Abstract
Background
Primary vesicoureteral reflux (PVUR) is the most common malformation of the kidney and urinary tract and reflux nephropathy is a major cause of chronic kidney disease in children. Recently, we reported mutations in tenascin XB (TNXB) as a cause of PVUR with joint hypermobility.
Methods
To define the role of rare variants in tenascin genes in the etiology of PVUR, we screened a cohort of patients with familial PVUR (FPVUR) and non-familial PVUR (NFPVUR) for rare missense variants in TNXB and tenascin C (TNC) genes after excluding mutations in ROBO2 and SOX17.
Results
We identified 134 individuals from 112 families with PVUR, we excluded two families with mutations in ROBO2. We found rare missense variants in TNXB in the remaining 110 families comprising of 5/55 (9%) of families with FPVUR and 2/55 (4%) of NFPVUR. There were no differences in high-grade reflux, or renal parenchymal scarring between patients with and without TNXB variants. All patients with TNXB rare variants that were tested exhibited joint hypermobility. Overall we were able to identify causes of FPVUR in 7/57 (12%) families (9% in TNXB and 3% in ROBO2).
Conclusions
In conclusion, a rare missense variant in TNXB in combination with a positive family history of VUR and joint hypermobility may represent a non-invasive method to diagnose PVUR and warrants further evaluation in other cohorts.
Keywords: VUR, Tenascin genes, Joint hypermobility, UTI, reflux nephropathy
Introduction
Primary vesicoureteral reflux (PVUR) is the retrograde flow of urine from the bladder into the upper urinary tract due to an abnormal ureterovesical junction [1-2]. It is the most common congenital anomaly of the kidneys and urinary tract (CAKUT) in children and it is also the most important risk factor for pyelonephritis2. The prevalence of PVUR in children is estimated to be 1-2% [3], but this is likely an underestimate because of phenotype heterogeneity and the lack of non-invasive diagnostic tools. In the pediatric age group, untreated PVUR is the single most important risk factor for renal parenchymal scarring (RPS) [1-2]. RPS due to PVUR (reflux nephropathy) is a major cause of end stage kidney disease requiring dialysis and kidney transplantation in children [4]. Although the pathogenesis of PVUR has not been fully elucidated, it is believed to be a developmental anomaly due to a defect in the reciprocal signaling interaction between metanephric mesenchyme and the ureteric bud during kidney development5. This interaction is controlled by a variety of genes during development. However, the entire repertoire of genes involved remains unknown [5]. Familial aggregation of PVUR has been reported in different studies, however few loci have been reported for PVUR and the genetic causes of familial PVUR are unknown in a large proportion of cases [6-8]. The variable expression of the disease often makes case ascertainment difficult and complex. In addition, the gold standard for diagnosing PVUR is a voiding cystourethrogram (VCUG), which requires catheterization of the child and radiation exposure, both undesirable interventions. Thus, ascertainment of large pedigrees with enough power for genome-wide linkage studies (GWLS) is often impossible. It is therefore not surprising that most of the loci that have been reported in the literature are from relatively small pedigrees, yielding findings that are inconclusive. We recently reported segregating mutations in the TNXB gene in a large kindred with PVUR and joint hypermobility [9]. The tenascin genes are a class of extracellular matrix proteins with a relatively similar structure comprising N-terminal assembly and C-terminal fibrinogen-like domains, epidermal growth factor (EGF)-like repeats, and fibronectin III domains [10-12]. These fibronectin domains control cell adhesion and migration during development [13].
The main objective of this study is to define the disease burden due to rare variants in TNXB and TNC (encoding for tenascin XB and tenascin C respectively) in a cohort of children with PVUR and define the relationship between rare variants in tenascin genes, PVUR severity, and joint hypermobility. We screened 110 families with familial or non-familial PVUR for rare variants (minor allele frequency <0.01) in TNXB and TNC genes and obtained clinical data including reflux grade and joint hypermobility score using the Beighton criteria14. We found rare missense variants in the TNXB gene in in 5/55 (9%) families with PVUR and 2/55 (4%) individuals with non-familial PVUR. We did not find any segregating rare variants in TNC. All the rare variants in TNXB were predicted to be damaging by at least one in silico prediction software program. All three individuals with rare TNXB variants who had formal joint examination were found to have significant joint hypermobility. Rare variants in TNXB are responsible for PVUR in 9% of families with familial PVUR and 4% of individuals with non-familial PVUR. These findings demonstrate that rare variants in TNXB and joint hypermobility may serve as a disease marker in familial PVUR and further reinforces the importance of genes encoding for extra cellular matrix proteins in the etiology and pathogenesis of PVUR.
