Abstract
High plasma levels of leptin, a major adipocytokine produced by adipocytes, are correlated with increased fat mass in obese state. Leptin is emerging as a key candidate molecule linking obesity with breast cancer. Acting via endocrine, paracrine, and autocrine manner, leptin impacts various stages of breast tumorigenesis from initiation and primary tumor growth to metastatic progression. Leptin also modulates the tumor microenvironment mainly through supporting migration of endothelial cells, neo-angiogenesis and sustaining recruitment of macrophage and monocytes. Various studies have shown that hyperactive leptin-signaling network leads to concurrent activation of multiple oncogenic pathways resulting in enhanced proliferation, decreased apoptosis, acquisition of mesenchymal phenotype, potentiated migration and enhanced invasion potential of tumor cells. Furthermore, the capability of leptin to interact with other molecular effectors of obese state including, estrogen, IGF-1, insulin, VEGF and inflammatory cytokines further increases its impact on breast tumor progression in obese state. This article presents an overview of the studies investigating the involvement of leptin in breast cancer.
Introduction
Many prospective epidemiological studies have demonstrated that obesity, defined as excessive fat accumulation, is a pandemic condition that greatly influences risk, prognosis and progression of various cancers including breast cancer [1–7]. The Million Women study examined breast cancer incidence and mortality in relation to body mass index and reported that approximately half of the cancers can be attributed to obesity in postmenopausal women [8]. A study focused on invasive breast cancer reported that advanced grade and stage including lymph node metastases were more prevalent in obese women [9]. Investigating the relationship of obesity with mortality from breast cancer, many studies report that obese women in the highest quintile of body mass index (BMI) have double the death rate from breast cancer when compared with women in the lowest quintile [1,10–13]. In addition, in women with BMI in the highest quintile, an increased proportion of tumors were ER negative, had a high S-phase fraction, histological grade, mitotic cell count, expression levels of proliferation markers, and a larger tumor size [14]. Similar findings were reported by Daling et al., in a population-based follow-up study showing that the women in the highest quintile of BMI are more likely to have high histological grade, increased size and ER negative breast tumors [15]. Taking a molecular approach to examine the link between obesity and breast cancer, Creighton et al., examined the effect of patient obesity on gene expression of primary breast tumors. Transcription profiles were conducted on 103 tumors for which the patient’s body mass index (BMI) was known and stratified into three groups according to BMI (normal, overweight and obese). Tumors from obese patients were compared to tumors from either normal or overweight patients, and the transcriptional level of 662 genes were found to be associated with the obese state defined as obesity-associated cancer gene signature. Obesity-associated cancer gene signature associated with worst patient outcome in meta-analysis [16]. Together, these studies have shown that obese women regardless of their menopausal status (in most cases) are likely to have metastatic breast cancer when they are first diagnosed, and to have a poor final outcome.
Several hypotheses have been proposed to explain this association and very popularly, particular emphasis has been placed on the increased production of estrogen from peripheral aromatization of androgens in adipose tissue. High levels of estrogen can promote the development of post-menopausal carcinoma of the breast but despite the relationship between obesity and high estrogenic activity, it has become evident that this cannot fully explain the associations between body weight and breast cancer risk and poor prognosis because, in studies of both premenopausal and postmenopausal women, large subgroups of obese women were identified with ER-negative breast cancer exhibiting rapid growth and aggressive metastatic biological character. According to the newer hypothesis, obesity is now considered as an endocrine disorder and is associated with increased circulating levels of insulin, bioavailable insulin-like growth factor (IGF-1), inflammatory cytokines, vascular integrity factors such as vascular endothelial growth factors (VEGF), plasminogen activator inhibitor (PAI)-1 and adipocytokines [10,11,17]. These mediators, their interacting partners and pathways form the complex molecular network by which obese state impacts pathological manifestation of carcinogenesis.
A typical feature of obese state is an excess fat mass as a consequence of hypertrophy and hyperplasia of adipocytes. With recent research elevating the status of adipocytes from ‘inert energy storing cells’ to ‘active endocrine organs’, there has been significant interest in the potential role of adipocytokines (a group of proteins synthesized in adipose tissues) in the development of obesity-associated cancers. Adipocytokines are mainly produced by the adipocytes and the stromal cells (fibroblasts) that can potentially differentiate to mature adipocytes and macrophages that infiltrate the adipose tissue. Acting by endocrine, paracrine, and autocrine mechanisms, adipocytokines affect various biological processes. [18,19]. Among various adipocytokines, leptin, owing to its myriad oncogenic effects on carcinogenesis have come to be recognized as an important mediator of molecular effects of obesity. In this review, we will focus on adipocytokine leptin, its normal role in breast, clinical and preclinical studies showing its association with breast cancer, notably its effect on breast cancer cells growth and metastatic properties. We will also emphasize the interaction of leptin with other important mediators of obesity-breast cancer axis with the hypothesis that only by studying the interconnected network of various biological mediators of obesity can a complete understanding be achieved.
Dysregulated Leptin Levels in Obesity and Breast Cancer
Leptin, a product of the obese (ob) gene, is a neuroendocrine hormone that has attracted attention since its identification in 1994 [20] as it launched a new field in obesity research. Leptin is synthesized and secreted in proportion to body mass predominantly from preadipocytes and adipocytes, and to a lesser extent from placenta, stomach and skeletal muscle. Circulating as a 16 kDa non-glycosylated protein partially bound to plasma proteins, leptin targets hypothalamus, and peripheral organs, including liver, skeletal muscle and pancreas [21,22]. In addition to its role as a satiety hormone research over the last few years provided important clues about its apheliotropic actions, its role in the pathogenesis of atherosclerotic vascular disease and importantly carcinogenesis [23–25]. Biological actions of leptin are mediated through binding to the extracellular domain of specific membrane receptor (leptin receptor) present in a variety of tissues localized to the cell membranes [26]. Leptin receptor are characterized as class I cytokine receptors typically containing a cytokine receptor homologous domain in the extracellular region [27]. Up to now, six isoforms of the leptin receptor have been identified. All isoforms have a similar extracellular ligand binding domain at the amino terminus but they differ at the intracellular carboxy-terminal domain. While all five short isoforms have transmembrane domains, only the long form has the intracellular motifs necessary for activation of signaling pathways [28].
Although leptin was found as an afferent satiety signal, regulating appetite and energy expenditure in both humans and rodents [29], obese state is not related to deficiency of leptin. In fact, high plasma levels of leptin are correlated with increased fat mass in obese state and decreased levels are found in lean humans and animals [30,31]. Multiple epidemiological studies have examined serum leptin levels in women with breast cancer over past few years. The relationship of circulating leptin levels with breast cancer appears to be complex with most studies reporting a clear positive association between increased serum leptin levels and elevated breast cancer risk while few others found no change or even reduced levels of leptin associated with breast cancer [32–35]. The contradiction is these results can be largely attributed to confounding factors such as disease stage, inclusion of small number of subjects, considering only premenopausal subjects or combining pre and post menopausal women. To address the contradictory results that have been reported regarding the association between leptin level and breast cancer, Niu et al., recently performed a meta-analysis utilizing data from 23 relevant studies involving 2058 breast cancer patients, 2078 healthy controls and 285 breast benign controls. They concluded that the circulating leptin levels were lowest in healthy people while increasing levels were found in different groups as follows: healthy people<breast benign diseases patients<breast cancer patients <lymph node metastasis positive patients [36]. Clear evidence has emerged for postmenopausal breast cancer patients with ER positive breast cancer where serum leptin levels significantly correlated with poor clinocopathological tumor classification [37]. A case control study investigating the relationships of plasma leptin level and anthropometric measures of adiposity with the risk of breast cancer reported that overall higher leptin concentrations were significantly associated with an increased risk of breast cancer. They also found that the associations of leptin with breast cancer risk persisted after adjustment for obesity indices suggesting that leptin may have an independent role in breast tumorigenesis [38]. Another study analyzing leptin and leptin receptor involvement in breast carcinoma showed positive correlation with tumor size [39]. Leptin signaling and breast cancer were linked in a clinical study showing that leptin receptors were not detectable in normal mammary epithelial cells by immunohistochemistry, whereas in 83% of cases, carcinoma cells showed positive staining for the leptin receptor [40]. Of importance, overexpression of leptin was observed in 92% of breast tumors examined but in none of the cases of normal breast epithelium. The positive correlation of both leptin and leptin receptor suggested that leptin can potentially act on breast cancer cells via an autocrine pathway [40]. Another important study reported a positive relationship between blood leptin levels and breast cancer risk. Also, the degree of leptin mRNA expression in the peritumoral adipose tissue was found to be significantly higher in the breast cancer patients than the control women [41]. Studies also indicated that leptin and leptin receptor are overexpressed in primary and metastatic invasive ductal breast carcinoma compared with non-cancer mammary tissue [42]. In a recent study, 78 obese patients with newly diagnosed breast cancer and without diabetes and 78 obese women without breast cancer and diabetes were evaluated for their leptin levels. Higher leptin levels were found in women with breast cancer and obesity in comparison to similar obese women without breast cancer (Romero-Figueroa, et al., Clinical Breast Cancer, 2013, In press).
