Abstract
We report on the tentative detection of trans ethyl methyl ether (tEME), t-CH3CH2OCH3, through the identification of a large number of rotational lines from each one of the spin states of the molecule towards Orion KL. We also search for gauche-trans-n-propanol, Gt-n-CH3CH2CH2OH, an isomer of tEME in the same source. We have identified lines of both species in the IRAM 30 m line survey and in the ALMA Science Verification data. We have obtained ALMA maps to establish the spatial distribution of these species. Whereas tEME mainly arises from the compact ridge component of Orion, Gt-n-propanol appears at the emission peak of ethanol (south hot core). The derived column densities of these species at the location of their emission peaks are ≤(4.0 ± 0.8) × 1015 cm−2 and ≤(1.0 ± 0.2)× 1015 cm−2 for tEME and Gt-n-propanol, respectively. The rotational temperature is ~100 K for both molecules. We also provide maps of CH3OCOH, CH3CH2OCOH, CH3OCH3, CH3OH, and CH3CH2OH to compare the distribution of these organic saturated O-bearing species containing methyl and ethyl groups in this region. Abundance ratios of related species and upper limits to the abundances of non-detected ethers are provided. We derive an abundance ratio N(CH3OCH3)/N(tEME) ≥ 150 in the compact ridge of Orion.
Keywords: ISM: abundances, ISM: clouds, ISM: individual objects: Orion KL, ISM: molecules, radio lines: ISM, surveys
1. Introduction
The spectral millimeter-wave survey of Orion KL carried out with the IRAM 30 m radio telescope (Tercero et al. 2010; Tercero 2012) shows more than 15 400 spectral features of which about 11 000 have been identified and attributed to 50 molecules (199 different isotopologues and vibrational modes). To date, there have been several works based on these data. As the result of a fruitful collaboration with spectroscopy laboratories, 3000 previously unidentified lines have been assigned to new species in the interstellar medium (ISM). We have detected in space 16 new isotopologues and vibrationally excited states of abundant molecules in Orion for the first time (Demyk et al. 2007; Margulès et al. 2009, 2010; Carvajal et al. 2009; Tercero et al. 2012; Motiyenko et al. 2012; Daly et al. 2013; Coudert et al. 2013; Haykal et al. 2014; López et al. 2014) as well as four new molecules (Tercero et al. 2013; Cernicharo et al. 2013; Kolesniková et al. 2014). These identifications reduce the number of unidentified lines and mitigate line confusion in the spectra. Nevertheless, many features still remain unidentified and correspond to new species that we have to search and identify. Formates, ethers, acetates, alcohols, and cyanides are the best candidates for this purpose in Orion.
The recent search for trans ethyl methyl ether (tEME) in selected hot cores (Sgr B2(N-LMH) and W51 e1/e2) by Carroll et al. (2015) only provides upper limits to tEME. Hence, the results from that work do not confirm the previous tentative identification of this species by Fuchs et al. (2005) towards W51 e1/e2.
A systematic line survey with most weeds removed permits us to address the problem of the abundances of isomers and derivatives of key species, such as methyl formate (A. López et al., in prep.), through combined IRAM and ALMA studies.
In this Letter, we report on the tentative detection of tEME towards the compact ridge (CR) of Orion KL. We have detected emission of features arising from the five spin states at 3, 2, and 1 mm with the IRAM 30 m telescope and the ALMA interferometer. In addition, several unidentified lines of these data have been identified as belonging to the gauche-trans conformer of n-propanol (an isomer of tEME). ALMA maps of organic saturated O-bearing species containing methyl, ethyl, and propyl groups, abundance ratios of related species, and upper limits to the column densities of non-detected ethers are presented and discussed in Sect. 4.
2. Observations
IRAM 30 m: new data of the IRAM 30 m telescope, which complement and improve those of Tercero et al. (2010), were collected in August 2013 and March 2014 towards Orion KL (see Tercero et al. 2010 and López et al. 2014, for information about the previous data set). Frequencies in the ranges 80.7–116, 122.7–161.2, 199.7–291.0, 291.4–306.7GHz, were observed with the EMIR receivers connected to the FFTS (200 kHz of spectral resolution) spectrometers. We pointed towards IRc2 source at α2000.0 = 5h35m 14s.5, δ2000.0 = −5°22′30″.0, corresponding to the survey position (see Sect. 4). We observed an additional position to target the CR: α2000.0 = 5h35m14s.3, δ2000.0 = −5°22′37″.0 (see Sect. 4). The observations were performed using the wobbler switching mode with a beam throw in azimuth of ±120″. The intensity scale was calibrated using the atmospheric transmission model (ATM, Cernicharo 1985; Pardo et al. 2001). Focus and pointing were checked every 1–2 h on planets or nearby quasars. System temperatures were in the range of 100–800 K from the lowest to highest frequencies. Half power beam width (HPBW) ranged from 31″ to 8″ from 80 to 307 GHz (HPBW[arcsec] = 2460/Freq.[GHz]). The data were reduced using the GILDAS package1.
ALMA SV: the ALMA Science Verification (SV) data2 were taken in January 2012 towards the IRc2 region in Orion. The observations were carried out with 16 antennas of 12 m in Band 6 (213.715–246.627GHz). The primary beam was ≃27″. Spectral resolution was 0.488 MHz corresponding to a velocity resolution of 0.64 km s−1. The observations were centred on coordinates: αJ2000 = 05h35m14s.35, δJ2000 = −05°22′35″.00. The CASA software3 was used for initial processing and then the visibilities were exported to the GILDAS package. The line maps were cleaned using the HOGBOM algorithm (Högbom 1974). The synthesized beam ranged from 2″.00 × 1″.48 with a PA of 176° at 214.0 GHz to 1″.75 × 1″.29 with a PA of 164× at 246.4 GHz. The brightness temperature to flux density conversion factor is 9 K for 1 Jy per beam.
3. Results
3.1. Search for trans ethyl methyl ether
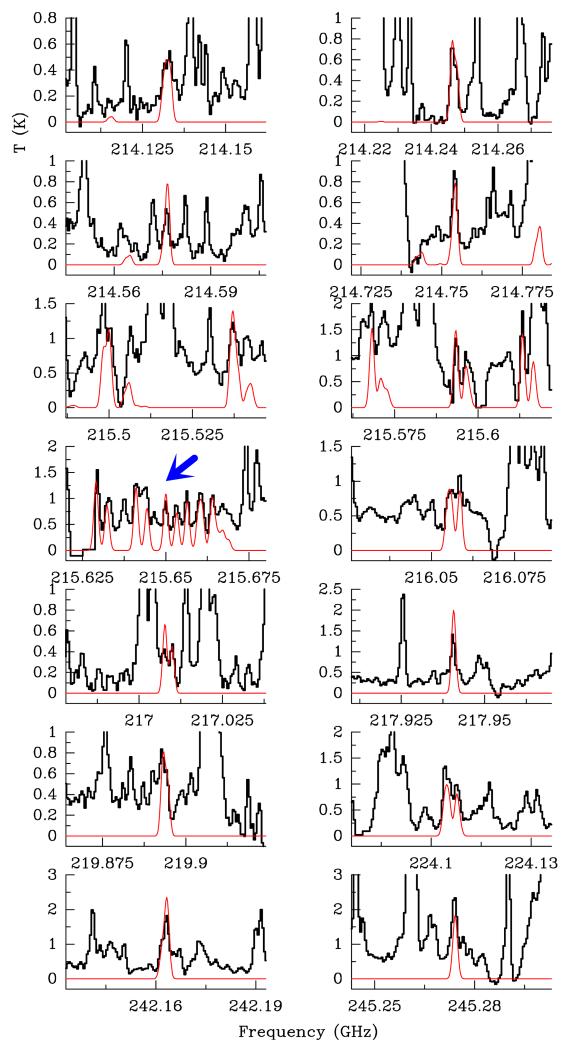
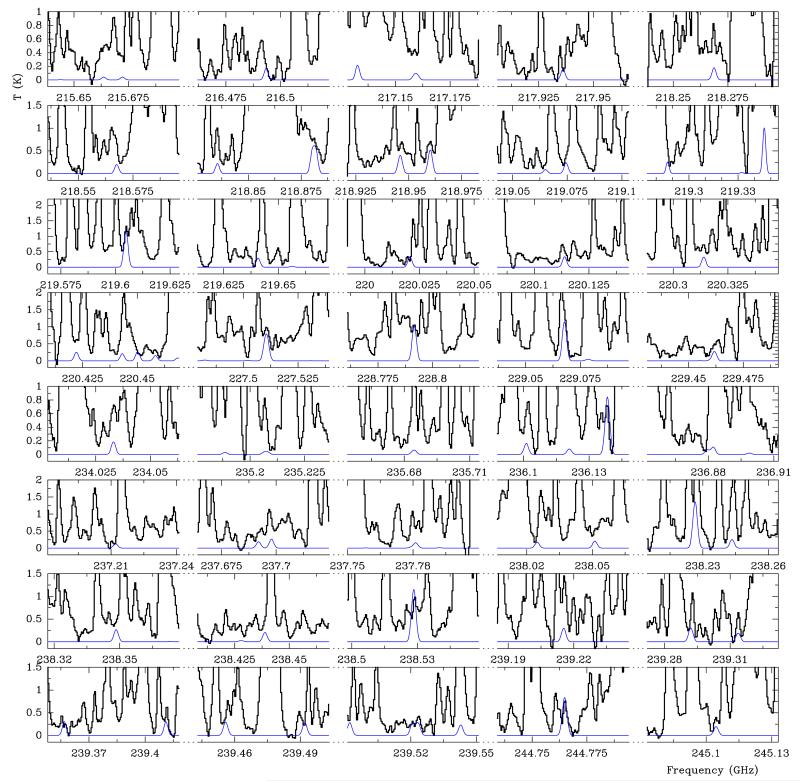
ALMA SV data: frequency predictions from Fuchs et al. (2003) and dipole moments measured by Hayashi & Kuwada (1975) of tEME were implemented in MADEX (Cernicharo 2012) to model the emission of this species and search for it towards Orion KL. Using the ALMA SV data, we extracted the averaged spectrum over 5 × 5 pixels (1″ × 1″) around the CH3OCH3 emission peak of the CR component (Position A; see Sect. 4). The advantage of ALMA with respect to single dish telescope data (see below) is the drastic reduction of the confusion limit. The ALMA SV data show the presence of tEME as shown in Fig. 1 (selected lines) and Fig. A.1 (all lines favourable for detection (corresponding to b-type transitions with upper level energies up to 300 K and large line strenghts, Sij ≥ 1) present in the ALMA SV frequency range). The model that best fits the data is shown with the red line. The assumed parameters are a source size of 3″, vLSR = +7.5 km s−1, Δv = 2.0 km s−1, and TK = 100 ± 20 K. Using MADEX and assuming local thermodynamic equilibrium (LTE), we obtain Ng.s.(tEME) ≤ (4.0 ± 0.8) × 1015 cm−2. In our models, rotation temperature and column density values are given with their corresponding uncertainty and we obtained them by fitting all available lines by eye. We adopted the source size in agreement with the emission of the maps (see below). In addition, a considerable number of unblended features allows us to fix the radial velocities and line widths. According to our model, in the ALMA frequency range only 33% of the detectable lines of tEME (102 lines) are totally hidden by the emission of stronger lines of other species. At least 46 lines (45% of the detectable lines) shown in Fig. A.1 are free of blending, i.e. these lines are present at the expected radial velocity and there are no other species with significant intensity at the same observed frequency (±3 MHz). Another point to ensure this tentative detection is that the forest of lines emitted by tEME between 215.5 and 215.7 GHz is not covered by lines of abundant molecules in the source allowing the detection of several lines that follow a straightened pattern (see Fig. 1). Hence, there are several clues that could reveal the presence of this species in the CR of Orion KL, but further analysis exploring new available ALMA data and modelling all the molecular content of the CR is needed to give the definitive detection in space of tEME. Table A.1 gives line parameters and blends of all lines of favourable transitions in the ALMA SV data. The spatial distribution of tEME is shown in Fig. 2. Lines that we found to be unblended at the Position A appear blended with emission from other components in the averaged spectrum (see the case of the 30 m data). We selected a line at 245.274 GHz, which is mixed with some emission from extreme velocities of 34SO2 and SO2. Nevertheless, the emission of tEME at Position A in Fig. 2 is not blended (see Sect. 4).
Fig. 1.
Selected lines of trans ethyl methyl ether, t-CH3CH2OCH3, towards Orion KL detected with the ALMA interferometer in Position A (see text). A vLSR of +7.5 km s−1 is assumed.
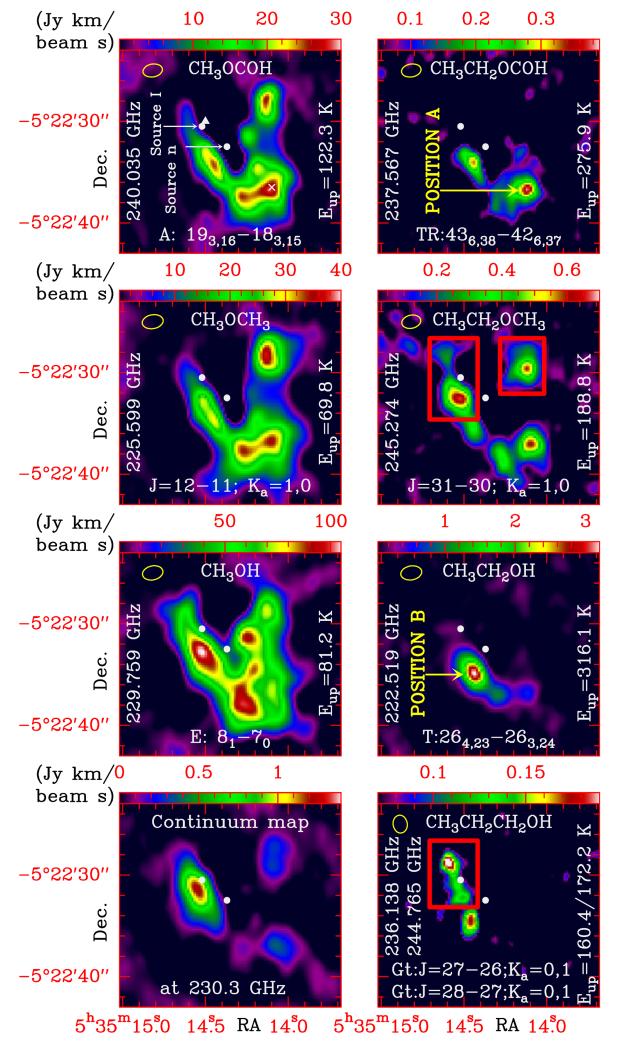
Fig. 2.
ALMA maps of organic saturated O-bearing molecules in Orion KL which have been detected containing both the methyl and the ethyl group, as well as a map of Gt-n-propanol and a continuum map at the central frequencies of the ALMA S V band (~230 GHz). Emission that probably arises from blended species in these maps is confined inside red rectangles. The yellow ellipse at the top left corner of the maps represents the ALMA synthetic beam. Triangle symbol: IRAM 30 m “survey position” (see Sect. 2). Cross symbol: IRAM 30 m compact ridge position (see Sect. 2). Position A: compact ridge (coordinates α2000.0 = 5h35m14s.1, δ2000.0 = −5°22′37″.9). Position B: south hot core (coordinates α2000.0 = 5h35m14s.4, δ2000.0 = −5°22″34″.9).
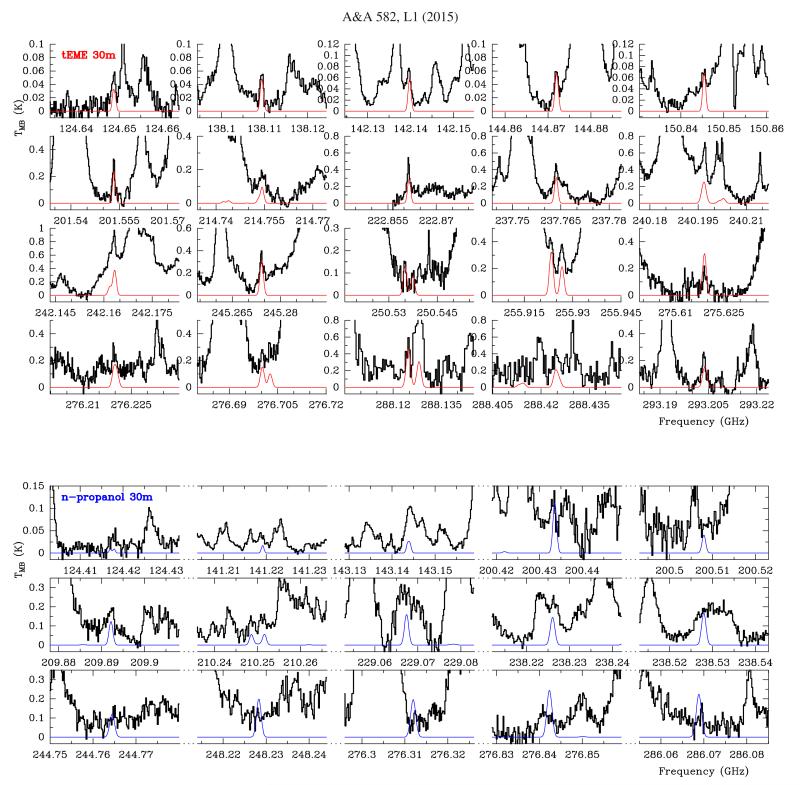
IRAM 30 m data: to search for tEME in the IRAM data, a synthetic spectra of tEME (red curve in Fig. A.2) was obtained with MADEX assuming LTE and adopting the following physical parameters: source diameter 3″, TK = 100 ± 30 K, vLSR = +7.5 km s−1, Δv = 1.5 km s−1; and a column density of (9±3)×1015 cm−2 for the ground state (g.s.) of tEME. According to our model, all favourable lines for detection in the 30 m data were detected or were blended with features from more abundant species. Nevertheless, owing to the weakness of the features (TMB < 0.1 K at 3 mm, TMB < 0.2 K at 2 mm, and TMB < 1 K at 1.3–0.9 mm) and the high level of line confusion at ~1 mm, only a few lines were mostly free of blending with other species in this domain. Whereas the synthetic beam of the ALMA SV is 1″.90 × 1″.40, in the 30 m the beam diameter ranging from 30″ to 8″. Therefore, in the 30 m data, the spectrum is a mix of all molecules from all source components (average spectrum over the beam) given rise to a high level of line blending and line confusion. Table A.2 shows line parameters, intensity provided by the model, and blends of all lines of favourable transitions in the 30 m data.
3.2. Search for gauche-trans-n-propanol
All lines of Gt-CH3CH2CH2OH, an isomer of C3H8O (as well as tEME), reported by Maeda et al. (2006) and the dipole moments from Abdurakhmanov et al. (1969) were used to derive its rotational constants and to implement this species in MADEX. We conducted the search for Gt-n-propanol in the ALMA SV data at two different positions: Position A and the position where the emission peak of ethanol is located (Position B; see Sect. 4). We assign several unidentified lines in the source at Position B to this species. According to our model (dsou = 3″, vLSR = +8.0 km s−1, Δv = 3.0 km s−1, TK = 100 ± 20 K, and Ng.s ≤ (1.0 ± 0.2) × 1015 cm−2), many of the lines are below the detection limit although the strongest features are detected. Unfortunately, several lines remain blended (see Fig. A.3). A few lines of this species are also detected in the IRAM 30 m data at the survey position (Fig. A.2 bottom panel; model parameters: dsou = 3″, vLSR = +8.0 km s−1, Δv = 1.5 km s−1, TK = 100 ± 20 K, and Ng.s ≤ (2.0 ± 0.4) × 1015 cm−2). Table A.3 shows line parameters for the detected lines. The derived upper limit to its column density (assuming the same physical parameters than those of the tEME ALMA model) at Position A is ≤(3.0 ± 0.6) × 1014 cm−2. The spatial distribution of this species around Position B is shown in Fig. 2. To perform the ALMA map, we averaged the emission between vLSR 6 and 9 km s−1 of two lines (lines at 236.138 and 244.765GHz). Emission around source I should be due to other less abundant species in Orion (we did not find Gt-n-propanol at these positions).
4. Discussion
Species containing the functional groups formate, alcohol, and ether have been detected in Orion with both the methyl and ethyl groups (methyl formate (MF), ethyl formate (EF), methanol, ethanol, dimethyl ether (DME), and tEME). ALMA maps for the spatial distribution of these species as well as Gt-n-propanol are shown in Fig. 2. To address the flux filtered out by ALMA and the accuracy of the maps in a larger energy range, the following discussion is also based on the maps shown in Fig. 5 of Feng et al. (2015; maps performed mixing SMA and IRAM 30 m data) with MF, DME, methanol, and ethanol. For MF, DME, and methanol the spatial distribution and the position of the emission peaks are in agreement with those of the maps presented in this work (note, however, that the ALMA maps provide a more detailed structure at small scales, i.e. ≤5″). For ethanol, we note a more extended spatial distribution in the map of Feng et al. (2015) mostly due to the lower energy of the transition involved. Nevertheless, the emission peak of ethanol is located at the same position.
For the methyl species, we note: i) a rather similar spatial structure: the three species present the V shape distribution of several clumps (at least six) studied by Favre et al. (2011) for the distribution of MF, which was mapped using data from the Plateau de Bure Interferometer (PdBI); ii) that although Brouillet et al. (2013) probed a striking similarity between the spatial distributions of CH3OCH3 and CH3OCOH, we found some differences in the relative intensities of both species. These differences could be mostly due to different excitation temperatures of the involved transitions; and iii) although methanol also follows this V shape structure, a displacement of the intensity peaks is observed with respect to MF. This behaviour suggests methanol as a possible precursor of MF and DME (see also Neill et al. 2011).
Comparing the methyl and ethyl species, we note: i) a reduced spatial distribution of the three ethyl species with respect to their methyl counterpart; ii) the two emission peaks of EF are correlated with those found in MF; iii) the emission peak of tEME is at the same position as the DME peak at the CR (Position A); and iv) the emission peak of ethanol (Position B) is displaced 2″ south-west from the methanol peak.
Concerning the ethyl and propyl species, we note: i) a close correlation between EF and tEME; and ii) ethanol also presents a “V” shape structure (see Fig. 5 of Feng et al. 2015) with the bulk of the emission located away from the CR and coinciding with that of Gt-n-propanol. The ethanol/propanol peak is displaced 1″.5 south from the ethylene glycol (CH2OH)2 peak (Brouillet et al. 2015), which is a double alcohol and we could naively expect to have the same spatial distribution. Whereas the ethylene glycol peak corresponds to the 13CH3OH peak, the ethanol/propanol peak is the same as that of deuterated methanol (CH2DOH; see Peng et al. 2012).
Table 1 shows derived column densities and ratios for related species. The derived ratios and the spatial distribution of these molecules suggest important gas phase processes after the evaporation of the mantles of dust grains in hot cores. Possible reactions of the methoxy radical (CH3O), detected recently in space (Cernicharo et al. 2012), with other species could lead to the increase of chemical complexity in hot cores and hot corinos (Balucani et al. 2015). The spatial stratification of the different species also suggests the time dependent effects on the chemistry of the gas. The detection of the less stable isomers of some species (Tercero et al. 2013) also points in this direction.
Table 1.
Column densities and ratios.
| Species | Ng.s. (×l015) [cm−2] | N Ratio |
|---|---|---|
| CH3OCH3 (DME) | 600 ± 120a,b | |
| CH3CH2OCH3 (tEME) | ≤4.0 ± 0.8a | DME/tEME ≥ 150 |
| CH3CH2OCH2CH3 | ≤1.0 ± 0.2a | DME/Tt-DEE ≥ 600 |
| (Tt-DEE)† | tEME/Tt-DEE ≥ 4 | |
| CH3OCHCH2 | ≤0.5 ± 0.1a | DME/cis-MVE ≥ 1200 |
| (cis-MVE)†† | tEME/cis-MVE ≥ 9 | |
| CH3OCOH (MF) | 240 ± 50a,b,c | |
| CH3CH2OCOH (EF) | 2.0 ± 0.4a,d | MF/EF ≃ 120 |
| CH3OH (MetOH) | 2700 ± 500b,e,f | |
| CH3CH2OH (EtOH) | 60 ± 10b,d,e | MetOH/EtOH ≃ 45 |
| Gt-CH3CH2CH2OH | 1.0 ± 0.2e | MetOH/PropOH ≃ 2700 |
| (PropOH) | EtOH/PropOH ≃ 60 |
Notes.
trans-trans diethyl ether.
cis methyl vinyl ether.
Position A; same physical parameters of the ALMA tEME model (see Sect. 3.1).
Three kinetic temperatures: 50 ± 10, 150 ± 30, and 250 ± 75 K.
b type lines fitted (a type lines are optically thick); another component has been included to properly fit the observed line profiles (vLSR = +9 km s−1, Δv = 4 km s−1, TK = 150 ± 30 K, Ng.s = (1.0 ± 0.2) × 1017cm−2).
trans+gauche.
Position B; assuming the same physical parameters of the ALMA Gt-n-propanol model (see Sect. 3.2).
12C/13C = 45 (Tercero et al. 2010).
