Abstract
Background
Patients who present with radiographically occult palpable breast abnormalities represent a diagnostic challenge. We hypothesized that fine needle aspiration cytology (FNAC) would be an accurate method for diagnosing and excluding malignancy in these patients.
Methods
The records of all patients undergoing FNAC at our institution between 2010 and 2012 were queried. 173 patients with 175 palpable breast masses without an imaging correlate were included.
Results
Of 175 FNAC performed, 2 (1%) were malignant, 16 (9%) were suspicious, and 157 (90%) were benign (n = 75) or non-diagnostic (n = 82). All 16 suspicious FNAC had additional biopsy, of which 4 were malignant. FNAC led to the identification of 6 (3.4%) occult malignancies. At a median follow-up of 16.3 months, 1 patient within the benign cohort was found to have an incidental 2.5 mm cancer identified on reduction mammaplasty, which was unrelated to the index mass. The negative predictive value of FNAC in benign patients was 100%.
Conclusion
FNAC detected malignancy in a small but significant percentage of patients with a palpable mass and negative breast imaging, while excluding carcinoma in the remaining patients. FNAC may be included in the evaluation of patients with radiographically occult palpable breast masses.
A palpable breast mass is the reason for consultation to a primary care physician in 42% of patients with breast symptoms,1 and accounts for more than half of breast complaints in women presenting to breast centers.2 When a mammographic or sonographic correlate to the palpable abnormality can be identified, the decision for biopsy is based on the imaging characteristics of the lesion. In 30 to 45% of patients with a palpable lump, there are no imaging findings to explain the palpable abnormality.3,4 Evaluation of these patients ranges from close clinical follow-up with imaging and physical examination, to open surgical biopsy, which is costly and not considered “best practice” for the initial diagnosis of breast lesions.5,6
Several studies have demonstrated that a normal mammogram and ultrasound in the setting of a palpable breast mass has a high specificity and can reliably exclude carcinoma.3,7,8 However, Dennis et al. acknowledge that biopsy avoidance in this setting requires a thorough ultrasound examination by a skilled technologist and radiologist, utilizing excellent near-field transducers to effectively exclude carcinoma.7 Furthermore, long-term clinical and imaging follow-up may be necessary in the setting of biopsy avoidance in order to avoid a “missed cancer”, with most studies reporting a minimum 2-year follow-up.3,7
Fine needle aspiration cytology (FNAC) is a minimally invasive biopsy technique that can be performed in the office under palpation guidance. Ariga et al. demonstrated excellent histopathologic correlation of FNAC with core biopsy, excisional biopsy, and surgical specimens among 1,158 women undergoing FNAC, with a sensitivity, specificity, and positive and negative predictive value of 98%, 98%, 99%, and 91%, respectively for the entire cohort.9 Compared to core needle biopsy, FNAC is less frequently used in the assessment of imaging abnormalities requiring biopsy because differentiation between in situ and invasive malignancies is not possible, and because immunohistochemical stains for prognostic markers may be less accurate when performed on cytology specimens. In patients with imaging occult palpable lesions, FNAC, with its reported high sensitivity and specificity,9,10 may be helpful in differentiating benign from malignant lesions.
Few studies have assessed the utility of FNAC for imaging occult palpable breast masses.11 The purpose of our study was to evaluate the accuracy of FNAC in diagnosing and excluding malignancy in patients with radiographically occult breast lumps.
MATERIALS AND METHODS
Following approval from our institutional review board, the radiology and pathology records of all patients with a palpable breast lump undergoing FNAC at our Comprehensive Breast Center between January 2010 and December 2012 were queried. A total of 569 patients were identified who underwent FNAC for a palpable breast abnormality. Of these, 396 were excluded because they had a breast lump that was visible by imaging, or because they had less than 3 months of follow-up. The remaining 173 patients had documented imaging without a focal mammographic or sonographic finding within 1 year of FNAC.