Evidence for the Role of Leptin in Mediating Obesity-Breast Cancer Link
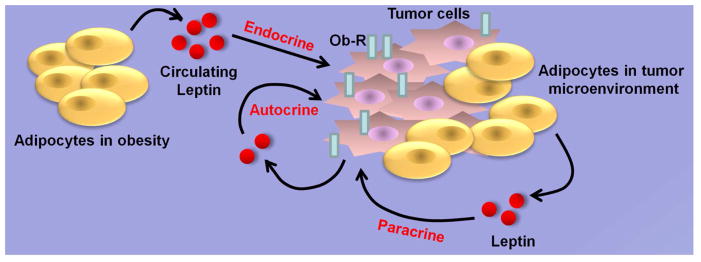
In breast microenvironment, adipokine leptin is secreted by adipocytes which form the bulk of the human breast being the most abundant cell type surrounding breast cancer cells and mammary epithelial cells which also produce leptin [43]. Several preclinical animal studies have also put forth the important role of leptin in breast tumorigenesis. In vivo studies examining the impact of leptin-axis on breast carcinogenesis utilizing genetic loss-of-function mutants for leptin or the leptin receptor showed that leptin or leptin receptor-deficient MMTV-Transforming growth factor-α (MMTV-TGF-α/Ob/Ob and MMTV-TGF-α/db/db) mice did not develop oncogene-induced mammary tumors [44,45]. These studies suggested that intact leptin-axis is required for spontaneous mammary tumorigenesis. In another approach, when hypothalamic Ob-Rb (long-form leptin receptor) reconstituted db/db (LEPR-null) mice (db/dbNse+/+) [46] were crossed with MMTV-PyMT mice, it was found that an Ob-Rb-mediated signaling promoted mammary tumor growth and metastasis [47]. Physiologically relevant high-fat diet-induced obese mouse models have also been used for manipulating leptin levels in vivo. Obese MMTV-TGFα mice certainly showed higher levels of leptin as well as accelerated growth of mammary tumors [48]. In contrast, obesity and elevated leptin levels did not affect the development of estrogen-negative breast tumors in MMTV-neu mice [49]. When xenografts of MMTV-Wnt1 tumors were transplanted in diet-induced obese mice, the tumors developed faster as compared to lean counterparts [50]. In coherence with the important role of leptin in breast tumorigenesis, xenografts of MMTV-Wnt1 cancer cells transplanted into leptin-deficient obese (Ob/Ob) mice displayed stunted growth [51]. In athymic-nude mice xenograft studies, leptin treatment significantly increased breast tumor growth [52]. Genetically obese Zucker rats which have a leptin receptor defect and lean, non-litter mates were injected with a chemical carcinogen methylnitrosourea and analyzed for the development of breast carcinoma. A smaller percentage of obese Zucker rats developed carcinomas as compared to the lean, non-litter mates [53]. High level of leptin circulating in the blood in obese state exerts its biological effects on responsive cells in a classical endocrine manner. In addition, produced by the adipocytes in the breast tumor microenvironment, leptin acts on breast cancer cells through paracrine pathways. It is important to note that breast cancer cells possess leptin receptors and secrete leptin which acts in an autocrine manner (Figure 1). The studies discussed above suggest the importance of endocrine, paracrine and autocrine effects of leptin and the importance of leptin receptor in breast carcinogenesis.
Figure 1. Endocrine, paracrine and autocrine actions of leptin.
Leptin circulates at high levels in obese conditions and impacts breast tumor growth in an endocrine manner. Resident adipocytes secrete leptin in breast tumor microenvironment and act on the infiltrating breast cancer cells exhibiting paracrine effect of leptin. A direct autocrine effect of leptin is observed when leptin secreted from breast tumor cells impacts tumor growth acting via leptin receptors present on the breast tumor cells.
Interaction of Leptin with Other Important Mediators of Breast Cancer
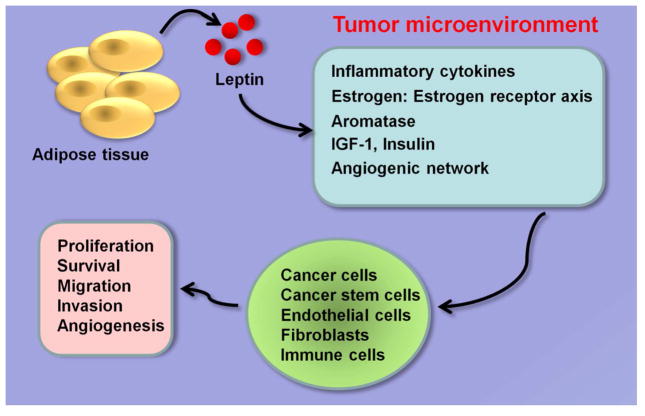
Leptin has been shown to interact with various important mediators of breast cancer in obese state including estrogen signaling network, insulin, IGF-1, angiogenic pathways and inflammatory cytokines impacting breast cancer initiation, growth and metastatic progression (Figure 2).
Figure 2. Leptin interacts with various important mediators of breast cancer.
Leptin produced by adipose tissue binds to leptin receptor expressing cells in the tumor microenvironment including breast cancer cells, breast cancer stem cells (BCSCs), endothelial cells, fibroblasts and immune cells. Leptin activates multiple oncogenic pathways and impacts various steps of tumor progression.
Estrogen and Aromatase: functional crosstalk with leptin
Estrogen plays an integral role in normal mammary gland development and is associated with breast tumor progression [54]. In postmenopausal women, adipose tissue serves as a major source of estrogens primarily produced by aromatization of androstenedione via cytochrome P450 19A1 (CYP19A1) also known as aromatase [55]. Functional crosstalk between leptin and estrogen signaling network further contributes to breast carcinogenesis exemplifying the interactions between resident adipocytes and breast epithelial cells. Leptin enhances aromatase mRNA expression, aromatase content, and its enzymatic activity in breast cancer cells in an ERK and Stat3-dependent manner as the presence of MAPK inhibitor, ERK2 dominant negative or Stat3 dominant negative mitigates the stimulatory effects of leptin [56]. Leptin-induced aromatase activity further leads to increased production of estradiol and estrogen receptor (ER) signaling in breast cancer cells [56]. Another mechanism by which leptin increases estrogen signaling in breast cancer cells is via direct transactivation of ER in the absence of cognate ligand. Leptin treatment induces classical features of ER activation including nuclear translocation, downregulation of ER mRNA and protein and upregulation of classical ER responsive genes which is abrogated in the presence of ERK inhibitor or dominant negative ERK2 [57]. It has been shown in multiple cancer cell lines that leptin activates phosphorylation of ERK [58–60]. ER is known to get phosphorylated on Ser-118 by ERK in response to upstream signaling events including estrogen, insulin/insulin like factor1 (IGF-1) and this phosphorylation is required for functional activation of ER [61]. In a feed-forward mechanism, leptin activates ERK which in turn phosphorylates ER resulting in its activation in breast cancer cells. Leptin mediated activation of ERK and Stat3 has also been associated with increased expression levels of ER in MCF7 breast cancer cells [62]. Leptin-mediated increased expression of ER was lost upon silencing of leptin receptor indicating a functional dependence. Analysis of breast cancer samples from 33 patients at different stages of disease showed a statistically significant correlation between the expression of leptin receptor and estrogen receptor [62]. Exogenous leptin treatment has been reported to increase ER expression in breast tumors in nude-mouse xenograft model [63]. Chronic treatment of breast cancer cells with leptin potentiates the mitogenic actions of estrogen and alters the ratio of ERα to ERβ [64] indicating that high levels of leptin in breast tumor microenvironment can enhance estrogen-signaling. According to these findings, leptin plays an important role in modulating E2-ER network in breast cancer cells. On one hand, leptin enhances estrogen levels by increasing the conversion of androstenedione to estrogen especially in post-menopausal conditions and on the other hand activates ER function in a ligand-independent manner. Interestingly, estrogen-signaling also in turn modulates leptin function as overexpression of estrogen receptor increases leptin-induced Stat3 activity in breast cancer cells [65]. Together, these studies indicate a biologically important crosstalk between estrogen and leptin signaling networks.
