Abstract
Most breast cancer genomes harbor complex mutational landscapes. Somatic alterations have been predominantly discovered in breast cancer patients of European ancestry; however, little is known about somatic aberration in patients of other ethnic groups including Asians. In the present study, whole-exome sequencing (WES) was conducted in DNA extracted from tumor and matched adjacent normal tissue samples from eleven early onset breast cancer patients who were included in the Shanghai Breast Cancer Study. We discovered 159 somatic missense and ten nonsense mutations distributed among 167 genes. The most frequent 50 somatic mutations identified by WES were selected for validation using Sequenom MassARRAY system in the eleven breast cancer patients and an additional 433 tumor and 921 normal tissue/blood samples from the Shanghai Breast Cancer Study. Among these 50 mutations selected for validation, 32 were technically validated. Within the validated mutations, somatic mutations in the TRPM6, HYDIN, ENTHD1, and NDUFB10 genes were found in two or more tumor samples in the replication stage. Mutations in the ADRA1B, CBFB, KIAA2022, and RBM25 genes were observed once in the replication stage. To summarize, this study identified some novel somatic mutations for breast cancer. Future studies will need to be conducted to determine the function of these mutations/genes in the breast carcinogenesis.
Keywords: Breast cancer, Somatic mutation, Exome sequencing, Chinese population
Introduction
Breast cancer is the most common malignancy among women worldwide. In the United States, there were 226,870 new cases diagnosed and 39,510 deaths in 2012 [1]. Despite improvements in survival, breast cancer remains the second leading cause of female cancer mortality. It is well-established that breast cancer genomes harbor complex mutational landscapes, often characterized by point mutations, small insertions and deletions (Indels), and large structure changes across the genome [2–7]. Such somatic DNA changes in the tumor genome may result in an inactivation of tumor suppressor genes and activation or deregulation of oncogenes. Comprehensive identification of these somatic changes is necessary to better understand biological mechanisms of carcinogenesis and cancer progression, which may help to predict prognosis and to advance the development of targeted therapies.
To date, large scale genome-wide sequencing projects in breast tumors have identified several key breast cancer related genes, such as TP53, PIK3CA, PTEN, AKT1, GATA3, CDH1, RB1, MAP3K1, and CDKN1B [3,6]. These studies, however, were conducted in breast cancer patients predominantly of European ancestry. Given the difference in breast cancer subtypes, clinical characteristics, incidence, and survival as well as risk profiles among women of different ethnic backgrounds, somatic aberrations may differ across ethnic groups [8]. For example, compared with lung cancer patients of European ancestry, Asian patients carry more EGFR mutations and exhibit higher clinical response rates to EGFR tyrosine kinase inhibitors [9]. Similarly, somatic EGFR activating mutations in breast cancer were specifically observed in Asian ancestry patients but not in European ancestry patients [10].
To identify novel somatic alternations of breast cancer, we performed whole-exome sequencing in the breast tumor and matched normal DNA samples from eleven Chinese patients. Novel mutations were further selected for replication in additional samples.
Materials and Methods
Patients and specimens
The subjects in this study were a subset of participants in the Shanghai Breast Cancer Study (SBCS), a population-based case-control study among Chinese women. Details of the study have been described elsewhere [11,12]. Briefly, cases were women diagnosed with breast cancer between August 1996 and March 1998, 25 to 64 years of age, without a previous cancer diagnosis, and alive at the time of interview. All cases were identified via the population-based Shanghai Cancer Registry. Medical charts were reviewed using a standard protocol to obtain information on cancer treatment, clinical stages, and cancer characteristics, such as estrogen and progesterone receptor status. Two senior pathologists reviewed all tissue slides to confirm the diagnosis. Among the SBCS, most study participants provided blood samples for germline based genetic studies [11,12]. We also collected fresh tumor tissue samples and adjacent non-tumor tissue samples from a portion of the breast cancer patients from the SBCS. During surgery, tumor tissue samples were obtained from the tumor and the adjacent normal tissue samples were obtained from the distal edge of the resection. These samples were snap-frozen in liquid nitrogen as soon as possible, typically within 10 minutes. Samples were stored at −80°C. All patients were interviewed at the time of recruitment.
