Abstract
Success of an immunotherapy for cancer often depends on the critical balance of T helper 1 (Th1) and T helper 2 (Th2) responses driven by antigen presenting cells, specifically dendritic cells (DCs). Th1-driven cytotoxic T cell (CTL) responses are key to eliminating tumor cells. It is well established that CpG oligonucleotides (ODN), a widely studied Toll-like receptor 9 (TLR9) agonist, used to enhance Th1 response, also induces high levels of the anti-inflammatory, Th2-promoting cytokine IL10, which could dampen the resulting Th1 response. Biomaterials-based immunomodulatory strategies that can reduce IL10 production while maintaining IL12 levels during CpG delivery could further enhance the Th1/Th2 cytokine balance and improve anti-tumor immune response. Here we report that dual-delivery of IL10-silencing siRNA along with CpG ODN to the same DCs using pathogen-mimicking microparticles (PMPs), significantly enhances their Th1/Th2 cytokine ratio through concurrent inhibition of CpG-induced IL10 production. Co-delivery of poly(I:C), a TLR3 agonist had only minor effects on IL10 levels. Further, simultaneous immunotherapy with CpG ODN and IL10 siRNA enhanced immune protection of an idiotype DNA vaccine in a prophylactic murine model of B cell lymphoma whereas co-delivery of poly(I:C) and CpG did not enhance protection. These results suggest that PMPs can be used to precisely modulate TLR ligand-mediated immune-stimulation in DCs, through co-delivery of cytokine-silencing siRNAs and thereby boost antitumor immunity.
Introduction
Since their first identification almost 40 years ago [1], dendritic cells (DCs) have emerged as one of the most important professional antigen presenting cells (APCs) bridging the two indispensable arms of the immune system i.e. innate and adaptive immunity [2, 3]. DCs play a central role in a series of immunological events during infection, immunization, and immunotherapies that eventually lead to adaptive T and B cell-mediated immunity. These include (a) migration of immature DCs to the site of infection (or antigen source) and sensing the pathogen or pathogen associated molecular patterns (PAMPs) using various receptors (pathogen recognition receptors, PRRs, e.g. toll-like receptors (TLRs)) [4], (b) antigen uptake, activation and maturation of the migrated DCs resulting in surface expression of co-stimulatory molecules and release of cytokines, and (c) migration of mature DCs to local lymph nodes, and antigen presentation to naïve T cells. Depending on the activation stimuli, cytokine profiles, maturation status, and antigen presentation mode (via MHCI or MHCII), DCs can drive naïve T cells to differentiate into various helper T cell phenotypes, namely T helper 1 (Th1), T helper 2 (Th2), T helper 17 (Th17), T follicular helper (Tfh), T regulatory (Treg) or cytotoxic T cells (CTLs) [5]. This unique ability to control specific types of cellular immune responses as well as the strength of those immune responses, makes DCs a prime target for ex vivo or in vivo manipulation (i.e. immunomodulation) to stimulate therapeutic immunity, especially against cancers [5] where Th1-type immune responses leading to tumor-specific CTLs are needed [6, 7].
With the discovery of TLRs and their specific agonists [8], it has been possible to enhance and modulate both innate and adaptive immunity against many diseases, including cancer, by simply using synthetic TLR ligands instead of whole pathogen/pathogen derived molecules. Specifically, unmethylated cytosine-phosphate-guanosine oligodeoxynucleotides (CpG ODN), a TLR9 agonist, and Poly (I:C), a synthetic double stranded RNA based TLR3 agonist, have been widely explored in cancer immunotherapy, either individually [9–12] or in tandem, due to potentially synergistic Th1 polarizing effects [13–15]. CpG ODN in particular is currently used in clinical trials for treatment of different types of cancers as a vaccine adjuvant or monotherapy [9, 10, 16, 17]. CpG ODN binds to TLR9 receptors on endosomal membranes of DCs and activates them to secrete various cytokines. This leads to DC maturation, increased expression of surface co-stimulatory and MHC molecules, and enhanced antigen presentation to naïve T cells. During these immunological cascades, cytokines released by CpG-induced, activated DCs play a critical role to polarize the immune response towards a specific T helper phenotype. Although CpG ODN is known to stimulate DCs to secrete high amounts of Th1-specific cytokine IL12, a significant amount of anti-inflammatory, immunosuppressive cytokine IL10 is also simultaneously secreted [18, 19] (Fig. 1C). This autocrine IL10 dampens the capacity of DCs to stimulate a stronger Th1 response [20]. IL10 is known to polarize immunity towards Th2 and help induce immunosuppressive Treg cells [21] (Fig. 1C), which ultimately leads to a poor outcome in cancer immunotherapy [22]. Silencing IL10 alone in DCs has been shown to enhance Th1 and CTL responses [23–26]. On the other hand, a combination of CpG and Poly (I:C) synergistically increases IL12 secretions of DCs and thus enhances the Th1 response and antitumor immunity [13–15].
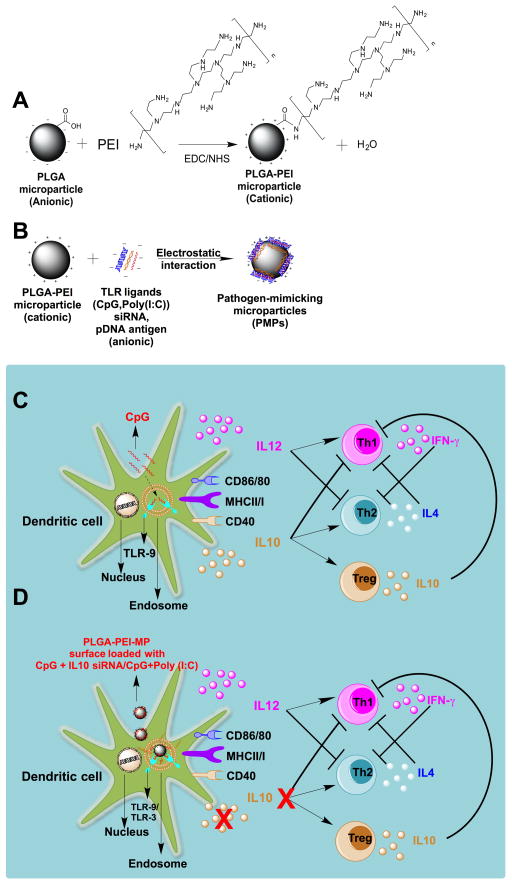
Fig. 1.
A) Reaction scheme showing surface functionalization of PLGA microparticle covalently with a cationic polymer, PEI through EDC/NHS chemistry; B) Diagram showing surface loading of various anionic nucleic acid based immunomodulatory molecules e.g., CpG, Poly (I:C), siRNA, pDNA on cationic PLGA-PEI microparticles by electrostatic interaction; C) Schematic illustrating binding of soluble CpG to TLR9 receptor of DCs leading to secretion of both IL12 and IL10 cytokines, which may ultimately polarize to Th1 due to IL12, and Th2/Treg due to IL10; D) Schematic showing the concept of co-delivery IL10 siRNA and CpG molecules surface loaded on cationic PLGA microparticle to DCs to switch CpG induced IL12/IL10 (Th1/Th2) cytokine balance by inhibiting IL10 secretion by silencing IL10 gene in DCs and ultimately resulting into an enhanced Th1 response.
We hypothesized that the Th1-specific immune-stimulatory effect of CpG ODN can be further enhanced by modulating the IL12/IL10 and IFN-γ/IL4 (Th1/Th2) cytokine balance in DCs through concurrent inhibition (gene silencing) of CpG-induced IL10 production during immunotherapy. To evaluate this, we developed surface-functionalized, cationic microparticles that can simultaneously present both antigen and PAMPs (e.g. TLR ligands) to immature DCs, thereby mimicking the immunostimulatory and cytokine-modulating properties of pathogens. These pathogen-mimicking particles (PMPs). were used to treat mouse bone marrow-derived primary dendritic cells (BMDCs) with CpG ODN+IL10-silencing siRNA or CpG ODN+Poly(I:C) with or without pDNA antigens. Effects of various PMP formulations on BMDC maturation and Th1/Th2 cytokine profiles were studied. For in-vivo studies PMPs were co-delivered with MIP3 alpha, a DC attracting chemokine, using an in-situ forming “synthetic immune priming center (sIPC)”, previously developed and characterized in our lab [25]. Specifically, PMPs carrying CpG ODN + IL10 siRNA + pDNA antigen and CpG ODN + poly(I:C) + pDNA-antigen were evaluated to determine how concurrent immune modulation through gene silencing or multiple TLR activation enhance protective immunity of an idiotype pDNA vaccine in a mouse model of B cell lymphoma.
2. Materials and Methods
All animal experiments were approved by The Institutional Animal Care and Use Committees (IACUC) at The University of Texas at Austin (Austin, TX), The University of Texas M. D. Anderson Cancer Center (Houston, TX) and Georgia Institute of Technology (Atlanta, GA).