Materials and Methods
Clinical Ascertainment
Institutional review board approval was obtained from Duke University Medical Center (Durham, NC). We obtained informed consent from parents and assent from children participating in the study. Study subjects were classified as previously described9. Briefly, study subjects were considered affected if they had VUR on voiding cystourethrogram (VCUG). Supportive evidence included history of recurrent urinary tract infection (UTI) and abnormal findings on renal ultrasonography. Unaffected individuals are those with no detectable VUR on screening VCUG performed as part of routine clinical care or if they were un-related married individuals in the family. Individuals classified as unknown are those with history of UTI but with no radiological investigations. We defined familial VUR as presence of at least two individuals with radiologically confirmed VUR in the same family.
Mutation Analysis
Genomic DNA was extracted from whole blood or saliva using standard methods. Mutation analysis was carried out by sequencing of both strands of all exons of TNXB, TNC, ROBO2 and SOX17 using exon-flanking primers; primer sequences are listed in Supplementary Table 1. All sequences were analyzed with the Sequencher software (Gene Codes Corp, Ann Arbor, Michigan).
Stratification of Variants and in silico prediction of impact of missense changes
All missense variants with minor allele frequency <0.01 were subjected to further analyses. In silico prediction of impact of amino acid substitution was determined using the SIFT and Polyphen 2 software [15-16]. The impact of amino acid change on secondary structure of the protein was assessed by the I-TASSER server (http://zhanglab.ccmb.med.umich.edu/I-TASSER/) housed at the University of Michigan, Ann Arbor, USA.
Joint Examination for Hypermobility
Joint examinations for hypermobility were carried out using the Beighton hypermobility score [14]. The examiners (CER and SE) were blinded to the detailed clinical and genotype data.
Data Analysis
The clinical data and frequency of rare missense variants in TNXB, TNC, ROBO2 and SOX17 were compared between the familial and non-familial group. All categorical data were compared by the χ2 test and Fisher’s exact test where indicated and p value <0.05 was considered significant.
Results
Clinical Data
We identified 134 individuals with PVUR; 79 individuals from 57 families were presumed to have familial VUR because there were two or more affected individuals in the family. The study population was predominantly Caucasian, with 74/79 (94%) of patients in the familial group and 48/55 (87%) of patients in the non-familial group self-identified as Caucasian. The majority 102/134 (76%) of the cohort were female. There was no significant difference in age at diagnosis between familial and non-familial PVUR patients (Table 1). Bilateral disease, distribution of reflux grades and presence of renal parenchymal scarring (RPS) on DMSA scan were similar between the familial and the non-familial groups (Table 2). Severe PVUR (VUR grade > 3) was present in 31/79 (39%) of the familial group and was similar in frequency to the non-familial group 14/55 or 26% (Fisher’s exact test, p = 0.14).
Table 1. Demographics of 134 individuals with Primary VUR.
| Parameters | Familial VUR# n=79 |
Non Familial VUR n=55 |
|---|---|---|
| Age at diagnosis median (range)* | 1.9 (0.02-10) | 1.56 (0.1-6.0) |
| Race (%) | ||
| Caucasian | 94 | 87 |
| African American | 4 | 7 |
| Asian | 1 | 2 |
| Hispanic | 1 | 4 |
| Gender (%) | ||
| Female | 76 | 76 |
| Male | 24 | 24 |
Information available on 117 individuals, 67 familial and 50 non familial,
VUR: Vesico ureteric reflux
Table 2. Clinical Phenotype of 134 patients with Primary VUR.