It has been suggested that patients with early-onset of disease may have a stronger genetic component. In the present study, only patients with early onset of breast cancer were selected for the whole exome sequencing in the discovery stage. The patients were 33 years on average when they were diagnosed with breast cancer. In the replication stage, additional 433 tumor samples and 921 normal tissue/blood samples from the SBCS participants were analyzed. These samples include 158 paired tumor-blood or tumor-normal tissue samples, 275 additional tumor tissue samples, 136 normal tissue samples, and 352 blood samples. Genomic DNA was extracted using a QIAamp DNA kit (Qiagen, Valencia, California) following the manufacturer's protocol. Approval of the study was granted by the relevant institutional review boards in both China and the United States.
Exome capture, library construction and sequencing
Libraries were constructed by shearing genomic DNA and ligating Illumina paired-end adaptors. The constructed DNA libraries were hybridized to Agilent Human All Exon Target Enrichment kit V1, which was designed to capture 165,637 coding exons and their flanking regions (37.8 million bases, 71.6% in exons with average length of 228 bp). The purified capture products were then amplified and subjected to 72 base paired-end sequencing on the Illumina GAII instrument according to Illumina's standard protocol.
Read mapping
First, we shifted the Illumina base quality scores (Phred + 64) to the Sanger scale (Phred + 33) [13]. Then, we performed alignment to the human reference genome (hg19) using Burrows-Wheeler Aligner (BWA) algorithm (version 0.7.0) [14] in default parameters. Aligned reads were processed and sorted with SAMtools [15]. We then marked duplicates with Picard (version 1.60) (http://picard.sourceforge.net) and carried out regional realignment and base quality score recalibration (BQSR) using Genome Analysis Toolkit (GATK, version 2.1.8) [16] with default setting. We used QPLOT in default requirement (http://genome.sph.umich.edu/wiki/QPLOT) for sequencing data quality assessment and statistics summary.
Somatic mutations calling
We conducted two sets of somatic mutations calling. First, we used GATK's Unified Genotyper to call variants simultaneously on all BAM files from the tumor/normal tissue DNA. To decrease the false positive discovery rate, we applied the following criteria to ensure that only high quality reads were used to call and only high quality variants were included in the final analyses: (1) only using reads with a mapping quality score (MAPQ) ≥ 20 and bases with base quality score (BQ) ≥ 20; (2) only including variants with genotype quality score (GQ) ≥ 30 and depth ≥ 30 in both tumor and normal tissue DNA; (3) only including variants exclusively observed in tumor samples.
We also used VarScan2 (v2.3.3) software [17] to identify somatic variants for each paired BAM files from the same patient with default parameters. We also included somatic variants within 100 bp of a target region. In the filter step, we used the somatic Filter command implemented in the VarScan2 program with the following options: -somatic-p-value=0.05 and a minimum of 10× coverage (−min-coverage 10) for both the tumor and matched normal samples. We additionally examined the read pileups for each mutation of each BAM file from both calling sets by using GATK’s Pileup option. Finally, we combined somatic variants called by both GATK toolkit and VarScan2 program for further analyses. We obtained the known somatic mutation dataset (Level 2, version 2.4) in breast cancer studies from TCGA data portal (https://tcga-data.nci.nih.gov/tcga/tcgaHome2.jsp). All known somatic mutations from the COSMIC (v62) were also downloaded. The somatic mutations that were identified in the current study and were not reported in these two datasets were regarded as novel mutations.
Functional prediction of mutations
All somatic mutations were annotated using SeattleSeq Annotation 137 (http://snp.gs.washington.edu/SeattleSeqAnnotation137/HelpAbout.jsp) and ANNOVAR (http://www.openbioinformatics.org/annovar/). For missense mutations, we combined the SeattleSeq Annotation 137 and SIFT (http://sift.jcvi.org/) to predict the possible impact of an amino acid substitution on the structure/function of the associated protein.