2.1 Materials
Acid end-capped PLGA RG502H (MW–11000) was purchased from Boehringer Ingelheim (Evonik, Germany). Poly (vinyl alcohol) (MW 31,000) was purchased from Fluka (Sigma-Aldrich, St. Louis, MO). Branched polyethyleneimine (PEI, MW 70,000) was obtained from Polysciences (Warrington, PA). 1-Ethyl-3-(3 dimethylaminopropyl) carbodiimide hydrochloride (EDC) and sulfo N hydroxysuccinimide (NHS) were purchased from Pierce Biotechnology (Rockford, IL). Dextran (average MW 15,000–20,000), divinyl sulfone (DVS; 97%, MW 118.15) and 3-mercaptopropionic acid (MW 106.14) were purchased from Sigma–Aldrich (St. Louis, MO). Tetra-functional PEG-thiol (PEG-4-SH) was purchased from Sunbio (Anyang City, South Korea). Dimethyl sulfoxide (DMSO), p-toluenesulfonic acid (pTSA) and tetrahydrofuran (THF, HPLC grade) were from Thermo Fisher Scientific. Vaccigrade phosphorothioated mouse CpG ODN 1826 (5′-TCCATGACGTTCCTGACGTT-3′) was purchased from Invivogen (San Diego, CA). Mouse IL10 siRNA (SMART pool: ON-TARGET plus IL10 siRNA) was purchased from Thermo Scientific Dharmacon (Pittsburg, PA). Vaccigrade Poly (I:C) was purchased from Invivogen (Sandiego, CA) Plasmid DNA encoding MCP3sFv20 was developed in the Kwak lab [27] and amplified by Aldevron (Fargo, ND).
2.2 Synthesis of PLGA microparticles and their surface functionalization with PEI
PLGA microparticles were prepared using a water-oil-water double emulsion, solvent evaporation method as reported earlier by our group [28, 29]. Briefly, 200 mg of PLGA (RG502H, Evonik, Germany) was first dissolved in 7 ml dichloromethane (DCM) and homogenized at 10,000 rpm speed for 2 minutes in the presence of 300 ul water. The emulsion was then immediately added to 50 ml of 1% PVA (Sigma-Aldrich, St. Louis, MO) and homogenized again for 2 minutes at 10,000 rpm and stirred for 3 hours for evaporation of DCM. The microparticles were then collected by centrifugation and washed 3 times with deionized water and lyophilized and stored at −20 °C until further use. Surface functionalization of the synthesized PLGA microparticles was done by covalent conjugation of branch PEI (MW 70,000, Polyscience, Warrington, PA) with the acid group on the surface of PLGA microparticles using EDC/NHS chemistry as also reported earlier by our group [28, 29]. The PEI functionalized particles were characterized for their size and zeta potential using a Zetasizer Nano ZS instrument (Malvern, MA).
2.3 Surface loading of different nucleic acid based immunomodulatory molecules and pDNA antigen onto PLGA-PEI microparticles
Different nucleic acid based immunomodulatory molecules such as CpG ODN 1826 (Invivogen, San Diego, CA), Poly (I:C) (Invivogen, San Diego, CA), IL10 siRNA (SMART pool: ON-TARGET plus IL10 siRNA, Thermo Scientific Dharmacon, Pittsburg, PA) and pDNA encoding MCP3sFv20 (developed in Kwak lab [27] and amplified by Aldevron, Fargo, DD) were surface loaded onto the PLGA-PEI microparticles by electrostatic interaction. PLGA-PEI particles at 5 mg/ml and different nucleic acids (individual or together) at 60 μg/ml concentration (at 1.2% weight of the microparticle) were suspended in phosphate buffer (10 mM, pH-6) in two separate DNase RNase free tubes. Then, the PLGA-PEI solution was added drop-wise to the nucleic acid solution while vortexing and the mixture was incubated on an end to over shaker overnight at 4°C. The particles surface loaded with nucleic acids were collected by centrifugation and the supernatant was analyzed for nucleic acid content at 260 nm wavelength using a NanoDrop 2000 UV-VIS spectrophotometer (Thermo scientific, Wilmington, DE). The amount of nucleic acid present in the supernatant was subtracted from the initially added amount to quantify the total amount of surface loaded nucleic acid onto PLGA-PEI microparticles. At the 1.2% wt/wt ratio, 100% surface loading of nucleic acid was achieved. To visualize surface loading of CpG/siRNA on PLGA-PEI microparticles, FITC labeled CpG (CpG-FITC, Invivogen, Sandiego, CA) or Cy3 labeled siRNA (siRNA-Cy3, Ambion, Austin, Texas) surface loaded microparticles prepared as described above and imaged using a confocal microscope (Prairie Technologies, WI, USA).
2.3 Bone marrow derived dendritic cell (BMDC) generation and culture
Murine BMDCs were generated from bone marrow progenitor cells of Balb/c mice (H-2d, 5–10 weeks old; Jackson Laboratories, Bar Harbor, ME) as described by Inaba et al. [30] with some modifications. Briefly, bone marrow progenitor cells were isolated from tibias and femurs of Balb/c mice and differentiated into BMDCs in RPMI 1640 Glutamax medium (Invitrogen, Carlsbad, CA) supplemented with 10% FBS, 25 ng/ml mouse granulocyte–macrophage colony-stimulating factor (GM-CSF) and 25 ng/ml IL-4 (Peprotech, Rocky Hill, NJ) for 6 days. On day 2 and 4, the medium was replaced with new medium containing GMCSF and IL4. On day 6, loosely adherent immature DCs were harvested and used for further in vitro experiments.
2.4 Uptake studies by flow cytometry and confocal microscopy
Uptake of soluble and PLGA-PEI microparticle loaded CpG and siRNA by BMDCs was assessed by flow cytometry and confocal microcopy. For flow cytometry experiment, immature 6-day-old BMDCs were plated at 5×105 cells/ml/well in a 24 well plate and treated with various soluble or surface loaded samples having CpG-FITC and/or Cy-3 siRNA (each at 1 ug/ml concentration). After 2 and 24 hrs of treatment, the BMDCs were collected and washed with PBS and incubated first with CD16/CD32 Fc block (at 4°C for 10 minutes, eBioscience, San Diego, CA) and then with fluorescent anit-mouse CD11c-APC antibody for 30 min at 4°C. Finally, the BMDCs were washed twice with fluorescent activated cell sorting (FACS) buffer (PBS with 2% FBS) and analyzed using a BD Accuri flow cytometer. CD11c+ cells were gated to quantify the uptake of CpG-FITC and/or Cy3-siRNA by BMDCs. The percentage and median fluorescent intensity (MFI) of CD11c+ BMDCs up-taking CpG-FITC and/or Cy-3 siRNA were compared across different groups. For confocal microscopy, BMDCs (immature 6-day-old) were grown on poly-l-lysine coated coverslips (BD BiocoatTM) in a 24 well plate and treated with either CpG-FITC loaded or siRNA-Cy3 loaded (each at 1 ug/ml) PLGA-PEI microparticles for 24 hrs. Then, the coverslips were washed twice with PBS and fixed with 4% paraformaldehyde solution and imaged using a confocal microscope (Prairie Technologies, WI, USA).
2.5 Analysis of BMDC activation using flow cytometry
BMDCs activation was evaluated by analyzing surface expression of different co-stimulatory molecules like CD86, MHCII, CD80 and CD40. Six day old immature BMDCs were plated at a density of 5×105 cells/ml/well in a 24 well plate and treated with different samples (soluble or surface loaded onto PLGA-PEI microparticles) having CpG ODN 1826, IL10 siRNA, Poly (I:C) and pDNA (MCP3sFv20) each at various concentration (1–2.5 ug/ml) for 48 hrs. Thereafter, the BMDCs were washed with PBS and harvested and incubated with anti-mouse CD16/CD32 Fc block (at 4°C for 10 minutes) to prevent nonspecific binding of antibodies. The BMDCs were stained with fluorescent anti-mouse CD86, MHCII, CD80, CD4, CD11c antibodies for 30 minutes at 4°C and washed twice FACS buffer and analyzed for surface expression of the co-stimulatory molecules in a BD Accuri flow cytometer. The flow cytometer data was further analyzed with Flowjo software. Percentage of CD11c+ cells and their median fluorescent intensity (MFI) was reported for comparison of activation among treated and untreated groups.
2.6 Analysis of cytokine production by BMDCs using ELISA
Similar experiments as activation studies were set up to quantify cytokine in the culture medium by enzyme-linked immune sorbent assay (ELISA). Briefly, after 48 hours of incubation of BMDCs with different samples, the culture medium was collected and quantities of IL10, IL12p70, IL12p40 and TNF-alpha were measured using ELISA Ready-Set-Go! Kits (eBioscience, San Diego, CA).
2.7 Analysis of cytokine gene expression of BMDCs using RT-PCR
Following 48 hrs of incubation of BMDCs with different samples as described above, the BMDCs were washed thoroughly with PBS and total RNA was extracted using RNAeasy kit (Qiagen, USA). Complementary DNA (cDNA) was synthesized from total RNA using the SuperScript® III First-Strand Synthesis System (Invitrogen, Carlsbad, CA), and real time q-PCR was performed using2 SYBR® green qPCR mastermix (Qiagen, USA) on an Applied Biosystems® ViiA™ 7 Real-Time PCR System. Specific primers for mouse IL10, IL12p35, IL12p40, and mouse β-actin (SABiosciences, USA) were used. β-actin was used as reference gene. Relative target gene expression was calculated following 2(−ΔΔCT) method [31] using the formula: , where ΔΔCT = [CT (target gene) – CT (reference gene)] of treatment – [CT (target gene) – CT (reference gene)] of control and the CT (threshold cycles) is the PCR cycle at which first signal of reporter fluorescence above a baseline signal is detected.
