Abstract
The current melting of glaciers and ice sheets is a consequence of climatic change and their turbid meltwaters are filling and enlarging many new proglacial and ice-contact lakes around the world, as well as affecting coastal areas. Paradoxically, very little is known on the ecology of turbid glacier-fed aquatic ecosystems even though they are at the origin of the most common type of lakes on Earth. Here, I discuss the consequences of those meltwaters for planktonic organisms. A remarkable characteristic of aquatic ecosystems receiving the discharge of meltwaters is their high content of mineral suspensoids, so-called glacial flour that poses a real challenge for filter-feeding planktonic taxa such as Daphnia and phagotrophic groups such as heterotrophic nanoflagellates. The planktonic food-web structure in highly turbid meltwater lakes seems to be truncated and microbially dominated. Low underwater light levels leads to unfavorable conditions for primary producers, but at the same time, cause less stress by UV radiation. Meltwaters are also a source of inorganic and organic nutrients that could stimulate secondary prokaryotic production and in some cases (e.g. in distal proglacial lakes) also phytoplankton primary production. How changes in turbidity and in other related environmental factors influence diversity, community composition and adaptation have only recently begun to be studied. Knowledge of the consequences of glacier retreat for glacier-fed lakes and coasts will be crucial to predict ecosystem trajectories regarding changes in biodiversity, biogeochemical cycles and function.
Keywords: climate change, glacial lakes, glacial flour, planktonic food-web, Daphnia, mixotrophy, grazing, bacteria, viruses, DOM
INTRODUCTION
The current rapid retreat of glaciers and ice sheets contitutes one of the most prominent signs of climate change (IPCC, 2007, 2013). Most glaciers are expected to significantly shrink within the next decades and, eventually, many low elevation glaciers will disappear in this century (Zemp et al., 2009). The retreat of glaciers and ice sheets is a global phenomenon and several areas of the world including the Alps, Greenland and the Central and Southern Andes are identified as particularly vulnerable (IPCC, 2007, 2013). In the Alps, for example, glaciers located below 3000 m above sea level are those showing the most significant reduction in area (Zemp et al., 2009). Changes in the area covered by glaciers are not exclusive of the 20–21th century and several periods of glacier retreat and expansion are known during Earth history (Solomina et al., 2015). An important difference, however, is that compared with the early mid-Holocene, current glacier retreat has a clear anthropogenic component (Marzeion et al., 2014) and rates seem to be higher than in the past (Solomina et al., 2015). Interestingly, the abrupt glacier retreat in the Alps after the mid-19th century maximum (i.e. at end of the Little Ice Age), is attributed to the increase in black carbon emissions, as a consequence of industrialization in Western Europe (Painter et al., 2013).
One obvious consequence of the melting of glaciers and ice sheets is the long-term loss of natural freshwater storage in frozen form (70% of the Earth’s freshwater is stored under glaciers and ice sheets) that will have, beside the alteration of the hydrological cycle, also a strong societal impact in many parts of the world (Hinkel et al., 2014; Kohler et al., 2014). The current rapid melting of glaciers and ice sheets has also resulted in the mobilization and transport of different inorganic and organic molecules stored for long periods (Fountain et al., 2012), as well as of pollutants. For example, the melting of rock glaciers in some mountain areas of the Alps has dramatically increased levels of nickel and other metals in lakes that now exceed thresholds for drinking water standards and have unknown hitherto biological effects (Thies et al., 2007). Similarly, the retreat of tropical mountain glaciers has strongly impaired water quality in areas like the Cordillera Blanca, Peru, due to high concentrations of lead and nickel (Fortner et al., 2011). Further, the retreat of glaciers makes available, soils, plant detritus and nutrient resources previously buried under the ice, which are important for both the succession of the biota in terrestrial and aquatic ecosystems. For instance, in glacier forelands, soil heterotrophic microbial communities rely on ancient and recalcitrant carbon sources accumulated in past periods of glacier fluctuations, before primary succession of vegetation takes place (Bardgett et al., 2007). Finally, the shrinking of glaciers is also affecting the biodiversity of rivers and streams (Jacobsen et al., 2012), where more studies are available compared with lakes.
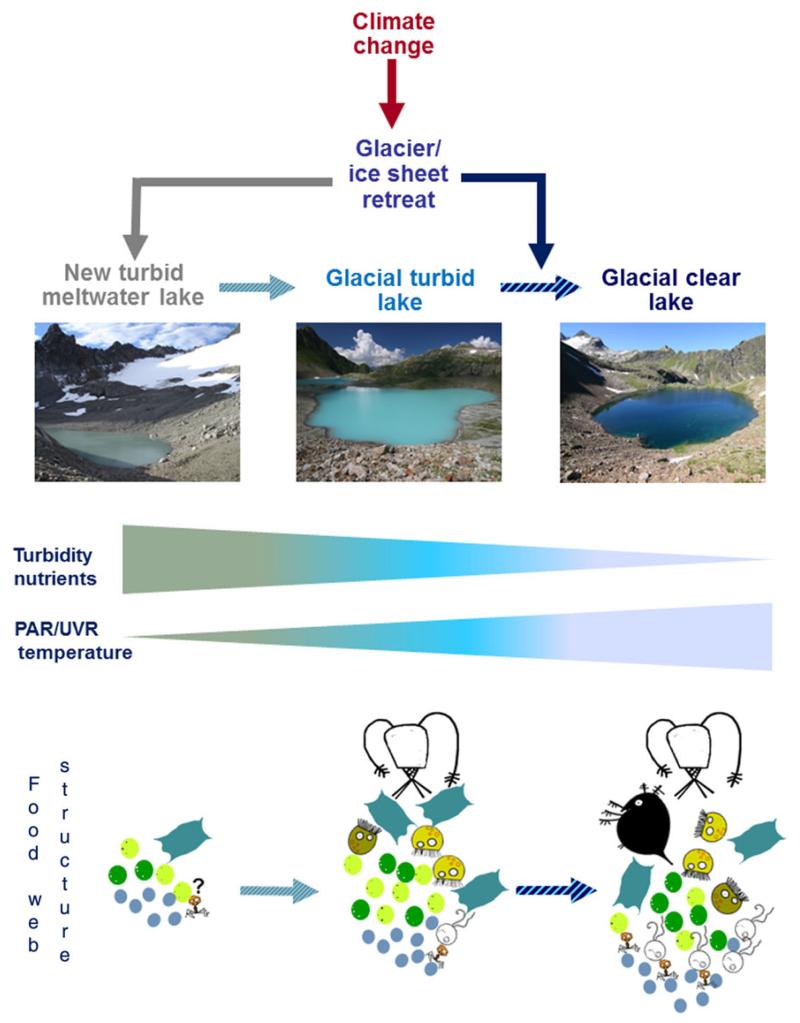
Yet, the melting of glaciers and ice sheets has another important consequence, that is, the creation of many new proglacial and ice-contact lakes (Fig. 1) around the world, where terrain topology allows (Carrivick and Tweed, 2013). Some of those newly created lakes are not stable and can be emptied through dangerous outburst floods. Nevertheless, the number of new lakes, as well as the increase in glacial lake area are relevant. For example, estimates based on a scenario where all glacier ice disappears in the Swiss Alps, predict ~500 new lakes (>1 ha) (Linsbauer et al., 2012), which represents ~30% more lentic systems for Switzerland. In other regions, such as in northern Patagonia, total glacial lake area has increased by ~65% from 1945 to 2011 (Loriaux and Casassa, 2013).
