Abstract
Objective
Cancer mortality is higher in individuals with schizophrenia, a finding that may be due, in part, to inequalities in care. We evaluated gaps in lung cancer diagnosis, treatment, and survival among elderly individuals with schizophrenia.
Methods
The Surveillance, Epidemiology, and End Results (SEER) database linked to Medicare records was used to identify primary non-small cell lung cancer (NSCLC) patients ≥66 years of age. Lung cancer stage, diagnostic evaluation, and rates of stage-appropriate treatment were compared among patients with and without schizophrenia using unadjusted and multiple regression analyses. Survival was compared among groups using Kaplan-Meier methods.
Results
Of the 96,702 NSCLC patients in SEER, 1,303 (1.3%) had schizophrenia. In comparison to the general population, patients with schizophrenia were less likely to present with late-stage disease after controlling for age, sex, marital status, race/ethnicity, income, histology, and comorbidities (odds ratio [OR]: 0.82; 95% confidence interval [CI]: 0.73-0.93) and were less likely to undergo appropriate evaluation (p<0.050 for all comparisons). Adjusting for similar factors, patients with schizophrenia were also less likely to receive stage-appropriate treatment (OR: 0.50, 95% CI: 0.43-0.58). Survival was decreased among patients with schizophrenia (mean survival 22.3 vs. 26.3 months, p=0.002), however no differences were observed after controlling for treatment received (p=0.4).
Conclusions
Elderly patients with schizophrenia present with earlier stages of lung cancer, but are less likely to undergo diagnostic evaluation or to receive stage appropriate treatment, resulting in poorer outcomes. Efforts to increase treatment rates for elderly patients with schizophrenia may lead to improved survival in this group.
Keywords: Schizophrenia, elderly, non-small cell lung cancer, health care disparities, cigarette smoking, health outcomes
Introduction
Schizophrenia is a chronic mental illness that affects approximately 1% of the population (1). In comparison to the general population, individuals with schizophrenia have a shorter lifespan(2), a gap that has widened in recent decades(3). After suicide, cancer is one of the leading causes of mortality among these individuals(4, 5) and a recent analysis showed that patients with schizophrenia are at a 30% increased risk of death from cancer(3). Lung cancer, the leading cause of cancer mortality in the United States (US) (6), is a disease primarily affecting older adults(7), and a significant concern for the elderly population with schizophrenia because of their increased disease burden due to high smoking rates(8). Furthermore, as the number of elderly patients with schizophrenia increases due to aging of the US population and improvement in psychotropic medications, the burden of lung cancer among these patients is expected to grow.
Evidence shows that individuals with schizophrenia are twice as likely to die from lung cancer in comparison to the general population(4). The increased burden of cancer mortality among these individuals may be a result of delayed diagnosis and suboptimal treatment(9, 10). This problem may be exacerbated among older patients who, even without significant mental illness, are often not appropriately staged or treated for lung cancer(11, 12). However, there is little data characterizing specific deficiencies in diagnosis, clinical evaluation and treatment of lung cancer that explains the poorer outcomes experienced by patients with schizophrenia. This information may provide specific targets for improvement in the care and outcomes of this vulnerable population.
Using a national cancer database linked with Medicare data, we evaluated disparities in lung cancer diagnosis, evaluation, treatment and survival in elderly patients with schizophrenia diagnosed with primary non-small cell lung cancer (NSCLC).
Methods
Study Population
We used the Surveillance, Epidemiology and End Results (SEER) database linked to Medicare claims to assemble the study cohort. The SEER registry is sponsored by the National Cancer Institute and integrates data from 17 regional cancer registries throughout the US(13). The SEER-Medicare database encompasses approximately 94% of individuals ≥65 years of age in the SEER registry(14).
Using the SEER-Medicare database, we identified individuals ≥66 years of age (so that they would have at least a year of Medicare claims available) with histologically confirmed primary NSCLC diagnosed between 1992 and 2007. Patients were excluded if their diagnoses were established post-mortem or if they lacked part B (outpatient) coverage or were insured by a Health Maintenance Organization(15).
Diagnosis of Schizophrenia and Other Covariates
We identified patients with schizophrenia, prior to lung cancer diagnosis, using Medicare inpatient, physician, and outpatient ICD-9 codes (295.xx), which have been shown to be 90-100% reliable in validation studies(16, 17). We ascertained Medicare claims for long-term care facility use to identify institutionalized patients with schizophrenia and used records of utilization of home services to assess their functional status(11). Additional markers of disease severity included episodes of emergency department (ED) and hospital admissions for schizophrenia and diagnosis codes signifying drug or alcohol abuse(11).
Sociodemographic information, such as age, gender, race/ethnicity, marital status and estimated income was obtained from the SEER and Medicare databases. We evaluated the number of primary care visits during the year prior to lung cancer diagnosis as a measure of health care utilization. The burden of comorbid illness (other than lung cancer and schizophrenia) was evaluated using the Deyo adaptation of the Charlson comorbidity index. From SEER data, we obtained information on tumor location and histology.