To summarize, a combined IRAM 30 m and ALMA SV data study allows us to provide a solid starting point to assess the identification of tEME in the ISM. In addition, some unidentified lines in the source have been assigned to another C3H8O isomer, Gt-n-propanol. ALMA maps show different spatial distributions for these species. Whereas tEME seems to mainly arises from the CR component (as well as EF) (Position A), emission from Gt-n-propanol could be located at the south hot core (at the same position as the emission peak of ethanol) (Position B). The CR is no longer the main host of all organic saturated O-bearing species in Orion (see also Peng et al. 2013, for the spatial distribution of acetone and A. López et al., in prep. for the acetic acid emission).
Acknowledgements
We thank Marcelino Agúndez for carefully reading the paper and providing useful comments and suggestions. B.T., J.C., and A.L. thank the Spanish MINECO for funding support under grants CSD2009-00038, AYA2009-07304, and AYA2012-32032 and also the ERC for funding support under grant ERC-2013-Syg-610256-NANOCOSMOS.
Appendix A: Online figures and tables
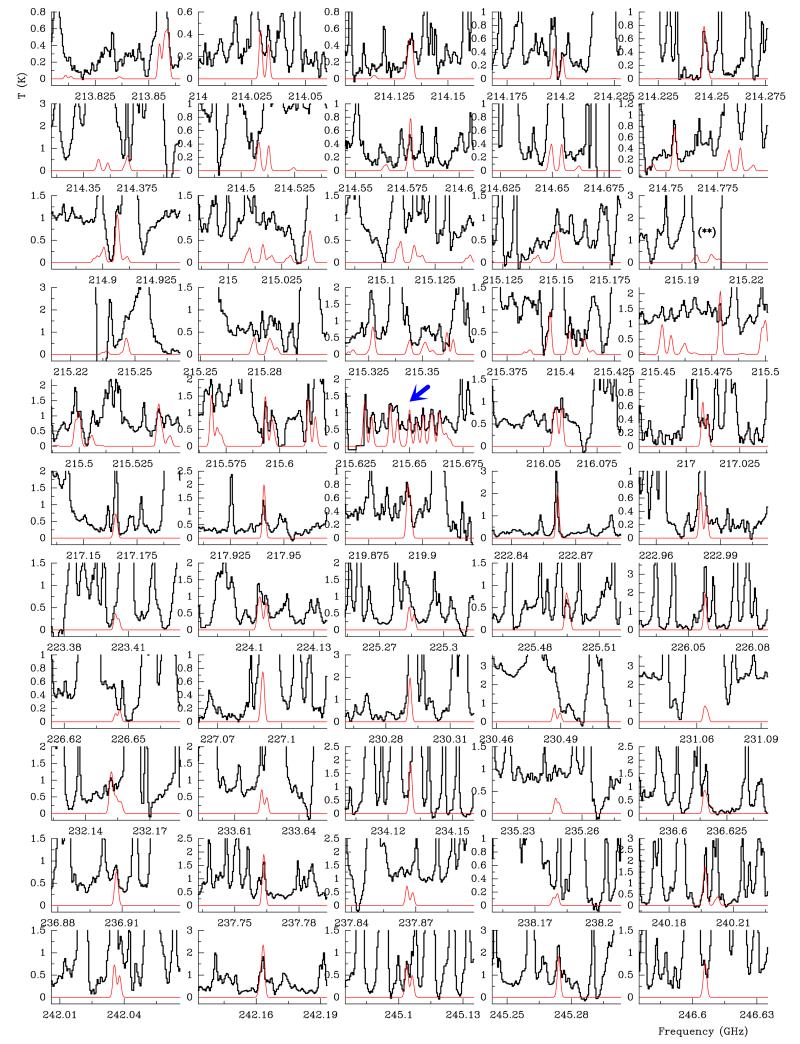
Fig. A.1.
Lines of trans ethyl methyl ether, t-CH3CH2OCH3, towards Orion KL detected with the ALMA interferometer in Position A (see text). (**): Features blended with SO (see Table A.1; artifacts in the spectrum due to the cleaning process). A vLSR of +7.5 km s−1 is assumed.
Fig. A.2.
Top panel: selected lines of trans ethyl methyl ether, t-CH3CH2OCH3, towards Orion KL detected with the IRAM 30 m telescope. Data in the frequency range 124–151 GHz are those of the survey position. From 201 to 293.5 GHz the data are those of the CR (see Sect. 2), where the emission peak of organic saturated O-rich species such as dimethyl ether (CH3OCH3) and methyl formate (CH3OCOH) is located (Favre et al. 2011; Brouillet et al. 2013). A vLSR of +7.5 km s−1 is assumed. Bottom panel: selected lines of gauche-trans-n-Propanol, Gt-n-CH3CH2CH2OH, towards Orion KL detected with the IRAM 30 m telescope. A vLSR of +7.5 km s−1 is assumed.
Fig. A.3.
Lines of gauche-trans-n-propanol, Gt-n-CH3CH2CH2OH, towards Orion KL detected with the ALMA interferometer in Position B (see text). A vLSR of +8 km s−1 is assumed.
Table A.1.
Lines of trans-CH3CH2OCH3 in ALMA SV data.
| Species | Transition |
Predicted frequency (MHz) |
Eupp (K) |
Sij | Observed frequency (MHz) |
T (K) |
Blends |
|---|---|---|---|---|---|---|---|
| tEME-EE’ | 315,27–314,28 | 213 854.674 | 220.5 | 16.34 | … | … | CH3CH2OH |
| tEME-EE | 315,27–314,28 | 213 854.982 | 220.5 | 16.44 | … | … | ” |
| tEME-AE | 315,27–314,28 | 213 855.220 | 220.5 | 16.40 | … | … | ” |
| tEME-EE | 285,23–284,24 | 213 856.708 | 185.7 | 14.17 | … | … | ” |
| tEME-AE | 285,23–284,24 | 213 857.473 | 185.7 | 13.94 | … | … | ” |
| tEME-EE’ | 285,23–284,24 | 213 857.509 | 185.7 | 13.69 | … | … | ” |
| tEME-EA | 315,27–314,28 | 213 858.239 | 220.5 | 16.52 | … | … | ” |
| tEME-AA | 315,27–314,28 | 213 858.632 | 220.5 | 16.53 | … | … | ” |
| tEME-EA | 285,23–284,24 | 213 859.015 | 185.7 | 14.70 | … | … | ” |
| tEME-AA | 285,23–284,24 | 213 859.367 | 185.7 | 14.73 | … | … | ” |
| tEME-EE’ | 305,26–304,27 | 214 028.729 | 208.6 | 15.58 | … | … | CH3COCH3 |
| tEME-EE | 305,26–304,27 | 214 029.157 | 208.6 | 15.76 | … | … | ” |
| tEME-AE | 305,26–304,27 | 214 029.345 | 208.6 | 15.68 | … | … | ” |
| tEME-EA | 305,26–304,27 | 214 032.539 | 208.6 | 15.91 | 214 032.3† | 0.50 | |
| tEME-AA | 305,26–304,27 | 214 032.539 | 208.6 | 15.91 | † | ||
| tEME-EE | 275,22–274,23 | 214 131.118 | 174.9 | 13.15 | 214 132.6 | 0.58 | |
| tEME-AE | 275,22–274,23 | 214 132.010 | 174.9 | 12.80 | † | ||
| tEME-EE’ | 275,22–274,23 | 214 132.166 | 174.9 | 12.46 | † | ||
| tEME-EA | 275,22–274,23 | 214 133.150 | 174.9 | 14.07 | † | ||
| tEME-AA | 275,22–274,23 | 214 133.486 | 174.9 | 14.14 | † | ||
| tEME-EE’ | 295,25–294,26 | 214 196.531 | 196.9 | 14.73 | 214 196.8 | 0.94 | |
| tEME-EE | 295,25–294,26 | 214 197.122 | 196.9 | 15.01 | † | ||
| tEME-AE | 295,25–294,26 | 214 197.239 | 196.9 | 14.88 | † | ||
| tEME-EA | 295,25–294,26 | 214 200.673 | 196.9 | 15.31 | 214 201.4 | 0.41 | |
| tEME-AA | 295,25–294,26 | 214 201.091 | 196.9 | 15.31 | † | ||
| tEME-EE’ | 212,20–201,19 | 214 246.202 | 93.7 | 7.76 | 214 246.5 | 0.72 | |
| tEME-EE | 212,20–201,19 | 214 246.202 | 93.7 | 7.76 | † | ||
| tEME-AE | 212,20–201,19 | 214 246.332 | 93.7 | 7.76 | † | ||
| tEME-EA | 212,20–201,19 | 214 247.602 | 93.7 | 7.76 | † | ||
| tEME-AA | 212,20–201,19 | 214 247.732 | 93.7 | 7.76 | † | ||
| tEME-EE’ | 265,22–264,22 | 214 355.828 | 164.4 | 2.47 | … | … | CH3CH2OH; SO |
| tEME-EE’ | 285,24–284,25 | 214 356.510 | 185.7 | 13.69 | … | … | ” |
| tEME-AE | 265,22–264,22 | 214 356.963 | 164.4 | 2.08 | … | … | ” |
| tEME-EE | 265,22–264,22 | 214 357.232 | 164.4 | 1.63 | … | … | ” |
| tEME-EE | 285,24–284,25 | 214 357.312 | 185.7 | 14.17 | … | … | ” |
| tEME-AE | 285,24–284,25 | 214 357.333 | 185.7 | 13.93 | … | … | ” |
| tEME-EA | 285,24–284,25 | 214 361.091 | 185.7 | 14.69 | … | … | CH3OCH3 |
| tEME-AA | 285,24–284,25 | 214 361.527 | 185.7 | 14.72 | … | … | ” |
| tEME-EE | 265,21–264,22 | 214 369.161 | 164.4 | 11.92 | … | … | CH3COOCH3 |
| tEME-AE | 265,21–264,22 | 214 370.185 | 164.4 | 11.47 | … | … | ” |
| tEME-EE’ | 265,21–264,22 | 214 370.456 | 164.4 | 11.08 | … | … | ” |
| tEME-EA | 265,21–264,22 | 214 370.843 | 164.4 | 13.42 | … | … | ” |
| tEME-AA | 265,21–264,22 | 214 371.154 | 164.4 | 13.55 | … | … | ” |
| tEME-EE’ | 275,23–274,24 | 214 507.462 | 174.9 | 12.45 | 214 508.5 | 0.61 | |
| tEME-AE | 275,23–274,24 | 214 508.414 | 174.9 | 12.79 | † | ||
| tEME-EE | 275,23–274,24 | 214 508.510 | 174.9 | 13.14 | † | ||
| tEME-EA | 275,23–274,24 | 214 512.591 | 174.9 | 14.06 | … | … | CH3OCOH vt = 1 |
| tEME-AA | 275,23–274,24 | 214 513.051 | 174.9 | 14.13 | … | … | ” |
| tEME-EE | 255,20–254,21 | 214 575.213 | 154.4 | 10.56 | 214 576.5 | 0.58 | |
| tEME-AE | 255,20–254,21 | 214 576.349 | 154.4 | 10.10 | † | ||
| tEME-EA | 255,20–254,21 | 214 576.498 | 154.4 | 12.69 | † | ||
| tEME-EE’ | 255,20–254,21 | 214 576.706 | 154.4 | 9.74 | † | ||
| tEME-AA | 255,20–254,21 | 214 576.769 | 154.4 | 12.96 | † | ||
| tEME-EE’ | 265,22–264,23 | 214 648.535 | 164.4 | 11.08 | … | … | CH3OCOH |
| tEME-AE | 265,22–264,23 | 214 649.611 | 164.4 | 11.47 | … | … | ” |
| tEME-EE | 265,22–264,23 | 214 649.829 | 164.4 | 11.92 | … | … | ” |
| tEME-EA | 265,22–264,23 | 214 654.288 | 164.5 | 13.41 | … | … | ” |
| tEME-AA | 265,22–264,23 | 214 654.781 | 164.5 | 13.54 | … | … | ” |
| tEME-EE | 245,19–244,20 | 214 753.118 | 144.7 | 9.23 | 214 754.0 | 0.94 | |
| tEME-EA | 245,19–244,20 | 214 754.023 | 144.7 | 11.84 | † | ||
| tEME-AA | 245,19–244,20 | 214 754.233 | 144.7 | 12.38 | † | ||
| tEME-AE | 245,19–244,20 | 214 754.329 | 144.7 | 8.86 | † | ||
| tEME-EE’ | 245,19–244,20 | 214 754.733 | 144.7 | 8.60 | † | ||
| tEME-EE’ | 255,21–254,22 | 214 779.208 | 154.4 | 9.73 | … | … | CH3OCOH |
| tEME-AE | 255,21–254,22 | 214 780.378 | 154.4 | 10.10 | … | … | ” |
| tEME-EE | 255,21–254,22 | 214 780.701 | 154.4 | 10.56 | … | … | ” |
| tEME-EA | 255.21–254,22 | 214 785.581 | 154.4 | 12.69 | … | … | ” |
| tEME-AA | 255,21–254,22 | 214 786.123 | 154.4 | 12.96 | … | … | ” |
| tEME-EE’ | 235,19–234,19 | 214 895.777 | 135.4 | 4.03 | … | … | CH3OCOH vt = 1 |
| tEME-AE | 235,19–234,19 | 214 897.184 | 135.4 | 3.90 | … | … | ” |
| tEME-EE | 235,19–234,19 | 214 897.739 | 135.4 | 3.67 | … | … | ” |
| tEME-EE’ | 245,20–244,21 | 214 899.228 | 144.7 | 8.61 | … | … | ” |
| tEME-AE | 245,20–244,21 | 214 900.454 | 144.7 | 8.87 | … | … | ” |
| tEME-EE | 245,20–244,21 | 214 900.844 | 144.7 | 9.23 | … | … | ” |
| tEME-EA | 235,19–234,19 | 214 903.639 | 144.7 | 1.05 | … | … | ” |
| tEME-EA | 245,20–244,21 | 214 906.128 | 144.7 | 11.83 | 214 906.7 | 1.30 | |
| tEME-EE | 235,18–234,19 | 214 906.260 | 135.4 | 8.13 | † | ||
| tEME-AA | 245,20–244,21 | 214 906.739 | 144.7 | 12.38 | † | ||
| tEME-EA | 235,18–234,19 | 214 906.872 | 135.4 | 10.76 | † | ||
| tEME-AA | 235,18–234,19 | 214 906.992 | 135.4 | 11.80 | † | ||
| tEME-AE | 235,18–234,19 | 214 907.507 | 135.4 | 7.91 | † | ||
| tEME-EE’ | 235,18–234,19 | 214 907.507 | 135.4 | 7.77 | † | ||
| tEME-EE | 245,19–244,21 | 214 909.858 | 144.7 | 11.83 | … | … | CH3CH2CN |
| tEME-EA | 245,19–244,21 | 214 910.548 | 144.7 | 0.55 | … | … | ” |
| tEME-AE | 245,19–244,21 | 214 911.166 | 144.7 | 3.51 | … | … | ” |
| tEME-EE’ | 245,19–244,21 | 214 911.685 | 144.7 | 3.77 | … | … | ” |
| tEME-EE’ | 235,19–234,20 | 215 008.545 | 135.4 | 7.77 | … | … | CH3OCOH vt = 2 |
| tEME-AE | 235,19–234,20 | 215 009.791 | 135.4 | 7.91 | … | … | ” |
| tEME-EE | 235,19–234,20 | 215 010.209 | 135.4 | 8.13 | … | … | ” |
| tEME-EA | 235,19–234,20 | 215 015.810 | 135.4 | 10.75 | 215 016.5 | 1.16 | |
| tEME-AA | 235,19–234,20 | 215 016.519 | 135.4 | 11.80 | † | ||
| tEME-EE | 235,18–234,20 | 215 018.730 | 135.4 | 3.67 | … | … | CH3OCOD |
| tEME-EA | 235,18–234,20 | 215 019.043 | 135.4 | 1.05 | … | … | ” |
| tEME-AE | 235,18–234,20 | 215 020.114 | 135.4 | 3.90 | … | … | ” |
| tEME-EE’ | 235,18–234,20 | 215 020.693 | 135.4 | 4.03 | … | … | ” |
| tEME-EE’ | 225,18–224,18 | 215 027.250 | 126.6 | 4.02 | … | … | U-line |
| tEME-AE | 225,18–224,18 | 215 028.719 | 126.6 | 3.99 | … | … | ” |
| tEME-EE | 225,18–224,18 | 215 029.330 | 126.6 | 4.00 | … | … | ” |
| tEME-EA | 225,18–224,18 | 215 035.544 | 126.6 | 1.78 | … | … | CH3OCOD,CH3COOCH3 |
| tEME-EE | 225,17–224,18 | 215 037.639 | 126.6 | 7.33 | … | … | ” |
| tEME-EA | 225,17–224,18 | 215 038.085 | 126.6 | 9.45 | … | … | ” |
| tEME-AA | 225,17–224,18 | 215 038.089 | 126.6 | 11.23 | … | … | CH3CH2CN |
| tEME-AE | 225,17–224,18 | 215 038.890 | 126.6 | 7.24 | … | … | ” |
| tEME-EE’ | 225,17–224,18 | 215 039.292 | 126.6 | 7.21 | … | … | ” |
| tEME-AA | 352,33–343,32 | 215 107.148 | 250.8 | 9.27 | … | … | CH3COOCH3, CH3OCOH vt = 2 |
| tEME-EA | 352,33–343,32 | 215 107.270 | 250.8 | 9.27 | … | … | ” |
| tEME-EE’ | 225,18–224,19 | 215 107.270 | 126.6 | 7.21 | … | … | ” |
| tEME-AE | 225,18–224,19 | 215 108.508 | 126.6 | 7.24 | … | … | ” |
| tEME-EE | 225,18–224,19 | 215 108.923 | 126.6 | 7.33 | … | … | ” |
| tEME-AE | 352,33–343,32 | 215 109.184 | 250.8 | 9.27 | … | … | ” |
| tEME-EE | 352,33–343,32 | 215 109.306 | 250.8 | 9.27 | … | … | ” |
| tEME-EE’ | 352,33–343,32 | 215 109.306 | 250.8 | 9.27 | … | … | ” |
| tEME-EA | 225,18–224,19 | 215 114.713 | 126.6 | 9.44 | … | … | CH3O13COH |
| tEME-AA | 225,18–224,19 | 215 115.545 | 126.6 | 11.22 | … | … | ” |
| tEME-EE | 225,17–224,19 | 215 117.232 | 126.6 | 3.90 | … | … | CH3CH2CN |
| tEME-EA | 225,17–224,19 | 215 117.254 | 126.6 | 1.78 | … | … | ” |
| tEME-AE | 225,17–224,19 | 215 118.679 | 126.6 | 3.99 | … | … | ” |
| tEME-EE’ | 225,17–224,19 | 215 119.312 | 126.6 | 4.02 | … | … | ” |
| tEME-EE’ | 215,17–214,17 | 215 139.474 | 118.0 | 3.75 | … | … | CH3O13COH vt = 1 |
| tEME-AE | 215,17–214,17 | 215 141.013 | 118.0 | 3.82 | 215 141.0 | 0.59 | CH3O13COH vt = 1 |
| tEME-EE | 215,17–214,17 | 215 141.682 | 118.0 | 3.85 | † | ||
| tEME-EA | 215,17–214,17 | 215 148.139 | 118.0 | 2.58 | 215 150.7 | 1.28 | |
| tEME-EE | 215,16–214,17 | 215 149.914 | 118.0 | 6.81 | † | ||
| tEME-AA | 215,16–214,17 | 215 150.202 | 118.0 | 10.66 | † | ||
| tEME-EA | 215,16–214,17 | 215 150.327 | 118.0 | 8.07 | † | ||
| tEME-AE | 215,16–214,17 | 215 151.142 | 118.0 | 6.84 | 215 150.7† | 1.28 | |
| tEME-EE’ | 215,16–214,17 | 215 151.503 | 118.0 | 6.91 | † | ||
| tEME-EE’ | 225,17–224,18 | 215 195.665 | 118.0 | 6.91 | … | … | SO |
| tEME-AE | 225,17–224,18 | 215 196.869 | 118.0 | 6.84 | … | … | ” |
| tEME-EE | 225,17–224,18 | 215 197.254 | 118.0 | 6.81 | … | … | ” |
| tEME-EA | 225,17–224,18 | 215 203.100 | 118.0 | 8.07 | … | … | ” |
| tEME-AA | 225,17–224,18 | 215 204.068 | 118.0 | 10.66 | … | … | ” |
| tEME-EA | 225,16–224,18 | 215 205.287 | 118.0 | 2.58 | … | … | ” |
| tEME-EE | 225,16–224,18 | 215 205.486 | 118.0 | 3.84 | … | … | ” |
| tEME-AE | 225,16–224,18 | 215 206.998 | 118.0 | 3.82 | … | … | ” |
| tEME-EE’ | 225,16–224,18 | 215 207.694 | 118.0 | 3.75 | … | … | ” |
| tEME-EE’ | 205,16–204,16 | 215 234.851 | 109.9 | 3.26 | … | … | ” |
| tEME-AE | 205,16–204,16 | 215 236.480 | 109.9 | 3.41 | … | … | ” |
| tEME-EE | 205,16–204,16 | 215 237.223 | 109.9 | 3.55 | … | … | ” |
| tEME-EA | 205,16–204,16 | 215 243.901 | 109.9 | 3.19 | … | … | CH3COOCH3 CH3O13COH, CH3CH2CN v13/v21 |
| tEME-EE | 205,15–204,16 | 215 245.438 | 109.9 | 6.54 | … | … | ” |
| tEME-AA | 205,15–204,16 | 215 245.691 | 109.9 | 10.09 | … | … | ” |
| tEME-EA | 205,15–204,16 | 215 245.934 | 109.9 | 6.89 | … | … | ” |
| tEME-AE | 205,15–204,16 | 215 246.615 | 109.9 | 6.67 | … | … | ” |
| tEME-EE’ | 205,15–204,16 | 215 246.907 | 109.9 | 6.83 | … | … | ” |
| tEME-EE’ | 205,16–204,17 | 215 274.126 | 109.9 | 6.83 | … | … | CH3COOCH3, U-line |
| tEME-AE | 205,16–204,17 | 215 275.268 | 109.9 | 6.67 | … | … | ” |
| tEME-EE | 205,16–204,17 | 215 275.595 | 109.9 | 6.54 | … | … | ” |
| tEME-EA | 205,16–204,17 | 215 281.378 | 109.9 | 6.89 | … | … | U-line |
| tEME-AA | 205,16–204,17 | 215 282.470 | 109.9 | 10.09 | … | … | ” |
| tEME-EA | 205,15–204,17 | 215 283.411 | 109.9 | 3.19 | … | … | ” |
| tEME-EE | 205,15–204,17 | 215 283.810 | 109.9 | 3.55 | … | … | ” |
| tEME-AE | 205,15–204,17 | 215 285.403 | 109.9 | 3.41 | … | … | ” |
| tEME-EE’ | 205,15–204,17 | 215 286.182 | 109.9 | 3.26 | … | … | ” |
| tEME-EE’ | 195,15–194,15 | 215 315.464 | 102.2 | 2.57 | … | … | U-line |
| tEME-AE | 195,15–194,15 | 215 317.213 | 102.2 | 2.79 | 215 317.4 | 0.54 | U-line |
| tEME-EE | 195,15–194,15 | 215 318.057 | 102.2 | 3.02 | † | ||
| tEME-EA | 195,15–194,15 | 215 324.992 | 102.2 | 3.49 | 215 327.2 | 1.96 | 13CH3OCOH vt = 1 |
| tEME-EE | 195,14–194,15 | 215 326.280 | 102.2 | 6.50 | † | ||
| tEME-AA | 195,14–194,15 | 215 326.637 | 102.2 | 9.52 | † | ||
| tEME-EA | 195,14–194,15 | 215 326.971 | 102.2 | 6.03 | † | ||
| tEME-AE | 195,14–194,15 | 215 327.373 | 102.2 | 6.72 | † | ||
| tEME-EE’ | 195,14–194,15 | 215 327.561 | 102.2 | 6.95 | † | ||
| tEME-EE’ | 195,15–194,16 | 215 343.169 | 102.2 | 6.95 | … | … | CH3OCOH |
| tEME-AE | 195,15–194,16 | 215 344.214 | 102.2 | 6.72 | … | … | ” |
| tEME-EE | 195,15–194,16 | 215 344.450 | 102.2 | 6.50 | … | … | ” |
| tEME-EA | 195,15–194,16 | 215 350.059 | 102.2 | 6.03 | 215 351.2 | 0.91 | CH3CH2CN (v = 0, v13/v21) |
| tEME-AA | 195,15–194,16 | 215 351.248 | 102.2 | 9.52 | † | ||
| tEME-EA | 195,14–194,16 | 215 352.037 | 102.2 | 3.49 | † | ||
| tEME-EE | 195,14–194,16 | 215 352.674 | 102.2 | 3.02 | † | ||
| tEME-AE | 195,14–194,16 | 215 354.374 | 102.2 | 2.79 | … | … | CH3CH2CN (v = 0, v13/v21) |
| tEME-EE’ | 195,14–194,16 | 215 355.266 | 102.2 | 2.57 | … | … | ” |
| tEME-EE’ | 123,10–112,9 | 215 361.252 | 40.5 | 4.16 | 215 361.7 | 0.43 | |
| tEME-EE | 123,10–112,9 | 215 361.484 | 40.5 | 4.18 | † | ||
| tEME-AE | 123,10–112,9 | 215 361.676 | 40.5 | 4.17 | † | ||
| tEME-EA | 123,10–112,9 | 215 364.224 | 40.5 | 4.19 | … | … | CH3CH2OH |
| tEME-AA | 123,10–112,9 | 215 364.533 | 40.5 | 4.20 | … | … | ” |
