SUMMARY
Discrepancy between subjective and objective measures of sleep is associated with insomnia and increasing age. Cognitive behavioral therapy for insomnia improves sleep quality and decreases subjective-objective sleep discrepancy. This study describes differences between older adults with insomnia and controls in sleep discrepancy, and tests the hypothesis that reduced sleep discrepancy following cognitive behavioral therapy for insomnia correlates with the magnitude of symptom improvement reported by older adults with insomnia. Participants were 63 adults >60 years of age with insomnia, and 51 controls. At baseline, participants completed sleep diaries for 7 days while wearing wrist actigraphs. After receiving cognitive behavioral therapy for insomnia, insomnia patients repeated this sleep assessment. Sleep discrepancy variables were calculated by subtracting actigraphic sleep onset latency and wake after sleep onset from respective self-reported estimates, pre- and post-treatment. Mean level and night-to-night variability in sleep discrepancy were investigated. Baseline sleep discrepancies were compared between groups. Pre- to post-treatment changes in Insomnia Severity Index score and sleep discrepancy variables were investigated within older adults with insomnia. Sleep discrepancy was significantly greater and more variable across nights in older adults with insomnia than controls, p ≤.001 for all. Treatment with cognitive behavioral therapy for insomnia was associated with significant reduction in Insomnia Severity Index score that correlated with changes in mean level and night-to-night variability in wake after sleep onset discrepancy, p <.001 for all. Study of sleep discrepancy patterns may guide more targeted treatments for late-life insomnia.
Keywords: actigraphy, self-report, sleep discrepancy, insomnia, older adults, Sleep Measurement, Cognitive-Behavioral Therapy, Late-Life Insomnia
INTRODUCTION
Insomnia increases with age (Bixler et al. 1979;Buysse et al. 1991;Foley et al. 1995) and has high personal and social cost (Botteman et al. 2007;Foley et al. 1995;Ozminkowski et al. 2007). Insomnia is a potentially potent target for improving quality of life in older adults. Cognitive behavioral therapy for insomnia (CBTI) is an effective treatment for late-life insomnia (Buysse et al. 2011;Edinger et al. 1992;McCurry et al. 2007;Morin et al. 1993;Rybarczyk et al. 2005) but the mechanisms of its therapeutic effects remain unknown. Self-reported treatment improvements in core insomnia symptoms including sleep onset latency (SOL) and wake after sleep onset (WASO) often exceed changes in corresponding objective measures (Okajima et al. 2011). Vanable et al. (2000) hypothesized that self-reported improvement following behavioral insomnia treatment may be partially explained by changes in perception of sleep features. The purpose of this study was to test this hypothesis in a sample of older adults with insomnia who received CBTI.
The perception of poor sleep is a central criterion for insomnia. On average, insomnia patients tend to report greater SOL and WASO, while reporting less total sleep time (TST) relative to objective measures (Frankel et al. 1976). Healthy sleepers, on the other hand, tend to have an opposite pattern (Frankel et al. 1976;O’Donnell et al., 2008). Several terms have been used to capture these phenomenon including sleep misperception, paradoxical insomnia, and subjective insomnia. However, these terms are burdened with unproven causal attribution (i.e., discrepancy may not be a perception error), poor empirical support (see Edinger et al. 2011), and lack of conceptual clarity (i.e., insomnia is, by definition, subjective). Another term that has been used is subjective-objective sleep discrepancy (McCall and Edinger 1992) and is defined as the time differences between subjective and objective measures of sleep features. Negative sleep discrepancy occurs when self-reports of sleep features are in the direction of greater sleep impairment than corresponding objectively measures. Positive sleep discrepancy occurs when self-reported sleep is in the direction of less sleep impairment than corresponding objective measures. In contrast to other perspectives which assume these discrepancies are primarily error on the part of the sleeper (Manconi et al. 2010), this study conceptualized objective and subjective measures as valid information on different aspects of sleep (Tryon et al., 2007). We posit sleep discrepancy reflects a unique aspect of sleep not fully captured by either objective or subjective measures.
Important differences exist between sleep discrepancy in older, compared to younger individuals (Vitiello et al. 2004). Although aging is associated with higher rates of insomnia, age-related deterioration of objectively measured sleep is often greater than older adults perceive or report (Floyd et al. 2000;van den Berg et al. 2008). Paradoxically, objective polysomnography (PSG) and actigraphy measures of sleep often fail to capture poor sleep in insomnia patients (Buysse et al. 1991). Moreover, while extreme sleep discrepancy is considered rare in the general population (Means et al. 2003), ~35% of older adults estimate their sleep to be >1 hr more or less than actigraphy sleep on average (van den Berg et al. 2008). Thus, reliance on a single sleep measurement tool, subjective or objective, can lead to inaccurate assessment, diagnosis, and treatment of late-life sleep problems. In addition, the direction of sleep discrepancy (positive vs. negative) is thought to be a consistent trait-like feature in the younger population (Means et al. 2003) but sleep discrepancy is highly variable from night-to-night among older adults (Kay et al. 2013). For example, on any given night older adults are as likely to have negative SOL discrepancy as they are to have positive SOL discrepancy (Williams et al. 2013). Kay et al. (2013) found that greater night-to-night variability in sleep discrepancy was associated with late-life insomnia complaints. Thus, consideration of both mean level and night-to-night variability in sleep discrepancy is particularly important in older adults. Actigraphy is a validated means for obtaining enough nights to accurately capture both mean level and night-tonight variability in sleep and to conduct these levels of analysis.
