Abstract
Children who met DSM-IV criteria for attention-deficit/hyperactivity disorder (ADHD) with functional impairment in at least one setting at 4–6 years of age were followed prospectively through age 18 years. On average, the 125 children (107 boys) with ADHD at baseline improved over time, but still continued to exhibit more symptoms, functional impairment, and risky behavior through adolescence than demographically matched healthy comparison children. These findings support the predictive validity of the diagnosis of ADHD at younger ages by demonstrating that the symptoms and impairment are enduring. Nonetheless, there were marked variations in developmental outcomes. Among children with ADHD, higher numbers of inattention and hyperactivity-impulsivity symptoms and higher number of concurrent symptoms (oppositional, conduct disorder, anxiety, and depression) measured at baseline each predicted higher future levels of the same dimension of symptoms. In addition, higher baseline levels of inattention, oppositional, conduct disorder, and anxiety symptoms predicted greater future functional impairment. Among children with ADHD, girls and children from families with lower family incomes had relatively poorer outcomes. Although outcomes varied along a continuum, approximately 10% of the children with ADHD at 4–6 years could be classified as functioning in the normative range on multiple measures during 15–18 years. Although this finding awaits replication, lower levels of hyperactivity-impulsivity symptoms at 4–6 years predicted more normative functioning during adolescence. These findings suggest that ADHD identified in early childhood predicts an increased likelihood of functional impairment through adolescence for most, but not all, children.
The diagnosis of attention-deficit/hyperactivity disorder (ADHD) during childhood and adolescence is valid in the fundamental sense of being strongly associated with impairment in adaptive functioning when confounded factors are controlled (Willcutt et al., 2012). There also is substantial evidence that ADHD is a relatively enduring and impairing condition when given to school-age children and adolescents, in spite of gradual improvements in symptoms with increasing age. In particular, school-aged children given the diagnosis of ADHD are particularly at risk for academic and occupational impairment, risky behavior and unintentional injuries, and for exhibiting both ADHD symptoms and symptoms of other forms of psychopathology in adolescence and early adulthood (Barkley, 2005; Biederman et al., 2006; Hinshaw, 1994; Kuriyan et al., 2013; Mannuzza, Klein, Bessler, Malloy, & LaPadula, 1998; Sibley et al., 2012; Weiss & Hechtman, 1993; Willcutt et al., 2012)
Very little is known about the stability and long-term consequences of ADHD when it is diagnosed at early ages, however. Although ADHD may be a validly diagnosed disorder in school-age children and adolescents, the same may not be true when the diagnosis of ADHD is given in early childhood, when it is typical for children to be active, impulsive, and have short attention spans. Given that the current clinical practice guidelines of the American Academy of Pediatrics recommend the diagnosis and treatment of ADHD may begin as early as 4 years of age (American Academy of Pediatrics, 2011), it is essential to understand the long-term outcomes of young children who exhibit ADHD.
Several short- and intermediate-term prospective studies of the stability of ADHD in diagnosed younger children have been conducted. These studies consistently show that more than half of 3–6 year old children who meet criteria for ADHD continue to meet criteria 18 months (Bunte, Schoemaker, Hessen, van der Heijden, & Matthys, 2014), 3 years (Bufferd, Dougherty, Carlson, Rose, & Klein, 2012), 6 years (Riddle et al., 2013), and 7 years (Law, Sideridis, Prock, & Sheridan, 2014) later. Similarly, we have followed a prospective sample of children who met DSM-IV criteria for the diagnosis of ADHD at ages 4–6 years to determine if the diagnosis has long-term consequences for the children. We previously reported that the diagnosis of ADHD was valid in the present sample in the sense of being significantly associated with concurrent functional impairment at the time of diagnosis, controlling for other concurrent dimensions of psychopathology, intelligence, and potential demographic confounds (Lahey, Pelham, et al., 1998). In addition, these young children with ADHD exhibited a high degree of persistence in both symptoms and functional impairment through 11–14 years of age (Lahey, Pelham, et al., 2004; Lee, Lahey, Owens, & Hinshaw, 2008), although they often shifted among the subtypes of ADHD over time (Lahey, Pelham, Loney, Lee, & Willcutt, 2005). Indeed, very few children with ADHD in this sample had shown significant improvement in functioning by 11–14 years of age (Lee et al., 2008). Thus, even at young ages, it is possible to identify children with relatively persistent and impairing levels of ADHD symptoms.
Little is known about the longer-term outcomes of children given the diagnosis of ADHD at young ages, however. This is important because of concerns that have been raised that symptoms of ADHD during the preschool years may only reflect transient problems that do not require labeling and treatment (Barkley, 2005). To our knowledge, no study has followed preschool children who meet diagnostic criteria for ADHD through adolescence to determine if they continue to exhibit symptoms of psychopathology and maladaptive functioning during this key developmental period. In this paper, we report the results of analyses of new waves of data to examine the persistence of ADHD through 18 years of age. We address three fundamental questions in these analyses:
Do children given the diagnosis of ADHD at 4–6 years show improvement in symptoms and functional impairment across childhood and during adolescence? This includes changes in symptoms of ADHD and other disorders, global measures of adaptive functioning, and more specific measures of functioning relevant to safety and academic functioning.
Do children given the diagnosis of ADHD at 4–6 years exhibit higher mean levels of symptoms and functional impairment over increasing age and continue to exhibit more dysfunction during adolescence than demographically matched healthy comparison children? We focus on maladaptive outcomes that have been found to be more common in older children given the diagnosis of ADHD (Barkley & Cox, 2007; Mannuzza, Klein, Bessler, Malloy, & LaPadula, 1993; Trampush, Miller, Newcorn, & Halperin, 2009).
Do the kinds of baseline variables that are measured in comprehensive structured clinical assessments (e.g., demographic variables, intelligence, and numbers of symptoms of a range of forms of psychopathology) predict individual differences in the developmental outcomes of young children with ADHD during adolescence?
Understanding the long-term outcomes of ADHD and predictors of future improvement will help the field understand the degree of persistence of the syndrome and improve long-term clinical prognosis and treatment planning for children given the diagnosis of ADHD at early ages.
METHOD
Participants
Two cohorts of 3.8–7.0 year old children were recruited in consecutive years in both Chicago and Pittsburgh for the present longitudinal study. Five potential participants were ineligible because of pervasive developmental disorder, mental retardation, or seizure disorder (Lahey, Pelham, et al., 2004). Of 310 eligible participants, 259 parents gave written informed consent and all children gave oral assent. Four of these 259 participants who had not been given the diagnosis of mental retardation were excluded from the present analyses because they were found to have an intelligence score less than 70. All participants lived with their biological mothers at the time of the first assessment. The diagnosis of ADHD was given in the first assessment to 125 children using the methods described below. In Chicago, children with ADHD were recruited from urban and suburban university child psychiatry clinics. In Pittsburgh, 42% of the children with ADHD were recruited from an urban university child psychiatry clinic and 58% were recruited through advertisements, but no differences were found between recruitment methods on demographics or functional impairment in wave 1 (Lahey, Loeber, et al., 1998). Comparison children (N=130) were recruited from similar schools and neighborhoods as probands and approximately matched with ADHD probands on sex, race-ethnicity, and age. Comparison children had never been referred for mental health problems, but were not excluded if they met criteria for a disorder other than ADHD at baseline. Four children who met symptom criteria for ADHD but were not reported to be impaired in any setting were part of the 130 comparison children. This made tests of differences in the long-term outcomes of children in the ADHD and comparison group more conservative.
Beginning with the initial assessment when the children were 4–6 years of age, 13 approximately annual assessments were conducted, except that assessments were not conducted in the fifth, tenth, and eleventh years of the study due to lack of funding. These are referred to as assessment waves A, B, C, D, F, G, H, I, L, M, N, O, and P. In each wave, the children are of different ages, e.g., 5–7 years old in wave B. Of the 125 children given the diagnosis of ADHD in wave A, 112 (89.6%) were assessed at least once during assessment waves L, M, N, O, and P (the 12th through 16th assessment years, when the participants were 15–18 years of age). Because participants were eligible only through 18 years of age, they participated in varying numbers of assessments during waves L-P, but 86.6% completed at least two adolescent assessments. A total of 68 of the comparison children also were followed until 18 years of age (94.7% completed at least two adolescent assessments). These 68 comparison children are a subset of the 130 comparison children identified at baseline. Their numbers in the adolescent follow-ups were reduced slightly by attrition, but primarily because nearly half of the comparison children were randomly eliminated from the sample in assessment waves L-P (reducing the numbers of assessments of comparison youth at 15–18 years of age) due to reduced funding. All comparison girls were retained in waves L-P, but 50% of the other comparison children within each year of age and within race-ethnicity groups were randomly dropped. If a retained comparison child declined assessment in Wave L, a dropped comparison child (randomly selected within age and race-ethnicity) was substituted and assessed in Wave L and beyond; no subsequent substitutions were made. The demographic characteristics of the sample and the participation rates in each wave for children with ADHD and comparison children are presented in Table 1.
Table 1.