| Parameters | Familial VUR# n=79 |
Non Familial VUR n=55 |
Fisher’s exact p |
|---|---|---|---|
| VUR Highest Grade (%) | |||
| Grade 1 | 6 | 5 | |
| Grade 2 | 27 | 29 | |
| Grade 3 | 28 | 40 | |
| Grade 4 | 21 | 20 | |
| Grade 5 | 18 | 6 | 0.24 |
| VUR > grade 3 any side (%) | 39 | 26 | 0.14 |
| Bilateral VUR (%) | 56 | 66 | 0.29 |
| Renal parenchymal scarring (%)* | 53 | 44 | 0.62 |
Information available on 65 individuals (38 familial and 27 non-familial)
VUR: Vesico ureteric reflux
Screening for mutations in ROBO2 and SOX17
Since mutations in ROBO2 and SOX17 have previously been associated with congenital anomalies of the kidney (CAKUT) and isolated VUR [17-21], we screened the cohort for mutations in these genes and identified two families with segregating mutations in ROBO2 (Table 3, Supplementary Figures 1 and 2 ) and one individual with a rare variant in SOX17. The two mutations found in ROBO2 are novel; they segregate with disease in the two families; they are not present in any public database, and both mutations are predicted to be damaging by in silico prediction software [15-16]. The pathogenicity of the SOX17 variant is unclear. We excluded individuals with ROBO2 mutations from the tenascin genes studies.
Table 3. Rare Variants in TNXB and ROBO2 in Families with PVUR.
| Family number |
Familial Y/N |
Gene | Variant | rs number | MAF (EVS) |
Frequency in house controls |
Polyphen score# |
Sift score* |
|---|---|---|---|---|---|---|---|---|
| 6606+ | Y | TNXB | c.9770C>T (T3257I) | NA | 0 | 0 | 0.99 | 0.15 |
| 6952+ | Y | TNXB | c.3991G>A (G1331R) | NA | 0 | 0 | 1.0 | 0 |
| 34324 | Y | TNXB | c.11227G>A (D3743N) | NA | 0 | 0 | 1.0 | 0.2 |
| 34324 | Y | TNXB | c.11581G>A (A3861T) | rs201121030 | 0 | 0 | 0.94 | 0.07 |
| 34439 | Y | TNXB | c.211G>T (V71L) | rs201922477 | 0.0002 | 0 | 0.98 | 0 |
| 34222 | Y | TNXB | c.619G>A (G207S) | rs139154852 | 0.0001 | 0 | 0.99 | 0 |
| 34334 | N | TNXB | c.113G>A (R38Q) | rs149502087 | 0.008 | 0 | 0.98 | 0 |
| 6866 | N | TNXB | c.8740G>A (G2914S) | rs200708257 | 0.0005 | 0 | 1.0 | 0.04 |
| 6836 | Y | ROBO2 | c.911 C>A (A304D) | NA | 0 | 0 | 0.99 | 0.01 |
| 34333 | Y | ROBO2 | c.2200 G>A (E734K) | NA | 0 | 0 | 0.99 | 0.003 |
#Polyphen score range 0 – 1.0 with 1.0 most damaging
SIFT score range 0 – 1.0 with 0 most damaging
The findings in Families 6606 and 6952 have previously been reported in reference 9.
Screening for rare variants in TNXB and TNC
Familial VUR
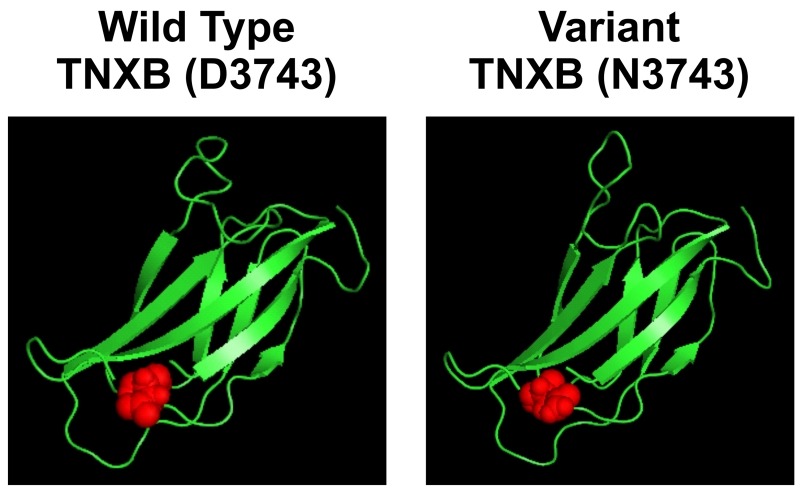
We found six segregating heterozygous rare variants in TNXB in 5/55 (9%) of families with familial VUR who did not carry mutations in ROBO2 or SOX17 (Supplementary Figures 1 and 2). We have previously reported two of these variants {c.9770C>T (T3257I) and c.3991G>A (G1331R) in families 6606 and 6952 respectively} [9]. All the TNXB variants were annotated using the transcript ENST00000375244 (Supplementary Figure 3). We found the rare variants in all the seven affected individuals screened from the three new families (Supplementary Figure 2). All the variants caused non-conservative amino acid changes, and the amino acid changes were predicted to be damaging by at least one in silico prediction software. Three of the six variants in the FPVUR families are not listed in dbSNP, 1000 Genomes or NHLBI Exome Variant Server database. The other three