Treatment studies suggest negative sleep discrepancy may improve with CBTI. One study in a small sample of older adults with insomnia reported that behavioral interventions improved both actigraphy and sleep diary WASO but these changes (subjective and objective) did not correlate within individuals (e.g., Brooks et al. 1993). A more recent study in older adults with insomnia (OAI) showed CBTI significantly decreased negative sleep discrepancy (PSG vs. sleep diary) in SOL but not WASO or TST (Lund et al. 2012). More importantly, pre- to post-treatment changes in sleep discrepancy mediated the association between stage N1 sleep changes and sleep efficiency (SE=percent of time in bed spent sleeping) improvements (Lund et al. 2012). These studies suggest negative sleep discrepancy may be modifiable via CBTI and may be related to improvement in insomnia severity. The present study built on prior work to explore the impact of CBTI on sleep discrepancy in older adults during in-home sleep using actigraphy in relation to changes in perceived insomnia severity. We hypothesized that OAI would have greater sleep discrepancy and more night-to-night variability in sleep discrepancy compared to older adults with good sleep (OAGS). In addition, we predicted that patterns of sleep discrepancy in OAI would become more like OAGS following CBTI and that the degree to which sleep discrepancy changed would be related to the amount of improvements in insomnia severity.
METHODS
This study reports the results of secondary analyses of data from an ongoing study of OAI and OAGS (P01 AG 20677-09). The primary aims of the parent project were to identify novel psychological, electrophysiological, genetic, and functional neuroanatomical correlates of insomnia in older adults. CBTI was used as a treatment probe to interrogate these potential mechanisms. Results of the parent study have not yet been published. Participants were recruited through community advertisement or referral and provided written informed consent in accordance with the Internal Review Board (IRB) at the University of Pittsburgh. Financial compensation was provided for participation. To determine eligibility, participants underwent an overnight PSG sleep screen to rule-out significant sleep apnea [presence of daytime symptoms and apnea/hypopnea index (AHI), number of breathing pauses or shallow breathing episodes per hour of sleep, >20], physical examination, medical review, and clinician-administered questionnaires. Eligible participants completed a baseline sleep assessment including the Insomnia Severity Index (ISI; Morin, 1993) and 7 days of in-home daily sleep diaries concurrent with actigraphy monitoring. Older adults with insomnia, but not OAGS, received 8 sessions of weekly individual CBTI, each lasting 45–50 minutes. Treatment components included psychoeducation, sleep hygiene, stimulus control, sleep restriction, fatigue management methods, and cognitive restructuring. Post-treatment, OAI (n=57) repeated the ISI and sleep diary with concurrent actigraphy for 7 consecutive days. Because this was not an efficacy trial there was no treatment control group and OAGS were not followed beyond baseline.
Participants
Eligible participants for these analyses included 63 OAI and 51 OAGS. Exclusion criteria were age <60 years, sleep disorder other than insomnia (restless leg syndrome, AHI >20, and periodic limb movement arousal index (PLMAI; periodic limb movement with arousal per hour of sleep) >20, shift work, recent history of untreated severe psychiatric condition (past 6 months), use of hypnotic medications or other medications that affect sleep within 1 month of baseline sleep assessment, excessive alcohol (>14 drinks per week or >6 drinks at a single sitting) or caffeine (>3 drinks per day) consumption, unstable medical condition, cognitive impairment based on Mini-Mental Status Exam (MMSE<27; Folstein et al. 1975), and central nervous system conditions. Insomnia participants met criteria for DSM-IV-TR primary insomnia, but without strict exclusions for medical conditions (American Psychiatric Association 2000), as well as criteria for ICSD-2 general insomnia (American Academy of Sleep Medicine et al. 2005). Criteria were derived from clinical interview and self-report questionnaires. The presence of insomnia was further confirmed by sleep diary measures of mean SOL + WASO >40 minutes, sleep efficiency <90%, and ISI >10 at the screening interview (Morin et al. 2011). Classification as an OAGS required absence of significant insomnia symptoms (ISI <7; Morin 1993). Group demographic and health features are presented in Table 1. In brief, our sample of older adults were primarily Caucasian, college-educated, retired, in a relationship, middle-high SES, and female. Groups were well-matched across demographic characteristics.
Table 1.
Characteristics of older adults with insomnia (OAI) compared to older adults with good sleep (OAGS) at initial screening.
| Variable | Total sample (N=114) | OAI (n=63) | OAGS (n=51) | F/χ2/Z | df | p-Value |
|---|---|---|---|---|---|---|
| Age, years | 67.77 (6.09) | 68.03(6.54) | 67.44(5.53) | Z=−0.15 | .890 | |
| Sex, % female | 65 | 71 | 57 | χ2=2.63 | 1 | .105 |
| Race, % Caucasian | 92 | 91 | 93 | χ2=0.19 | 1 | .661 |
| Employment status, % working | 39 | 33 | 45 | χ2=1.60 | 1 | .206 |
| Subjective socioeconomic status†,‡ | 3.49(1.71) | 3.40(1.78) | 3.60(1.63) | 0.49 | 95 | .487 |
| Married or living with partner, % | 62 | 59 | 64 | χ2=0.28 | 1 | .597 |
| Education, % ≥college | 87 | 83 | 89 | χ2=1.30 | 1 | .254 |
| Mini-Mental Status Exam§ | 29.36(0.98) | 29.32(1.07) | 29.40(0.86) | Z=−0.12 | .911 | |
| Insomnia Severity Index | 9.86(78.90) | 16.70(3.50) | 1.40(2.01) | 8.69 | 112 | <.001*** |
| Apnea hypopnea index | 5.62(5.62) | 5.51(5.33) | 5.77(6.01) | 0.06 | 112 | .815 |
| Periodic limb movement arousal index | 7.20 (5.80) | 7.50 (5.89) | 6.83 (5.89) | 0.53 | 112 | .530 |
Measures
Participants were characterized in terms of socioeconomic status (SES), psychological status (mental status, anxiety, and depression), and general health status using validated questionnaires and interviews (see Table 1 for references). Sleep features were assessed with questionnaires, daily sleep diaries, and actigraphy.