Demographic characteristics of the sample and rates of participation at each age for children with attention-deficit/hyperactivity disorder in wave 1 (4–6 years of age) and healthy comparison children matched on age, sex, and race-ethnicity.
| Mean Age | Child’s Sex | Race-ethnicity | Mean Income | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | (SD) | (% male) | (% Afr-Am) | (%Hisp/Asian) | (SD) | ||||||||||
| ADHD1 | 125 | 5.24 (0.71) | 85.60 | 30.40 | 6.40 | 39,672 (34,940) | |||||||||
| Comparison | 130 | 5.16 (0.78) | 80.77 | 30.77 | 5.38 | 47,647 (35,034) | |||||||||
|
| |||||||||||||||
| % Assessed at Each Age in Years | |||||||||||||||
| 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
| ADHD | |||||||||||||||
| % Participate | 100 | 97.30 | 96.80 | 96.00 | 96.19 | 91.55 | 95.95 | 92.00 | 88.00 | 89.52 | 84.31 | 80.00 | 89.19 | 81.60 | 67.20 |
| %Parent | 100 | 97.30 | 96.80 | 96.00 | 96.19 | 91.55 | 91.89 | 88.80 | 87.20 | 87.62 | 84.31 | 80.00 | 89.19 | 80.80 | 60.81 |
| %Teacher | 100 | 95.95 | 92.00 | 85.60 | 82.86 | 81.69 | 87.84 | 75.20 | 68.00 | 71.43 | 62.75 | 70.00 | 64.86 | 47.20 | 14.86 |
| %Youth | 100 | 97.30 | 96.80 | 92.80 | 89.52 | 87.32 | 87.84 | 86.40 | 85.60 | 86.67 | 82.35 | 75.00 | 85.14 | 77.60 | 60.81 |
| Comparison | |||||||||||||||
| % Participate | 100 | 97.47 | 96.92 | 93.08 | 96.00 | 96.30 | 88.61 | 90.77 | 90.00 | 92.00 | 92.16 | 56.67 | 50.63 | 50.77 | 44.62 |
| %Parent | 100 | 97.47 | 96.92 | 93.08 | 96.00 | 95.06 | 87.34 | 90.77 | 87.69 | 91.00 | 92.16 | 53.33 | 49.37 | 50.77 | 39.24 |
| %Teacher | 100 | 93.67 | 89.23 | 90.00 | 85.00 | 79.01 | 81.01 | 76.15 | 74.62 | 73.00 | 66.67 | 43.33 | 40.51 | 36.15 | 10.13 |
| %Youth | 100 | 96.20 | 95.38 | 92.31 | 95.00 | 93.83 | 86.08 | 90.00 | 86.15 | 90.00 | 92.16 | 53.33 | 49.37 | 50.00 | 40.51 |
Differences between groups in demographic characteristics not significant at p < .05 (uncorrected).
Note: Participate = at least one informant assessed; ADHD = given the diagnosis of ADHD in wave 1 (4–6 years of age); N = number of children in each group in wave 1 when they were 4–6 years of age; Hisp = Hispanic or Asian ethnicity; italics = ages at which approximately half of comparison children were dropped from the study due to funding limitations.
Measures
Assessment of Psychopathology
The Diagnostic Interview Schedule for Children (DISC) (Shaffer, Fisher, Piacentini, Schwab- Stone, & Wicks, 1993) was administered to mothers in each assessment by trained interviewers. Information was obtained on DSM-IV symptoms of ADHD, oppositional defiant disorder (ODD), conduct disorder (CD), anxiety disorders (separation anxiety disorder, specific phobia, social phobia, agoraphobia, panic attacks, overanxious disorder, and generalized anxiety disorder) and major depression and dysthymia during the last 12 months (Lahey, Loeber, et al., 1998). In each assessment, the child’s primary or English/Language Arts teacher completed the DSM-IV version of the DBD Rating Scale, which queried symptoms of ADHD, ODD, and CD (Pelham, Gnagy, Greenslade, & Milich, 1992) by mail. Following standard procedures (Pelham et al., 1992), teacher-reported symptoms rated “pretty much” or “very much” were scored as present. Beginning in wave F, when all children were at least 9 years of age, the children also were directly interviewed using the DISC regarding CD and depression symptoms and assessments of anxiety using youth reports began in wave G. At these ages, youth are reliable and valid informants for these dimensions of symptoms (Bird, Gould, & Staghezza, 1992; Hart, Lahey, Loeber, & Hanson, 1994; Jensen et al., 1999). Counts of the numbers of symptoms in each of these dimensions were calculated in each wave. For anxiety symptoms, this was the total number of symptoms of all assessed anxiety disorders. Parent and teacher reports of ADHD, ODD, and CD symptoms were combined according to the standard ‘or’ rule, meaning that each symptom was counted if reported by either or both informants (Piacentini, Cohen, & Cohen, 1992).
Diagnosis of ADHD
The diagnosis of DSM-IV ADHD in year 1 was based on counts of symptoms reported by either the parent or the teacher (Piacentini et al., 1992) and both parent and teacher reports of impairment. Functional impairment was assessed in two ways to make the diagnosis of ADHD. First, the parent was asked in the DISC if the child’s ADHD symptoms had caused problems (a) at home or with friends, or (b) at school. Second, parents and teachers completed the Impairment Rating Scale (IRS) (Fabiano et al., 2006), in which the child’s need for treatment in multiple areas was rated using 7-point scales ranging from “No problem; definitely does not need treatment” (=0) to “Extreme problem; definitely needs treatment” (=6). Parents rated the child on problems in relations with peers, siblings, and parents, academic progress at school, self-esteem, and impact on the family. Teachers rated problems in relations with classmates and teachers, academic progress, classroom disruption, and self-esteem using the IRS. Both respondents rated the child’s overall need for treatment. For the year 1 diagnosis of ADHD, based on previous studies, children were said to be impaired if they received a rating of ≥ 3 on at least one IRS scale (Fabiano et al., 2006). Children were said to exhibit ADHD in this study if they met DSM-IV symptom criteria and exhibited impairment in at least one setting. We relaxed the DSM-IV requirement of impairment in two settings because previous findings based on this sample suggested that 4–6 year olds who met symptom criteria sometimes did not exhibit impairment in school until later ages, but nearly all children who met criteria for ADHD with impairment in one setting later exhibited impairment in two settings (Lahey et al., 2005).
Intelligence
Intelligence was assessed using the standard four-test Short Form of the Stanford-Binet Intelligence Scale, Fourth Edition (Thorndike et al., 1986). The short form was administered in the initial assessment and again in the second annual assessment, with the average of the two scores used as the intelligence score. The examiners were trained and observed in the administration of the intelligence test by doctoral-level clinical child/school psychologists. All children were off stimulant medication at the time of the intellectual assessments.
Measures of Functional Impairment over Time
To measure the functional outcomes of children with ADHD, a range of measures of global adaptive functioning and specific aspects of functional impairment were obtained in each assessment. Notably, these measures were different from the impairment measures used to make the diagnosis of ADHD. In every assessment, ratings of functioning were obtained separately from the parent and the interviewer who administered the DISC to the parent using the Non-clinician Version of the Children’s Global Assessment Scale (CGAS; Setterberg et al., 1992). The CGAS consists of a thermometer-like rating scale of general adaptive functioning that ranges from 1–100. At each decile, a phrase is presented that describes the child’s functioning at that level. Raters were asked to assign a single rating that represented the child’s lowest level of functioning during the past 6 months. In addition, a number of specific aspects of impairment and risky behavior were assessed. In each wave, the parent was also asked if the child had suffered an injury more serious than a scratch, bruise, or bump on the head that the parent attributed to the child’s carelessness, impulsiveness, or poor judgment. Beginning with the wave F assessment, the parent and youth were each asked if the youth had been arrested in the previous 12 months. Beginning in wave L, the youth completed a set of questions regarding the operation of motor vehicles in the last 12 months, including questions about driving without a license or permit, riding a motorcycle without a helmet, being in an accident or hitting a bicyclist or pedestrian while driving, and receiving a ticket for a moving violation.
Statistical Analyses
Cross-sectional comparisons, such as tests of differences between participants who were retained or dropped out of the study were conducted using generalized linear models (Nelder & Wedderburn, 1972), specifying working distributions appropriate to the data (e.g., Poisson for count data) and using robust variance estimators in PROC GENMOD in SAS 9.2. Our primary analyses were longitudinal analyses of change over time, tests of differences between diagnostic groups over time, and tests of predictive associations between demographic and clinical measures (i.e., diagnosis, symptoms, and intelligence test scores) collected in the initial structured clinical assessment at 4–6 years of age with outcome variables over time. Data on all children participating in any assessments across the covered range of ages were included in these analyses. Unless noted, all analyses included fixed covariates controlling site, cohort (referring to participants recruited in two consecutive years), sex, race-ethnicity (two contrasts for African American and other groups versus non-Hispanic white), and the log of total family income. Some analyses of future outcomes involved longitudinal analyses of data collected on the children in repeated assessments of the same variables over increasing age. All such longitudinal analyses were conducted in general estimating equations (GEE) using robust variance estimators (Zeger & Liang, 1986). GEE is an application of generalized linear models (Nelder & Wedderburn, 1972) to the analysis of repeated measures of the same variable in longitudinal studies. It models the mean response in each assessment and does not depend on the correct specification of the within-person covariance structure over time. GEE is robust to observations that are missing completely at random (Zeger & Liang, 1986). For counts of symptoms, Poisson distributions were specified, but repeated CGAS ratings were treated as normally distributed response variables.