Outcomes
We analyzed inequalities in lung cancer care between elderly patients with and without schizophrenia with respect to four primary outcomes: 1) stage at diagnosis; 2) diagnostic and staging evaluation; 3) stage-appropriate treatment; and 4) survival. Lung cancer stage was determined based on the most recent Tumor, Node and Metastasis staging system of the American Joint Commission on Cancer using SEER data. Patients with stage III or IV lung cancer were classified as diagnosed with advanced-stage disease. The extent of diagnostic evaluation was examined using Medicare claims. Procedures considered relevant to the diagnostic evaluation of all patients, regardless of stage, included bronchoscopy, fine needle aspiration (FNA), chest computed tomography (CT) scans, positron emission topography (PET) scans, and mediastinoscopy(18). For individuals with stage II-IV, we assessed use of brain magnetic resonance imaging (MRI). For individuals that did not undergo a PET scan, we assessed for use of an abdominal CT for all stages and bone scans for those with stage III-IV disease. Additionally, we evaluated whether patients had at least one visit with a surgeon (stage I-IIIA), a medical oncologist (stage IB-IV), and/or a radiation oncologist (unresected stage I-IIIA and stage IIIB).
Treatment with surgery and radiation therapy (RT) was ascertained from SEER and Medicare data. Administration of chemotherapy was established using Medicare data. We evaluated rates of appropriate treatment, as specified by current guidelines(19), including surgery for stages I-IIIA (followed by adjuvant chemotherapy for stage II-IIIA disease), combined chemotherapy and RT for stage IIIB disease, and chemotherapy for stage IV NSCLC. As some elderly patients may not be candidates for surgery or aggressive chemoradiation, we also evaluated the number of unresected patients with stage I-II NSCLC treated with RT or stage IIIB patients who underwent RT or chemotherapy alone.
Overall survival was determined using Medicare data. Survival time was estimated from the time of diagnosis until the date of death or last follow-up (December 31, 2009). The Institutional Review Board of Mount Sinai School of Medicine exempted this study from an ethics board approval.
Statistical Analysis
Differences in sociodemographic characteristics between patients with and without schizophrenia were evaluated using chi-square and t-tests, as appropriate. Unadjusted differences in lung cancer stage, diagnostic evaluation, and treatment were assessed using a chi-square test. We used logistic regression analysis to assess the association between these outcomes and a diagnosis of schizophrenia after controlling for age, sex, marital status, race/ethnicity, income, histology, and comorbidity status. Adjustments for cancer stage were made in the analyses assessing diagnostic evaluation or treatment. We used Kaplan-Meier methods to estimate survival among lung cancer patients with and without schizophrenia. We then stratified the cohort by use of stage-appropriate treatment, and repeated survival analyses within each strata, comparing outcomes in patients with and without schizophrenia. To assess differences in survival accounting for potential confounders, we fitted a Cox model evaluating overall survival with schizophrenia as our primary exposure of interest, adjusting for age, sex, race/ethnicity, marital status, median income in zip code area of residence, comorbidity score, tumor histology, tumor stage, and tumor site. In a second model, we further adjusted for stage-appropriate lung cancer treatment.
Analyses were performed with SPSS statistical package (IBM, Chicago, IL) using two tailed p-values.
Results
Of the 96,702 patients with NSCLC in SEER-Medicare, 1,303 (1.3%) had a diagnosis of schizophrenia. Overall, elderly lung cancer patients with schizophrenia were younger and more likely to be female, black, unmarried, and reside in areas with the lowest income quartile (Table 1). Middle lobe tumors and squamous cell histology were also more frequent among patients with schizophrenia. Additionally, individuals with schizophrenia had more visits with their PCP in the year prior to diagnosis and experienced a greater burden of comorbid illness.
Table 1. Characteristics of Study Patients.
| No Schizophrenia N = 95,399 |
Schizophrenia N = 1,303 |
|
|---|---|---|
| Age, years, mean (SD) | 75.1 (6.1) | 73.9 (5.6) |
| Female, N (%) | 43,080 (45.2) | 720 (55.3) |
| Marital Status, N (%) | 50,555 (53.0) | 346 (26.6) |
| Race/Ethnicity, N (%) | ||
| White | 79,615 (83.4) | 965 (74.1) |
| Black | 7,881 (8.3) | 240 (18.4) |
| Hispanic | 3,203 (3.4) | 54 (4.1) |
| Other | 4,700 (4.9) | 44 (3.4) |
| Income Quartiles, N (%) | ||
| Quartile 1 (Lowest) | 23,992 (25.2) | 443 (34.0) |
| Quartile 2 | 23,849 (25.0) | 317 (24.3) |
| Quartile 3 | 23,762 (24.9) | 320 (24.6) |
| Quartile 4 (Highest) | 23,693 (24.9) | 222 (17.1) |
| Histology, N (%) | ||
| Adenocarcinoma | 45,832 (48.0) | 540 (41.5) |
| Squamous Cell | 30,603 (32.1) | 520 (39.9) |
| Large Cell | 6,569 (6.9) | 85 (6.5) |
| Other | 12,395 (13.0) | 158 (12.1) |
| Tumor Location, N (%) | ||
| Upper Lobe | 47,291 (49.6) | 643 (49.4) |
| Middle Lobe | 3,779 (4.0) | 73 (5.6) |
| Lower Lobe | 25,375 (26.6) | 325 (24.9) |
| Other | 18,954 (19.9) | 262 (20.1) |
|
Total PCP1
visits in year prior to diagnosis, mean (SD) |
6.2 (6.4) | 8.3 (7.9) |
| Comorbidity Score, N (%) | ||
| ≤1.0 | 36,346 (38.0) | 314 (24.1) |
| 1.0 – 2.0 | 26,969 (28.3) | 382 (29.3) |
| 2.0 – 4.0 | 21,611 (22.7) | 365 (28.0) |
| ≥4.0 | 10,473 (11.0) | 242 (18.6) |
PCP=primary care provider
Stage at Diagnosis and Evaluation
Patients with schizophrenia were more likely to be diagnosed with early-stage (I-II) lung cancer compared to the general population (34.9% vs. 30.6%, respectively; p<0.001; Table 2). Adjusted analysis (including number of PCP visits in the last year) also showed that patients with schizophrenia had 0.82 (95% Confidence Interval [CI]: 0.73-0.93) lower odds of being diagnosed with late-stage (III- IV) NSCLC compared with the general population.