Fig. 1.
The retreat of glaciers and ice sheets caused by climate change creates new highly turbid meltwater proglacial/ice-contact lakes that during its ontogeny became less turbid (bright turquoise color) until they lose hydrological connectivity with the glacier and shift to a clear condition with high water transparency. The rapid glacier retreat also accelerates the shift from a turbid to a clear state (blue solid arrow). Along this lake’s ontogeny, strong gradients in turbidity, potentially also in nutrient concentrations, as well as in PAR/UVR and water temperature are found. The planktonic food-web in the highly turbid proglacial lakes is largely dominated by microbes with few metazoans present such as rotifers. As soon as turbidity and other in-lake conditions change (see text for more explanation), higher abundance of a more diverse planktonic food-web including copepods are feasible, but usually cladocerans such as Daphnia are found only when turbidity substantially decrease or the ratio of food to glacial particles improves. The food-web structure should be considered as a transition representation rather than a fixed one. Further steps in lake’s ontogeny, for example, when dissolved organic carbon (DOC) inputs from the catchment make lakes less UV transparent are not shown. Color-coded cartoons in the food-web structure: Blue, prokaryotes; light green, mixotrophic phytoplankton; dark green, phototrophic phytoplankton; yellow, mixotrophic ciliates; brown, heterotrophic ciliates; white, heterotrophic nanoflagellates. Depiction of rotifers, copepods, Daphnia and viruses are self-evident.
Lakes of glacial origin are probably the most numerous on Earth (Wetzel, 2001), but surprisingly, little is known about their plankton and general ecology at creation and during their early ontogeny, particularly when turbidity levels were high. Thus, the newly created lakes offer an opportunity to understand for example, the structure and functioning of the food-web, who are the first colonizers and what environmental factors shape these communities. The current retreat of glaciers also offers almost natural laboratory conditions in the form of deglaciation chronosequences that help us to elucidate what changes take place when turbid glacial lakes shift to a clear condition after they lose connectivity with the glacier. The study of deglaciation chronosequences, for instance, has been for many decades a crucial research strategy to understand primary succession in terrestrial ecosystems (e.g. William et al., 1971; Kaufmann, 2002; Raffl et al., 2006).
In this Horizons article, I will discuss how the melting of glaciers and ice sheets can influence planktonic organisms and processes and then, identify open questions that may guide future research in this area. Though most of the information is based on lakes, examples of coastal areas affected by glacier meltwaters discharge are also included.
Why are aquatic ecosystems affected by meltwaters unique?
A singularity of proglacial and ice-contact lakes, as well as of coastal areas receiving discharge of glacier meltwaters is the presence of a high concentration of mineral suspensoids. These particles, so-called glacial flour or glacial milk, resulting from subglacial erosion of the bedrock (actually it should be named “rock flour”) are delivered to recipient aquatic ecosystems by proglacial streams/rivers or by runoff (Smith, 1978; Hodder et al., 2007). The mineralogical characteristics of glacial flour depends largely on the lithology of the underlying bedrock (Ashley, 2002), but its size range falls typically within that of clays and fine silt (Smith, 1978; Gallegos et al., 2008; Chanudet and Filella, 2009; Sommaruga and Kandolf, 2014). Thus, the fine-sized mineral suspensoids give waters a typical “milky” look (Fig. 1), as well as a high reflectance (Gallegos et al., 2008). Glacial flour in young proglacial/ice-contact lakes gives a characteristic gray hue to the water, which is probably the water color at the origin of glacial lakes (Fig. 1). Later, as lakes become less turbid and more productive, their color shifts to a bright turquoise (Fig. 1).
Several factors influence turbidity levels in aquatic ecosystems receiving glacier meltwaters, such as for example, glacier proximity (proximal or distal proglacial lakes), the glacier to lake area ratio, water residence time, maximum depth of the water body, wind fetch (resuspension), ice-cover duration, thermal/salinity stratification and the relative contribution of glacier runoff and proglacial streams to the total water inflow from the catchment. Under exceptional runoff conditions, turbidity can reach extreme values, as observed for example, in reservoirs of the Swiss Alps where up to 644 nephelometric turbidity units (NTU) have been recorded [Bonalumi et al., 2011, in the original paper formazin turbidity units (FTU) are used]. However, even at turbidity values that are one order of magnitude lower, the abundance of mineral suspensoids is very high. For example, at 14 NTU [corresponding to a 1% depth for photosynthetically active radiation (PAR) of ~0.5 m], the abundance of glacial particles in the size range 0.7–4 μm is ~2.8 × 106 mL−1 (Sommaruga and Kandolf, 2014). This is one order of magnitude higher than the bacterial abundance and three orders of magnitude higher than the phytoplankton abundance, typically found in oligotrophic aquatic ecosystems (Wetzel, 2001). Similarly important from a food-web perspective, is the overlap in size of glacial and food particles (pico- and nanoplankton) available to filter-feeding planktonic species. Further, in contrast to inorganic particles found in productive aquatic ecosystems (typically estuaries or shallow eutrophic lakes), particles in glacial flour tend to have very low organic coating and no or very few attached bacteria (Sommaruga and Kandolf, 2014). Analyses by scanning electron microscopy of glacial flour from a proglacial mountain lake also indicate that particles have sharp edges and, thus, have a potential for causing physical damage when ingested (Sommaruga and Kandolf, 2014). Altogether, these characteristics make aquatic ecosystems affected by glacier meltwaters to be very special.