Unless noted, all longitudinal analyses also included time-varying covariates to control for whether the interviewer in each wave was blind to all data obtained from the parent and youth in the previous wave, and the number of parent and teacher informants providing data in that assessment, (when outcome variables were based on the combination of parent and teacher data). With the exception of age, sex, and family income, these fixed and time-varying control variables are treated as covariates of no interest, meaning that statistical tests of associations of these covariates with outcomes are not evaluated, reported, or considered when adjusting for the number of statistical tests. That is, they are used as covariates to improve the analyses of tested associations, but are not the focus of statistical tests themselves. All longitudinal analyses were repeated using parent reports of the child’s receipt of psychosocial services or use of medication for problems of emotion or behavior during the last 12 months as time-varying covariates in preliminary analyses. This was done to determine if treatment was related to improved outcomes, in which case treatments also would need to be controlled when assessing change over time and predictors of outcomes.
When predictions were tested from baseline measures to adolescent outcomes that were defined by the mean of all assessments during 15–18 years of age (e.g. mean counts of symptoms in all assessments in that age range), generalized linear models were used with scale correction. Predictions from baseline measures to binary outcome variables measured during 15–18 years of age (e.g., ever driving a motorcycle without a helmet during adolescence) were tested using logistic regression.
RESULTS
Tests of Attrition Bias
As shown in Table 1, not all children participated in the assessments at all ages. This was due to gaps in funded assessments for waves F, J, and K; the required reduction of comparison children by half beginning in wave L; but also from attrition from the study. Among children who met criteria for ADHD at baseline, no differences in any methodological control variables (site and cohort), demographic variables (age in wave 1, sex, and race-ethnicity), parent or interviewer CGAS scores, or counts of ADHD and other dimensions of concurrent symptoms in wave 1 were found between the 13 children not assessed at any age during the adolescent assessments at 15–18 years and the 112 who were assessed at least once during adolescence at p < .05 in generalized linear models. The sole exception was that children lost to attrition during adolescence had slightly but significantly fewer anxiety symptoms at baseline than children who were retained (p < .05), but baseline anxiety is controlled in all prospective analyses in which baseline symptoms are predictors. Comparing the 68 comparison children assessed during adolescence to the 62 healthy controls lost by attrition or by randomly reducing the control group, the only significant difference was that a slightly smaller proportion of the cohort recruited in the second year of participant recruitment was retained during adolescence than in the first cohort (p < .05). Cohort was controlled in all prospective analyses, however.
In addition, Table 1 shows that both adult informants (parent and teacher) did not participate in every assessment. To assess potential biases in the outcomes of ADHD symptoms and other outcome variables measured by the combination of parent and teacher reports, we examined the number of total number of parent + teacher informants (0, 1, or 2) participating in all adolescent assessments among children who participated in at least one assessment at 15–18 years. Controlling all methodologic and demographic covariates, children with ADHD were assessed by fewer informants in each adolescent wave, β = 0.57, χ2 = 4.63, p < .05. In addition, more informants participated during adolescence for girls (β = 0.12, χ2 = 4.85, p < .05), and fewer informants participated for youth who were older in wave 1 (β = −0.31, χ2 = 107.80, p < .0001. Therefore, to minimize any biases in comparisons of outcomes, the number of parent and teacher informants in each wave was included in all longitudinal models as a time-varying covariate when the outcome variables was assessed by the combination of these informants. Because GEE models means in each assessment, it is robust to missing data due to nonparticipation in a given wave (Zeger & Liang, 1986).
Correlations among Baseline Variables
As shown in Table 2, the correlations among the covariates and predictor variables measured at baseline, when the children were 4–6 years of age, reveal the relative independence of the methodologic covariates and most demographic variables, but intelligence was moderately correlated with multiple dimensions of psychopathology at baseline and, therefore, was included as a covariate in all longitudinal analyses. Consistent with studies of population-based samples (Lahey, Applegate, et al., 2004; Lahey et al., 2008), the various dimensions of psychopathology were correlated.
Table 2.
Correlations among wave 1 variables and in the full sample (N=255).
| Cohort | Age | Sex | Afr Amer | Other ethnic | Income | Intelligence | Inatt | Hyper | ODD | CD | Depress | Anxiety | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||
| Site | 0.14* | −0.09 | 0.06 | −0.25*** | 0.00 | −0.17** | −0.12 | −0.02 | −0.01 | −0.06 | 0.02 | −0.19** | −0.18** |
| Cohort | −0.11 | 0.00 | 0.12* | 0.03 | 0.04 | 0.02 | −0.16* | −0.22** | −0.18** | −0.15* | −0.17** | −0.16** | |
| Age in wave 1 | 0.03 | 0.10 | −0.02 | −0.04 | 0.11 | 0.07 | −0.01 | 0.04 | −0.01 | 0.15* | 0.08 | ||
| Sex (female =1; male =0); | −0.05 | 0.02 | 0.01 | 0.01 | −0.07 | −0.09 | −0.10 | −0.13* | −0.03 | −0.02 | |||
| Afr Amer (= 1) | −0.17** | −0.32*** | −0.28*** | 0.07 | 0.03 | 0.05 | 0.07 | 0.20** | 0.12 | ||||
| Other ethnic (= 1) | 0.04 | −0.03 | 0.03 | 0.03 | −0.01 | −0.03 | −0.03 | −0.05 | |||||
| Income1 | 0.40*** | −0.14* | −0.11 | −0.12* | −0.13* | −0.14* | −0.17** | ||||||
| Intelligence | −0.44*** | −0.36*** | −0.26*** | −0.19** | −0.24*** | −0.19** | |||||||
| Inatt | 0.83*** | 0.65*** | 0.43*** | 0.46*** | 0.42*** | ||||||||
| Hyper | 0.70*** | 0.49*** | 0.39*** | 0.33*** | |||||||||
| ODD | 0.61*** | 0.48*** | 0.45*** | ||||||||||
| CD | 0.27*** | 0.29*** | |||||||||||
| Depress | 0.57*** | ||||||||||||
p < .05;
p < .01;
p < .0001 (not corrected for multiple comparisons)
Total family income in wave 1; N = 251
Note: Income = log of ordinal income categories; Afr Amer = African American; Other ethnic = other race-ethnic group; Inatt = inattention; Hyper = hyperactivity-impulsivity; ODD = oppositional defiant disorder; CD = conduct disorder; Depress = depression.
Tests of Time-Varying Associations with Treatments
The results of all longitudinal analyses in GEE were virtually identical when terms for medication and psychosocial treatment were or were not included as time-varying covariates. Although the terms for medication and psychosocial treatment (scored 0 = no, 1 = yes) were both significant in nearly every longitudinal model, the results always indicated that children exhibited greater symptoms and impairment during waves in which they either took medication or received psychosocial treatment. We interpret this as reflecting the family’s treatment-seeking response to the child’s problems in that wave. Therefore, we dropped medication and psychosocial treatment as covariates to avoid overcontrol.
Age-Related Changes in Symptoms and Global Impairment across 4–18 Years
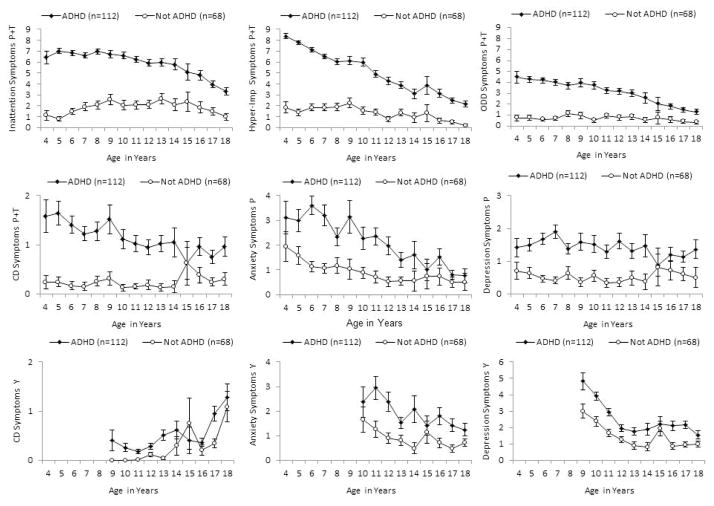
The first goal of these analyses is to describe changes in symptoms and impairment over increasing age in children given the diagnosis of ADHD at 4–6 years and comparison children using longitudinal analyses in GEE. As reported in in columns 2 and 3 of Table 3, and as shown in Figure 1, there were significant linear declines in counts of symptoms of most dimensions of psychopathology over increasing age in the full sample (i.e., both children with ADHD at baseline and comparison children combined). For example, the β coefficient on the log scale for age indicates that each one-year increase in age is associated with 100 x −.08 = 8% fewer symptoms of hyperactivity-impulsivity. The exceptions were parent- and teacher-rated depression symptoms, which did not show significant linear age-related change, and youth-reported CD symptoms, which significantly increased during late childhood and adolescence in the full sample. In the full sample, there were corresponding significant increases in CGAS ratings from both parents and interviewers over time, indicating improved functioning over increasing age.
Table 3.
