Abstract
BACKGROUND
Human ventricular tachycardia (VT) after myocardial infarction usually occurs because of subendocardial reentrant circuits originating in scar tissue that borders surviving myocardial bundles. Several preclinical large animal models have been used to further study postinfarct reentrant VT, but with varied experimental methodologies and limited evaluation of the underlying substrate or induced arrhythmia mechanism.
OBJECTIVE
We aimed to develop and characterize a swine model of scar-related reentrant VT.
METHODS
Thirty-five Yorkshire swine underwent 180-minute occlusion of the left anterior descending coronary artery. Thirty-one animals (89%) survived the 6–8-week survival period. These animals underwent cardiac magnetic resonance imaging followed by electrophysiology study, detailed electroanatomic mapping, and histopathological analysis.
RESULTS
Left ventricular (LV) ejection fraction measured using CMR imaging was 36% ± 6.6% with anteroseptal wall motion abnormality and late gadolinium enhancement across 12.5% ± 4.1% of the LV surface area. Low voltage measured using endocardial electroanatomic mapping encompassed 11.1% ± 3.5% of the LV surface area (bipolar voltage ≤1.5 mV) with anterior, anteroseptal, and anterolateral involvement. Reentrant circuits mapped were largely determined by functional rather than fix anatomical barriers, consistent with “pseudo-block” due to anisotropic conduction. Sustained monomorphic VT was induced in 28 of 31 swine (90%) (67 VTs; 2.4 ± 1.1; range 1–4) and characterized as reentry. VT circuits were subendocardial, with an arrhythmogenic substrate characterized by transmural anterior scar with varying degrees of fibrosis and myocardial fiber disarray on the septal and lateral borders.
CONCLUSION
This is a well-characterized swine model of scar-related subendocardial reentrant VT. This model can serve as the basis for further investigation in the physiology and therapeutics of humanlike postinfarction reentrant VT.
Keywords: Myocardial infarction, Mapping, Ventricular tachycardia, Ablation
Introduction
The pathophysiology of infarct-related ventricular tachycardia (VT) includes structural remodeling that occurs after myocardial cell death, resulting in inhomogeneous scarring with varying degrees of survived myocardial tissue contiguous with dense fibrosis, forming the so-called arrhythmogenic substrate.1
This arrhythmogenic substrate is characterized by zones of slow conduction due to nonuniform anisotropy resulting in fixed and/or functional regions of conduction block. This facilitates reentry as it generates enough time for tissue in the circuit to recover its excitability to allow the excitation wavefront to reenter the initial site of block, thereby creating a circuit. Both clinical and experimental data provide convincing evidence for reentry as the major underlying mechanism of postinfarction human VT.2–6
Large animal models have been used to study postinfarct arrhythmogenic substrate and VT for decades. The selected animal species, designated coronary artery, and myocardial ischemia induction method have significantly varied across investigations.7 These experimental characteristics are critical in determining whether or not arrhythmias occur and also define their inherent characteristics when they do. Arrhythmias may not occur without the presence of surviving myocardial fibers in the infarcted region, and their spatial arrangement is important for developing a milieu able to support reentry.8
Despite significant advancements in this field, there is still a need for humanlike models of postinfarct reentrant VT. These models can assist in the development of improved methodologies to identify and differentiate arrhythmogenic scar from nonarrhythmogenic scar and also to validate new diagnostic and therapeutic tools in the preclinical setting. For these reasons, we sought to develop and characterize a model of healed myocardial infarction (MI) with humanlike subendocardial reentrant VT by using a consistent experimental approach.
Methods
Swine preinfarct preparation
The research protocol was approved by the Institutional Animal Care and Use Committee and conformed to the Position of the American Heart Association on Research Animal Use as well as to the Declaration of Helsinki. The research was performed at the Beth Israel Deaconess Medical Center, Experimental Electrophysiology Laboratory in Boston, MA. Swine (male, 30–35 kg) were received 5–7 days before the planned infarction day to allow adjustment and acclimation to the indoor animal facility. Animals were treated with amiodarone at a dose of 800 mg twice daily for 3–4 days before the infarction procedure to reduce the incidence of ventricular arrhythmias during the peri-infarct period.
Induction of MI and survival period
After a 12-hour fasting period, sedation was initiated with 1.4 mg/kg intramuscular injection of Telazol (tiletamine/ zolazepam hydrochloride). Endotracheal intubation was then performed, and general anesthesia was maintained with isoflurane inhalation (1.5%–2.5%). Ventilation was maintained between 10 and 16 breaths/min with tidal volumes between 300 and 500 mL. Hemodynamic assessment including core body temperature, heart rate, oxygen saturation, and arterial blood pressure were continuously monitored. Percutaneous femoral arterial and venous access was guided by ultrasound (Siemens Acuson, Mountain View, CA) and obtained using a micropuncture needle (Cook Medical, Bloomington, IN) to minimize vascular trauma. After vascular access, 10,000 units of unfractionated heparin were administered intravenously with maintenance boluses (2000–3000 units) as needed to maintain activated clotting time (ACT) of 250–350 seconds. Intravenous lidocaine (50– 100 mg bolus, 1 mg/min continuous drip) and metoprolol (1 mg) were administered to reduce the incidence of malignant ventricular arrhythmias.
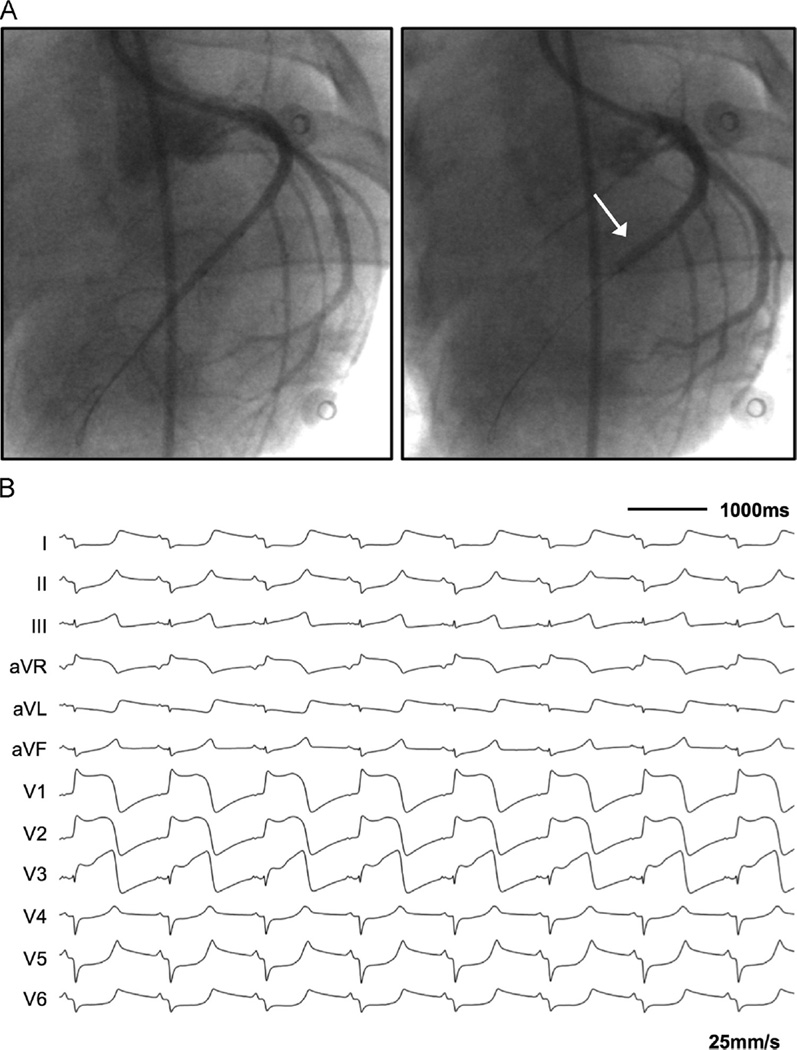
Under fluoroscopic guidance, a 6F Hockey stick guide catheter (Cordis Corporation, Fremont, CA) was positioned in the left main ostium. A 0.18-in, 180-cm ChoICE PT angioplasty wire (Boston Scientific, Marlborough, MA) was carefully advanced into the left anterior descending (LAD) coronary artery. A rapid-exchange 2.5 × 12 mm angioplasty balloon (Apex, Boston Scientific, Marlborough, MA) was placed over the angioplasty wire in the mid-LAD. Serial coronary angiography was performed to position the angioplasty balloon immediately distal to the second diagonal branch of the LAD. The angioplasty balloon was inflated and maintained at 12–14 atm throughout the infarct procedure. After initial balloon inflation, repeat coronary angiography was performed to confirm adequate distal occlusion of the LAD. Uninterrupted coronary occlusion was maintained for 180 minutes, with confirmation of acute MI by ST-segment elevation in the precordial electrocardiographic (ECG) leads (Figure 1).