| tEME-EA | 185,14–184,14 | 215 393.299 | 94.8 | 3.48 | 215 395.5 | 1.01 | |
| tEME-EE | 185,13–184,14 | 215 394.256 | 94.8 | 6.65 | † | ||
| tEME-AA | 185,13–184,14 | 215 394.875 | 94.8 | 8.95 | † | ||
| tEME-AE | 185,13–184,14 | 215 395.227 | 94.8 | 6.94 | † | ||
| tEME-EA | 185,13–184,14 | 215 395.264 | 94.8 | 5.47 | † | ||
| tEME-EE’ | 185,13–184,14 | 215 395.280 | 94.8 | 7.19 | † | ||
| tEME-EE’ | 185,14–184,15 | 215 403.394 | 94.8 | 7.19 | 215 404.5 | 0.91 | |
| tEME-AE | 185,14–184,15 | 215 404.308 | 94.8 | 6.94 | † | ||
| tEME-EE | 185,14–184,15 | 215 404.417 | 94.8 | 6.65 | † | ||
| tEME-EA | 185,14–184,15 | 215 409.728 | 94.8 | 5.47 | 215 411.2 | 0.59 | |
| tEME-AA | 185,14–184,15 | 215 410.979 | 94.8 | 8.95 | † | ||
| tEME-EA | 185,14–184,15 | 215 411.693 | 94.8 | 3.48 | † | ||
| tEME-EE | 185,14–184,15 | 215 412.657 | 94.8 | 2.31 | † | ||
| tEME-EA | 175,13–174,13 | 215 450.467 | 87.9 | 3.22 | 215 451.7 | 1.65 | |
| tEME-EE | 175,12–174,13 | 215 450.974 | 87.9 | 6.90 | † | ||
| tEME-EE’ | 175,12–174,13 | 215 451.703 | 87.9 | 7.39 | † | ||
| tEME-AE | 175,12–174,13 | 215 451.799 | 87.9 | 7.18 | † | ||
| tEME-AA | 175,12–174,13 | 215 452.025 | 87.9 | 8.39 | † | ||
| tEME-EA | 175,12–174,13 | 215 452.436 | 87.9 | 5.17 | † | ||
| tEME-EE’ | 175,13–174,13 | 215 455.422 | 87.9 | 7.39 | 215 456.2 | 1.60 | 13CH3OCOH vt = 1 |
| tEME-EE | 175,13–174,13 | 215 456.151 | 87.9 | 6.90 | † | ||
| tEME-AE | 175,13–174,13 | 215 456.193 | 87.9 | 7.18 | † | ||
| tEME-EA | 175,13–174,14 | 215 461.025 | 87.9 | 5.17 | 215 462.2 | 1.81 | U-line |
| tEME-AA | 175,13–174,14 | 215 462.304 | 87.9 | 8.39 | † | ||
| tEME-EA | 175,12–174,14 | 215 462.994 | 87.9 | 3.22 | † | ||
| tEME-EE’ | 271,27–260,26 | 215 478.840 | 144.2 | 21.60 | 215 479.5 | 1.81 | |
| tEME-EE | 271,27–260,26 | 215 478.840 | 144.2 | 21.60 | † | ||
| tEME-AE | 271,27–260,26 | 215 478.854 | 144.2 | 21.60 | † | ||
| tEME-EA | 271,27–260,26 | 215 479.025 | 144.2 | 21.60 | † | ||
| tEME-AA | 271,27–260,26 | 215 479.039 | 144.2 | 21.60 | † | ||
| tEME-EE | 165,11–164,12 | 215 497.897 | 81.3 | 7.05 | 215 498.7 | 1.96 | CH3CH2CN v13/v21 |
| tEME-EA | 165,12–164,12 | 215 497.943 | 81.3 | 2.74 | † | ||
| tEME-EE’ | 165,11–164,12 | 215 498.372 | 81.3 | 7.37 | † | ||
| tEME-AE | 165,11–164,12 | 215 498.590 | 81.3 | 7.23 | † | ||
| tEME-AA | 165,11–164,12 | 215 499.524 | 81.3 | 7.82 | 215 500.2† | 1.22 | |
| tEME-EE’ | 165,12–164,13 | 215 499.830 | 81.3 | 7.37 | † | ||
| tEME-EA | 165,11–164,12 | 215 499.921 | 81.3 | 5.08 | † | ||
| tEME-EE | 165,12–164,13 | 215 500.305 | 81.3 | 7.05 | † | ||
| tEME-AE | 165,12–164,13 | 215 500.485 | 81.3 | 7.24 | † | ||
| tEME-EA | 165,12–164,13 | 215 504.636 | 81.3 | 5.08 | 215 506.2 | 1.22 | CH3C13CH |
| tEME-AA | 165,12–164,13 | 215 505.905 | 81.3 | 7.82 | † | ||
| tEME-EA | 165,11–164,13 | 215 506.613 | 81.3 | 2.74 | † | ||
| tEME-EE | 155,10–154,11 | 215 536.404 | 75.1 | 6.94 | 215 537.7 | 1.33 | |
| tEME-EE’ | 155,10–154,11 | 215 536.720 | 75.1 | 7.08 | † | ||
| tEME-EA | 155,11–154,11 | 215 537.002 | 75.1 | 2.05 | † | ||
| tEME-AE | 155,10–154,11 | 215 537.010 | 75.1 | 7.03 | † | ||
| tEME-EE’ | 155,11–154,12 | 215 537.169 | 75.1 | 7.08 | † | ||
| tEME-EE | 155,11–154,12 | 215 537.485 | 75.1 | 6.94 | † | ||
| tEME-AE | 155,11–154,12 | 215 537.757 | 75.1 | 7.08 | † | ||
| tEME-AA | 155,10–154,11 | 215 538.650 | 75.1 | 7.25 | † | ||
| tEME-EA | 155,10–154,11 | 215 538.990 | 75.1 | 5.21 | † | ||
| tEME-EA | 155,11–154,12 | 215 541.270 | 75.1 | 5.21 | 215 541.5† | 1.06 | CH3COOCH3 |
| tEME-AA | 155,11–154,12 | 215 542.488 | 75.1 | 7.25 | † | ||
| tEME-EA | 155,10–154,12 | 215 543.258 | 75.1 | 2.05 | … | ||
| tEME-EE | 145,9–144,10 | 215 567.750 | 69.3 | 6.58 | 215 567.9 | 2.13 | |
| tEME-EE’ | 145,9–144,10 | 215 567.993 | 69.3 | 6.63 | † | ||
| tEME-EE’ | 145,10–144,11 | 215 568.043 | 69.3 | 6.63 | † | ||
| tEME-EE | 145,10–144,11 | 215 568.286 | 69.3 | 6.58 | † | ||
| tEME-AE | 145,9–144,10 | 215 568.316 | 69.3 | 6.61 | † | ||
| tEME-AE | 145,10–144,11 | 215 568.602 | 69.3 | 6.61 | 215 571.7 | 1.89 | CH3OCOH vt = 1 |
| tEME-AE | 145,10–144,10 | 215 568.774 | 69.3 | 1.23 | † | ||
| tEME-AA | 145,9–144,10 | 215 570.539 | 69.3 | 6.68 | † | ||
| tEME-EA | 145,9–144,10 | 215 570.772 | 69.3 | 5.45 | † | ||
| tEME-EA | 145,10–144,11 | 215 571.650 | 69.3 | 5.45 | † | ||
| tEME-AA | 145,10–144,11 | 215 572.766 | 69.3 | 6.68 | † | ||
| tEME-EA | 145,9–144,11 | 215 573.648 | 69.3 | 1.23 | † | ||
| tEME-EE | 135,8–134,9 | 215 593.014 | 63.9 | 6.08 | 215 593.5 | 1.41 | |
| tEME-EE’ | 135,9–134,10 | 215 593.138 | 63.9 | 6.09 | † | ||
| tEME-EE’ | 135,8–134,9 | 215 593.228 | 63.9 | 6.09 | † | ||
| tEME-EE | 135,9–134,10 | 215 593.353 | 63.9 | 6.08 | † | ||
| tEME-AE | 135,8–134,9 | 215 593.566 | 63.9 | 6.09 | † | ||
| tEME-AE | 135,9–134,10 | 215 593.687 | 63.9 | 6.09 | † | ||
| tEME-EA | 135,9–134,9 | 215 594.282 | 63.9 | 0.53 | † | ||
| tEME-AA | 135,8–134,9 | 215 596.207 | 63.9 | 6.11 | 215 596.5 | 1.12 | |
| tEME-EA | 135,8–134,9 | 215 596.289 | 63.9 | 5.58 | † | ||
| tEME-EA | 135,9–134,10 | 215 596.478 | 63.9 | 5.58 | † | ||
| tEME-AA | 135,9–134,10 | 215 597.447 | 63.9 | 6.11 | † | ||
| tEME-EA | 135,8–134,10 | 215 598.486 | 63.9 | 0.53 | † | ||
| tEME-EE | 125,7–124,8 | 215 613.102 | 58.9 | 5.52 | 215 613.7 | 1.89 | |
| tEME-EE’ | 125,8–124,9 | 215 613.172 | 58.9 | 5.53 | † | ||
| tEME-EE’ | 125,7–124,8 | 215 613.308 | 58.9 | 5.53 | † | ||
| tEME-EE | 125,8–124,9 | 215 613.378 | 58.9 | 5.52 | † | ||
| tEME-AE | 125,7–124,8 | 215 613.651 | 58.9 | 5.52 | † | ||
| tEME-AE | 125,8–124,9 | 215 613.720 | 58.9 | 5.52 | † | ||
| tEME-EA | 125,8–124,9 | 215 616.405 | 58.9 | 5.37 | … | … | 13CH3OCOH vt = 1, CH3CH2CN |
| tEME-EA | 125,7–124,8 | 215 616.490 | 58.9 | 5.37 | … | … | ” |
| tEME-AA | 125,7–124,8 | 215 616.564 | 58.9 | 5.53 | … | … | ” |
| tEME-AA | 125,8–124,9 | 215 617.221 | 58.9 | 5.53 | … | … | ” |
| tEME-EE | 115,6–114,7 | 215 628.800 | 54.3 | 4.94 | 215 629.7 | 1.60 | |
| tEME-EE’ | 115,7–114,8 | 215 628.855 | 54.3 | 4.94 | † | ||
| tEME-EE’ | 115,6–114,7 | 215 629.003 | 54.3 | 4.94 | † | ||
| tEME-EE | 115,7–114,8 | 215 629.058 | 54.3 | 4.94 | † | ||
| tEME-AE | 115,6–114,7 | 215 629.349 | 54.3 | 4.94 | † | ||
| tEME-AE | 115,7–114,8 | 215 629.404 | 54.3 | 4.94 | † | ||
| tEME-EA | 115,7–114,8 | 215 632.056 | 54.3 | 4.91 | 215 632.7 | 1.06 | |
| tEME-EA | 115,6–114,7 | 215 632.229 | 54.3 | 4.91 | † | ||
| tEME-AA | 115,6–114,7 | 215 632.425 | 54.3 | 4.94 | † | ||
| tEME-AA | 115,7–114,8 | 215 632.754 | 54.3 | 4.94 | † | ||
| tEME-EE | 105,5–104,6 | 215 640.803 | 50.0 | 4.35 | 215 641.1 | 1.31 | |
| tEME-EE’ | 105,6–104,7 | 215 640.854 | 50.0 | 4.35 | † | ||
| tEME-EE’ | 105,5–104,6 | 215 641.007 | 50.0 | 4.35 | † | ||
| tEME-EE | 105,6–104,7 | 215 641.058 | 50.0 | 4.35 | † | ||
| tEME-AE | 105,5–104,6 | 215 641.354 | 50.0 | 4.35 | † | ||
| tEME-AE | 105,6–104,7 | 215 641.405 | 50.0 | 4.35 | † | ||
| tEME-EA | 105,6–104,7 | 215 644.051 | 50.0 | 4.34 | 215 644.1 | 1.26 | 13CH3OCOH vt = 1 |
| tEME-EA | 105,5–104,6 | 215 644.248 | 50.0 | 4.34 | † | ||
| tEME-AA | 105,5–104,6 | 215 644.522 | 50.0 | 4.35 | † | ||
| tEME-AA | 105,6–104,7 | 215 644.676 | 50.0 | 4.35 | † | ||
| tEME-EE | 95,4–94,5 | 215 649.740 | 46.1 | 3.73 | 215 650.2 | 1.02 | |
| tEME-EE’ | 95,5–94,6 | 215 649.791 | 46.1 | 3.73 | † | ||
| tEME-EE’ | 95,4–94,5 | 215 649.944 | 46.1 | 3.73 | † | ||
| tEME-EE | 95,5–94,6 | 215 649.995 | 46.1 | 3.73 | † | ||
| tEME-AE | 95,4–94,5 | 215 650.292 | 46.1 | 3.73 | † | ||
| tEME-AE | 95,5–94,6 | 215 650.343 | 46.1 | 3.73 | † | ||
| tEME-EA | 95,5–94,6 | 215 652.990 | 46.1 | 3.73 | 215 653.2 | 0.92 | |
| tEME-EA | 95,4–94,5 | 215 653.193 | 46.1 | 3.73 | † | ||
| tEME-AA | 95,4–94,5 | 215 653.509 | 46.1 | 3.73 | † | ||
| tEME-AA | 95,5–94,6 | 215 653.575 | 46.1 | 3.73 | † | ||
| tEME-EE | 85,3–84,4 | 215 656.176 | 42.7 | 3.10 | 215 656.2 | 1.16 | |
| tEME-EE’ | 85,4–84,5 | 215 656.227 | 42.7 | 3.10 | † | ||
| tEME-EE’ | 85,3–84,4 | 215 656.380 | 42.7 | 3.10 | † | ||
| tEME-EE | 85,4–84,5 | 215 656.432 | 42.7 | 3.10 | † | ||
| tEME-AE | 85,3–84,4 | 215 656.730 | 42.7 | 3.10 | † | ||
| tEME-AE | 85,4–84,5 | 215 656.781 | 42.7 | 3.10 | † | ||
| tEME-EA | 85,4–84,5 | 215 659.431 | 42.7 | 3.10 | 215 660.8 | 1.16 | |
| tEME-EA | 85,3–84,4 | 215 659.635 | 42.7 | 3.10 | † | ||
| tEME-AA | 85,3–84,4 | 215 659.972 | 42.7 | 3.10 | † | ||
| tEME-AA | 85,4–84,5 | 215 659.998 | 42.7 | 3.10 | † | ||
| tEME-EE | 75,2–74,3 | 215 660.620 | 39.6 | 2.43 | † | ||
| tEME-EE’ | 75,3–74,4 | 215 660.672 | 39.6 | 2.43 | † | ||
| tEME-EE’ | 75,2–74,3 | 215 660.825 | 39.6 | 2.43 | † | ||
| tEME-EE | 75,3–74,4 | 215 660.877 | 39.6 | 2.43 | † | ||
| tEME-AE | 75,2–74,3 | 215 661.175 | 39.6 | 2.43 | † | ||
| tEME-AE | 75,3–74,4 | 215 661.227 | 39.6 | 2.43 | † | ||
| tEME-EE | 65,1–64,2 | 215 663.523 | 36.9 | 1.72 | 215 664.6 | 1.11 | |
| tEME-EE’ | 65,2–64,3 | 215 663.575 | 36.9 | 1.72 | † | ||
| tEME-EE’ | 65,1–64,2 | 215 663.728 | 36.9 | 1.72 | † | ||
| tEME-EE | 65,2–64,3 | 215 663.780 | 36.9 | 1.72 | † | ||
| tEME-EA | 75,3–74,4 | 215 663.879 | 39.6 | 2.43 | † | ||
| tEME-AE | 65,1–64,2 | 215 664.080 | 36.9 | 1.72 | † | ||
| tEME-EA | 75,2–74,3 | 215 664.084 | 39.6 | 2.43 | † | ||
| tEME-AE | 65,2–64,3 | 215 664.131 | 36.9 | 1.72 | † | ||
| tEME-AA | 75,2–74,3 | 215 664.430 | 39.6 | 2.43 | † | ||
| tEME-AA | 75,3–74,4 | 215 664.439 | 39.6 | 2.43 | † | ||
| tEME-EE | 55,0–54,1 | 215 665.279 | 34.6 | 0.92 | † | ||
| tEME-EE’ | 55,1–54,2 | 215 665.331 | 34.6 | 0.92 | † | ||
| tEME-EE’ | 55,0–54,1 | 215 665.485 | 34.6 | 0.92 | † | ||
| tEME-EE | 55,1–54,2 | 215 665.537 | 34.6 | 0.92 | † | ||
| tEME-AE | 55,0–54,1 | 215 665.837 | 34.6 | 0.92 | † | ||
| tEME-AE | 55,1–54,2 | 215 665.889 | 34.6 | 0.92 | † | ||
| tEME-EA | 65,2–64,3 | 215 666.786 | 36.9 | 1.72 | 215 667.6 | 0.97 | CH3CH2CN |
| tEME-AE | 65,1–64,2 | 215 666.991 | 36.9 | 1.72 | † | ||
| tEME-AE | 65,1–64,2 | 215 667.342 | 36.9 | 1.72 | † | ||
| tEME-EA | 65,2–64,3 | 215 667.344 | 36.9 | 1.72 | † | ||
| tEME-AE | 55,1–54,2 | 215 668.545 | 34.6 | 0.92 | † | ||
| tEME-AA | 55,0–54,1 | 215 668.751 | 34.6 | 0.92 | † | ||
| tEME-AA | 55,0–54,1 | 215 669.103 | 34.6 | 0.92 | † | ||
| tEME-AE | 55,1–54,2 | 215 669.103 | 34.6 | 0.92 | † | ||
| tEME-EE’ | 64,3–53,3 | 216 054.535 | 26.5 | 3.73 | 216 056.4 | 0.95 | |
| tEME-EE | 64,3–53,3 | 216 054.868 | 26.5 | 3.73 | † | ||
| tEME-AE | 64,3–53,3 | 216 055.095 | 26.5 | 3.73 | † | ||
| tEME-EE | 64,2–53,2 | 216 055.759 | 26.5 | 3.73 | † | ||
| tEME-EE’ | 64,2–53,2 | 216 056.093 | 26.5 | 3.73 | † | ||
| tEME-AE | 64,2–53,2 | 216 056.319 | 26.5 | 3.73 | † | ||
| tEME-EA | 64,3–53,3 | 216 058.222 | 26.5 | 3.69 | 216 059.3† | 1.14 | |
| tEME-EA | 64,2–53,2 | 216 058.519 | 26.5 | 3.69 | † | ||
| tEME-AA | 64,3–53,2 | 216 058.597 | 26.5 | 3.73 | † | ||
| tEME-AA | 64,2–53,3 | 216 058.930 | 26.5 | 3.73 | † | ||
| tEME-EE | 123,9–112,10 | 217 007.530 | 40.5 | 4.14 | 217 007.6 | 0.49 | |
| tEME-EE’ | 123,9–112,10 | 217 007.763 | 40.5 | 4.12 | † | ||
| tEME-AE | 123,9–112,10 | 217 007.939 | 40.5 | 4.13 | † | ||
| tEME-EA | 123,9–112,10 | 217 009.762 | 40.5 | 4.16 | 217 010.6 | 0.54 | |
| tEME-AA | 123,9–112,10 | 217 010.054 | 40.5 | 4.16 | † | ||
| tEME-AA | 301,29–292,28 | 217 164.465 | 182.5 | 13.55 | 217 165.1 | 2.68 | CH3OCOH |
| tEME-EA | 301,29–292,28 | 217 164.508 | 182.5 | 13.55 | † | ||
| tEME-AE | 301,29–292,28 | 217 165.360 | 182.5 | 13.55 | † | ||
| tEME-EE | 301,29–292,28 | 217 165.402 | 182.5 | 13.55 | † | ||
| tEME-EE’ | 301,29–292,28 | 217 165.402 | 182.5 | 13.55 | † | ||
| tEME-AA | 280,28–271,27 | 217 940.650 | 154.7 | 22.59 | 217 940.7 | 1.46 | |
| tEME-EA | 280,28–271,27 | 217 940.651 | 154.7 | 22.59 | † | ||
| tEME-AE | 280,28–271,27 | 217 940.758 | 154.7 | 22.59 | † | ||
| tEME-EE | 280,28–271,27 | 217 940.759 | 154.7 | 22.59 | † | ||
| tEME-EE’ | 280,28–271,27 | 217 940.759 | 154.7 | 22.59 | † | ||
| tEME-EE’ | 222,21–211,20 | 219 893.140 | 102.2 | 8.31 | 219 893.0 | 0.86 | |
| tEME-EE | 222,21–211,20 | 219 893.140 | 102.2 | 8.31 | † | ||
| tEME-AE | 222,21–211,20 | 219 893.263 | 102.2 | 8.31 | † | ||
| tEME-EA | 222,21–211,20 | 219 894.511 | 102.2 | 8.31 | 219 894.5† | 0.66 | |
| tEME-AA | 222,21–211,20 | 219 894.635 | 102.2 | 8.31 | † | ||
| tEME-EE’ | 281,28–270,27 | 222 861.487 | 154.8 | 22.63 | 222 861.2 | 3.06 | CH3OCHO |
| tEME-EE | 281,28–270,27 | 222 861.487 | 154.8 | 22.63 | † | ||
| tEME-AE | 281,28–270,27 | 222 861.500 | 154.8 | 22.63 | † | ||
| tEME-EA | 281,28–270,27 | 222 861.655 | 154.8 | 22.63 | † | ||
| tEME-AA | 281,28–270,27 | 222 861.668 | 154.8 | 22.63 | † | ||
| tEME-EE’ | 133,11–122,10 | 222 980.574 | 45.5 | 4.38 | … | … | CH3O13COH |
| tEME-EE | 133,11–122,10 | 222 980.720 | 45.5 | 4.39 | … | … | ” |
| tEME-AE | 133,11–122,10 | 222 980.951 | 45.5 | 4.39 | … | … | ” |
| tEME-EA | 133,11–122,10 | 222 983.368 | 45.5 | 4.40 | … | … | ” |
| tEME-AA | 133,11–122,10 | 222 983.672 | 45.5 | 4.40 | … | … | ” |
| tEME-EE | 162,14–151,15 | 223 403.761 | 57.4 | 2.48 | 223 403.5 | 0.82 | CH3OCH3 |
| tEME-EE’ | 162,14–151,15 | 223 403.762 | 57.4 | 2.48 | † | ||
| tEME-AE | 162,14–151,15 | 223 404.001 | 57.4 | 2.48 | † | ||
| tEME-EA | 162,14–151,15 | 223 405.432 | 57.4 | 2.48 | … | … | CH3OCH3 |
| tEME-AA | 162,14–151,15 | 223 405.671 | 57.4 | 2.48 | … | … | |
| tEME-EE’ | 74,4–63,4 | 224 103.753 | 29.2 | 3.89 | 224 104.2 | 1.49 | |
| tEME-EE | 74,4–63,4 | 224 104.093 | 29.2 | 3.88 | † | ||
| tEME-AE | 74,4–63,4 | 224 104.315 | 29.2 | 3.89 | † | ||
| tEME-EE | 74,3–63,3 | 224 104.926 | 29.2 | 3.88 | † | ||
| tEME-EE’ | 74,3–63,3 | 224 105.266 | 29.2 | 3.89 | † | ||
| tEME-AE | 74,3–63,3 | 224 105.490 | 29.2 | 3.89 | † | ||
| tEME-AA | 74,4–63,3 | 224 107.456 | 29.2 | 3.90 | 224 108.0† | 1.12 | |
| tEME-EA | 74,4–63,4 | 224 107.546 | 29.2 | 3.58 | † | ||
| tEME-EA | 74,3–63,3 | 224 107.581 | 29.2 | 3.58 | † | ||
| tEME-AA | 74,3–63,4 | 224 108.457 | 29.2 | 3.90 | † | ||
| tEME-EE | 133,10–122,11 | 225 283.674 | 45.5 | 4.33 | 225 283.2 | 1.41 | CH3CH2OH |
| tEME-EE’ | 133,10–122,11 | 225 283.820 | 45.5 | 4.33 | † | ||
| tEME-AE | 133,10–122,11 | 225 284.043 | 45.5 | 4.33 | † | ||
| tEME-EA | 133,10–122,11 | 225 285.990 | 45.5 | 4.34 | † | ||
| tEME-AA | 133,10–122,11 | 225 286.286 | 45.5 | 4.34 | † | ||
| tEME-EE’ | 232,22–221,21 | 225 494.508 | 111.0 | 8.90 | 225 494.6 | 0.72 | |
| tEME-EE | 232,22–221,21 | 225 494.508 | 111.0 | 8.90 | † | ||
| tEME-AE | 232,22–221,21 | 225 494.625 | 111.0 | 8.90 | † | ||
| tEME-EA | 232,22–221,21 | 225 495.848 | 111.0 | 8.90 | † | ||
| tEME-AA | 232,22–221,21 | 225 495.965 | 111.0 | 8.90 | † | ||
| tEME-EA | 290,29–281,28 | 226 057.836 | 165.7 | 23.62 | 226 058.4 | 3.50 | CH3CH2OH |
| tEME-AA | 290,29–281,28 | 226 057.836 | 165.7 | 23.62 | † | ||
| tEME-EE | 290,29–281,28 | 226 057.928 | 165.7 | 23.62 | † | ||
| tEME-EE’ | 290,29–281,28 | 226 057.928 | 165.7 | 23.62 | † | ||
| tEME-AE | 290,29–281,28 | 226 057.928 | 165.7 | 23.62 | † | ||
| tEME-AA | 311,30–302,29 | 227 090.552 | 194.5 | 14.51 | … | … | CH3OH |