In total, 175 FNAC were performed in 173 patients. FNAC was performed in the office by the surgeon under palpation guidance. The skin was cleansed with alcohol. Approximately 0.5 cc of 1% lidocaine was infiltrated into the skin overlying the palpable abnormality with a 25 gauge needle. Then, using a 22 gauge needle attached to a 10 cc syringe, 3 to 4 passes were made into the lesion with constant negative pressure applied to the syringe. The cellular aspirate was placed in a methanol-water preservative solution (CytoLyt Solution, Cytyc Corporation, Boxborough, MA), and sent for cytologic evaluation. In line with available resources, cytologic specimens were reviewed post-procedure and were not assessed in real time by pathologists to determine specimen adequacy.
Cytology findings were grouped into 4 categories: benign, non-diagnostic, suspicious, or malignant. Specimen adequacy was defined by our cytopathologists, based on the Bethesda conference on breast cytology guidelines.12,13 An adequate benign specimen required at least 6 well-visualized cell groups. A hypocellular or sparsely cellular specimen was considered unsatisfactory or non-diagnostic. A specimen was considered suspicious if the cellular findings were suggestive, but not diagnostic of malignancy; additional tissue biopsy was recommended in these cases. A malignant diagnosis was made when sufficient well-preserved malignant cells were identified.
Information regarding the treating surgeon’s clinical suspicion of the palpable mass was obtained from the medical record and was available for 171/175 (98%) breast masses. Patients with benign and non-diagnostic aspirates were followed with clinical exam and/or imaging evaluation at the discretion of the treating surgeon. All patients with a suspicious or malignant aspirate underwent additional tissue biopsy with either core biopsy or surgical biopsy (Fig 1).
Fig 1.
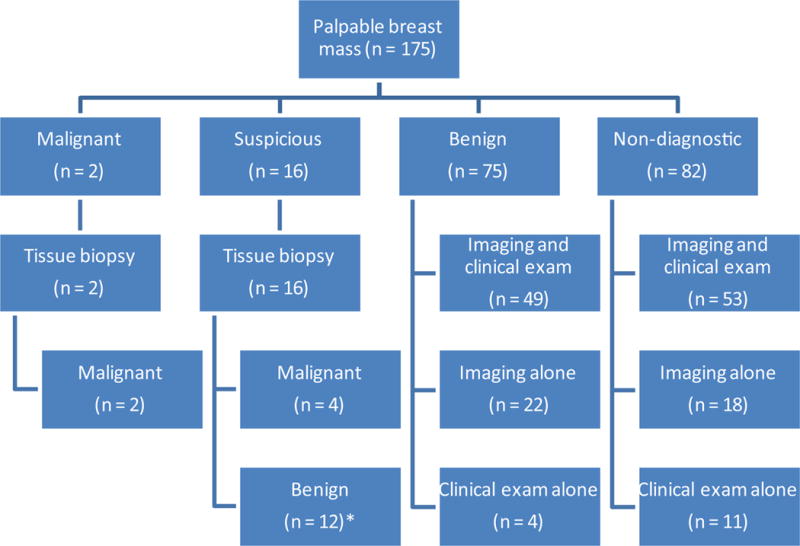
Breakdown of 175 FNAC by cytologic diagnosis and clinical follow-up. *Includes 1 patient with lobular neoplasia
RESULTS
Initial imaging and cytologic findings of the palpable mass stratified by age
Median age was 45 years (range, 17–82 years). Of 173 patients, 47 (27%) were < 40 years of age, while 126 (73%) were ≥ 40. All 173 patients had imaging without an identifiable lesion within 12 months of FNAC, with 153 (88%) occurring within 6 months of biopsy. Most patients (85%) had imaging prior to FNAC, while 26 (15%) had imaging after FNAC (as these patients presented to the surgeon prior to an imaging study being performed). Table 1 describes the initial imaging evaluation: a) for the entire cohort, and b) broken down by age < 40 years and ≥ 40. Most patients had imaging with mammography (n = 158, 91%). Information regarding mammographic technique was available for 136 (86%) of the 158 mammograms performed. The majority had diagnostic mammography (n = 115, 85%), while 21 (15%) had screening mammography. Of 15 patients imaged with ultrasound alone, 14 (93%) were < 40 years of age.
Table 1.