Age-related changes in levels of symptoms and group differences in mean counts of symptoms and mean global functional impairment ratings across 4–18 years of age between 125 children who met symptom criteria for attention-deficit/hyperactivity disorder with impairment in at least one setting at 4–6 years and 130 demographically matched healthy comparison children.
| Response variable | β linear age1 | Z | β ADHD versus comparison1 | Z | β ADHD x linear age2 | Z |
|---|---|---|---|---|---|---|
| Inattention (P+T) | −0.02 | −3.99*** | 1.14 | 11.09*** | −0.07 | −5.50*** |
| Hyperactivity- Impulsivity (P+T) | −0.08 | −11.86*** | 1.39 | 14.36*** | −0.00 | −0.11 |
| ODD (P+T) | −0.06 | −6.89*** | 1.47 | 9.93*** | −0.02 | −0.98 |
| CD (P+T) | −0.03 | −2.14* | 1.72 | 6.84*** | −0.06 | −1.80 |
| Anxiety (P) | −0.11 | −8.07*** | 0.91 | 4.91*** | −0.00 | −0.12 |
| Depression (P) | −0.02 | −1.73 | 1.10 | 5.73*** | −0.05 | −1.82 |
| CD (Y) | 0.26 | 8.08*** | 0.52 | 2.21* | 0.17 | −2.43* |
| Anxiety (Y) | −0.12 | −6.27*** | 0.66 | 4.21*** | −0.00 | −0.12 |
| Depression (Y) | 0.13 | −10.35*** | 0.45 | 4.72*** | 0.04 | 1.55 |
| CGAS (P) | 0.54 | 5.78*** | −16.87 | −13.16*** | 0.66 | 3.87*** |
| CGAS (I) | 0.56 | 5.81*** | −17.46 | −13.43*** | 0.69 | 3.83*** |
Tested in a set of models including the demographic and methodologic covariates and the diagnosis of ADHD at baseline; the ADHD-by-age interaction term was not included.
Tested in a set of models including the demographic and methodologic covariates and the linear term for age and the diagnosis of ADHD at baseline.
p < .05;
p < .01;
p < .0001 (not corrected for multiple comparisons)
Note: P = parent informant; T = teacher informant; Y = youth informant; I = interviewer informant; hyper-Imp = hyperactivity-impulsivity; ODD = oppositional defiant disorder; CD = conduct disorder; CGAS = Children’s Global Assessment Scale (higher scores indicate better functioning); covariates of no interest in all multiple regression models were site, cohort, blindness of interviewer to previous interviews of parent and youth, number of informants (for variables based on parent + teacher informants), sex of the child, and race-ethnicity.
Figure 1.
Mean numbers of nine dimensions of symptoms across ages 4–18 years in children who met DSM-IV symptom criteria for ADHD and exhibited impairment in at least one setting at 4–6 years of age and in non-ADHD comparison children who were approximately matched on age, sex, and race-ethnicity.
In a separate set of analyses, both the linear and quadratic terms for age were jointly tested in longitudinal GEE among only children with ADHD at 4–6 years to examine non-linear developmental changes in symptoms in this group. These models controlled the same demographic and methodologic covariates as the models reported in Table 3. As can be seen in Figure 1, the quadratic terms for age were significant for parent- and teacher-rated inattention, β = −0.00, χ2 = −4.69, p < .0001, and ODD symptoms, β = −0.01, χ2 = −3.67, p < .0002, and for parent-rated anxiety symptoms, β = −0.01, χ2 = −3.33, p < .0001. In each case, these significant terms reflect increasingly more rapid declines in symptoms at older ages. The quadratic term for age also was significant for youth-rated depression symptoms, β = 0.02, χ2 = 7.17, p < .0001, but this term reflected a steeper decline in depression symptoms across younger than older ages. The quadratic terms for age were not significant at p < .05 for parent- and teacher-rated CD, youth-rated CD, parent-rated depression symptoms, or either CGAS score.
Differences in Outcomes between Children with and without ADHD at Baseline
The second goal of the study was to compare the outcomes of young children with and without ADHD through adolescence. These groups are first compared across the entire span of 4–18 years and then only during adolescence using GEE.
Differences in Symptoms and Global Impairment across 4–18 Years
Counts of symptoms and measures of functional impairment at each age across 4–18 years were compared between the children given the diagnosis of ADHD at ages 4–6 years and the healthy comparison children. As reported in columns 4 and 5 in the top rows shown of Table 3, and as shown in Figure 1, the longitudinal analyses of outcome data across ages 4–18 years revealed that children given the diagnosis of ADHD exhibited greater numbers of symptoms of every dimension of psychopathology (i.e., inattention, hyperactivity-impulsivity, ODD, CD, anxiety, and depression) reported by parents and teachers or by the youth themselves. In addition, the tests of diagnosis-by-age interactions (columns 6 and 7 in the top rows of Table 3) revealed significantly steeper age-related declines in inattention symptoms in children with ADHD than in comparison children. Conversely, there was a less steep increase in youth-reported CD symptoms among children with ADHD than among the comparison children, whose childhood levels of CD symptoms were low.
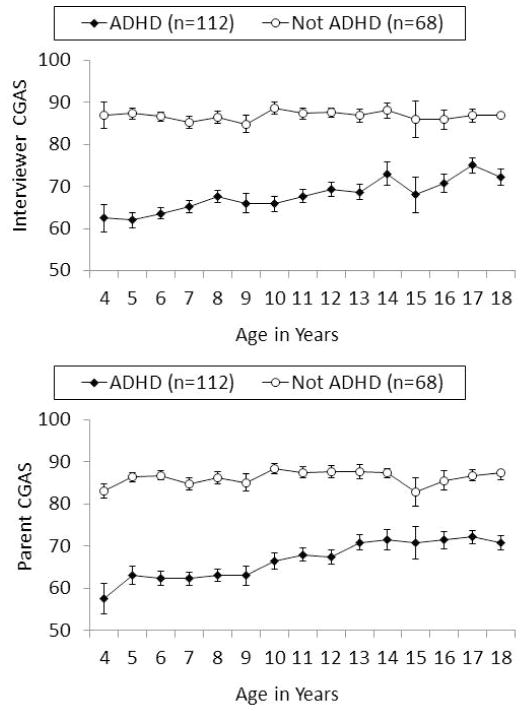
As reported at the bottom rows of Table 3 and shown in Figure 2, the longitudinal analyses based on the assumption of normally distributed global functioning scores also revealed marked differences between children with ADHD and comparison children on both parent- and interviewer-rated CGAS scores across 4–18 years. For example, the linear coefficient (β) for group differences in with parent-reported CGAS ratings indicated an expected 16.87 points lower rating across these ages for children with ADHD than children without ADHD. In addition, the significant age-by-group interactions reflected significantly steeper increases in both CGAS scores over increasing age in the ADHD than the comparison group.
Figure 2.
Mean parent and interviewer ratings on the Children’s Global Assessment Scale (CGAS) across ages 4–18 years in children who met DSM-IV symptom criteria for ADHD and exhibited impairment in at least one setting at 4–6 years of age and in non-ADHD comparison children who were approximately matched on age, sex, and race-ethnicity.
Diagnostic Group Differences in Symptoms and Functioning during Adolescence
In spite of improvements in symptoms across ages 4–18 years in children with ADHD at baseline, relative to the comparison group, the ADHD group still exhibited significantly higher mean numbers of parent-and teacher-reported symptoms of inattention (β = 0.82, χ2 = 18.88, p < 0.0001), hyperactivity-impulsivity (β = 1.62, χ2 = 31.39, p < 0.0001), ODD (β = 1.22, χ2 = 15.57, p < 0.0001), and CD (β = 1.01, χ2 = 8.98, p < 0.01), and greater youth-reported symptoms of anxiety (β = 0.84, χ2 = 11.04, p < 0.001) and depression (β = 0.60, χ2 = 9.63, p < 0.01), during ages 15–18 years. There were no significant ADHD group differences at p < .05 in mean parent-reported symptoms of anxiety or depression or youth-reported CD during 15–18 years of age, however. Because the difference in global ratings of impairment narrowed between children with ADHD and comparison children with increasing age, we evaluated differences in measures of impaired functioning during only adolescence (15–18 years). Children with ADHD at 4–6 years were given significantly lower mean parent CGAS ratings (M = 73.10, SD= 13.74) than comparison children (M = 86.25, SD= 11.19), β = −13.56, χ2 = 38.52, p < .0001 (Cohen’s d = −1.05), and significantly lower mean interviewer CGAS scores (M = 72.03, SD = 15.34) than comparison children (M = 85.83, SD = 12.78), β = −13.35, χ2 = 36.49, p < .0001 (Cohen’s d = −0.98), averaged across 15–18 years to create a composite measure.
Furthermore, during 15–18 years of age, more children with ADHD at 4–6 years were arrested at least once (29.5%) than comparison children (8.8%), adjusted OR = 4.31; 95% CI: 1.70 – 10.95. The percent of children who had obtained a driver’s license or permit by age 18 years did not reliably differ between those with ADHD (64.3%) and comparison children (77.9%), adjusted OR = 0.49; 95% CI: 0.20 – 1.17. Furthermore, the percent of children who had ever operated a motor vehicle by age 18 years did not reliably differ between those with ADHD (89.7%) and comparison children (90.2%), adjusted OR = 1.02; 95% CI: 0.35 – 3.02. Nonetheless, children with ADHD were more likely to operate a motor vehicle without a valid license or permit (51.8%) than comparison children (32.8%), adjusted OR = 2.79; 95% CI: 1.31 – 5.97, and were more likely to both ride a motorcycle and do so without a helmet (33.3%) than comparison children (7.5%), adjusted OR = 4.78; 95% CI: 1.77 – 12.91. Similarly, during waves L-P (15–18 years), more children with ADHD (21.4%) than comparison children (2.9%) experienced at least one unintentional injury attributed to the adolescent’s behavior, adjusted OR = 12.19; 95% CI: 2.36 – 62.89. In contrast, during adolescence, youth with ADHD at baseline were not more likely to report receiving a ticket for a moving violation (23.8%) than comparison children (16.4%), adjusted OR = 1.42; 95% CI: 0.59 – 3.43, and were not more likely to report being in a motor vehicle accident when driving (27.8%) than comparison children (31.3%) (adjusted OR = 0.90; 95% CI: 0.44 – 1.86). During adolescence, 13.4% of children with ADHD at baseline and 4.4% of comparison children had dropped out of school, but this difference did not exceed chance levels (adjusted OR = 2.39; 95% CI: 0.62 – 9.24).