2.8 Cytokine profile during allogenic MLR (mixed lymphocyte reaction) between BMDCs and CD4+ T cells
An allogenic MLR between activated BMDCs from Balb/C and CD4+ naïve T cells from C57BL/6 mice was set up to quantify IFN-γ (Th1 cytokine) and IL4 (Th2 cytokine) by ELISA. Briefly, 6 day old BMDCs differentiated from Balb/c mice were first activated with different samples for 48 hrs and then cultured in a 96 well plate at a 1:2 ratio with 2×105 CD4+ naïve T cells isolated from the spleen of C57BL/6 mice using CD4 MicroBeads with MACS columns and MACS separators (Miltenyi Bioetch, Germany) in 200 ul culture medium. After 96 hrs, the culture medium was analyzed for IFN-γ (Th1) and IL4 (Th2) cytokines using ELISA Ready-Set-Go! Kits (eBioscience, San Diego, CA).
2.9 Preparation of in-situ crosslinking hydrogel for immunization in vivo
In-situ crosslinkable hydrogels were prepared using the Michael type addition reaction between dextran vinyl-sulfone (DextranVS) and tetra-thiolated polyethylyneglycol (PEG-4-SH) as reported earlier by our group [32]. DextranVS with degree of substitution 2 (DS2) was synthesized by reacting dextran with vinyl sulfone and purified as reported earlier [32]. The degree of substitution was calculated from the number of vinylsulfone substituted per 100 anhydroglucosidic rings of dextran as determined by nuclear magnetic resonance (NMR) spectroscopy [32]. To prepare the hydrogel, 10 % w/v DextranVS (DS2) and 10% w/v PEG-4-SH were resuspended in 0.3 M triethanolammine (TEA) buffer (pH 7.8, 0.22 μm membrane filtered) separately in two different tubes and sonicated briefly. MIP-3α (50 ng, Peprotech, Rocky Hill, NJ), an immature DC attracting chemokine, was mixed with PEG-4-SH solution and different surface loaded PLGA-PEI microparticle formulations were suspended in DextranVS solution in tubes that were then kept on ice. Lastly, DextranVS and PEG-4-SH solutions were mixed and loaded in a 271/2 G syringe immediately and injected in mice.
2.10 Immunization in an A20 B cell lymphoma mouse model
To evaluate the efficacy of different surface loaded PLGA-PEI microparticle formulations for immune protection against lethal A20 tumor challenge, Balb/c mice (n=10–20 per group) were immunized 3 times with various formulations at 2 week intervals as reported by the Kwak lab [33] and our group earlier [25, 29]. In situ crosslinking hydrogel containing MIP-3α and various formulations were injected intramuscularly (quadriceps and anterior tibialis muscles) with an equivalent dose of 100 μg of pDNA (MCP3sFv20 encoding), 50 μg CpG ODN 1826, 50 ug IL10 siRNA and 50 ug Poly (I:C). Naked pDNA encoding MCP3sFv20 (100 ug), and TEA buffer and PBS (negative controls) were comparative controls. Following 2 weeks of the final booster dose, the mice were challenged with 2×105 (lethal dose) A20 murine B-cell lymphoma cells intraperitoneally (IP). Tumor development and mice survival was followed till 80 days after tumor challenge. Median survival and Kaplan-Meier curves were generated for comparative analysis of mice survival and a log rank p test was done for statistical analysis using GraphPad Prism 6 software.
2.11 Statistical analysis
An unpaired, two-tailed T test was used to compare between pairs of groups to establish significant difference using GraphPad Prism 6 software. P < 0.05 (denoted *) and P<0.01 (denoted **) were considered to be significant.
3. Results
The schematic shown in Fig. 1 illustrates the concept of delivering multiple nucleic acid based immunomodulatory molecules to DCs, thereby modifying their cytokine milieu and switching the immunological balance robustly towards a Th1 phenotype. A cationic PLGA microparticle-based delivery system was fabricated by covalent conjugation of surface available acid groups of PLGA with amine groups of a branched cationic polymer, PEI using NHS/EDC chemistry (Fig. 1A). Various nucleic acid based anionic immunomodulatory molecules e.g. CpG ODN 1826, IL10 siRNA, poly (I:C), and pDNA based antigen can be loaded on the surface on the cationic PLGA-PEI microparticles via electrostatic interaction (Fig. 1B). We hypothesized, that when CpG and IL10 siRNA are simultaneously delivered to DCs, CpG induced IL10 production from DCs will be minimized due to simultaneous silencing of IL10 production resulting in an overall increase in the Th1/Th2 cytokine balance (Fig. 1D), which would enhance efficacy of cancer vaccines.
3.1 Preparation and characterization of cationic PLGA-PEI microparticles and optimization of surface loading of CpG, siRNA and pDNA
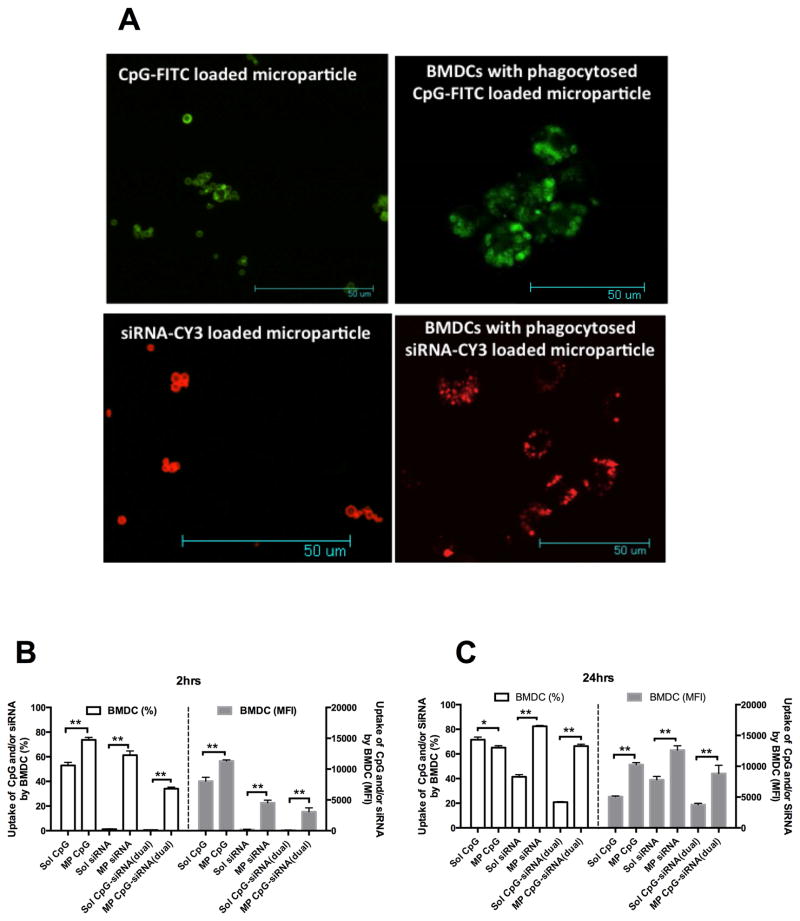
PLGA microparticles with a slightly negative zeta potential (−7 mV) were prepared by a water-oil-water emulsion method using acid capped PLGA polymer (RG502H resomer from Evonik, Germany). Covalent conjugation of branched PEI (MW 70,000) to the surface available acid group of PLGA microparticles using EDC/NHS chemistry resulted in cationic PLGA-PEI microparticles with an average diameter of 1.18 ± 0.28 μm and an average zeta potential of 34.13±3.85 mV (see Table-1). These cationic microparticles were used for surface loading of CpG, IL10 siRNA, Poly (I:C) and pDNA antigen individually, dual or triple components together using a phosphate buffer at pH-6 showing best loading efficiency. With increasing wt % of nucleic acids to PLGA-PEI microparticles, a decrease in loading efficiency was observed. Up to 1.2% of total nucleic acid feeding ratio to PLGA-PEI microparticles, 100% loading efficiency for each type of nucleic acids was observed. Also, loading efficiency did not change when dual (CpG+IL10 siRNA or CpG+Poly (I:C)) or triple (CpG + IL10 siRNA+pDNAor CpG+Poly (I:C)+pDNA) nucleic acids were surface loaded onto PLGA-PEI microparticles at 1.2 wt% total nucleic acid ratio (see Table 1). From confocal microscopic images (Fig. 2A, left panel), it was further confirmed that the fluorescent CpG and siRNA could be efficiently surface loaded individually onto PLGA-PEI microparticles.
Table 1.