Mutation verification
To confirm exome sequencing findings, the top 50 somatic mutations ranked by the sequencing depth and frequency were selected for technical validation in the eleven pairs of tumor-normal tissue samples using the Sequenom MassARRAY platform (Sequenom, San Diego, CA). Among these 50 mutations, 32 were validated. They were further investigated in an additional 433 tumor samples and 921 normal tissue/blood samples. PCR primers and allelic-specific extension primers were designed with the MassARRAY Assay Design 4.0 software, and alleles of each mutation were detected through matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry. All calls from Sequenom Typer Analyzer software (version 4.0.20) were manually confirmed by examining the spectra. Peaks for two alleles were checked against the background of each well, and a mutation call was confirmed if the peak was unique to that allele. Blinded duplicates and negative controls (distilled water) were included in each of the 384-well plates.
Results
Patient characteristics and sequencing statistics
Clinical characteristics of breast cancer patients included in the present study are listed in Table 1. Patients included in the discovery stage of whole exome sequencing were early-onset with the average diagnostic age of 33 years. Among them, seven were ER+, and three were ER−. Summary statistics for the exome sequencing data are presented in Table S1. For the tumor samples, we obtained an average of 78.28 (range 75.31–82.06) million reads per sample, with a mean depth of 63.39 for the target sequencing regions. On average, 99.34% (99.28–99.46%) of the reads were aligned to the human reference genome. Similarly, for the DNA samples from normal tissue from the same individuals, we got an average of 74.10 (range 61.79–82.27) million reads per sample, with a mean depth of 61.1 across the target regions.
Table 1.
Clinical characteristics of breast cancer patients in the discovery and replication stages.
| Characteristics | Discovery stage | Replication stage |
|---|---|---|
| No. of breast cancer patients with tumor tissue samples | 11 | 433 |
| Age of diagnosis (median, range) | 33.1 (28–42) | 49.4 (26.1–74.2) |
| TNM (%) | ||
| 0–I | 27.27 | 30.02 |
| II | 54.55 | 55.89 |
| III | 18.18 | 7.62 |
| IV | 0.00 | 0.46 |
| Unknown | 0.00 | 6.00 |
| ER status (%) | ||
| Positive | 63.64 | 61.20 |
| Negative | 27.27 | 27.25 |
| Unknown | 9.09 | 11.55 |
| PR status (%) | ||
| Positive | 72.73 | 56.58 |
| Negative | 18.18 | 31.18 |
| Unknown | 9.09 | 12.24 |
| Chemotherapy (%) | ||
| Yes | 90.91 | 91.22 |
| No | 9.09 | 5.08 |
| Unknown | 0.00 | 3.70 |
| Radiotherapy (%) | ||
| Yes | 36.36 | 36.26 |
| No | 36.36 | 51.96 |
| Unknown | 27.27 | 11.78 |
| Hormonal therapy (%) | ||
| Yes | 54.55 | 57.97 |
| No | 18.18 | 32.33 |
| Unknown | 27.27 | 9.70 |
Somatic mutation profiling
We identified 588 unique somatic point mutations in the study samples (Tables 2 and S2). Each tumor sample carried an average of 63 somatic mutations with a range of 32–133 mutations. The number is comparable to the previous TCGA observation with the mean of 56 mutations [18].
Table 2.
Number of somatic mutations identified in the discovery stage using whole exome sequencing.