Prognosis: Predictors of Functional Outcomes among Children with ADHD at Baseline
The third goal of the study was to assess the possibility of making accurate long-term prognoses from the kinds of clinical measures obtained in structured diagnostic assessments at 4–6 years. To do so, tests were conducted to identify baseline variables that predict future individual differences in the global and specific measures of functional impairment among only children with ADHD using GEE. Baseline predictors were first tested for future outcomes across 7–18 years of age (after the baseline assessments at 4–6 years) and then tested as predictors of mean outcomes during the adolescence years only using generalized linear models for continuous outcomes and logistic regression for binary outcomes.
Predictors of Outcomes across 7–18 Years of Age among Children with ADHD
As summarized in Table 4, there was robust continuity over many years, with each dimension of symptoms at baseline significantly predicting itself in future years when the dimension was measured by the same informant (i.e., parent) or informants (i.e., parents and teachers) over time. In addition, baseline ODD symptoms predicted future levels of hyperactivity-impulsivity symptoms, baseline anxiety symptoms predicted future ODD and depression symptoms, and baseline CD symptoms predicted future depression symptoms. In addition, tests of the predictive associations of baseline variables with future youth-reported symptoms reported in Table 5 showed that parent- and teacher-reported CD and anxiety symptoms at baseline predicted youth reports of the same dimension of symptoms across 9–18 years, but no dimension of symptoms at baseline significantly predicted youth-reported symptoms of depression through age 18 years.
Table 4.
Prediction of adult-reported symptoms across 7–18 years of age from variables measured at 4–6 years of age, controlling for all other predictors and covariates among 125 children who met symptom criteria for attention-deficit/hyperactivity disorder with impairment in at least one setting at 4–6 years.
| Predictors at 4–6 years | β | χ2 |
|---|---|---|
| Outcome: Inattention Symptoms (P+T) | ||
| Longitudinal Age | −0.05 | −7.25*** |
| Female sex | −0.02 | −0.26 |
| Total family income (log) | −0.06 | −2.28* |
| Intelligence | 0.00 | 0.24 |
| Inattention symptoms (P+T) | 0.08 | 3.39** |
| Hyper-imp symptoms (P+T) | 0.02 | 0.98 |
| ODD symptoms (P+T) | 0.00 | 0.08 |
| CD symptoms (P+T) | 0.03 | 1.45 |
| Depression symptoms (P) | 0.00 | 0.08 |
| Anxiety symptoms (P) | −0.00 | −0.27 |
| Outcome: Hyper-Imp Symptoms (P+T) | ||
| Longitudinal Age | −0.08 | −10.31*** |
| Female sex | 0.02 | 0.20 |
| Total family income (log) | −0.10 | −2.97** |
| Intelligence | −0.00 | −0.23 |
| Inattention symptoms (P+T) | −0.01 | −0.57 |
| Hyper-imp symptoms (P+T) | 0.13 | 5.28*** |
| ODD symptoms (P+T) | 0.04 | 2.71** |
| CD symptoms (P+T) | −0.00 | −0.16 |
| Depression symptoms (P) | −0.01 | −0.61 |
| Anxiety symptoms (P) | 0.01 | 1.00 |
| Outcome: ODD Symptoms (P+T) | ||
| Longitudinal Age | −0.08 | −7.82*** |
| Female sex | 0.24 | 2.14* |
| Total family income (log) | −0.13 | −3.01** |
| Intelligence | 0.00 | 0.49 |
| Inattention symptoms (P+T) | 0.00 | 0.08 |
| Hyper-imp symptoms (P+T) | 0.01 | 0.36 |
| ODD symptoms (P+T) | 0.10 | 3.86*** |
| CD symptoms (P+T) | 0.06 | 1.77 |
| Depression symptoms (P) | −0.00 | −0.04 |
| Anxiety symptoms (P) | 0.03 | 2.84** |
| Outcome: CD Symptoms (P+T) | ||
| Longitudinal Age | −0.04 | −2.37* |
| Female sex | 0.23 | 1.05 |
| Total family income (log) | −0.19 | −2.59** |
| Intelligence | 0.00 | 0.69 |
| Inattention symptoms (P+T) | 0.01 | 0.18 |
| Hyper-imp symptoms (P+T) | 0.01 | 0.37 |
| ODD symptoms (P+T) | 0.03 | 0.96 |
| CD symptoms (P+T) | 0.18 | 4.53*** |
| Depression symptoms (P) | 0.03 | 0.51 |
| Anxiety symptoms (P) | 0.05 | 1.99* |
| Outcome: Anxiety Symptoms (P) | ||
| Longitudinal Age | −0.12 | −6.15*** |
| Female sex | 0.62 | 2.78** |
| Total family income (log) | 0.01 | 0.14 |
| Intelligence | −0.00 | −0.26 |
| Inattention symptoms (P+T) | −0.02 | −0.34 |
| Hyper-imp symptoms (P+T) | 0.04 | 0.58 |
| ODD symptoms (P+T) | 0.03 | 0.68 |
| CD symptoms (P+T) | 0.07 | 1.76 |
| Depression symptoms (P) | 0.10 | 1. 96* |
| Anxiety symptoms (P) | 0.08 | 3.97*** |
| Outcome: Depression Symptoms (P) | ||
| Longitudinal Age | −0.03 | −2.04* |
| Female sex | 0.50 | 2.58** |
| Total family income (log) | −0.02 | −0.28 |
| Intelligence | 0.00 | 0.52 |
| Inattention symptoms (P+T) | 0.02 | 0.65 |
| Hyper-imp symptoms (P+T) | 0.02 | 0.38 |
| ODD symptoms (P+T) | 0.00 | 0.09 |
| CD symptoms (P+T) | 0.08 | 2.56* |
| Depression symptoms (P) | 0.13 | 3.08** |
| Anxiety symptoms (P) | 0.03 | 2.05* |
p < .05;
p < .01;
p < .0001 (not corrected for multiple comparisons)
Note: P = parent informant; T = teacher informant; Hyper-Imp = hyperactivity-impulsivity; ODD = oppositional defiant disorder; CD = conduct disorder; covariates of no interest in all multiple regression models were site, cohort, blindness of interviewer to previous interviews of parent and youth, number of informants (for variables based on parent + teacher informants), sex of the child, race-and ethnicity contrasts.
Table 5.
Prediction of youth-reported symptoms across 9–18 years of age from variables measured at 4–6 years of age, controlling for all other predictors and covariates among 125 children who met symptom criteria for attention-deficit/hyperactivity disorder with impairment in at least one setting at 4–6 years.
| Predictors at 4–6 years | β | χ2 |
|---|---|---|
| Outcome: CD Symptoms (Y) | ||
| Longitudinal Age | 0.20 | 5.61*** |
| Female sex | −0.04 | −0.12 |
| Total family income (log) | −0.06 | −0.51 |
| Intelligence | 0.01 | 0.70 |
| Inattention symptoms (P+T) | 0.07 | 1.07 |
| Hyper-imp symptoms (P+T) | −0.00 | −0.04 |
| ODD symptoms (P+T) | −0.04 | −0.70 |
| CD symptoms (P+T) | 0.15 | 3.07** |
| Depression symptoms (P) | 0.06 | 0.95 |
| Anxiety symptoms (P) | 0.04 | 1.27 |
| Outcome: Anxiety Symptoms (Y) | ||
| Longitudinal Age | −0.12 | −4.94*** |
| Female sex | 0.84 | 4.17*** |
| Total family income (log) | 0.04 | 0.54 |
| Intelligence | −0.03 | −3.89*** |
| Inattention symptoms (P+T) | 0.02 | 0.44 |
| Hyper-imp symptoms (P+T) | 0.02 | 0.39 |
| ODD symptoms (P+T) | −0.00 | −0.13 |
| CD symptoms (P+T) | −0.04 | −0.62 |
| Depression symptoms (P) | −0.06 | −1.38 |
| Anxiety symptoms (P) | 0.07 | 2.67** |
| Outcome: Depression Symptoms (Y) | ||
| Longitudinal Age | −0.12 | −7.63*** |
| Female sex | 0.21 | 1.18 |
| Total family income (log) | 0.02 | 0.44 |
| Intelligence | −0.01 | −2. 18* |
| Inattention symptoms (P+T) | 0.01 | 0.40 |
| Hyper-imp symptoms (P+T) | −0.01 | −0.37 |
| ODD symptoms (P+T) | 0.03 | 1.00 |
| CD symptoms (P+T) | −0.00 | −0.11 |
| Depression symptoms (P) | 0.00 | 0.05 |
| Anxiety symptoms (P) | 0.03 | 1.67 |
p < .05;
p < .01;
p < .0001
Note: P = parent informant; T = teacher informant; Y = youth informant; Hyper-Imp = hyperactivity-impulsivity; ODD = oppositional defiant disorder; CD = conduct disorder; covariates of no interest in all multiple regression models were site, cohort, blindness of interviewer to previous interviews of parent and youth, number of informants (for variables based on parent + teacher informants), race-and ethnicity contrasts (Bonferroni corrected alpha for 30 tests .002).