Characterization of PLGA-PEI microparticles
| Size | 1.18 ± 0.28 μm | |
| Zeta potential | 34.13 ± 3.85 mV | |
| Single loading (1.2 wt% nucleic acid to particle ratio) | CpG/IL10-siRNA/Poly(I:C)/pDNA | 12 ug/mg of microparticles (100% loading) |
| Dual loading (1.2 wt% nucleic acid to particle) | CpG + IL10-siRNA/CpG+Poly(I:C) | 6+6 ug/mg of microparticles (100% loading) |
| Triple loading (1.2 wt% nucleic acid to particle ratio) | CpG +IL10-siRNA+pDNA/ CpG+Poly(I:C)+pDNA | 4+4+4 ug/mg of microparticles (100% loading) |
Fig. 2.
A) Confocal micrographs at the left panel showing surface loading of CpG-FITC/siRNA-Cy3 on PLGA microparticles and at the right panel showing uptake of CpG-FITC/siRNA-Cy3 surface loaded PLGA microparticles by BMDC. B) Uptake of CpG-FITC and/or siRNA-Cy3 by CD11c+ BMDC at 2 hrs, and C) 24 hrs intervals as determined by flow cytometry; *P<0.05 and **P<0.01 when compared between the groups.
3.2 Uptake of CpG and siRNA surface loaded PLGA-PEI microparticles by BMDCs
Fig. 2B and 2C show the uptake of fluorescent CpG and siRNA loaded microparticles by BMDCs at 2 and 24 hr intervals respectively as determined by flow cytometry. After 2 hrs, BMDCs show a significantly higher uptake for CpG, siRNA and dual (CpG and siRNA on the same particles) loaded MP compared to their soluble counterpart both in terms of percentage and median fluorescent intensity (MFI) of BMDCs. A similar trend in uptake was observed for the 24 hr interval except that the percentage of BMDCs internalizing CpG loaded MP (described as MP CpG in the graph) was slightly less than the soluble CpG (described as sol CpG). However, MFI of BMDCs for MP CpG was significantly higher than soluble CpG indicating a higher amount of uptake per cell. Interestingly, when soluble CpG is mixed with soluble siRNA, the uptake efficiency decreases considerably compared to CpG alone. From confocal micrographs (Fig 2A, right panel), fluorescent CpG and siRNA loaded MP can be seen within the BMDCs confirming their efficient uptake.
3.3 Activation and maturation studies of BMDCs
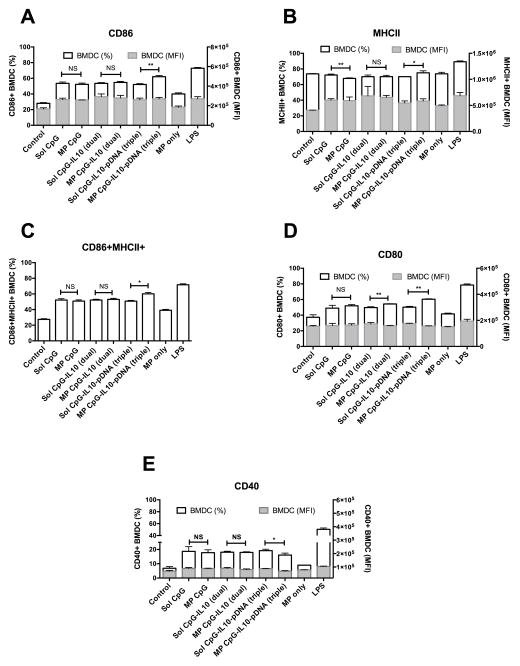
Activation and maturation of CD11c+ gated BMDCs following treatment of different formulations were evaluated by surface expression of different co-stimulatory molecules using flow cytometry. As shown in Fig. 3A, D and E, various co-stimulatory surface markers such as CD86, CD80, and CD40 were expressed at a significantly higher percentage on BMDCs for all the microparticle formulation treated groups as compared to untreated (Control) and only microparticle (MP only) treated BMDCs. However, there was no significant difference between soluble and microparticle formulated CpG and IL10 siRNA either in their individual or dual loaded forms (except for CpG and IL10 siRNA dual loaded microparticle treated BMDCs showing significantly higher expression of CD80 compared to the dual soluble group). When these molecules were triple loaded with pDNA encoding MCP3sv20 on the microparticles, a significantly higher expression of CD86 (P<0.01) and CD80 (P<0.01) was observed compared to the triple soluble group. Surface expression of MHCII, an antigen-presenting molecule, was found to be higher for all the groups without any major difference in terms of percentage of BMDCs among different formulation treated and untreated groups. However, a significant increase in MFI of BMDCs was observed for all the formulation containing soluble or microparticle loaded CpG as compared to untreated or only microparticle treated groups. Also, all the formulation treated groups resulted in significantly high dual CD86+MHCII+ BMDCs as compared to untreated and only microparticle treated groups. Notably, the triple microparticle formulated group resulted in a significantly (P<0.05) higher percentage of CD86+MHCII+ BMDCs compared to the triple soluble groups. The positive control LPS showed a high level of expression for CD86, 80, 40 and MHCII in BMDCs.
Fig. 3.
Surface expression of activation and maturation markers in primary BMDCs. Bar graph showing percentage and median fluorescent intensity (MFI) of CD11c+ BMDCs expressing A) CD86, B) MHCII, C) both CD6 and MHCII, D) CD80 and E) CD40 following the treatment with different formulations of CpG, IL10-siRNA and pDNA each at 1 ug/ml concentration for 48hrs; *P<0.05 and **P<0.01 when compared between the groups.
We also evaluated surface expression of co-stimulatory markers of BMDCs following treatment of microparticle formulations comprising of CpG and Poly (I:C). From our in vitro experiments, it was evident that although soluble Poly (I:C) at 1 ug/ml concentration activated BMDCs significantly over untreated cells, secretion of IL12p70 was not significantly higher than untreated BMDCs (data not shown). However, at 2.5 ug/ml concentration, significantly higher secretion of IL12p70 (P<0.01) was observed as compared to the untreated BMDCs (see Fig. 5). Hence, we report BMDC activation studies of Poly (I:C) and CpG at 2.5 ug/ml concentration (1:1 CpG to poly(I:C) dose is maintained as in the case for siRNA). As shown in supplementary Fig. 1, soluble CpG, soluble Poly (I:C), and soluble CpG-Poly (I:C) dual at 2.5 ug/ml concentration showed a significantly higher percentage of CD86+ and CD86+MCHII+ BMDCs as compared to the untreated one. However, MP CpG and MP CpG-Poly (I:C) dual did not show significant differences in the percentage of CD86+ and CD86+MCHII+ BMDCs as compared to their soluble counter groups. MP- Poly (I:C) showed a significantly higher percentage of CD86+ and CD86+MCHII+ BMDCs as compared to soluble Poly (I:C). We did not include a microparticle loaded CpG-Poly (I:C)-pDNA triple group due to microparticle loading limitations and dose constraint in vitro. To achieve the desired immunostimulatory concentration of each of CpG, Poly (I:C), and pDNA, we had to use a very high dose of microparticle, i.e. more than 500 ug of microparticle/500,000 BMDCs per well of a 24 well plate.
Fig. 5.
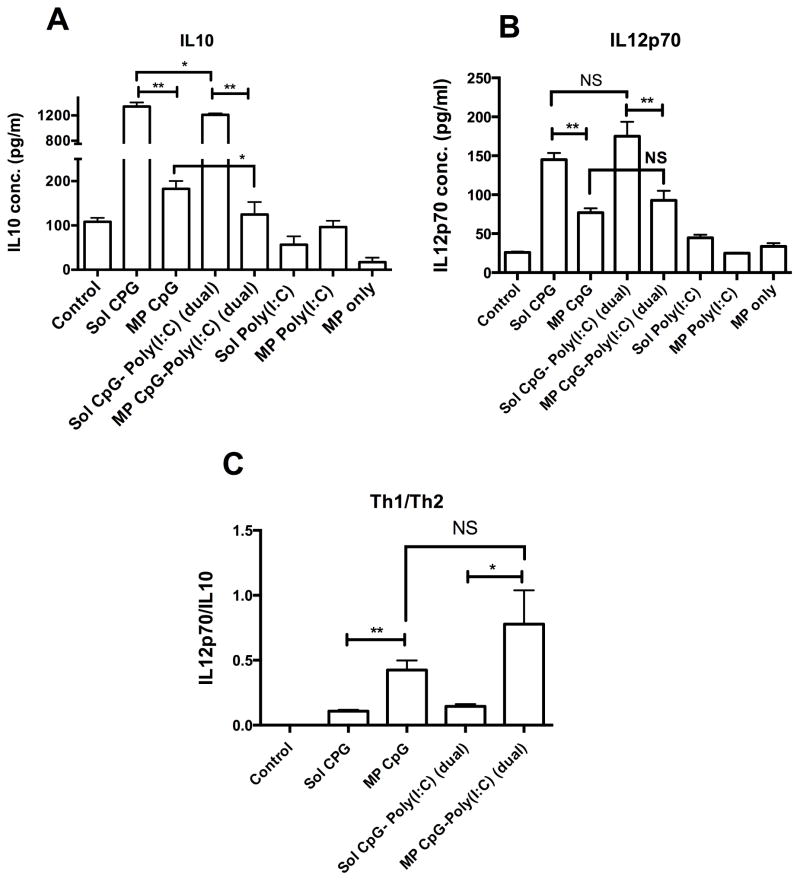
Cytokine profile of BMDCs. Bar graph showing concentration of A) IL10, and B) IL12p70 in the culture supernatant of BMDCs after treatment with various formulations of CpG, and Poly (I:C) each at 2.5 ug/ml concentration for 48hrs. C) Bar graph showing ratio of Th1 cytokine, IL12p70 and Th2 cytokine IL10; *P<0.05 and **P<0.01 when compared between the groups.