| tEME-EA | 311,30–302,29 | 227 090.589 | 194.5 | 14.51 | … | … | ” |
| tEME-AE | 311,30–302,29 | 227 091.390 | 194.5 | 14.51 | … | … | ” |
| tEME-EE | 311,30–302,29 | 227 091.426 | 194.5 | 14.51 | … | … | ” |
| tEME-EE’ | 311,30–302,29 | 227 091.426 | 194.5 | 14.51 | … | … | ” |
| tEME-EE’ | 291,29–280,28 | 230 291.194 | 165.8 | 23.66 | … | … | CH3OH, CH3OCOH |
| tEME-EE | 291,29–280,28 | 230 291.194 | 165.8 | 23.66 | … | … | ” |
| tEME-AE | 291,29–280,28 | 230 291.205 | 165.8 | 23.66 | … | … | ” |
| tEME-EA | 291,29–280,28 | 230 291.345 | 165.8 | 23.66 | … | … | ” |
| tEME-AA | 291,29–280,28 | 230 291.357 | 165.8 | 23.66 | … | … | ” |
| tEME-EE’ | 143,12–132,11 | 230 486.881 | 50.9 | 4.59 | 230 487.1 | 0.58 | CO |
| tEME-EE | 143,12–132,11 | 230 486.975 | 50.9 | 4.59 | † | ||
| tEME-AE | 143,12–132,11 | 230 487.227 | 50.9 | 4.59 | † | ||
| tEME-EA | 143,12–132,11 | 230 489.569 | 50.9 | 4.59 | … | … | CO |
| tEME-AA | 143,12–132,11 | 230 489.869 | 50.9 | 4.59 | … | … | ” |
| tEME-EE’ | 242,23–231,22 | 231 063.540 | 120.2 | 9.52 | … | … | OCS |
| tEME-EE | 242,23–231,22 | 231 063.540 | 120.2 | 9.52 | … | … | ” |
| tEME-AE | 242,23–231,22 | 231 063.652 | 120.2 | 9.52 | … | … | ” |
| tEME-EA | 242,23–231,22 | 231 064.846 | 120.2 | 9.52 | … | … | ” |
| tEME-EA | 242,23–231,22 | 231 064.958 | 120.2 | 9.52 | … | … | ” |
| tEME-EE’ | 84,5–73,5 | 232 151.207 | 32.3 | 4.03 | 232 151.8 | 1.16 | |
| tEME-EE | 84,5–73,5 | 232 151.590 | 32.3 | 3.98 | † | ||
| tEME-AE | 84,5–73,5 | 232 151.787 | 32.3 | 4.01 | † | ||
| tEME-EE | 84,4–73,4 | 232 152.098 | 32.3 | 3.98 | † | ||
| tEME-EE’ | 84,4–73,4 | 232 152.480 | 32.3 | 4.03 | † | ||
| tEME-EA | 84,5–73,4 | 232 152.521 | 32.3 | 1.00 | † | ||
| tEME-AE | 84,4–73,4 | 232 152.685 | 32.3 | 4.01 | † | ||
| tEME-AA | 84,5–73,4 | 232 154.033 | 32.3 | 4.08 | † | ||
| tEME-EA | 84,4–73,4 | 232 154.362 | 32.3 | 3.08 | † | ||
| tEME-EA | 84,5–73,5 | 232 155.426 | 32.3 | 3.08 | † | ||
| tEME-AA | 84.4–73.5 | 232 156.540 | 32.3 | 4.08 | … | … | CH3OCOH vt = 1 |
| tEME-EA | 84.4–73.5 | 232 157.268 | 32.3 | 1.00 | … | … | ” |
| tEME-EE | 143,11–132,12 | 233 622.462 | 51.0 | 4.51 | 233 622.5 | 2.10 | CH3CH2CN v13/v21 |
| tEME-EE’ | 143,11–132,12 | 233 622.556 | 51.0 | 4.51 | † | ||
| tEME-AE | 143,11–132,12 | 233 622.806 | 51.0 | 4.51 | † | ||
| tEME-EA | 143.11–132.12 | 233 624.824 | 51.0 | 4.51 | … | … | CH3OCOH vt = 0,1 |
| tEME-AA | 143,11–132,12 | 233 625.121 | 51.0 | 4.51 | … | … | ” |
| tEME-EA | 300,30–291.29 | 234 130.523 | 177.0 | 24.66 | … | … | CH3OCOH |
| tEME-AA | 300,30–291.29 | 234 130.524 | 177.0 | 24.66 | … | … | ” |
| tEME-EE | 300,30–291,29 | 234 130.601 | 177.0 | 24.66 | … | … | ” |
| tEME-EE’ | 300,30–291,29 | 234 130.601 | 177.0 | 24.66 | … | … | ” |
| tEME-AE | 300,30–291,29 | 234 130.602 | 177.0 | 24.66 | … | … | ” |
| tEME-EE | 172,15–161,16 | 235 247.484 | 64.0 | 2.37 | 235 247.5 | 0.49 | U-line |
| tEME-EE’ | 172,15–161,16 | 235 247.485 | 64.0 | 2.37 | † | ||
| tEME-AE | 172,15–161,16 | 235 247.731 | 64.0 | 2.37 | † | ||
| tEME-EA | 172,15–161,16 | 235 249.156 | 64.0 | 2.37 | 235 249.6 | 0.41 | U-line |
| tEME-AA | 172,15–161,16 | 235 249.403 | 64.0 | 2.37 | † | ||
| tEME-EE’ | 252,24–241,23 | 236 614.358 | 129.8 | 10.19 | 236 614.8 | 1.89 | CH3O13COH vt = 1 |
| tEME-EE | 252,24–241,23 | 236 614.358 | 129.8 | 10.19 | † | ||
| tEME-AE | 252,24–241,23 | 236 614.464 | 129.8 | 10.19 | † | ||
| tEME-EA | 252,24–241,23 | 236 615.628 | 129.8 | 10.19 | † | ||
| tEME-AA | 252,24–241,23 | 236 615.733 | 129.8 | 10.19 | † | ||
| tEME-AA | 321,31–312,30 | 236 906.877 | 206.9 | 15.50 | 236 907.6 | 0.99 | |
| tEME-EA | 321,31–312,30 | 236 906.908 | 206.9 | 15.50 | † | ||
| tEME-AE | 321,31–312,30 | 236 907.656 | 206.9 | 15.50 | † | ||
| tEME-EE | 321,31–312,30 | 236 907.687 | 206.9 | 15.50 | † | ||
| tEME-EE’ | 321,31–312,30 | 236 907.687 | 206.9 | 15.50 | † | ||
| tEME-EE’ | 301,30–290,29 | 237 763.517 | 177.1 | 24.69 | 237 763.2 | 1.76 | |
| tEME-EE | 301,30–290,29 | 237 763.517 | 177.1 | 24.69 | † | ||
| tEME-AE | 301,30–290,29 | 237 763.527 | 177.1 | 24.69 | † | ||
| tEME-EA | 301,30–290,29 | 237 763.653 | 177.1 | 24.69 | † | ||
| tEME-AA | 301,30–290,29 | 237 763.664 | 177.1 | 24.69 | † | ||
| tEME-EE’ | 153,13–142, 12 | 237 865.715 | 56.7 | 4.79 | 237 866.1 | 1.40 | |
| tEME-EE | 153,13–142,12 | 237 865.778 | 56.7 | 4.80 | † | ||
| tEME-AE | 153,13–142,12 | 237 866.042 | 56.7 | 4.80 | † | ||
| tEME-EA | 153,13–142,12 | 237 868.339 | 56.7 | 4.80 | … | … | U-line |
| tEME-AA | 153,13–142,12 | 237 868.635 | 56.7 | 4.80 | … | … | ” |
| tEME-EA | 94,6–83,5 | 240 195.817 | 35.8 | 1.64 | 240 196.7 | 2.66 | 13CH3OCOH |
| tEME-EE | 94,5–83,5 | 240 196.101 | 35.8 | 3.88 | † | ||
| tEME-EE’ | 94,6–83,6 | 240 196.396 | 35.8 | 3.88 | † | ||
| tEME-EE’ | 94,5–83,5 | 240 196.642 | 35.8 | 3.88 | † | ||
| tEME-AE | 94,5–83,5 | 240 196.777 | 35.8 | 3.88 | † | ||
| tEME-EE | 94,6–83,6 | 240 196.937 | 35.8 | 3.88 | † | ||
| tEME-AE | 94,6–83,6 | 240 197.045 | 35.8 | 3.88 | † | ||
| tEME-AA | 94.6–83,5 | 240 197.196 | 35.8 | 3.88 | † | ||
| tEME-EA | 94,5–83,5 | 240 197.654 | 35.8 | 3.88 | † | ||
| tEME-EA | 94,6–83,6 | 240 201.478 | 35.8 | 2.63 | … | … | CH3OCOH vt = 1 |
| tEME-AA | 94,5–83,6 | 240 202.719 | 35.8 | 4.28 | … | … | ” |
| tEME-EA | 94,5–83,6 | 240 203.315 | 35.8 | 1.64 | … | … | ” |
| tEME-EE | 153,12–142,13 | 242 035.318 | 56.8 | 4.68 | … | … | CH2DOH; CH2DCN |
| tEME-EE’ | 153,12–142,13 | 242 035.380 | 56.8 | 4.68 | … | … | ” |
| tEME-AE | 153,12–142,13 | 242 035.647 | 56.8 | 4.68 | … | … | ” |
| tEME-EA | 153,12–142,13 | 242 037.704 | 56.8 | 4.68 | … | … | CH2DOCOH |
| tEME-AA | 153,12–142,13 | 242 038.002 | 56.8 | 4.68 | … | … | ” |
| tEME-EE’ | 262,25–251,24 | 242 161.785 | 139.8 | 10.89 | 242 163.3 | 1.86 | |
| tEME-EE | 262.25–251,24 | 242 161.785 | 139.8 | 10.89 | † | ||
| tEME-AE | 262,25–251,24 | 242 161.884 | 139.8 | 10.89 | † | ||
| tEME-EA | 262,25–251,24 | 242 163.015 | 139.8 | 10.89 | † | ||
| tEME-AA | 262,25–251,24 | 242 163.114 | 139.8 | 10.89 | † | ||
| tEME-EA | 310,31–301,30 | 242 163.369 | 188.7 | 25.69 | † | ||
| tEME-AA | 310,31–301,30 | 242 163.371 | 188.7 | 25.69 | † | ||
| tEME-EE | 310,31–301,30 | 242 163.434 | 188.7 | 25.69 | † | ||
| tEME-EE’ | 310,31–301,30 | 242 163.434 | 188.7 | 25.69 | † | ||
| tEME-AE | 310,31–301,30 | 242 163.436 | 188.7 | 25.69 | † | ||
| tEME-EE’ | 163,14–152,13 | 245 103.550 | 62.9 | 4.99 | 245 103.4 | 0.92 | |
| tEME-EE | 163,14–152,13 | 245 103.592 | 62.9 | 4.99 | † | ||
| tEME-AE | 163,14–152,13 | 245 103.864 | 62.9 | 4.99 | † | ||
| tEME-EA | 163,14–152,13 | 245 106.133 | 62.9 | 4.99 | 245 106.4† | 1.13 | CH3OCOH vt = 2 |
| tEME-EA | 163,14–152,13 | 245 106.426 | 62.9 | 5.00 | † | ||
| tEME-EE’ | 311,31–300,30 | 245 274.088 | 188.8 | 25.71 | 245 274.0 | 2.44 | |
| tEME-EE | 311,31–300,30 | 245 274.088 | 188.8 | 25.71 | † | ||
| tEME-AE | 311,31–300,30 | 245 274.098 | 188.8 | 25.71 | † | ||
| tEME-EA | 311,31–300,30 | 245 274.211 | 188.8 | 25.71 | † | ||
| tEME-AA | 311,31–300,30 | 245 274.221 | 188.8 | 25.71 | † | ||
| tEME-AA | 331,32–322,31 | 246 605.346 | 219.6 | 16.51 | 246 605.6 | 0.92 | |
| tEME-EA | 331,32–322,31 | 246 605.372 | 219.6 | 16.51 | † | ||
| tEME-AE | 331,32–322,31 | 246 606.066 | 219.6 | 16.51 | † | ||
| tEME-EE | 331,32–322,31 | 246 606.092 | 219.6 | 16.51 | † | ||
| tEME-EE’ | 331,32–322,31 | 246 606.092 | 219.6 | 16.51 | † |
Notes. Lines of trans-CH3CH2OCH3 (tEME) ground state present in the spectral scan of Orion KL from the ALMA interferometer. Column 1 indicates the species, Col. 2 gives the transition, Col. 3 the predicted frequency, Col. 4 upper level energy, Col. 5 the line strength, Col. 6 observed frequency at the peak channel of the line (relative to a vLSR of +7.5 km s−1), Col. 7 brightness temperature at the peak channel of the line, and Col. 8 shows blends with other molecular species.
Blended with previous line.
Table A.2.
Lines of trans-CH3CH2OCH3 in 30 m data.
| Species | Transition |
Predicted frequency (MHz) |
Eupp (K) |
Sij | Observed1 frequency (MHz) |
Observed1 TMB (K) |
Model2 TMB (K) |
Blends |
|---|---|---|---|---|---|---|---|---|
| tEME-AA | 130,13–121,12 | 88 665.592 | 35.0 | 7.66 | 88 665.8 | 0.05 | 0.01 | U-line |
| tEME-EA | 130,13–121,12 | 88 665.628 | 35.0 | 7.66 | † | |||
| tEME-AE | 130,13–121,12 | 88 666.042 | 35.0 | 7.66 | † | |||
| tEME-EE | 130,13–121,12 | 88 666.078 | 35.0 | 7.66 | † | |||
| tEME-EE’ | 130,13–121,12 | 88 666.078 | 35.0 | 7.66 | † | |||
| tEME-EE’ | 101,10–90,9 | 97 575.502 | 22.0 | 6.04 | … | … | 0.01 | CH3OH |
| tEME-EE | 101,10–90,9 | 97 575.502 | 22.0 | 6.04 | † | |||
| tEME-AE | 101,10–90,9 | 97 575.556 | 22.0 | 6.04 | † | |||
| tEME-EA | 101,10–90,9 | 97 576.027 | 22.0 | 6.04 | † | |||
| tEME-AA | 101,10–90,9 | 97 576.081 | 22.0 | 6.04 | † | |||
| tEME-AA | 140,14–131,13 | 97 678.316 | 40.4 | 8.52 | … | … | 0.01 | CH3OH, CH3CH2CN v13/v21 |
| tEME-EA | 140,14–131,13 | 97 678.350 | 40.4 | 8.52 | † | |||
| tEME-AE | 140,14–131,13 | 97 678.746 | 40.4 | 8.52 | † | |||
| tEME-EE | 140,14–131,13 | 97 678.779 | 40.4 | 8.52 | † | |||
| tEME-EE’ | 140,14–131,13 | 97 678.779 | 40.4 | 8.52 | † | |||
| tEME-EE | 263,23–262,24 | 101 017.882 | 146.4 | 17.08 | … | … | 0.01 | U-line |
| tEME-EE’ | 263,23–262,24 | 101 017.884 | 146.4 | 17.08 | † | |||
| tEME-AE | 263,23–262,24 | 101 018.111 | 146.4 | 17.08 | † | |||
| tEME-EE | 253,22–252,23 | 102 684.892 | 136.2 | 16.03 | 102 684.6 | 0.07 | 0.01 | 18OCS |
| tEME-EE’ | 253,22–252,23 | 102 684.894 | 136.2 | 16.03 | † | |||
| tEME-AE | 253,22–252,23 | 102 685.127 | 136.2 | 16.03 | † | |||
| tEME-EE | 243,21–242,22 | 104 364.955 | 126.5 | 15.03 | … | … | 0.01 | CH3CH2CN |
| tEME-EE’ | 243,21–242,22 | 104 364.959 | 126.5 | 15.03 | † | |||
| tEME-AE | 243,21–242,22 | 104 365.198 | 126.5 | 15.03 | † | |||
| tEME-EE’ | 111,11–100,10 | 104 401.631 | 26.2 | 6.73 | … | … | 0.02 | CH2CHCN |
| tEME-EE | 111,11–100,10 | 104 401.631 | 26.2 | 6.73 | † | |||
| tEME-AE | 111,11–100,10 | 104 401.683 | 26.2 | 6.73 | † | |||
| tEME-EA | 111,11–100,10 | 104 402.145 | 26.2 | 6.73 | † | |||
| tEME-AA | 111,11–100,10 | 104 402.196 | 26.2 | 6.73 | † | |||
| tEME-EE | 233,20–232,21 | 106 030.362 | 117.2 | 14.09 | … | … | 0.01 | CH3OCOH |
| tEME-EE’ | 233,20–232,21 | 106 030.367 | 117.2 | 14.09 | † | |||
| tEME-AE | 233,20–232,21 | 106 030.611 | 117.2 | 14.09 | † | |||
| tEME-AA | 150,15–141,14 | 106 666.950 | 46.1 | 9.41 | … | … | 0.02 | CH3OCOH |
| tEME-EA | 150,15–141,14 | 106 666.980 | 46.1 | 9.41 | † | |||
| tEME-AE | 150,15–141,14 | 106 667.358 | 46.1 | 9.41 | † | |||
| tEME-EE | 150,15–141,14 | 106 667.388 | 46.1 | 9.41 | † | |||
| tEME-EE’ | 150,15–141,14 | 106 667.388 | 46.1 | 9.41 | † | |||
| tEME-EE | 213,18–212,19 | 109 216.897 | 99.8 | 12.35 | 109 215.1 | 0.02 | 0.01 | U-line |
| tEME-EE’ | 213,18–212,19 | 109 216.904 | 99.8 | 12.35 | † | |||
| tEME-AE | 213,18–212,19 | 109 217.159 | 99.8 | 12.35 | † | |||
| tEME-EA | 213,18–212,19 | 109 219.323 | 99.8 | 12.35 | 109 219.6 | 0.01 | 0.01 | |
| tEME-AA | 213,18–212,19 | 109 219.581 | 99.8 | 12.35 | † | |||
| tEME-EE | 203,17–202,18 | 110 694.695 | 91.6 | 11.56 | … | … | 0.02 | CH3CN v8 = 1 |
| tEME-EE’ | 203,17–202,18 | 110 694.705 | 91.6 | 11.56 | † | |||
| tEME-AE | 203,17–202,18 | 110 694.963 | 91.6 | 11.56 | † | |||
| tEME-EA | 203,17–202,18 | 110 697.132 | 91.6 | 11.56 | … | … | 0.01 | CH3CN v8 = 1 |
| tEME-AA | 203,17–202,18 | 110 697.395 | 91.6 | 11.56 | † | |||
| tEME-EE’ | 121,12–110,11 | 111 178.747 | 30.8 | 7.46 | 111 178.7 | 0.03 | 0.02 | |
| tEME-EE | 121,12–110,11 | 111 178.747 | 30.8 | 7.46 | † | |||
| tEME-AE | 121,12–110,11 | 111 178.796 | 30.8 | 7.46 | † | |||
| tEME-EA | 121,12–110,11 | 111 179.248 | 30.8 | 7.46 | † | |||
| tEME-AA | 121,12–110,11 | 111 179.297 | 30.8 | 7.46 | † | |||
| tEME-EE | 193,16–192,17 | 112 072.074 | 83.9 | 10.80 | 112 072.3 | 0.03 | 0.02 | |
| tEME-EE’ | 193,16–192,17 | 112 072.088 | 83.9 | 10.80 | † | |||
| tEME-AE | 193,16–192,17 | 112 072.349 | 83.9 | 10.80 | † | |||
| tEME-EA | 193,16–192,17 | 112 074.518 | 83.9 | 10.80 | 112 074.5 | 0.03 | 0.01 | |
| tEME-AA | 193,16–192,17 | 112 074.786 | 83.9 | 10.80 | † | |||
| tEME-EE | 183,15–182,16 | 113 336.061 | 76.5 | 10.08 | 113 336.2 | 0.02 | 0.02 | |
| tEME-EE’ | 183,15–182,16 | 113 336.081 | 76.5 | 10.08 | † | |||
| tEME-AE | 183,15–182,16 | 113 336.343 | 76.5 | 10.08 | † | |||
| tEME-EA | 183,15–182,16 | 113 338.507 | 76.5 | 10.08 | 113 338.7 | 0.03 | 0.01 | |
| tEME-AA | 183,15–182,16 | 113 338.779 | 76.5 | 10.08 | † | |||
| tEME-EE | 173,14–172,15 | 114 477.615 | 69.5 | 9.39 | 114 477.9 | 0.05 | 0.02 | U-line |
| tEME-EE’ | 173,14–172,15 | 114 477.643 | 69.5 | 9.39 | † | |||
| tEME-AE | 173,14–172,15 | 114 477.905 | 69.5 | 9.39 | † | |||
| tEME-EA | 173,14–172,15 | 114 480.057 | 69.5 | 9.39 | … | … | 0.01 | CH3COOCH3 |
| tEME-AA | 173,14–172,15 | 114 480.333 | 69.5 | 9.39 | † | |||
| tEME-AE | 201,19–192,18 | 114 717.852 | 83.4 | 6.11 | 114 718.0 | 0.06 | 0.01 | CH3CHO vt = 1 |
| tEME-EE | 201,19–192,18 | 114 717.961 | 83.4 | 6.11 | † | |||
| tEME-EE’ | 201,19–192,18 | 114 717.962 | 83.4 | 6.11 | † | |||
| tEME-EE | 163,13–162,14 | 115 491.673 | 62.9 | 8.73 | … | … | 0.02 | CH3CHO |
| tEME-EE’ | 163,13–162,14 | 115 491.713 | 62.9 | 8.73 | † | |||
| tEME-AE | 163,13–162,14 | 115 491.972 | 62.9 | 8.73 | † | |||
| tEME-EA | 163,13–162,14 | 115 494.106 | 62.9 | 8.73 | … | … | 0.02 | CH3CHO |
| tEME-AA | 163,13–162,14 | 115 494.385 | 62.9 | 8.73 | † | |||
| tEME-AA | 160,16–151,15 | 115 618.006 | 52.2 | 10.34 | 115 618.4 | 0.07 | 0.04 | |
| tEME-EA | 160,16–151,15 | 115 618.033 | 52.2 | 10.34 | † | |||
| tEME-AE | 160,16–151,15 | 115 618.391 | 52.2 | 10.34 | † | |||
| tEME-EE | 160,16–151,15 | 115 618.417 | 52.2 | 10.34 | † | |||
| tEME-EE’ | 160,16–151,15 | 115 618.417 | 52.2 | 10.34 | † | |||
| tEME-EE’ | 193,17–192,18 | 123 318.746 | 83.8 | 10.04 | 123 318.8 | 0.02 | 0.02 | |
| tEME-EE | 193,17–192,18 | 123 318.760 | 83.8 | 10.04 | † | |||
| tEME-AE | 193,17–192,18 | 123 319.043 | 83.8 | 10.04 | † | |||
| tEME-EA | 193,17–192,18 | 123 321.168 | 83.8 | 10.04 | 123 321.3 | 0.04 | 0.01 | CH3O13COH |
| tEME-AA | 193,17–192,18 | 123 321.458 | 83.8 | 10.04 | † | |||
| tEME-EE’ | 203,18–202,19 | 124 043.337 | 91.6 | 10.58 | 124 043.5 | 0.03 | 0.02 | CH3COOHvt = 1 |
| tEME-EE | 203,18–202,19 | 124 043.347 | 91.6 | 10.58 | † | |||
| tEME-AE | 203,18–202,19 | 124 043.631 | 91.6 | 10.58 | † | |||
| tEME-EA | 203,18–202,19 | 124 045.738 | 91.6 | 10.58 | 124 046.0 | 0.02 | 0.01 | |
| tEME-AA | 203,18–202,19 | 124 046.027 | 91.6 | 10.58 | † | |||
| tEME-AA | 170,17–161,16 | 124 519.803 | 58.7 | 11.29 | 124 519.5 | 0.19 | 0.05 | U-line |
| tEME-EA | 170,17–161,16 | 124 519.826 | 58.7 | 11.29 | † | |||
| tEME-AE | 170,17–161,16 | 124 520.163 | 58.7 | 11.29 | † | |||
| tEME-EE | 170,17–161,16 | 124 520.186 | 58.7 | 11.29 | † | |||
| tEME-EE’ | 170,17–161,16 | 124 520.186 | 58.7 | 11.29 | † | |||
| tEME-EE’ | 141,14–130,13 | 124 648.594 | 41.0 | 9.02 | 124 649.0 | 0.06 | 0.04 | |
| tEME-EE | 141,14–130,13 | 124 648.594 | 41.0 | 9.02 | † | |||