Connection between insulin, IGF1 and leptin
Hyperinsulinemia or insulin resistance is associated with obese conditions and increases the risk and progression of breast cancer [66]. Insulin, a peptide hormone produced by the beta cells of the pancreas and released in response to increased blood glucose, is reported to exert antiapoptotic effects at normal levels while supraphysiological levels of insulin, as evident in obese state, have mitogenic effects. Insulin mediates its tumor-promoting effects directly via the insulin receptor (IR) or insulin-like growth factor 1 receptor (IGF-1R). Elevated insulin levels induce extracellular-signal regulated kinase (ERK) and phosphatidylinositol-3 kinase (PI3K) pathways in breast cancer cells leading to downstream activation of several pathways and oncogenic processes [67]. Studies examining the relationship between obesity related stimuli including insulin and leptin in breast cancer reported a positive correlation between leptin and insulin as high concentrations of insulin stimulated leptin mRNA in both ERα-positive MCF-7 and ERα-negative MDA-MB 231 cell lines [42,68]. Elevated level of IGF-1, a peptide growth factor that shares ~50% sequence homology with insulin, observed in obese state is positively associated with increased breast cancer risk [69]. IGF-I receptors are known to mediate the proliferative effects of insulin as the insulin receptor (IR) and the receptor for IGF-I (IGF-IR) exhibit more than 50% of overall sequence homology and the tyrosine kinase domain of the α-subunit share 84% homology. Also, their respective ligands, insulin, and IGF-I share 40–50% homology supporting interaction of insulin and IGF-1 with both IR and IGF-1R [66]. IGF-1 overexpression leads to excessive proliferation and survival signals for the breast tumor development [70]. IGF-1R is overexpressed in ~50% of primary breast tumors compared with normal tissue indicating that these carcinomas have enhanced responses to the mitogenic and anti-apoptotic effects of IGF-1[71] and inactivation of IGF-1R results in reduced breast tumor growth and metastasis in vivo [72]. Studies from our group and others have shown interactions between leptin signaling network and IGF-1[73,74]. We demonstrated that combined treatment with leptin and IGF-1 significantly increased proliferation as well as invasion and migration of breast cancer cells. A novel bidirectional crosstalk between leptin and IGF-1 signaling was found; IGF-1 treatment induced remarkable phosphorylation of leptin receptor (Ob-Rb) and leptin treatment induced tyrosine phosphorylation of IGF-1 receptor (IGF-1R) while co-treatment induced synergistic phosphorylation of both receptors and association of Ob-Rb and IGF-1R along with activation of downstream effectors, Akt, ERK, IRS-1, and IRS-2. Interestingly, the biological effects of this crosstalk were mediated by epidermal growth factor receptor (EGFR) activation depending on proteolytic release of EGFR ligands as broad-spectrum matrix metalloproteinase inhibitor, GM6001 could inhibit this effect. Our study further showed that inhibition of EGFR activation using clinically relevant EGFR inhibitors, erlotinib and lapatinib, inhibited leptin and IGF-1 induced invasion and migration of breast cancer cells [74]. Together, these studies suggest that interactions between leptin, insulin and IGF-1 could contribute to breast cancer initiation, growth and metastatic progression in obese state.
Impact of leptin on angiogenic network
Vascular endothelial growth factor (VEGF), possessing angiogenic, mitogenic, and vascular permeability-enhancing activities specific for endothelial cells, is a key angiogenic factor [75]. VEGF is considered an adipokine due to its synthesis in adipose tissue and is positively correlated with increased BMI [76]. High circulating levels and increased tumoral expression of VEGF is associated with poor prognosis [77,78]. Recent studies have shown that leptin is a pro-angiogenic cytokine promoting angiogenesis, upregulating VEGF and VEGF receptors in breast cancer. Leptin induces angiogenesis in normal rat corneas but not in corneas of rats genetically lacking leptin receptors [79] indicating the involvement of leptin-signaling network. Using various in vitro and in vivo approaches, various studies have shown that leptin increases proliferation of human umbilical venous endothelial cells with a potency comparable to VEGF treatment, induces capillary-like tube formation and stimulates angiogenesis in chorioallantoic membrane (CAM) assay and disc implantation assay [80–82]. Synergistic effects of leptin, VEGF and bFGF have also been shown to stimulate angiogenesis [83]. Leptin directly upregulates VEGF in breast cancer cells via mediating various canonical and non-canonical signaling pathways [84,85]. Leptin-mediated increased Stat3, ERK, and Akt phosphorylation leads to upregulation of VEGF mRNA and protein in mouse mammary cancer cells (MT) and inhibition of these canonical signaling pathways using specific small-molecule inhibitors abrogates the effect of leptin on VEGF levels. Leptin-mediated VEGF upregulation also involves modulation of HIF1α and NF-κB and inhibitors of these transcription factors such as NS398 (for HIF1α) and inhibitor IKK antagonist (for NF-κB) negatively impact leptin-mediated VEGF expression [84]. Leptin treatment has also been reported to increase the expression of VEGF receptor 2 (VEGFR2) independent of VEGF [86] resulting in promotion of mammary tumor growth. Using multiple breast cancer cells, Ray et al., show that leptin treatment results in elevated levels of VEGF [87]. Upregulation of VEGF and VEGFR2 by leptin have important implications in breast carcinogenesis as neoangiogenesis is required to fulfill the nutrients and oxygen needs of growing tumors. In addition to modulating VEGF/VEGFR2 axis to support neoangiogenesis, leptin induces the expression of several other molecules including MMP-2, MMP-9, leukemia inhibitory factor (LIF), interleukin-1 (IL-1), and also αvβ3 integrin in various model systems to further increase tumor angiogenesis. Leptin stimulates MMP-2 activity in breast cancer cells via Jun N-terminal kinase activation [88]. Leptin also regulates IL-1, LIF and their respective receptors, IL-1R tI and LIFR in benign (primary and HES) and cancerous-endometrial epithelial cells (An3Ca, SK-UT2 and Ishikawa), and induces a greater increase in LIF levels in cancer as compared to benign cells [89]. Furthermore, leptin modulates fibroblasts and macrophages to stimulate increased secretion of pro-angiogenic factors. In tumor microenvironment, breast tumors produce a high level of IL-1 in vivo which triggers leptin expression in stromal cells and infiltrating immune cells [90]. Leptin is also shown to induce several signaling pathways for transcriptional and translational expression of IL-1 and associated components in breast cancer cells and inhibition of IL-1 abrogates leptin-induced VEGF expression [91]. Further research is needed to delineate the molecular mechanisms by which leptin crosstalks with various components of angiogenic network and exerts its effect on promotion of angiogenesis in breast cancer.
Leptin and inflammatory cytokines
Inflammatory cytokines such as tumor necrosis factor-α (TNF-α) and IL-6 are primarily produced by infiltrating macrophages while small amounts are synthesized by adipocytes in obese state. Increased plasma concentration of TNF-α, IL-17 and IL-6 are associated with high BMI levels [92,93]. Tumor associated macrophages express multiple receptors including leptin-receptor (Ob-R), toll-like receptor (TLR4), and play an important role in breast tumor progression mediating crosstalk between tumor cells and adipocytes [94]. Adipocytes secrete adipokine leptin which binds to its receptor on macrophages and stimulate the secretion of multiple pro-inflammatory and pro-angiogenic cytokines such as IL-1, TNF-α, IL-6, IL-11 and nitric oxide modulating macrophage phenotype (anti-inflammatory M2 and pro-inflammatory M1). M1-polarized or classically activated macrophages colonize adipose tissue [95]. Adipose tissue macrophages exhibit increased inflammatory properties during diet-induced obesity [96]. Several studies have reported leptin-mediated modulations of proinflammatory cytokines in multiple systems. Leptin treatment induces IL-17 expression in CD4+ cells while macrophages are shown to produce IL-6 and TNF-α in response to leptin. Dendritic cells exhibit increased production of multiple cytokines including IL-1, IL-6, IL-12, TNF-α and MIP-1α upon leptin stimulation [97–99]. Leptin upregulates IL-1 and TNF-α in macrophages and tissue factor (TF) in breast cancer cells via TNF-α [100,101]. Although high circulating levels of some cytokines and leptin are known to be negative prognostic markers for breast cancer and some studies have shown interactions between them, further studies are needed to clarify their cell-specific molecular link and importance in breast tumor progression.
How Does Leptin Influence Breast Cancer Growth and Metastasis?
Activation of oncogenic pathways: multipartite leptin network
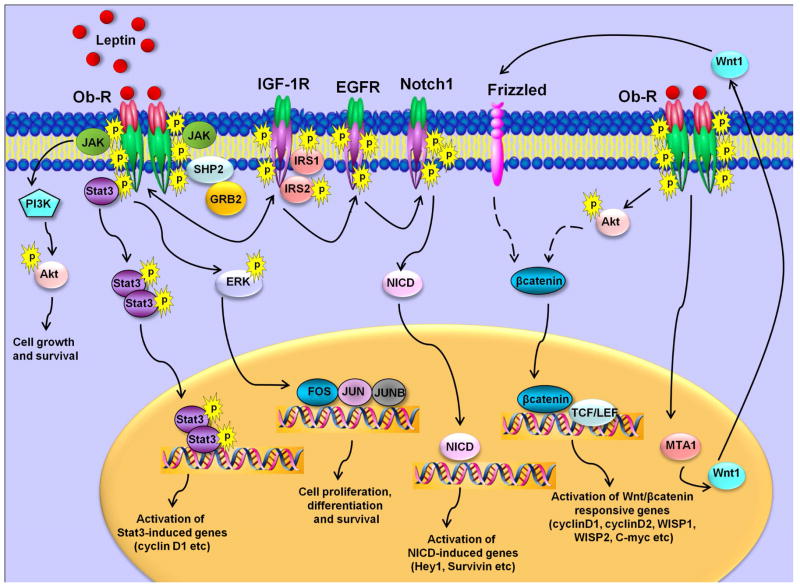
Various research groups have been studying the oncogenic role of leptin in breast cancer. In vitro studies from our lab and others have shown that leptin regulates various molecules and oncogenic pathways involved in proliferation, adhesion, invasion, migration, inflammation and angiogenesis, such as cyclin D1, survivin, β3 integrin, interleukin-1 (IL-1), IL-1 receptor, vascular endothelial growth factor and its receptor type 2 in breast carcinogenesis [52,58,74,86,102–106]. Concomitant activation of multiple signaling pathways including ERK, Akt and Stat3 network via leptin has been reported in breast cancer [58,59,74,102,107]. Leptin modulates the expression of various important genes implicated in breast carcinogenesis via Stat3 activation. It is interesting to note that leptin can directly manipulate the transactivation function of certain coactivator molecules leading to alterations in local chromatin structure. For example, leptin induces cyclin D1 expression increasing histone acetylation at the cyclin D1 promoter. Genes in repressed state have been shown to be associated with methylation of K9-dimethylated H3 whereas the active state is associated with increased methylation of K4-dimethylated H3 [108]. Leptin increases H3-K4 methylation and decreases H3-K9 methylation creating a permissive environment for the recruitment of specific coactivator complexes including Med1 and SRC1 resulting in increased breast cancer cell growth [102]. Leptin also regulates cyclin D1 expression in several other breast cancer cells [87,109,110] and luminal epithelial cells of mouse MMTV-Wnt1 mammary tumors [111]. Leptin-mediated increased expression of survivin, a member of inhibitor-of-apoptosis proteins (IAP) family, has been reported in breast cancer cells [52,112,113]. Our recent work showing leptin-mediated increased survivin expression implicates a complex upstream network involving activation of EGFR-Notch1 axis. These studies show that leptin activates Notch1 and induces recruitment of NICD (transcriptionally active intracellular Notch) to survivin promoter. Interestingly, Notch 1 activation by leptin is mediated via epidermal growth factor receptor (EGFR) transactivation implicating another oncogenic pathway in leptin network [52]. In addition, involvement of EGFR transactivation in leptin function has also been reported in gastric cancer cells [114], and oesophageal adenocarcinomac cells [115]. Several studies have reported multiple crosstalks between leptin and other contributors of mammary tumorigenesis and progression including IGF1, IL6, Notch and sex hormones [52,62,74,116] resulting in enhanced growth and metastatic properties of breast cancer cells. Hence, highly-active leptin-induced signaling-network (Figure 3) could contribute to various aspects of breast tumorigenesis and metastasis by modulating various molecular mediators and key pathways.