As reported in Tables 4 and 5, girls had higher levels of parent- and teacher-reported ODD and parent-reported depression and anxiety symptoms and girls had higher levels of self-reported anxiety symptoms across 7–18 years. Lower family income at baseline significantly predicted higher future levels of parent- and teacher-reported inattention, hyperactivity-impulsivity, ODD, and CD symptoms, but did not predict youth-reported CD or internalizing symptoms. Child intelligence scores did not independently predict any dimension of future symptoms reported by adults, but were inversely related to youth-reported anxiety and depression across 9–18 years (the ages at which youth were interviewed about themselves).
In addition, baseline clinical measures at 4–6 years were tested as predictors of global and specific measures of functional impairment among only children with ADHD across 7–18 years. As reported in Table 6, among children with ADHD, higher family income predicted higher (i.e., better) parent CGAS ratings and female sex predict lower (i.e., poorer) parent CGAS scores across 7–18 years, but only family income predicted interviewer CGAS ratings. In addition, greater numbers of inattention, ODD, and CD symptoms each significantly predicted lower parent CGAS ratings across 7–18 years and greater numbers of anxiety and ODD symptoms predicted lower interviewer CGAS ratings.
Table 6.
Prediction of parent- and interviewer-rated global functioning across 7–18 years of age from variables measured at 4–6 years of age, simultaneously controlling for all other predictors and covariates among 125 children who met symptom criteria for attention-deficit/hyperactivity disorder with impairment in at least one setting at 4–6 years.
| Predictors at 4–6 years | β | χ2 |
|---|---|---|
| Outcome: Parent CGAS 7–18 Years | ||
| Longitudinal Age | 0.82 | 5.04*** |
| Female sex | −6.39 | −2.83** |
| Total family income (log) | 2.62 | 2.57* |
| Intelligence | −0.08 | −1.09 |
| Inattention symptoms (P+T) | −1.06 | −2.43* |
| Hyper-imp symptoms (P+T) | 0.03 | 0.06 |
| ODD symptoms (P+T) | −1.24 | −2.77** |
| CD symptoms (P+T) | −1.26 | −2.16* |
| Depression symptoms (P) | 0.46 | 0.90 |
| Anxiety symptoms (P) | −0.41 | −1.62 |
| Outcome: Interviewer CGAS 7–18 Years | ||
| Longitudinal Age | 0.97 | 5.86*** |
| Female sex | −4.21 | −1.86 |
| Total family income (log) | 2.41 | 2.43* |
| Intelligence | 0.05 | 0.64 |
| Inattention symptoms (P+T) | −0.85 | −1.81 |
| Hyper-imp symptoms (P+T) | −0.71 | −1.49 |
| ODD symptoms (P+T) | −1.08 | −2.39* |
| CD symptoms (P+T) | −0.80 | −1.29 |
| Depression symptoms (P) | −0.36 | −0.63 |
| Anxiety symptoms (P) | −0.70 | −2.59** |
p < .05;
p < .01;
p < .0001
Note: CGAS = Children’s Global Assessment Scale; P = parent informant; T = teacher informant; Hyper-Imp = hyperactivity-impulsivity; ODD = oppositional defiant disorder; CD = conduct disorder; covariates of no interest in all multiple regression models were site, cohort, blindness of interviewer to previous interviews of parent and youth, race-and ethnicity contrasts (Bonferroni corrected alpha for 20 tests = .0025).
Predictors of Outcomes across 15–18 Years of Age among Children with ADHD
The same baseline variables also were tested as predictors of the mean of adolescent CGAS ratings averaged across 15–18 years (not tabled) among the 112 youth who met criteria ADHD at baseline and were assessed at least once during adolescence. Again, females with ADHD at baseline had significantly lower parent (β = −8.89, χ2 = 6.97, p < 0.01) and interviewer (β = −9.48, χ2 = 8.67, p < 0.01) CGAS scores by about 9 points during adolescence. There were no other significant predictors of mean parent CGAS scores during adolescence, but baseline anxiety symptoms (β = −0.94, χ2 = 5.49, p < 0.05) and CD symptoms (β = −1.64, χ2 = 5.50, p < .05) each inversely predicted mean interviewer CGAS ratings during adolescence. In addition, children with ADHD at 4–6 years who had higher numbers of inattention symptoms were found to be more likely to experience at least one unintentional injury across 15–18 years (β = 0.38, χ2 = 3.97, p < .05) and those with more CD symptoms were more likely to drive without a license, drop out of school, and be arrested at least once during adolescence (all p < .05) using logistic regression. For example, the odds ratio of 1.57 (95% CI: 1.15 – 2.15) adjusted for all methodologic and demographic covariates and all other dimensions of symptoms at baseline indicated that the odds of arrest during 15–18 years were 57% greater at each greater number of CD symptoms (observed range of CD symptoms = 0 – 9).
Children with Functioning in the Normative Range during Adolescence
We identified a small group of children who were arguably functioning in the normative range during ages 15–18 years in a series of steps. First, 17 of the 112 children with ADHD at baseline who were assessed at least once during 15–18 years were reported to exhibit an average of ≤ 1 ADHD symptoms (inattention and hyperactivity-impulsivity combined) across 15–18 years; 66.18% of the controls who participated in at least one assessment at these ages exhibited the same low level of ADHD symptoms. Of this group of 17 children with a diagnosis of ADHD at baseline with few symptoms of ADHD during adolescence, 12 also showed no evidence of serious functional impairment, defined as having both mean parent and mean interviewer CGAS scores of ≥ 71i, no unintentional injuries, no arrests, and no suicide attempts during ages 15–18 years, suggesting normative functioning.
We tested the possibility that the 12 children with the best outcomes simply had fewer informants involved fewer adolescent assessments using generalized linear models. The total number of informants participating in the assessment across ages 15–18 years in the best outcomes group (M = 5.17; SD = 2.62) did not differ at .05 from the rest of the children with ADHD (M = 5.67; SD = 2.40). We also tested the association between membership in the best outcomes group and the number of situations in which impaired functioning was reported in the diagnostic assessment of ADHD at 4–6 years. Only one of the 12 children in the best outcomes group was reported to be impaired in only one setting at baseline with the remainder being reported to be impaired in two or more settings, OR = 0.22 (95% CI: 0.02 – 2.68. Controlling for the methodologic and demographic covariates, the number years of taking medication also was not significantly related to a best outcome, but the number of years of psychosocial treatment was inversely related to best outcomes (p < .05). As shown in Figure 3, logistic regression analysis showed that the only baseline demographic or symptom predictor of membership in the best outcomes group among children with ADHD was a lower number of hyperactivity-impulsivity symptoms (OR = 0.59, 95% CI: 0.38 – 0.93) at 4–6 years of age. We examined the sensitivity and specificity of this predictive association to determine if it could be of clinical utility in predicting the outcomes of individual children. Although Figure 3 shows that children with 9 hyperactivity-impulsivity symptoms are much less likely than other children to have a best outcome than other children with ADHD at baseline, the sensitivity (.17) and specificity (.53) of this predictor-outcome association are weak.
Figure 3.
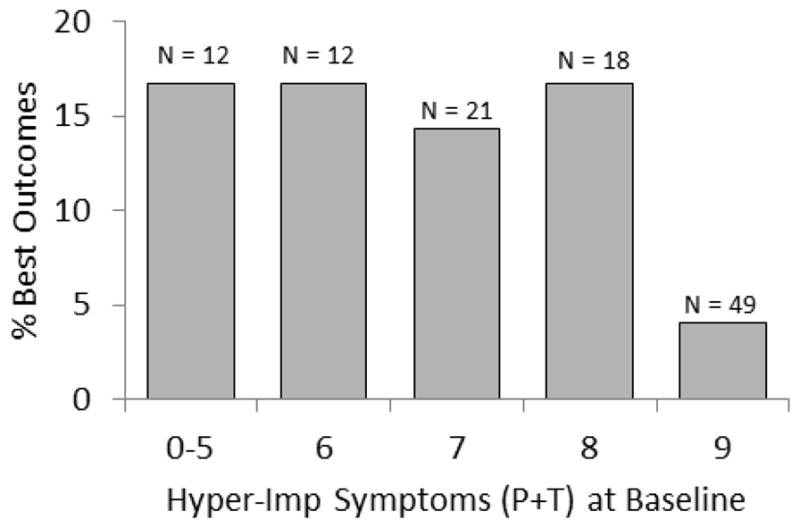
The proportions of children who met criteria for ADHD at 4–6 years of age who were classified as functioning in the normative range (defined in text) at 15–18 years of age at each level of parent- and teacher-reported hyperactivity-impulsivity symptoms (left panel) and parent-reported anxiety symptoms (right panel) measured in the baseline assessment.
CONCLUSIONS
Across a broad range of outcome variables, longitudinal analyses across ages 4–18 revealed declining mean levels of symptoms and improving average levels of global functioning in children given the diagnosis of ADHD at baseline.ii Despite their improved functioning, on average, children with ADHD at 4–6 years of age continued to exhibit more symptoms of a broad range of dimensions of psychopathology and substantially lower global ratings of adaptive functioning across childhood and through adolescence than healthy controls.