Sleep questionnaires
Maladaptive thoughts about sleep were assessed with the Dysfunctional Beliefs about Sleep scale (DBAS) (Morin et al. 1993). The ISI (Bastien et al. 2001) was used to quantify baseline insomnia symptom severity and treatment response.
Sleep diary
The Pittsburgh Sleep Diary was used to measure daily SOL, WASO, TST, and SE (Monk et al. 1994). To calculate TST, SOL and WASO were subtracted from the amount of time between in-bed-time to out-of-bed-time. SE was calculated as [(TST/time in bed) x 100].
Actigraphy
Participants wore an Actiwatch 2® (Philips Respironics, Bend, OR) on the non-dominant wrist. Data were recorded continuously for 7 consecutive days before being downloaded to a PC and then analyzed by Actiware 5 software (Philips Respironics, Bend, OR), using the medium sensitivity setting. A validated algorithm for sleep scoring each 30 second epoch was used (Cole et al. 1992;Oakley 1997). Objective SOL was determined by the amount of time between the start of the rest interval and actigraphy defined sleep onset. There is no scientifically established method to derive the start of the rest cycle (Morgenthaler et al. 2007). In accordance with Actical® manual recommendations, all available data was utilized to determine the start of the rest interval including the marker press, sleep diaries, activity patterns, and ambient light patterns. Actigraphy defined sleep onset was determined using the epoch detection method provided by the device software. Actigraphic WASO was determined by total amount of time scored as wake between sleep onset and offset.
Sleep discrepancy variables
Daily sleep discrepancy variables were calculated by subtracting actigraphy estimates of SOL and WASO from respective diary self-reports. Mean level SOL discrepancy and WASO discrepancy were calculated for both pre- and post-treatment time points for each participant. Night-to-night variability was determined by calculating the within-person standard deviations for both SOL and WASO discrepancy across the 7-day baseline and post-treatment time points. Because computation of TST and SE relied on self-reported SOL and WASO, TST discrepancy and SE discrepancy could not be directly determined in this study.
Statistical analysis
Assumptions for missingness of data and normality were assessed. About 9–13% of actigraphy and sleep diary data, respectively, were missing which was managed with multiple imputations. All variables, with the exception of the DBAS, had non-normal distributions. In the total sample, transformations did not normalize distributions for STAI, MMSE, and all SOL and WASO variables. Extreme values in SOL and WASO variables were visually determined to be within the range of natural fluctuations relative to previous studies in older adults (Lund et al. 2012; Kay et al., 2013). Where appropriate, non-parametric tests were used.
Differences between OAI and OAGS in demographic, sleep, and health variables were tested using one-way analysis of variance, Mann-Whitney U with the Monte Carlo method, or χ2 tests. Mann-Whitney U tests using the Monte Carlo method were utilized to compare sleep features of OAI and OAGS. Wilcoxon using the Monte Carlo method was utilized to investigate pre- to post-CBTI changes in sleep features among OAI. Time-point (pre- vs. post-treatment) was the independent variable.
Mixed modeling was used to investigate whether changes in sleep discrepancy following CBTI were related to insomnia improvements measured by the ISI. The independent variables were time-point (pre- vs. post-treatment) and each individuals mean centered pre- and post-ISI score. Pre- and post-DBAS score was included in the analysis as a covariate. Pre- to post-CBTI change in mean level and night-to-night variability in SOL and WASO discrepancy were the dependent variables in four separate mixed models. The first order autoregressive AR(1) error structure was utilized, which assumed correlations between items decreased with repeated measurement. Sensitivity analysis was conducted to confirm results were not driven by extreme sleep discrepancy values. All analyses were performed using IBM SPSS 21 (IBM Corp., Armonk, NY, USA).
RESULTS
Table 2 shows group (OAI vs. OAGS) differences in sleep and health features. At baseline, OAI had significantly greater problems with anxiety, depression, and perceived poor health than controls. In addition, OAI endorsed more dysfunctional cognitions about sleep. Subjective sleep was considerably poorer in OAI. Although statistically significant for WASO, there were only small differences in objective sleep between groups. OAI had considerable negative sleep discrepancy that was marked by a highly variable pattern night-to-night, while OAGS tended to vary night-to-night between relative concordance between sleep diary and actigraphy to having positive sleep discrepancy.
Table 2.
Clinical features of older adults with insomnia (OAI) compared to older adults with good sleep (OAGS).