Figure 3. Complex leptin signaling network.
Leptin binds to long-form of leptin receptor (Ob-R) resulting in conformational changes and receptor oligomerization. Leptin receptor gets phosphorylated followed by JAK activation which in turn phosphorylates Ob-R. These early events trigger activation of multiple pathways such as Akt activation, ERK phosphorylation and Stat3 activation. Bidirectional crosstalk occurs between Ob-R and IGF-1R. Leptin also transactivates EGFR and activates Notch1 resulting in release of NICD. Leptin increases the expression of MTA1 resulting in increased levels of Wnt1 and activation of Wnt1/β-catenin network. Abbreviations: AKT, protein kinase B; GRB2, growth factor receptor-bound protein 2; JAK, Janus kinase; Ob-R, leptin receptor; MAPK, mitogen-activated protein kinase; PI3K, phosphatidylinositol 3 kinase; SHP2, Src homology 2-containing tyrosine phosphatase; STAT3, signal transducer and activator of transcription 3; insulin-like growth factor 1 receptor, IGF-1R; epidermal growth factor receptor, EGFR; transcriptionally active intracellular Notch, NICD; metastasis associated protein 1, MTA1.
Leptin, epithelial-mesenchymal transition and breast cancer stem cells
Epithelial mesenchymal transition (EMT) is a crucial step in the induction of cell motility and enhanced survival during physiological processes such as embryonic development and wound healing as well as in pathological situations like malignant cells undergoing invasion and metastasis [117]. Recent studies from our lab present a central role of leptin in acquisition of mesenchymal characteristics and aggressive behavior in breast cancer. Leptin treatment induces breast cancer cells to undergo a transition from epithelial to spindle-like mesenchymal morphology. The key mechanism to account for this important function of leptin is that it increases β-catenin stabilization and nuclear translocation. Leptin-mediated stabilization of β-catenin is achieved by concomitant activation of Akt/GSK3 and MTA1/Wnt1 signaling leading to destruction of LKB1-GSK3β-Axin complex. We also provide molecular evidence supporting the regulatory role of MTA1 in leptin-induced Wnt1 upregulation, β-catenin stabilization and nuclear translocation. These studies implicate a previously unrecognized crosstalk between leptin and MTA1/Wnt signaling in epithelial-mesenchymal transition of breast cancer cells [118]. Two other studies report involvement of leptin-signaling in EMT: role of leptin in endocardial cushion formation by modulating EMT [119] and activating hedgehog pathway to play a role in EMT in hepatic stellate cells [120]. Given that EMT plays an integral role in metastatic progression of breast tumors as well as sustaining the breast cancer stem cells (BCSCs), it is of interest to discuss the current knowledge regarding the impact of leptin on breast cancer stem cells.
BCSCs, known for their characteristic ability to undergo self-renewal as well as tumor differentiation, play an important role in breast cancer initiation, growth and metastatic manifestation. Several signaling pathways including Notch, Hedgehog, Wnt/β-catenin and TGF-β play an essential role in stem cell function during embryogenesis as well as oncogenesis [121]. It is interesting to note that leptin has been reported to regulate many signaling pathways and transcription factors implicated in BCSCs (discussed in previous section). Recently, Zheng et al. observed that MMTV-Wnt1 xenografts grow poorly in leptin knockout ob/ob mice in comparison to wild-type mice. Analysis of these tumor samples led to the identification of a leptin-responsive cell population that was present only in the tumors from wild-type mice. Fluorescent-activated cell sorting (FACS) for CSC markers including CD29 (integrin β1), CD49f (integrin α6), and CD24 (heat stable antigen) show that CD29+CD24− and leptin receptor expressing CSC population is highly increased in the presence of leptin. These studies indicate the involvement of leptin in CSC survival [51]. Utilizing leptin-deficient ob/ob and leptin-receptor-deficient db/db, another study show that leptin-receptor (Ob-R) is a characteristic feature of tumor-initiating stem cells (TISCs) and is regulated by the core pluripotency-associated transcription factors OCT4 and SOX2. Also, TISCs demonstrate increased phosphorylation of pluripotency-associated oncogene Stat3 and induction of OCT4 and SOX2 in response to leptin [122]. It is suggested that leptin receptor plays an important role in the expression of Nanog, OCT4 and SOX2 in BCSCs, viability of BCSCs, proliferation and tumor-initiating activity [123]. These findings raise the possibility that obesity and its associated increased level of leptin-network can act as a mechanistic link between obesity, increased maintenance of cancer stem cells, augmented cancer recurrence, increased distant metastasis and overall poor survival. Leptin signaling has also been implicated in mediating interactions between K303R mutant estrogen receptor-expression breast cancer cells and cancer-associated fibroblasts (CAFs). It is proposed that leptin plays an integral role in a bidirectional crosstalk between breast cancer cells and the “educated” CAFs driving tumor progression [124].
Concluding Remarks
Breast cancer progression, a multistep process involving tumor initiation, primary tumor growth, invasion, metastatic progression, involves complex interaction with various stromal components including endothelial cells, immune cells, fibroblasts and adipocytes. Adipocytes are the major component in breast cancer microenvironment and are known to secrete various adipokines. Adipokine leptin is produced by adipocytes as well as breast cancer cells and acts in a paracrine, autocrine as well as endocrine manner. Various studies over the past few years have shown that leptin impacts breast tumor progression directly by interacting with breast tumor cells and indirectly by influencing various components of the tumor microenvironment. Hyperactive leptin-signaling network during obese state leads to concurrent activation of multiple oncogenic pathways resulting in: enhanced proliferation, decreased apoptosis, acquisition of mesenchymal phenotype, potentiated migration and enhanced invasion potential of tumor cells. In addition, leptin supports neo-angiogenesis and sustained recruitment of macrophages and monocytes, which upon leptin-stimulation, secrete VEGF and proinflammatory cytokines. Leptin also crosstalks with several other molecular effectors of obese state including, estrogen, IGF-1, insulin, VEGF and inflammatory cytokines, many times, potentiating their activities. Recent research advances have also implicated leptin in cancer initiation and breast cancer stem cells though further research is needed to strengthen these conclusions and elucidate the underlying molecular mechanisms. Given all the potential roles of leptin in various steps of breast cancer progression and its strong link with obesity, the leptin-signaling network emerges as an attractive therapeutic target for the obese breast cancer patients.
Acknowledgments
Financial Support: This work was supported by NIDDK NIH, K01DK076742 and R03DK089130 (to NKS); NCI NIH R01CA131294, Avon Foundation, Breast Cancer Research Foundation (BCRF) 90047965 (to DS).