variants have low minor allele frequency between 0.000 and 0.0002 in at least 6,000 chromosomes. Furthermore, we did not find any of the variants in at least 200 in-house ethnicity matched control chromosomes (Table 3). Interestingly, in one family (34324) we found two segregating heterozygous variants. Both variants are inherited in-cis from one of the parents. Four out of the six variants are located in the fibronectin III domains or linker regions between the fibronectin domains. Modeling one of the novel rare variant D3743N using the I-TASSER software (http://zhanglab.ccmb.med.umich.edu/I-TASSER/) results in perturbation of the secondary structure of TNXB fibronectin III domain 28 encoded by the exon carrying this rare variant (C-scores 0.15-0.19) (Figure 2). The phenotype associated with families with rare variants in TNXB gene is described in Table 4. We did not find segregating variants in TNC in any of the families studied.
Figure 2. Missense variant D3743N in TNXB resulted in perturbation of 28th fibronectin III domain of the protein.
I-TASSER was used to model the structure of TNXB FnIII domain 28. The model was predicted to be reliable with a robust C-scores 0.15-0.19. Residue D3743 (in red) is predicted to reside in fibronectin domain 28. Structural modeling of the 28th fibronectin III domain containing the D3743N variant (highlighted in red) resulted in, perturbation of the secondary structure of the protein.
Table 4. Phenotype of Patients with PVUR and Rare Variants in TNXB.
| ID | TNXB variants | Sex | Age (yrs) |
Bilateral VUR Y/N |
VUR ≥ Grade 3 Y/N (Side) |
Reflux nephropathy Y/N |
Joint hypermobility Y/N |
|---|---|---|---|---|---|---|---|
| 6606-01+ | c.9770C>T (T3257I) | F | 4.0 | N | N | Y | Y |
| 6606-132+ | c.9770C>T (T3257I) | F | 0.5 | N | N | N | U |
| 6606-103+ | c.9770C>T (T3257I) | F | 1.0 | Y | Y (L) | Y | U |
| 6952+ | c.3991G>A (G1331R) | F | 6.0 | N | N | Y | U |
| c.11227G>A (D3743N) | |||||||
| 34324-01 | c.11581G>A (A3861T) | M | 0.3 | Y | Y (R&L) | Y | U |
| 34439-01 | c.211G>T (V71L) | F | 3.25 | N | N | U | Y |
| 34439-100 | c.211G>T (V71L) | F | 0.1 | Y | Y (R&L) | U | |
| 34222 | c.619G>A (G207S) | F | 3.0 | Y | Y (L) | U | U |
| 34334 | c.113G>A (R38Q) | F | 0.9 | Y | Y (R&L) | U | Y |
| 6866 | c.8740G>A (G2914S) | F | 0.5 | N | N | N | U |
N= No, Y= Yes, U= Unknown, L=Left, R= Right, F= Female, M= Male
The findings in Families 6606 and 6952 have previously been reported in reference 9.
Non-Familial VUR
Since we were not certain about the pathogenicity of the SOX17 variant found in one individual with non-familial VUR, we screened all the 55 individuals with non-familial PVUR for rare variants in tenascin genes. We found rare variants (MAF: 0.008, 0.0005) in TNXB in 2/55 (4%) of patients in this cohort. Similar to the familial cohort, the two variants were predicted to be damaging by in silico modeling (Table 3).
Joint Hypermobility in TNXB Rare Variants
We have previously reported that VUR co-segregates with joint hypermobility in a family with TNXB mutation [9]. To provide further evidence that the rare variants identified by us are likely to be pathogenic, we performed joint hypermobility testing on three individuals with rare variants in TNXB. All three individuals met the criteria for joint hypermobility with Beighton score of ≥4 (Table 3).