Figure 1.
Induction of myocardial infarction via percutaneous balloon occlusion of the left anterior descending (LAD) coronary artery. A: Coronary angiography performed in the anterior-posterior view before and after balloon inflation with confirmation of distal occlusion immediately after the second diagonal branch of the mid-LAD (white arrow). B: Twelve-lead electrocardiogram shortly after balloon inflation, exhibiting significant ST-segment elevation in leads V1–V3 with reciprocal ST-segment depressions in leads I and aVL.
Animals were recovered and survived for 6–8 weeks after the MI. Amiodarone at a dose of 800 mg twice daily was continued for another 5–6 days after the infarct procedure up to a total dose of 14,400–16,000 mg. Swine that completed the survival period without complication underwent in vivo cardiac magnetic resonance (CMR) imaging 4–7 days before the terminal electrophysiology study.
CMR imaging
CMR imaging was performed using a 1.5T magnetic resonance imaging scanner (Phillips Achieva, Best, NL) with a 32-element cardiac phased-array receiver coil. During imaging, each animal was sedated, intubated, and mechanically ventilated. Cardiac function and anatomy was assessed using a cardiac cine examination in short-axis view with steady-state free-precession imaging sequence with use of the following imaging parameters: repetition time = 3.2 ms, echo time =1.6 ms, flip angle = 60°, field of view = 320 × 320 mm2 , spatial resolution = 2.7 × 2.7 mm2, slice thickness = 8 mm, slice number =10, slice gap = 2 mm, temporal resolution = 48 ms, and acquired/reconstructed number of cardiac phase = 20/30. Late gadolinium enhancement (LGE) images were acquired 10–20 minutes after infusion of a bolus (2 mL/s) of 0.2 mmol/kg of gadobenate dimeglumine (MultiHance, Bracco, Rome, Italy). The optimal inversion time was selected to null the LV myocardial signal by using a Look-Locker sequence before LGE imaging. A free-breathing electrocardiography-triggered navigator-gated inversion-recovery gradient-echo sequence was used for 3-dimensional LGE. Imaging parameters were as follows: repetition time = 6.1 ms, echo time = 2.7 ms, flip angle = 25°, field of view = 270 × 270 × 112 mm3, spatial resolution =1×1×1 mm3, and compressed sensing acceleration factor = 4.9 A respiratory navigator (2-dimensional spiral pencil beam) placed on the dome of the right hemidiaphragm was used for respiratory motion compensation with use of prospective real-time correction with a 5-mm end-expiration gating window. Saturation bands along the phase-encoding direction were used to reduce fold-over artifacts.10–12
Image analysis was performed off-line using an in-house platform.13 Left ventricular ejection fraction (LVEF) was measured using CMR imaging.14 LGE LV myocardial volume, LV cavity volume, scar volume, and scar transmurality index were measured using LGE data. Endocardial and epicardial contours of the myocardium were manually delineated in all slices, and LV myocardial volume and LV cavity volume were measured directly from both endocardial and epicardial contours. Average (Sremote) and standard deviation (SDremote) of a “healthy” myocardial signal was measured over a remote region of interest manually positioned within an area of “healthy” myocardium. Voxels with signal intensity above Sremote + (3 × SDremote) were classified as part of the scar area, and the scar volume was computed. Scar transmurality was finally visually assessed for each of the 17 American Heart Association (AHA) myocardial segments as the maximum scar transmurality in each segment. 15
Electrophysiology study
Recording and mapping
The LabSystem Pro EP Recording System (Bard, Boston Scientific, Lowell, MA) was used for surface ECG and intracardiac electrogram (EGM) recordings. A Bloom DTU 215B (Fisher Medical Technologies, Broomfiled, CO) was used for electrical stimulation. Electroanatomic mapping (EAM) was performed using Carto 3 (Biosense Webster, Johnson & Johnson, Diamond Bar, CA) or Rhythmia (Boston Scientific, Cambridge, MA).
Unfractionated heparin was administered throughout the procedure to maintain an ACT of 250–350 seconds. A 6F pentapolar diagnostic catheter (Bard EP, Lowell, MA) was placed in the right ventricular (RV) apex and/or the RV outflow tract to allow recording and pacing. Intracardiac echocardiography (Acuson Acunav, Siemens, Mountain View, CA) was used for the assessment of LV dimension, function, and catheter contact during EAM. Mapping of the LV using a retrograde transaortic approach was performed using a 5-spline multielectrode catheter with 20 electrodes and 2-mm interelectrode spacing (Pentaray, Biosense Webster, Diamond Bar, CA) or a 64-electrode mini-basket catheter with 2.5-mm interelectrode spacing (Orion, Boston Scientific, Cambridge, MA). Bipolar EGMs were filtered at 30–250 Hz and unipolar EGMs were filtered at 0.5 and 250 Hz and displayed at a sweep speed of 200 mm/s. All recordings were stored in a digital format for off-line annotation and analysis.
Ventricular stimulation and mechanism characterization
Electrical stimulation was performed from the RV apex using a current strength twice the capture threshold and a pulse width of 2.0 ms. Burst pacing and ventricular extra-stimulation at a paced cycle length of 400–500 ms with 1–4 extrastimuli down to ventricular effective refractory period were performed in an attempt to induce VT. Upon induction of sustained monomorphic VT, pacing maneuvers were used to characterize the arrhythmia mechanism. If the VT was not hemodynamically tolerated, it was terminated by pacing or electrical cardioversion. In cases of hemodynamically non-tolerated VTs, inotropic support (phenylephrine bolus of 5–10 mg) was administered to increase and stabilize the blood pressure. In addition, procainamide (5 mg/kg bolus followed by infusion at a rate of 2–4 mg/min) was administered to increase the cycle length of the VT. If electrical stimulation from the RV apex failed to induce VT, stimulation was repeated from the RV outflow tract followed by the LV.
The mechanism of the VT was determined on the basis of the following: (1) reproducible initiation by programmed stimulation, (2) response to single and multiple extrastimuli (analysis of the resetting response), (3) response to overdrive pacing (suppression vs acceleration, and/or termination) and entrainment, and (4) pattern of ventricular activation. A reentrant mechanism was considered in the presence of one or more of the following: (1) reproducible initiation with extrastimuli, (2) overdrive pacing from remote sites demonstrating fixed and progressive fusion (ie, entrainment), (3) resetting response pattern of constant return cycles at longer coupling intervals followed by increasing return cycles in response to progressively shorter coupling intervals, and (4) activation mapping demonstrating reentrant excitation.16–20
EAM
The mitral and aortic annuli were mapped, as well as the entire LV. The mapping density was sufficient to allow detailed endocardial surface geometric reconstruction with a fill threshold of ≤ 5 mm in regions of low bipolar voltage and ≤ 10 mm elsewhere, thus limiting interpolation between points to less than 5 and 10 mm, respectively. Abnormal bipolar voltage was defined using conventional cutoff values (low bipolar voltage ≤ 1.5 mV and very low bipolar voltage ≤0.5 mV).21 The low-voltage and very low-voltage LV surface area and percentage were quantified using the mapping system surface area measurement utility. All EGMs were analyzed off-line using fixed gain and fixed high- and low-pass filters to examine the presence of fractionated signals and isolated late potentials (ILPs). ILPs display an additional EGM recorded after the end of the QRS complex, separated from the local ventricular EGM by an isoelectric interval of ≥ 20 ms.22–24
Activation mapping during the VT was also performed with the multielectrode or basket catheter during 22 sustained VTs. The mean number of points obtained during sinus rhythm was 8460 ± 2460 and 5333 ± 3127 during VT. This allowed detailed and high-density mapping data. The catheters are steerable and allowed navigation throughout the entire chamber. EGMs were selected for local time annotation if their bipolar voltage amplitude was > 0.06 mV (2-fold higher than the noise level in our laboratory) and had distinct and reproducible near-field potentials. Activation maps were considered complete when the putative circuit was identified. Overdrive pacing was performed in order to entrain the tachycardia and further characterize the circuit as described in the previous section.