References
- 1.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 2.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–91. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 3.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–78. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 4.Wolin KY, Carson K, Colditz GA. Obesity and cancer. Oncologist. 2010;15:556–65. doi: 10.1634/theoncologist.2009-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Azambuja E, McCaskill-Stevens W, Francis P, Quinaux E, Crown JP, Vicente M, Giuliani R, Nordenskjold B, Gutierez J, Andersson M, Vila MM, Jakesz R, Demol J, Dewar J, Santoro A, Lluch A, Olsen S, Gelber RD, Di Leo A, Piccart-Gebhart M. The effect of body mass index on overall and disease-free survival in node-positive breast cancer patients treated with docetaxel and doxorubicin-containing adjuvant chemotherapy: the experience of the BIG 02-98 trial. Breast Cancer Res Treat. 2010;119:145–53. doi: 10.1007/s10549-009-0512-0. [DOI] [PubMed] [Google Scholar]
- 6.Majed B, Moreau T, Senouci K, Sigal B, Salmon RJ, Fourquet A, Asselain B. Stoutness and prognosis of female non-metastatic breast cancer: results from a French observational cohort study. Bull Cancer. 2009;96:531–41. doi: 10.1684/bdc.2009.0856. [DOI] [PubMed] [Google Scholar]
- 7.Majed B, Moreau T, Senouci K, Salmon RJ, Fourquet A, Asselain B. Is obesity an independent prognosis factor in woman breast cancer? Breast Cancer Res Treat. 2008;111:329–42. doi: 10.1007/s10549-007-9785-3. [DOI] [PubMed] [Google Scholar]
- 8.Reeves GK, Pirie K, Beral V, Green J, Spencer E, Bull D. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ. 2007;335:1134. doi: 10.1136/bmj.39367.495995.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Porter GA, Inglis KM, Wood LA, Veugelers PJ. Effect of obesity on presentation of breast cancer. Ann Surg Oncol. 2006;13:327–32. doi: 10.1245/ASO.2006.03.049. [DOI] [PubMed] [Google Scholar]
- 10.Rose DP, Komninou D, Stephenson GD. Obesity, adipocytokines, and insulin resistance in breast cancer. Obes Rev. 2004;5:153–65. doi: 10.1111/j.1467-789X.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- 11.Ray A, Cleary MP. Obesity and breast cancer: a clinical biochemistry perspective. Clin Biochem. 2012;45:189–97. doi: 10.1016/j.clinbiochem.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 12.Dal Maso L, Zucchetto A, Talamini R, Serraino D, Stocco CF, Vercelli M, Falcini F, Franceschi S. Effect of obesity and other lifestyle factors on mortality in women with breast cancer. Int J Cancer. 2008;123:2188–94. doi: 10.1002/ijc.23747. [DOI] [PubMed] [Google Scholar]
- 13.Dawood S, Broglio K, Gonzalez-Angulo AM, Kau SW, Islam R, Hortobagyi GN, Cristofanilli M. Prognostic value of body mass index in locally advanced breast cancer. Clin Cancer Res. 2008;14:1718–25. doi: 10.1158/1078-0432.CCR-07-1479. [DOI] [PubMed] [Google Scholar]
- 14.Vona-Davis L, Rose DP, Hazard H, Howard-McNatt M, Adkins F, Partin J, Hobbs G. Triple-negative breast cancer and obesity in a rural Appalachian population. Cancer Epidemiol Biomarkers Prev. 2008;17:3319–24. doi: 10.1158/1055-9965.EPI-08-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daling JR, Malone KE, Doody DR, Johnson LG, Gralow JR, Porter PL. Relation of body mass index to tumor markers and survival among young women with invasive ductal breast carcinoma. Cancer. 2001;92:720–9. doi: 10.1002/1097-0142(20010815)92:4<720::aid-cncr1375>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 16.Creighton CJ, Sada YH, Zhang Y, Tsimelzon A, Wong H, Dave B, Landis MD, Bear HD, Rodriguez A, Chang JC. A gene transcription signature of obesity in breast cancer. Breast Cancer Res Treat. 2012;132:993–1000. doi: 10.1007/s10549-011-1595-y. [DOI] [PubMed] [Google Scholar]
- 17.Hursting SD, Berger NA. Energy balance, host-related factors, and cancer progression. J Clin Oncol. 2010;28:4058–65. doi: 10.1200/JCO.2010.27.9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vona-Davis L, Howard-McNatt M, Rose DP. Adiposity, type 2 diabetes and the metabolic syndrome in breast cancer. Obes Rev. 2007;8:395–408. doi: 10.1111/j.1467-789X.2007.00396.x. [DOI] [PubMed] [Google Scholar]
- 19.Vona-Davis L, Rose DP. Adipokines as endocrine, paracrine, and autocrine factors in breast cancer risk and progression. Endocr Relat Cancer. 2007;14:189–206. doi: 10.1677/ERC-06-0068. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 21.Chinookoswong N, Wang JL, Shi ZQ. Leptin restores euglycemia and normalizes glucose turnover in insulin-deficient diabetes in the rat. Diabetes. 1999;48:1487–92. doi: 10.2337/diabetes.48.7.1487. [DOI] [PubMed] [Google Scholar]
- 22.Pallett AL, Morton NM, Cawthorne MA, Emilsson V. Leptin inhibits insulin secretion and reduces insulin mRNA levels in rat isolated pancreatic islets. Biochem Biophys Res Commun. 1997;238:267–70. doi: 10.1006/bbrc.1997.7274. [DOI] [PubMed] [Google Scholar]
- 23.Singhal A, Farooqi IS, Cole TJ, O’Rahilly S, Fewtrell M, Kattenhorn M, Lucas A, Deanfield J. Influence of leptin on arterial distensibility: a novel link between obesity and cardiovascular disease? Circulation. 2002;106:1919–24. doi: 10.1161/01.cir.0000033219.24717.52. [DOI] [PubMed] [Google Scholar]
- 24.Wolk R, Berger P, Lennon RJ, Brilakis ES, Johnson BD, Somers VK. Plasma leptin and prognosis in patients with established coronary atherosclerosis. J Am Coll Cardiol. 2004;44:1819–24. doi: 10.1016/j.jacc.2004.07.050. [DOI] [PubMed] [Google Scholar]
- 25.Somasundar P, McFadden DW, Hileman SM, Vona-Davis L. Leptin is a growth factor in cancer. J Surg Res. 2004;116:337–49. doi: 10.1016/j.jss.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Houseknecht KL, Mantzoros CS, Kuliawat R, Hadro E, Flier JS, Kahn BB. Evidence for leptin binding to proteins in serum of rodents and humans: modulation with obesity. Diabetes. 1996;45:1638–43. doi: 10.2337/diab.45.11.1638. [DOI] [PubMed] [Google Scholar]
- 27.Tartaglia LA. The leptin receptor. J Biol Chem. 1997;272:6093–6. doi: 10.1074/jbc.272.10.6093. [DOI] [PubMed] [Google Scholar]
- 28.Ahima RS, Osei SY. Leptin signaling. Physiol Behav. 2004;81:223–41. doi: 10.1016/j.physbeh.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 29.Muoio DM, Lynis Dohm G. Peripheral metabolic actions of leptin. Best Pract Res Clin Endocrinol Metab. 2002;16:653–66. doi: 10.1053/beem.2002.0223. [DOI] [PubMed] [Google Scholar]
- 30.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–5. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 31.Ostlund RE, Jr, Yang JW, Klein S, Gingerich R. Relation between plasma leptin concentration and body fat, gender, diet, age, and metabolic covariates. J Clin Endocrinol Metab. 1996;81:3909–13. doi: 10.1210/jcem.81.11.8923837. [DOI] [PubMed] [Google Scholar]
- 32.Coskun U, Gunel N, Toruner FB, Sancak B, Onuk E, Bayram O, Cengiz O, Yilmaz E, Elbeg S, Ozkan S. Serum leptin, prolactin and vascular endothelial growth factor (VEGF) levels in patients with breast cancer. Neoplasma. 2003;50:41–6. [PubMed] [Google Scholar]
- 33.Stattin P, Soderberg S, Biessy C, Lenner P, Hallmans G, Kaaks R, Olsson T. Plasma leptin and breast cancer risk: a prospective study in northern Sweden. Breast Cancer Res Treat. 2004;86:191–6. doi: 10.1023/B:BREA.0000036782.11945.d7. [DOI] [PubMed] [Google Scholar]
- 34.Woo HY, Park H, Ki CS, Park YL, Bae WG. Relationships among serum leptin, leptin receptor gene polymorphisms, and breast cancer in Korea. Cancer Lett. 2006;237:137–42. doi: 10.1016/j.canlet.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 35.Hancke K, Grubeck D, Hauser N, Kreienberg R, Weiss JM. Adipocyte fatty acid-binding protein as a novel prognostic factor in obese breast cancer patients. Breast Cancer Res Treat. 2010;119:367–7. doi: 10.1007/s10549-009-0577-9. [DOI] [PubMed] [Google Scholar]