Consequences of glacier meltwaters for planktonic organisms
Sediment records for Lake Baikal show a widespread negative effect of glacier meltwaters on the food-web during the last glacial maximum. For example, diatoms, chrysophyte cysts and zooplankton are absent during this period despite being found before it (Karabanov et al., 2004). Similarly, the discharge of glacier meltwaters at the start of the Younger Dryas has been argued to largely affect the survival of lake organisms (Birks et al., 2000). A high concentration of glacial flour certainly represents a challenge for filter- and interception-feeding planktonic organisms, particularly, if they cannot discriminate against them. This is because either they will largely fill their gut with inorganic particles or they will need to invest more energy in finding a suitable food particle among the “cloud” of mineral suspensoids. This in turn, will results in a decrease in feeding efficiency, as well as in the allocation of energy to somatic growth and reproduction. If one adds the effect of glacial flour to the oligotrophic condition of proglacial and ice-contact lakes, the resulting scenario concerning food resources is extreme. One pioneering study on the effect of glacial flour on lake biota is that of Koenings et al. (Koenings et al. 1990), who in laboratory experiments demonstrated that growth and survival of Daphnia is significantly reduced in the presence of mineral suspensoids. Similarly, Sommaruga and Kandolf (Sommaruga and Kandolf 2014) found in experiments with a natural heterotrophic nanoflagellate (HNF) community from a clear alpine lake and with single phagotrophic HNF species that the addition of glacial flour negatively affects growth and survival depending on the concentration of particles. They also established that the negative effect is due to the interference of glacial flour with the efficient uptake of bacteria (i.e. it causes a decrease in clearance rates) rather than to physical damage. High turbidity levels caused by the discharge of glacier meltwaters into clear lakes have been found to also alter trophic interactions between planktivorous fish species and Daphnia with an overall positive effect for the prey (Jönsson et al., 2011).
The discharge of meltwaters from glaciers or ice sheets into a lake or coastal area can affect planktonic organisms by several other mechanisms that do not involve a direct effect of glacial flour. For example, the massive discharge of glacier meltwaters into coastal fjords has been implicated in extensive zooplankton mortality (Weslawski and Legezyriska, 1998). However, whether this is consequence of the load of glacial particles or of changes in salinity and osmotic stress remained unanswered (Weslawski and Legezyriska, 1998). Another physical factor affected by the discharge of glacier meltwaters into recipient waterbodies is temperature (Svendsen et al., 2002;Slemmons et al., 2013). The average water temperature in the water column of glacier-fed lakes is typically lower than in clear ones (Gallegos et al., 2008), though in distal proglacial alpine lakes, maximum temperatures at the surface can reach similar values. It is noteworthy that, during strong glacier runoff and precipitation events, holomixis in glacier-fed alpine lakes can occur several times during summer (Peter and Sommaruga, unpublished). This “polymictic” kind of thermal regime contrasts with the typical dimictic one, known for clear alpine lakes located in the temperate zone. Further, the discharge of cold waters with a high content of glacial flour can cause density underflows in the recipient lakes (Gilbert and Crookshanks, 2009; Bonalumi et al., 2011).
The discharge of turbid glacier meltwaters also significantly reduces the extent of the euphotic zone (Modenutti et al., 2000; Svendsen et al., 2002; Hylander et al., 2011; Rose et al., 2014) and this in turn, is expected to reduce photosynthetic rates and integrated primary production rates as found, for example, in fjords receiving glacier runoff (Piwosz et al., 2009). Paleolimnological studies also provide evidence that primary production is reduced during prolonged periods of glacier meltwater discharge caused by climatic warming (Vinebrooke et al., 2010). However, measurements of primary production conducted in distal glacier-fed alpine lakes of the central Rocky Mountains, USA, showed the opposite effect (Slemmons and Saros, 2012). In a comparison with lakes receiving runoff from both snow and glacier meltwaters with those that are fed only by snow meltwater, higher primary production rates were found in the former type of lakes (Slemmons and Saros, 2012). This was attributed mainly to the stimulatory effect of the higher supply of reactive nitrogen provided by glacier meltwaters in that area (Saros et al., 2010; Slemmons and Saros, 2012). Further, the glaciers in this case are distant enough from the lakes, so that lake primary production is not impaired by inorganic turbidity (Slemmons and Saros, 2012).
The reduction in PAR by glacial flour may also have consequences for the stoichiometry of phytoplankton and grazers (Laspoumaderes et al., 2013). Following the predictions of the light:nutrient ratio hypothesis (Sterner et al., 1997), these authors found that the food quality of primary producers and the competition outcome between herbivorous zooplankton with different phosphorus requirements, such as Daphnia and the copepod Boeckella, are affected by the strong horizontal gradient in water transparency formed by the discharge of glacier meltwaters into a clear lake. Along this transparency gradient, the light:phosphorus supply ratio positively covaries with the seston C:P ratio (i.e. a higher C:P means a lower nutritional quality) and results in a decrease of the relative abundance of P-rich Daphnia to low-P Boeckella when light penetration increases. In other words, the turbid conditions improve the nutritional quality of phytoplankton during this time-window (Laspoumaderes et al., 2013).
The horizontal gradient in water transparency observed in lakes receiving the discharge of turbid glacier meltwaters affects also the vertical distribution of phytoplankton and zooplankton in the water column (Hylander et al., 2011). In this context, it is important to consider that not only is PAR rapidly attenuated in a turbid system, but also UV radiation (UVR, Svendsen et al., 2002; Rose et al., 2014; Tartarotti et al., 2014), which in high-mountain and polar areas, is a relevant environmental stress factor (Sommaruga, 2001; Vincent et al., 2007). Thus, the discharge of glacier meltwaters protects primary producers from UV stress (Martyniuk et al., 2014). Since UVR is also important in regulating daytime depth distribution of zooplankton (Williamson et al., 2011), species living in turbid glacier-fed lakes probably do not follow the typical daytime escape reaction from surface waters observed in clear alpine lakes (Fisher et al., 2014, B. Tartarotti et al. in prep.). Further, the rapid UV attenuation in the water column will minimize negative effects on sensitive processes, such as on heterotrophic bacterial production (Sommaruga et al., 1997). Though the high scattering of short UV wavelengths caused by glacial flour (Rose et al., 2014) can result in higher UV to PAR ratios and eventually impair sensitive organisms (Leavitt et al., 2003), this effect is expected to be only significant very close to the water surface, where UV irradiance (particularly of the short wavelengths UV-B) is high. In fact, direct measurements of backscattering in turbid glacier-fed lakes (“turquoise” lakes) show low values in comparison to those expected based on reflectance (Gallegos et al., 2008).