Histopathogical analysis
After the completion of mapping procedures, animals were euthanized and the heart was harvested and placed in a 10% buffered formalin solution for > 72 hours for tissue fixation. After tissue fixation, the hearts were serially sectioned parallel to the atrioventricular groove into 1-cm-thick slices. The tissue samples were paraffin embedded and sectioned at 5-µm thickness perpendicular to the epicardial surface so that each section showed the full thickness of the ventricular wall from the epicardium to the endocardium. Slides at intervals of 100 µm were stained with hematoxylin and eosin stain as well as with Masson trichrome stain (MTS) for collagen. Slides were digitized using a Philips Ultra Fast Scanner, and the whole slide images were displayed via https://slide-atlas.org, a high-performance Web-based viewer platform. Tools were developed for local adaptive alignment of the images of serial sections and incorporated into the software system. Aligned whole slide images were displayed side by side and could be quickly manipulated (eg, rotated) and stepped through. Regions of interest could be compared simultaneously in adjacent sections, allowing the precise characterization of the locations of myocardial fibrosis and surviving myofibrils. Digital images of the MTS tissue samples were imported into the open source ImageJ software v1.48 (National Institutes of Health, Bethesda, MD) to calculate the percentage of collagen tissue utilizing a color threshold macro.25 Immunofluorescence was performed in a similar fashion as H&E and MTS described above. Diluted mouse anti-connexin 43 monoclonal primary antibody (3512, Cell Signaling Technologies, Danvers, MA) was applied overnight at 4°C followed by application of CCC secondary antibody for 2 hours. Sections were imaged with a Zeiss LSM 510 Meta Upright Confocal System (Carl Zeiss Microscopy, Thornwood, NY). The gap junction expression and distribution were assessed across the septal, anterior, and lateral walls throughout the scar region, with a particular focus on the subendocardial level.
Statistical analysis
Statistical analyses were performed with Stata/MP version 13 (StataCorp, College Station, TX). Continuous variables were reported as mean ± SD, and distribution of discrete variables were reported as percentages for each group. Continuous variables were compared using the Mann-Whitney-Wilcoxon test. Correlation of CMR and EAM scar size was evaluated using linear regression analysis. P values of < .05 were considered statistically significant.
Results
Experimental demographics
Thirty-four of the 35 animals (97%) survived the MI procedure. One animal developed refractory ventricular fibrillation 45 minutes after intracoronary balloon occlusion that persisted despite balloon deflation, defibrillation, and antiarrhythmic drug therapy. Thirty-one of the remaining animals (91%) completed the survival period of 47.7 ± 12.4 days without complication. One animal developed a spontaneous episode of sustained monomorphic VT 6 days after the MI that resulted in congestive heart failure. This animal was successfully converted to sinus rhythm, but was subsequently euthanized because of cardiogenic shock. Two animals died suddenly 38 and 45 days after the MI procedure without preceding signs and/or symptoms of heart failure. The mean swine weight at the end of the survival period was 62.8 ± 11.7 kg. The experimental demographics and data from imaging, mapping, and electrophysiology study are displayed in Table 1.
Table 1.
Experimental results (N = 35)
| Demographics | |
| Acute mortality | 1 (3) |
| VF during infarct | 8 (27) |
| Study completion | 31 (91) |
| Postinfarct period (d) | 47.7 ± 12.4 |
| Final weight (kg) | 62.8 ± 11.7 |
| CMR imaging data | |
| LV end-diastolic volume (mL) | 142 ± 37 |
| LVEF (%) | 36 ± 6.6 |
| Total scar area (%) | 12.5 ± 4.1 |
| LV mapping | |
| LV chamber volume (mL) | 149 ± 50 |
| Low bipolar voltage (≤1.5 mV) area (%) | 11.1 ± 3.5 |
| Very low bipolar voltage (≤0.5 mV) area (%) | 3.8 ± 1.8 |
| Fractionated EGM density (%) | 65.2 ± 9.6 |
| ILP EGM density (%) | 16.6 ± 3.8 |
| VT | |
| VT induction | 28/31 (90) |
| Distinct VT morphologies | 2.4 ± 1.1 (range 1–4) |
| Tachycardia cycle length (ms) | 256 ± 42 (range 185–356) |
| VT QRSd (ms) | 137 ± 26 |
| VT duration (s) | 396 ± 186 |
Values are presented as mean ± SD or as n (%). CMR = cardiac magnetic resonance; EGM = electrogram; ILP = isolated late potential; LV = left ventricle; LVEF = left ventricular ejection fraction; QRSd = QRS duration; VF = ventricular fibrillation; VT = ventricular tachycardia.
CMR imaging
The LV end-diastolic volume was 142 ± 37 mL, with an LVEF of 36% ± 6.6%. All animals had anteroseptal wall motion abnormality and corresponding myocardial thinning (Figure 2). LV scar as assessed with LGE was present in 12.5% ± 4.1% of the chamber and involved an average of 4.5 ± 0.9 (10.8 ± 3.5 mL) myocardial segments; 92% of these segments had a component of transmural scar. The remaining segments (8%) had only subendocardial scar with preserved midmyocardial and subepicardial tissues. VT circuits correlated with the presence of scar that was adjacent to areas of preserved subendocardial tissue, potentially representing “conduction channels.” However, similar scar architecture was also identified in areas that were not part of the reentrant circuit.
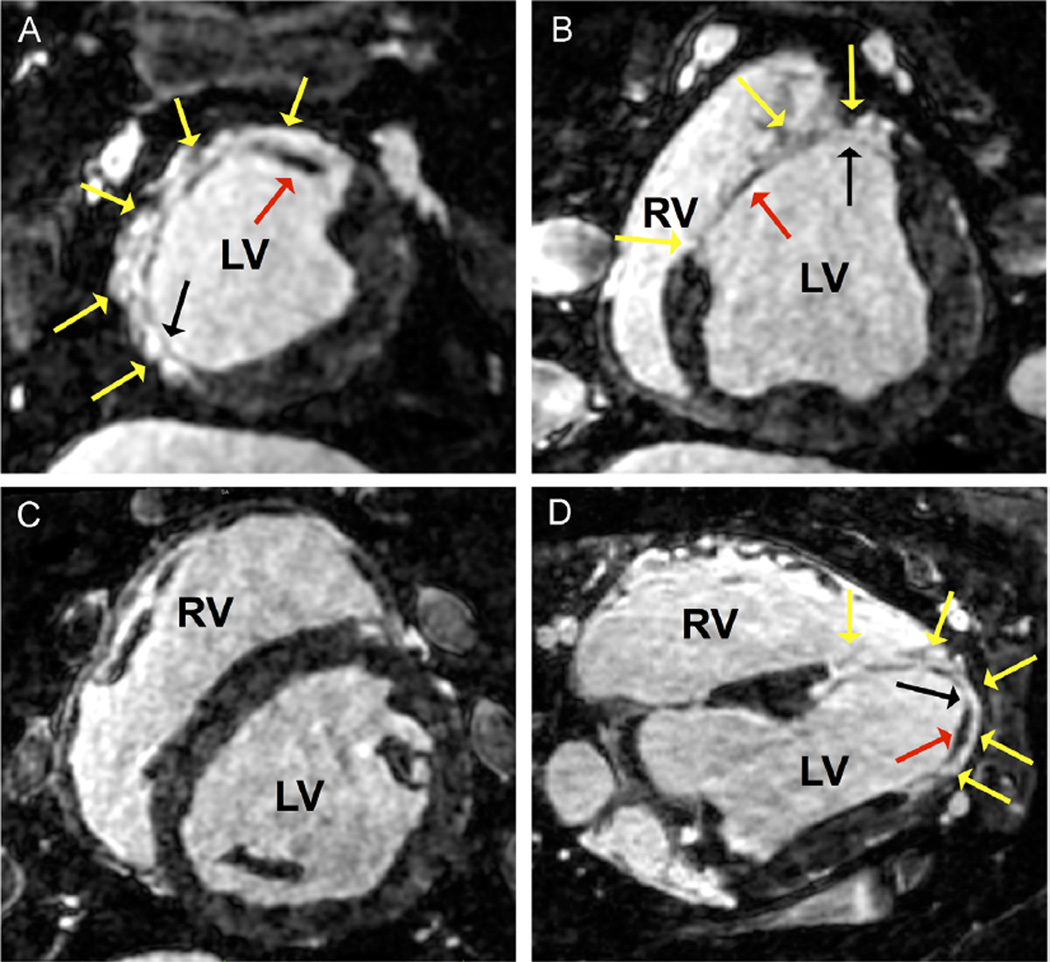
Figure 2.