- 36.Niu J, Jiang L, Guo W, Shao L, Liu Y, Wang L. The Association between Leptin Level and Breast Cancer: A Meta-Analysis. PLoS One. 2013;8:e67349. doi: 10.1371/journal.pone.0067349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maccio A, Madeddu C, Gramignano G, Mulas C, Floris C, Massa D, Astara G, Chessa P, Mantovani G. Correlation of body mass index and leptin with tumor size and stage of disease in hormone-dependent postmenopausal breast cancer: preliminary results and therapeutic implications. J Mol Med (Berl) 2010;88:677–86. doi: 10.1007/s00109-010-0611-8. [DOI] [PubMed] [Google Scholar]
- 38.Wu MH, Chou YC, Chou WY, Hsu GC, Chu CH, Yu CP, Yu JC, Sun CA. Circulating levels of leptin, adiposity and breast cancer risk. Br J Cancer. 2009;100:578–82. doi: 10.1038/sj.bjc.6604913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jarde T, Caldefie-Chezet F, Damez M, Mishellany F, Penault-Llorca F, Guillot J, Vasson MP. Leptin and leptin receptor involvement in cancer development: a study on human primary breast carcinoma. Oncol Rep. 2008;19:905–11. [PubMed] [Google Scholar]
- 40.Ishikawa M, Kitayama J, Nagawa H. Enhanced expression of leptin and leptin receptor (OB-R) in human breast cancer. Clin Cancer Res. 2004;10:4325–31. doi: 10.1158/1078-0432.CCR-03-0749. [DOI] [PubMed] [Google Scholar]
- 41.Tessitore L, Vizio B, Jenkins O, De Stefano I, Ritossa C, Argiles JM, Benedetto C, Mussa A. Leptin expression in colorectal and breast cancer patients. Int J Mol Med. 2000;5:421–6. doi: 10.3892/ijmm.5.4.421. [DOI] [PubMed] [Google Scholar]
- 42.Garofalo C, Koda M, Cascio S, Sulkowska M, Kanczuga-Koda L, Golaszewska J, Russo A, Sulkowski S, Surmacz E. Increased expression of leptin and the leptin receptor as a marker of breast cancer progression: possible role of obesity-related stimuli. Clin Cancer Res. 2006;12:1447–53. doi: 10.1158/1078-0432.CCR-05-1913. [DOI] [PubMed] [Google Scholar]
- 43.Smith-Kirwin SM, O’Connor DM, De Johnston J, Lancey ED, Hassink SG, Funanage VL. Leptin expression in human mammary epithelial cells and breast milk. J Clin Endocrinol Metab. 1998;83:1810–3. doi: 10.1210/jcem.83.5.4952. [DOI] [PubMed] [Google Scholar]
- 44.Cleary MP, Phillips FC, Getzin SC, Jacobson TL, Jacobson MK, Christensen TA, Juneja SC, Grande JP, Maihle NJ. Genetically obese MMTV-TGF-alpha/Lep(ob)Lep(ob) female mice do not develop mammary tumors. Breast Cancer Res Treat. 2003;77:205–15. doi: 10.1023/a:1021891825399. [DOI] [PubMed] [Google Scholar]
- 45.Cleary MP, Juneja SC, Phillips FC, Hu X, Grande JP, Maihle NJ. Leptin receptor-deficient MMTV-TGF-alpha/Lepr(db)Lepr(db) female mice do not develop oncogene-induced mammary tumors. Exp Biol Med (Maywood) 2004;229:182–93. doi: 10.1177/153537020422900207. [DOI] [PubMed] [Google Scholar]
- 46.Chua SC, Jr, Liu SM, Li Q, Sun A, DeNino WF, Heymsfield SB, Guo XE. Transgenic complementation of leptin receptor deficiency. II. Increased leptin receptor transgene dose effects on obesity/diabetes and fertility/lactation in lepr-db/db mice. Am J Physiol Endocrinol Metab. 2004;286:E384–92. doi: 10.1152/ajpendo.00349.2003. [DOI] [PubMed] [Google Scholar]
- 47.Park J, Kusminski CM, Chua SC, Scherer PE. Leptin receptor signaling supports cancer cell metabolism through suppression of mitochondrial respiration in vivo. Am J Pathol. 2010;177:3133–44. doi: 10.2353/ajpath.2010.100595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dogan S, Hu X, Zhang Y, Maihle NJ, Grande JP, Cleary MP. Effects of high-fat diet and/or body weight on mammary tumor leptin and apoptosis signaling pathways in MMTV-TGF-alpha mice. Breast Cancer Res. 2007;9:R91. doi: 10.1186/bcr1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cleary MP, Grande JP, Juneja SC, Maihle NJ. Diet-induced obesity and mammary tumor development in MMTV-neu female mice. Nutr Cancer. 2004;50:174–80. doi: 10.1207/s15327914nc5002_7. [DOI] [PubMed] [Google Scholar]
- 50.Nunez NP, Perkins SN, Smith NC, Berrigan D, Berendes DM, Varticovski L, Barrett JC, Hursting SD. Obesity accelerates mouse mammary tumor growth in the absence of ovarian hormones. Nutr Cancer. 2008;60:534–41. doi: 10.1080/01635580801966195. [DOI] [PubMed] [Google Scholar]
- 51.Zheng Q, Dunlap SM, Zhu J, Downs-Kelly E, Rich J, Hursting SD, Berger NA, Reizes O. Leptin deficiency suppresses MMTV-Wnt-1 mammary tumor growth in obese mice and abrogates tumor initiating cell survival. Endocr Relat Cancer. 2011;18:491–503. doi: 10.1530/ERC-11-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Knight BB, Oprea-Ilies GM, Nagalingam A, Yang L, Cohen C, Saxena NK, Sharma D. Survivin upregulation, dependent on leptin-EGFR-Notch1 axis, is essential for leptin-induced migration of breast carcinoma cells. Endocr Relat Cancer. 2011;18:413–28. doi: 10.1530/ERC-11-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee WM, Lu S, Medline A, Archer MC. Susceptibility of lean and obese Zucker rats to tumorigenesis induced by N-methyl-N-nitrosourea. Cancer Lett. 2001;162:155–60. doi: 10.1016/s0304-3835(00)00635-2. [DOI] [PubMed] [Google Scholar]
- 54.Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354:270–82. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- 55.Simpson ER, Mahendroo MS, Means GD, Kilgore MW, Hinshelwood MM, Graham-Lorence S, Amarneh B, Ito Y, Fisher CR, Michael MD, et al. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocr Rev. 1994;15:342–55. doi: 10.1210/edrv-15-3-342. [DOI] [PubMed] [Google Scholar]
- 56.Catalano S, Marsico S, Giordano C, Mauro L, Rizza P, Panno ML, Ando S. Leptin enhances, via AP-1, expression of aromatase in the MCF-7 cell line. J Biol Chem. 2003;278:28668–76. doi: 10.1074/jbc.M301695200. [DOI] [PubMed] [Google Scholar]
- 57.Catalano S, Mauro L, Marsico S, Giordano C, Rizza P, Rago V, Montanaro D, Maggiolini M, Panno ML, Ando S. Leptin induces, via ERK1/ERK2 signal, functional activation of estrogen receptor alpha in MCF-7 cells. J Biol Chem. 2004;279:19908–15. doi: 10.1074/jbc.M313191200. [DOI] [PubMed] [Google Scholar]
- 58.Saxena NK, Sharma D, Ding X, Lin S, Marra F, Merlin D, Anania FA. Concomitant activation of the JAK/STAT, PI3K/AKT, and ERK signaling is involved in leptin-mediated promotion of invasion and migration of hepatocellular carcinoma cells. Cancer Res. 2007;67:2497–507. doi: 10.1158/0008-5472.CAN-06-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sharma D, Saxena NK, Vertino PM, Anania FA. Leptin promotes the proliferative response and invasiveness in human endometrial cancer cells by activating multiple signal-transduction pathways. Endocr Relat Cancer. 2006;13:629–40. doi: 10.1677/erc.1.01169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Taliaferro-Smith L, Nagalingam A, Knight BB, Oberlick E, Saxena NK, Sharma D. Integral role of PTP1B in adiponectin-mediated inhibition of oncogenic actions of leptin in breast carcinogenesis. Neoplasia. 2013;15:23–38. doi: 10.1593/neo.121502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, Masushige S, Gotoh Y, Nishida E, Kawashima H, Metzger D, Chambon P. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science. 1995;270:1491–4. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- 62.Fusco R, Galgani M, Procaccini C, Franco R, Pirozzi G, Fucci L, Laccetti P, Matarese G. Cellular and molecular crosstalk between leptin receptor and estrogen receptor-{alpha} in breast cancer: molecular basis for a novel therapeutic setting. Endocr Relat Cancer. 2010;17:373–82. doi: 10.1677/ERC-09-0340. [DOI] [PubMed] [Google Scholar]
- 63.Yu W, Gu JC, Liu JZ, Wang SH, Wang Y, Zhang ZT, Ma XM, Song MM. Regulation of estrogen receptors alpha and beta in human breast carcinoma by exogenous leptin in nude mouse xenograft model. Chin Med J (Engl) 2010;123:337–43. [PubMed] [Google Scholar]
- 64.Valle A, Sastre-Serra J, Oliver J, Roca P. Chronic leptin treatment sensitizes MCF-7 breast cancer cells to estrogen. Cell Physiol Biochem. 2011;28:823–32. doi: 10.1159/000335796. [DOI] [PubMed] [Google Scholar]
- 65.Binai NA, Damert A, Carra G, Steckelbroeck S, Lower J, Lower R, Wessler S. Expression of estrogen receptor alpha increases leptin-induced STAT3 activity in breast cancer cells. Int J Cancer. 2010;127:55–66. doi: 10.1002/ijc.25010. [DOI] [PubMed] [Google Scholar]