3.4 Cytokine secretion profile of BMDCs
Various Th1 (IL12p70, IL12p40 and TNF-α) and Th2 (IL10) specific cytokines in the BMDC culture supernatant were measured using ELISA after 48 hrs of treatment of different formulations. As shown in Fig. 4A, BMDCs treated with soluble CpG secreted high amounts of IL10. However, when the CpG was loaded onto microparticles, the IL10 amount was significantly (P<0.01) low. Moreover, when CpG was delivered along with IL10 siRNA on the same microparticle (sample name: MP CpG-IL10 (dual)), the IL10 secretion was significantly (P<0.01) decreased further to a minimal level. Also, when a pDNA encoding A20 idiotype antigen was delivered along with CpG and IL10 siRNA on microparticles (sample name: MP CpG-IL10-pDNA), IL10 secretion was minimal similar to the MP CpG-IL10 group. The soluble form of CpG, IL10-siRNA, and pDNA when delivered together did show, however, a significantly (P<0.01) higher secretion of IL10 as compared to their microparticle formulations. Untreated control BMDCs and only microparticle treated BMDCs secreted a very low amount of IL10, underscoring the fact that CpG itself induces IL10 production.
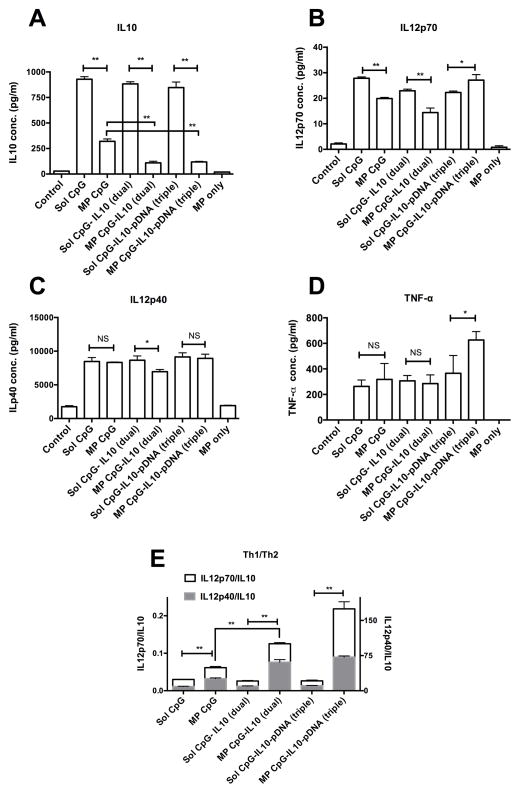
Fig. 4.
Cytokine profile of BMDCs. Bar graph showing concentration of A) IL10, B) IL12p70, C) IL12p40, D) TNF-α in the culture supernatant of BMDCs after treatment with various formulations of CpG, IL10, and pDNA each at 1 ug/ml concentration for 48hrs. E) Bar graph showing ratio of Th1 cytokines, IL12p70 and IL12p40 and Th2 cytokine IL10; *P<0.05 and **P<0.01 when compared between the groups.
IL12p70 and IL12p40 secretion profiles of BMDCs are shown in Fig. 4B and C. BMDCs treated with MP CpG showed significantly (P<0.01) lower IL12p70 secretion compared with the sol CpG. A similar trend was observed for MP CpG-IL10 (dual) loaded groups. However, MP CpG-IL10-pDNA (triple) group showed significantly (P<0.05) higher secretion of IL12p70 compared with the soluble triple group. IL12p40 secretion was relatively higher across all the formulation treated groups except for the MP CpG-IL10 (dual) group where a slight but significantly low (P<0.05) secretion of IL12p40 was observed. TNF-α secretion by BMDCs was also high for all the formulation treated groups with the MP CpG-IL10-pDNA showing the highest amount of secreted TNF-α among all the groups.
Fig. 4E shows Th1/Th2 cytokine balance for BMDCs treated with various formulations. The ratios of Th1/Th2 cytokine i.e. IL12p70/IL10 and IL12p40/IL10 significantly increased when CpG was delivered on microparticles along with IL10 siRNA and pDNA antigen as dual or triple components surface loaded on the same microparticles. However, the ratios were low and similar for all the groups treated with their soluble counterpart including CpG, IL10 siRNA and pDNA antigen.
We also assessed cytokine profile of BMDCs treated with various formulations containing CpG and Poly(I:C) each at 2.5 ug/ml concentration. As shown in Fig. 5A, sol CpG treated BMDCs secreted a high level of IL10 but its microparticle formulation (i.e. MP CpG) significantly decreased the secretion of IL10. However, IL10 secretion with MP CpG was still significantly higher than untreated BMDCs. IL10 secretion due to soluble Poly(I:C) was similar to the control group. There were only minimal differences (though statistically, p < 0.05) in IL10 levels between sol CpG alone, and sol CpG- Poly(I:C) dual groups and similarly between MP CpG and MP CpG-Poly(I:C) groups, indicating that Poly(I:C) does not affect IL10 levels as much as co-delivery of IL10-silencing siRNA. On the other hand, both sol CpG and Poly(I:C) at 2.5 ug/ml concentration significantly increased the IL12p70 level as compared to the control (Fig. 5B). Moreover, the sol CpG- Poly(I:C) dual group showed slight increase in IL12p70 level over the control group. However, all the microparticle formulated groups had significantly decreased IL12p70 levels as compared to their soluble counter groups. However, when the ratio of IL12p70/IL10 were compared (Fig. 5C), MP CpG and MP CpG- Poly(I:C) dual groups showed significant increases in the pro vs. anti-inflammatory cytokine balance as compared to their soluble counter groups. But no significant difference in the IL12p70/IL10 ratio was observed between MP CpG and MP CpG- Poly(I:C) groups.
3.5 Cytokine gene expression in BMDCs
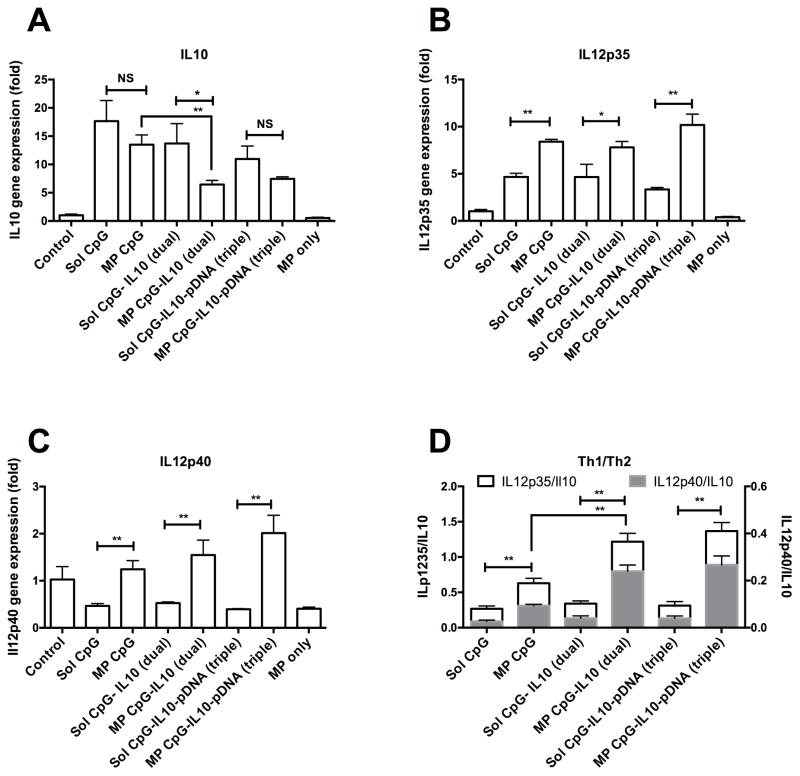
Fig. 6 shows IL10, IL12p35 and IL12p40 gene expression in BMDCs 48hrs following treatment of different formulations. As shown in Fig. 6A, soluble CpG triggered IL10 gene expression in BMDCs, which was about 18 fold higher as compared to the untreated control BMDCs. When CpG was delivered on microparticles, IL10 gene expression did not vary much compared with soluble CpG. However, the MP CpG-IL10 (dual) group, which delivered microparticle loaded with both CpG and IL10 siRNA together, down regulated IL10 gene expression by about 50% (P <0.01) compared to the MP CpG group. The MP CpG-IL10-pDNA group also had lower IL10 gene expression as compared to its corresponding soluble group, i.e. Sol CpG-IL10-pDNA (triple) (P=0.056) and MP CpG group (P<0.01). Microparticle only treated group did not show any activation in IL10 gene expression.