| Sleep and sleep-related features | Total sample (N=114) | OAI (n=63) | OAGS (n=51) | Z/F | p-Value |
|---|---|---|---|---|---|
| Questionnaires | |||||
| State-Trait Inventory-Form Y†,‡ | 27.75(7.86) | 30.67 (8.60) | 24.14(4.86) | −4.43 | <.001*** |
| Inventory of Depressive Symptomotology-Self Report§,¶ | 1.79(2.31) | 2.11 (2.22) | 1.39(2.38) | F=4.98 | .028* |
| Perceived health (MOS-36)††,‡‡ | 84. 17(14.36) | 82.98(15.98) | 87.57(11.05) | F=2.95 | .041* |
| Insomnia Severity Index | 7.83(7.15) | 13.65(4.06) | 0.76(1.31) | −8.70 | <.001*** |
| Dysfunctional Beliefs and Attitudes about Sleep | 27.70(11.23) | 29.22(10.10) | 24.16(9.65) | F=15.83 | <.001*** |
| Actigraphy | |||||
| SOL, M | 11.00(10.38) | 11.02(8.54) | 10.95(12.34) | −0.72 | .482 |
| WASO, M | 43.57(18.70) | 48.00(18.13) | 38.19(18.13) | −3.35 | .001** |
| TST, M | 405.54(50.17) | 404.06(54.32) | 407.35(45.07) | −0.08 | .989 |
| SE, % | 85.99(5.18) | 84.70(5.24) | 87.56(4.69) | −3.29 | .001** |
| Sleep diary | |||||
| SOL diary, M | 22.49(19.68) | 30.39(21.74) | 12.87(10.92) | −5.59 | <.001*** |
| WASO diary, M | 44.13(41.91) | 65.89(44.10) | 17.67(16.13) | −7.00 | <.001*** |
| TST, M | 381.21(70.08) | 343.17(65.18) | 427.46(42.92) | −6.73 | <.001*** |
| SE, % | 77.40(13.99) | 68.58(12.40) | 88.11(6.14) | −8.08 | <.001*** |
| Sleep discrepancy variable | |||||
| SOL discrepancy, M | 11.51(19.61) | 19.41(19.22) | 1.89(15.46) | −5.16 | <.001*** |
| SOL discrepancy, ISD | 16.26(20.91) | 21.67(23.24) | 9.67(15.49) | −4.40 | <.001*** |
| WASO discrepancy, M | 0.61(39.63) | 17.95(43.88) | −20.47(18.50) | −5.73 | <.001*** |
| WASO discrepancy, ISD | 30.11(22.14) | 40.16(20.74) | 17.90(17.19) | −5.49 | <.001*** |
Note: ISD=individual standard deviation, SOL=sleep onset latency, WASO=wake after sleep onset, TST=total sleep time, SE=sleep efficiency. Values for SOL, WASO, and TST are reported in minutes.
Spielberger et al. 1983;
p <.05,
p <.01,
p<.001
Table 3 shows pre- to post-treatment differences in sleep and mood features among OAI. Following CBTI, OAI had significantly lower ISI score, dysfunctional cognitions about sleep, objective TST, objective WASO, subjective SOL and WASO. They also had significantly increased sleep quality and SE following treatment. At post-treatment, mean level of SOL discrepancy and WASO discrepancy were significantly lower compared to pre-treatment. Night-to-night variability in WASO discrepancy was also significantly lower following treatment.
Table 3.
Differences in sleep features pre- to post-treatment with cognitive behavioral therapy for insomnia (CBTI) in older adults with insomnia.
| Sleep feature | Pre- to post-CBTI (n=54)
|
Z | p-Value | |
|---|---|---|---|---|
| Pre-treatment | Post-treatment | |||
| Questionnaires | ||||
| Insomnia Severity Index | 13.37(4.16) | 4.28(4.37) | −6.56 | <.001*** |
| Dysfunctional Beliefs and Attitudes about Sleep | 30.99(11.06) | 22.38(9.98) | −5.90 | <.001*** |
| Actigraphy | ||||
| SOL, M | 10.90(8.75) | 9.52(8.38) | −0.58 | .566 |
| WASO, M | 49.38(18.82) | 35.85(16.36) | −5.35 | <.001*** |
| TST, M | 400.46(55.31) | 359.97(42.70) | −4.74 | <.001*** |
| SE, M | 84.39(5.51) | 86.89(4.40) | 3.93 | <.001*** |
| Sleep diary | ||||
| SOL diary, M | 28.66(20.51) | 20.29(24.08) | −4.70 | <.001*** |
| WASO diary, M | 64.41(44.74) | 28.54(30.53) | −5.46 | <.001*** |
| TST, min | 343.33(65.51) | 357.25(52.01) | −1.62 | .099 |
| SE | 69.41(12.24) | 86.73(9.37) | −6.62 | <.001*** |
| Sleep discrepancy variable | ||||
| SOL discrepancy, M | 17.82(18.02) | 6.69(12.01) | −4.28 | <.001*** |
| SOL discrepancy, ISD | 22.77(20.23) | 18.80(15.96) | −1.38 | .173 |
| WASO discrepancy, M | 15.11(43.53) | −7.32(34.09) | −4.43 | <.001*** |
| WASO discrepancy, ISD | 39.82(20.53) | 24.49(17.95) | −4.36 | <.001*** |
Note: ISD=individual standard deviation, SOL=sleep onset latency, WASO=wake after sleep onset, TST=total sleep time, SE=sleep efficiency. Values for SOL, WASO, and TST are reported in minutes.
p <.001
Mixed model analyses revealed that greater change in ISI, pre- to post-treatment, was correlated with the amount of change in mean level WASO discrepancy, F(1, 91.73) = 13.75, p < .001 (Figure 1) and change in WASO discrepancy variability, F(1, 40.84) = 18.57, p < .001 (Figure 2). There was a trend for greater change in SOL discrepancy being associated with greater change in ISI score pre- to post-treatment, F(1, 85.87) = 2.99, p = .088. Variability in SOL discrepancy was not significantly associated with ISI change, F(1, 67.26) = 0.27, p = .606. Pre- to post-treatment change in dysfunctional beliefs about sleep was not significantly related to pre- to post-treatment changes in any sleep discrepancy variable.
Figure 1.