Table 2. Stage at Diagnosis of Patients with and without Schizophrenia.
| Stage | No Schizophrenia N (%) |
Schizophrenia N (%) |
P-value1 |
|---|---|---|---|
| I | 24,899 (26.1) | 398 (30.5) | <0.001 |
| II | 4,292 (4.5) | 57 (4.4) | |
| III | 29,501 (30.9) | 423 (32.5) | |
| IV | 35,596 (37.3) | 413 (31.7) | |
| Unstaged | 1,111 (1.2) | 12 (0.9) |
Chi-square test
Use of chest CT (p=0.01), PET scan (p<0.001), mediastinoscopy (p=0.01), abdominal CT (p<0.001), and bone scan (p<0.001) was less frequent among elderly patients with schizophrenia (Table 3). However, no significant differences were observed in the use of bronchoscopy, FNA, and brain MRI (p>0.05 for all comparisons). In analyses adjusting for sociodemographics, comorbid illness, and cancer stage, bronchoscopy was also less likely to be used in the diagnostic evaluation of patients with schizophrenia (OR: 0.88; 95% CI: 0.79-0.98; Table 3). Results similar to our unadjusted analysis were observed for other diagnostic tests.
Table 3. Diagnostic Evaluation of Patients with and without Schizophrenia.
| Test | No Schizophrenia N (%) |
Schizophrenia N (%) |
Test Use among Patients with Schizophrenia | |
|---|---|---|---|---|
| Unadjusted2 OR1 (95% CI) |
Adjusted3 OR (95% CI) |
|||
| All Stages: | ||||
| Bronchoscopy | 47,895 (50.2) | 631 (48.4) | 0.93 (0.84-1.04) | 0.88 (0.79-0.98) |
| Fine Needle Aspiration | 39,677 (41.6) | 542 (41.6) | 1.00 (0.90-1.12) | 1.01 (0.90-1.13) |
| Positron Emission Test | 11,428 (12.0) | 111 (8.5) | 0.68 (0.56-0.83) | 0.68 (0.56-0.83) |
| Chest Computed Tomography | 80,640 (84.5) | 1,069 (82.0) | 0.84 (0.73-0.96) | 0.84 (0.73-0.98) |
| Mediastinoscopy | 7,658 (8.0) | 77 (5.9) | 0.72 (0.57-0.91) | 0.75 (0.59-0.95) |
| Stage II-IV: | ||||
| Brain Magnetic Resonance Imaging | 144 (0.2) | ≤11 (0.2) | 1.08 (0.27-4.36) | 1.00 (0.25-4.06) |
| If no PET: | ||||
| Abdominal Computed Tomography | 32,054 (38.2) | 373 (31.3) | 0.74 (0.65-0.83) | 0.78 (0.69-0.88) |
| Bone Scan (stages III-IV) | 28,625 (49.1) | 316 (41.2) | 0.73 (0.63-0.84) | 0.74 (0.64-0.86) |
OR = odds ratio
Logistic regression model, unadjusted
Logistic regression model, adjusted for age, gender, marital status, race/ethnicity, income, histology, comorbidities, and stage
Lung cancer patients with schizophrenia were less likely to be evaluated by a surgeon (58.2% vs. 53.5%; p=0.02) or to have visited a medical oncologist (58.5% vs. 49.4%; p<0.001; Table 4). Patients with schizophrenia were 4% less likely to visit with a radiation oncologist however, this difference was not statistical significant (37.1% vs. 41.2%, p=0.06).
Table 4. Cancer Specialist Visits among Patients with and without Schizophrenia.
| Specialist | No Schizophrenia N (%) |
Schizophrenia N (%) |
Specialist Visit among Patients with Schizophrenia | |
|---|---|---|---|---|
| Unadjusted2 OR1 (95% CI) |
Adjusted3 OR (95% CI) |
|||
| Surgeon | 22,729 (58.2) | 319 (53.5) | 0.83 (0.70-0.97) | 0.98 (0.74-1.04) |
| Medical Oncologist | 42,727 (58.5) | 462 (49.4) | 0.69 (0.61-0.79) | 0.73 (0.64-0.83) |
| Radiation Oncologist | 14,234 (41.2) | 202 (37.1) | 0.84 (0.71-1.00) | 0.80 (0.67-0.96) |
OR = odds ratio
Logistic regression model, unadjusted
Logistic regression model, adjusted for age, gender, marital status, race/ethnicity, income, histology, comorbidities, and stage
Analyses adjusting for year of diagnosis showed similar associations between a diagnosis of schizophrenia with disease stage and diagnostic evaluation.