| Mutation class |
Breast cancer patients | Sum | Total unique |
Percent age (%) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | ||||
| Missense | 12 | 5 | 22 | 10 | 3 | 5 | 6 | 35 | 14 | 49 | 13 | 174 | 159 | 27 |
| Splice site | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 4 | 4 | 0.7 |
| Nonsense | 0 | 0 | 0 | 4 | 0 | 0 | 1 | 2 | 1 | 2 | 0 | 10 | 10 | 1.7 |
| Synonymous | 4 | 2 | 7 | 2 | 4 | 3 | 3 | 15 | 8 | 17 | 5 | 70 | 64 | 10.9 |
| Intron | 30 | 21 | 29 | 16 | 32 | 18 | 13 | 63 | 29 | 50 | 27 | 328 | 309 | 52.6 |
| 3'/5' UTR | 1 | 1 | 4 | 3 | 2 | 0 | 2 | 5 | 1 | 3 | 2 | 24 | 24 | 4.1 |
| Intergenic | 4 | 2 | 4 | 0 | 0 | 0 | 1 | 2 | 5 | 0 | 0 | 18 | 18 | 3 |
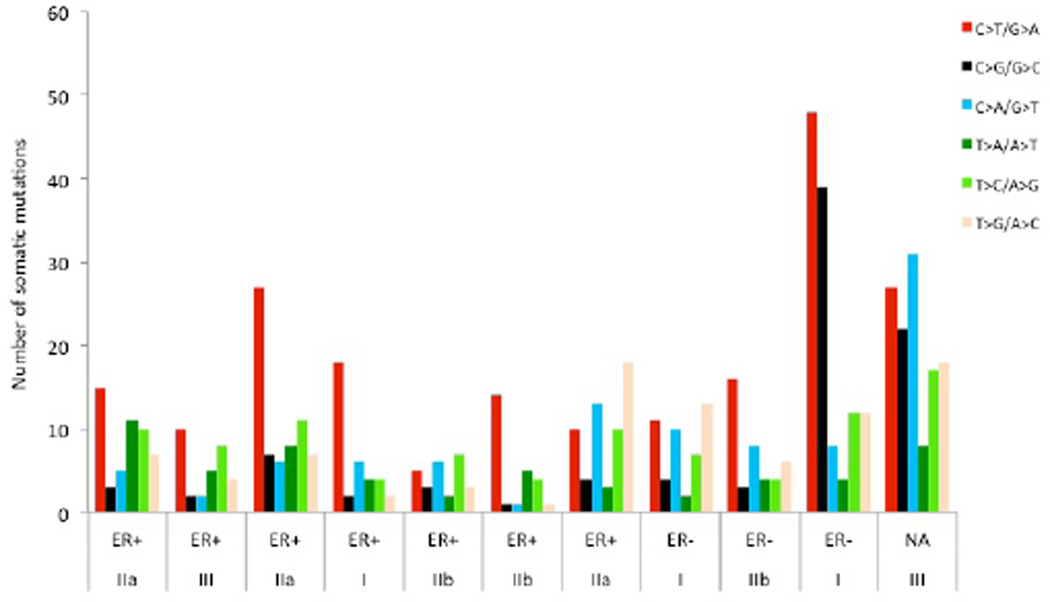
As expected, most mutations (93%) were observed only once. Of the 588 mutations observed, 40.3% were located in coding regions, 4.1% in UTRs, 52.6% in introns, and 3.0% in intergenic regions (Table 2). Among the 237 protein-coding related mutations, 159 were missense, 10 nonsense, 4 splicing, and 64 synonymous mutations (Table 2). Among the six possible mutation classes, including C>T/G>A, C>G/G>C, C>A/G>T, T>C/A>G, T>A/A>T, and T>G/A>C, the C>T/G>A transition dominated the mutation spectra, accounting for 34.2% of the total mutations. The T>A/A>T and C>G/G>C mutations were the least frequent (Figure 1). Such phenomena were observed in ER+ but not in ER− breast cancers. Although only three ER− tumors were surveyed in this study, a similar pattern was reported elsewhere [19]. In addition, we did not find any association between the frequency of somatic mutations and tumor stage (Figure 1). We then focused on the 159 unique missense mutations. They were located in 158 genes, including the known breast cancer genes PIK3CA, HLA-DPA1, ACACB, FOXD4, KDM3A, CCDC108, ACVR2A, TP53, and ATM (Table S3). The A>G mutation (p.H1047R) and A>T mutation (p.H1047L) in the PIK3CA gene were detected in three patients and one patient, respectively, in the discovery stage of whole exome sequencing.
Figure 1.
Distribution of somatic mutations with regard to estrogen receptor (ER) status in the discovery stage. The number of mutation types in the target regions is plotted against the ER status and tumor stage. ER+, ER− and NA denote the ER-positive, ER-negative and no available ER status, respectively.