As mentioned before, glaciers, at least in some regions, supply inorganic nutrients such as nitrogen accumulated from local and distant sources over time, to recipient water bodies. It is becoming clear, however, that glacier meltwaters also supply significant amounts of organic carbon in dissolved (Hood et al., 2009, 2015; Singer et al., 2012) and in particulate form (e.g. as estimated for Greenland, Hood et al., 2015). These recent estimates indicate that global glacier/ice sheet runoff contributes 1.04 ± 0.18 TgC year−1 to proglacial freshwater systems and coastal areas of the world (Hood et al., 2015). Interestingly, glacier-derived dissolved organic carbon (DOC) appears to be highly biologically available (Hood et al., 2009, 2015). Another essential nutrient supplied by glacier meltwaters is phosphorus (Hodson, 2006). Most of the phosphorus is probably not biologically available (being adsorbed in the mineral clay phases), but if a small fraction is made available as reactive phosphorus, the large supply of glacial flour in glacial meltwaters could compensate for its adsorption and low availability (Hodson et al., 2008) and stimulate production. Experimental evidence indicates that the addition of dry glacial flour to a natural bacterial glacier meltwater community significantly stimulates bacterial production when incubated at water temperatures found in proglacial lakes (Mindl et al., 2007). Actually, many of the prokaryotes occurring in proglacial lakes may derive from the glacier itself (Anesio and Laybourn-Parry, 2012) and find better growing conditions (e.g. higher water temperatures) in the recipient water body (Mindl et al., 2007). Anesio et al. (2009), however, observed that bacteria living in cryoconite holes in glaciers can attain high activity, even at low water temperatures. Thus, nutrients supplied by glacier meltwater can compensate for low water temperatures.
The planktonic food-web structure of turbid glacier-fed lakes
In newly created proglacial/ice-contact lakes with high glacial flour content, the structure of the planktonic food-web is probably simple and dominated by microbes (Fig. 1) and characterized by very low predatory or grazing losses (Koenings et al., 1990; Sommaruga and Kandolf, 2014). The low phytoplankton abundance mainly allows for the establishment of cold-adapted rotifers (Fig. 1). Nondiscriminating zooplankton filter-feeding species such as Daphnia, Bosmina and Holepedium (Koenings et al., 1990), but also HNF, that are the main predators on bacteria, are excluded or present at low abundance in those very turbid young glacier-fed lakes (Sommaruga and Kandolf, 2014). The exclusion of key-stone species such as Daphnia suggests that the planktonic food-web is truncated at this stage (Fig. 1). Perhaps the only functional group that can act as an important trophic link to bacteria is that of mixotrophic phytoplankton because the combination of photosynthesis and phagotrophy, makes them less sensitive to the negative effect of glacial flour, like for example found for Dinobryon (Sommaruga and Kandolf, 2014).
Another source of mortality for the aquatic microbial community is viruses. However, it is unknown whether viruses could act instead as a shaping force of the composition and diversity of microbial communities in highly turbid glacier-fed lakes. Indeed, the occurrence of high number of glacial particles can potentially impair the establishment of viruses in these ecosystems, because the high ratio of these particles to bacteria will presumably result in a low encounter rate with the potential host. Further, if nonspecific adsorption to glacial flour particles occurs, encounter rates will be further reduced because this process is one of the major causes of viral inactivation and decay (Murray and Jackson, 1992). Thus, lakes receiving high loads of glacial flour may represent a unique type of aquatic ecosystem where microbial mortality by viruses and grazers is significantly reduced. If this is confirmed, theory predicts a reduced microbial diversity (Thingstad, 2000). However, it may be possible that prokaryotes colonizing turbid proglacial lakes carry viral DNA/RNA (i.e. lysogenic life cycle) and a lytic cycle is triggered when they meet suitable conditions for growth. So, there may exist “hot moments” in those lakes, when turbidity decreases (e.g. at times of low runoff) and viral encounter rates with the host increase, so that lytic infection is possible. Further, because organic coating of glacial flour particles is very low, perhaps viral loss or inactivation by adsorption is not as relevant as it is with organic-coated particles (Murray and Jackson, 1992).
Changes during the first steps in lake’s ontogeny and when glacier connectivity is lost
Most of the information on what changes occurred in the biota during lake’s ontogeny after glaciers receded is limited to studies on remnants found in the sediment. Unfortunately, in many cases, it is difficult to extrapolate results from benthic (sometimes littoral) indicator species to the planktonic realm because light, temperature, nutrient conditions, and trophic interactions are different. Nevertheless, results from paleolimnological studies suggest that when lake turbidity decreases and light conditions improve, relatively rapid changes in species composition take place (e.g. Lotter et al., 2006). Paleolimnological studies also identify the importance of considering the effects of succession in terrestrial vegetation for proglacial lakes. For example, the initial phase after deglaciation in many areas is characterized by lake oligotrophication resulting from nutrient sequestration by the slow establishment of terrestrial vegetation (Anderson et al., 2008). Further, from studies of deglaciation chronosequences, we know that the succession in the terrestrial environment significantly affects the chemistry of lakes and their biota (Engstrom et al., 2000). For example, in lakes located along a chronosequence (but without measureable levels of glacial flour turbidity) in Glacier Bay, Alaska, zooplankton species richness increased only when lakes reach a certain degree of UV protection given by the input of terrestrially-derived DOC, thus, allowing colonization by additional UV-sensitive species (Williamson et al., 2001).
For how long taxa such as Daphnia remain excluded from highly turbid glacial lakes will largely depend on how rapidly the ratio of glacial flour to food particles decreases (Koenings et al., 1990; Sommaruga and Kandolf, 2014). Thus, any potential lake nutrient fertilization, for example, by bird droppings as typically observed in the Arctic, could promote phytoplankton growth and accelerate this process. When conditions improve, then colonization is possible providing that a seed population can immigrate to the system and species sorting can take place. Paleolimnological evidence from Kråkenes Lake, Norway, indicates that once glacial flour settled, colonization by planktonic algae and Daphnia was relatively rapid (Birks et al., 2000). Certainly, the increase in primary productivity when turbidity levels decrease (Vinebrooke et al., 2010) allows for the establishment of larger populations of zooplankton selective feeders (Fig. 1) such as copepods and rotifers (Kirk, 1991; Koenings et al., 1990).