- 66.Gunter MJ, Hoover DR, Yu H, Wassertheil-Smoller S, Rohan TE, Manson JE, Li J, Ho GY, Xue X, Anderson GL, Kaplan RC, Harris TG, Howard BV, Wylie-Rosett J, Burk RD, Strickler HD. Insulin, insulin-like growth factor-I, and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 2009;101:48–60. doi: 10.1093/jnci/djn415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Renehan AG, Frystyk J, Flyvbjerg A. Obesity and cancer risk: the role of the insulin-IGF axis. Trends Endocrinol Metab. 2006;17:328–36. doi: 10.1016/j.tem.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 68.Bartella V, Cascio S, Fiorio E, Auriemma A, Russo A, Surmacz E. Insulin-dependent leptin expression in breast cancer cells. Cancer Res. 2008;68:4919–27. doi: 10.1158/0008-5472.CAN-08-0642. [DOI] [PubMed] [Google Scholar]
- 69.Jernstrom H, Barrett-Connor E. Obesity, weight change, fasting insulin, proinsulin, C-peptide, and insulin-like growth factor-1 levels in women with and without breast cancer: the Rancho Bernardo Study. J Womens Health Gend Based Med. 1999;8:1265–72. doi: 10.1089/jwh.1.1999.8.1265. [DOI] [PubMed] [Google Scholar]
- 70.Pacher M, Seewald MJ, Mikula M, Oehler S, Mogg M, Vinatzer U, Eger A, Schweifer N, Varecka R, Sommergruber W, Mikulits W, Schreiber M. Impact of constitutive IGF1/IGF2 stimulation on the transcriptional program of human breast cancer cells. Carcinogenesis. 2007;28:49–59. doi: 10.1093/carcin/bgl091. [DOI] [PubMed] [Google Scholar]
- 71.Shimizu C, Hasegawa T, Tani Y, Takahashi F, Takeuchi M, Watanabe T, Ando M, Katsumata N, Fujiwara Y. Expression of insulin-like growth factor 1 receptor in primary breast cancer: immunohistochemical analysis. Hum Pathol. 2004;35:1537–42. doi: 10.1016/j.humpath.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 72.Sachdev D, Hartell JS, Lee AV, Zhang X, Yee D. A dominant negative type I insulin-like growth factor receptor inhibits metastasis of human cancer cells. J Biol Chem. 2004;279:5017–24. doi: 10.1074/jbc.M305403200. [DOI] [PubMed] [Google Scholar]
- 73.Ozbay T, Nahta R. A novel unidirectional cross-talk from the insulin-like growth factor-I receptor to leptin receptor in human breast cancer cells. Mol Cancer Res. 2008;6:1052–8. doi: 10.1158/1541-7786.MCR-07-2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Saxena NK, Taliaferro-Smith L, Knight BB, Merlin D, Anania FA, O’Regan RM, Sharma D. Bidirectional crosstalk between leptin and insulin-like growth factor-I signaling promotes invasion and migration of breast cancer cells via transactivation of epidermal growth factor receptor. Cancer Res. 2008;68:9712–22. doi: 10.1158/0008-5472.CAN-08-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Byrne AM, Bouchier-Hayes DJ, Harmey JH. Angiogenic and cell survival functions of vascular endothelial growth factor (VEGF) J Cell Mol Med. 2005;9:777–94. doi: 10.1111/j.1582-4934.2005.tb00379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Miyazawa-Hoshimoto S, Takahashi K, Bujo H, Hashimoto N, Saito Y. Elevated serum vascular endothelial growth factor is associated with visceral fat accumulation in human obese subjects. Diabetologia. 2003;46:1483–8. doi: 10.1007/s00125-003-1221-6. [DOI] [PubMed] [Google Scholar]
- 77.Liu Y, Tamimi RM, Collins LC, Schnitt SJ, Gilmore HL, Connolly JL, Colditz GA. The association between vascular endothelial growth factor expression in invasive breast cancer and survival varies with intrinsic subtypes and use of adjuvant systemic therapy: results from the Nurses’ Health Study. Breast Cancer Res Treat. 2011;129:175–84. doi: 10.1007/s10549-011-1432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Makey KL, Patterson SG, Robinson J, Loftin M, Waddell DE, Miele L, Chinchar E, Huang M, Smith AD, Weber M, Gu JW. Increased plasma levels of soluble vascular endothelial growth factor receptor 1 (sFlt-1) in women by moderate exercise and increased plasma levels of vascular endothelial growth factor in overweight/obese women. Eur J Cancer Prev. 2013;22:83–9. doi: 10.1097/CEJ.0b013e328353ed81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sierra-Honigmann MR, Nath AK, Murakami C, Garcia-Cardena G, Papapetropoulos A, Sessa WC, Madge LA, Schechner JS, Schwabb MB, Polverini PJ, Flores-Riveros JR. Biological action of leptin as an angiogenic factor. Science. 1998;281:1683–6. doi: 10.1126/science.281.5383.1683. [DOI] [PubMed] [Google Scholar]
- 80.Bouloumie A, Drexler HC, Lafontan M, Busse R. Leptin, the product of Ob gene, promotes angiogenesis. Circ Res. 1998;83:1059–66. doi: 10.1161/01.res.83.10.1059. [DOI] [PubMed] [Google Scholar]
- 81.Park HY, Kwon HM, Lim HJ, Hong BK, Lee JY, Park BE, Jang Y, Cho SY, Kim HS. Potential role of leptin in angiogenesis: leptin induces endothelial cell proliferation and expression of matrix metalloproteinases in vivo and in vitro. Exp Mol Med. 2001;33:95–102. doi: 10.1038/emm.2001.17. [DOI] [PubMed] [Google Scholar]
- 82.Artwohl M, Roden M, Holzenbein T, Freudenthaler A, Waldhausl W, Baumgartner-Parzer SM. Modulation by leptin of proliferation and apoptosis in vascular endothelial cells. Int J Obes Relat Metab Disord. 2002;26:577–80. doi: 10.1038/sj.ijo.0801947. [DOI] [PubMed] [Google Scholar]
- 83.Cao R, Brakenhielm E, Wahlestedt C, Thyberg J, Cao Y. Leptin induces vascular permeability and synergistically stimulates angiogenesis with FGF-2 and VEGF. Proc Natl Acad Sci U S A. 2001;98:6390–5. doi: 10.1073/pnas.101564798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gonzalez-Perez RR, Xu Y, Guo S, Watters A, Zhou W, Leibovich SJ. Leptin upregulates VEGF in breast cancer via canonic and non-canonical signalling pathways and NFkappaB/HIF-1alpha activation. Cell Signal. 2010;22:1350–62. doi: 10.1016/j.cellsig.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Caldefie-Chezet F, Dubois V, Delort L, Rossary A, Vasson MP. Leptin: Involvement in the pathophysiology of breast cancer. Ann Endocrinol (Paris) 2013;74:90–101. doi: 10.1016/j.ando.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 86.Gonzalez RR, Cherfils S, Escobar M, Yoo JH, Carino C, Styer AK, Sullivan BT, Sakamoto H, Olawaiye A, Serikawa T, Lynch MP, Rueda BR. Leptin signaling promotes the growth of mammary tumors and increases the expression of vascular endothelial growth factor (VEGF) and its receptor type two (VEGF-R2) J Biol Chem. 2006;281:26320–8. doi: 10.1074/jbc.M601991200. [DOI] [PubMed] [Google Scholar]
- 87.Ray A, Nkhata KJ, Cleary MP. Effects of leptin on human breast cancer cell lines in relationship to estrogen receptor and HER2 status. Int J Oncol. 2007;30:1499–509. [PubMed] [Google Scholar]
- 88.McMurtry V, Simeone AM, Nieves-Alicea R, Tari AM. Leptin utilizes Jun N-terminal kinases to stimulate the invasion of MCF-7 breast cancer cells. Clin Exp Metastasis. 2009;26:197–204. doi: 10.1007/s10585-008-9231-x. [DOI] [PubMed] [Google Scholar]
- 89.Carino C, Olawaiye AB, Cherfils S, Serikawa T, Lynch MP, Rueda BR, Gonzalez RR. Leptin regulation of proangiogenic molecules in benign and cancerous endometrial cells. Int J Cancer. 2008;123:2782–90. doi: 10.1002/ijc.23887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kumar S, Kishimoto H, Chua HL, Badve S, Miller KD, Bigsby RM, Nakshatri H. Interleukin-1 alpha promotes tumor growth and cachexia in MCF-7 xenograft model of breast cancer. Am J Pathol. 2003;163:2531–41. doi: 10.1016/s0002-9440(10)63608-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhou W, Guo S, Gonzalez-Perez RR. Leptin pro-angiogenic signature in breast cancer is linked to IL-1 signalling. Br J Cancer. 2011;104:128–37. doi: 10.1038/sj.bjc.6606013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Himmerich H, Fulda S, Linseisen J, Seiler H, Wolfram G, Himmerich S, Gedrich K, Pollmacher T. TNF-alpha, soluble TNF receptor and interleukin-6 plasma levels in the general population. Eur Cytokine Netw. 2006;17:196–201. [PubMed] [Google Scholar]
- 93.Sunderkotter C, Steinbrink K, Goebeler M, Bhardwaj R, Sorg C. Macrophages and angiogenesis. J Leukoc Biol. 1994;55:410–22. doi: 10.1002/jlb.55.3.410. [DOI] [PubMed] [Google Scholar]
- 94.Ando S, Catalano S. The multifactorial role of leptin in driving the breast cancer microenvironment. Nat Rev Endocrinol. 2012;8:263–75. doi: 10.1038/nrendo.2011.184. [DOI] [PubMed] [Google Scholar]