Furthermore, children given the diagnosis of ADHD at young ages were at substantially greater risk than controls to be arrested, to experience unintentional injuries, and to engage in risky behaviors with motor vehicles during adolescence. These findings are consistent with previous prospective studies of the long-term outcomes of school-aged children given the diagnosis of ADHD (Barkley, Fischer, Smallish, & Fletcher, 2004; Barkley, Guevremont, Anastopoulos, Dupaul, & Shelton, 1993; Mannuzza et al., 1998). We did not detect a greater risk of motor vehicle accidents in the ADHD children during adolescence in this study, but we did not follow them into adulthood as in previous research (Barkley & Cox, 2007; Fischer, Barkley, Smallish, & Fletcher, 2002, 2007; Thompson, Molina, Pelham, & Gnagy, 2007). Like previous studies of older youth with ADHD (Kent et al., 2011; Trampush et al., 2009), but unlike Kuriyan et al. (2013), we did not find that ADHD in early childhood predicted dropping out of school by age 18. The present findings should be interpreted along with those of another recent report from the same sample in which the children given the diagnosis of ADHD at 4–6 years also were more likely to use cigarettes, alcohol, and marijuana and to progress to heavier use than comparison children (Sibley et al., 2014).
Together, these findings strongly suggest that the diagnosis of ADHD is valid in young children in the sense of predicting robustly elevated levels of symptoms and impairment over long periods of time in most children who were given the diagnosis at 4–6 years. Because the children in the present naturalistic study received a variety of clinical treatments over time, it is not clear if the present findings accurately estimate the adverse outcomes of untreated young children with ADHD. Studies like the present one do not allow unconfounded assessments of the effects of treatments. Nonetheless, the treatments sought by families for their children were not associated with fewer symptoms and lesser impairment over time, suggesting that improving symptoms and impairment are not due solely to treatments, if at all.
Predictors of Individual Differences in Outcomes
In spite of the poor adolescent outcomes of young children with ADHD on average, there were marked individual differences in these outcomes. As reported in Tables 3–6 and the results section, measures like those obtained in a comprehensive clinical assessment provided statistically significant prediction of individual differences in outcomes at the group level. In particular, the number of hyperactivity-impulsivity symptoms at baseline inversely predicted functioning in the normative range during adolescence. Consistent with a previous longitudinal study showing persisting deficits in girls with ADHD during adolescence (Hinshaw et al., 2012; Owens, Hinshaw, Lee, & Lahey, 2009), girls with the diagnosis of ADHD at baseline had poorer long-term outcomes than boys with ADHD. In addition, consistent with other findings, among children with ADHD at baseline, lower family income predicted less adaptive outcomes (Law et al., 2014).
Statistical Issues in the Interpretation of the Present Findings
This comprehensive evaluation of the adolescent outcomes of ADHD diagnosed at early ages necessarily involved a large number of statistical tests. Counting only the number of statistical tests reported in Tables 1–5, 239 tests were conducted. In addition, 32 tests of potential attrition biases, 22 preliminary tests of treatment variables were conducted, and 70 additional tests were reported in this manuscript. Thus, a total of 363 individual statistical tests were reported. If these tests were evaluated using a strict Bonferroni-correction (which is designed to avoid a single false discovery in a group of tests), alpha would be .00014. Notably, the 69 tests (among the 309 substantive tests tabled and reported in the text) that were significant at p <.0001 still would be considered to be significant even according to this strict standard. Furthermore, interpretations of the likelihood that a statistically significant association reflects a true association in the population should be based on both the p value and the prior probability of observing an association (Sellke, Bayarri, & Berger, 2001). In this paper, there were few findings that were not consistent with previous findings of studies conducted over shorter periods of time. Thus, although every finding requires replication, the interpretations of the findings of the present study as a whole are adequately founded. Nonetheless, the predictions of best outcomes during adolescence among children with ADHD at 4–6 years by adult reports of anxiety and hyperactivity-impulsivity symptoms (and not other symptom dimensions) at baseline were not based on previous findings and should be interpreted cautiously until attempts at replication are completed.
Limitations
It is important to note that a reliable and valid structured diagnostic assessment of symptoms was conducted in each wave of the present study. Therefore, the present results may not apply to unstructured clinical assessments. Like essentially all longitudinal studies of ADHD, particularly studies with a focus on diagnoses at an early age, this study ascertained a sample that cannot be said to be representative of children given the diagnosis of ADHD. The sample size provided reasonable statistical power for all analyses related to the goals of the study, but a larger sample size could have resulted in the detection of more associations with adverse outcomes. In particular, a larger sample may have allowed identification of more significant interactions between changes over increasing age and diagnostic groups. This is particularly the case because the lack of steady and fully adequate funding required the omission of some annual assessments and a reduction in the number of the healthy comparison children who could be followed through adolescence.
Clinical Implications
A primary goal of clinical assessments is to determine if the risks inherent in labeling and treatment exceed the risks inherent in not intervening. That decision requires knowledge of both the adverse consequences of labeling and treating (Hinshaw, 2006) and knowledge of the adverse outcomes expected for children who meet criteria for ADHD in the absence of effective treatment. The present findings suggest that the great majority of children given the diagnosis of ADHD at 4–6 years of age continue to exhibit more symptoms and greater functional impairment in a variety of global and specific domains through adolescence than healthy comparison children. Thus, the outcomes of young children with the diagnosis of ADHD in the present study are of value in balancing the risks and benefits of labeling and treatment against the expectation of long-term symptoms and impairment if interpreted with caution.
Even if these several findings are closely replicated, however, the degree of prediction of future outcomes based on demographic factors and numbers of these symptoms probably would not allow clinical prognosis that is accurate enough at the level of the individual child to make treatment decisions. That is, based on the kinds of measures obtained in even comprehensive and standardized clinical assessments at 4–6 years, the level of prediction of adaptive adolescent outcomes does not appear to be accurate enough to withhold diagnosis or treatment from some young children with ADHD symptoms, particularly if the treatments are low-risk interventions.
Lay summary.
This study provides evidence that the diagnostic criteria for attention-deficit/hyperactivity disorder (ADHD) can be used validly with 4–6 year old children. A small percentage of young children with ADHD appear to function in the normative range during adolescence, but which children will improve to that degree cannot be accurately predicted at present. Because the great majority of young children with ADHD children continue to be impaired in their functioning and to be at risk for accidents throughout childhood and adolescence, the risks inherent in treating may be lower than the risks inherent in not treating.
Acknowledgments
This study was supported by grant R01 MH053554. We sincerely appreciate the essential contributions to this study of Heidi Kipp and the project directors, interviewers, and families over the years. We also greatly appreciate the helpful statistical advice from Paul J. Rathouz.
Footnotes
This threshold was based on face validity: The descriptor for CGAS ratings of 71–80 is “doing all right” with the text explanation stating that “Any problem in functioning is temporary and mild.” In contrast, the descriptor for ratings of 61–70 is “some problems,” with the statement that “… persons who know her/him well could be concerned.”
The sole exception was an age-related increase in symptoms of CD across late childhood and adolescence in both children with ADHD and comparison children.
Contributor Information
Benjamin B. Lahey, University of Chicago
Steve S. Lee, University of California, Los Angeles
Margaret H. Sibley, Florida International University
Brooks Applegate, Western Michigan University.