Stage-appropriate Treatment
Elderly patients with schizophrenia were less likely to receive stage-appropriate NSCLC treatment compared to those without schizophrenia (38.4% vs. 49.1%, respectively; p<0.001; Table 5). When analyzed according to stage, patients with schizophrenia were less likely to undergo surgery for stages I-IIIA (p<0.001), receive combined RT and chemotherapy for stage IIIB (p<0.001) or chemotherapy for stage IV (p<0.001) NSCLC. Among stage I-II patients who were not treated with surgical resection, RT was also less frequently used among patients with schizophrenia (p=0.001). However, no differences were observed in the use of adjuvant chemotherapy for resected stage II-IIIA disease (p=0.52). Adjusted analysis showed that patients with schizophrenia had half (95% CI: 0.43-0.58) the odds of receiving stage-appropriate treatment compared with the patients without schizophrenia. Similar results were obtained when year of diagnosis was included in the model, thus controlling for potential temporal changes in the treatment of lung cancer.
Table 5. Stage-Appropriate Treatment among Patients with and without Schizophrenia.
| Stage | No Schizophrenia N (%) |
Schizophrenia N (%) |
Treatment among Patients with Schizophrenia | |
|---|---|---|---|---|
| Unadjusted2 OR1 (95% CI) |
Adjusted3 OR (95% CI) |
|||
| All Stages | 45,365 (49.1) | 484 (38.4) | 0.65 (0.58-0.73) | 0.50 (0.43-0.58) |
| Stage I-IIIA | ||||
| Surgery | 23,519 (60.5) | 313 (53.0) | 0.73 (0.62-0.86) | 0.82 (0.68-0.98) |
| Resected Stage II-IIIA | ||||
| Adjuvant Chemotherapy | 1,132 (17.7) | ≤ 11 (14.9) | 0.81 (0.43-1.54) | 0.84 (0.44-1.62) |
| Unresected Stage I-II | ||||
| Radiation Therapy | 5,261 (62.7) | 84 (51.5) | 0.63 (0.46-0.86) | 0.62 (0.45-0.85) |
| Stage IIIB | ||||
| Radiation and Chemotherapy | 4,225 (22.2) | 27 (10.2) | 0.39 (0.26-0.60) | 0.39 (0.26-0.58) |
| Radiation or Chemotherapy | 7,689 (40.4) | 91 (34.5) | ||
| Neither | 7,106 (37.4) | 146 (55.3) | ||
| Stage IV | ||||
| Chemotherapy | 12,981 (36.5) | 70 (16.9) | 0.36 (0.28-0.48) | 0.39 (0.30-0.50) |
OR = odds ratio
Logistic regression model, unadjusted
Logistic regression model, adjusted for age, gender, marital status, race/ethnicity, income, histology, and comorbidities
Long-term care facility use (OR: 0.62; 95% CI: 0.38-0.99), and ED visits (OR: 0.59; 95% CI: 0.40-0.87) or hospitalizations (OR: 0.59; 95% CI: 0.41-0.85) for schizophrenia were significantly associated with receiving suboptimal treatment in unadjusted and adjusted analyses.
Survival
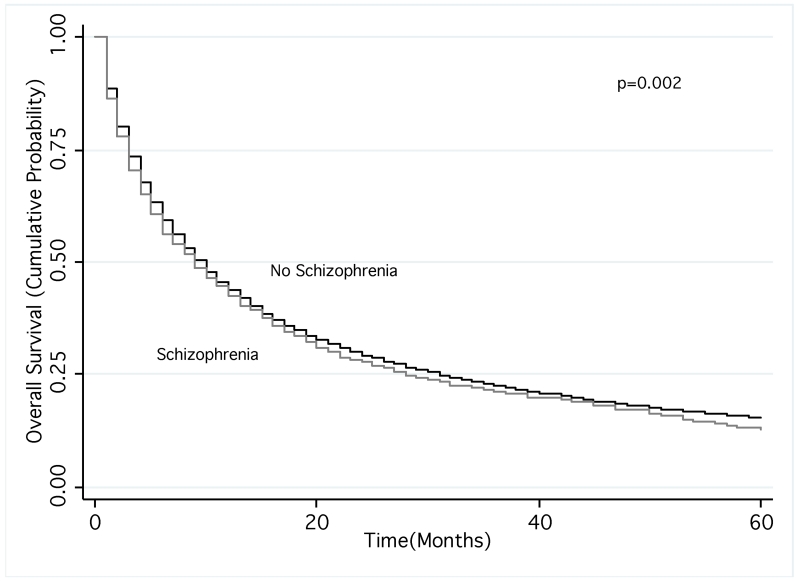
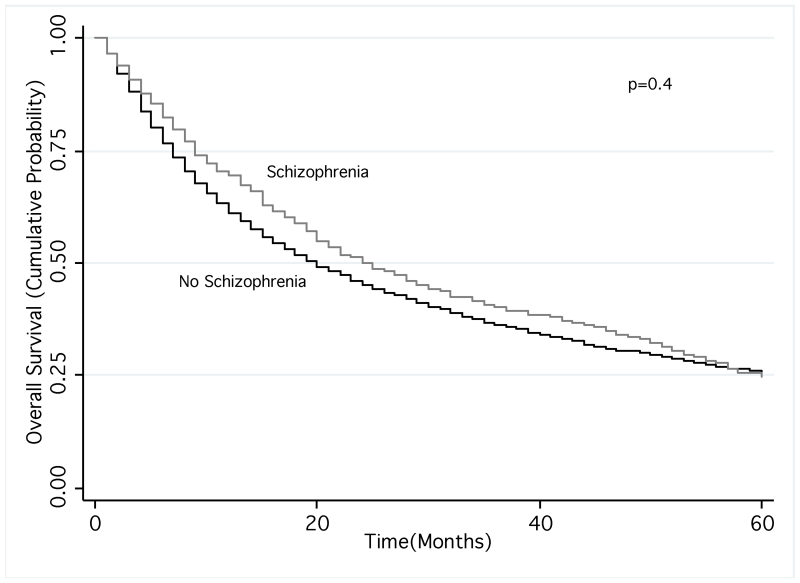
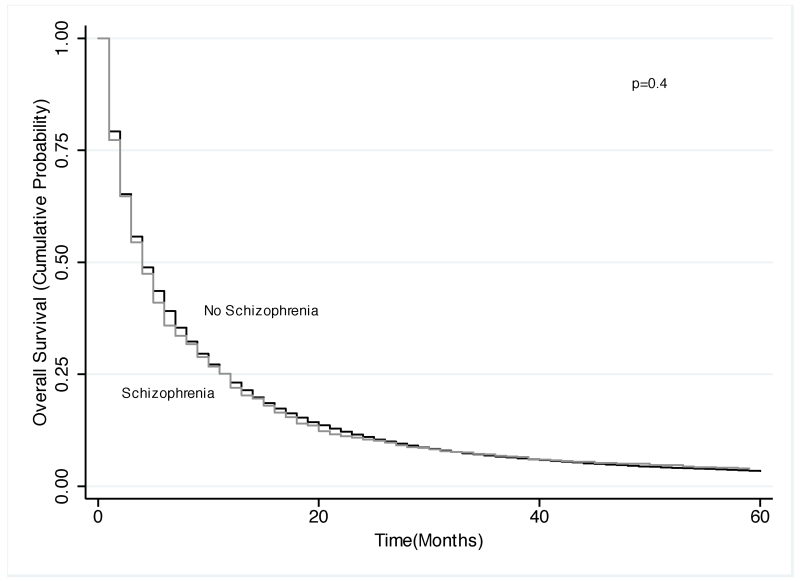
Kaplan-Meier analysis revealed that NSCLC patients with schizophrenia had significantly shorter overall survival (mean survival 22.3 vs. 26.3 months; p=0.002). After stratifying by use of stage-appropriate treatment, patients had similar survival times (mean survival 41.5 vs. 43.1 months for appropriately treated patients and 9.8 vs. 10.3 months for not appropriately treated patients with and without schizophrenia, respectively; for both comparisons p=0.4). Our Cox model also showed schizophrenia was associated with an increased hazard of death (hazard ratio [HR]: 1.07; 95% CI: 1.01-1.13), after adjustment for potential confounders except lung cancer treatment. After including stage-appropriate treatment in the model, there was no significant difference in overall survival (HR: 1.02; 95% CI: 0.96-1.09).