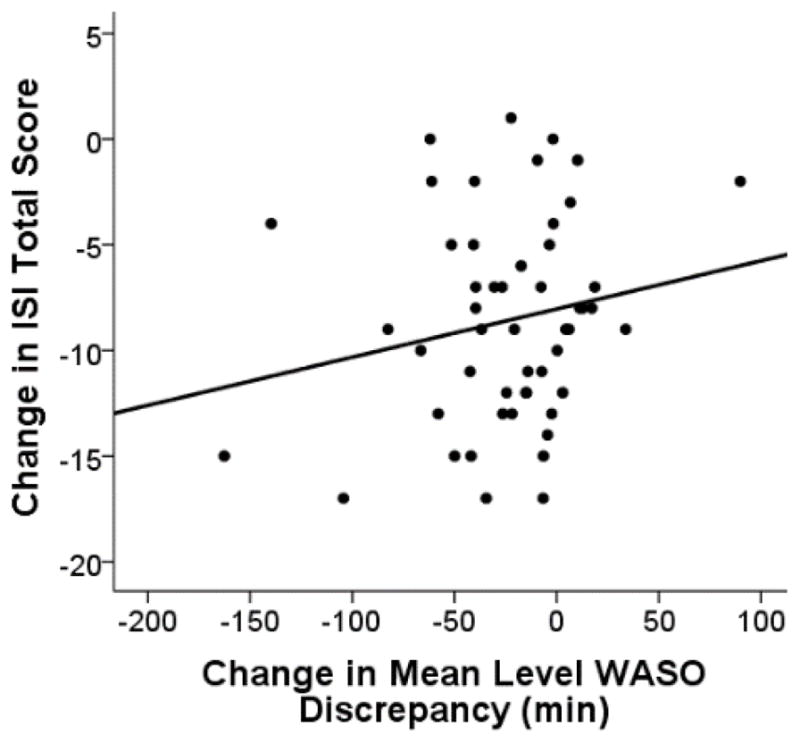
Data points represent individuals’ change in Insomnia Severity Index (ISI) total score in relation to the change in minutes of wake after sleep onset (WASO) discrepancy (post- minus pre- treatment values). Negative values indicate a decline from pre- to post-treatment. Individuals who had a greater shift in the direction of reporting less WASO than was objectively measured had a greater reduction in insomnia severity measured by the ISI.
Figure 2.
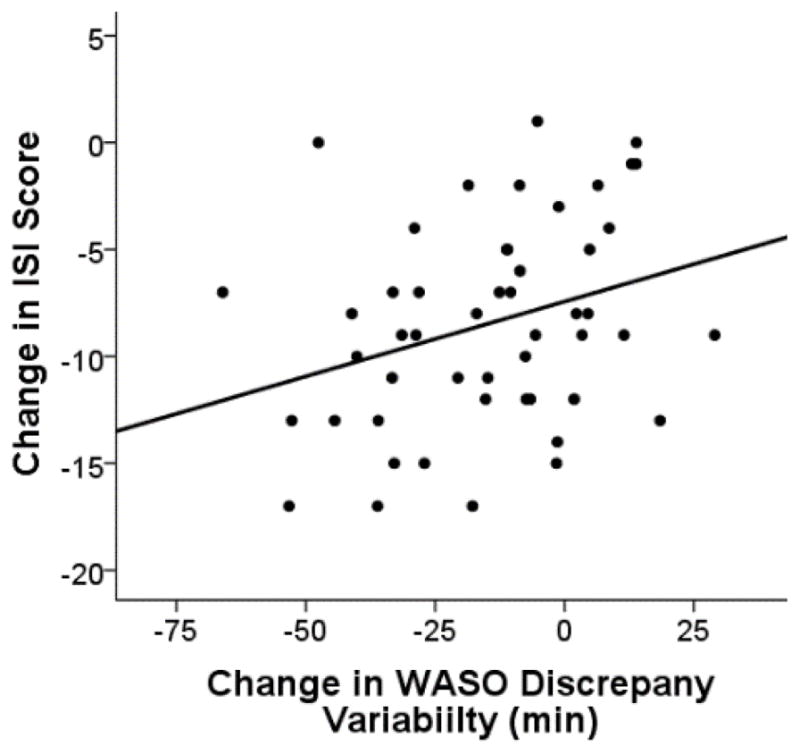
Data points represent individuals’ change in Insomnia Severity Index (ISI) total score in relation to the change in minutes of night-to-night variability in wake after sleep onset (WASO) discrepancy (post- minus pre- treatment values). Negative values indicate a decline from pre- to post-treatment. Individuals who had a greater decline in night-to-night variability in WASO discrepancy had a greater reduction in insomnia severity measured by the Insomnia Severity Index.
DISCUSSION
The primary findings from this study were: 1) OAI had greater negative sleep discrepancy than OAGS; 2) sleep discrepancy became less negative and less variable in OAI following CBTI. Changes in WASO discrepancy were most pronounced with a 148% change in the direction of positive discrepancy. There was also a 38% decrease in WASO discrepancy variability and an 83% change in SOL discrepancy; and 3) Greater pre-to post-treatment change in insomnia severity was associated with greater pre-post treatment changes in the mean and night-to-night variability in WASO discrepancy. These results replicate and extend prior research showing OAI have greater negative sleep discrepancy and night-to-night variability in SOL and WASO compared to OAGS (Williams et al. 2013;Kay et al. 2013). Unlike patients with insomnia, OAGS reported significantly less sleep disturbance than was measured objectively. Importantly, positive WASO discrepancy emerged on average in OAI following CBTI, suggesting they became more like OAGS in terms of perceived wakefulness during the night. These findings are consistent with Lund et al., (2012) which employed PSG to investigate pre- to post-CBTI mean level sleep discrepancy.
Several hypotheses have been proposed to explain sleep discrepancy in insomnia, the majority of which have limited scientific support (Harvey and Tang 2012). This study supports the hypothesis that sleep discrepancy is temporally related to sleep quality. Some evidence suggests that training participants to report subjective estimates of SOL and WASO to match objective measures may have therapeutic effects (Downey, III and Bonnet 1992;Mercer et al. 2002;Tang and Harvey 2006). These studies did not include a follow-up assessment to determine long-term effects. Simply changing individuals’ perception of time spent in SOL or WASO does not guarantee that the underlying sleep problem will resolve, or that not perceiving the presence of a true sleep problem minimizes the negative long-term consequences of poor sleep. These considerations are particularly important in OAI because changes in sleep discrepancy following CBTI are not a function of change in dysfunctional beliefs about sleep, as reported herein and by Lund et al. (2012). Because subjective reports of sleep are most predictive of long-term health outcomes in older adults, care should be taken that OAI are not led to perceive a subjective sleep problem as no need for concern, particularly given the general proclivity of older adults to under-report sleep problems.