In vivo high-resolution late gadolinium enhancement (LGE) magnetic resonance imaging performed 6 weeks after myocardial infarction. LGE images were acquired in the axial view with isotropic spatial resolution of 1 mm3. A and B: Reformatted short-axis views extending from the apex (panel A) to the base (panel B) are shown with anterior-septal LGE (yellow arrows). Variable LGE signal intensities were observed within the scar area including regions of dark signal representative of survived myocardial tissue (red arrow). These regions of viable tissue were predominantly preserved to the subendocardial rather than subepicardial tissue. Regions of confluent LGE signal intensity indicative of transmural scar were also observed (black arrow). C: Short-axis view further toward the base without evidence of LGE. D: Reformatted 4-chamber view with anterior, septal, and lateral scar with variable LGE intensities (yellow arrows). Viable septal and lateral subendocardial tissue is present (red arrow) and surrounds a segment of transmural anterior scar (black arrow). LV = left ventricle; RV = right ventricle.
Electrophysiology study
Sustained monomorphic VT was initiated in 28 of 31 animals (90%) with programmed stimulation from the RV or LV. The number of extrastimuli required for the initiation was 2–5 (median 3). A total of 67 VTs were induced (2.4 ± 1.1; range 1–4) with a left bundle branch block type pattern in 51 (76%) and a right bundle branch block type pattern in 16 (24%). The higher prevalence of VTs with a left bundle branch block type pattern is likely due to the relative rightward position of the porcine heart with standard ECG lead configuration. The mean tachycardia cycle length (TCL) was 256.4 ± 41.6 ms (range 185–356 ms), with a mean QRS duration of 137.5 ± 25.6 ms and a mean event duration of 396 ±186 seconds (range 28–946 seconds) before arrhythmia termination. Eight VTs (12%) were hemodynamically tolerated in 4 swine (13%) without any pharmacological therapy. The remainder required inotropic support to allow VT mapping and characterization as described in the Methods section. Thirty-eight (57%) terminated during catheter mapping or with pacing maneuvers, 18 (27%) degenerated into polymorphic VT during mapping requiring external defibrillation, and 11 (16%) required RV overdrive pacing to terminate the VT in the setting of progressive hemodynamic compromise. Nonsustained VTs were not included for analysis because of the incomplete assessment of the mechanism.
In addition to the mode of initiation, a reentrant mechanism was determined in 20 VTs by overdrive pacing from remote sites demonstrating fixed and progressive fusion (Figure 3A), resetting response pattern with a mixed resetting response pattern with flat return at long coupling intervals and increasing response return during short coupling intervals in 3 VTs, and activation mapping demonstrated reentrant excitation in 37 VTs (Figure 4). Overdrive pacing from the LV endocardial area with diastolic electrical activity demonstrated concealed fusion with a postpacing interval that was 0–25 ms longer than the TCL in 19 VTs (Figure 3B).
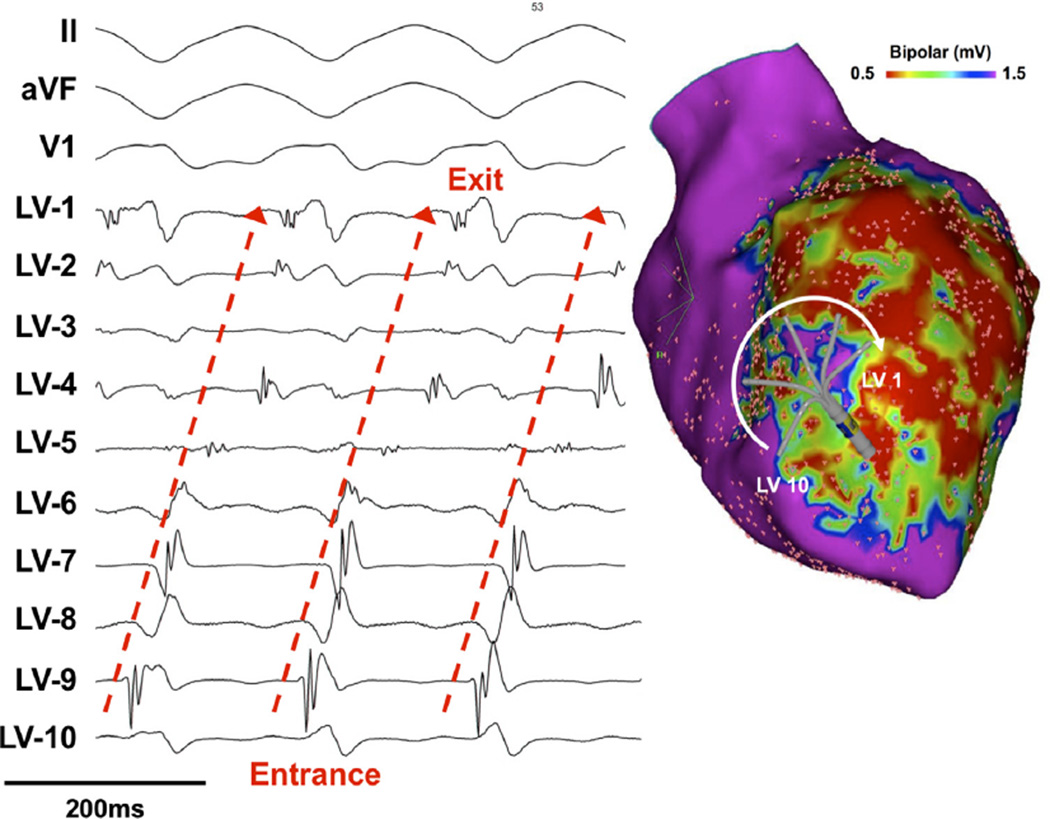
Figure 3.
A: Overdrive pacing at incremental cycle lengths from the right ventricular outflow tract (RVOT) showed evidence of progressive fusion. B: Overdrive pacing from a multielectrode catheter positioned within the reentrant circuit at the anteroseptal endocardium. Pacing at 30 ms faster than the tachycardia cycle length (the paced electrode is not shown because of significant artifact) resulted in entrainment of the tachycardia with minimal fusion on the 12-lead electrocardiogram and similar wavefront propagation on the multielectrode catheter, consistent with orthodromic capture. VT = ventricular tachycardia.
Figure 4.
Endocardial recordings using a multielectrode catheter during ventricular tachycardia. The catheter was positioned in the anteroseptum and recorded continuous electrical activity consistent with “reentrant excitation.” LV = left ventricular.