The shift in water transparency that took place in the past after the last deglaciation is occurring currently at rapid pace as a consequence of glacier retreat. Though the time needed for such a shift will depend on the specific geographical area, the particular topology (e.g. mountain vs. lowland coastal terrains), but also on glacier size, runoff and other factors, it may occur in some cases very rapidly (25–30 years), as for example, in Lake Grünau, Austrian Alps (R. Psenner, personal communication). Now, independent of the duration of this process, it is reasonable to argue that such a shift in water transparency will impact the biota that colonized and established under the originally turbid condition. So, we may expect ecological (among species) and evolutionary responses (within species) of the biota to take place (Urban et al., 2012). As mentioned before, changes in UV water transparency determine the chances of UV-sensitive species establishing a population in newly created lakes (Williamson et al., 2001). By analogy, it is arguable that depending on the depth refuge available in a lake, some species are lost during the shift from turbid (i.e. UV-protected) to a clear and high UVenvironment, and that changes in community composition take place, though plausibly, this is not the only environmental factor that will be responsible for such a change (Sommaruga and Casamayor, 2009). Recent comparative studies in clear and turbid glacier-fed lakes using deep-sequencing analysis of 18S rDNA from microbial planktonic communities provide evidence for such a shift in community composition. For example, significant differences in protistan plankton community composition have been found between clear and turbid lakes in two different biogeographical areas (Kammerlander et al., 2015). Similarly, bacterial community structure from three interconnected glacier-turbid lakes and from one adjacent lake that lost its connection with the same glacier largely differs (H. Peter et al., submitted).
A recent comparative study of populations of the copepod Cyclops abyssorum tatricus, living in clear alpine and turbid glacier-fed lakes, illustrates what are the adaptive mechanisms allowing survival in a high UVenvironment. In this species, investment in UV photoprotection and efficiency in repairing DNA damage are part of those mechanisms (Tartarotti et al., 2014). Similarly, when conditions make colonization of lakes by Daphnia possible, these are typically melanized, a phenotypic trait, that probably appeared after Pleistocene glaciations and made possible colonization of vacant shallow high UV habitats (Colbourne et al., 1997).
Conclusions and perspectives
The information discussed here supports the view that turbid glacier-fed lakes provide contrasting growth conditions (e.g. reduced predation/grazing and PAR/UVR availability, increased supply of inorganic and organic nutrients) for planktonic organisms compared with those found in lakes that became clear at a later stage. Because predation and resource availability/productivity are recognized as strong structuring forces at the community level (Hairston et al., 1960; Pace and Funke, 1991; Currie et al., 1999), it remains an open question how these contrasting conditions shape the structure of the planktonic community under turbid conditions and what level of diversity can be attained. For example, is microbial (both prokaryotic and eukaryotic) diversity in glacier-fed systems low due to reduced predation/grazing/viral lysis or can they attain similar or even higher diversity because of the input of microbes by glacier runoff as observed for bacteria in glacier-fed streams (Wilhelm et al., 2013). Further, what percentage of that diversity is metabolically active in glacier-fed systems or are just few dominant active taxa present? Testing what mechanisms explain differences in diversity and community composition between turbid and clear lakes or along a turbidity gradient remains challenging, because beside the change in water transparency, several other factors including the occur-rence of keystone taxa such as Daphnia, known to shape the microbial food-web, also change (Fig. 1). So, here experimental work is needed to disentangle the effects along the environmental gradient during early lake’s ontogeny. Regarding the effects of glacier meltwaters on production, though clearly more data are needed to draw any firm conclusion, the supply of limiting resources and nutrients such as DOC and phosphorus/nitrogen has the potential to stimulate prokaryotic secondary production in recipient fresh and marine waters. In the case of primary producers, more data on how glacier meltwaters affect primary production is needed, as well as on how the contrasting effect of PAR/UVR reduction and of potential higher nutrient supply balance under turbid conditions. Further, how important mixotrophy is among phytoplankton and other groups such as ciliates needs to be assessed together with the role of viruses. Comparative studies between turbid and clear lakes on the stoichiometry of (mixotrophic) plankton and how this relates to zooplankton growth will provide data on more integrative aspects of the effect of glacier meltwaters. A major effort is needed to understand which and how organisms adapt during early lake’s ontogeny (Fig. 1) and on how colonization and genetic/phenotypic variation in plankton are related along lake deglaciation chronosequences. Studies based on chronosequences will be also crucial to disentangle the relevance of the time component in succession and diversity patterns.
It is evident that the magnitude and impact of hydrological variability caused by the melting of glaciers on the food-web also needs to be evaluated. The melting of glaciers and ice sheets subjects recipient waters to large interseasonal and interannual hydrological variability (Casassa et al., 2009; Carrivick and Tweed, 2013). However, it is unknown how this variability influences the dynamics and distribution of planktonic organisms in those ecosystems. In addition, physical processes (e.g. changes in water density and holomixis) will have consequences for the spatial distribution of planktonic organisms, particularly, of those with low motility, but this has not yet been considered.
Since alpine and polar lakes are excellent sentinels of global changes (Adrian et al., 2009), further studies on how glacier meltwaters impact those ecosystems will allow us to better define their past and future ecological trajectories, as well as to learn how those unique environments function. Finally, even long after the disappearance of glaciers, stochastic events such as resuspension of glacial particles, for example, by large storms could reset succession and cause regime shifts similar to those known to occur in other aquatic ecosystems (Scheffer et al., 2001).
ACKNOWLEDGEMENTS
I thank Erik Jeppesen, Hannes Peter, Roland Psenner, and John Dolan for their comments, and Clara Ruiz Gonzalez for providing some of the cartoons of planktonic members in Fig. 1.
FUNDING
This work as supported by the Austrian Science Fund (FWF) through the Project BACK-ALP (#P24442 to R.S.) and the Carlsberg Foundation (Project 2013_01_0535 to E. Jeppesen) that made possible my research stay in Greenland.