| tEME-AE | 141,14–130,13 | 124 648.637 | 41.0 | 9.02 | † | |||
| tEME-EA | 141,14–130,13 | 124 649.062 | 41.0 | 9.02 | † | |||
| tEME-AA | 141,14–130,13 | 124 649.106 | 41.0 | 9.02 | † | |||
| tEME-EE’ | 213,19–212,20 | 124 866.195 | 99.7 | 11.10 | … | … | 0.02 | SO2 |
| tEME-EE | 213,19–212,20 | 124 866.202 | 99.7 | 11.10 | † | |||
| tEME-AE | 213,19–212,20 | 124 866.487 | 99.7 | 11.10 | † | |||
| tEME-EA | 213,19–212,20 | 124 868.576 | 99.7 | 11.10 | … | … | 0.01 | SO2 |
| tEME-AA | 213,19–212,20 | 124 868.864 | 99.7 | 11.10 | † | |||
| tEME-AA | 211,20–202,19 | 125 001.226 | 91.6 | 6.68 | … | … | 0.01 | CH3OCOH |
| tEME-EA | 211,20–202,19 | 125 001.329 | 91.6 | 6.68 | † | |||
| tEME-AE | 211,20–202,19 | 125 002.535 | 91.6 | 6.68 | … | … | 0.02 | CH3OCOH |
| tEME-EE | 211,20–202,19 | 125 002.638 | 91.6 | 6.68 | † | |||
| tEME-EE’ | 211,20–202,19 | 125 002.638 | 91.6 | 6.68 | † | |||
| tEME-EE’ | 72,6–61,5 | 125 433.445 | 15.4 | 2.61 | … | … | 0.01 | CH3CH2CN, SO2 |
| tEME-EE | 72,6–61,5 | 125 433.472 | 15.4 | 2.61 | † | |||
| tEME-AE | 72,6–61,5 | 125 433.646 | 15.4 | 2.61 | † | |||
| tEME-EE’ | 223,20–222,21 | 125 793.386 | 108.2 | 11.61 | … | … | 0.02 | CH3CH2CN v13/v21 |
| tEME-EE | 223,20–222,21 | 125 793.392 | 108.2 | 11.61 | † | |||
| tEME-AE | 223,20–222,21 | 125 793.677 | 108.2 | 11.61 | † | |||
| tEME-EA | 223,20–222,21 | 125 795.748 | 108.2 | 11.61 | … | … | 0.01 | CH3CH2CN v13/v21 |
| tEME-AA | 223,20–222,21 | 125 796.036 | 108.2 | 11.61 | † | |||
| tEME-EE’ | 233,21–232,22 | 126 830.671 | 117.1 | 12.11 | … | … | 0.02 | HC13CCN |
| tEME-EE | 233,21–232,22 | 126 830.675 | 117.1 | 12.11 | † | |||
| tEME-AE | 233,21–232,22 | 126 830.960 | 117.1 | 12.11 | † | |||
| tEME-EA | 233,21–232,22 | 126 833.013 | 117.1 | 12.11 | … | … | 0.01 | HC13CCN |
| tEME-AA | 233,21–232,22 | 126 833.301 | 117.1 | 12.11 | † | |||
| tEME-EE’ | 243,22–242,23 | 127 983.452 | 126.4 | 12.59 | 127 983.6 | 0.07 | 0.02 | U-line |
| tEME-EE | 243,22–242,23 | 127 983.456 | 126.4 | 12.59 | † | |||
| tEME-AE | 243,22–242,23 | 127 983.741 | 126.4 | 12.59 | † | |||
| tEME-EA | 243,22–242,23 | 127 985.776 | 126.4 | 12.59 | 127 986.1 | 0.06 | 0.01 | NH2CHO v12 = 1 |
| tEME-AA | 243,22–242,23 | 127 986.063 | 126.4 | 12.59 | † | |||
| tEME-EE’ | 253,23–252,24 | 129 256.739 | 136.1 | 13.06 | 129 256.8 | 0.04 | 0.02 | |
| tEME-EE | 253,23–252,24 | 129 256.741 | 136.1 | 13.06 | † | |||
| tEME-AE | 253,23–252,24 | 129 257.027 | 136.1 | 13.06 | † | |||
| tEME-EA | 253,23–252,24 | 129 259.044 | 136.1 | 13.06 | 129 259.3 | 0.04 | 0.01 | |
| tEME-AA | 253,23–252,24 | 129 259.331 | 136.0 | 13.06 | † | |||
| tEME-EE’ | 263,24–262,25 | 130 655.101 | 146.1 | 13.52 | … | … | 0.02 | U-line |
| tEME-EE | 263,24–262,25 | 130 655.103 | 146.1 | 13.52 | † | |||
| tEME-AE | 263,24–262,25 | 130 655.389 | 146.1 | 13.52 | † | |||
| tEME-EA | 263,24–262,25 | 130 657.388 | 146.1 | 13.52 | … | … | 0.01 | U-line |
| tEME-AA | 263,24–262,25 | 130 657.674 | 146.1 | 13.52 | † | |||
| tEME-EE’ | 151,15–140,14 | 131 372.619 | 46.7 | 9.86 | 131 372.7 | 0.05 | 0.05 | |
| tEME-EE | 151,15–140,14 | 131 372.619 | 46.7 | 9.86 | † | |||
| tEME-AE | 151,15–140,14 | 131 372.660 | 46.7 | 9.86 | † | |||
| tEME-EA | 151,15–140,14 | 131 373.069 | 46.7 | 9.86 | † | |||
| tEME-AA | 151,15–140,14 | 131 373.110 | 46.7 | 9.86 | † | |||
| tEME-EE’ | 273,25–272,26 | 132 182.639 | 156.6 | 13.95 | 132 182.8 | 0.04 | 0.02 | |
| tEME-EE | 273,25–272,26 | 132 182.640 | 156.6 | 13.95 | † | |||
| tEME-AE | 273,25–272,26 | 132 182.927 | 156.6 | 13.95 | † | |||
| tEME-EA | 273,25–272,26 | 132 184.906 | 156.6 | 13.95 | 132 185.5 | 0.01 | 0.01 | |
| tEME-AA | 273,25–272,26 | 132 185.194 | 156.6 | 13.95 | † | |||
| tEME-EE’ | 82,7–71,6 | 132 547.336 | 18.5 | 2.87 | 132 547.2 | 0.02 | 0.02 | |
| tEME-EE | 82,7–71,6 | 132 547.352 | 18.5 | 2.87 | † | |||
| tEME-AE | 82,7–71,6 | 132 547.529 | 18.5 | 2.87 | † | |||
| tEME-EA | 82,7–71,6 | 132 548.982 | 18.5 | 2.87 | 132 549.0 | 0.02 | 0.01 | |
| tEME-AA | 82,7–71,6 | 132 549.167 | 18.5 | 2.87 | † | |||
| tEME-AA | 180,18–171,17 | 133 362.763 | 65.6 | 12.27 | … | … | 0.06 | O13CS, CH2CHCN v11 = 1 |
| tEME-EA | 180,18–171,17 | 133 362.784 | 65.6 | 12.27 | † | |||
| tEME-AE | 180,18–171,17 | 133 363.098 | 65.6 | 12.27 | † | |||
| tEME-EE | 180,18–171,17 | 133 363.118 | 65.6 | 12.27 | † | |||
| tEME-EE’ | 180,18–171,17 | 133 363.118 | 65.6 | 12.27 | † | |||
| tEME-EE’ | 283,26–282,27 | 133 842.950 | 167.4 | 14.37 | … | … | 0.02 | CH2DOH |
| tEME-EE | 283,26–282,27 | 133 842.951 | 167.4 | 14.37 | † | |||
| tEME-AE | 283,26–282,27 | 133 843.238 | 167.4 | 14.37 | † | |||
| tEME-EA | 283,26–282,27 | 133 845.198 | 167.4 | 14.37 | … | … | 0.01 | CH2DOH |
| tEME-AA | 283,26–282,27 | 133 845.486 | 167.4 | 14.37 | † | |||
| tEME-AA | 221,21–212,20 | 135 315.986 | 100.2 | 7.28 | 135 316.0 | 0.07 | 0.01 | U-line |
| tEME-EA | 221,21–212,20 | 135 316.082 | 100.2 | 7.28 | † | |||
| tEME-AE | 221,21–212,20 | 135 317.259 | 100.2 | 7.28 | 135 317.5 | 0.05 | 0.02 | CH3CHO vt = 1 |
| tEME-EE | 221,21–212,20 | 135 317.355 | 100.2 | 7.28 | † | |||
| tEME-EE’ | 221,21–212,20 | 135 317.355 | 100.2 | 7.28 | † | |||
| tEME-EE’ | 293,27–292,28 | 135 639.103 | 178.6 | 14.77 | … | … | 0.02 | CH3OCOH vt = 1 |
| tEME-EE | 293,27–292,28 | 135 639.104 | 178.6 | 14.77 | † | |||
| tEME-AE | 293,27–292,28 | 135 639.392 | 178.6 | 14.77 | † | |||
| tEME-EA | 293,27–292,28 | 135 641.333 | 178.6 | 14.77 | … | … | 0.01 | CH3OCOH vt = 1 |
| tEME-AA | 293,27–292,28 | 135 641.621 | 178.6 | 14.77 | † | |||
| tEME-EE’ | 303,28–302,29 | 137 573.618 | 190.2 | 15.14 | 137 573.7 | 0.04 | 0.02 | |
| tEME-EE | 303,28–302,29 | 137 573.618 | 190.2 | 15.14 | † | |||
| tEME-AE | 303,28–302,29 | 137 573.907 | 190.2 | 15.14 | † | |||
| tEME-EA | 303,28–302,29 | 137 575.829 | 190.2 | 15.14 | 137 575.9 | 0.05 | 0.02 | CH3COOCH3 |
| tEME-AA | 303,28–302,29 | 137 576.118 | 190.2 | 15.14 | † | |||
| tEME-EE’ | 161,16–150,15 | 138 109.231 | 52.7 | 10.73 | 138 109.7 | 0.06 | 0.06 | |
| tEME-EE | 161,16–150,15 | 138 109.231 | 52.7 | 10.73 | † | |||
| tEME-AE | 161,16–150,15 | 138 109.269 | 52.7 | 10.73 | † | |||
| tEME-EA | 161,16–150,15 | 138 109.661 | 52.7 | 10.73 | † | |||
| tEME-AA | 161,16–150,15 | 138 109.699 | 52.7 | 10.73 | † | |||
| tEME-EE’ | 92,8–81,7 | 139 530.181 | 22.0 | 3.15 | … | … | 0.02 | CH2DCN |
| tEME-EE | 92,8–81,7 | 139 530.191 | 22.0 | 3.15 | † | |||
| tEME-AE | 92,8–81,7 | 139 530.369 | 22.0 | 3.15 | † | |||
| tEME-EA | 92,8–81,7 | 139 531.807 | 22.0 | 3.15 | … | … | 0.01 | CH2DCN |
| tEME-AA | 92,8–81,7 | 139 531.989 | 22.0 | 3.15 | † | |||
| tEME-EE’ | 313,29–312,30 | 139 648.445 | 202.2 | 15.50 | … | … | 0.02 | CH3COCH3 |
| tEME-EE | 313,29–312,30 | 139 648.445 | 202.2 | 15.50 | † | |||
| tEME-AE | 313,29–312,30 | 139 648.735 | 202.2 | 15.50 | † | |||
| tEME-EA | 313,29–312,30 | 139 650.637 | 202.2 | 15.50 | … | … | 0.01 | CH3COCH3 |
| tEME-AA | 313,29–312,30 | 139 650.927 | 202.2 | 15.50 | † | |||
| tEME-EE | 82,6–71,7 | 140 527.950 | 18.5 | 2.43 | 140 528.1 | 0.02 | 0.02 | |
| tEME-EE’ | 82,6–71,7 | 140 527.966 | 18.5 | 2.43 | † | |||
| tEME-AE | 82,6–71,7 | 140 528.160 | 18.5 | 2.43 | † | |||
| tEME-EA | 82,6–71,7 | 140 529.589 | 18.5 | 2.43 | 140 529.6 | 0.03 | 0.01 | U-line |
| tEME-AA | 82,6–71,7 | 140 529.791 | 18.5 | 2.43 | † | |||
| tEME-EE’ | 323,30–322,31 | 141 864.954 | 214.6 | 15.83 | … | … | 0.02 | CH3COOH vt = 1 |
| tEME-EE | 323,30–322,31 | 141 864.954 | 214.6 | 15.83 | † | |||
| tEME-AE | 323,30–322,31 | 141 865.246 | 214.6 | 15.83 | † | |||
| tEME-EA | 323,30–322,31 | 141 867.127 | 214.6 | 15.83 | … | … | 0.01 | CH3COOH vt = 1 |
| tEME-AA | 323,30–322,31 | 141 867.419 | 214.6 | 15.83 | † | |||
| tEME-AA | 190,19–181,18 | 142 139.587 | 72.8 | 13.27 | 142 139.7 | 0.08 | 0.06 | |
| tEME-EA | 190,19–181,18 | 142 139.605 | 72.8 | 13.27 | † | |||
| tEME-AE | 190,19–181,18 | 142 139.896 | 72.8 | 13.27 | † | |||
| tEME-EE | 190,19–181,18 | 142 139.914 | 72.8 | 13.27 | † | |||
| tEME-EE’ | 190,19–181,18 | 142 139.914 | 72.8 | 13.27 | † | |||
| tEME-EE’ | 33,1–22,1 | 143 977.261 | 12.7 | 2.45 | 143 977.8 | 0.06 | 0.02 | U-line |
| tEME-EE | 33,1–22,1 | 143 977.759 | 12.7 | 2.41 | † | |||
| tEME-AE | 33.1–22.1 | 143 977.810 | 12.7 | 2.44 | † | |||
| tEME-EA | 33,1–22,0 | 143 979.010 | 12.7 | .72 | 143 980.0 | 0.05 | 0.02 | CH3COCH3 |
| tEME-EE | 33,0–22,0 | 143 979.276 | 12.7 | 2.41 | † | |||
| tEME-EE’ | 33,0–22,0 | 143 979.774 | 12.7 | 2.45 | † | |||
| tEME-AE | 33,0–22,0 | 143 979.832 | 12.7 | 2.44 | † | |||
| tEME-AA | 33,1–22,0 | 143 980.192 | 12.7 | 2.50 | † | |||
| tEME-EA | 33,0–22,0 | 143 980.533 | 12.7 | 1.78 | † | |||
| tEME-EE’ | 333,31–332,32 | 144 223.924 | 227.3 | 16.14 | 144 224.0 | 0.02 | 0.01 | |
| tEME-EE | 333,31–332,32 | 144 223.925 | 227.3 | 16.14 | † | |||
| tEME-AE | 333,31–332,32 | 144 224.218 | 227.3 | 16.14 | † | |||
| tEME-EE’ | 171,17–160,16 | 144 871.829 | 59.2 | 11.63 | 144 872.1 | 0.06 | 0.06 | |
| tEME-EE | 171,17–160,16 | 144 871.829 | 59.2 | 11.63 | † | |||
| tEME-AE | 171,17–160,16 | 144 871.864 | 59.2 | 11.63 | † | |||
| tEME-EA | 171,17–160,16 | 144 872.238 | 59.2 | 11.63 | † | |||
| tEME-AA | 171,17–160,16 | 144 872.273 | 59.2 | 11.63 | † | |||
| tEME-AA | 231,22–222,21 | 145 647.137 | 109.2 | 7.92 | 145 647.4 | 0.03 | 0.01 | |
| tEME-EA | 231,22–222,21 | 145 647.226 | 109.2 | 7.92 | † | |||
| tEME-AE | 231,22–222,21 | 145 648.372 | 109.2 | 7.92 | 145 648.6 | 0.03 | 0.02 | |
| tEME-EE | 231,22–222,21 | 145 648.461 | 109.2 | 7.92 | † | |||
| tEME-EE’ | 231,22–222,21 | 145 648.462 | 109.2 | 7.92 | † | |||
| tEME-EE’ | 102,9–91,8 | 146 383.619 | 25.8 | 3.44 | 146 384.0 | 0.03 | 0.02 | |
| tEME-EE | 102,9–91,8 | 146 383.626 | 25.8 | 3.44 | † | |||
| tEME-AE | 102,9–91,8 | 146 383.802 | 25.8 | 3.44 | † | |||
| tEME-EA | 102,9–91,8 | 146 385.228 | 25.8 | 3.44 | 146 385.5 | 0.03 | 0.02 | |
| tEME-AA | 102,9–91,8 | 146 385.408 | 25.8 | 3.44 | † | |||
| tEME-EE | 364,32–363,33 | 146 397.039 | 276.3 | 21.88 | … | … | 0.01 | SO2, CH3OCH3 |
| tEME-EE’ | 364,32–363,33 | 146 397.042 | 276.3 | 21.88 | † | |||
| tEME-AE | 364,32–363,33 | 146 397.310 | 276.3 | 21.88 | † | |||
| tEME-EE’ | 343,32–342,33 | 146 725.545 | 240.5 | 16.44 | 146 725.6 | 0.03 | 0.01 | SO18O |
| tEME-EE | 343,32–342,33 | 146 725.545 | 240.5 | 16.44 | † | |||
| tEME-AE | 343,32–342,33 | 146 725.840 | 240.5 | 16.44 | † | |||
| tEME-AE | 292,27–283,26 | 146 736.865 | 174.4 | 6.43 | … | … | 0.01 | U-line |
| tEME-EE | 292,27–283,26 | 146 737.046 | 174.4 | 6.43 | † | |||
| tEME-EE’ | 292,27–283,26 | 146 737.048 | 174.4 | 6.43 | † | |||
| tEME-EE | 354,31–353,32 | 148 578.427 | 262.3 | 20.95 | … | … | 0.01 | CH3OCOH vt = 1 |
| tEME-EE’ | 354,31–353,32 | 148 578.431 | 262.3 | 20.95 | † | |||
| tEME-AE | 354,31–353,32 | 148 578.707 | 262.3 | 20.95 | † | |||
| tEME-EE’ | 353,33–352,34 | 149 369.412 | 254.0 | 16.71 | … | … | 0.01 | CH3OCOH vt = 1 |
| tEME-EE | 353,33–352,34 | 149 369.412 | 254.0 | 16.71 | † | |||
| tEME-AE | 353,33–352,34 | 149 369.709 | 254.0 | 16.71 | † | |||
| tEME-EE | 92,7–81,8 | 149 921.139 | 22.0 | 2.54 | … | … | 0.02 | CH3OCOH |
| tEME-EE’ | 92,7–81,8 | 149 921.149 | 22.0 | 2.54 | † | |||
| tEME-AE | 92,7–81,8 | 149 921.348 | 22.0 | 2.54 | † | |||
| tEME-EA | 92,7–81,8 | 149 922.787 | 22.0 | 2.54 | … | … | 0.01 | CH3OCOH |
| tEME-AA | 92,7–81,8 | 149 922.992 | 22.0 | 2.54 | † | |||
| tEME-EE | 344,30–343,31 | 150 661.347 | 248.7 | 20.06 | 150 661.4 | 0.04 | 0.02 | U-line |
| tEME-EE’ | 344,30–343,31 | 150 661.353 | 248.7 | 20.06 | † | |||
| tEME-AE | 344,30–343,31 | 150 661.636 | 248.7 | 20.06 | † | |||
| tEME-EA | 344,30–343,31 | 150 664.261 | 248.7 | 20.06 | 150 664.4 | 0.03 | 0.02 | U-line |
| tEME-AA | 344,30–343,31 | 150 664.547 | 248.7 | 20.06 | † | |||
| tEME-AA | 200,20–191,19 | 150 845.281 | 80.4 | 14.28 | 150 845.4 | 0.08 | 0.09 | |
| tEME-EA | 200,20–191,19 | 150 845.296 | 80.4 | 14.28 | † | |||
| tEME-AE | 200,20–191,19 | 150 845.565 | 80.4 | 14.28 | † | |||
| tEME-EE | 200,20–191,19 | 150 845.580 | 80.4 | 14.28 | † | |||
| tEME-EE’ | 200,20–191,19 | 150 845.580 | 80.4 | 14.28 | † | |||
| tEME-EE’ | 181,18–170,17 | 151 672.109 | 66.0 | 12.56 | 151 672.4 | 0.06 | 0.09 | |
| tEME-EE | 181,18–170,17 | 151 672.109 | 66.0 | 12.56 | † | |||
| tEME-AE | 181,18–170,17 | 151 672.141 | 66.0 | 12.56 | † | |||
| tEME-EA | 181,18–170,17 | 151 672.495 | 66.0 | 12.56 | † | |||
| tEME-AA | 181,18–170,17 | 151 672.527 | 66.0 | 12.56 | † | |||
| tEME-EE’ | 112,10–101,9 | 153 109.700 | 30.1 | 3.73 | 153 109.7 | 0.05 | 0.03 | |
| tEME-EE | 112,10–101,9 | 153 109.705 | 30.1 | 3.73 | † | |||
| tEME-AE | 112,10–101,9 | 153 109.879 | 30.1 | 3.73 | † | |||
| tEME-EA | 112,10–101,9 | 153 111.294 | 30.1 | 3.73 | 153 111.4 | 0.05 | 0.02 | U-line |
| tEME-AA | 112,10–101,9 | 153 111.471 | 30.1 | 3.73 | † | |||
| tEME-EE | 324,28–323,29 | 154 467.850 | 222.8 | 18.42 | 154 468.1 | 0.02 | 0.02 | |
| tEME-EE’ | 324,28–323,29 | 154 467.860 | 222.8 | 18.42 | † | |||
| tEME-AE | 324,28–323,29 | 154 468.157 | 222.8 | 18.42 | † | |||
| tEME-AA | 241,23–232,22 | 155 980.202 | 118.5 | 8.61 | … | … | 0.03 | CH3CH2CN v12 = 1 |
| tEME-EA | 241,23–232,22 | 155 980.284 | 118.5 | 8.61 | † | |||
| tEME-AE | 241,23–232,22 | 155 981.396 | 118.5 | 8.61 | … | … | 0.03 | CH3CH2CN v12 = 1 |
| tEME-EE | 241,23–232,22 | 155 981.478 | 118.5 | 8.61 | † | |||
| tEME-EE’ | 241,23–232,22 | 155 981.479 | 118.5 | 8.61 | † | |||
| tEME-EE | 314,27–313,28 | 156 169.103 | 210.3 | 17.65 | … | … | 0.02 | CH3CH2CN |
| tEME-EE’ | 314,27–313,28 | 156 169.114 | 210.3 | 17.65 | † | |||
| tEME-AE | 314,27–313,28 | 156 169.418 | 210.3 | 17.65 | † | |||
| tEME-EE | 304,26–313,27 | 157 727.158 | 198.3 | 16.91 | 157 727.3 | 0.03 | 0.02 | |
| tEME-EE’ | 304,26–313,27 | 157 727.174 | 198.3 | 16.91 | † | |||
| tEME-AE | 304,26–313,27 | 157 727.482 | 198.3 | 16.91 | † | |||
| tEME-EE’ | 191,19–180,18 | 158 519.756 | 73.2 | 13.52 | … | … | 0.10 | CH3OCOH vt = 1, CH3COCH3 |
| tEME-EE | 191,19–180,18 | 158 519.756 | 73.2 | 13.52 | † | |||
| tEME-AE | 191,19–180,18 | 158 519.786 | 73.2 | 13.52 | † | |||
| tEME-EA | 191,19–180,18 | 158 520.119 | 73.2 | 13.52 | † | |||
| tEME-AA | 191,19–180,18 | 158 520.149 | 73.2 | 13.52 | † | |||
| tEME-EE | 294,25–293,26 | 159 140.100 | 186.7 | 16.20 | … | … | 0.03 | U-line |
| tEME-EE’ | 294,25–293,26 | 159 140.121 | 186.7 | 16.19 | † | |||
| tEME-AE | 294,25–293,26 | 159 140.432 | 186.7 | 16.20 | † | |||
| tEME-EA | 294,25–293,26 | 159 143.035 | 186.7 | 16.20 | … | … | 0.02 | U-line |
| tEME-AA | 294,25–293,26 | 159 143.357 | 186.7 | 16.20 | † | |||
| tEME-AA | 210,21–201,20 | 159 477.061 | 88.4 | 15.31 | 159 477.1 | 0.15 | 0.10 | CH2CN |
| tEME-EA | 210,21–201,20 | 159 477.074 | 88.4 | 15.31 | † | |||
| tEME-AE | 210,21–201,20 | 159 477.319 | 88.4 | 15.31 | † | |||
| tEME-EE | 210,21–201,20 | 159 477.332 | 88.4 | 15.31 | † | |||
| tEME-EE’ | 210,21–201,20 | 159 477.332 | 88.4 | 15.31 | † | |||
| tEME-EE | 102,8–91,9 | 159 548.654 | 25.9 | 2.61 | 159 548.8 | 0.09 | 0.02 | CH3COOH vt = 2 |
| tEME-EE’ | 102,8–91,9 | 159 548.661 | 25.9 | 2.61 | † | ” | ” | |