Validation of novel mutations
We selected 50 missense mutations for validation using the Sequenom MassARRAY system, and 32 of them (64%) were confirmed (Table S4). Furthermore, the PIK3CA H1047R mutation was observed in additional 53 patients in the replication stage, including 50 heterozygous and 3 homozygous mutations (Table 3). Of the remaining 31 experimentally validated mutations, three were located in the genes performing mRNA binding and metabolic processes (KHDRBS1, RBM25, SF3B3) and four were located in the genes related to phosphate metabolic process (NDUFB10, TEK, PGK2 and TRPM6). Besides PIK3CA gene, eight of these mutations were observed in independent tumor samples in the replication stage (Table 3).
Table 3.
Replication of the somatic mutations in independent breast cancer patients.
| Gene | Chromosome | Position | Mutation | Amino acid change |
No. § |
|---|---|---|---|---|---|
| ADRA1B | 5 | 159344316 | G>A | p.Ser135Asn | 1 |
| CBFB | 16 | 67070567 | T>A | p.Leu64Gln | 1 |
| ENTHD1 | 22 | 40140031 | T>C | p.Ile493Val | 3 |
| HYDIN | 16 | 70896126 | C>T | p.Thr3867Ala | 3 |
| KIAA2022 | X | 73964178 | G>A | p.Pro72Ser | 1 |
| NDUFB10 | 16 | 2011243 | G>A | p.Glu74Lys | 1 (1)* |
| PIK3CA | 3 | 178952085 | A>G | p.His1047Arg | 50 (3)* |
| RBM25 | 14 | 73572910 | A>G | p.Lys462Glu | 1 |
| TRPM6 | 9 | 77390837 | C>T | p.Arg1122Gln | 5 |
No. of patients carrying the mutation
The number of homozygote detected.
Of note, the Arg1122Gln mutation in the TRPM6 (transient receptor potential cation channel, subfamily M, member 6) gene was observed in five samples. Both mutations, Ile493Val in the ENTHD1 (ENTH domain containing 1) gene and Thr3867Ala in the HYDIN (axonemal central pair apparatus protein) gene, were observed in an additional three samples. Mutation Glu74Lys in the NDUFB10 (NADH dehydrogenase (ubiquinone) 1 beta subcomplex, 10, 22kDa) gene was observed in two additional samples, one heterozygote and one homozygote. The other four mutations were observed once in the independent patients in the replication stage, including Ser135Asn in the ADRA1B (adrenoceptor alpha 1B), Leu64Gln in the CBFB (core-binding factor, beta subunit), Pro72Ser in the KIAA2022, and Lys462Glu in the RBM25 (RNA binding motif protein 25) (Table 3).
Discussion
In the present study, we systematically searched somatic alternations in the coding region using samples from eleven Chinese breast cancer patients. We identified 588 mutations with 40% located in coding regions. Of the 50 mutations selected for validation, 32 were technically validated. Nine mutations were detected in independent samples in the replication stage. Especially, mutations in the PIK3CA, TRPM6, HYDIN, ENTHD1 and NDUFB10 genes were observed in multiple samples in the replication stage.
One of the most significant findings was the recurrent R1122Q mutation in the TRPM6 gene, which was detected in 6 of 444 breast cancer tumor samples in the present study. The TRPM6 gene plays a crucial role in magnesium homeostasis and epithelial magnesium transport. Both basic and pre-clinical studies show that the magnesium flux by TRP magnesium channels is highly related with tumor cell proliferation and cycle, angiogenesis, tumor growth and reprogramming, as well as metastasis [20]. The R1122Q mutation occurs at the topological domain of TRPM6 towards the cytoplasm, according to the Uniprot database annotation (http://www.uniprot.org/uniprot/Q9BX84). Thereby, dysfunction of the TRPM6 protein may affect the cation homeostasis leading to the tumor development. Recently, Stephens et al. reported two somatic mutations (p.T1822A and p.A1765T) in the TRPM6 gene from screening 100 breast cancer patients of European ancestry [6]. However, the mutation R1122Q that was observed in the present study was not reported in the study of Stephens et al. [6]. Meanwhile, another study, which included 108 breast cancer patients from Mexico and Vietnam using whole-genome and whole-exome sequencing, did not discover any mutations in the TRPM6 gene [21]. Mutations in other members of the TRPM gene family were also reported in breast cancer tumor genome, including three mutations in each of the TRPM8, TRPM3, and TRPM1 genes [6]. In addition, mutations in the TRPM6 gene were also discovered in patients of non-tumor diseases, such as hypomagnesemia with secondary hypocalcemia [22].