Endocardial LV EAM and EGM characteristics
The mean LV chamber volume was 149.4 ± 49.8 mL and correlated well with the chamber volume measured using CMR imaging (95% CI 0.13–0.62; P = .005). The low bipolar voltage area, defined as ≤ 1.5 mV, primarily involved the anterior LV wall and extended to both the septal and lateral walls (Figure 5A). The area of low bipolar voltage comprised 11.1% ± 3.5% (18.1 ± 8.2 cm2) of the total LV surface area. Very low bipolar voltage (≤0.5 mV) comprised 3.8% ± 1.8% (6.3 ± 4.0 cm2) and was generally limited to the center of the anterior wall scar and correlated with transmural scar as visualized using CMR imaging. The septal and lateral borders of the scar recorded higher and variable bipolar voltage amplitudes (septal 0.75 ± 0.55 mV vs lateral 0.87 ± 0.45 mV; P = .78). These areas correlated with nontransmural scar as visualized using CMR imaging. The variability in bipolar voltage in these areas was partially related to the presence or absence of subendocardial fibrosis rather than to the absolute degree of scarring. In particular, subendocardial fibrosis resulted in lower endocardial bipolar voltage amplitude as compared with a similar extent of fibrosis limited to the mid- or subepicardial layers. In 25 of 31 swine (80%), regions of relatively higher bipolar voltage amplitude (≥ 1.0 mV) transected or immediately bordered the area of very low bipolar voltage representing possible subendocardial VT circuit “channels” (Figure 5B).
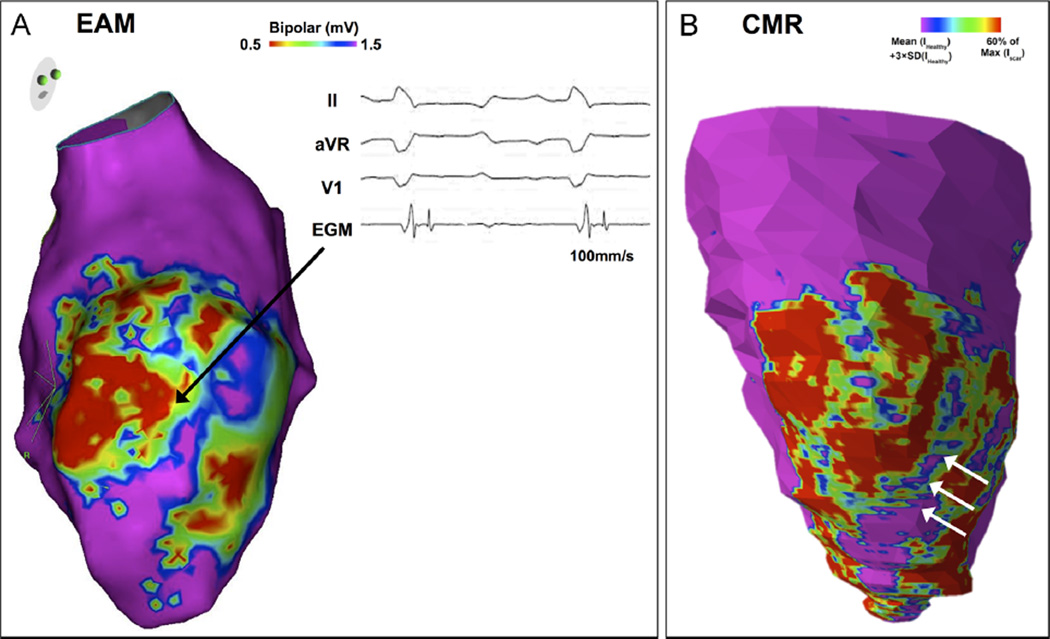
Figure 5.
A: Endocardial left ventricular (LV) bipolar voltage map (voltage range displayed 0.5–1.5 mV) in the right anterior oblique (RAO) projection 6 weeks after myocardial infarction with anterior low-voltage regions extending to the septum and lateral LV. A region of preserved endocardial bipolar voltage (>1.5 mV) is observed transecting vertically through the anterior scar. The adjacent low-voltage electrograms often displayed fractioned and/or late potentials (black arrow). B: RAO view of in vivo late gadolinium enhancement (LGE) magnetic resonance imaging (MRI) reconstruction of the same animal acquired before the mapping procedure. The reconstruction depicts variable LGE intensities in the 1-mm subendocardial layer across the LV. The LGE MRI map shows scar distribution corresponding to the voltage map (panel A). In particular, a region within the scar area where LGE was not present (white arrows) corresponded to the area of preserved endocardial bipolar voltage. CMR = cardiac magnetic resonance; EAM = electroanatomic mapping; EGM = electrogram.
The percentage of LV low bipolar voltage (≤ 1.5 mV) was significantly lower in the 3 animals that were noninducible (3.9% ± 2.6% vs 11.6% ± 2.8%, respectively; P < .001). These animals may represent a subgroup that, for unknown reasons, has a more favorable post-MI remodeling process.
Bipolar EGMs in the healthy, noninfarcted LV areas (inferior and lateral wall) were characterized by an amplitude of 5.1 ± 2.9 mV, bi- or triphasic signals, and a duration of <80 ms (64.1 ± 8.9 ms; range 46–78 ms). In contrast, abnormal EGM morphology in the low bipolar voltage region was frequently present and included both fractionated EGMs (65.2% ± 9.6%), ILPs (16.6% ± 3.8%), and prolonged EGM duration (113.8 ± 20.2 ms; range 90–160 ms).
Correlation between reentrant VT circuit and EGMs during sinus rhythm
Using small multielectrode catheters, the entire reentrant circuit including boundaries of the isthmus was defined in 22 VTs (TCL 278 ± 211 ms) mapped in 15 swine. On average, isthmuses were 27.3 ± 7 mm long and 9.2 ± 5 mm wide. The axis of the isthmus was oriented parallel to the myocardial fibers (long axis of the ventricle) in all the mapped VTs. During sinus rhythm, endocardial EGMs at sites of the protected isthmus showed multicomponent fractionated signals (20 of 22 VTs [91%]), split signals (17 of 22 VTs [77%]), and ILPs (5 of 22 VTs [23%]). While multicomponent fractionated signals were most common, they had low specificity as they were also commonly recorded in other sites in and around the scar. Split potentials, although observed in only 77% of the mapped VTs, were more specific to isthmus sites, only occasionally recorded at other sites (3 of 15 swine [20%]). Activation during sinus rhythm showed that reentrant circuits mapped were largely determined by functional rather than fix anatomical barriers, consistent with “pseudo-block” due to anisotropic conduction as previously described by Andy Wit.2 Even in areas of split potentials (>50 ms between 2 potentials separated by an isoelectric segment), slow conduction was possible during sinus rhythm or pacing.
Endocardial RV and epicardial EAM
Endocardial RV and epicardial EAM was performed in a limited number of swine (n = 3). RV bipolar voltage amplitude abnormalities (≤ 1.5 mV) were identified and localized to the apical septum and comprised only 5.3% ± 2.1% (9.8 ± 4.7 cm2) of the RV surface (Figure 6A). There was minimal RV EGM fractionation present (14.8% ± 4.2%) within the low bipolar voltage region and no detectable ILPs. Epicardial low voltage (≤ 1.0 mV) was also present opposite the anterior LV endocardium along the trajectory of the LAD with both EGM fractionation and ILPs present in the low-voltage area representative of actual fibrosis as opposed to the presence of epicardial fat (Figure 6B).26
Figure 6.
A: Endocardial right ventricular (RV) and left ventricular (LV) bipolar voltage map (voltage range 0.5–1.5 mV) in the anterior-posterior projection after myocardial infarction. B: Epicardial bipolar voltage map (voltage range 0.5–1.0 mV) in the anterior-posterior projection in the same animal with epicardial anterior low voltage with abnormal electrograms (EGMs) consistent with scar as opposed to epicardial fat.