Discussion
PVUR is the most important risk factor for pyelonephritis and renal parenchymal scarring (RPS) in the pediatric age group [2]. Unlike other CAKUT, the genetic causes of PVUR are unknown in most cases [1-3, 22]. Mutations in ROBO2 and SOX17 have been reported as causes of isolated PVUR [17-21], and more recently we reported mutations in TNXB as a cause of PVUR [9]. In the present study, we performed mutation analysis in ROBO2, SOX17, TNXB and TNC in a cohort of patients with familial and sporadic PVUR. We found that rare variants in TNXB and ROBO2 account for 12% (9% TNXB and 3% ROBO2) of all cases of familial PVUR in this cohort and rare variants in TNXB for 4% of non-familial PVUR. Thus based on this modest cohort, rare variants in TNXB appeared to be the most common cause of autosomal dominant PVUR. Overall, we were able to identify a genetic cause of PVUR in 7/57 (12%) of families with familial PVUR. This is similar to 7% (47/650) in a cohort of 650 families with CAKUT reported by Hwang et al [21]. In their study, participants were screened for mutations in 17 known dominant genes that have been associated with CAKUT in both humans and animal models [19]. It should be noted that the panel of 17 genes in the Hwang study did not include TNXB. We did not find differences in bilateral disease, high-grade reflux, and RPS between patients with familial and non-familial diseases. The reason for this is unknown, but it may be due to the limited sample size of the present cohort. In addition, we did not have access to detailed prospective data on history of recurrent UTI, therapy and other factors that may influence outcome in PVUR in all the patients in our cohort.
Further analysis of the families with rare variants in TNXB demonstrated that the prevalence of bilateral disease and high-grade reflux is similar to the prevalence in the entire cohort. Most of the variants found in TNXB are likely to be pathogenic because they segregate with the disease. Some are not in any public database and have extremely low MAF in the general population. In addition, all are predicted to be damaging by at least one in silico prediction software. Furthermore, all the individuals with the TNXB variants who had joint mobility examination done have significant joint hypermobility as assessed by Beighton score [14].
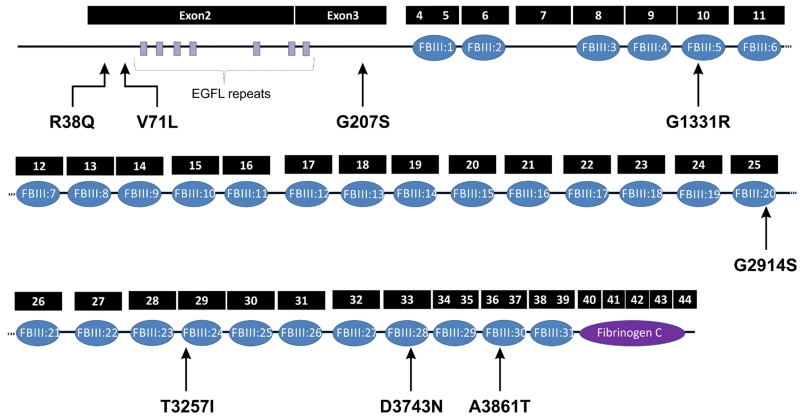
TNXB belongs to a family of large extracellular matrix proteins that is characterized by N-terminal assembly domains, EGF-like repeats, multiple fibronectin III domains and a C-terminal fibrinogen-like domain [9-13]. TNXB is expressed in kidneys and the urinary tract during development and it is highly expressed at a critical period when the ureterovesical junction (UVJ) is being formed [9]. We did not find any specific clustering of the variants in any exons or domains (Figure 1); however the majority of the variants (5/8) are located in the fibronectin III domains or linker regions between the fibronectin domains. The mechanism by which variants in TNXB cause PVUR has not been elucidated. However, we have shown in previous studies that fibroblast cell lines from an individual with T3257I missense mutation in TNXB displayed reduced cell motility in response to platelet derived growth factor and reduced expression of phosphorylated focal adhesion kinase suggesting persistent and enhanced cell adhesion and defective disassembly of focal adhesion [9]. Future studies will address the mechanisms by which the variants may cause defective formation of the UVJ during development.
Figure 1. Rare missense variants in TNXB found in patients with PVUR.
Exons of TNXB gene (filled boxes) and protein domains (filled oval). Five of the eight missense variants are located in the fibronectin III or linker regions between the domains.