| tEME-AE | 102,8–91,9 | 159 548.866 | 25.9 | 2.61 | † | ” | ” | |
| tEME-EE’ | 122,11–111,10 | 159 710.946 | 34.7 | 4.05 | … | … | 0.03 | CH3CH2CN v12/v21 |
| tEME-EE | 122,11–111,10 | 159 710.950 | 34.7 | 4.05 | † | |||
| tEME-AE | 122,11–111,10 | 159 711.121 | 34.7 | 4.05 | † | |||
| tEME-EA | 122,11–111,10 | 159 712.526 | 34.7 | 4.05 | … | … | 0.02 | CH3CH2CN v12/v21 |
| tEME-AA | 122,11–111,10 | 159 712.699 | 34.7 | 4.05 | † | |||
| tEME-EE | 284,24–283,25 | 160 409.193 | 175.5 | 15.50 | … | … | 0.03 | CH3COCH3 |
| tEME-EE’ | 284,24–283,25 | 160 409.221 | 175.5 | 15.50 | † | |||
| tEME-AE | 284,24–283,25 | 160 409.535 | 175.5 | 15.50 | † | |||
| tEME-EA | 284,24–283,25 | 160 412.123 | 175.5 | 15.50 | … | … | 0.02 | CH3OCOH vt = 1 |
| tEME-AA | 284,24–283,25 | 160 412.450 | 175.5 | 15.50 | † | |||
| tEME-EE’ | 103,8–92,7 | 199 842.884 | 31.6 | 3.55 | 199 843.5 | … | 0.05 | CH3OCOH vt = 2 |
| tEME-EE | 103,8–92,7 | 199 843.513 | 31.6 | 3.65 | † | |||
| tEME-AE | 103,8–92,7 | 199 843.521 | 31.6 | 3.61 | † | |||
| tEME-EA | 103,8–92,7 | 199 846.703 | 31.6 | 3.78 | … | … | 0.04 | CH3OCOH vt = 1 |
| tEME-AA | 103,8–92,7 | 199 847.033 | 31.6 | 3.78 | † | |||
| tEME-EE’ | 44,1–33,1 | 199 953.072 | 22.3 | 3.50 | 199 953.5 | 0.18 | 0.06 | CH3CH2OH |
| tEME-EE | 44,1–33,1 | 199 953.406 | 22.3 | 3.50 | † | |||
| tEME-AE | 44,1–33,1 | 199 953.633 | 22.3 | 3.50 | † | |||
| tEME-EE | 44,0–33,0 | 199 954.307 | 22.3 | 3.50 | 199 954.7 | 0.12 | 0.06 | CH3CH2OH |
| tEME-EE’ | 44,0–33,0 | 199 954.642 | 22.3 | 3.50 | † | |||
| tEME-AE | 44,0–33,0 | 199 954.869 | 22.3 | 3.50 | † | |||
| tEME-EA | 44,1–33,1 | 199 956.750 | 22.3 | 3.50 | 199 957.2 | 0.19 | 0.08 | CH2CHCN v15 = 1 |
| tEME-EA | 44,0–33,0 | 199 957.085 | 22.3 | 3.50 | † | |||
| tEME-AA | 44,1–33,0 | 199 957.305 | 22.3 | 3.50 | † | |||
| tEME-AA | 44,0–33,1 | 199 957.317 | 22.3 | 3.50 | † | |||
| tEME-EE | 103,7–92,8 | 200 603.336 | 31.6 | 3.64 | … | … | 0.05 | U-line |
| tEME-AE | 103,7–92,8 | 200 603.932 | 31.6 | 3.59 | † | |||
| tEME-EE’ | 103,7–92,8 | 200 603.965 | 31.6 | 3.54 | † | |||
| tEME-EA | 103,7–92,8 | 200 605.130 | 31.6 | 3.76 | … | … | 0.04 | CH3OCOH |
| tEME-AA | 103,7–92,8 | 200 605.404 | 31.6 | 3.77 | † | |||
| tEME-EE | 142,12–131,13 | 200 820.096 | 45.3 | 2.62 | … | … | 0.04 | CH3OH |
| tEME-EE’ | 142,12–131,13 | 200 820.098 | 45.3 | 2.62 | † | |||
| tEME-AE | 142,12–131,13 | 200 820.324 | 45.3 | 2.62 | † | |||
| tEME-EA | 142,12–131,13 | 200 821.765 | 45.3 | 2.62 | … | … | 0.03 | CH3OH |
| tEME-AA | 142,12–131,13 | 200 821.991 | 45.3 | 2.62 | † | |||
| tEME-EE’ | 251,25–240,24 | 200 871.974 | 124.2 | 19.55 | 200 872.2 | 0.59 | 0.20 | CH2CHCN v11 = 2 |
| tEME-EE | 251,25–240,24 | 200 871.975 | 124.2 | 19.55 | † | |||
| tEME-AE | 251,25–240,24 | 200 871.991 | 124.2 | 19.55 | † | |||
| tEME-EA | 251,25–240,24 | 200 872.199 | 124.2 | 19.55 | † | |||
| tEME-AA | 251,25–240,24 | 200 872.216 | 124.2 | 19.55 | † | |||
| tEME-AA | 260,26–251,25 | 201 553.272 | 133.9 | 20.51 | 201 553.4 | 0.32 | 0.21 | |
| tEME-EA | 260,26–251,25 | 201 553.275 | 133.9 | 20.51 | † | |||
| tEME-AE | 260,26–251,25 | 201 553.416 | 133.9 | 20.51 | † | |||
| tEME-EE | 260,26–251,25 | 201 553.419 | 133.9 | 20.51 | † | |||
| tEME-EE’ | 260,26–251,25 | 201 553.419 | 133.9 | 20.51 | † | |||
| tEME-EE’ | 192,18–181,17 | 202 767.815 | 77.9 | 6.76 | … | … | 0.08 | H13CCCN, CH3CN v8 = 1 |
| tEME-EE | 192,18–181,17 | 202 767.815 | 77.9 | 6.76 | † | |||
| tEME-AE | 192,18–181,17 | 202 767.956 | 77.9 | 6.76 | † | |||
| tEME-EA | 192,18–181,17 | 202 769.267 | 77.9 | 6.76 | … | … | 0.05 | H13CCCN, CH3CN v8 = 1 |
| tEME-AA | 192,18–181,17 | 202 769.407 | 77.9 | 6.76 | † | |||
| tEME-AA | 291,28–282,27 | 207 138.789 | 170.9 | 12.63 | 207 139.6 | 0.07 | 0.07 | |
| tEME-EA | 291,28–282,27 | 207 138.838 | 170.9 | 12.63 | † | |||
| tEME-AE | 291,28–282,27 | 207 139.740 | 170.9 | 12.63 | † | |||
| tEME-EE | 291,28–282,27 | 207 139.789 | 170.9 | 12.63 | † | |||
| tEME-EE’ | 291,28–282,27 | 207 139.789 | 170.9 | 12.63 | † | |||
| tEME-EE’ | 113,9–102,8 | 207 643.771 | 35.9 | 3.90 | … | … | 0.07 | CH3CH2CN |
| tEME-EE | 113,9–102,8 | 207 644.152 | 35.9 | 3.94 | † | |||
| tEME-AE | 113,9–102,8 | 207 644.276 | 35.9 | 3.92 | † | |||
| tEME-EA | 113,9–102,8 | 207 647.053 | 35.9 | 3.99 | 207 647.4 | 0.20 | 0.05 | CH3CH2CN |
| tEME-AA | 113,9–102,8 | 207 647.369 | 35.9 | 3.99 | † | |||
| tEME-EE’ | 54,2–43,2 | 208 004.136 | 24.2 | 3.60 | … | … | 0.07 | CH3CH2CN, CH3CH2OH |
| tEME-EE | 54,2–43,2 | 208 004.469 | 24.2 | 3.60 | † | |||
| tEME-AE | 54,2–43,2 | 208 004.696 | 24.2 | 3.60 | † | |||
| tEME-EE | 54,1–43,1 | 208 005.368 | 24.2 | 3.60 | † | |||
| tEME-EE’ | 54,1–43,1 | 208 005.702 | 24.2 | 3.60 | † | |||
| tEME-AE | 54,1–43,1 | 208 005.929 | 24.2 | 3.60 | † | |||
| tEME-EA | 54,2–43,2 | 208 007.812 | 24.2 | 3.60 | … | … | 0.09 | CH3CH2CN, CH3CH2OH |
| tEME-EA | 54,1–43,1 | 208 008.143 | 24.2 | 3.60 | † | |||
| tEME-AA | 54,2–43,1 | 208 008.330 | 24.2 | 3.60 | † | |||
| tEME-AA | 54,1–43,2 | 208 008.413 | 24.2 | 3.60 | † | |||
| tEME-EE’ | 261,26–250,25 | 208 147.627 | 134.1 | 20.57 | 208 147.7 | 0.58 | 0.22 | CH2CHCN v11 = 1 |
| tEME-EE | 261,26–250,25 | 208 147.627 | 134.1 | 20.57 | † | |||
| tEME-AE | 261,26–250,25 | 208 147.642 | 134.1 | 20.57 | † | |||
| tEME-EA | 261,26–250,25 | 208 147.831 | 134.1 | 20.57 | † | |||
| tEME-AA | 261,26–250,25 | 208 147.846 | 134.1 | 20.57 | † | |||
| tEME-EE’ | 202,19–191,18 | 208 541.474 | 85.6 | 7.24 | 208 541.5 | 0.19 | 0.08 | |
| tEME-EE | 202,19–191,18 | 208 541.474 | 85.6 | 7.24 | † | |||
| tEME-AE | 202,19–191,18 | 208 541.609 | 85.6 | 7.24 | † | |||
| tEME-EA | 202,19–191,18 | 208 542.901 | 85.6 | 7.24 | 208 543.0 | 0.16 | 0.05 | |
| tEME-AA | 202,19–191,18 | 208 543.036 | 85.6 | 7.24 | † | |||
| tEME-EE | 113,8–102,9 | 208 783.787 | 35.9 | 3.92 | … | … | 0.07 | CH3OCOH |
| tEME-EE’ | 113,8–102,9 | 208 784.168 | 35.9 | 3.88 | † | |||
| tEME-AE | 113,8–102,9 | 208 784.265 | 35.9 | 3.90 | † | |||
| tEME-EA | 113,8–102,9 | 208 785.864 | 35.9 | 3.96 | … | … | 0.05 | CH3OCOH |
| tEME-AA | 113,8–102,9 | 208 786.150 | 35.9 | 3.96 | † | |||
| tEME-AA | 270,27–261,26 | 209 774.111 | 144.1 | 21.55 | 209 774.0 | 0.35 | 0.22 | CH2CHCN v15 = 1 |
| tEME-EA | 270,27–261,26 | 209 774.113 | 144.1 | 21.55 | † | |||
| tEME-AE | 270,27–261,26 | 209 774.236 | 144.1 | 21.55 | † | |||
| tEME-EE | 270,27–261,26 | 209 774.238 | 144.1 | 21.55 | † | |||
| tEME-EE’ | 270,27–261,26 | 209 774.238 | 144.1 | 21.55 | † | |||
| tEME-EE | 152,13–141,14 | 211 933.388 | 51.2 | 2.56 | … | … | 0.04 | CH3OCOH |
| tEME-EE’ | 152,13–141,14 | 211 933.389 | 51.2 | 2.56 | † | |||
| tEME-AE | 152,13–141,14 | 211 933.621 | 51.2 | 2.56 | † | |||
| tEME-EA | 152,13–141,14 | 211 935.057 | 51.2 | 2.56 | … | … | 0.03 | CH3OCOH |
| tEME-AA | 152,13–141,14 | 211 935.290 | 51.2 | 2.56 | † | |||
| tEME-EE | 325,27–324,28 | 212 287.353 | 232.9 | 17.12 | … | … | 0.04 | CH3COCH3 |
| tEME-EE’ | 325,27–324,28 | 212 287.576 | 232.9 | 17.07 | † | |||
| tEME-AE | 325,27–324,28 | 212 287.827 | 232.9 | 17.10 | † | |||
| tEME-EA | 325,27–324,28 | 212 290.165 | 232.9 | 17.17 | … | … | 0.03 | CH3COCH3 |
| tEME-AA | 325,27–324,28 | 212 290.528 | 232.9 | 17.17 | † | |||
| tEME-EE | 315,26–314,27 | 212 762.787 | 220.6 | 16.46 | … | … | 0.03 | CH3OCH3 |
| tEME-EE’ | 315,26–314,27 | 212 763.095 | 220.6 | 16.37 | † | |||
| tEME-AE | 315,26–314,27 | 212 763.306 | 220.6 | 16.42 | † | |||
| tEME-EA | 315,26–314,27 | 212 765.533 | 220.6 | 16.55 | 212 766.9 | 0.14 | 0.03 | CH3OCH3 |
| tEME-AA | 315,26–314,27 | 212 765.897 | 220.6 | 16.55 | † | |||
| tEME-EE | 305,25–304,26 | 213 178.397 | 208.6 | 15.77 | … | … | 0.04 | U-line |
| tEME-EE’ | 305,25–304,26 | 213 178.825 | 208.6 | 15.61 | † | |||
| tEME-AE | 305,25–304,26 | 213 178.976 | 208.6 | 15.70 | † | |||
| tEME-EA | 305,25–304,26 | 213 181.046 | 208.6 | 15.93 | 213 181.4 | 0.21 | 0.03 | CH2OHCHO |
| tEME-AA | 305,25–304,26 | 213 181.410 | 208.6 | 15.94 | † | |||
| tEME-EE’ | 335,29–334,30 | 213 495.962 | 245.7 | 17.69 | 213 495.8 | 0.09 | 0.04 | U-line |
| tEME-EE | 335,29–334,30 | 213 496.124 | 245.7 | 17.72 | † | |||
| tEME-AE | 335,29–334,30 | 213 496.418 | 245.7 | 17.71 | † | |||
| tEME-EA | 335,29–334,30 | 213 499.211 | 245.7 | 17.75 | 213 499.3 | 0.10 | 0.03 | U-line |
| tEME-AA | 335,29–334,30 | 213 499.587 | 245.7 | 17.75 | † | |||
| tEME-EE | 295,24–294,25 | 213 540.983 | 196.9 | 15.03 | 213 541.4 | 0.16 | 0.05 | CH2CHCN v11 = 2 |
| tEME-EE’ | 295,24–294,25 | 213 541.573 | 196.9 | 14.74 | † | |||
| tEME-AE | 295,24–294,25 | 213 541.643 | 196.9 | 14.89 | † | |||
| tEME-EA | 295,24–294,25 | 213 543.491 | 196.9 | 15.32 | … | … | 0.04 | CH2CHCN v11 = 2 |
| tEME-AA | 295,24–294,25 | 213 543.850 | 196.9 | 15.33 | † | |||
| tEME-EE’ | 325,28–324,29 | 213 676.308 | 232.9 | 17.03 | 213 676.5 | 0.20 | 0.04 | CH3CH2OH |
| tEME-EE | 325,28–324,29 | 213 676.530 | 232.9 | 17.09 | † | |||
| tEME-AE | 325,28–324,29 | 213 676.802 | 232.9 | 17.06 | † | |||
| tEME-EA | 325,28–324,29 | 213 679.692 | 232.9 | 17.13 | 213 680.0 | 0.14 | 0.03 | CH3COOCH3 |
| tEME-AA | 325,28–324,29 | 213 680.076 | 232.9 | 17.14 | † | |||
| tEME-EE’ | 315,27–314,28 | 213 854.674 | 220.5 | 16.34 | … | … | 0.05 | CH3CH2OH |
| tEME-EE | 315,27–314,28 | 213 854.982 | 220.5 | 16.44 | † | |||
| tEME-AE | 315,27–314,28 | 213 855.220 | 220.5 | 16.39 | † | |||
| tEME-EE | 285,23–284,24 | 213 856.708 | 185.7 | 14.18 | … | … | 0.05 | CH3CH2OH |
| tEME-AE | 285,23–284,24 | 213 857.473 | 185.7 | 13.94 | † | |||
| tEME-EE’ | 285,23–284,24 | 213 857.509 | 185.7 | 13.70 | † | |||
| tEME-EA | 315,27–314,28 | 213 858.239 | 220.5 | 16.52 | … | … | 0.05 | CH3CH2OH |
| tEME-AA | 315,27–314,28 | 213 858.632 | 220.5 | 16.53 | † | |||
| tEME-EA | 285,23–284,24 | 213 859.015 | 185.7 | 14.70 | † | |||
| tEME-AA | 285,23–284,24 | 213 859.367 | 185.7 | 14.73 | † | |||
| tEME-EE’ | 305,26–304,27 | 214 028.729 | 208.6 | 15.59 | … | … | 0.05 | CH3COCH3 |
| tEME-EE | 305,26–304,27 | 214 029.157 | 208.6 | 15.76 | † | |||
| tEME-AE | 305,26–304,27 | 214 029.345 | 208.6 | 15.68 | † | |||
| tEME-EA | 305,26–304,27 | 214 032.539 | 208.6 | 15.91 | … | … | 0.04 | |
| tEME-AA | 305,26–304,27 | 214 032.943 | 208.6 | 15.92 | † | |||
| tEME-EE | 275,22–274,23 | 214 131.118 | 174.9 | 13.15 | … | … | 0.04 | CH3CH213CN, CH3COOH vt = 1 |
| tEME-AE | 275,22–274,23 | 214 132.010 | 174.9 | 12.80 | † | |||
| tEME-EE’ | 275,22–274,23 | 214 132.166 | 174.9 | 12.45 | † | |||
| tEME-EA | 275,22–274,23 | 214 133.150 | 174.9 | 14.07 | … | … | 0.04 | CH3CH213CN, CH3COOH vt = 1 |
| tEME-AA | 275,22–274,23 | 214 133.486 | 174.9 | 14.14 | † | |||
| tEME-EE’ | 295,25–294,25 | 214 196.531 | 196.9 | 14.72 | … | … | 0.05 | CH313CH2CN, CH3O13COH vt = 1 |
| tEME-EE | 295,25–294,25 | 214 197.122 | 196.9 | 15.02 | † | |||
| tEME-AE | 295,25–294,25 | 214 197.239 | 196.9 | 14.88 | † | |||
| tEME-EA | 295,25–294,25 | 214 200.673 | 196.9 | 15.30 | … | … | 0.04 | CH313CH2CN, CH3O13COH vt = 1 |
| tEME-AA | 295,25–294,25 | 214 201.091 | 196.9 | 15.32 | † | |||
| tEME-EE’ | 212,20–201,19 | 214 246.202 | 93.7 | 7.76 | 214 246.2 | 0.15 | 0.09 | 13CH3CN |
| tEME-EE | 212,20–201,19 | 214 246.202 | 93.7 | 7.76 | † | |||
| tEME-AE | 212,20–201,19 | 214 246.332 | 93.7 | 7.76 | † | |||
| tEME-EA | 212,20–201,19 | 214 247.602 | 93.7 | 7.76 | 214 247.7 | 0.11 | 0.06 | 13CH3CN |
| tEME-AA | 212,20–201,19 | 214 247.732 | 93.7 | 7.76 | † | |||
| tEME-EE’ | 265,22–264,22 | 214 355.828 | 164.4 | 2.47 | … | … | 0.05 | SO, 13CH3CN |
| tEME-EE’ | 285,24–284,25 | 214 356.510 | 185.7 | 13.69 | † | |||
| tEME-AE | 265,22–264,22 | 214 356.963 | 164.4 | 2.08 | † | |||
| tEME-EE | 265,22–264,22 | 214 357.232 | 164.4 | 1.63 | † | |||
| tEME-EE | 285,24–284,25 | 214 357.312 | 185.7 | 14.17 | † | |||
| tEME-AE | 285,24–284,25 | 214 357.333 | 185.7 | 13.93 | † | |||
| tEME-EA | 285,24–284,25 | 214 361.091 | 185.7 | 14.69 | … | … | 0.04 | SO, 13CH3CN |
| tEME-AA | 285,24–284,25 | 214 361.527 | 185.7 | 14.72 | † | |||
| tEME-EE | 265,21–264,22 | 214 369.161 | 164.4 | 11.92 | … | … | 0.07 | SO, 13CH3CN |
| tEME-AE | 265,21–264,22 | 214 370.185 | 164.4 | 11.47 | † | |||
| tEME-EE’ | 265,21–264,22 | 214 370.456 | 164.4 | 11.08 | † | |||
| tEME-EA | 265,21–264,22 | 214 370.843 | 164.4 | 13.42 | † | |||
| tEME-AA | 265,21–264,22 | 214 371.154 | 164.4 | 13.55 | † | |||
| tEME-EE’ | 275,23–274,24 | 214 507.462 | 174.9 | 12.45 | … | … | 0.04 | CH3CH2CN |
| tEME-AE | 275,23–274,24 | 214 508.414 | 174.9 | 12.79 | † | |||
| tEME-EE | 275,23–274,24 | 214 508.510 | 174.9 | 13.15 | † | |||
| tEME-EA | 275,23–274,24 | 214 512.591 | 174.9 | 14.07 | … | … | 0.04 | CH3CH2CN |
| tEME-AA | 275,23–274,24 | 214 513.051 | 174.9 | 14.13 | † | |||
| tEME-EE | 255,20–254,21 | 214 575.213 | 154.4 | 10.55 | … | … | 0.09 | 13C17O |
| tEME-AE | 255,20–254,21 | 214 576.349 | 154.4 | 10.10 | † | |||
| tEME-EA | 255,20–254,21 | 214 576.498 | 154.4 | 12.69 | † | |||
| tEME-EE’ | 255,20–254,21 | 214 576.706 | 154.4 | 9.74 | † | |||
| tEME-AA | 255,20–254,21 | 214 576.769 | 154.4 | 12.96 | † | |||
| tEME-EE’ | 265,22–264,23 | 214 648.535 | 164.4 | 11.08 | … | … | 0.04 | CH3OCOH |
| tEME-AE | 265,22–264,23 | 214 649.611 | 164.4 | 11.47 | † | |||
| tEME-EE | 265,22–264,23 | 214 649.829 | 164.4 | 11.92 | † | |||
| tEME-EA | 265,22–264,23 | 214 654.288 | 164.5 | 13.41 | … | … | 0.04 | CH3OCOH |
| tEME-AA | 265,22–264,23 | 214 654.781 | 164.5 | 13.54 | † | |||
| tEME-EE | 245,19–244,20 | 214 753.118 | 144.7 | 9.23 | 214 754.3 | 0.20 | 0.09 | |
| tEME-EA | 245,19–244,20 | 214 754.023 | 144.7 | 11.84 | † | |||
| tEME-AA | 245,19–244,20 | 214 754.233 | 144.7 | 12.38 | † | |||
| tEME-AE | 245,19–244,20 | 214 754.329 | 144.7 | 8.86 | † | |||
| tEME-EE’ | 245,19–244,20 | 214 754.733 | 144.7 | 8.60 | † | |||
| tEME-EE’ | 255,21–254,22 | 214 779.208 | 154.4 | 9.74 | … | … | 0.04 | CH3OCOH |
| tEME-AE | 255,21–254,22 | 214 780.378 | 154.4 | 10.10 | † | |||
| tEME-EE | 255,21–254,22 | 214 780.701 | 154.4 | 10.55 | † | |||
| tEME-EA | 255,21–254,22 | 214 785.581 | 154.4 | 12.69 | … | … | 0.04 | CH3OCOH |
| tEME-AA | 255,21–254,22 | 214 786.123 | 154.4 | 12.96 | † | |||
| tEME-EE’ | 245,20–244,21 | 214 899.228 | 144.7 | 8.61 | … | … | 0.04 | CH3OCOH vt = 1 |
| tEME-AE | 245,20–244,21 | 214 900.454 | 144.7 | 8.87 | † | |||
| tEME-EE | 245,20–244,21 | 214 900.844 | 144.7 | 9.23 | † | |||
| tEME-EA | 245,20–244,21 | 214 906.128 | 144.7 | 11.83 | … | … | 0.11 | CH3CH2CN |
| tEME-EE | 235,18–234,19 | 214 906.260 | 135.4 | 8.13 | † | |||
| tEME-AA | 245,20–244,21 | 214 906.739 | 144.7 | 12.38 | † | |||
| tEME-EA | 235,18–234,19 | 214 906.872 | 135.4 | 10.76 | † | |||
| tEME-AA | 235,18–234,19 | 214 906.992 | 135.4 | 11.80 | † | |||
| tEME-AE | 235,18–234,19 | 214 907.507 | 135.4 | 7.91 | † | |||
| tEME-EE’ | 235,18–234,19 | 214 907.924 | 135.4 | 7.77 | † | |||
| tEME-EE | 245,19–244,21 | 214 909.858 | 144.7 | 3.15 | † | |||
| tEME-EA | 245,19–244,21 | 214 910.548 | 144.7 | 0.55 | † | |||
| tEME-AE | 245,19–244,21 | 214 911.166 | 144.7 | 3.51 | † | |||
| tEME-EE’ | 245,19–244,21 | 214 911.685 | 144.7 | 3.77 | † | |||