REFERENCES
- Adrian R, O’Reilly CM, Zagarese H, Baines SB, Hessen DO, Keller W, Livingstone DM, Sommaruga R, et al. Lakes as sentinels of climate change. Limnol. Oceanogr. 2009;54:2283–2297. doi: 10.4319/lo.2009.54.6_part_2.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson NJ, Brodersen KP, Ryves DB, McGowan S, Johansson LS, Jeppesen E, Leng MJ. Climate versus in-lake processes as controls on the development of community structure in a low-Arctic lake (south-west Greenland) Ecosystems. 2008;11:307–324. [Google Scholar]
- Anesio AM, Hodson AJ, Fritz A, Psenner R, Sattler B. High microbial activity on glaciers: importance to the global carbon cycle. Global Change Biol. 2009;15:955–960. [Google Scholar]
- Anesio AM, Laybourn-Parry J. Glaciers and ice sheets as a biome. Trends Ecol. Evol. 2012;27:219–225. doi: 10.1016/j.tree.2011.09.012. [DOI] [PubMed] [Google Scholar]
- Ashley GM. Glaciolacustrine environments. In: Menzies J, editor. Modern and Past Glacial Environments. Butterworth Heinemann; UK: 2002. pp. 335–359. [Google Scholar]
- Bardgett RD, Richter A, Bol R, Garnett MH, Bäumler R, Xu X, Lopez-Capel E, Manning DA, et al. Heterotrophic microbial communities use ancient carbon following glacial retreat. Biol. Lett. 2007;3:487–490. doi: 10.1098/rsbl.2007.0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birks HH, Battarbee RW, Birks HJB. The development of the aquatic ecosystem at Kråkenes Lake, western Norway, during the late glacial and early Holocene—a synthesis. J. Paleolimnol. 2000;23:91–114. [Google Scholar]
- Bonalumi M, Anselmetti FS, Kaegi R, Wüest A. Particle dynamics in high-Alpine proglacial reservoirs modified by pumped-storage operation. Water Resour. Res. 2011;47:W09523. doi: 10.1029/2010WR010262. [DOI] [Google Scholar]
- Carrivick JL, Tweed FS. Proglacial lakes: character, behaviour and geological importance. Quat. Sci. Rev. 2013;78:34–52. [Google Scholar]
- Casassa G, López P, Pouyaud B, Escobar F. Detection of changes in glacial run-off in alpine basins: examples from North America, the Alps, central Asia and the Andes. Hydrol. Process. 2009;23:31–41. [Google Scholar]
- Chanudet V, Filella M. Size and composition of inorganic colloids in a peri-alpine, glacial flour-rich lake. Geochim. Cosmochim. Acta. 2009;72:1466–1479. [Google Scholar]
- Colbourne JK, Hebert PDN, Taylor DJ. Evolutionary origins of phenotypic diversity in Daphnia. In: Givnish TJ, Sytsma KJ, editors. Molecular Evolution and Adaptive Radiation. Cambridge University Press; Cambridge: 1997. pp. 163–188. [Google Scholar]
- Currie DJ, Dilwortth-Christie P, Chapleau F. Assessing the strength of top-down influences on plankton abundance in unmanipulated lakes. Can. J. Fish. Aquat. Sci. 1999;56:427–436. [Google Scholar]
- Engstrom DR, Fritz SC, Almendinger JE, Juggins S. Chemical and biological trends during lake evolution in recently deglaciated terrain. Nature. 2000;408:161–166. doi: 10.1038/35041500. [DOI] [PubMed] [Google Scholar]
- Fischer JM, Olson MH, Theodore N, Williamson CE, Rose KC, Hwang J. Diel vertical migration of copepods in mountain lakes: The changing role of ultraviolet radiation across a transparency gradient. Limnol. Oceanogr. 2015;60:252–262. [Google Scholar]
- Fortner S, Mark BG, McKenzie JM, Bury J, Trierweiler A, Baraer M, Burns PJ, Munk L. Elevated stream metal concentrations in the foreland of a tropical glacier. Appl. Geochem. 2011;26:1792–1801. [Google Scholar]
- Fountain AG, Campbell JL, Schuur EAG, Stammerjohn SE, Williams MW, Ducklow HW. The disappearing cryosphere: impacts and ecosystem responses to rapid cryosphere loss. Bioscience. 2012;62:405–415. [Google Scholar]
- Gallegos CL, Davies-Colley RJ, Gall M. Optical closure in lakes with contrasting extremes of reflectance. Limnol. Oceanogr. 2008;53:2021–2034. [Google Scholar]
- Gilbert R, Crookshanks S. Sediment waves in a modern high-energy glacial lacustrine environment. Sedimentology. 2009;56:645–659. [Google Scholar]
- Hairston NG, Smith FE, Slobodkin LB. Community structure, population control, and competition. Am. Nat. 1960;44:421–425. [Google Scholar]
- Hinkel J, Lincke D, Vafeidis AT, Perrette M, Nicholls RJ, Tol RSJ, Marzeion B, Fettweis X, et al. Coastal flood damage and adaptation costs under 21st century sea-level rise. Proc. Nat. Acad. Sci. USA. 2014;111:3292–3297. doi: 10.1073/pnas.1222469111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodder K, Gilbert R, Desloges JR. Assessment of varved glaciolacustrine sedimentary records as hydroclimatic proxy. J. Paleolimnol. 2007;38:365–394. [Google Scholar]
- Hodson AJ. Phosphorus in glacial meltwaters. In: Knight PG, editor. Glacier Science and Environmental Change. Blackwell Publication; Malden, MA: 2006. pp. 81–82. [Google Scholar]
- Hodson AJ, Anesio AM, Tranter M, Fountain A, Osborn M, Priscu J, Laybourn-Parry J, Sattler B. Glacial ecosystems. Ecol. Monogr. 2008;78:41–67. [Google Scholar]
- Hood E, Battin T, Fellman J, O’Neel S, Spencer RGM. Storage and release of organic carbon from glaciers and ice sheets. Nat. Geosci. 2015 doi: 10.1038/ngeo2331. [DOI] [Google Scholar]
- Hood E, Fellman J, Spencer RGM, Hernes PJ, Edwards R, D’Amore D, Scott D. Glaciers as a source of ancient and labile organic matter to the marine environment. Nature. 2009;462:1044–1047. doi: 10.1038/nature08580. [DOI] [PubMed] [Google Scholar]
- Hylander S, Jephson T, Lebret K, Von Einem J, Fagerberg T, Balseiro E, Modenutti B, Souza M-S, et al. Climate-induced input of turbid glacial meltwater affects vertical distribution and community composition of phyto- and zooplankton. J. Plankton Res. 2011;33:1239–1248. [Google Scholar]