We did not find any possible disease-causing variants in >80% of individuals with familial PVUR, thus further confirming that PVUR is genetically heterogeneous. There is significant need for further studies to identify other genetic causes of PVUR in order to develop a robust molecular diagnosis platform for PVUR. The clinical implications of our findings suggest that the presence of PVUR and joint hypermobility in a child presenting in clinic may warrant screening for rare missense variants in TNXB and other extracellular matrix protein genes in order to make a molecular diagnosis. If a pathogenic variant is identified in the index child presenting with PVUR, screening for rare missense variants in TNXB in asymptomatic family members may be enough to make a presumptive diagnosis of VUR.
In conclusion, familial PVUR is due to rare variants in TNXB and ROBO2 in 12% of all families with familial PVUR studied in this cohort. If the findings in this study are confirmed in an independent cohort, rare missense variants in TNXB and clinical findings such as joint hypermobility may represent a non-invasive diagnostic tool for PVUR.
Supplementary Material
Acknowledgements
This study was supported by the National Institutes of Health (NIH) and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant R21DK096200 and the Bayden Collins Pediatric Kidney Disease Research Fund, Duke University Medical Center. RG is the recipient of the Doris Duke Clinical Research Mentorship grant. We would like to thank the personnel of the genomic core of the Duke Molecular Physiology Institute (DMPI) and most importantly the participants in the study. This study is dedicated to the memory of Michelle P. Winn, MD for her seminal contribution to the field of nephrology and, most importantly, for being a great teacher and an outstanding mentor.
References
- 1.Mak RH, Kuo HJ. Primary ureteral reflux: Emerging insights from molecular and genetic studies. Curr Opin Pediatr. 2004;15:181–185. doi: 10.1097/00008480-200304000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Smellie JM, Normand IC. Bacteriuria, reflux, and renal scarring. Arch Dis Child. 1975;50:581–585. doi: 10.1136/adc.50.8.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arant BS., Jr Vesicoureteric reflux and renal injury. Am J Kidney Dis. 1991;17:491–511. doi: 10.1016/s0272-6386(12)80490-2. [DOI] [PubMed] [Google Scholar]
- 4.North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) Annual Transplant Report. Boston, MA, USA: 2010. https://web.emmes.com/study/ped/annlrept/2010_Report.pdf. [Google Scholar]
- 5.Kelly H, Molony CM, Darlow JM, Pirker ME, Yoneda A, Green AJ, Puri P, Barton DE. A genome-wide scan for genes involved in primary vesicoureteric reflux. J Med Genet. 2007;44:710–717. doi: 10.1136/jmg.2007.051086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feather SA, Malcolm S, Woolf AS, Wright V, Blaydon D, Reid CJ, Flinter FA, Proesmans W, Devriendt K, Carter J, Warwicker P, Goodship TH, Goodship JA. Primary, non-syndromic vesicoureteric reflux and its nephropathy is genetically heterogeneous, with a locus on chromosome 1. Am J Hum Genet. 2000;66:1420–1425. doi: 10.1086/302864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weng PL, Sanna-Cherchi S, Hensle T, Shapiro E, Werzberger A, Caridi G, Izzi C, Konka A, Reese AC, Cheng R, Werzberger S, Schlussel RN, Burk RD, Lee JH, Ravazzolo R, Scolari F, Ghiggeri GM, Glassberg K, Gharavi AG. A recessive gene for primary vesicoureteral reflux maps to chromosome 12p11-q13. J AM Soc Nephrol. 2009;20:1633–1640. doi: 10.1681/ASN.2008111199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ashraf S, Hoskins BE, Chaib H, Hoefele J, Pasch A, Saisawat P, Trefz F, Hacker HW, Nuernberg G, Nuernberg P, Otto EA, Hildebrandt F. Mapping of a new locus for congenital anomalies of the kidney and urinary tract on chromosome 8q24. Nephrol. Dial. Transplant. 