Discussion
The burden of lung cancer is increased in elderly patients with schizophrenia. Using a large population-based registry, we demonstrated that individuals with schizophrenia presented with earlier stages of lung cancer, but were less likely to undergo standard diagnostic evaluation or to receive stage-appropriate treatment. In addition, survival was decreased among elderly patients with schizophrenia, a finding potentially explained, in part, by differences in utilization of stage-appropriate treatment. These results show the need to assess the reasons and develop interventions to eliminate these inequalities in care, thus improving the outcomes of this vulnerable population.
Elderly cancer patients with schizophrenia are an understudied population. Both the elderly as well as individuals with mental illness are reported to receive suboptimal health care and to have poorer outcomes(12, 20-23); however, there is a paucity of information pertaining to the care of cancer in general and lung cancer specifically among elderly patients with schizophrenia. Recognition of inequalities in lung cancer care in this population is important because it will help advance necessary policy changes to promote better outcomes for these patients. Additionally, similar inequalities may exist for other cancers, highlighting the need for further research.
Elderly patients with schizophrenia in our study were more likely to present with early-stage lung cancer, a somewhat unexpected result. This finding may be due to an increased utilization of primary care services by individuals with schizophrenia, a previously reported phenomenon(24), which may be more prominent in Medicare beneficiaries. It is also possible that physicians treating patients with schizophrenia have a heightened level of suspicion for lung cancer due to increased rates of smoking in this population. Patients with schizophrenia presenting with pulmonary comorbidities may have undergone more aggressive evaluation, thus, finding cancer at an earlier stage. Another mechanism for the increase rate of early disease among patients with schizophrenia may be related to antipsychotic medication use. Several large epidemiological studies and, more recently, laboratory data suggest that antipsychotics may have an anti-cancer effect(25-28). If these data are correct, lung cancer may progress slower in patients with schizophrenia treated with antipsychotic medications, increasing the opportunities for diagnosis at an early stage. Lastly, individuals with schizophrenia in our study were more likely to have squamous cell carcinomas, a centrally located cancer that, due to compression of central airways and blood vessels, may produce symptoms and, ultimately, earlier diagnosis.