Initial imaging evaluation of the palpable abnormality with breakdown by age.
| IMAGING | Total (n = 173) |
< 40 years (n = 47) |
≥ 40 years (n = 126) |
|---|---|---|---|
| Mammogram + ultrasound | 108 (62%) | 29 (62%) | 79 (63%) |
| Mammogram alone | 50 (29%) | 4 (8%) | 46 (36%) |
| Ultrasound alone | 15 (9%) | 14 (30%) | 1 (1%) |
Table 2 demonstrates the initial cytology results for the 175 FNAC, stratified by: a) age, and b) clinical suspicion of the palpable finding. 90% of the cohort had a benign or non-diagnostic FNAC, with a similar incidence of benign cytology in patients < 40 (40%) versus ≥ 40 years of age (44%). Of 171 breast masses where clinical suspicion was documented, 168 (98%) were considered to be of low clinical suspicion. Only 3 masses were of moderate (n = 2) or high suspicion (n = 1), of which 2 were ultimately malignant.
Table 2.
Initial cytology results for the entire cohort (n = 175) stratified by a) age and b) clinical suspicion.
| A. | |||
|---|---|---|---|
|
| |||
| Cytology results | Total (n = 175) |
< 40 years (n = 48) |
≥ 40 years (n = 127) |
| Benign | 75 (43%) | 19 (40%) | 56 (44%) |
| Non-diagnostic | 82 (47%) | 22 (46%) | 60 (47%) |
| Suspicious | 16 (9%) | 6 (12%) | 10 (8%) |
| Malignant | 2 (1%) | 1 (2%) | 1 (1%) |
| B. | ||||
|---|---|---|---|---|
|
| ||||
| Cytology results | Clinical Suspicion | |||
|
| ||||
| Low (n = 168) |
Moderate (n = 2) |
High (n = 1) |
Not documented (n = 4) |
|
|
|
||||
| Benign (n = 75) | 74 | 0 | 0 | 1 |
| Non-diagnostic (n = 82) | 78 | 1 | 0 | 3 |
| Suspicious (n = 16) | 15* | 1* | 0 | 0 |
| Malignant (n = 2) | 1 | 0 | 1 | 0 |
3/15 patients with low clinical suspicion and 1/1 with moderate clinical suspicion and suspicious cytology were subsequently diagnosed with malignancy.
Malignant/Suspicious cytology
Two patients (1%) had malignant FNAC and 16 (9%) had suspicious FNAC; all 16 suspicious FNAC had additional tissue biopsy (core, n = 13; surgical, n = 3), of which 4 were malignant. Of the 12 remaining suspicious FNAC, 1 patient had lobular neoplasia on excision, while 11 had benign pathology without atypia. None of the 12 initial “suspicious” FNAC that were benign by additional tissue biopsy have developed malignancy at a median of 13 months from initial biopsy. In total, FNAC led to the identification of 6 (3.4%) occult malignancies. The clinical and tumor characteristics of the 6 patients with occult malignancy identified by FNAC are summarized in Table 3.
Table 3.
Clinical characteristics of patients with occult malignancy (n = 6) diagnosed by FNAC
| Case no. | Age (years) | Imaging study | FNAC finding | Additional biopsy method | Histology | Tumor size (cm) | Tumor stage |
|---|---|---|---|---|---|---|---|
| 20† | 77 | Mammogram | Suspicious | Core | IDC | 4.2 | IV |
| 96 | 49 | Mammogram | Suspicious | Core | ILC | 1.6 | IA |
| 111† | 51 | Mammogram | Malignant | N/A‡ | IDC | 0.9 | IA |
| 119 | 30 | US | Malignant | Core | DCIS | 1.3 | 0 |
| 121 | 43 | Mammogram | Suspicious | Core | IDC | 3.6 | IIA |
| 134 | 32 | Mammogram, US | Suspicious | Open | IDC | 1.7 | IIIC |
Personal history of ipsilateral breast cancer
Patient had definitive surgical therapy with lumpectomy following FNAC
US, ultrasound; FNAC, fine needle aspiration cytology; IDC, invasive ductal carcinoma; ILC invasive lobular carcinoma; DCIS, ductal carcinoma in situ
Benign/non-diagnostic cytology
Table 4 demonstrates the pathologic findings in the 157 benign/non-diagnostic aspirates performed in 156 patients. Median follow-up in the benign/non-diagnostic cohort was 16.3 months (range, 3.6–43.4 months), with 105/156 patients (67%) having at least 12 months of follow-up.