| tEME-EE’ | 235,19–234,20 | 215 008.545 | 135.4 | 7.77 | … | … | 0.02 | CH3CH2CN v13/v21 |
| tEME-AE | 235,19–234,20 | 215 009.791 | 135.4 | 7.91 | … | … | 0.04 | CH3CH2CN v13/v21 |
| tEME-EE | 235,19–234,20 | 215 010.209 | 135.4 | 8.13 | † | |||
| tEME-EA | 235,19–234,20 | 215 015.810 | 135.4 | 10.75 | 215 016.7 | 0.05 | 0.04 | |
| tEME-AA | 235,19–234,20 | 215 016.519 | 135.4 | 11.80 | † | |||
| tEME-EA | 225,18–224,18 | 215 035.544 | 126.6 | 1.78 | … | … | 0.07 | CH3CH2CN |
| tEME-EE | 225,17–224,18 | 215 037.639 | 126.6 | 7.33 | † | |||
| tEME-EA | 225,17–224,18 | 215 038.085 | 126.6 | 9.45 | † | |||
| tEME-AA | 225,17–224,18 | 215 038.089 | 126.6 | 11.23 | † | |||
| tEME-AE | 225,17–224,18 | 215 038.890 | 126.6 | 7.24 | † | |||
| tEME-EE’ | 225,17–224,18 | 215 039.292 | 126.6 | 7.21 | † | |||
| tEME-AA | 352,33–343,32 | 215 107.148 | 250.8 | 9.27 | … | … | 0.03 | CH3CH2CN |
| tEME-EA | 352,33–343,32 | 215 107.270 | 250.8 | 9.27 | † | |||
| tEME-EE’ | 225,18–224,19 | 215 107.270 | 126.6 | 7.21 | † | |||
| tEME-AE | 225,18–224,19 | 215 108.508 | 126.6 | 7.24 | † | |||
| tEME-EE | 225,18–224,19 | 215 108.923 | 126.6 | 7.33 | … | … | 0.05 | CH3CH2CN |
| tEME-AE | 352,33–343,32 | 215 109.184 | 250.8 | 9.27 | † | |||
| tEME-EE | 352,33–343,32 | 215 109.306 | 250.8 | 9.27 | † | |||
| tEME-EE’ | 352,33–343,32 | 215 109.306 | 250.8 | 9.27 | † | |||
| tEME-EA | 225,18–224,19 | 215 114.713 | 126.6 | 9.45 | … | … | 0.04 | CH3CH2CN |
| tEME-AA | 225,18–224,19 | 215 115.545 | 126.6 | 11.23 | † | |||
| tEME-EE | 225,17–224,19 | 215 117.232 | 126.6 | 3.90 | † | |||
| tEME-EA | 225,17–224,19 | 215 117.254 | 126.6 | 1.78 | † | |||
| tEME-AE | 225,17–224,19 | 215 118.679 | 126.6 | 3.99 | † | |||
| tEME-EE’ | 225,17–224,19 | 215 119.312 | 126.6 | 4.02 | † | |||
| tEME-EA | 215,17–214,17 | 215 148.139 | 118.0 | 2.58 | 215 150.3 | 0.25 | 0.07 | CH3COCH3 |
| tEME-EE | 215,16–214,17 | 215 149.914 | 118.0 | 6.81 | † | |||
| tEME-AA | 215,16–214,17 | 215 150.202 | 118.0 | 10.66 | † | |||
| tEME-EA | 215,16–214,17 | 215 150.327 | 118.0 | 8.07 | † | |||
| tEME-AE | 215,16–214,17 | 215 151.142 | 118.0 | 6.84 | † | |||
| tEME-EE’ | 215,16–214,17 | 215 151.503 | 118.0 | 6.91 | † | |||
| tEME-EE’ | 215,17–214,18 | 215 195.665 | 118.0 | 6.91 | … | … | 0.04 | SO |
| tEME-AE | 215,17–214,18 | 215 196.869 | 118.0 | 6.84 | † | |||
| tEME-EE | 215,17–214,18 | 215 197.254 | 118.0 | 6.81 | † | |||
| tEME-EA | 215,17–214,18 | 215 203.100 | 118.0 | 8.07 | … | … | 0.04 | SO |
| tEME-AA | 215,17–214,18 | 215 204.068 | 118.0 | 10.66 | † | |||
| tEME-EA | 215,16–214,18 | 215 205.287 | 118.0 | 2.58 | † | |||
| tEME-EE | 215,16–214,18 | 215 205.486 | 118.0 | 3.85 | † | |||
| tEME-EA | 205,16–204,16 | 215 243.901 | 109.9 | 3.19 | … | … | 0.07 | SO |
| tEME-EE | 205,15–204,16 | 215 245.438 | 109.9 | 6.54 | † | |||
| tEME-AA | 205,15–204,16 | 215 245.691 | 109.9 | 10.09 | † | |||
| tEME-EA | 205,15–204,16 | 215 245.934 | 109.9 | 6.89 | † | |||
| tEME-AE | 205,15–204,16 | 215 246.615 | 109.9 | 6.68 | † | |||
| tEME-EE’ | 205,15–204,16 | 215 246.907 | 109.9 | 6.83 | † | |||
| tEME-EE’ | 205,16–204,17 | 215 274.126 | 109.9 | 6.83 | … | … | 0.04 | CH3CH2CN v20 = 1 |
| tEME-AE | 205,16–204,17 | 215 275.268 | 109.9 | 6.68 | † | |||
| tEME-EE | 205,16–204,17 | 215 275.595 | 109.9 | 6.54 | † | |||
| tEME-EA | 205,16–204,17 | 215 281.378 | 109.9 | 6.89 | … | … | 0.04 | CH3CH2CN v13/v21 |
| tEME-AA | 205,16–204,17 | 215 282.470 | 109.9 | 10.09 | † | |||
| tEME-EA | 205,15–204,17 | 215 283.411 | 109.9 | 3.19 | † | |||
| tEME-EE | 205,15–204,17 | 215 283.810 | 109.9 | 3.55 | † | |||
| tEME-EA | 195,15–194,15 | 215 324.992 | 102.2 | 3.49 | 215 326.9 | 0.20 | 0.08 | CH3OCOH |
| tEME-EE | 195,14–194,15 | 215 326.280 | 102.2 | 6.50 | † | |||
| tEME-AA | 195,14–194,15 | 215 326.637 | 102.2 | 9.52 | † | |||
| tEME-EA | 195,14–194,15 | 215 326.971 | 102.2 | 6.03 | † | |||
| tEME-AE | 195,14–194,15 | 215 327.373 | 102.2 | 6.72 | † | |||
| tEME-EE’ | 195,14–194,15 | 215 327.561 | 102.2 | 6.95 | † | |||
| tEME-EE’ | 195,15–194,16 | 215 343.169 | 102.2 | 6.95 | 215 344.3 | 0.20 | 0.05 | CH3OCOH vt = 1 |
| tEME-AE | 195,15–194,16 | 215 344.214 | 102.2 | 6.72 | † | |||
| tEME-EE | 195,15–194,16 | 215 344.450 | 102.2 | 6.50 | † | |||
| tEME-EA | 195,15–194,16 | 215 350.059 | 102.2 | 6.03 | … | … | 0.05 | CH3CH2CN |
| tEME-AA | 195,15–194,16 | 215 351.248 | 102.2 | 9.52 | † | |||
| tEME-EE’ | 123,10–112,9 | 215 361.252 | 40.5 | 4.16 | … | … | 0.07 | CH3CH2CN v13/v21 |
| tEME-EE | 123,10–112,9 | 215 361.484 | 40.5 | 4.18 | † | |||
| tEME-AE | 123,10–112,9 | 215 361.676 | 40.5 | 4.17 | † | |||
| tEME-EA | 123,10–112,9 | 215 364.224 | 40.5 | 4.19 | … | … | 0.05 | CH3CH2CN v13/v21 |
| tEME-AA | 123,10–112,9 | 215 364.533 | 40.5 | 4.20 | † | |||
| tEME-EE | 185,13–184,14 | 215 394.256 | 94.8 | 6.65 | … | … | 0.11 | CH3CH2CN |
| tEME-AA | 185,13–184,14 | 215 394.875 | 94.8 | 8.95 | † | |||
| tEME-AE | 185,13–184,14 | 215 395.227 | 94.8 | 6.94 | † | |||
| tEME-EA | 185,13–184,14 | 215 395.264 | 94.8 | 5.47 | † | |||
| tEME-EE’ | 185,13–184,14 | 215 395.280 | 94.8 | 7.19 | † | |||
| tEME-EE’ | 185,14–184,15 | 215 403.394 | 94.8 | 7.19 | … | … | 0.06 | CH3CH2CN |
| tEME-AE | 185,14–184,15 | 215 404.308 | 94.8 | 6.94 | † | |||
| tEME-EE | 185,14–184,15 | 215 404.417 | 94.8 | 6.65 | † | |||
| tEME-EA | 185,14–184,15 | 215 409.728 | 94.8 | 5.47 | … | … | 0.09 | CH3CH2CN v = 0; v13/v21 |
| tEME-AA | 185,14–184,15 | 215 410.979 | 94.8 | 8.95 | † | |||
| tEME-EA | 175,13–174,13 | 215 450.467 | 87.9 | 3.22 | 215 451.8 | 0.47 | 0.11 | CH3CH2CN v20 = 1, NH2CHO v12 = 1 |
| tEME-EE | 175,13–174,13 | 215 450.974 | 87.9 | 6.90 | † | |||
| tEME-EE’ | 175,13–174,13 | 215 451.703 | 87.9 | 7.39 | † | |||
| tEME-AE | 175,13–174,13 | 215 451.799 | 87.9 | 7.18 | † | |||
| tEME-AA | 175,13–174,13 | 215 452.025 | 87.9 | 8.39 | † | |||
| tEME-EA | 175,13–174,13 | 215 452.436 | 87.9 | 5.17 | † | |||
| tEME-EE’ | 175,13–174,13 | 215 455.422 | 87.9 | 7.39 | … | … | 0.07 | CH3CH2CN v13/v21, CH3CH2OH |
| tEME-EE | 175,13–174,13 | 215 456.151 | 87.9 | 6.90 | † | |||
| tEME-AE | 175,13–174,13 | 215 456.193 | 87.9 | 7.18 | † | |||
| tEME-EA | 175,13–174,14 | 215 461.025 | 87.9 | 5.17 | … | … | 0.04 | CH3CH2CN v13/v21, CH3CH2OH |
| tEME-AA | 175,13–174,14 | 215 462.304 | 87.9 | 8.39 | † | |||
| tEME-EA | 175,12–174,14 | 215 462.994 | 87.9 | 3.22 | † | |||
| tEME-EE’ | 271,27–260,26 | 215 478.840 | 144.2 | 21.60 | … | … | 0.24 | CH3CH2CN v13/v21 |
| tEME-EE | 271,27–260,26 | 215 478.840 | 144.2 | 21.60 | † | |||
| tEME-AE | 271,27–260,26 | 215 478.854 | 144.2 | 21.60 | † | |||
| tEME-EA | 271,27–260,26 | 215 479.025 | 144.2 | 21.60 | † | |||
| tEME-AA | 271,27–260,26 | 215 479.039 | 144.2 | 21.60 | † | |||
| tEME-EE | 165,11–164,12 | 215 497.897 | 81.3 | 7.05 | … | … | 0.09 | CH3OCH3, CH3CH2CN v13/v21 |
| tEME-EA | 165,12–164,12 | 215 497.943 | 81.3 | 2.74 | † | |||
| tEME-EE’ | 165,11–164,12 | 215 498.372 | 81.3 | 7.37 | † | |||
| tEME-AE | 165,11–164,12 | 215 498.590 | 81.3 | 7.24 | † | |||
| tEME-AA | 165,11–164,12 | 215 499.524 | 81.3 | 7.82 | … | … | 0.11 | CH3OCH3, CH3CH2CN v13/v21 |
| tEME-EE’ | 165,12–164,13 | 215 499.830 | 81.3 | 7.37 | † | |||
| tEME-EA | 165,11–164,12 | 215 499.921 | 81.3 | 5.08 | † | |||
| tEME-EE | 165,12–164,13 | 215 500.305 | 81.3 | 7.05 | † | |||
| tEME-AE | 165,12–164,13 | 215 500.485 | 81.3 | 7.24 | † | |||
| tEME-EA | 165,11–164,12 | 215 504.636 | 81.3 | 5.08 | … | … | 0.05 | CH3OCH3, CH3CH2CN v13/v21, 33SH2 |
| tEME-AA | 165,12–164,13 | 215 505.905 | 81.3 | 7.82 | † | |||
| tEME-EA | 165,12–164,13 | 215 506.613 | 81.3 | 2.74 | † | |||
| tEME-EE | 155,10–154,11 | 215 536.404 | 75.1 | 6.94 | 215 536.9 | 0.25 | 0.15 | CH3OCOH vt = 1 |
| tEME-EE’ | 155,10–154,11 | 215 536.720 | 75.1 | 7.08 | † | |||
| tEME-EA | 155,11–154,11 | 215 537.002 | 75.1 | 2.05 | † | |||
| tEME-AE | 155,10–154,11 | 215 537.010 | 75.1 | 7.03 | † | |||
| tEME-EE’ | 155,11–154,12 | 215 537.169 | 75.1 | 7.08 | † | |||
| tEME-EE | 155,11–154,12 | 215 537.485 | 75.1 | 6.94 | † | |||
| tEME-AE | 155,11–154,12 | 215 537.757 | 75.1 | 7.03 | † | |||
| tEME-AA | 155,10–154,11 | 215 538.650 | 75.1 | 7.25 | … | … | 0.04 | CH3OCOH vt = 1, CH3CH2CN v13/v21 |
| tEME-EA | 155,10–154,11 | 215 538.990 | 75.1 | 5.21 | † | |||
| tEME-AA | 155,10–154,11 | 215 538.650 | 75.1 | 7.25 | † | |||
| tEME-EA | 155,10–154,11 | 215 538.990 | 75.1 | 5.21 | † | |||
| tEME-EA | 155,11–154,12 | 215 541.270 | 75.1 | 5.21 | † | |||
| tEME-AA | 155,11–154,12 | 215 542.488 | 75.1 | 7.25 | † | |||
| tEME-EE | 145,9–144,10 | 215 567.750 | 69.3 | 6.58 | … | … | 0.17 | CH3CH2CN v20 = 1 |
| tEME-EE’ | 145,9–144,10 | 215 567.993 | 69.3 | 6.63 | † | |||
| tEME-EE’ | 145,10–144,11 | 215 568.043 | 69.3 | 6.63 | † | |||
| tEME-EE | 145,10–144,11 | 215 568.286 | 69.3 | 6.58 | † | |||
| tEME-AE | 145,9–144,10 | 215 568.316 | 69.3 | 6.61 | † | |||
| tEME-AE | 145,10–144,11 | 215 568.602 | 69.3 | 6.61 | † | |||
| tEME-EA | 145,10–144,10 | 215 568.774 | 69.3 | 1.23 | † | |||
| tEME-AA | 145,9–144,10 | 215 570.539 | 69.3 | 6.68 | … | … | 0.06 | CH3CH2CN v13/v21 |
| tEME-EA | 145,9–144,10 | 215 570.772 | 69.3 | 5.45 | † | |||
| tEME-EA | 145,10–144,11 | 215 571.650 | 69.3 | 5.45 | † | |||
| tEME-AA | 145,10–144,11 | 215 572.766 | 69.3 | 6.68 | … | … | 0.04 | CH3CH2CN v13/v21 |
| tEME-EA | 145,9–144,11 | 215 573.648 | 69.3 | 1.23 | † | |||
| tEME-EE | 135,8–134,9 | 215 593.014 | 63.9 | 6.08 | … | … | 0.17 | CH3CH2CN v20 = 1, SiO v = 1 |
| tEME-EE’ | 135,9–134,10 | 215 593.138 | 63.9 | 6.09 | † | |||
| tEME-EE’ | 135,8–134,9 | 215 593.228 | 63.9 | 6.09 | † | |||
| tEME-EE | 135,9–134,10 | 215 593.353 | 63.9 | 6.08 | † | |||
| tEME-AE | 135,8–134,9 | 215 593.566 | 63.9 | 6.09 | † | |||
| tEME-AE | 135,9–134,10 | 215 593.687 | 63.9 | 6.09 | † | |||
| tEME-AA | 135,8–134,9 | 215 596.207 | 63.9 | 6.11 | … | … | 0.09 | CH3CH213CN, SiO v = 1 |
| tEME-EA | 135,8–134,9 | 215 596.289 | 63.9 | 5.58 | † | |||
| tEME-EA | 135,9–134,10 | 215 596.478 | 63.9 | 5.58 | † | |||
| tEME-EE | 125,7–124,8 | 215 613.102 | 58.9 | 5.52 | … | … | 0.18 | CH3CH2OH, CH3OCOH vt = 1, CH3CH2CN |
| tEME-EE’ | 125,8–124,9 | 215 613.172 | 58.9 | 5.53 | † | |||
| tEME-EE’ | 125,7–124,8 | 215 613.308 | 58.9 | 5.53 | † | |||
| tEME-EE | 125,8–124,9 | 215 613.378 | 58.9 | 5.52 | † | |||
| tEME-AE | 125,7–124,8 | 215 613.651 | 58.9 | 5.52 | † | |||
| tEME-AE | 125,8–124,9 | 215 613.720 | 58.9 | 5.52 | † | |||
| tEME-EA | 125,8–124,9 | 215 616.405 | 58.9 | 5.37 | … | … | 0.10 | CH3CH2CN |
| tEME-EA | 125,7–124,8 | 215 616.490 | 58.9 | 5.37 | † | |||
| tEME-AA | 125,7–124,8 | 215 616.564 | 58.9 | 5.53 | † | |||
| tEME-AA | 125,8–124,9 | 215 617.221 | 58.9 | 5.53 | † | |||
| tEME-EE | 115,6–114,7 | 215 628.800 | 54.3 | 4.94 | … | … | 0.16 | CH3CH2CN |
| tEME-EE’ | 115,7–114,8 | 215 628.855 | 54.3 | 4.94 | † | |||
| tEME-EE’ | 115,6–114,7 | 215 629.003 | 54.3 | 4.94 | † | |||
| tEME-EE | 115,7–114,8 | 215 629.058 | 54.3 | 4.94 | † | |||
| tEME-AE | 115,6–114,7 | 215 629.349 | 54.3 | 4.94 | † | |||
| tEME-AE | 115,7–114,8 | 215 629.404 | 54.3 | 4.94 | … | … | 0.10 | CH3CH2CN |
| tEME-EA | 115,7–114,8 | 215 632.056 | 54.3 | 4.91 | † | |||
| tEME-EA | 115,6–114,7 | 215 632.229 | 54.3 | 4.91 | † | |||
| tEME-AA | 115,6–114,7 | 215 632.425 | 54.3 | 4.94 | † | |||
| tEME-AA | 115,7–114,8 | 215 632.754 | 54.3 | 4.94 | † | |||
| tEME-EE | 105,5–104,6 | 215 640.803 | 50.0 | 4.35 | 215 641.1 | 0.18 | 0.13 | CH3COOH |
| tEME-EE’ | 105,6–104,7 | 215 640.854 | 50.0 | 4.35 | † | |||
| tEME-EE’ | 105,5–104,6 | 215 641.007 | 50.0 | 4.35 | † | |||
| tEME-EE | 105,6–104,7 | 215 641.058 | 50.0 | 4.35 | † | |||
| tEME-AE | 105,5–104,6 | 215 641.354 | 50.0 | 4.35 | † | |||
| tEME-AE | 105,6–104,7 | 215 641.405 | 50.0 | 4.35 | † | |||
| tEME-EA | 105,6–104,7 | 215 644.051 | 50.0 | 4.34 | 215 644.4 | 0.21 | 0.10 | CH3COOH |
| tEME-EA | 105,5–104,6 | 215 644.248 | 50.0 | 4.34 | † | |||
| tEME-AA | 105,5–104,6 | 215 644.522 | 50.0 | 4.35 | † | |||
| tEME-AA | 105,6–104,7 | 215 644.676 | 50.0 | 4.35 | † | |||
| tEME-EE | 95,4–94,5 | 215 649.740 | 46.1 | 3.73 | 215 650.1 | 0.42 | 0.12 | CH3CH2CN v20 = 1 |
| tEME-EE’ | 95,5–94,6 | 215 649.791 | 46.1 | 3.73 | † | |||
| tEME-EE’ | 95,4–94,5 | 215 649.944 | 46.1 | 3.73 | † | |||
| tEME-EE | 95,5–94,6 | 215 649.995 | 46.1 | 3.73 | † | |||
| tEME-AE | 95,4–94,5 | 215 650.292 | 46.1 | 3.73 | † | |||
| tEME-AE | 95,5–94,6 | 215 650.343 | 46.1 | 3.73 | † | |||
| tEME-EA | 95,5–94,6 | 215 652.990 | 46.1 | 3.73 | 215 653.4 | 0.42 | 0.08 | CH3CH2CN v20 = 1 |
| tEME-EA | 95,4–94,5 | 215 653.193 | 46.1 | 3.73 | † | |||
| tEME-AA | 95,4–94,5 | 215 653.509 | 46.1 | 3.73 | † | |||
| tEME-AA | 95,5–94,6 | 215 653.575 | 46.1 | 3.73 | † | |||
| tEME-EE | 85,3–84,4 | 215 656.176 | 42.7 | 3.10 | 215 656.4 | 0.43 | 0.11 | CH3CH2CN v20 = 1 |
| tEME-EE’ | 85,4–84,5 | 215 656.227 | 42.7 | 3.10 | † | |||
| tEME-EE’ | 85,3–84,4 | 215 656.380 | 42.7 | 3.10 | † | |||
| tEME-EE | 85,4–84,5 | 215 656.432 | 42.7 | 3.10 | † | |||
| tEME-AE | 85,3–84,4 | 215 656.730 | 42.7 | 3.10 | † | |||
| tEME-AE | 85,4–84,5 | 215 656.781 | 42.7 | 3.10 | † | |||
| tEME-EA | 85,4–84,5 | 215 659.431 | 42.7 | 3.10 | 215 659.8 | 0.36 | 0.09 | CH3CH2CN v20 = 1 |
| tEME-EA | 85,3–84,4 | 215 659.635 | 42.7 | 3.10 | † | |||
| tEME-AA | 85,3–84,4 | 215 659.972 | 42.7 | 3.10 | † | |||
| tEME-AA | 85,4–84,5 | 215 659.998 | 42.7 | 3.10 | † | |||
| tEME-EE | 75,2–74,3 | 215 660.620 | 39.6 | 2.43 | 215 660.8 | 0.34 | 0.11 | CH3CH2CN v20 = 1 |
| tEME-EE’ | 75,3–74,4 | 215 660.672 | 39.6 | 2.43 | † | |||
| tEME-EE’ | 75,2–74,3 | 215 660.825 | 39.6 | 2.43 | † | |||
| tEME-EE | 75,3–74,4 | 215 660.877 | 39.6 | 2.43 | † | |||
| tEME-AE | 75,2–74,3 | 215 661.175 | 39.6 | 2.43 | † | |||
| tEME-AE | 75,3–74,4 | 215 661.227 | 39.6 | 2.43 | † | |||
| tEME-EE | 65,1–64,2 | 215 663.523 | 36.9 | 1.72 | 215 663.9 | 0.30 | 0.11 | CH3CH2CN v20 = 1 |
| tEME-EE’ | 65,2–64,3 | 215 663.575 | 36.9 | 1.72 | † | |||
| tEME-EE’ | 65,1–64,2 | 215 663.728 | 36.9 | 1.72 | † | |||
| tEME-EE | 65,2–64,3 | 215 663.780 | 36.9 | 1.72 | † | |||
| tEME-EA | 75,3–74,4 | 215 663.879 | 39.6 | 2.43 | † | |||
| tEME-AE | 65,1–64,2 | 215 664.080 | 36.9 | 1.72 | † | |||
| tEME-EA | 75,2–74,3 | 215 664.084 | 39.6 | 2.43 | † | |||
| tEME-AE | 65,2–64,3 | 215 664.131 | 36.9 | 1.72 | † | |||
| tEME-AA | 75,2–74,3 | 215 664.430 | 39.6 | 2.43 | † | |||
| tEME-AA | 75,3–74,4 | 215 664.439 | 39.6 | 2.43 | † | |||
| tEME-EE | 55,0–54,1 | 215 665.279 | 34.6 | 0.92 | † | |||
| tEME-EE’ | 55,1–54,2 | 215 665.331 | 34.6 | 0.92 | † | |||
| tEME-EE’ | 55,0–54,1 | 215 665.485 | 34.6 | 0.92 | † | |||
| tEME-EE | 55,1–54,2 | 215 665.537 | 34.6 | 0.92 | † | |||
| tEME-AE | 55,0–54,1 | 215 665.837 | 34.6 | 0.92 | † | |||
| tEME-AE | 55,1–54,2 | 215 665.889 | 34.6 | 0.92 | † | |||
| tEME-EA | 65,2–64,2 | 215 666.786 | 36.9 | 1.72 | … | … | 0.05 | CH3CH2CN v = 0, v20 = 1 |
| tEME-EA | 65,1–64,2 | 215 666.991 | 36.9 | 1.72 | † | |||
| tEME-EA | 55,1–54,2 | 215 668.545 | 34.6 | 0.92 | † | |||
| tEME-EA | 55,0–54,1 | 215 668.751 | 34.6 | 0.92 | † | |||