Despite an earlier stage at diagnosis, patients with schizophrenia were less likely to receive stage-appropriate diagnostic evaluation and treatment. Inequalities in disease management for individuals with mental illnesses have been reported in the literature and range from suboptimal preventative medical care (29) to poor quality management of chronic diseases and surgical interventions (30, 31). In a meta-analysis of cardiovascular disease management, patients with severe mental illnesses were less likely to receive appropriate prescriptions for cardiovascular disease or to undergo basic cardiac procedures (31). Similarly, another study showed that individuals with mental illnesses received substandard diabetic care and had more diabetic complications(30). In one study assessing the receipt of coronary artery bypass graft surgery among patients in New York State, individuals with any mental disorder were more likely to be treated by a low-quality surgeon(32). Our results extend these findings to the treatment of lung cancer, an important cause of death in this population.
The reasons for these disparities in the care of patients with schizophrenia are complex and not well understood. One potential explanation may be related to difficulties for patients in navigating the health system(33, 34). Lung cancer treatment requires patients to make specialist appointments, schedule and complete multiple diagnostic tests, and, for radiation and chemotherapy, attend multiple treatment sessions. These tasks can be overwhelming, particularly for patients with schizophrenia, who may experience varying levels of cognitive dysfunction. If indeed this is a major barrier for appropriate lung cancer care, future initiatives may evaluate the effectiveness of patient navigators, who have been found to effectively address racial disparities in cancer care(35).
Another reason for these inequalities may be that individuals with schizophrenia were unable to consent or less likely adhere to diagnostic tests and lung cancer treatments. This barrier may be related to difficulties understanding treatments and outcomes. We found that the severity of schizophrenia was significantly associated with receiving suboptimal treatment, suggesting that more impaired patients are at particular risk for undertreatment. In addition, it is possible that providers may contribute to these inequalities because of uncertainty about the impact of lung cancer treatments on the outcomes of patients with schizophrenia that have, in general, a reduced life expectancy.
Our analysis has some strengths and limitations that are worth mentioning. Our study was limited to elderly patients, thus these findings may not be generalizable to younger populations. However, lung cancer is predominantly a disease of older adults, and elderly patients with schizophrenia are an understudied population. We were unable to evaluate the smoking history of study patients due to lack of data on tobacco use in the SEER-Medicare database. The number of years of tobacco consumption is an important indicator of lung cancer survival among the schizophrenic population. However, more than 90% of all lung cancer patients have a history of smoking(36), potentially attenuating differences in smoking patterns among patients with schizophrenia compared to the general population. Given the relatively rare treatment of nicotine dependence in patients with severe mental illness compared to the general population, exploring the impact of smoking status and cessation should be considered in future studies(37). We were also unable to identify the underlying reasons for suboptimal evaluation and treatment of patients with schizophrenia, as this data is not available in the SEER-Medicare database. However, use of the SEER-Medicare data allowed us to evaluate the patterns of care for a large, nationally representative cohort of lung cancer patients with schizophrenia. One study that analyzed lung cancer treatment among 29 patients with schizophrenia found that treatment decisions were not based solely on whether the patient had schizophrenia but rather a combination of the patients’ comorbidity status and mental capacity(38). This finding, while promising, should be analyzed in a larger patient population.
In summary, our study provides evidence of considerable inequalities in the lung cancer care received by elderly patients with schizophrenia, a finding that translates into worse survival of these vulnerable individuals. Future explorations into the underlying reasons for this disparity are necessary to improve lung cancer care and outcomes in for these patients.
Figure 1.
Acknowledgments
Source of Funding: This study was supported by funding from the Doris Duke Foundation (CB), and the National Center for Research Resources (KL2TR000069 to KS).
The authors acknowledge the efforts of the Applied Research Branch, Division of Cancer Prevention and Population Science, National Cancer Institute; the Office of Information Services, and the Office of Strategic Planning, Health Care Finance Administration; Information Management Services (IMS), Inc.; and the SEER Program tumor registries in the creation of the SEER-Medicare Database. The interpretation and reporting of these data are the sole responsibilities of the authors.
Abbreviations
- NSCLC
Non-small cell lung cancer
- SEER
Surveillance Epidemiology and End-Results
- ICD-9
International Classification of Disease, Ninth Edition
- FNA
Fine needle aspiration
- CT
Computed tomography
- PET
Positron emission topography
- MRI
Magnetic resonance imaging
- PCP
Primary care provider
- ED
Emergency department
- RT
Radiation Therapy
Footnotes
Conflicts of Interest: Dr. Wisnivesky is a member of the research Board of EHE International, has received honorarium from Merck, IMS Health, and UBS, and a research grant from GlaxoSmithKline. Other authors report no conflicts of interest related to this study.