Several authors have linked negative sleep discrepancy to heightened brain activity during PSG defined sleep (Perlis et al. 2001) suggesting an underlying sleep disturbance may accurately be perceived by patients but require more refined objective measures to observe scientifically (St-Jean et al. 2013). Changes in sleep discrepancy may shadow improved sleep due to treatment. Recognition of current evidence and better understanding of the underlying physiological source of sleep discrepancy in OAI may inform novel treatments aimed at underlying pathophysiology.
LIMITATIONS
The present study utilized a set of inclusive criteria for late-life insomnia that may limit generalizability of results to more strict classifications of insomnia. However, given the similarity of results to previous reports which utilized slightly different criteria, we feel these results can reasonably be generalized to OAI generally.
This study obtained a single night of in-lab PSG during baseline and post-treatment. However, given the high degree of variability in sleep discrepancy and the limited window of PSG, these data provide limited insight to the questions of interest in this study and were not included in this study. Relative to PSG, actigraphy tends to underestimate SOL and WASO among older adults, and the relationship between these objective measures becomes less related with increased sleep disturbance (Sivertsen et al. 2006). Nevertheless, because of its utility in collecting multiple nights of naturalistic sleep, actigraphy may provide a more accurate picture of sleep in older adults than 1–2 nights of PSG. Indeed, continuous monitoring with actigraphy provides valuable information not captured by standard PSG measurement. For example, recently it was found that a brief behavioral treatment for late-life insomnia resulted in significant reductions in sleep diary and actigraphy measures of SOL and WASO but that PSG measurement of these variables did not significantly change (Buysse et al. 2011). Findings from this study are consistent with prior studies which utilized PSG (Lund et al. 2012) and actigraphy (Kay et al. 2012) to study sleep discrepancy in older adults. Both PSG and actigraphy methods are commiserate for further understand this phenomenon in OAI.
The sleep diary in this study did not query participants on perception of nightly total sleep time which prevented us from quantifying TST discrepancy. Future studies investigating sleep discrepancy should include an explicit question on perceived sleep length.
The parent project study did not follow controls into post-treatment and did not include an insomnia treatment control group limiting the extent to which results can account for changes in sleep discrepancy over time. This limitation is minimized by the chronic nature of insomnia in late-life. It is unlikely that results are due to the passing of time. Moreover, the study participants were Caucasian, well-educated, and relatively healthy. Therefore, generalizability of results may be specific to community-dwelling older adults with these demographic features.
Acknowledgments
Supported by P01 AG020677-09 (AgeWise, PI Monk). Dr. Kay is supported by HL082610 (T32, PI Buysse). We thank Jean Miewald and Mary Fletcher for database management and Annette Wood for actigraphy data management, and the AgeWise team for subject recruitment, screening, and data collection.
Footnotes
Conflicts of Interest: None
Author contributorship: Dr. Kay developed the hypotheses tested in this article, conducted the statistical analyses, and wrote the first draft of the paper. Dr. Monk, co-developed and led the program project (AG 20677) in which the data were collected. Drs. Buysse, Germain, Hall and Monk designed and led the studies which collected the data reported in the paper. All authors contributed to the writing and revisions of the manuscript.
References
- Hauri PJ, Sateia MJ. American Academy of Sleep Medicine. The International Classification of Sleep Disorders, Second Edition (ICSD-2): Diagnostic and Coding Manual. 2005. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) 4. American Psychiatric Association; Washington, DC: 2000. Text Revision edition. [Google Scholar]
- Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- Bixler EO, Kales A, Soldatos CR, Kales JD, Healey S. Prevalence of sleep disorders in the Los Angeles metropolitan area. Am J Psychiatry. 1979;136:1257–1262. doi: 10.1176/ajp.136.10.1257. [DOI] [PubMed] [Google Scholar]
- Botteman MF, Ozminkowski RJ, Wang S, Pashos CL, Schaefer K, Foley DJ. Cost effectiveness of long-term treatment with eszopiclone for primary insomnia in adults: a decision analytical model. CNS Drugs. 2007;21:319–334. doi: 10.2165/00023210-200721040-00005. [DOI] [PubMed] [Google Scholar]
- Brooks JO, Friedman L, Bliwise DL, Yesavage JA. Use of the wrist actigraph to study insomnia in older adults. Sleep. 1993;16:151–155. doi: 10.1093/sleep/16.2.151. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Cheng Y, Germain A, et al. Night-to-night sleep variability in older adults with and without chronic insomnia. Sleep Med. 2010;11:56–64. doi: 10.1016/j.sleep.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Germain A, Moul DE, Franzen PL, Brar LK, Fletcher, et al. Efficacy of brief behavioral treatment for chronic insomnia in older adults. Archives of Internal Medicine. 2011;171:887–895. doi: 10.1001/archinternmed.2010.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, III, Monk TH, Hoch CC, Yeager AL, Kupfer DJ. Quantification of subjective sleep quality in healthy elderly men and women using the Pittsburgh Sleep Quality Index (PSQI) Sleep. 1991;14:331–338. [PubMed] [Google Scholar]