Histopathological analysis and connexin 43 immunofluorescence expression
Transmural endocardial to epicardial fibrosis was observed only on the anterior-septal wall, not on the lateral component of the scar (Figure 7). The septal and lateral borders of the transmural scar had significantly less fibrosis present (anterior 92.3% ± 1.8% vs septal 49.7% ± 3.9%; P < .001 and lateral 50.4% ± 5.5%; P < .001), with both subendocardial and subepicardial survived tissue present. However, the preserved subendocardial layer on both septal and lateral borders included a heterogeneous patchy substrate of fibrosis with fiber disarray and loss of normal parallel alignment (Figure 7C). The subepicardial preserved tissue in these regions consisted of minimal fibrosis with limited instances of transverse tissue fiber orientation. There were also substantial differences in the extent of connexin 43 gap junction expression and remodeling within these distinct regions. As opposed to a region remote to scar region, the connexin 43 expression was predominantly absent in the anterior transmural scar and redistributed to the lateral cell margins at both the septal and the lateral heterogeneous subendocardial layer.
Figure 7.
A: Mason’s trichrome stained histology section of the anterior wall including the septal and lateral borders (magnification 2.25 ×). The blue stained tissue indicates the extent of fibrosis. The yellow box encloses the anterior wall with transmural endocardial to epicardial fibrosis. The red subendocardial tissue indicated by the yellow arrows is red blood cell accumulation from tissue sectioning. The survived layer of myocardial fibers in disarray was commonly observed at the septal and lateral borders of the scar (black arrows). B: Magnified image (magnification 10×) of the subendocardial region demonstrating predominant fibrotic tissue with red blood cell distribution. C: Magnified image (magnification 10×) of the lateral border of the transmural scar with survived subendocardial tissue. The viable tissue demonstrates loss of the parallel bundle orientation with myocardial fiber disarray and varying degrees of fibrosis.
Discussion
In this study, we performed balloon occlusion of the mid-LAD for 180 minutes in 35 swine to determine the incidence of VT inducibility, evaluate the VT mechanism, and characterize the arrhythmogenic substrate in postinfarct healed LV scar.
Major findings
The major findings include the following: (1) mid-LAD balloon occlusion for 180 minutes results in the reproducible development of a humanlike postinfarct arrhythmogenic scar in a majority of cases. This includes a transmural component of anterior/anteroseptal fibrosis with adjacent septal and lateral borders of preserved subendocardial tissue. These regions contribute to slow conduction and nonuniform anisotropy underlying reentrant VT. (2) Reproducible and sustained monomorphic reentrant VT can be induced in all swine that develop this arrhythmogenic substrate after the postinfarct healing phase. Only in rare cases can VT not be induced and is most likely a reflection of a more favorable ventricular remodeling process. (3) Activation mapping during the VT is highly suggestive of a subendocardial VT circuit consistent with the aforementioned subendocardial substrate.
Comparison to other animal models
A number of animal models have been developed to study the arrhythmogenic substrate and mechanisms associated with postinfarct VT.7 The assorted species and methodologies used have translated into variable results, including infarct size, location, and the extent of surviving myocardial fibers. These anatomical features are critical in determining whether arrhythmias occur and also define their inherent characteristics when they do. Arrhythmias may not occur without the presence of surviving myocardial fibers in the infarcted region, and their spatial arrangement is important for developing a milieu able to support reentry.8 For example, canine coronary occlusion has been used for decades as a model to investigate scar and ventricular arrhythmias. Survived myocardial fibers are located on the epicardial and endocardial surface, but with reentrant VT circuits more frequently involving the epicardial surface in the canine MI model.27–30 As such, reentrant VT circuits more often involve the epicardial surface in the canine MI model. In contrast, swine have a predominant endocardial/midmyocardial system and absent epicardial collateral system more favorable for a subendocardial reentrant VT model.31,32
The mid-LAD ischemia reperfusion technique used in our study was adapted and based on the extensive experience reported by Sasano et al,33 who characterized the swine LV remodeling process with serial transthoracic echocardiograms (TEEs) and also performed programmed extrastimulation each week to evaluate VT induction rate. The authors reported a 100% monomorphic VT induction rate following 150-minute balloon occlusion after a 4-week survival period. LV mapping with a 64-pole basket noncontact system showed earliest activation at the subendocardial septal infarct border in a majority of VTs. Detailed endocardial and epicardial mapping was performed in 6 animals, but detailed voltage and VT circuit characteristics are not described, as this was not the primary objective of the authors. Histology from the septal “border zone” of this region demonstrated surviving strands of the myocardium surrounded by fibrotic tissue.33
Ashikaga et al34 performed a similar technique of 150-minute LAD balloon occlusion with a 10–12-week survival period, followed by CMR imaging and either endocardial (n = 5) or epicardial (n = 6) VT mapping. The authors also reported a complex CMR substrate with preserved survived tissue with a corresponding subendocardial and subepicardial substrate. The authors hypothesize that a majority of mapped VTs were subendocardial in nature, but did not perform endocardial mapping in swine that underwent epicardial mapping. This study also did not report bipolar voltage mapping and/or histopathologic correlates to the CMR data.34
Tung et al35 performed obtuse–marginal balloon occlusion for 90 minutes in swine and evaluated the distribution of fibrosis after a 12-week survival period with complete LV endocardial and epicardial voltage mapping, ex vivo CMR imaging, and histopathologic correlates. While endocardial and epicardial abnormalities were present, VT induction was not performed.35
We also used an ischemia-reperfusion model, but performed LAD balloon occlusion for 180 minutes in 35 swine followed by a 6–8-week survival period. Animals underwent in vivo CMR imaging, detailed electrophysiology study with high-resolution EAM, and histopathological analysis. This multimodality characterization allowed us to evaluate and confirm the distribution, quality, and relevance of scar in this preclinical setting. We were able to reproducibly induce reentrant monomorphic VT in 90% of animals.
Arrhythmias occurring in hearts with healing and healed infarcts are, to a large extent, the result of reentrant excitation; however, delayed after depolarization–dependent triggered activity is another possible mechanism of arrhythmia documented in both human and experimental models of infarction. To distinguish reentry from triggered activity, we used properties of the 2 mechanisms, including the response to stimulation. Sustained monomorphic VT was induced in all animals using programmed stimulation. While this characteristic alone is not indicative of either triggered activity caused by delayed after depolarization or reentrant excitation, it eliminates both automaticity (normal and abnormal) and triggered activity caused by early after-depolarization, as both cannot be initiated by stimulation. Programmed stimulation may establish slow conduction and transient conduction block for the initiation of reentry or may increase delayed after depolarization amplitude for the initiation of triggered activity. In order to distinguish between the 2, we examined the effect of rapid pacing vs premature stimulation, the response to resetting and/or entrainment, as well as analyzed mapping data of sustained VTs. In our model, programmed stimulation with 1–3 premature stimuli at a relatively high basic pacing rate but not rapid pacing was a reproducible mode of arrhythmia initiation, supporting a reentrant mechanism. The pattern of resetting response of the tachycardia to premature stimuli showed a mixed curve with a fairly short flat curve during long premature intervals and a longer increasing curve as the coupling interval was further decreased, an observation consistent with a reentrant circuit. This observation suggests the presence of a relatively small fully excitable gap as reflected by a narrow window of the flat response curve (20–60 ms) and a predominant partial excitable gap as reflected by the increasing cycle length after the premature impulse (increasing curve). This pattern suggests that there is slowing in conduction in the reentrant circuit, which is dependent on prematurity. Although circuits having an increased response curve have an excitable gap allowing the stimulated impulse to enter the circuit, the slowing of conduction indicates that the gap is not fully excitable and that the premature impulse conducts in increasingly relatively refractory tissue in the circuit, as it enters the circuit earlier and earlier. This can also occur in anatomical circuits or functional circuits caused by anisotropic conduction. Pacing from the RV demonstrated entrainment with progressive fusion (Figure 3A), consistent with reentrant mechanism. In addition, activation mapping showed reentrant excitation with continuous diastolic electrical activity. Taken together, these data suggest that sustained ventricular arrhythmias induced in our model were predominantly reentrant.