Table 4.
Cytologic findings among 157 benign/non-diagnostic aspirates
| Cytologic Diagnosis
|
Benign (n= 75)
|
Non-Diagnostic (n=82)
|
|---|---|---|
| Fibrocystic change | 35 (46.7%) | 3 (3.7%)* |
| Fat necrosis | 6 (8%) | 1 (1.2%)† |
| Fat | 10 (13%) | 30 (36.6%) |
| Fibroadenoma | 1 (1.3%) | 0 (0%) |
| Lymphoid tissue | 1 (1.3%) | 0 (0%) |
| Inflammation | 0 (0%) | 1 (1.2%) |
| No diagnosis specified | 22 (29.3%)‡ | 47 (57.3%) |
Insufficient material for diagnosis but suggestive of fibrocystic change
Insufficient material for diagnosis but suggestive of fat necrosis
Denotes benign clusters of ductal cells without a specific pathologic finding
Of the 157 benign/non-diagnostic FNAC, 102 (65%) had combined clinical and imaging follow-up, 40 (25%) had imaging follow-up alone, and 15 (10%) had clinical follow-up alone (Fig 1). 99 of 117 benign/non-diagnostic FNAC with clinical follow-up had a stable or improved exam with no additional intervention. Conversely, 18 patients had additional tissue sampling due to the presence of a prominent, persistent palpable mass. The majority had repeat FNAC (n = 17) and 1 had surgical excision (with benign results) due to moderate clinical suspicion. The second FNAC was benign in 10 and non-diagnostic in 7. Ultimately, 1 patient, within the benign cohort, was found to have an incidental 2.5 mm cancer identified on reduction mammaplasty, which was unrelated to the index palpable breast mass. The negative predictive value of FNAC in the 75 patients in the benign cohort was 100%.
Cytologic findings in patients with uniform initial imaging (combined mammography and ultrasound)
Evaluating results for patients who had both mammogram and ultrasound (initially) to evaluate the palpable mass (n = 108), FNAC was benign in 48 (44%), non-diagnostic in 54 (50%), and suspicious in 6 (6%). One of the 6 patients with suspicious cytology was found to have a malignancy with additional tissue biopsy, for a cancer detection rate of 0.9% in this subset of patients with more uniform pre-biopsy imaging.
DISCUSSION
Despite significant advances in breast imaging, approximately one-third of patients complaining of a palpable breast mass have no imaging correlate.4 Reasons that a palpable breast mass may not be seen by imaging include mammographically dense breasts, benign changes in the breast that produce no discernible imaging finding, or insidious tumor growth pattern making detection by standard imaging difficult. The National Comprehensive Cancer Network (NCCN) guidelines recommend that for patients with a palpable breast mass and negative imaging, the clinician can opt for clinical observation every 3–6 months for 1–2 years (when clinical suspicion is low) or perform tissue biopsy.14 However, accurate assessment of clinical suspicion in patients with radiographically occult masses may be challenging. In our own study, 98% of occult breast masses were considered to be of low (clinical) suspicion. In patients with malignancy (n = 6), only 2 were of moderate (n = 1) or high (n = 1) suspicion. Due to the poor discrimination of malignant lesions based on clinical assessment alone observed in this study, tissue biopsy of occult lesions provides additional information to diagnose or exclude malignancy. Open surgical biopsy is not considered “best practice” for initial diagnosis and should be reserved for patients with diagnostic uncertainty after initial minimally invasive biopsy. FNAC and core biopsy are both appropriate options; however, FNAC requires a pathologist experienced in cytology.14 In our study, FNAC was the initial diagnostic approach for evaluating patients with radiographically occult palpable breast masses due to its technical simplicity and short procedure time; here we evaluate the accuracy of FNAC in this setting.