- Cole RJ, Kripke DF, Gruen W, Mullaney DJ, Gillin JC. Automatic sleep/wake identification from wrist activity. Sleep. 1992;15:461–469. doi: 10.1093/sleep/15.5.461. [DOI] [PubMed] [Google Scholar]
- Downey R, III, Bonnet MH. Training subjective insomniacs to accurately perceive sleep onset. Sleep. 1992;15:58–63. doi: 10.1093/sleep/15.1.58. [DOI] [PubMed] [Google Scholar]
- Edinger JD, Hoelscher TJ, Marsh GR, Lipper S, Ionescu-Pioggia M. A cognitive-behavioral therapy for sleep-maintenance insomnia in older adults. Psychol Aging. 1992;7:282–289. doi: 10.1037//0882-7974.7.2.282. [DOI] [PubMed] [Google Scholar]
- Edinger JD, Wyatt JK, Stepanski E, Olsen MK, Stechuchak KM, Carney CE, Chiang A, Crisostomo MI, Lineberger MD, Means MK, Radtke RA, Wohlgemuth WK, Krystal AD. Testing the reliability and validity of DSM-IV-TR and ICSD-2 insomnia diagnosis: results of a multi-method/multi-trait analysis. Archives of General Psychiatry. 2011;68:992–1002. doi: 10.1001/archgenpsychiatry.2011.64. [DOI] [PubMed] [Google Scholar]
- Floyd JA, Medler SM, Ager JW, Janisse JJ. Age-related changes in initiation and maintenance of sleep: a meta-analysis. Res Nurs Health. 2000;23:106–117. doi: 10.1002/(sici)1098-240x(200004)23:2<106::aid-nur3>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Foley DJ, Monjan AA, Brown SL, Simonsick EM, Wallace RB, Blazer DG. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18:425–432. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SW, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Frankel BL, Coursey RD, Buchbinder R, Snyder F. Recorded and reported sleep in chronic primary insomnia. Arch Gen Psychiatry. 1976;33:615–623. doi: 10.1001/archpsyc.1976.01770050067011. [DOI] [PubMed] [Google Scholar]
- Harvey AG, Tang NK. (Mis)perception of sleep in insomnia: a puzzle and a resolution. Psychol Bull. 2012;138:77–101. doi: 10.1037/a0025730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch CC, Reynolds CF, Kupfer DJ, Berman SR, Houck PR, Stack JA. Empirical note: Self-report vs. recorded sleep in healthy seniors. Psychophysiology. 1987;24:293–299. doi: 10.1111/j.1469-8986.1987.tb00298.x. [DOI] [PubMed] [Google Scholar]
- Horne JA, Pankhurst FL, Reyner LA, Hume K, Diamond ID. A field study of sleep disturbance: effects of aircraft noise and other factors on 5,742 nights of actimetrically monitored sleep in a large subject sample. Sleep. 1994;17:146–159. doi: 10.1093/sleep/17.2.146. [DOI] [PubMed] [Google Scholar]
- Kay DB, Dzierzewski JM, Rowe M, McCrae CS. Greater night-to-night variability in sleep discrepancy among older adults with a sleep complaint compared to noncomplaining older adults. Behav Sleep Med. 2013;11:76–90. doi: 10.1080/15402002.2011.602775. [DOI] [PubMed] [Google Scholar]
- Lund HG, Rybarczyk BD, Perrin PB, Leszczyszyn D, Stepanski E. The discrepancy between subjective and objective measures of sleep in older adults receiving CBT for comorbid insomnia. J Clin Psychol. 2012;69:1108–1120. doi: 10.1002/jclp.21938. [DOI] [PubMed] [Google Scholar]
- Manconi M, Ferri R, Sagrada C, Punjabi NM, Tettamanzi E, Zucconi M, Oldani A, Castronovo V, Ferini-Strambi L. Measuring the error in sleep estimation in normal subjects and in patients with insomnia. J Sleep Res. 2010;19:478–486. doi: 10.1111/j.1365-2869.2009.00801.x. [DOI] [PubMed] [Google Scholar]
- Mangione CM, Marcantonio ER, Goldman L, Cook EF, Donaldson MC, Sugarbaker DJ, Poss R, Lee TH. Influence of age on measurement of health status in patients undergoing elective surgery. J Am Geriatr Soc. 1993;41:377–383. doi: 10.1111/j.1532-5415.1993.tb06944.x. [DOI] [PubMed] [Google Scholar]
- McCall WV, Edinger JD. Subjective total insomnia: An example of sleep state misperception. Sleep. 1992;15:71–73. [PubMed] [Google Scholar]
- McCurry SM, Logsdon RG, Teri L, Vitiello MV. Evidence-based psychological treatments for insomnia in older adults. Psychol Aging. 2007;22:18–27. doi: 10.1037/0882-7974.22.1.18. [DOI] [PubMed] [Google Scholar]
- Means MK, Edinger JD, Glenn DM, Fins AI. Accuracy of sleep perceptions among insomnia sufferers and normal sleepers. Sleep Med. 2003;4:285–296. doi: 10.1016/s1389-9457(03)00057-1. [DOI] [PubMed] [Google Scholar]
- Mercer JD, Bootzin RR, Lack LC. Insomniacs’ perception of wake instead of sleep. Sleep. 2002;25:564–571. [PubMed] [Google Scholar]
- Monk TH, Reynolds CF, Kupfer, et al. The Pittsburgh Sleep Diary. J Sleep Res. 1994;3:111–120. [PubMed] [Google Scholar]
- Morin CM, Kowatch RA, Barry T, Walton E. Cognitive-behavior therapy for late-life insomnia. J Consult Clin Psychol. 1993;61:137–46. doi: 10.1037//0022-006x.61.1.137. [DOI] [PubMed] [Google Scholar]
- Morin CM. Insomnia: Psychological Assessment and Management. The Guilford Press; New York-London: 1993. [Google Scholar]