Substrate assessment using CMR imaging, EAM, and histopathology in these swine showed a large infarct region with transmural anterior components and septal and lateral border zones with subendocardial and subepicardial preserved tissue. However, the subendocardial component was more heterogeneous with both fibrosis and survived fibers than was the subepicardial layer. The geometrical arrangement of the survived subendocardial myocardial bundles showed a greater extent of fiber disarray with loss of parallel orientation and redistribution of connexin 43 gap junctions in sampled regions, resulting in a greater degree of nonuniform anisotropy. Nonuniform anisotropic conduction was evident across the infarct region and marked by fractioned, split, and isolated late potentials during sinus rhythm. This is consistent with our subendocardial characterization of VT circuits and demonstrated by continuous diastolic electrical activity during LV endocardial activation mapping.
Comparison to human pathophysiology
The majority of sustained VTs in humans with healed MI originate in the subendocardial region, particularly in hearts with anteroseptal infarcts and ventricular aneurysms. The endocardial border zone of these infarcts contains bundles of ventricular muscle as well as Purkinje fibers. The survived muscle bundles provide the necessary substrate for the formation of reentrant circuits due to the physical and electrical ventricular remodeling that occurs after MI. The consequential nonuniform anisotropy properties create the necessary slow conduction required for reentry.1,2 Our swine model of healed infarct resembles the human pathophysiology with subendocardial arrhythmia origin and characteristics of nonuniform anisotropic conduction as suggested by fractionated EGMs. In addition, swine frequently developed anteroseptal aneurysm after MI, a known and common phenomenon that also occurs in humans.
Study limitations
Although our findings demonstrate similar LV remodeling and arrhythmia mechanisms to humans, our studies were performed 6–8 weeks after infarction and may differ from humans with healed MI beyond this time period. The process of LV remodeling in humans and probably in swine continues far beyond this time point.36 Nonetheless, we found no significant differences in the anatomical or electro-physiological substrate between animals that were evaluated at the 6-week time point vs 8-week time point.
As the layer of survived myocardial fibers in swine is in the subendocardium, we recorded signals only from the endocardium. We recorded continuous electrical activity consistent with a subendocardial reentrant circuit; however, we cannot exclude a midmyocardial or epicardial component of the circuit. This will require simultaneous epicardial mapping or mapping using needle plunges. In addition, data on connexin 43 were not quantified, thus representing anecdotal data performed to confirm the findings of previous, more detailed, investigations in this field.
In addition, EAM was performed with 2 mapping modalities with slightly different interelectrode spacing (Pentaray 2 mm vs Orion 2.5 mm interelectrode spacing). This can create variability in detection of voltage and abnormal signals.
Conclusion
Percutaneous balloon occlusion of the LAD for 180 minutes followed by a 6–8-week survival period results in a swine model of LV scar and reproducible initiation of reentrant VT similar to humans. This model can be used to further improve our understanding of infarct-related VT in addition to the development and validation of new pharmacological or ablative therapeutics.
Acknowledgments
We are thankful to the Animal Research Staff of Beth Israel Deaconess Medical Center for their assistance during this study and for providing excellent care to our “patients.”
Biosense Webster and Boston Scientific provided partial funding for this study in the form of an investigator-initiated study. This study was also partially funded by the National Institutes of Health (grant no. 1R21HL127650-01). Dr Anter receives research grants and speaking honoraria from Biosense Webster and Boston Scientific. Dr Buxton receives research grants from Biosense Webster and Medtronic. Dr Josephson receives speaking honoraria from Medtronic. Mr Tschabrunn receives research grants from Biosense Webster.
ABBREVIATIONS
- CMR
cardiac magnetic resonance
- EAM
electroanatomic mapping
- ECG
electrocardiogram/electrocardiographic
- EGM
electrogram
- ILP
isolated late potential
- LAD
left anterior descending
- LV
left ventricle/ ventricular
- MI
myocardial infarction
- MTS
Masson trichrome stain
- RV
right ventricle/ventricular
- TCL
tachycardia cycle length
- VT
ventricular tachycardia
References
- 1.de Bakker JM, van Capelle FJ, Janse MJ, Tasseron S, Vermeulen JT, de Jonge N, Lahpor JR. Slow conduction in the infarcted human heart. ‘Zigzag’ course of activation. Circulation. 1993;88:915–926. doi: 10.1161/01.cir.88.3.915. [DOI] [PubMed] [Google Scholar]
- 2.Dillon SM, Allessie MA, Ursell PC, Wit AL. Influences of anisotropic tissue structure on reentrant circuits in the epicardial border zone of subacute canine infarcts. Circ Res. 1988;63:182–206. doi: 10.1161/01.res.63.1.182. [DOI] [PubMed] [Google Scholar]
- 3.de Bakker JM, Janse MJ, Van Capelle FJ, Durrer D. Endocardial mapping by simultaneous recording of endocardial electrograms during cardiac surgery for ventricular aneurysm. J Am Coll Cardiol. 1983;2:947–953. doi: 10.1016/s0735-1097(83)80244-7. [DOI] [PubMed] [Google Scholar]
- 4.Miller JM, Harken AH, Hargrove WC, Josephson ME. Pattern of endocardial activation during sustained ventricular tachycardia. J Am Coll Cardiol. 1985;6:1280–1287. doi: 10.1016/s0735-1097(85)80214-x. [DOI] [PubMed] [Google Scholar]
- 5.Josephson ME, Wit AL. Fractionated electrical activity and continuous electrical activity: fact or artifact? Circulation. 1984;70:529–532. doi: 10.1161/01.cir.70.4.529. [DOI] [PubMed] [Google Scholar]
- 6.Waldo AL, Kaiser GA. A study of ventricular arrhythmias associated with acute myocardial infarction in the canine heart. Circulation. 1973;47:1222–1228. doi: 10.1161/01.cir.47.6.1222. [DOI] [PubMed] [Google Scholar]
- 7.Wit AL, Janse MJ. The Ventricular Arrhythmias of Ischemia and Infarction: Electrophysiolgical Mechanisms. Mount Kisco, NY: Futura Publishing; 1993. [Google Scholar]
- 8.Euler DE, Prood CE, Spear JF, Moore EN. The interruption of collateral blood flow to the ischemic canine myocardium by embolization of a coronary artery with latex: effects on conduction delay and ventricular arrhythmias. Circ Res. 1981;49:97–108. doi: 10.1161/01.res.49.1.97. [DOI] [PubMed] [Google Scholar]
- 9.Akcakaya M, Rayatzadeh H, Basha TA, Hong SN, Chan RH, Kissinger KV, Hauser TH, Josephson ME, Manning WJ, Nezafat R. Accelerated late gadolinium enhancement cardiac MR imaging with isotropic spatial resolution using compressed sensing: initial experience. Radiology. 2012;264:691–699. doi: 10.1148/radiol.12112489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Basha TA, Roujol S, Kissinger KV, Goddu B, Berg S, Manning WJ, Nezafat R. Free-breathing cardiac MR stress perfusion with real-time slice tracking. Magn Reson Med. 2014;72:689–698. doi: 10.1002/mrm.24977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roujol S, Foppa M, Basha TA, Akcakaya M, Kissinger KV, Goddu B, Berg S, Nezafat R. Accelerated free breathing ECG triggered contrast enhanced pulmonary vein magnetic resonance angiography using compressed sensing. J Cardiovasc Magn Reson. 2014;16:91. doi: 10.1186/s12968-014-0091-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weingartner S, Akcakaya M, Roujol S, Basha T, Tschabrunn C, Berg S, Anter E, Nezafat R. Free-breathing combined three-dimensional phase sensitive late gadolinium enhancement and T mapping for myocardial tissue characterization. Magn Reson Med. 2014 doi: 10.1002/mrm.25495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roujol S, Basha TA, Tan A, Khanna V, Chan RH, Moghari MH, Rayatzadeh H, Shaw JL, Josephson ME, Nezafat R. Improved multimodality data fusion of late gadolinium enhancement MRI to left ventricular voltage maps in ventricular tachycardia ablation. IEEE Trans Biomed Eng. 2013;60:1308–1317. doi: 10.1109/TBME.2012.2233738. [DOI] [PubMed] [Google Scholar]