Of 175 FNAC, 10% had a suspicious or malignant diagnosis, of which 3.4% were confirmed malignant by additional tissue biopsy. These findings are similar to published data by Rajan et al., which demonstrated suspicious FNAC in 5 (3.5%) of the 142 patients with a palpable abnormality and no radiologic correlate. Of those, 2 patients, or 1.4% of the cohort had a malignancy on further biopsy.11 In the 2 aforementioned studies, the incidence of malignancy in patients with imaging occult breast masses is lower than the 27% incidence (of malignancy) reported following minimally invasive biopsy for mammographically or sonographically visible breast masses.15 Despite the lower incidence of malignancy seen in patients with imaging occult breast masses, it is notable that the rate of malignancy is substantial enough to warrant investigation with FNAC, particularly in this setting where assessment of clinical suspicion is limited. Although initial diagnostic imaging was not uniform for the entire cohort, the numbers of suspicious (6%) and malignant biopsies (1%) in the subset that had both mammogram and ultrasound are slightly lower, but similar to that of the entire cohort (with less uniform imaging). Therefore, the small but significant percentage of patients diagnosed with malignancy by FNAC provides justification for consideration of its use in patients with imaging occult palpable breast masses, provided that a pathologist experienced in cytology is available. Notably, our study was carried out in a community hospital, highlighting that FNAC can be performed across multiple practice settings, as cytology experience was gained with familiarity of the technique.
The clinical significance of non-diagnostic cytology for a palpable breast abnormality in the context of negative imaging has not been well studied. In patients with a mammographically or sonographically visible mass, a biopsy result that does not explain the imaging finding is considered discordant, and repeat biopsy or excision is usually recommended.6 However, concordance is more difficult to determine in the absence of an imaging finding, as assessment of clinical suspicion based on clinical exam alone can be challenging. The Bethesda Breast Cytology Consensus Statement reports that non-diagnostic cytology may be explained by the fact that non-proliferative breast changes such as fibrosis found in fibrocystic changes, atrophy, lipomas, and hyalinized fibroadenomas presenting as a palpable mass, yield very few or no epithelial cells, even with repeat aspiration.13 In our study, 82 (47%) of the 175 FNAC were considered non-diagnostic, which is comparable to a study by Patel et al., who reported an overall unsatisfactory aspirate rate of 41.6%.16 Similarly, Rajan et al. reported “insufficient” cytologic findings in 110 (78%) of 142 patients with a palpable breast mass and negative imaging. In their study, at 4 weeks of follow-up, only 31/142 patients had a persistent palpable abnormality for which repeat FNAC (n = 26) or core biopsy (n = 5) was performed. Of the 26 repeat FNAC, 8 were benign and 18 were insufficient. These patients were ultimately discharged back to their primary care physician. Despite the high incidence of insufficient cytology in the study by Rajan et al, no cancers were diagnosed in their cohort of patients at a median follow-up of 61 months.11 Similar to Rajan’s study, none of the patients with non-diagnostic cytology (n = 82) in our study have developed a malignancy at the site of the index lesion at a median follow-up of 16.3 months. However, caution should be taken when interpreting our results, as our follow-up is too short to definitively exclude a “missed” cancer in this patient cohort. Importantly, additional tissue biopsy, should be performed in any patient with a clinically suspicious breast mass and non-diagnostic cytology, or in a patient with a documented clinical change in a breast mass previously considered to be of low clinical suspicion.
As such, we propose an algorithm for the evaluation of patients with radiographically occult breast masses (Fig 2). Prior to biopsy, level of clinical suspicion based on clinical characteristics of the lesion should be documented on all patients. Patients with clinically suspicious breast masses and benign or non-diagnostic cytology should be considered for additional tissue biopsy due to clinical-pathological discordance. Patients with low-suspicion lesions and benign or non-diagnostic cytology should have at least one short-interval follow-up to document the presence or absence of a mass. If the mass is no longer palpable, the patient can be safely discharged to routine screening (similar to the study by Rajan et al.). However, until studies with longer follow-up are performed, the decision to discharge a patient back to routine screening with benign/non-diagnostic cytology and a stable breast mass should be made cautiously.
Fig 2.