- 95.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–84. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007;56:16–23. doi: 10.2337/db06-1076. [DOI] [PubMed] [Google Scholar]
- 97.Won HY, Lee JA, Park ZS, Song JS, Kim HY, Jang SM, Yoo SE, Rhee Y, Hwang ES, Bae MA. Prominent bone loss mediated by RANKL and IL-17 produced by CD4+ T cells in TallyHo/JngJ mice. PLoS One. 2011;6:e18168. doi: 10.1371/journal.pone.0018168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pantschenko AG, Pushkar I, Miller LJ, Wang YP, Anderson K, Peled Z, Kurtzman SH, Kreutzer DL. In vitro demonstration of breast cancer tumor cell sub-populations based on interleukin-1/tumor necrosis factor induction of interleukin-8 expression. Oncol Rep. 2003;10:1011–7. [PubMed] [Google Scholar]
- 99.Mattioli B, Straface E, Quaranta MG, Giordani L, Viora M. Leptin promotes differentiation and survival of human dendritic cells and licenses them for Th1 priming. J Immunol. 2005;174:6820–8. doi: 10.4049/jimmunol.174.11.6820. [DOI] [PubMed] [Google Scholar]
- 100.Napoleone E, Cutrone A, Cugino D, Latella MC, Zurlo F, Iacoviello L, de Gaetano G, Donati MB, Lorenzet R. Leptin upregulates tissue factor expression in human breast cancer MCF-7 cells. Thromb Res. 2012;129:641–7. doi: 10.1016/j.thromres.2011.07.037. [DOI] [PubMed] [Google Scholar]
- 101.Gonzalez RR, Simon C, Caballero-Campo P, Norman R, Chardonnens D, Devoto L, Bischof P. Leptin and reproduction. Hum Reprod Update. 2000;6:290–300. doi: 10.1093/humupd/6.3.290. [DOI] [PubMed] [Google Scholar]
- 102.Saxena NK, Vertino PM, Anania FA, Sharma D. leptin-induced growth stimulation of breast cancer cells involves recruitment of histone acetyltransferases and mediator complex to CYCLIN D1 promoter via activation of Stat3. J Biol Chem. 2007;282:13316–25. doi: 10.1074/jbc.M609798200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gonzalez RR, Leavis P. Leptin upregulates beta3-integrin expression and interleukin-1beta, upregulates leptin and leptin receptor expression in human endometrial epithelial cell cultures. Endocrine. 2001;16:21–8. doi: 10.1385/ENDO:16:1:21. [DOI] [PubMed] [Google Scholar]
- 104.Pinteaux E, Inoue W, Schmidt L, Molina-Holgado F, Rothwell NJ, Luheshi GN. Leptin induces interleukin-1beta release from rat microglial cells through a caspase 1 independent mechanism. J Neurochem. 2007;102:826–33. doi: 10.1111/j.1471-4159.2007.04559.x. [DOI] [PubMed] [Google Scholar]
- 105.Gonzalez RR, Devoto L, Campana A, Bischof P. Effects of leptin, interleukin-1alpha, interleukin-6, and transforming growth factor-beta on markers of trophoblast invasive phenotype: integrins and metalloproteinases. Endocrine. 2001;15:157–64. doi: 10.1385/ENDO:15:2:157. [DOI] [PubMed] [Google Scholar]
- 106.Perera CN, Chin HG, Duru N, Camarillo IG. Leptin-regulated gene expression in MCF-7 breast cancer cells: mechanistic insights into leptin-regulated mammary tumor growth and progression. J Endocrinol. 2008;199:221–33. doi: 10.1677/JOE-08-0215. [DOI] [PubMed] [Google Scholar]
- 107.Housa D, Housova J, Vernerova Z, Haluzik M. Adipocytokines and cancer. Physiol Res. 2006;55:233–44. doi: 10.33549/physiolres.930848. [DOI] [PubMed] [Google Scholar]
- 108.Sharma D, Blum J, Yang X, Beaulieu N, Macleod AR, Davidson NE. Release of methyl CpG binding proteins and histone deacetylase 1 from the Estrogen receptor alpha (ER) promoter upon reactivation in ER-negative human breast cancer cells. Mol Endocrinol. 2005;19:1740–51. doi: 10.1210/me.2004-0011. [DOI] [PubMed] [Google Scholar]
- 109.Chen C, Chang YC, Liu CL, Chang KJ, Guo IC. Leptin-induced growth of human ZR-75-1 breast cancer cells is associated with up-regulation of cyclin D1 and c-Myc and down-regulation of tumor suppressor p53 and p21WAF1/CIP1. Breast Cancer Res Treat. 2006;98:121–32. doi: 10.1007/s10549-005-9139-y. [DOI] [PubMed] [Google Scholar]
- 110.Okumura M, Yamamoto M, Sakuma H, Kojima T, Maruyama T, Jamali M, Cooper DR, Yasuda K. Leptin and high glucose stimulate cell proliferation in MCF-7 human breast cancer cells: reciprocal involvement of PKC-alpha and PPAR expression. Biochim Biophys Acta. 2002;1592:107–16. doi: 10.1016/s0167-4889(02)00276-8. [DOI] [PubMed] [Google Scholar]
- 111.Zheng Q, Hursting SD, Reizes O. Leptin regulates cyclin D1 in luminal epithelial cells of mouse MMTV-Wnt-1 mammary tumors. J Cancer Res Clin Oncol. 2012;138:1607–12. doi: 10.1007/s00432-012-1252-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jiang H, Yu J, Guo H, Song H, Chen S. Upregulation of survivin by leptin/STAT3 signaling in MCF-7 cells. Biochem Biophys Res Commun. 2008;368:1–5. doi: 10.1016/j.bbrc.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 113.Palianopoulou M, Papanikolaou V, Stefanou N, Tsezou A. The activation of leptin-mediated survivin is limited by the inducible suppressor SOCS-3 in MCF-7 cells. Exp Biol Med (Maywood) 2011;236:70–6. doi: 10.1258/ebm.2010.010224. [DOI] [PubMed] [Google Scholar]
- 114.Shida D, Kitayama J, Mori K, Watanabe T, Nagawa H. Transactivation of epidermal growth factor receptor is involved in leptin-induced activation of janus-activated kinase 2 and extracellular signal-regulated kinase 1/2 in human gastric cancer cells. Cancer Res. 2005;65:9159–63. doi: 10.1158/0008-5472.CAN-05-0598. [DOI] [PubMed] [Google Scholar]
- 115.Ogunwobi OO, Beales IL. Leptin stimulates the proliferation of human oesophageal adenocarcinoma cells via HB-EGF and Tgfalpha mediated transactivation of the epidermal growth factor receptor. Br J Biomed Sci. 2008;65:121–7. doi: 10.1080/09674845.2008.11732814. [DOI] [PubMed] [Google Scholar]
- 116.Guo S, Gonzalez-Perez RR. Notch, IL-1 and leptin crosstalk outcome (NILCO) is critical for leptin-induced proliferation, migration and VEGF/VEGFR-2 expression in breast cancer. PLoS One. 2011;6:e21467. doi: 10.1371/journal.pone.0021467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–54. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 118.Yan D, Avtanski D, Saxena NK, Sharma D. Leptin-induced epithelial-mesenchymal transition in breast cancer cells requires beta-catenin activation via Akt/GSK3- and MTA1/Wnt1 protein-dependent pathways. J Biol Chem. 2012;287:8598–612. doi: 10.1074/jbc.M111.322800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nath AK, Brown RM, Michaud M, Sierra-Honigmann MR, Snyder M, Madri JA. Leptin affects endocardial cushion formation by modulating EMT and migration via Akt signaling cascades. J Cell Biol. 2008;181:367–80. doi: 10.1083/jcb.200708197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Choi SS, Syn WK, Karaca GF, Omenetti A, Moylan CA, Witek RP, Agboola KM, Jung Y, Michelotti GA, Diehl AM. Leptin promotes the myofibroblastic phenotype in hepatic stellate cells by activating the hedgehog pathway. J Biol Chem. 2010;285:36551–60. doi: 10.1074/jbc.M110.168542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Takebe N, Warren RQ, Ivy SP. Breast cancer growth and metastasis: interplay between cancer stem cells, embryonic signaling pathways and epithelial-to-mesenchymal transition. Breast Cancer Res. 2011;13:211. doi: 10.1186/bcr2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Feldman DE, Chen C, Punj V, Tsukamoto H, Machida K. Pluripotency factor-mediated expression of the leptin receptor (OB-R) links obesity to oncogenesis through tumor-initiating stem cells. Proc Natl Acad Sci U S A. 2012;109:829–34. doi: 10.1073/pnas.1114438109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zheng Q, Banaszak L, Fracci S, Basali D, Dunlap SM, Hursting SD, Rich JN, Hjlemeland AB, Vasanji A, Berger NA, Lathia JD, Reizes O. Leptin receptor maintains cancer stem-like properties in triple negative breast cancer cells. Endocr Relat Cancer. 2013;20:797–808. doi: 10.1530/ERC-13-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Barone I, Catalano S, Gelsomino L, Marsico S, Giordano C, Panza S, Bonofiglio D, Bossi G, Covington KR, Fuqua SA, Ando S. Leptin mediates tumor-stromal interactions that promote the invasive growth of breast cancer cells. Cancer Res. 2012;72:1416–27. doi: 10.1158/0008-5472.CAN-11-2558. [DOI] [PMC free article] [PubMed] [Google Scholar]