- IPCC . Contribution of working groups I to the fourth assessment report of the intergovernmental panel on climate change. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL, editors. Climate Change 2007: The Physical Science Basis. Cambridge University Press; Cambridge, United Kingdom and New York, NY: 2007. p. 996. [Google Scholar]
- IPCC . Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change. In: Stocker TF, Plattner G-K, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM, editors. Climate Change 2013: The Physical Science Basis. Cambridge University Press; Cambridge, United Kingdom and New York, NY: 2013. p. 1535. [Google Scholar]
- Jacobsen D, Milner AM, Brown LE, Dangles O. Biodiversity under threat in glacier-fed river systems. Nat. Clim. Change. 2012;2:361–364. [Google Scholar]
- Jönsson M, Ranåker L, Nicolle A, Ljungberg P, Fagerberg T, Hylander S, Jephson T, Lebret K, et al. Glacial clay affects foraging performance in a Patagonian fish and cladoceran. Hydrobiologia. 2011;663:101–108. [Google Scholar]
- Kammerlander B, Breiner H-W, Filker S, Sommaruga R, Sonntag B, Stoeck T. High diversity of protistan plankton communities in remote high mountain lakes in the European Alps and the Himalaya mountains. FEMS Microbiol. Ecol. 2015 doi: 10.1093/femsec/fiv010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karabanov E, Williams D, Kuzmin M, Sideleva V, Khursevich G, Prokopenko A, Solotchina E, Tkachenko L, et al. Ecological collapse of Lake Baikal and Lake Hovsgol ecosystems during the Last Glacial and consequences for aquatic species diversity. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2004;209:227–243. [Google Scholar]
- Kaufmann R. Glacier foreland colonisation: distinguishing between short-term and long-term effects of climate change. Oecologia. 2002;130:470–475. doi: 10.1007/s00442-001-0815-2. [DOI] [PubMed] [Google Scholar]
- Kirk KL. Inorganic particles alter competition in grazing plankton: the role of selective feeding. Ecology. 1991;72:915–923. [Google Scholar]
- Koenings JP, Burkett RD, Edmundson JM. The exclusion of limnetic cladocera from turbid glacier-meltwater lakes. Ecology. 1990;71:57–67. [Google Scholar]
- Kohler T, Wehrli A, Jurek M, editors. Mountains and climate change: a global concern. Centre for Development and Environment (CDE), Swiss Agency for Development and Cooperation (SDC) and Geographica Bernensia, Bern; Switzerland: 2014. p. 136. ( Sustainable Mountain Development Series ). [Google Scholar]
- Laspoumaderes C, Modenutti B, Souza MS, Bastidas Navarro M, Cuassolo F, Balseiro E. Glacier melting and stoichiometric implications for lake community structure: zooplankton species distributions across a natural light gradient. Global Change Biol. 2013;19:316–326. doi: 10.1111/gcb.12040. [DOI] [PubMed] [Google Scholar]
- Leavitt PR, Cumming BF, Smol JP, Reasoner M, Pienitz R, Hodgson DA. Climatic control of ultraviolet radiation effects on lakes. Limnol. Oceanogr. 2003;48:2062–2069. [Google Scholar]
- Linsbauer A, Paul F, Haeberli W. Modeling glacier thickness distribution and bed topography over entire mountain ranges with GlabTop: application of a fast and robust approach. J. Geophys. Res. 2012;117 doi: 10.1029/2011JF002313. [DOI] [Google Scholar]
- Loriaux T, Casassa G. Evolution of glacial lakes from the Northern Patagonia Icefield and terrestrial water storage in a sea-level rise context. Global Planet. Change. 2013;113:33–40. [Google Scholar]
- Lotter AF, Heiri O, Hofmann W, van der Knaap WO, van Leeuwen JFN, Walker IR, Wick L. Holocene timber-line dynamics at Bachalpsee, a lake at 2265 m a.s.l. in the northern Swiss Alps. Veget. Hist. Archaeobot. 2006;15:295–307. [Google Scholar]
- Martyniuk N, Modenutti B, Balseiro EG. Can increased glacial melting resulting from global change provide attached algae with transient protection against high irradiance? Freshwater Biol. 2014;59:2290–2302. [Google Scholar]
- Marzeion B, Cogley JG, Richter K, Parkes D. Attribution of global glacier mass loss to anthropogenic and natural causes. Science. 2014;345:919–921. doi: 10.1126/science.1254702. [DOI] [PubMed] [Google Scholar]
- Mindl B, Anesio AM, Meirer K, Hodson AH, Laybourn-Parry J, Sommaruga R, Sattler B. Factors influencing bacterial dynamics along a transect from supraglacial runoff to proglacial lakes of a high Arctic glacier. FEMS Microbiol. Ecol. 2007;59:307–317. doi: 10.1111/j.1574-6941.2006.00262.x. [DOI] [PubMed] [Google Scholar]
- Modenutti B, Pérez G, Balseiro E, Queimaliños C. The relationship between light attenuation, chlorophyll a and total suspended solids in a Southern Andes glacial lake. Verh. Int. Ver. Limnol. 2000;27:1–4. [Google Scholar]
- Murray AG, Jackson GA. Viral dynamics: a model of the effects of size, shape, motion and abundance of single-celled planktonic organisms and other particles. Mar. Ecol. Prog. Ser. 1992;89:103–116. [Google Scholar]
- Pace ML, Funke E. Regulation of planktonic microbial communities by nutrients and herbivores. Ecology. 1991;72:904–914. [Google Scholar]
- Painter TH, Flanner MG, Kaser G, Marzeion B, VanCuren RA, Abdalati W. End of the Little Ice Age in the Alps forced by industrial black carbon. Proc. Nat. Acad. Sci. USA. 2013;110:15216–15221. doi: 10.1073/pnas.1302570110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piwosz K, Walkusz W, Hapter R, Wieczorek P, Hop H, Wiktor J. Comparison of productivity and phytoplankton in a warm (Kongsfjorden) and a cold (Hornsund) Spitsbergen fjord in mid-summer 2002. Polar Biol. 2009;32:549–559. [Google Scholar]
- Raffl C, Mallaun M, Mayer R, Erschbamer B. Vegetation succession pattern and diversity changes in a glacier valley, central Alps, Austria. Arct. Antarct. Alp. Res. 2006;38:421–428. [Google Scholar]
- Rose KC, Hamilton DP, Williamson CE, McBride CG, Fischer JM, Olson MH, Saros JE, Allan MG, et al. Light attenuation characteristics of glacially-fed lakes. J. Geophys. Res. Biogeosci. 2014;119:1446–1457. [Google Scholar]