In addition to the observed recurrent missense mutation (T3867A) in the HYDIN gene in the present study, ten other mutations have been reported in previous studies [6,21], though each was observed only once in 100 breast cancer patients. The mutation site (T3867A) observed in the present study shows evolutionarily high conservation across all vertebrates with 4.26 of Genomic Evolutionary Rate Profiling (GERP) score, implying potentially functional constraint of this mutation site in the HYDIN gene. However, there is less direct evidence linking the biological role of the HYDIN gene to breast carcinogenesis. It will be of interest to determine the mechanistic and phenotypic consequences of HYDIN mutations in the breast and other tumor cells. We also detected a recurrent mutation (I493V) in the ENTHD1 gene of four patients. However, in the previously two exome sequencing studies of breast cancer, no ENTHD1 mutations were observed [6]. The functional feature of this gene is largely unknown. A recent study showed that the ENTHD1 protein might interact with HDAC6 [23], suggesting that the ENTHD1 may be associated with the histone acetyltransferase activity.
In the present study, we also found recurrent novel missense mutations in the CBFB, ADRA1B, KHDRBS1, NDUFB10, RBM25, and KIAA2022 genes. Among them, the CBFB oncogene has been documented into the COSMIC cancer Gene Census, a gene category for which mutations have been causally implicated in cancer [24]. The CBFB gene plays a critical role in tumor cell growth and proliferation [25]. Other mutations in this gene were recently reported in breast cancer patients, including nonsense mutations at E152 and R83 and truncating frame shift mutations at E16, Q67 and Y96 [6, 21]. The CBFB mutations were found to be accompanied by deletions of the gene encoding its binding partner, RUNX1, in some breast cancers [21]. For the ADRA1B gene, which encodes adrenoceptor alpha 1B, a previous study showed this gene is highly expressed in breast tumors with increased tumor recurrence and poor clinical outcome [26], suggesting this gene may perform a cancer-promoting role. The KHDRBS1 gene encodes KH domain containing, RNA binding, signal transduction associated 1. The protein appears to have many functions and may be involved in a variety of cellular processes, including alternative splicing, cell cycle regulation, RNA 3'-end formation, and tumorigenesis [27]. The NDUFB10 gene encodes NADH dehydrogenase (ubiquinone) 1 beta subcomplex 10, an integral factor in Complex 1 on the mitochondrial inner membrane [28]. Phosphorylation of NDUFB10 by Src kinase could participate in the development of the proliferative phenotype of glycolytic cancer cells, such as 143B and DU145 cells by preserving complex I activity [29]. However, no mutations were observed in the previous breast cancer exome sequencing data [6]. The RBM25 gene encodes the RNA binding motif protein 25, one of the RNA-binding regulators that direct the alternative splicing of apoptotic factors such as Bcl-x [30]. Three other mutations, R708Q, H6D, and G130A, were observed in one study [21] but not in another study [6]. A different mutation, R348Q, in the KIAA2022 gene is observed in breast cancer tumor in a previous study [21]. Taken together, the discovery of some novel somatic mutations partially indicate the difference of somatic aberrations across ethnic groups of patients. Further comprehensive comparison in genomic aberrations may help to develop cancer therapeutics strategies for the ethnic-specific groups of patients.
There are several limitations in this study. The small sample size in the discovery stage of the present study limits the power to detect driver mutations and compare the mutation profiles stratified by disease subtype. Biological samples used in the present study were collected a couple of years ago, which may affect somatic mutations discovery. However, in the present study, we observed those recurrent somatic mutations such as the H1047R mutation in the PIK3CA gene. Another limitation is that only a small portion of mutations were selected for replication. In addition, there are some differences in patient characteristics between the discovery and replication stage, such as age and ER/PR status. Small Indels as well as large structure changes were not investigated in the present study due to the high false positive rate using exome sequencing [31]. No data were available for tumor purity, which may affect the mutation discovery and validation. Similar to other exome sequencing projects, although the high density of read coverage across target region is obtained from the whole-exome sequencing, with over 60-fold on average, there is a small portion of the coding regions in low or no read coverage due to the capturing design.