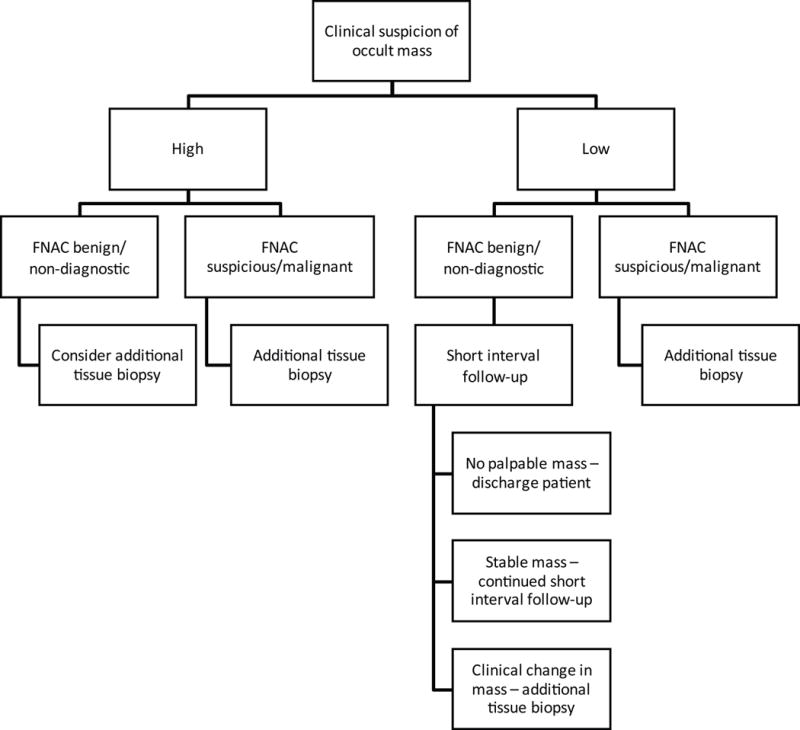
Algorithm for the management of patients with radiographically occult breast masses based on initial clinical suspicion of the mass
Our study has several limitations. It is a retrospective, non-randomized study of a small cohort of patients who were selected for biopsy with FNAC. In addition, our follow-up time is short and may not be sufficiently long enough to capture patients who may eventually present with a “missed” cancer following a benign or non-diagnostic aspirate. Patient follow-up was inconsistent among the cohort, with some patients undergoing clinical and imaging follow-up, imaging follow-up alone, or clinical exam alone; this could result in variable detection of malignancy in the follow-up period. Another limitation is that pre-biopsy imaging was not uniform for all patients undergoing FNAC, in that only 62% had both initial mammographic and sonographic evaluation. Furthermore, mammographic technique varied among the cohort, with 15% (of 136 with available data) receiving screening mammogram. Taking this into consideration, additional diagnostic imaging may have identified a correlate to the palpable abnormality, thereby reducing the potential benefit of FNAC in this setting.
In conclusion, FNAC detected malignancy in a small but significant percentage of patients with a palpable lump and negative breast imaging, while effectively excluding carcinoma in the remaining patients, including patients with non-diagnostic cytology. Discrimination of benign and malignant lesions based on clinical suspicion alone may be challenging. FNAC, in conjunction with clinical judgment, is an accurate diagnostic tool and can be included in the standard work-up of patients with radiographically occult palpable breast lumps.
Acknowledgments
Funding: This work received no funding.
Abbreviations
- FNAC
fine needle aspiration cytology
Footnotes
Conflicts of interest: The authors declare that they have no competing interests.