2010;25:1496–1501. doi: 10.1093/ndt/gfp650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gbadegesin RA, Brophy PD, Adeyemo A, Hall G, Gupta IR, Hains D, Bartkowiak B, Rabinovich CE, Chandrasekharappa S, Homstad A, Westreich K, Wu G, Liu Y, Holanda D, Clarke J, Lavin P, Selim A, Miller S, Wiener JS, Ross SS, Foreman J, Rotimi C, Winn MP. TNXB mutations can cause vesicoureteral reflux. J Am Soc Nephrol. 2013;24:1313–1322. doi: 10.1681/ASN.2012121148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zweers MC, Bristow J. Haploinsufficiency of TNXB is associated with hypermobility type of Ehlers-Danlos syndrome. Am J Hum Genet. 2003;73:214–217. doi: 10.1086/376564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schaller MD, Parsons JT. Focal adhesion kinase and associated proteins. Curr Opin Cell Biol. 1994;6:705–710. doi: 10.1016/0955-0674(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 12.Petersen JW, Douglas JY. Tenascin-X, collagen, and Ehlers-Danlos syndrome: tenascin-X gene defects can protect against adverse cardiovascular events. Med Hypotheses. 2013;81:443–447. doi: 10.1016/j.mehy.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiquet-Ehrismann R, Tucker RP. Tenascins and the importance of adhesion modulation. Cold Spring Harb Perspect Biol. 2011;3:11–19. doi: 10.1101/cshperspect.a004960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smits-Engelsman B, Klerks M, Kirby A. Beighton score: a valid measure for generalized hypermobility in children. J Pediatr. 2011;158:119–123. doi: 10.1016/j.jpeds.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 15.Sunyaev S, Ramensky V, Koch I, Lathe W, 3rd, Kondrashov AS, Bork P. Prediction of deleterious human alleles. Hum Mol Genet. 2001;10:591–597. doi: 10.1093/hmg/10.6.591. [DOI] [PubMed] [Google Scholar]
- 16.Kumar P, Henikoff S, Ng PC. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc. 2009;4:1073–1081. doi: 10.1038/nprot.2009.86. [DOI] [PubMed] [Google Scholar]
- 17.Lu W, van Eerde AM, Fan X, Quintero-Rivera F, Kulkarni S, Ferguson H, Kim HG, Fan Y, Xi Q, Li QG, Sanlaville D, Andrews W, Sundaresan V, Bi W, Yan J, Giltay JC, Wijmenga C, de Jong TP, Feather SA, Woolf AS, Rao Y, Lupski JR, Eccles MR, Quade BJ, Gusella JF, Morton CC, Maas RL. Disruption of ROBO2 is associated with urinary tract anomalies and confers risk of vesicoureteral reflux. Am J Hum Genet. 2007;80:616–632. doi: 10.1086/512735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bertoli-Avella AM, Conte ML, Punzo F, de Graaf BM, Lama G, La Manna A, Polito C, Grassia C, Nobili B, Rambaldi PF, Oostra BA, Perrotta S. ROBO2 gene variants are associated with familial vesicoureteral reflux. J Am Soc Nephrol. 2008;19:825–831. doi: 10.1681/ASN.2007060692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gimelli S, Caridi G, Beri S, McCracken K, Bocciardi R, Zordan P, Dagnino M, Fiorio P, Murer L, Benetti E, Zuffardi O, Giorda R, Wells JM, Gimelli G, Ghiggeri GM. Mutations in SOX17 are associated with congenital anomalies of the kidney and the urinary tract. Hum Mutat. 2010;31:1352–1359. doi: 10.1002/humu.21378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Combes P, Planche V, Eymard-Pierre E, Sarret C, Rodriguez D, Boespflug-Tanguy O, Vaurs-Barriere C. Relevance of SOX17 variants for hypomyelinating leukodystrophies and congenital anomalies of the kidney and urinary tract (CAKUT) Ann Hum Genet. 2012;76:261–267. doi: 10.1111/j.1469-1809.2011.00702.x. [DOI] [PubMed] [Google Scholar]
- 21.Hwang DY, Dworschak GC, Kohl S, Saisawat P, Vivante A, Hilger AC, Reutter HM, Soliman NA, Bogdanovic R, Kehinde EO, Tasic V, Hildebrandt F. Mutations in 12 known dominant disease-causing genes clarify many congenital anomalies of the kidney and urinary tract. Kidney Int. 2014;85:1429–1433. doi: 10.1038/ki.2013.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ichikawa I, Kuwayama F, Pope JC, 4th, Stephens FD, Miyazaki Y. Paradigm shift from classic anatomic theories to contemporary cell biological views of CAKUT. Kidney Int. 2002;61:889–898. doi: 10.1046/j.1523-1755.2002.00188.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.