In summary, we discovered novel somatic mutations in the TRPM6, HYDIN, ENTHD1 and NDUFB10 genes in early onset Chinese breast cancer patients through whole exome sequencing. These mutations were replicated in independent breast cancer patients. Our findings of these novel mutations may provide new clues regarding the molecular mechanism of breast cancer carcinogenesis.
Acknowledgments
The authors wish to thank all individuals who took part in these studies. We also thank Regina Courtney, Jie Wu, Jiajun Shi, and Jing He for their help with sample preparation and genotyping, sequencing data process and analysis for the project. This work was partly supported by R01CA158473 and R01CA158473 as well as Ingram Professorship funds. DNA sample preparation and Sequenom genotyping were conducted at the Survey and Biospecimen Shared Resource, which is supported in part by the Vanderbilt-Ingram Cancer Center (P30CA068485). Data analyses were conducted using the Advanced Computing Center for Research and Education (ACCRE) at Vanderbilt University.
Abbreviations
- BQ
Base Quality Score
- BQSR
Base Quality Score Recalibration
- BP
Base pairs
- GQ
Genotype Quality Score
- Indels
Inserts and deletions
- MAPQ
Mapping Quality Score
- SBCS
Shanghai Breast Cancer Study
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA: A Cancer Journal for Clinicians. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Curtis C, Shah SP, Chin SF, Turashvili G, Rueda OM, et al. The genomic and transcriptomic architecture of ,000 breast tumours reveals novel subgroups. Nature. 2012;486:346–352. doi: 10.1038/nature10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 5.Sjöblom T, Jones S, Wood LD, Parsons DW, Lin J, et al. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314:268–274. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- 6.Stephens PJ, Tarpey PS, Davies H, Van Loo P, Greenman C, et al. The landscape of cancer genes and mutational processes in breast cancer. Nature. 2012;486:400–404. doi: 10.1038/nature11017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wood LD, Parsons DW, Jones S, Lin J, Sjöblom T, et al. The genomic landscapes of human breast and colorectal cancers. Science. 2007;318:1108–1113. doi: 10.1126/science.1145720. [DOI] [PubMed] [Google Scholar]
- 8.Henderson BE, Lee NH, Seewaldt V, Shen H. The influence of race and ethnicity on the biology of cancer. Nat Rev Cancer. 2012;12:648–653. doi: 10.1038/nrc3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broët P, Dalmasso C, Tan EH, Alifano M, Zhang S, et al. Genomic profiles specific to patient ethnicity in lung adenocarcinoma. Clin Cancer Res. 2011;17:3542–3550. doi: 10.1158/1078-0432.CCR-10-2185. [DOI] [PubMed] [Google Scholar]
- 10.Lamy PJ, Jacot W. Worldwide variations in EGFR somatic mutations: a challenge for personalized medicine. Diagn Pathol. 2012;7:13. doi: 10.1186/1746-1596-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao YT, Shu XO, Dai Q, Potter JD, Brinton LA, et al. Association of menstrual and reproductive factors with breast cancer risk: Results from the Shanghai breast cancer study. International Journal of Cancer. 2000;87:295–300. doi: 10.1002/1097-0215(20000715)87:2<295::aid-ijc23>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 12.Zheng W, Long J, Gao YT, Li C, Zheng Y, et al. Genome-wide association study identifies a new breast cancer susceptibility locus at 6q25.1. Nat Genet. 2009;41:324–328. doi: 10.1038/ng.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cock PJA, Fields CJ, Goto N, Heuer ML, Rice PM. The Sanger FASTQ file format for sequences with quality scores, and the Solexa/Illumina FASTQ variants. Nucleic Acids Research. 2010;38:1767–1771. doi: 10.1093/nar/gkp1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DePristo MA, Banks E, Poplin R, Garimella KV, Maguire JR, et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. 