References
- 1.Freedman R. Schizophrenia. N Engl J Med. 2003;349:1738–49. doi: 10.1056/NEJMra035458. [DOI] [PubMed] [Google Scholar]
- 2.Brown S. Excess mortality of schizophrenia. A meta-analysis. Br J Psychiatry. 1997;171:502–8. doi: 10.1192/bjp.171.6.502. [DOI] [PubMed] [Google Scholar]
- 3.Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry. 2007;64:1123–31. doi: 10.1001/archpsyc.64.10.1123. [DOI] [PubMed] [Google Scholar]
- 4.Tran E, Rouillon F, Loze JY, Casadebaig F, Philippe A, Vitry F, Limosin F. Cancer mortality in patients with schizophrenia: an 11-year prospective cohort study. Cancer. 2009;115:3555–62. doi: 10.1002/cncr.24383. [DOI] [PubMed] [Google Scholar]
- 5.Bushe CJ, Taylor M, Haukka J. Mortality in schizophrenia: a measurable clinical endpoint. J Psychopharmacol. 2010;24:17–25. doi: 10.1177/1359786810382468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 7.SEER . Surveillance Epidemiology and End Results. National Cancer Institute; 2000-2008. [Google Scholar]
- 8.McClave AK, McKnight-Eily LR, Davis SP, Dube SR. Smoking characteristics of adults with selected lifetime mental illnesses: results from the 2007 National Health Interview Survey. Am J Public Health. 2010;100:2464–72. doi: 10.2105/AJPH.2009.188136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inagaki T, Yasukawa R, Okazaki S, Yasuda H, Kawamukai T, Utani E, Hayashida M, Mizuno S, Miyaoka T, Shinno H, Horiguchi J. Factors disturbing treatment for cancer in patients with schizophrenia. Psychiatry Clin Neurosci. 2006;60:327–31. doi: 10.1111/j.1440-1819.2006.01509.x. [DOI] [PubMed] [Google Scholar]
- 10.Wildgust HJ, Beary M. Are there modifiable risk factors which will reduce the excess mortality in schizophrenia? J Psychopharmacol. 2010;24:37–50. doi: 10.1177/1359786810384639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wisnivesky JP, Smith CB, Packer S, Strauss GM, Lurslurchachai L, Federman A, Halm EA. Survival and risk of adverse events in older patients receiving postoperative adjuvant chemotherapy for resected stages II-IIIA lung cancer: observational cohort study. BMJ. 2011;343:d4013. doi: 10.1136/bmj.d4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jazieh AR, Kyasa MJ, Sethuraman G, Howington J. Disparities in surgical resection of early-stage non-small cell lung cancer. J Thorac Cardiovasc Surg. 2002;123:1173–6. doi: 10.1067/mtc.2002.122538. [DOI] [PubMed] [Google Scholar]
- 13.SEER Cancer Statistics Review, 1975-2006 [database on the Internet] National Cancer Institute; Bethesda, MD: [Google Scholar]
- 14.Potosky AL, Riley GF, Lubitz JD, Mentnech RM, Kessler LG. Potential for cancer related health services research using a linked Medicare-tumor registry database. Med Care. 1993;31:732–48. [PubMed] [Google Scholar]
- 15.Wisnivesky JP, Halm EA, Bonomi M, Smith C, Mhango G, Bagiella E. Postoperative radiotherapy for elderly patients with stage III lung cancer. Cancer. 2012;118:4478–85. doi: 10.1002/cncr.26585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frayne SM, Miller DR, Sharkansky EJ, Jackson VW, Wang F, Halanych JH, Berlowitz DR, Kader B, Rosen CS, Keane TM. Using administrative data to identify mental illness: what approach is best? Am J Med Qual. 2010;25:42–50. doi: 10.1177/1062860609346347. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz AH, Perlman BB, Paris M, Schmidt K, Thornton JC. Psychiatric diagnoses as reported to Medicaid and as recorded in patient charts. Am J Public Health. 1980;70:406–8. doi: 10.2105/ajph.70.4.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lathan CS, Neville BA, Earle CC. The effect of race on invasive staging and surgery in non-small-cell lung cancer. J Clin Oncol. 2006;24:413–8. doi: 10.1200/JCO.2005.02.1758. [DOI] [PubMed] [Google Scholar]
- 19.Ettinger DS, Akerley W, Borghaei H, Chang AC, Cheney RT, Chirieac LR, D’Amico TA, Demmy TL, Ganti AK, Govindan R, Grannis FW, Jr., Horn L, Jahan TM, Jahanzeb M, Kessinger A, Komaki R, Kong FM, Kris MG, Krug LM, Lennes IT, Loo BW, Jr., Martins R, O’Malley J, Osarogiagbon RU, Otterson GA, Patel JD, Pinder-Schenck MC, Pisters KM, Reckamp K, Riely GJ, Rohren E, Swanson SJ, Wood DE, Yang SC, Hughes M, Gregory KM. Non-small cell lung cancer. J Natl Compr Canc Netw. 2012;10:1236–71. doi: 10.6004/jnccn.2012.0130. [DOI] [PubMed] [Google Scholar]
- 20.Viron MJ, Stern TA. The impact of serious mental illness on health and healthcare. Psychosomatics. 2010;51:458–65. doi: 10.1176/appi.psy.51.6.458. [DOI] [PubMed] [Google Scholar]