Fig. 6.
Cytokine gene expression in BMDCs. Bar graph showing A) IL10, B) IL12p35 and C) IL12p40 gene expression in BMDCs treated with different samples for 48hrs. D) Bar graph showing ratio of Th1 cytokine genes, IL12p70 and IL12p40 and Th2 cytokine gene IL10; *P<0.05 and **P<0.01 when compared between the groups.
From Fig. 6B, it can be observed that soluble CpG up-regulated IL12p35 gene expression in BMDCs, which was significantly (P<0.01) increased further with its delivery on microparticle. Similar differences in IL12p35 expression between soluble and microparticle were observed for dual and triple combination of CpG with IL10 and pDNA antigen. However, no significant difference was observed between MP-CPG and MP-CpG-IL10 (dual) groups indicating that co-delivery of IL-10 silencing siRNA does not alter the immune-stimulatory effect of CpG delivery. A similar trend was observed for IL12p40 gene expression (Fig. 6 C) in BMDCs. When ratios of IL12p35/IL10 and ILp40/IL10 were compared (Fig. 6D), all the microparticle formulated groups resulted in a significantly (P<0.01) higher ratio as compared to their soluble counterpart. Most importantly, the Th1/Th2 cytokine ratio was significantly (P<0.01) enhanced when IL10 siRNA was included along with CpG in microparticle formulation and was unaffected even after inclusion of pDNA antigen in the formulation.
3.6 Cytokine profile of allogenic MLR
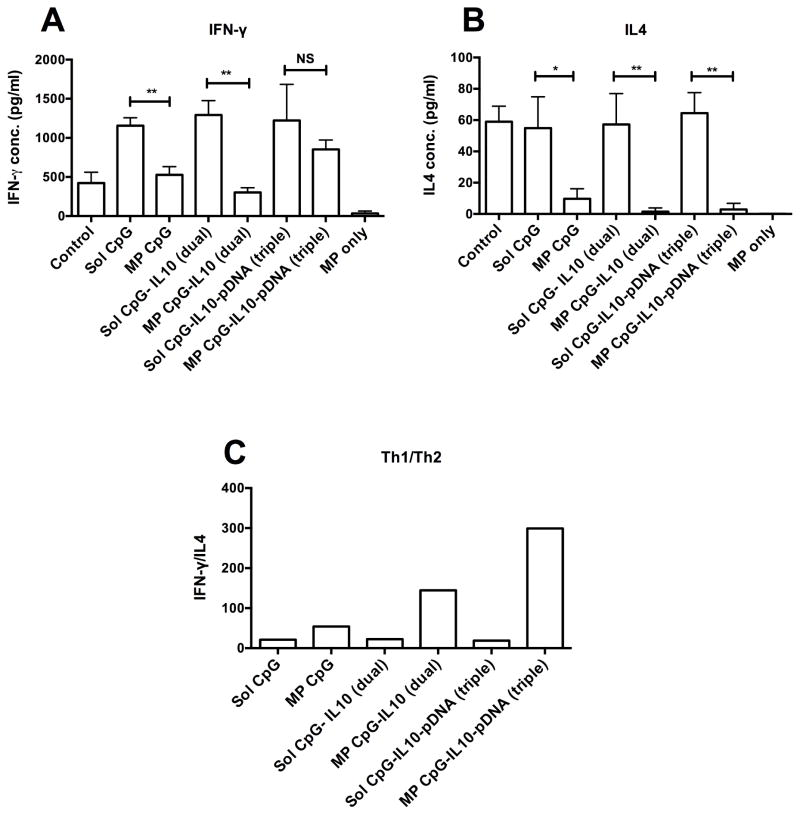
An allogenic MLR between BMDCs (Balb/C origin) and CD4+ T cells (C57BL/6) was set up to evaluate the immunogenic potential of BMDCs activated with various formulations to stimulate production of IFN-γ and IL4 cytokines by activated CD4+ T cells. As shown in Fig. 7A and B, sol CpG treated groups caused significant increases in IFN-γ production as compared to the untreated control group. Simultaneously, both the sol CpG treated and control groups showed higher IL4 secretion. However, MP loaded CpG and/or IL10 siRNA showed significantly low levels of both IFN-γ and IL4 as compared to their soluble counterparts. In contrast, the MP CpG-IL10-pDNA (triple) group had a comparable IFN-γ level to sol CpG containing groups but showed a very low IL4 level. When ratios of means of IFN-γ and IL4 (indicator of Th1/Th2 balance, Fig. 7C) were compared, all of the surface loaded microparticle groups, i.e. MP CpG, MP CpG-IL10 (dual) and MP-CpG-IL10-pDNA (triple), showed higher ratios as compared to their corresponding soluble single, dual or triple groups. Moreover, MP-CpG-IL10 (dual) had a higher ratio than the MP CpG group. The ratio is further markedly enhanced for the MP-CpG-IL10-pDNA (triple) group, which showed the highest IFN-γ/IL4 ratios among all groups.
Fig. 7.
Cytokine profile of allogenic MLR. Bar Graph showing A) IFN-γ and B) IL4 secretion in culture supernatant after 96 hrs of an allogenic MLR between CD4+ T cells (C57BL/6 origin) and BMDCs (Balb/C origin) treated with different samples for 48 hrs. C) Bar graph showing ratio of Th1 cytokine, IFN-γ and Th2 cytokine, IL4; *P<0.05 and **P<0.01 when compared between the groups.
3.7 Immune protection against A20 B lymphoma
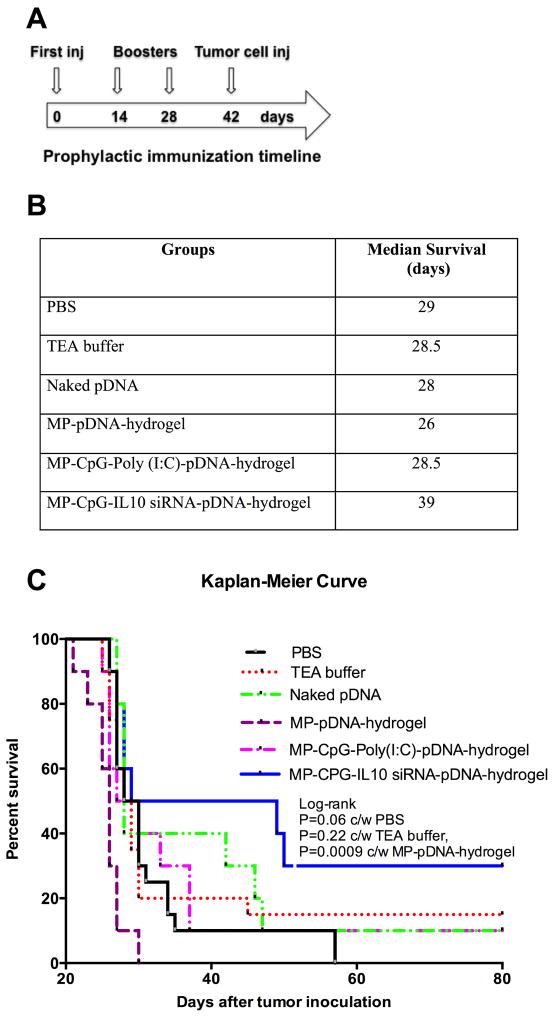
Fig. 8 shows median survival and Kaplan-Meier curves for Balb/C mice prophylactically immunized with various formulations and then challenged with a lethal dose of A20 B-cell lymphoma. Immunization and tumor challenge schedules are shown in Fig 8a. As indicated in Fig. 8B, median survival of controls (PBS/TEA buffer), naked pDNA, and various formulation treated groups were within a range of 26–29 days except for MP-CpG-IL10 siRNA-pDNA-hydrogel group where median survival was extended up to 39 days. Similarly, from the Kaplan-Meier curves (Fig. 8C), it is evident that the MP-CpG-IL10 siRNA-pDNA-hydrogel group performed better than all other groups (P=0.06 c/w PBS, P=0.22 c/w TEA buffer and P=0.0009 c/w MP-pDNA-hydrogel group), including the MP-CpG-poly(I:C)-pDNA-hydrogel group in extending survival of mice against a lethal dose of A20 B lymphoma.
Fig. 8.
Protection against A20 B-Lymphoma. A) Time line for prophylactic immunization and tumor inoculation, B) Median survival table, and C) Kaplan-Meier survival curve for immunized Balb/C mice with various formulations after lethal challenge of A20 B-lymphoma cells (2 ×105 cells/mice IP).