2011;43:491–498. doi: 10.1038/ng.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koboldt DC, Zhang Q, Larson DE, Shen D, McLellan MD, et al. VarScan 2: somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Res. 2012;22:568–576. doi: 10.1101/gr.129684.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cancer Genome Atlas Research Network. Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489:519–525. doi: 10.1038/nature11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nik-Zainal S, Alexandrov LB, Wedge DC, Van Loo P, Greenman CD, et al. Mutational processes molding the genomes of 21 breast cancers. Cell. 2012;149:979–993. doi: 10.1016/j.cell.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Futreal PA, Coin L, Marshall M, Down T, Hubbard T, et al. A census of human cancer genes. Nat Rev Cancer. 2004;4:177–183. doi: 10.1038/nrc1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banerji S, Cibulskis K, Rangel-Escareno C, Brown KK, Carter SL, et al. Sequence analysis of mutations and translocations across breast cancer subtypes. Nature. 2012;486:405–409. doi: 10.1038/nature11154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chubanov V, Waldegger S, Mederos y Schnitzler M, Vitzthum H, Sassen MC, et al. Disruption of TRPM6/TRPM7 complex formation by a mutation in the TRPM6 gene causes hypomagnesemia with secondary hypocalcemia. Proc Natl Acad Sci U S A. 2004;101:2894–2899. doi: 10.1073/pnas.0305252101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin YY, Kiihl S, Suhail Y, Liu SY, Chou YH, et al. Functional dissection of lysine deacetylases reveals that HDAC1 and p300 regulate AMPK. Nature. 2012;482:251–255. doi: 10.1038/nature10804. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Futreal PA, Coin L, Marshall M, Down T, Hubbard T, et al. A census of human cancer genes. Nat Rev Cancer. 2004;4:177–183. doi: 10.1038/nrc1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davis JN, Rogers D, Adams L, Yong T, Jung JS, et al. Association of core-binding factor β with the malignant phenotype of prostate and ovarian cancer cells. J Cell Physiol. 2010;225:875–887. doi: 10.1002/jcp.22298. [DOI] [PubMed] [Google Scholar]
- 26.Powe DG, Voss MJ, Habashy HO, Zänker KS, Green AR, et al. Alpha- and beta-adrenergic receptor (AR) protein expression is associated with poor clinical outcome in breast cancer: an immunohistochemical study. Breast Cancer Res Treat. 2011;130:457–463. doi: 10.1007/s10549-011-1371-z. [DOI] [PubMed] [Google Scholar]
- 27.Bielli P, Roberta B, Paronetto MP, Sette C. The RNA-binding protein Sam68 is a multifunctional player in human cancer. Endocrine-Related Cancer. 2011;18:R91–R102. doi: 10.1530/ERC-11-0041. [DOI] [PubMed] [Google Scholar]
- 28.Su YH, Lee YL, Chen SF, Lee YP, Hsieh YH, et al. Essential role of Î2-human 8-oxoguanine DNA glycosylase 1 in mitochondrial oxidative DNA repair. Environ Mol Mutagen. 2013;54:54–64. doi: 10.1002/em.21742. [DOI] [PubMed] [Google Scholar]
- 29.Hebert-Chatelain E, Jose C, Gutierrez Cortes N, Dupuy JW, Rocher C, et al. Preservation of NADH ubiquinone-oxidoreductase activity by Src kinase-mediated phosphorylation of NDUFB10. Biochim Biophys Acta. 2012;1817:718–725. doi: 10.1016/j.bbabio.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 30.Zhou A, Ou AC, Cho A, Benz EJ, Jr, Huang SC. Novel splicing factor RBM25 modulates Bcl-x pre-mRNA 5' splice site selection. Mol Cell Biol. 2008;28:5924–5936. doi: 10.1128/MCB.00560-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bamshad MJ, Ng SB, Bigham AW, Tabor HK, Emond MJ, et al. Exome sequencing as a tool for Mendelian disease gene discovery. Nat Rev Genet. 2011;12:745–755. doi: 10.1038/nrg3031. [DOI] [PubMed] [Google Scholar]