- Saros JE, Rose KC, Clow DW, Stephens VC, Nurse AB, Arnett HA, Stone JR, Williamson CE, et al. Melting alpine glaciers enrich high-elevation lakes with reactive nitrogen. Environ. Sci. Technol. 2010;44:4891–4896. doi: 10.1021/es100147j. [DOI] [PubMed] [Google Scholar]
- Scheffer M, Carpenter S, Foley JA, Folke C, Walker B. Catastrophic shifts in ecosystems. Nature. 2001;413:591–596. doi: 10.1038/35098000. [DOI] [PubMed] [Google Scholar]
- Singer GA, Fasching C, Wilhelm L, Niggemann J, Steier P, Dittmar T, Battin TJ. Biogeochemically diverse organic matter in Alpine glaciers and its downstream fate. Nat. Geosci. 2012;5:710–714. [Google Scholar]
- Slemmons KEH, Saros JE. Implications of nitrogen-rich glacial meltwater for phytoplankton diversity and productivity in alpine lakes. Limnol. Oceanogr. 2012;57:1651–1663. [Google Scholar]
- Slemmons KEH, Saros JE, Simon K. The influence of glacial meltwater on alpine aquatic ecosystems: a review. Environ. Sci. Process. Impacts. 2013;15:1794–1806. doi: 10.1039/c3em00243h. [DOI] [PubMed] [Google Scholar]
- Smith ND. Sedimentation processes and patterns in a glacier-fed lake with low sediment input. Can. J. Earth Sci. 1978;15:741–756. [Google Scholar]
- Solomina ON, Bradley RS, Hodgson DA, Ivy-Ochs S, Jomelli V, Mackintosh AN, Nesje A, Owen LA, et al. Holocene glacier fluctuations. Quat. Sci. Rev. 2015;111:9–34. [Google Scholar]
- Sommaruga R. The role of UV radiation in the ecology of alpine lakes. J. Photochem. Photobiol. B. 2001;62:35–42. doi: 10.1016/s1011-1344(01)00154-3. [DOI] [PubMed] [Google Scholar]
- Sommaruga R, Casamayor E. Bacterial “cosmopolitanism” and importance of local environmental factors for community composition in remote high-altitude lakes. Freshwater Biol. 2009;54:994–1005. doi: 10.1111/j.1365-2427.2008.02146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommaruga R, Kandolf G. Negative consequences of glacial turbidity for the survival of freshwater planktonic heterotrophic flagellates. Sci. Rep. 2014;4 doi: 10.1038/srep04113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommaruga R, Obernosterer I, Herndl GJ, Psenner R. Inhibitory effect of solar radiation on thymidine and leucine incorporation by freshwater and marine bacterioplankton. Appl. Environ. Microbiol. 1997;63:4178–4184. doi: 10.1128/aem.63.11.4178-4184.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterner RW, Elser JJ, Fee EJ, Guildford SJ, Chrzanowski TH. The light: nutrient ratio in lakes: the balance of energy and materials affects ecosystem structure and process. Am. Nat. 1997;150:663–684. doi: 10.1086/286088. [DOI] [PubMed] [Google Scholar]
- Svendsen H, Beszczynska-Møller A, Hagen JO, Lefauconnier B, Tverberg V, Gerland S, Ørbæk JB, Bischof K, et al. The physical environment of Kongsfjorden-Krossfjorden, an Arctic fjord system in Svalbard. Polar Res. 2002;21:133–166. [Google Scholar]
- Tartarotti B, Saul N, Chakrabarti S, Trattner F, Steinberg CEW, Sommaruga R. UV-induced DNA damage in Cyclops abyssorum tatricus populations from clear and turbid alpine lakes. J. Plankton Res. 2014;36:557–566. doi: 10.1093/plankt/fbt109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thies H, Nickus U, Mair V, Tessadri R, Tait D, Thaler B, Psenner R. Unexpected response of high alpine lake waters to climate warming. Environ. Sci. Technol. 2007;41:7424–7429. doi: 10.1021/es0708060. [DOI] [PubMed] [Google Scholar]
- Thingstad TF. Elements of a theory for the mechanisms controlling abundance, diversity, and biogeochemical role of lytic bacterial viruses in aquatic systems. Limnol. Oceanogr. 2000;45:1320–1328. [Google Scholar]
- Urban MC, De Meester L, Vellend M, Stoks R, Vanoverbeke J. A crucial step toward realism: responses to climate change from an evolving metacommunity perspective. Evol. Appl. 2012;5:154–167. doi: 10.1111/j.1752-4571.2011.00208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent W, Rautio M, Pienitz R. Climate control of biological UV exposure in polar and alpine aquatic ecosystems. In: Ørbaek JB, et al., editors. Arctic Alpine Ecosystems and People in a Changing Environment. Springer; Germany: 2007. pp. 227–249. [Google Scholar]
- Vinebrooke RD, Thompson PL, Hobbs W, Luckman WBH, Graham MD, Wolfe AP. Glacially mediated impacts of climate warming on alpine lakes of the Canadian Rocky Mountains. Verh. Int. Ver. Limnol. 2010;30:1449–1452. [Google Scholar]
- Weslawski JM, Legezyriska J. Glaciers caused zooplankton mortality? J. Plankton Res. 1998;20:1233–1240. [Google Scholar]
- Wetzel RG. Limnology: Lake and River Ecosystems. 3rd ed. Elsevier; San Diego: 2001. [Google Scholar]
- Wilhelm L, Singer GA, Fasching C, Battin TJ, Besemer K. Microbial biodiversity in glacier-fed streams. ISME J. 2013;7:1651–1660. doi: 10.1038/ismej.2013.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- William AR, Worley IA, Lawrence DB. Plant diversity in a chronosequence at Glacier Bay, Alaska. Ecology. 1971;52:55–69. [Google Scholar]
- Williamson CE, Fischer JM, Bollens SM, Overholt EP, Breckenridge JK. Towards a more comprehensive theory of zooplankton diel vertical migration: integrating ultraviolet radiation and water transparency into the biotic paradigm. Limnol. Oceanogr. 2011;56:1603–1623. [Google Scholar]
- Williamson CE, Olson OG, Lott SE, Walker ND, Engstrom DR, Hargreaves BR. Ultraviolet radiation and zoo-plankton community structure following deglaciation in Glacier Bay, Alaska. Ecology. 2001;82:1748–1760. [Google Scholar]
- Zemp M, Hoelzle M, Haeberli W. Six decades of glacier mass-balance observations: a review of the worldwide monitoring network. Ann. Glaciol. 2009;50:101–111. [Google Scholar]