4. Discussion
In recent years, cancer immunotherapy has gained much interest as monotherapy or as combination therapy due to its long-term independent and complementary clinical benefit to other standard modes of cancer treatments. A promising approach is to manipulate DCs to help improve efficacy of cancer vaccines [5]. In this study, our goal was to inhibit CpG-induced production of IL10 by co-delivering IL10 siRNA to DCs and thereby switching their IL12/IL10 (Th1/Th2) cytokine balance. Since both CpG and IL10 siRNA are highly anionic, we synthesized cationic PLGA-PEI microparticles, to enable efficient surface loading of CpG, IL10 siRNA and pDNA antigens, either individually or as dual or triple-loaded components. On the other hand, intrigued by reported synergistic effects of Poly (I:C) and CpG on dendritic cells [13–15], we also included Poly (I:C) in microparticle loaded CpG formulations to see their effect on switching IL12/IL10 cytokine balance and adjuvant effect on pDNA vaccine against B cell lymphoma. 100% loading efficiency was achieved for CpG, IL10 siRNA, Poly (I:C), and pDNA antigen individually or in their dual or triple combinations at a total feed ratio of 1.2 wt% nucleic acids to PLGA-PEI particles. These microparticles were able to rapidly deliver both CpG and IL10 siRNA into BMDCs with significantly higher efficacy than their soluble forms. Expression of various activation and maturation markers such as CD86, CD40, MHCII were up-regulated on BMDCs for all the microparticle formulations having surface adsorbed CpG, and the degree of activation and maturation were comparable to their soluble counterparts.
BMDCs activated by soluble CpG secrete high amounts of Th1-promoting cytokines such as IL12p70, IL12p40, and TNF-α. However, they also secrete high amounts of the Th2-promoting cytokine IL10 [11,12] thereby inhibiting their Th1/Th2 polarization. In cancer immunotherapy, Th1 cell-driven CTL generation that could lead to tumor cell killing is highly desirable [6, 7]. Thus strategies to reduce CpG-induced IL-10 production and enhancing the Th1/Th2 polarization could be beneficial. Our data indicates that when IL10 siRNA and CpG were co-delivered on PLGA-PEI microparticles to BMDCs, IL-10 production was reduced by almost 8 fold compared to cells exposed to soluble CpG. Although IL12p70 and IL12p40 secretions were also significantly decreased as compared to the soluble CpG+ soluble IL10 siRNA group, the overall IL-12/1L-10 polarization significantly improved. When the antigen was co-delivered using a triple-loaded combination of CpG, IL10 siRNA and pDNA antigen (encoding the idiotype protein of A20 B cell lymphoma) on PLGA-PEI particles, IL12p70 (a bioactive heterodimer form of IL12p40 and IL12p35) and IL12p40 secretion increased while IL10 secretion remained very low resulting in a significant improvement of the IL12/IL10 ratio i.e. Th1/Th2 polarization. The corresponding soluble groups had low IL12/IL10 ratios. Also, it is very interesting to see that surface loading of CpG on microparticles alone skew the IL12/IL10 balance significantly over soluble CpG by inhibiting IL10 secretion by BMDCs.
Further, from the cytokine gene expression profile, we saw that CpG induced IL10 gene expression was significantly down regulated when CpG and IL10 siRNA were co-delivered on microparticles. Surprisingly, IL12p35 and IL12p40 gene expressions were significantly enhanced for microparticle formulations of CpG, CpG+IL10 and CpG+IL10+pDNA over their corresponding soluble groups, in contrast to their protein counterparts like IL12p70 or IL12p40 levels. This lack of correlation would need further investigation. It would be interesting to see the kinetics of cytokine gene expression and protein production by these microparticle formulations in future studies. When we compared cytokine gene expression ratios between IL12p70 or IL12p40 (Th1) and IL10 (Th2), we observed that the Th1/Th2 cytokine balances were significantly enhanced due to down regulation of CpG induced IL10 gene in IL10 siRNA containing dual and triple loaded microparticle groups. This confirmed that Th1/Th2 cytokine balance in dendritic cells could be efficiently enhanced both at cytokine gene and protein levels by microparticle mediated delivery of CpG and IL10 siRNA to dendritic cells.
In a parallel experiment, cytokine profile of the CpG and Poly (I:C) combination was evaluated. In the in vitro experiments where a combination of CpG and IL10 siRNA was used, we used a concentration of 1 ug/ml of each, and at this concentration both CpG and IL10 siRNA were active in modulating the balance of IL10 and IL12p70. But, at 1 ug/ml concentration, the IL12p70 level due to Poly (I:C) activation of BMDCs was similar to the control group. In order to obtain a significant increase in IL12p70 secretion by BMDCs over the control group, a concentration of 2.5 ug/ml of Poly (I:C) was used. Also, to match 1:1 (wt/wt) in vivo dose ratio of CpG and Poly (I:C), we used CpG at 2.5 ug/ml as well for in vitro experiment. As reported in literature [13–15], we also observed an increase in the level of IL12p70 secretion due to the combined effect of CpG and Poly (I:C) in their soluble and microparticulate forms. However, the increase in IL12p70 levels due to soluble or MP loaded CpG and Poly (I:C) combination were not significantly higher than the soluble or MP loaded CpG only. On the other hand, Poly (I:C) did not show any effect on IL10 secretion, and the addition of Poly (I:C) to CpG did not have any prominent effect on switching CpG induced IL10 level in BMDCs. When IL12p70/IL10 ratios were compared between MP CpG and MP CpG+Poly (I:C) dual groups, only a nonsignificant increase was observed. In contrast, the IL12p70/IL10 ratio was increased significantly (P<0.01) when CpG was combined with IL10 siRNA in their surface loaded microparticle formulation. Thus, our in vitro results confirmed that co delivery of IL10 siRNA with CpG on microparticles could be a better strategy to switch IL12p70 or IL12p40/IL10 (Th1/Th2 cytokine) balance than co-delivery of TLR-3 agonists with CpG on microparticles to dendritic cells.
Further, the cytokine profile of allogenic MLR between microparticle-treated BMDCs and CD4+ T cells indicated that IL4 secretion by CD4+ T cells was almost completely inhibited when IL10-siRNA was co delivered with CpG and/or pDNA antigen on microparticle to BMDCs. However, these groups also showed a significant decrease in IFN-γ secretion. Although the ratio of IFN-γ/IL4 (Th1/Th2) was markedly improved in the microparticle-formulated groups as compared to their corresponding soluble groups, overall decrease in IFN-γ secretion might not provide adequate CTL generation. An increased IFN-γ response coupled with decreased IL4 would be an ideal cytokine profile for a maximum Th1 response. With the current PLGA-PEI microparticle system, co-delivery of surface loaded CpG and IL10 siRNA to DCs seems to primarily benefit IL10 inhibition. A more effective system needs to maximize the Th1/Th2 balance by also increasing IL12p70 and IFN-γ production while silencing IL10.
Lastly, we wanted to see whether co-delivery of CpG and IL10 siRNA or poly(I:C) along with antigens on PLGA-PEI microparticles could significantly improve pDNA vaccine efficacy in vivo. We chose a prophylactic pDNA-vaccine model for A20 murine B cell-lymphoma. We have previously reported that a pDNA-vaccine encoding for the idiotype protein could provide moderate efficacy as a monotherapy against lethal challenge with A20 tumor cells [33]. We have also reported that a hydrogel-based, injectable synthetic immune-priming center (sIPC) that creates inflammatory/infection-mimicking microenvironment at the site of immunization and slowly releases the chemokine MIP-3α, can attract and efficiently deliver pathogen-mimicking microparticles to numerous immature DCs [32]. In the current study, triple loaded microparticles (CpG+IL10 siRNA+idiotype pDNA) delivered in sIPCs, outperformed other groups by - extending the median survival of mice to 39 days against lethal tumor challenge as compared to PBS/TEA buffer, naked pDNA and other formulation groups where median survival was 26–29 days. Notably, 30% mice in the MP-CpG-IL10 siRNA-hydrogel group were tumor free and alive even 80 days after lethal tumor challenge as compared to only 10–15% tumor free mice in naked pDNA and MP-CpG-Poly (I:C)-pDNA-hydrogel groups.. This result indicates that PLGA-PEI microparticles surface loaded with CpG and IL10 siRNA were able to enhance immune protection against B cell lymphoma better than naked pDNA antigen or CpG +Poly (I:C)+pDNA antigen loaded microparticles groups. Although both CpG+IL10 siRNA and CpG+poly(I:C) improves Th1/Th2 cytokine ratios in BMDCs, IL10-silencing appears to be more effective strategy to boost protective anti-tumor immunity in-vivo. Although, CpG ODN and IL10 siRNA loaded microparticles showed moderate immune protection and increase in median survival against B lymphoma, admittedly the percentage of protected mice over the long term was still low. This could be due to the immunosuppressive nature of the tumor microenvironment with high levels of inhibitory immunomodulators e.g. IL10, transforming growth factor - β (TGF-β), IDO etc. along with T cell inhibitors like programmed cell death 1 ligand 1 (PDL1) [34]. Future studies in immunotherapy of B cell lymphoma need to address both enhancement of systemic immune response through peripheral immunization while at the same time modulation of the tumor microenvironment to reduce its immunosuppressive effects.
5. Conclusion
We have shown here that CpG induced IL10 secretion in DCs can be inhibited by co-delivery of IL10 siRNA on cationic PLGA-PEI microparticles. This approach, especially when combined with pDNA antigens delivered on the same particles, significantly enhances the Th1/Th2 cytokine balance of DCs. Co-delivery of CpG and IL10 siRNA on microparticles encapsulated in an in-situ crosslinking immune-priming center, showed better immune protection of an idiotype DNA vaccine in a mouse model of B cell lymphoma compared to microparticle formulations having no TLR agonists or those having CpG+Poly (I:C)+pDNA antigen. Thus, siRNA-mediated silencing of CpG induced IL-10 production could be an attractive strategy to improve anti-tumor immune response of TLR9 driven immunotherapies.
