Jr, Laws ER., Jr Postoperative care following pituitary surgery. J Intensive Care Med. 2005;20:127–140. doi: 10.1177/0885066605275247. [DOI] [PubMed] [Google Scholar]
- 11.Vance ML. Perioperative management of patients undergoing pituitary surgery. Endocrinol Metab Clin North Am. 2003;32:355–365. doi: 10.1016/s0889-8529(03)00003-3. [DOI] [PubMed] [Google Scholar]
- 12.Wiseman JE, Mossanen M, Ituarte PH, Bath JM, Yeh MW. An algorithm informed by the parathyroid hormone level reduces hypocalcemic complications of thyroidectomy. World J Surg. 2010;34:532–537. doi: 10.1007/s00268-009-0348-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdulla AG, Ituarte PH, Wiggins R, Teisberg EO, Harari A, Yeh MW. Endocrine surgery as a model for value-based health care delivery. Surg Neurol Int. 2012;3:163. doi: 10.4103/2152-7806.105102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inder WJ, Hunt PJ. Glucocorticoid replacement in pituitary surgery: guidelines for perioperative assessment and management. J Clin Endocrinol Metab. 2002;87:2745–2750. doi: 10.1210/jcem.87.6.8547. [DOI] [PubMed] [Google Scholar]
- 15.McLaughlin N, Cohan P, Barnett P, Eisenberg A, Chaloner C, Kelly DF. Early morning cortisol levels as predictors of short-term and long-term adrenal function after endonasal transsphenoidal surgery for pituitary adenomas and Rathke’s cleft cysts. World Neurosurg. 2013;80:569–575. doi: 10.1016/j.wneu.2012.07.034. [DOI] [PubMed] [Google Scholar]
- 16.Little AS, Chapple K, Jahnke H, White WL. Comparative inpatient resource utilization for patients undergoing endoscopic or microscopic transsphenoidal surgery for pituitary lesions. J Neurosurg. 2014;121:84–90. doi: 10.3171/2014.2.JNS132095. [DOI] [PubMed] [Google Scholar]
- 17.Leach P, Abou-Zeid AH, Kearney T, Davis J, Trainer PJ, Gnanalingham KK. Endoscopic transsphenoidal pituitary surgery: evidence of an operative learning curve. Neurosurgery. 2010;67:1205–1212. doi: 10.1227/NEU.0b013e3181ef25c5. [DOI] [PubMed] [Google Scholar]
- 18.Dusick JR, Esposito F, Mattozo CA, Chaloner C, McArthur DL, Kelly DF. Endonasal transsphenoidal surgery: the patient’s perspective-survey results from 259 patients. Surg Neurol. 2006;65:332–341. doi: 10.1016/j.surneu.2005.12.010. discussion 341–342. [DOI] [PubMed] [Google Scholar]
- 19.Dehdashti AR, Ganna A, Karabatsou K, Gentili F. Pure endoscopic endonasal approach for pituitary adenomas: early surgical results in 200 patients and comparison with previous microsurgical series. Neurosurgery. 2008;62:1006–1015. doi: 10.1227/01.neu.0000325862.83961.12. discussion 1015–1017. [DOI] [PubMed] [Google Scholar]
- 20.Dallapiazza R, Bond AE, Grober Y, et al. Retrospective analysis of a concurrent series of microscopic versus endoscopic transsphenoidal surgeries for Knosp Grades 0–2 nonfunctioning pituitary macroadenomas at a single institution. J Neurosurg. 2014;121:511–517. doi: 10.3171/2014.6.JNS131321. [DOI] [PubMed] [Google Scholar]
- 21.Mamelak AN, Carmichael J, Bonert VH, Cooper O, Melmed S. Single-surgeon fully endoscopic endonasal transsphenoidal surgery: outcomes in three-hundred consecutive cases. Pituitary. 2013;16:393–401. doi: 10.1007/s11102-012-0437-1. [DOI] [PubMed] [Google Scholar]
- 22.Ciric I, Ragin A, Baumgartner C, Pierce D. Complications of transsphenoidal surgery: results of a national survey, review of the literature, and personal experience. Neurosurgery. 1997;40:225–236. doi: 10.1097/00006123-199702000-00001. discussion 236–237. [DOI] [PubMed] [Google Scholar]
- 23.Swearingen B, Barker FG, 2nd, Katznelson L, et al. Long-term mortality after transsphenoidal surgery and adjunctive therapy for acromegaly. J Clin Endocrinol Metab. 1998;83:3419–3426. doi: 10.1210/jcem.83.10.5222. [DOI] [PubMed] [Google Scholar]
- 24.Forbes JA, Wilkerson J, Chambless L, et al. Safety and cost effectiveness of early discharge following microscopic trans-sphenoidal resection of pituitary lesions. Surg Neurol Int. 2011;2:66. doi: 10.4103/2152-7806.81723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loh JA, Verbalis JG. Diabetes insipidus as a complication after pituitary surgery. Nat Clin Pract Endocrinol Metab. 2007;3:489–494. doi: 10.1038/ncpendmet0513. [DOI] [PubMed] [Google Scholar]
- 26.Zada G, Liu CY, Fishback D, Singer PA, Weiss MH. Recognition and management of delayed hyponatremia following transsphenoidal pituitary surgery. J Neurosurg. 2007;106:66–71. doi: 10.3171/jns.2007.106.1.66. [DOI] [PubMed] [Google Scholar]
- 27.Kelly DF, Laws ER, Jr, Fossett D. Delayed hyponatremia after transsphenoidal surgery for pituitary adenoma. Report of nine cases. J Neurosurg. 1995;83:363–367. doi: 10.3171/jns.1995.83.2.0363. [DOI] [PubMed] [Google Scholar]
- 28.Kristof RA, Rother M, Neuloh G, Klingmüller D. Incidence, clinical manifestations, and course of water and electrolyte metabolism disturbances following transsphenoidal pituitary adenoma surgery: a prospective observational study. J Neurosurg. 2009;111:555–562. doi: 10.3171/2008.9.JNS08191. [DOI] [PubMed] [Google Scholar]
- 29.Fatemi N, Dusick JR, Mattozo C, et al. Pituitary hormonal loss and recovery after transsphenoidal adenoma removal. Neurosurgery. 2008;63:709–718. doi: 10.1227/01.NEU.0000325725.77132.90. discussion 718–719. [DOI] [PubMed] [Google Scholar]
- 30.Marko NF, Gonugunta VA, Hamrahian AH, Usmani A, Mayberg MR, Weil RJ. Use of morning serum cortisol level after transsphenoidal resection of pituitary adenoma to predict the need for long-term glucocorticoid supplementation. J Neurosurg. 2009;111:540–544. doi: 10.3171/2008.12.JNS081265. [DOI] [PubMed] [Google Scholar]
- 31.Molitch ME, Clemmons DR, Malozowski S, Merriam GR, Vance ML. Endocrine Society. Evaluation and treatment of adult growth hormone deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1587–1609. doi: 10.1210/jc.2011-0179. [DOI] [PubMed] [Google Scholar]