References
- 1.Salzman B, Fleegle S, Tully A. Common Breast Problems. Am Fam Physician. 2012;86:343–9. [PubMed] [Google Scholar]
- 2.Bleicher RJ. Management of the palpable breast mass. In: Harris JR, Lippman ME, Morrow M, Osborne CK, editors. Diseases of the breast. Fifth. Philadelphia: Wolters Kluwer Health; 2014. pp. 29–37. [Google Scholar]
- 3.Shetty M, Shah Y, Sharman R. Prospective evaluation of the value of negative sonographic and mammographic findings in patients with palpable abnormalities of the breast. J Ultrasound Med. 2003;22:263–8. doi: 10.7863/jum.2002.21.11.1211. [DOI] [PubMed] [Google Scholar]
- 4.Moy L, Slanetz PJ, Moore R, Satija S, Yeh ED, McCarthy KA, et al. Specificity of mammography and US in the evaluation of a palpable abnormality: Retrospective review. Radiology. 2002;225:176–81. doi: 10.1148/radiol.2251010999. [DOI] [PubMed] [Google Scholar]
- 5.Gutwein LG, Ang N, Liu H, Marshall JK, Hochwald SN, Copeland EM, et al. Utilization of minimally invasive breast biopsy for the evaluation of suspicious breast lesions. Am J Surg. 2011;202(2):127–32. doi: 10.1016/j.amjsurg.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Silverstein MJ, Recht A, Lagios MD, Bleiweiss IJ, Blumencranz PW, Gizienski T, et al. Special report: Consensus conference III. Image-detected breast cancer: state-of-the-art diagnosis and treatment. J Am Coll Surg. 2009;209(4):504–20. doi: 10.1016/j.jamcollsurg.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Dennis MA, Parker SH, Klaus AJ, Stavros AT, Kaske TI, Clark SB. Breast biopsy avoidance: The value of normal mammograms and normal sonograms in the setting of a palpable lump. Radiology. 2001;219(1):186–91. doi: 10.1148/radiology.219.1.r01ap35186. [DOI] [PubMed] [Google Scholar]
- 8.Gumus H, Gumus M, Mills P, Fish D, Devalia H, Jones SE, et al. Clinically palpable breast abnormalities with normal imaging: Is clinically guided biopsy still required? Clinical Radiology. 2012;67(5):437–40. doi: 10.1016/j.crad.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 9.Ariga R, Bloom K, Reddy VB, Kluskens L, Francescatti D, Dowlat K, et al. Fine needle aspiration of clinically suspicious palpable breast masses with histopathologic correlation. Am J Surg. 2002;184(5):410–3. doi: 10.1016/s0002-9610(02)01014-0. [DOI] [PubMed] [Google Scholar]
- 10.Ishikawa T, Hamaguchi Y, Tanabe M, Momiyama N, Chisima T, Nakatani Y, et al. False-positive and false-negative cases of fine needle aspiration cytology for palpable breast lesions. Breast Cancer. 2007;14(4):388–92. doi: 10.2325/jbcs.14.388. [DOI] [PubMed] [Google Scholar]
- 11.Rajan S, White J, Peckham-Cooper A, Lane S, Lansdown M. Management of palpable but radiologically occult breast abnormalities. Acta Cytol. 2012;56(3):266–70. doi: 10.1159/000337435. [DOI] [PubMed] [Google Scholar]
- 12.College of American Pathologists. Defining adequacy in nongynecologic cytology. 2003 Available: http://www.cap.org/apps//cap.portal [Accessed June 17, 2014]
- 13.Special Communication: The uniform approach to breast fine needle aspiration biopsy. Diagnostic Cytotechnology. 1997;16:295–311. doi: 10.1002/(sici)1097-0339(1997)16:4<295::aid-dc1>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 14.National Comprehensive Cancer Network guidelines version 1. Breast Cancer Screening and Diagnosis (BSCR-6); 2014. Available at: http://www.nccn.org/professionals/physician_gls/pdf/breast-screening.pdf [Accessed June 20, 2014] [Google Scholar]
- 15.Manjoros DT, Collett AE, Alberty-Oller JJ, Frazier TG, Barrio AV. The value of 6-month interval imaging after benign radiologic-pathologic concordant minimally invasive breast biopsy. Ann Surg Oncol. 2013;20(10):3163–8. doi: 10.1245/s10434-013-3114-3. [DOI] [PubMed] [Google Scholar]
- 16.Patel JJ, Gartell PC, Smallwood JA, Herbert A, Royle GT, Buchanan R, et al. Fine needle aspiration cytology of breast masses: An evaluation of its accuracy and reasons for diagnostic failure. Ann R Coll Surg Engl. 1987;69:156–9. [PMC free article] [PubMed] [Google Scholar]