- Morin CM, Stone J, Trinkle D, Mercer J, Remsberg S. Dysfunctional beliefs and attitudes about sleep among older adults with and without insomnia complaints. Psychol Aging. 1993;8:463–467. doi: 10.1037//0882-7974.8.3.463. [DOI] [PubMed] [Google Scholar]
- Morin CM, Belleville G, Bélanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34:601–608. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa M, Magnusson Hanson LL, Theorell T, Westerlund H. Subjective social status: its determinants and association with health in the Swedish working population (the SLOSH study) Eur J Public Health. 2012;22:593–597. doi: 10.1093/eurpub/ckr064. [DOI] [PubMed] [Google Scholar]
- Oakley NR. Validation of the Sleepwatch Sleep/Wake Scoring Algorithm Used by the Actiware Activity Monitoring System. Mini Mitter Co. Inc; Bend, OR: 1997. [Google Scholar]
- O’Bryant SE, Humphreys JD, Smith GE, Ivnik RJ, Graff-Radford NR, Petersen RC, Lucas JA. Detecting dementia with the mini-mental state examination in highly educated individuals. Arch Neurol. 2008;65:963–967. doi: 10.1001/archneur.65.7.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell D, Silva EJ, Münch M, Ronda JM, Wang W, Duffy JF. Comparison of subjective and objective assessments of sleep in healthy older subjects without sleep complaints. J Sleep Res. 2009;18:254–263. doi: 10.1111/j.1365-2869.2008.00719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okajima I, Komada Y, Inoue Y. A meta-analysis on the treatment effectiveness of cognitive behavioral therapy for primary insomnia. Sleep Biol Rhythms. 2011;9:24–34. [Google Scholar]
- Ostrove JM, Adler NE, Kuppermann M, Washington AE. Objective and subjective assessments of socioeconomic status and their relationship to self-rated health in an ethnically diverse sample of pregnant women. Health Psychol. 2000;19:613–618. doi: 10.1037//0278-6133.19.6.613. [DOI] [PubMed] [Google Scholar]
- Ozminkowski RJ, Wang S, Walsh JK. The direct and indirect costs of untreated insomnia in adults in the United States. Sleep. 2007;30:263–273. doi: 10.1093/sleep/30.3.263. [DOI] [PubMed] [Google Scholar]
- Perlis ML, Smith MT, Andrews PJ, Orff H, Giles DE. Beta/Gamma EEG activity in patients with primary and secondary insomnia and good sleeper controls. Sleep. 2001;24:110–117. doi: 10.1093/sleep/24.1.110. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med. 1996;26:477–486. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- Rybarczyk B, Stepanski E, Fogg L, Lopez M, Barry P, Davis A. A placebo-controlled test of cognitive-behavioral therapy for comorbid insomnia in older adults. J Consult Clin Psychol. 2005;73:1164–1174. doi: 10.1037/0022-006X.73.6.1164. [DOI] [PubMed] [Google Scholar]
- Sivertsen B, Omvik S, Havik OE, et al. A comparison of actigraphy and polysomnography in older adults treated for chronic primary insomnia. Sleep. 2006;29:1353–1358. doi: 10.1093/sleep/29.10.1353. [DOI] [PubMed] [Google Scholar]
- St-Jean G, Turcotte I, Perusse AD, Bastien CH. REM and NREM power spectral analysis on two consecutive nights in psychophysiological and paradoxical insomnia sufferers. Int J Psychophysiol. 2013;89:181–194. doi: 10.1016/j.ijpsycho.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Stanley MA, Beck JG, Zebb BJ. Psychometric properties of four anxiety measures in older adults. Behaviour Research and Therapy. 1996 doi: 10.1016/0005-7967(96)00064-2. [DOI] [PubMed] [Google Scholar]
- Tang NK, Harvey AG. Altering misperception of sleep in insomnia: behavioral experiment versus verbal feedback. J Consult Clin Psychol. 2006;74:767–776. doi: 10.1037/0022-006X.74.4.767. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Ibrahim HM, Carmody TJ, Biggs MM, Suppes T, Crismon ML, Shores-Wilson K, Toprac MG, Dennehy EB, Witte B, Kashner TM. The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C) and Self-Report (IDS-SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self-Report (QIDS-SR) in public sector patients with mood disorders: a psychometric evaluation. Psychol Med. 2004;34:73–82. doi: 10.1017/s0033291703001107. [DOI] [PubMed] [Google Scholar]
- van den Berg JF, Van Rooij FJ, Vos H, et al. Disagreement between subjective and actigraphic measures of sleep duration in a population-based study of elderly persons. J Sleep Res. 2008;17:295–302. doi: 10.1111/j.1365-2869.2008.00638.x. [DOI] [PubMed] [Google Scholar]
- Vanable PA, Aikens JE, Tadimeti L, Caruana-Montaldo B, Mendelson WB. Sleep latency and duration estimates among sleep disorder patients: variability as a function of sleep disorder diagnosis, sleep history, and psychological characteristics. Sleep. 2000;23:71–79. [PubMed] [Google Scholar]
- Vitiello MV, Larsen LH, Moe KE. Age-related sleep change: Gender and estrogen effects on the subjective-objective sleep quality relationships of healthy, noncomplaining older men and women. J Psychosom Res. 2004;56:503–510. doi: 10.1016/S0022-3999(04)00023-6. [DOI] [PubMed] [Google Scholar]
- Ware JE, Snow KK, Kosinski M. SF-36 Health Survey: Manual and Interpretation Guide. Health Institute, New England Medical Center; Boston, MA: 1993. [Google Scholar]
- Williams JM, Kay DB, Rowe M, McCrae CS. Sleep discrepancy, sleep complaint, and poor sleep among older adults. J Gerontol B Psychol Sci Soc Sci. 2013;68:712–720. doi: 10.1093/geronb/gbt030. [DOI] [PMC free article] [PubMed] [Google Scholar]