- 21.Horton JK, Gleason JF, Jr., Klepin HD, Isom S, Fried DB, Geiger AM. Age-related disparities in the use of radiotherapy for treatment of localized soft tissue sarcoma. Cancer. 2011;117:4033–40. doi: 10.1002/cncr.25996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen HL, Goldberg RJ, Gore JM, Fox KA, Eagle KA, Gurfinkel EP, Spencer FA, Reed G, Quill A, Anderson FA., Jr Age and sex differences, and changing trends, in the use of evidence-based therapies in acute coronary syndromes: perspectives from a multinational registry. Coron Artery Dis. 2010;21:336–44. doi: 10.1097/MCA.0b013e32833ce07c. [DOI] [PubMed] [Google Scholar]
- 23.Kisely S, Crowe E, Lawrence D. Cancer-related mortality in people with mental illness. JAMA Psychiatry. 2013;70:209–17. doi: 10.1001/jamapsychiatry.2013.278. [DOI] [PubMed] [Google Scholar]
- 24.Dickerson FB, McNary SW, Brown CH, Kreyenbuhl J, Goldberg RW, Dixon LB. Somatic healthcare utilization among adults with serious mental illness who are receiving community psychiatric services. Med Care. 2003;41:560–70. doi: 10.1097/01.MLR.0000053440.18761.F0. [DOI] [PubMed] [Google Scholar]
- 25.Carrillo JA, Benitez J. Are antipsychotic drugs potentially chemopreventive agents for cancer? Eur J Clin Pharmacol. 1999;55:487–8. doi: 10.1007/s002280050661. [DOI] [PubMed] [Google Scholar]
- 26.Dalton SO, Johansen C, Poulsen AH, Norgaard M, Sorensen HT, McLaughlin JK, Mortensen PB, Friis S. Cancer risk among users of neuroleptic medication: a population-based cohort study. Br J Cancer. 2006;95:934–9. doi: 10.1038/sj.bjc.6603259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones GR. Cancer therapy: phenothiazines in an unexpected role. Tumori. 1985;71:563–9. doi: 10.1177/030089168507100608. [DOI] [PubMed] [Google Scholar]
- 28.Mortensen PB. Neuroleptic medication and reduced risk of prostate cancer in schizophrenic patients. Acta Psychiatr Scand. 1992;85:390–3. doi: 10.1111/j.1600-0447.1992.tb10325.x. [DOI] [PubMed] [Google Scholar]
- 29.Lord O, Malone D, Mitchell AJ. Receipt of preventive medical care and medical screening for patients with mental illness: a comparative analysis. Gen Hosp Psychiatry. 2010;32:519–43. doi: 10.1016/j.genhosppsych.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 30.Mai Q, Holman CD, Sanfilippo FM, Emery JD, Preen DB. Mental illness related disparities in diabetes prevalence, quality of care and outcomes: a population-based longitudinal study. BMC Med. 2011;9:118. doi: 10.1186/1741-7015-9-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitchell AJ, Lord O. Do deficits in cardiac care influence high mortality rates in schizophrenia? A systematic review and pooled analysis. J Psychopharmacol. 2010;24:69–80. doi: 10.1177/1359786810382056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y, Glance LG, Cai X, Mukamel DB. Are patients with coexisting mental disorders more likely to receive CABG surgery from low-quality cardiac surgeons? The experience in New York State. Med Care. 2007;45:587–93. doi: 10.1097/MLR.0b013e31803d3b54. [DOI] [PubMed] [Google Scholar]
- 33.Goff DC, Cather C, Evins AE, Henderson DC, Freudenreich O, Copeland PM, Bierer M, Duckworth K, Sacks FM. Medical morbidity and mortality in schizophrenia: guidelines for psychiatrists. J Clin Psychiatry. 2005;66:183–94. 273–4. doi: 10.4088/jcp.v66n0205. quiz 47. [DOI] [PubMed] [Google Scholar]
- 34.Goldman LS. Medical illness in patients with schizophrenia. J Clin Psychiatry. 1999;60(Suppl 21):10–5. [PubMed] [Google Scholar]
- 35.Christie J, Itzkowitz S, Lihau-Nkanza I, Castillo A, Redd W, Jandorf L. A randomized controlled trial using patient navigation to increase colonoscopy screening among low-income minorities. J Natl Med Assoc. 2008;100:278–84. doi: 10.1016/s0027-9684(15)31240-2. [DOI] [PubMed] [Google Scholar]
- 36.Wingo PA, Ries LA, Giovino GA, Miller DS, Rosenberg HM, Shopland DR, Thun MJ, Edwards BK. Annual report to the nation on the status of cancer, 1973-1996, with a special section on lung cancer and tobacco smoking. J Natl Cancer Inst. 1999;91:675–90. doi: 10.1093/jnci/91.8.675. [DOI] [PubMed] [Google Scholar]
- 37.Prochaska JJ. Smoking and mental illness--breaking the link. N Engl J Med. 2011;365:196–8. doi: 10.1056/NEJMp1105248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mateen FJ, Jatoi A, Lineberry TW, Aranguren D, Creagan ET, Croghan GA, Jett JR, Marks RS, Molina JR, Richardson RL. Do patients with schizophrenia receive state-of-the-art lung cancer therapy? A brief report. Psychooncology. 2008;17:721–5. doi: 10.1002/pon.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]