Brooke S. G. Molina, University of Pittsburgh
William E. Pelham, Florida International University
References
- American Academy of Pediatrics. ADHD: Clinical Practice Guideline for the Diagnosis, Evaluation, and Treatment of Attention-Deficit/Hyperactivity Disorder in Children and Adolescents. Pediatrrics. 2011;128:1–16. doi: 10.1542/peds.2011-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley RA. Attention deficit hyperactivity disorder: A handbook for diagnosis and treatment. 3. New York: Guilford; 2005. [Google Scholar]
- Barkley RA, Cox D. A review of driving risks and impairments associated with attention-deficit/hyperactivity disorder and the effects of stimulant medication on driving performance. Journal of Safety Research. 2007;38:113–128. doi: 10.1016/j.jsr.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Fischer M, Smallish L, Fletcher K. Young adult follow-up of hyperactive children: Antisocial activities and drug use. Journal of Child Psychology and Psychiatry. 2004;45:195–211. doi: 10.1111/j.1469-7610.2004.00214.x. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Guevremont DC, Anastopoulos AD, Dupaul GJ, Shelton TL. Driving-related risks and outcomes of attention-deficity hyperactivity disorder in adolescents and young adults: A 3-year to 5-year follow-up survey. Pediatrics. 1993;92:212–218. [PubMed] [Google Scholar]
- Biederman J, Monuteaux MC, Mick E, Spencer T, Wilens TE, Silva JM, Faraone SV. Young adult outcome of attention deficit hyperactivity disorder: a controlled 10-year follow-up study. Psychol Med. 2006;36:167–179. doi: 10.1017/S0033291705006410. [DOI] [PubMed] [Google Scholar]
- Bird HR, Gould MS, Staghezza B. Aggregating data from multiple informants in child psychiatry epidemiological research. Journal of the American Academy of Child & Adolescent Psychiatry. 1992;31:78–85. doi: 10.1097/00004583-199201000-00012. [DOI] [PubMed] [Google Scholar]
- Bufferd SJ, Dougherty LR, Carlson GA, Rose S, Klein DN. Psychiatric disorders in preschoolers: Continuity from ages 3 to 6. American Journal of Psychiatry. 2012;169:1157–1164. doi: 10.1176/appi.ajp.2012.12020268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunte TL, Schoemaker K, Hessen DJ, van der Heijden PGM, Matthys W. Stability and change of ODD, CD and ADHD diagnosis in referred preschool children. Journal of Abnormal Child Psychology. 2014;42:1213–1224. doi: 10.1007/s10802-014-9869-6. [DOI] [PubMed] [Google Scholar]
- Fabiano GA, Pelham WE, Waschbusch DA, Gnagy EM, Lahey BB, Chronis AM, Burrows-MacLean L. A practical measure of impairment: Psychometric properties of the impairment rating scale in samples of children with attention deficit hyperactivity disorder and two school-based samples. Journal of Clinical Child and Adolescent Psychology. 2006;35:369–385. doi: 10.1207/s15374424jccp3503_3. [DOI] [PubMed] [Google Scholar]
- Fischer M, Barkley RA, Smallish L, Fletcher K. Young adult follow-up of hyperactive children: Self-reported psychiatric disorders, comorbidity, and the role of childhood conduct problems and teen CD. Journal of Abnormal Child Psychology. 2002;30:463–475. doi: 10.1023/a:1019864813776. [DOI] [PubMed] [Google Scholar]
- Fischer M, Barkley RA, Smallish L, Fletcher K. Hyperactive children as young adults: Driving abilities, safe driving behavior, and adverse driving outcomes. Accident Analysis and Prevention. 2007;39:94–105. doi: 10.1016/j.aap.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Hart EL, Lahey BB, Loeber R, Hanson KS. Criterion validity of informants in the diagnosis of disruptive behavior disorders in children: A preliminary study. Journal of Consulting and Clinical Psychology. 1994;62:410–414. doi: 10.1037/0022-006X.62.2.410. [DOI] [PubMed] [Google Scholar]
- Hinshaw SP. Attention deficits and hyperactivity in children. Los Angeles: Sage; 1994. [Google Scholar]
- Hinshaw SP. The mark of shame: Stigma of mental illness and an agenda for change. New York: Oxford University Press; 2006. [Google Scholar]
- Hinshaw SP, Owens EB, Zalecki C, Huggins SP, Montenegro-Nevado AJ, Schrodek E, Swanson EN. Prospective follow-up of girls with attention-deficit/hyperactivity disorder Into early adulthood: Continuing impairment Includes elevated risk for suicide attempts and self-injury. Journal of Consulting and Clinical Psychology. 2012;80:1041–1051. doi: 10.1037/a0029451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen PS, Rubio-Stipec M, Canino G, Bird HR, Dulcan MK, Schwab-Stone ME, Lahey BB. Parent and child contributions to diagnosis of mental disorder: Are both informants always necessary? Journal of the American Academy of Child & Adolescent Psychiatry. 1999;38:1569–1579. doi: 10.1097/00004583-199912000-00019. [DOI] [PubMed] [Google Scholar]
- Kent KM, Pelham WE, Jr, Molina BSG, Sibley MH, Waschbusch DA, Yu J, Karch KM. The academic experience of male high school students with ADHD. Journal of Abnormal Child Psychology. 2011;39:451–462. doi: 10.1007/s10802-010-9472-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyan AB, Pelham WE, Molina BSG, Waschbusch DA, Gnagy EM, Sibley MH, Kent KM. Young adult educational and vocational outcomes of children diagnosed with ADHD. Journal of Abnormal Child Psychology. 2013;41:27–41. doi: 10.1007/s10802-012-9658-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey BB, Applegate B, Waldman ID, Loft JD, Hankin BL, Rick J. The structure of child and adolescent psychopathology: Generating new hypotheses. Journal of Abnormal Psychology. 2004;113:358–385. doi: 10.1037/0021-843X.113.3.358. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Loeber R, Quay HC, Applegate B, Shaffer D, Waldman I, Bird H. Validity of DSM-IV subtypes of conduct disorder based on age of onset. Journal of the American Academy of Child and Adolescent Psychiatry. 1998;37:435–442. doi: 10.1097/00004583-199804000-00022. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Pelham WE, Loney J, Kipp H, Ehrhardt A, Lee SS, Massetti G. Three-year predictive validity of DSM-IV attention deficit/hyperactivity disorder in children diagnosed at 4–6 years of age. American Journal of Psychiatry. 2004;161:2014–2020. doi: 10.1176/appi.ajp.161.11.2014. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Pelham WE, Loney J, Lee SS, Willcutt E. Instability of the DSM-IV subtypes of ADHD from preschool through elementary school. Archives of General Psychiatry. 2005;62:896–902. doi: 10.1001/archpsyc.62.8.896. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Pelham WE, Stein MA, Loney J, Trapani C, Nugent K, Baumann B. Validity of DSM-IV attention-deficit/hyperactivity disorder for younger children. Journal of the American Academy of Child and Adolescent Psychiatry. 1998;37:695–702. doi: 10.1097/00004583-199807000-00008. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Rathouz PJ, Applegate B, Hulle CV, Garriock HA, Urbano RC, Waldman ID. Testing structural models of DSM-IV symptoms of common forms of child and adolescent psychopathology. Journal of Abnormal Child Psychology. 2008;36:187–206. doi: 10.1007/s10802-007-9169-5. [DOI] [PubMed] [Google Scholar]
- Law EC, Sideridis GD, Prock LA, Sheridan MA. Attention-deficit/hyperactivity disorder in young children: Predictors of diagnostic stability. Pediatrics. 2014;133:659–667. doi: 10.1542/peds.2013-3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS, Lahey BB, Owens EB, Hinshaw SP. Few preschool boys and girls with ADHD are well-adjusted during adolescence. Journal of Abnormal Child Psychology. 2008;36:373–383. doi: 10.1007/s10802-007-9184-6. [DOI] [PubMed] [Google Scholar]
- Mannuzza S, Klein RG, Bessler A, Malloy P, LaPadula M. Adult outcome of hyperactive boys: Educational achievement, occupational rank, and psychiatric status. Archives of General Psychiatry. 1993;50:565–576. doi: 10.1001/archpsyc.1993.01820190067007. [DOI] [PubMed] [Google Scholar]
- Mannuzza S, Klein RG, Bessler A, Malloy P, LaPadula M. Adult psychiatric status of hyperactive boys grown up. Am J Psychiatry. 1998;155:493–498. doi: 10.1176/ajp.155.4.493. [DOI] [PubMed] [Google Scholar]
- Nelder J, Wedderburn R. Generalized linear models. Journal of the Royal Statistical Society, Series A. 1972;135:370–384. [Google Scholar]
- Owens EB, Hinshaw SP, Lee SS, Lahey BB. Few girls With childhood attention-deficit/hyperactivity disorder show positive adjustment during adolescence. Journal of Clinical Child and Adolescent Psychology. 2009;38:132–143. doi: 10.1080/15374410802575313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham WE, Gnagy EM, Greenslade KE, Milich R. Teacher ratings of DSM-III-R symptoms of the disruptive behavior disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 1992;31:210–218. doi: 10.1097/00004583-199203000-00006. [DOI] [PubMed] [Google Scholar]
- Piacentini JC, Cohen P, Cohen J. Combining discrepant diagnostic information from multiple sources: Are complex algorithms better than simple ones? Journal of Abnormal Child Psychology. 1992;20:51–62. doi: 10.1007/BF00927116. [DOI] [PubMed] [Google Scholar]
- Riddle MA, Yershova K, Lazzaretto D, Paykina N, Yenokyan G, Greenhill L, Posner K. The Preschool Attention-Deficit/Hyperactivity Disorder Treatment Study (PATS) 6-year follow-up. Journal of the American Academy of Child and Adolescent Psychiatry. 2013;52:264–278. doi: 10.1016/j.jaac.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellke T, Bayarri MJ, Berger JO. Calibration of p values for testing precise null hypotheses. American Statistician. 2001;55:62–71. [Google Scholar]
- Shaffer D, Fisher P, Piacentini J, Schwab-Stone M, Wicks J. Diagnostic Interview Schedule for Children. New York: Columbia University; 1993. [Google Scholar]
- Sibley MH, Pelham WE, Jr, Molina BSG, Gnagy EM, Waschbusch DA, Garefino AC, Karch KM. Diagnosing ADHD in adolescence. Journal of Consulting and Clinical Psychology. 2012;80:139–150. doi: 10.1037/a0026577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley MH, Pelham WE, Molina BSG, Coxe S, Kipp H, Gnagy EM, Lahey BB. The role of early childhood ADHD and subsequent CD in the initiation and escalation of adolescent cigarette, alcohol, and marijuana use. Journal of Abnormal Psychology. 2014;123:362–374. doi: 10.1037/a0036585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AL, Molina BSG, Pelham W, Gnagy EM. Risky driving in adolescents and young adults with childhood ADHD. Journal of Pediatric Psychology. 2007;32:745–759. doi: 10.1093/jpepsy/jsm002. [DOI] [PubMed] [Google Scholar]
- Trampush JW, Miller CJ, Newcorn JH, Halperin JM. The impact of childhood ADHD on dropping out of high school in urban adolescents/young adults. Journal of Attention Disorders. 2009;13:127–136. doi: 10.1177/1087054708323040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss G, Hechtman L. Hyperactive children grown up: ADHD in children, adolescents, and adults 1993 [Google Scholar]
- Willcutt EG, Nigg JT, Pennington BF, Solanto MV, Rohde LA, Tannock R, Lahey BB. Validity of DSM-IV attention deficit/hyperactivity disorder symptom dimensions and subtypes. Journal of Abnormal Psychology. 2012;121:991–1010. doi: 10.1037/a0027347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]