Supplementary Material
Bar graph showing percentage of CD11c+ BMDCs expressing A) CD86, B) both CD6 and MHCII following treatment with various formulations of CpG and Poly (I:C) each at 2.5 ug/ml concentration for 48hrs; *P<0.05 and **P<0.01 when compared between the groups.
Acknowledgments
We acknowledge financial support from the Cancer Prevention and Research Institute of Texas (CPRIT) through Grant number RP100735 (KR and LWK) and support from the National Science Foundation Graduate Research Fellowship Program to JAL. The confocal/2P microscope described in this manuscript was funded by award number S10RR027950 from the National Center For Research Resources. The content of this manuscript are solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973;137:1142–62. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reis e Sousa C. Activation of dendritic cells: translating innate into adaptive immunity. Curr Opin Immunol. 2004;16:21–5. doi: 10.1016/j.coi.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Steinman RM, Hemmi H. Dendritic cells: translating innate to adaptive immunity. Curr Top Microbiol Immunol. 2006;311:17–58. doi: 10.1007/3-540-32636-7_2. [DOI] [PubMed] [Google Scholar]
- 4.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–45. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 5.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. NatRev Cancer. 2012;12:265–77. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang H, Hao SG, Li F, Ye ZM, Yang JB, Xiang J. CD4(+) Th1 cells promote CD8(+) Tc1 cell survival, memory response, tumor localization and therapy by targeted delivery of interleukin 2 via acquired pMHC I complexes. Immunology. 2007;120:148–59. doi: 10.1111/j.1365-2567.2006.02452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knutson KL, Disis ML. Tumor antigen-specific T helper cells in cancer immunity and immunotherapy. Cancer Immunol Immunother. 2005;54:721–8. doi: 10.1007/s00262-004-0653-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 9.Krieg AM. Development of TLR9 agonists for cancer therapy. J Clin Invest. 2007;117:1184–94. doi: 10.1172/JCI31414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bode C, Zhao G, Steinhagen F, Kinjo T, Klinman DM. Cpg DNA as a Vaccine Adjuvant. Expert Rev Vaccines. 2011;10:499–511. doi: 10.1586/erv.10.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salem ML, Kadima AN, Cole DJ, Gillanders WE. Defining the antigen-specific T-cell response to vaccination and poly(I:C)/TLR3 signaling: evidence of enhanced primary and memory CD8 T-cell responses and antitumor immunity. JImmunother. 2005;28:220–8. doi: 10.1097/01.cji.0000156828.75196.0d. [DOI] [PubMed] [Google Scholar]
- 12.Currie AJ, van der Most RG, Broomfield SA, Prosser AC, Tovey MG, Robinson BW. Targeting the effector site with IFN-alphabeta-inducing TLR ligands reactivates tumor-resident CD8 T cell responses to eradicate established solid tumors. J Immunol. 2008;180:1535–44. doi: 10.4049/jimmunol.180.3.1535. [DOI] [PubMed] [Google Scholar]
- 13.Krummen M, Balkow S, Shen LM, Heinz S, Loquai C, Probst HC, et al. Release of IL-12 by dendritic cells activated by TLR ligation is dependent on MyD88 signaling, whereas TRIF signaling is indispensable for TLR synergy. J Leukoc Biol. 2010;88:189–99. doi: 10.1189/jlb.0408228. [DOI] [PubMed] [Google Scholar]
- 14.Zheng RX, Cohen PA, Paustian CA, Johnson TD, Lee WT, Shu SY, et al. Paired toll-like receptor agonists enhance vaccine therapy through induction of interleukin-12. Cancer Res. 2008;68:4045–9. doi: 10.1158/0008-5472.CAN-07-6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat Immunol. 2005;6:769–76. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pashenkov M, Goess G, Wagner C, Hormann M, Jandl T, Moser A, et al. Phase II trial of a Toll-like receptor 9-activating oligonucleotide in patients with metastatic melanoma. J Clin Oncol. 2006;24:5716–24. doi: 10.1200/JCO.2006.07.9129. [DOI] [PubMed] [Google Scholar]
- 17.Friedberg JW, Kim H, McCauley M, Hessel EM, Sims P, Fisher DC, et al. Combination immunotherapy with a CpG oligonucleotide (1018 ISS) and rituximab in patients with non-Hodgkin lymphoma: increased interferon-alpha/beta-inducible gene expression, without significant toxicity. Blood. 2005;105:489–95. doi: 10.1182/blood-2004-06-2156. [DOI] [PubMed] [Google Scholar]
- 18.Krummen M, Balkow S, Shen L, Heinz S, Loquai C, Probst HC, et al. Release of IL-12 by dendritic cells activated by TLR ligation is dependent on MyD88 signaling, whereas TRIF signaling is indispensable for TLR synergy. J Leukoc Biol. 2010;88:189–99. doi: 10.1189/jlb.0408228. [DOI] [PubMed] [Google Scholar]
- 19.Dearman RJ, Cumberbatch M, Maxwell G, Basketter DA, Kimber I. Toll-like receptor ligand activation of murine bone marrow-derived dendritic cells. Immunology. 2009;126:475–84. doi: 10.1111/j.1365-2567.2008.02922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corinti S, Albanesi C, la Sala A, Pastore S, Girolomoni G. Regulatory activity of autocrine IL-10 on dendritic cell functions. J Immunol. 2001;166:4312–8. doi: 10.4049/jimmunol.166.7.4312. [DOI] [PubMed] [Google Scholar]
- 21.Saraiva M, O’garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10:170–81. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 22.Asadullah K, Sterry W, Volk HD. Interleukin-10 therapy--review of a new approach. Pharmacol Rev. 2003;55:241–69. doi: 10.1124/pr.55.2.4. [DOI] [PubMed] [Google Scholar]
- 23.Liu G, Ng H, Akasaki Y, Yuan X, Ehtesham M, Yin D, et al. Small interference RNA modulation of IL-10 in human monocyte-derived dendritic cells enhances the Th1 response. Eur J Immunol. 2004;34:1680–7. doi: 10.1002/eji.200425081. [DOI] [PubMed] [Google Scholar]
- 24.Chhabra A, Chakraborty NG, Mukherji B. Silencing of endogenous IL-10 in human dendritic cells leads to the generation of an improved CTL response against human melanoma associated antigenic epitope, MART-1 27–35. Clin Immunol. 2008;126:251–9. doi: 10.1016/j.clim.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh A, Qin H, Fernandez I, Wei J, Lin J, Kwak LW, et al. An injectable synthetic immune-priming center mediates efficient T-cell class switching and T-helper 1 response against B cell lymphoma. J Control Release. 2011;155:184–92. doi: 10.1016/j.jconrel.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 26.Singh A, Nie H, Ghosn B, Qin H, Kwak LW, Roy K. Efficient modulation of T-cell response by dual-mode, single-carrier delivery of cytokine-targeted siRNA and DNA vaccine to antigen-presenting cells. Mol Ther. 2008;16:2011–21. doi: 10.1038/mt.2008.206. [DOI] [PubMed] [Google Scholar]
- 27.Qin H, Cha SC, Neelapu SS, Lou Y, Wei J, Liu YJ, et al. Vaccine site inflammation potentiates idiotype DNA vaccine-induced therapeutic T cell-, and not B cell-, dependent antilymphoma immunity. Blood. 2009;114:4142–9. doi: 10.1182/blood-2009-05-219683. [DOI] [PubMed] [Google Scholar]
- 28.Kasturi SP, Sachaphibulkij K, Roy K. Covalent conjugation of polyethyleneimine on biodegradable microparticles for delivery of plasmid DNA vaccines. Biomaterials. 2005;26:6375–85. doi: 10.1016/j.biomaterials.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 29.Pai Kasturi S, Qin H, Thomson KS, El-Bereir S, Cha SC, Neelapu S, et al. Prophylactic anti-tumor effects in a B cell lymphoma model with DNA vaccines delivered on polyethylenimine (PEI) functionalized PLGA microparticles. J Control Release. 2006;113:261–70. doi: 10.1016/j.jconrel.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 30.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 32.Singh A, Suri S, Roy K. In-situ crosslinking hydrogels for combinatorial delivery of chemokines and siRNA-DNA carrying microparticles to dendritic cells. Biomaterials. 2009;30:5187–200. doi: 10.1016/j.biomaterials.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Biragyn A, Tani K, Grimm MC, Weeks S, Kwak LW. Genetic fusion of chemokines to a self tumor antigen induces protective, T-cell dependent antitumor immunity. Nat Biotechnol. 1999;17:253–8. doi: 10.1038/6995. [DOI] [PubMed] [Google Scholar]
- 34.Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012;12:237–51. doi: 10.1038/nrc3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Bar graph showing percentage of CD11c+ BMDCs expressing A) CD86, B) both CD6 and MHCII following treatment with various formulations of CpG and Poly (I:C) each at 2.5 ug/ml concentration for 48hrs; *P<0.05 and **P<0.01 when compared between the groups.