- 14.Kramer CM, Barkhausen J, Flamm SD, Kim RJ, Nagel E. Society for Cardiovascular Magnetic Resonance Board of Trustees Task Force on Standardized Protocols. Standardized cardiovascular magnetic resonance imaging (CMR) protocols, Society for Cardiovascular Magnetic Resonance Board of Trustees Task Force on Standardized Protocols. J Cardiovasc Magn Reson. 2008;10:35. doi: 10.1186/1532-429X-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS. American Heart Association Writing Group on Myocardial Segmentation and Registration for Cardiac Imaging. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 16.Josephson ME. Clinical Cardiac Electrophysiology: Techniques and Interpretations. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. Recurrent ventricular tachycardia; pp. 446–642. [Google Scholar]
- 17.Almendral JM, Rosenthal ME, Stamato NJ, Marchlinski FE, Buxton AE, Frame LH, Miller JM, Josephson ME. Analysis of the resetting phenomenon in sustained uniform ventricular tachycardia: incidence and relation to termination. J Am Coll Cardiol. 1986;8:294–300. doi: 10.1016/s0735-1097(86)80043-2. [DOI] [PubMed] [Google Scholar]
- 18.Almendral JM, Stamato NJ, Rosenthal ME, Marchlinski FE, Miller JM, Josephson ME. Resetting response patterns during sustained ventricular tachycardia: relationship to the excitable gap. Circulation. 1986;74:722–730. doi: 10.1161/01.cir.74.4.722. [DOI] [PubMed] [Google Scholar]
- 19.Josephson ME, Horowitz LN, Farshidi A. Continuous local electrical activity: a mechanism of recurrent ventricular tachycardia. Circulation. 1978;57:659–665. doi: 10.1161/01.cir.57.4.659. [DOI] [PubMed] [Google Scholar]
- 20.Waldo AL, Akhtar M, Brugada P, Henthorn RW, Scheinman MM, Ward DE, Wellens HJ. The minimally appropriate electrophysiologic study for the initial assessment of patients with documented sustained monomorphic ventricular tachycardia. J Am Coll Cardiol. 1985;6:1174–1177. doi: 10.1016/s0735-1097(85)80329-6. [DOI] [PubMed] [Google Scholar]
- 21.Marchlinski FE, Callans DJ, Gottlieb CD, Zado E. Linear ablation lesions for control of unmappable ventricular tachycardia in patients with ischemic and nonischemic cardiomyopathy. Circulation. 2000;101:1288–1296. doi: 10.1161/01.cir.101.11.1288. [DOI] [PubMed] [Google Scholar]
- 22.Josephson ME, Simson MB, Harken AH, Horowitz LN, Falcone RA. The incidence and clinical significance of epicardial late potentials in patients with recurrent sustained ventricular tachycardia and coronary artery disease. Circulation. 1982;66:1199–1204. doi: 10.1161/01.cir.66.6.1199. [DOI] [PubMed] [Google Scholar]
- 23.Simson MB, Untereker WJ, Spielman SR, Horowitz LN, Marcus NH, Falcone RA, Harken AH, Josephson ME. Relation between late potentials on the body surface and directly recorded fragmented electrograms in patients with ventricular tachycardia. Am J Cardiol. 1983;51:105–112. doi: 10.1016/s0002-9149(83)80020-4. [DOI] [PubMed] [Google Scholar]
- 24.de Bakker JM, van Capelle FJ, Janse MJ, Tasseron S, Vermeulen JT, de Jonge N, Lahpor JR. Fractionated electrograms in dilated cardiomyopathy: origin and relation to abnormal conduction. J Am Coll Cardiol. 1996;27:1071–1078. doi: 10.1016/0735-1097(95)00612-5. [DOI] [PubMed] [Google Scholar]
- 25.Hadi AM, Mouchaers KT, Schalij I, Grunberg K, Meijer GA, Vonk-Noordegraaf A, van der Laarse WJ, Belien JA. Rapid quantification of myocardial fibrosis: a new macro-based automated analysis. Cell Oncol (Dordr) 2011;34:343–354. doi: 10.1007/s13402-011-0035-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cano O, Hutchinson M, Lin D, Garcia F, Zado E, Bala R, Riley M, Cooper J, Dixit S, Gerstenfeld E, Callans D, Marchlinski FE. Electroanatomic substrate and ablation outcome for suspected epicardial ventricular tachycardia in left ventricular nonischemic cardiomyopathy. J Am Coll Cardiol. 2009;54:799–808. doi: 10.1016/j.jacc.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 27.El-Sherif N, Hope RR, Scherlag BJ, Lazzara R. Re-entrant ventricular arrhythmias in the late myocardial infarction period. 2. Patterns of initiation and termination of re-entry. Circulation. 1977;55:702–719. doi: 10.1161/01.cir.55.5.702. [DOI] [PubMed] [Google Scholar]
- 28.El-Sherif N, Scherlag BJ, Lazzara R, Hope RR. Re-entrant ventricular arrhythmias in the late myocardial infarction period. 1. Conduction characteristics in the infarction zone. Circulation. 1977;55:686–702. doi: 10.1161/01.cir.55.5.686. [DOI] [PubMed] [Google Scholar]
- 29.Kramer JB, Saffitz JE, Witkowski FX, Corr PB. Intramural reentry as a mechanism of ventricular tachycardia during evolving canine myocardial infarction. Circ Res. 1985;56:736–754. doi: 10.1161/01.res.56.5.736. [DOI] [PubMed] [Google Scholar]
- 30.Wit AL, Allessie MA, Bonke FI, Lammers W, Smeets J, Fenoglio JJ., Jr Electrophysiologic mapping to determine the mechanism of experimental ventricular tachycardia initiated by premature impulses: experimental approach and initial results demonstrating reentrant excitation. Am J Cardiol. 1982;49:166–185. doi: 10.1016/0002-9149(82)90292-2. [DOI] [PubMed] [Google Scholar]
- 31.Wit AL, Allessie JJ, Fenoglio JJ, Jr, Bonke FIM, Lammers WJEP, Smeets J. Significance of the endocardial and epicardial border zones in the genesis of myocardial infarction arrhythmias. In: Harrison DC, Hall GK, editors. Cardiac Arrhythmias: A Decade of Progress. Boston: Medical Publishers; 1981. pp. 39–68. [Google Scholar]
- 32.Ursell PC, Gardner PI, Albala A, Fenoglio JJ, Jr, Wit AL. Structural and electrophysiological changes in the epicardial border zone of canine myocardial infarcts during infarct healing. Circ Res. 1985;56:436–451. doi: 10.1161/01.res.56.3.436. [DOI] [PubMed] [Google Scholar]
- 33.Sasano T, McDonald AD, Kikuchi K, Donahue JK. Molecular ablation of ventricular tachycardia after myocardial infarction. Nat Med. 2006;12:1256–1258. doi: 10.1038/nm1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ashikaga H, Sasano T, Dong J, et al. Magnetic resonance-based anatomical analysis of scar-related ventricular tachycardia: implications for catheter ablation. Circ Res. 2007;101:939–947. doi: 10.1161/CIRCRESAHA.107.158980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tung R, Nakahara S, Ramirez R, Lai C, Fishbein MC, Shivkumar K. Distinguishing epicardial fat from scar: analysis of electrograms using high-density electroanatomic mapping in a novel porcine infarct model. Heart Rhythm. 2010;7:389–395. doi: 10.1016/j.hrthm.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaudron P, Eilles C, Kugler I, Ertl G. Progressive left ventricular dysfunction and remodeling after myocardial infarction: potential mechanisms and early predictors. Circulation. 1993;87:755–763. doi: 10.1161/01.cir.87.3.755. [DOI] [PubMed] [Google Scholar]