| tEME-AA | 55,0–54,1 | 215 669.103 | 34.6 | 0.92 | † | |||
| tEME-AA | 55,1–54,2 | 215 669.103 | 34.6 | 0.92 | † | |||
| tEME-EE’ | 64,3–53,3 | 216 054.535 | 26.5 | 3.73 | 216 054.9 | 0.30 | 0.08 | CH3CH2CN v13/v21 |
| tEME-EE | 64,3–53,3 | 216 054.868 | 26.5 | 3.73 | † | |||
| tEME-AE | 64,3–53,3 | 216 055.095 | 26.5 | 3.73 | † | |||
| tEME-EE | 64,2–53,2 | 216 055.759 | 26.5 | 3.73 | † | |||
| tEME-EE’ | 64,2–53,2 | 216 056.093 | 26.5 | 3.73 | 216 056.4 | 0.26 | 0.08 | CH3CH2CN v13/v21 |
| tEME-AE | 64,2–53,2 | 216 056.319 | 26.5 | 3.73 | † | |||
| tEME-EA | 64,3–53,3 | 216 058.222 | 26.5 | 3.69 | 216 058.4 | 0.25 | 0.10 | CH3CH2CN v13/v21 |
| tEME-EA | 64,2–53,2 | 216 058.519 | 26.5 | 3.69 | † | |||
| tEME-AA | 64,3–53,2 | 216 058.597 | 26.5 | 3.73 | † | |||
| tEME-AA | 64,2–53,3 | 216 058.930 | 26.5 | 3.73 | † | |||
| tEME-EE | 123,9–112,10 | 217 007.530 | 40.5 | 4.14 | 217 007.7 | 0.13 | 0.08 | HDCS, CH3OCOH |
| tEME-EE’ | 123,9–112,10 | 217 007.763 | 40.5 | 4.12 | † | |||
| tEME-AE | 123,9–112,10 | 217 007.939 | 40.5 | 4.13 | † | |||
| tEME-EA | 123,9–112,10 | 217 009.762 | 40.5 | 4.16 | 217 010.0 | 0.13 | 0.05 | HDCS, CH3OCOH |
| tEME-AA | 123,9–112,10 | 217 010.054 | 40.5 | 4.16 | † | |||
| tEME-AA | 301,29–292,28 | 217 164.465 | 182.5 | 13.55 | … | … | 0.07 | CH3OCOH |
| tEME-EA | 301,29–292,28 | 217 164.508 | 182.5 | 13.55 | † | |||
| tEME-AE | 301,29–292,28 | 217 165.360 | 182.5 | 13.55 | † | |||
| tEME-EE | 301,29–292,28 | 217 165.402 | 182.5 | 13.55 | † | |||
| tEME-EE’ | 301,29–292,28 | 217 165.402 | 182.5 | 13.55 | † | |||
| tEME-AA | 280,28–271,27 | 217 940.650 | 154.7 | 22.59 | 217 940.7 | 0.44 | 0.25 | c-C3H2, HCC13CN v7 = 1,CH3COOCH3 |
| tEME-EA | 280,28–271,27 | 217 940.651 | 154.7 | 22.59 | † | |||
| tEME-AE | 280,28–271,27 | 217 940.758 | 154.7 | 22.59 | † | |||
| tEME-EE | 280,28–271,27 | 217 940.759 | 154.7 | 22.59 | † | |||
| tEME-EE’ | 280,28–271,27 | 217 940.759 | 154.7 | 22.59 | † | |||
| tEME-EE’ | 222,21–211,20 | 219 893.140 | 102.2 | 8.31 | … | … | 0.09 | SO |
| tEME-EE | 222,21–211,20 | 219 893.140 | 102.2 | 8.31 | † | |||
| tEME-AE | 222,21–211,20 | 219 893.263 | 102.2 | 8.31 | † | |||
| tEME-EA | 222,21–211,20 | 219 894.511 | 102.2 | 8.31 | … | … | 0.06 | SO |
| tEME-AA | 222,21–211,20 | 219 894.635 | 102.2 | 8.31 | † | |||
| tEME-EE’ | 281,28–270,27 | 222 861.487 | 154.8 | 22.63 | 222 861.3 | 0.55 | 0.26 | CH3OCOH |
| tEME-EE | 281,28–270,27 | 222 861.487 | 154.8 | 22.63 | † | |||
| tEME-AE | 281,28–270,27 | 222 861.500 | 154.8 | 22.63 | † | |||
| tEME-EA | 281,28–270,27 | 222 861.655 | 154.8 | 22.63 | † | |||
| tEME-AA | 281,28–270,27 | 222 861.668 | 154.8 | 22.63 | † | |||
| tEME-EE’ | 133,11–122,10 | 222 980.574 | 45.5 | 4.38 | 222 980.7 | 0.22 | 0.09 | CH3O13COH |
| tEME-EE | 133,11–122,10 | 222 980.720 | 45.5 | 4.39 | † | |||
| tEME-AE | 133,11–122,10 | 222 980.951 | 45.5 | 4.39 | † | |||
| tEME-EA | 133,11–122,10 | 222 983.368 | 45.5 | 4.40 | 222 983.7 | 0.16 | 0.06 | CH3O13COH |
| tEME-AA | 133,11–122,10 | 222 983.672 | 45.5 | 4.40 | † | |||
| tEME-EE | 162,14–151,15 | 223 403.761 | 57.4 | 2.48 | … | … | 0.04 | CH3OCH3, CH3OCOH vt = 1 |
| tEME-EE’ | 162,14–151,15 | 223 403.762 | 57.4 | 2.48 | † | |||
| tEME-AE | 162,14–151,15 | 223 404.001 | 57.4 | 2.48 | † | |||
| tEME-EA | 162,14–151,15 | 223 405.432 | 57.4 | 2.48 | … | … | 0.03 | CH3OCH3 |
| tEME-AA | 162,14–151,15 | 223 405.671 | 57.4 | 2.48 | † | |||
| tEME-EE’ | 74,4–63,4 | 224 103.753 | 29.2 | 3.89 | 224 103.98 | 0.63 | 0.10 | CH3CH2CN v13/v21 |
| tEME-EE | 74,4–63,4 | 224 104.093 | 29.2 | 3.88 | † | |||
| tEME-AE | 74,4–63,4 | 224 104.315 | 29.2 | 3.89 | † | |||
| tEME-EE | 74,3–63,3 | 224 104.926 | 29.2 | 3.88 | † | |||
| tEME-EE’ | 74,3–63,3 | 224 105.266 | 29.2 | 3.89 | 224 105.2 | 0.47 | 0.10 | CH3CH2CN v20 = 1 |
| tEME-AE | 74,3–63,3 | 224 105.490 | 29.2 | 3.89 | † | |||
| tEME-AA | 74,4–63,3 | 224 107.456 | 29.2 | 3.90 | 224 107.7 | 0.52 | 0.10 | CH3CH2CN v20 = 1 |
| tEME-EA | 74,4–63,4 | 224 107.546 | 29.2 | 3.58 | † | |||
| tEME-EA | 74,3–63,3 | 224 107.581 | 29.2 | 3.58 | † | |||
| tEME-AA | 74,3–63,4 | 224 108.457 | 29.2 | 3.90 | † | |||
| tEME-EE | 133,10–122,11 | 225 283.674 | 45.5 | 4.33 | … | … | 0.09 | CH3CH2OH |
| tEME-EE’ | 133,10–122,11 | 225 283.820 | 45.5 | 4.33 | † | |||
| tEME-AE | 133,10–122,11 | 225 284.043 | 45.5 | 4.33 | † | |||
| tEME-EA | 133,10–122,11 | 225 285.990 | 45.5 | 4.34 | 225 286.28 | 0.29 | 0.06 | CH3CH2OH |
| tEME-AA | 133,10–122,11 | 225 286.286 | 45.5 | 4.34 | † | |||
| tEME-EE’ | 232,22–221,21 | 225 494.508 | 111.0 | 8.90 | 225 494.4 | 0.12 | 0.10 | |
| tEME-EE | 232,22–221,21 | 225 494.508 | 111.0 | 8.90 | † | |||
| tEME-AE | 232,22–221,21 | 225 494.625 | 111.0 | 8.90 | † | |||
| tEME-EA | 232,22–221,21 | 225 495.848 | 111.0 | 8.90 | 225 495.9 | 0.12 | 0.07 | |
| tEME-AA | 232,22–221,21 | 225 495.965 | 111.0 | 8.90 | † | |||
| tEME-EA | 290,29–281,28 | 226 057.836 | 165.7 | 23.62 | 226 057.9 | 0.65 | 0.26 | CH3CH2OH |
| tEME-AA | 290,29–281,28 | 226 057.836 | 165.7 | 23.62 | † | |||
| tEME-EE | 290,29–281,28 | 226 057.928 | 165.7 | 23.62 | † | |||
| tEME-EE’ | 290,29–281,28 | 226 057.928 | 165.7 | 23.62 | † | |||
| tEME-AE | 290,29–281,28 | 226 057.928 | 165.7 | 23.62 | † | |||
| tEME-AA | 311,30–302,29 | 227 090.552 | 194.5 | 14.51 | … | … | 0.09 | CH3OH |
| tEME-EA | 311,30–302,29 | 227 090.589 | 194.5 | 14.51 | † | |||
| tEME-AE | 311,30–302,29 | 227 091.390 | 194.5 | 14.51 | † | |||
| tEME-EE | 311,30–302,29 | 227 091.426 | 194.5 | 14.51 | † | |||
| tEME-EE’ | 311,30–302,29 | 227 091.426 | 194.5 | 14.51 | † | |||
| tEME-EE’ | 291,29–280,28 | 230 291.194 | 165.8 | 23.66 | … | … | 0.27 | CH3OH |
| tEME-EE | 291,29–280,28 | 230 291.194 | 165.8 | 23.66 | † | |||
| tEME-AE | 291,29–280,28 | 230 291.205 | 165.8 | 23.66 | † | |||
| tEME-EA | 291,29–280,28 | 230 291.345 | 165.8 | 23.66 | † | |||
| tEME-AA | 291,29–280,28 | 230 291.357 | 165.8 | 23.66 | † | |||
| tEME-EE’ | 143,12–132,11 | 230 486.881 | 50.9 | 4.59 | … | … | 0.09 | CO |
| tEME-EE | 143,12–132,11 | 230 486.975 | 50.9 | 4.60 | † | |||
| tEME-AE | 143,12–132,11 | 230 487.227 | 50.9 | 4.59 | † | |||
| tEME-EA | 143,12–132,11 | 230 489.569 | 50.9 | 4.60 | … | … | 0.07 | CO |
| tEME-AA | 143,12–132,11 | 230 489.869 | 50.9 | 4.60 | † | |||
| tEME-EE’ | 242,23–231,22 | 231 063.540 | 120.2 | 9.52 | … | … | 0.11 | OCS |
| tEME-EE | 242,23–231,22 | 231 063.540 | 120.2 | 9.52 | † | |||
| tEME-AE | 242,23–231,22 | 231 063.652 | 120.2 | 9.52 | † | |||
| tEME-EA | 242,23–231,22 | 231 064.846 | 120.2 | 9.52 | … | … | 0.08 | OCS |
| tEME-AA | 242,23–231,22 | 231 064.958 | 120.2 | 9.52 | † | |||
| tEME-EE’ | 84,5–73,5 | 232 151.207 | 32.3 | 4.03 | 232 152.1 | 0.47 | 0.11 | 13CH3CN,CH3OCOH vt =1 |
| tEME-EE | 84,5–73,5 | 232 151.590 | 32.3 | 3.98 | † | |||
| tEME-AE | 84,5–73,5 | 232 151.787 | 32.3 | 4.01 | † | |||
| tEME-EE | 84,4–73,4 | 232 152.098 | 32.3 | 3.98 | † | |||
| tEME-EE’ | 84,4–73,4 | 232 152.480 | 32.3 | 4.03 | † | |||
| tEME-EA | 84,5–73,4 | 232 152.521 | 32.3 | 1.00 | † | |||
| tEME-AE | 84,4–73,4 | 232 152.685 | 32.3 | 4.01 | † | |||
| tEME-AA | 84,5–73,4 | 232 154.033 | 32.3 | 4.08 | … | … | 0.06 | 13CH3CN,CH3OCOH vt =1 |
| tEME-EA | 84,4–73,4 | 232 154.362 | 32.3 | 3.08 | † | |||
| tEME-EA | 84,5–73,5 | 232 155.426 | 32.3 | 3.08 | † | |||
| tEME-AA | 84,4–73,5 | 232 156.540 | 32.3 | 4.08 | … | … | 0.04 | 13CH3CN,CH3OCOH vt =1 |
| tEME-EA | 84,4–73,5 | 232 157.268 | 32.3 | 1.00 | † | |||
| tEME-EE | 143,11–132,12 | 233 622.462 | 51.0 | 4.51 | … | … | 0.10 | CH3OCOH vt = 0,1 |
| tEME-EE’ | 143,11–132,12 | 233 622.556 | 51.0 | 4.51 | † | |||
| tEME-AE | 143,11–132,12 | 233 622.806 | 51.0 | 4.51 | † | |||
| tEME-EA | 143,11–132,12 | 233 624.824 | 51.0 | 4.52 | … | … | 0.06 | CH3OCOH vt = 0,1 |
| tEME-AA | 143,11–132,12 | 233 625.121 | 51.0 | 4.52 | † | |||
| tEME-EA | 300,30–291,29 | 234 130.523 | 177.0 | 24.66 | … | … | 0.27 | CH3CH2CN v13/v21 |
| tEME-AA | 300,30–291,29 | 234 130.524 | 177.0 | 24.66 | † | |||
| tEME-EE | 300,30–291,29 | 234 130.601 | 177.0 | 24.66 | † | |||
| tEME-EE’ | 300,30–291,29 | 234 130.601 | 177.0 | 24.66 | † | |||
| tEME-AE | 300,30–291,29 | 234 130.602 | 177.0 | 24.66 | † | |||
| tEME-EE | 172,15–161,16 | 235 247.484 | 64.0 | 2.37 | 235 247.6 | 0.09 | 0.05 | |
| tEME-EE’ | 172,15–161,16 | 235 247.485 | 64.0 | 2.37 | † | |||
| tEME-AE | 172,15–161,16 | 235 247.731 | 64.0 | 2.37 | † | |||
| tEME-EA | 172,15–161,16 | 235 249.156 | 64.0 | 2.37 | 235 249.6 | 0.14 | 0.03 | U-line |
| tEME-AA | 172,15–161,16 | 235 249.403 | 64.0 | 2.37 | † | |||
| tEME-EE’ | 252,24–241,23 | 236 614.358 | 129.8 | 10.19 | 236 614.4 | 0.30 | 0.11 | CH3COOCH3 |
| tEME-EE | 252,24–241,23 | 236 614.358 | 129.8 | 10.19 | † | |||
| tEME-AE | 252,24–241,23 | 236 614.464 | 129.8 | 10.19 | † | |||
| tEME-EA | 252,24–241,23 | 236 615.628 | 129.8 | 10.19 | † | 0.08 | CH3COOCH3 | |
| tEME-AA | 252,24–241,23 | 236 615.733 | 129.8 | 10.19 | † | |||
| tEME-AA | 321,31–312,30 | 236 906.877 | 206.9 | 15.50 | 236 907.2 | 0.30 | 0.10 | CH3SH, HC3N v6 = 1 |
| tEME-EA | 321,31–312,30 | 236 906.908 | 206.9 | 15.50 | † | |||
| tEME-AE | 321,31–312,30 | 236 907.656 | 206.9 | 15.50 | † | |||
| tEME-EE | 321,31–312,30 | 236 907.687 | 206.9 | 15.50 | † | |||
| tEME-EE’ | 321,31–312,30 | 236 907.687 | 206.9 | 15.50 | † | |||
| tEME-EE’ | 301,30–290,29 | 237 763.517 | 177.1 | 24.69 | 237 763.6 | 0.48 | 0.28 | CH2CHCN |
| tEME-EE | 301,30–290,29 | 237 763.517 | 177.1 | 24.69 | † | |||
| tEME-AE | 301,30–290,29 | 237 763.527 | 177.1 | 24.69 | † | |||
| tEME-EA | 301,30–290,29 | 237 763.653 | 177.1 | 24.69 | † | |||
| tEME-AA | 301,30–290,29 | 237 763.664 | 177.1 | 24.69 | † | |||
| tEME-EE’ | 153,13–142,12 | 237 865.715 | 56.7 | 4.79 | … | … | 0.10 | CH2CHCN |
| tEME-EE | 153,13–142,12 | 237 865.778 | 56.7 | 4.80 | † | |||
| tEME-AE | 153,13–142,12 | 237 866.042 | 56.7 | 4.80 | † | |||
| tEME-EA | 153,13–142,12 | 237 868.339 | 56.7 | 4.80 | … | … | 0.08 | CH2CHCN |
| tEME-AA | 153,13–142,12 | 237 868.635 | 56.7 | 4.80 | † | |||
| tEME-EA | 94,6–83,5 | 240 195.817 | 35.8 | 1.64 | … | … | 0.10 | CH2CHCN v15 = 1 |
| tEME-EE | 94,5–83,5 | 240 196.101 | 35.8 | 3.88 | † | |||
| tEME-EE’ | 94,6–83,6 | 240 196.396 | 35.8 | 3.88 | † | |||
| tEME-EE’ | 94,5–83,5 | 240 196.642 | 35.8 | 3.88 | † | |||
| tEME-AE | 94,5–83,5 | 240 196.777 | 35.8 | 3.88 | † | |||
| tEME-EE | 94,6–83,6 | 240 196.937 | 35.8 | 3.88 | † | |||
| tEME-AE | 94,6–83,6 | 240 197.045 | 35.8 | 3.88 | † | |||
| tEME-AA | 94,6–83,5 | 240 197.196 | 35.8 | 3.88 | † | |||
| tEME-EA | 94,5–83,5 | 240 197.654 | 35.8 | 3.88 | † | |||
| tEME-EA | 94,6–83,6 | 240 201.478 | 35.8 | 2.63 | … | … | 0.05 | CH2CHCN v15 = 1 |
| tEME-AA | 94,5–83,6 | 240 202.719 | 35.8 | 4.28 | † | |||
| tEME-EA | 94,5–83,6 | 240 203.315 | 35.8 | 1.64 | † | |||
| tEME-EE | 153,12–142,13 | 242 035.318 | 56.8 | 4.68 | … | … | 0.10 | CH2DCN |
| tEME-EE’ | 153,12–142,13 | 242 035.380 | 56.8 | 4.68 | † | |||
| tEME-AE | 153,12–142,13 | 242 035.647 | 56.8 | 4.68 | † | |||
| tEME-EA | 153,12–142,13 | 242 037.704 | 56.8 | 4.68 | 0.07 | CH2DCN | ||
| tEME-EA | 153,12–142,13 | 242 038.002 | 56.8 | 4.68 | † | |||
| tEME-EE’ | 262,25–251,24 | 242 161.785 | 139.8 | 10.89 | … | … | 0.32 | CH3CH2CN v20 = 1 |
| tEME-EE | 262,25–251,24 | 242 161.785 | 139.8 | 10.89 | † | |||
| tEME-AE | 262,25–251,24 | 242 161.884 | 139.8 | 10.89 | † | |||
| tEME-EA | 262,25–251,24 | 242 163.015 | 139.8 | 10.89 | † | |||
| tEME-AA | 262,25–251,24 | 242 163.114 | 139.8 | 10.89 | † | |||
| tEME-EA | 310,31–301,30 | 242 163.369 | 188.7 | 25.69 | † | |||
| tEME-AA | 310,31–301,30 | 242 163.371 | 188.7 | 25.69 | † | |||
| tEME-EE | 310,31–301,30 | 242 163.434 | 188.7 | 25.69 | † | |||
| tEME-EE’ | 310,31–301,30 | 242 163.434 | 188.7 | 25.69 | † | |||
| tEME-AE | 310,31–301,30 | 242 163.436 | 188.7 | 25.69 | † | |||
| tEME-EE’ | 163,14–152,13 | 245 103.550 | 62.9 | 4.99 | … | … | 0.10 | CH3CH213CN |
| tEME-EE | 163,14–152,13 | 245 103.592 | 62.9 | 4.99 | † | |||
| tEME-AE | 163,14–152,13 | 245 103.864 | 62.9 | 4.99 | † | |||
| tEME-EA | 163,14–152,13 | 245 106.133 | 62.9 | 4.99 | 245 106.4 | 0.32 | 0.07 | CH2OHCHO |
| tEME-AA | 163,14–152,13 | 245 106.426 | 62.9 | 5.00 | † | |||
| tEME-EE’ | 311,31–300,30 | 245 274.088 | 188.8 | 25.71 | 245 274.3 | 0.40 | 0.29 | 34SO2 |
| tEME-EE | 311,31–300,30 | 245 274.088 | 188.8 | 25.71 | † | |||
| tEME-AE | 311,31–300,30 | 245 274.098 | 188.8 | 25.71 | † | |||
| tEME-EA | 311,31–300,30 | 245 274.211 | 188.8 | 25.71 | † | |||
| tEME-AA | 311,31–300,30 | 245 274.221 | 188.8 | 25.71 | † | |||
| tEME-AA | 331,32–322,31 | 246 605.346 | 219.6 | 16.51 | 246 606.0 | 0.36 | 0.11 | CH3OCH3 |
| tEME-EA | 331,32–322,31 | 246 605.372 | 219.6 | 16.51 | † | |||
| tEME-AE | 331,32–322,31 | 246 606.066 | 219.6 | 16.51 | † | |||
| tEME-EE | 331,32–322,31 | 246 606.092 | 219.6 | 16.51 | † | |||
| tEME-EE’ | 331,32–322,31 | 246 606.092 | 219.6 | 16.51 | † | |||
| tEME-EE | 182,16–171,17 | 247 478.246 | 71.1 | 2.26 | 247 478.0 | 0.16 | 0.05 | CH3OCOH vt = 1 |
| tEME-EE’ | 182,16–171,17 | 247 478.247 | 71.1 | 2.26 | † | |||
| tEME-AE | 182,16–171,17 | 247 478.501 | 71.1 | 2.26 | † | |||
| tEME-EA | 182,16–171,17 | 247 479.920 | 71.1 | 2.26 | 247 480.0 | 0.16 | 0.03 | CH3OCOH vt = 1 |
| tEME-AA | 182,16–171,17 | 247 480.175 | 71.1 | 2.26 | † | |||
| tEME-EE’ | 272,26–261,25 | 247 721.097 | 150.2 | 11.63 | 247 721.2 | 0.23 | 0.11 | |
| tEME-EE | 272,26–261,25 | 247 721.097 | 150.2 | 11.63 | † | |||
| tEME-AE | 272,26–261,25 | 247 721.190 | 150.2 | 11.63 | † | |||
| tEME-EA | 272,26–261,25 | 247 722.285 | 150.2 | 11.63 | † | |||
| tEME-AA | 272,26–261,25 | 247 722.378 | 150.2 | 11.63 | † | |||
| tEME-EA | 104,7–93,6 | 248 234.223 | 39.7 | 2.12 | 248 235.7 | 0.31 | 0.15 | U-line |
| tEME-EE | 104,6–93,6 | 248 235.232 | 39.7 | 3.50 | † | |||
| tEME-AA | 104,7–93,6 | 248 235.506 | 39.7 | 4.48 | † | |||
| tEME-EA | 104,6–93,6 | 248 236.059 | 39.7 | 2.36 | † | |||
| tEME-AE | 104,6–93,6 | 248 236.076 | 39.7 | 3.66 | † | |||
| tEME-EE’ | 104,6–93,6 | 248 236.093 | 39.7 | 3.79 | † | |||
| tEME-EE’ | 104,7–93,7 | 248 239.115 | 39.7 | 3.79 | 248 239.8 | 0.07 | 0.09 | U-line |
| tEME-AE | 104,7–93,7 | 248 239.913 | 39.7 | 3.66 | † | |||
| tEME-EE | 104,7–93,7 | 248 239.977 | 39.7 | 3.50 | † | |||
| tEME-EA | 104,7–93,7 | 248 245.234 | 39.7 | 2.36 | … | … | 0.06 | CH3COCH3 |
| tEME-AA | 104,6–93,7 | 248 246.569 | 39.7 | 4.48 | † | |||
| tEME-EA | 104,6–93,7 | 248 247.070 | 39.7 | 2.12 | † | |||
| tEME-EE | 104,6–93,7 | 248 248.602 | 39.7 | 0.98 | † | |||
| tEME-AE | 104,6–93,7 | 248 250.368 | 39.7 | 0.82 | † | |||
| tEME-EE’ | 104,6–93,7 | 248 251.399 | 39.7 | 0.69 | † | |||
| tEME-EA | 320,32–311,31 | 250 160.753 | 200.8 | 26.72 | … | … | 0.29 | 34SO2 |
| tEME-AA | 320,32–311,31 | 250 160.756 | 200.8 | 26.72 | † | |||
| tEME-EE | 320,32–311,31 | 250 160.807 | 200.8 | 26.72 | † | |||
| tEME-EE’ | 320,32–311,31 | 250 160.807 | 200.8 | 26.72 | † | |||
| tEME-AE | 320,32–311,31 | 250 160.810 | 200.8 | 26.72 | † | |||
| tEME-EE | 163,13–152,14 | 250 534.903 | 62.9 | 4.83 | 250 535.0 | 0.16 | 0.11 | |
| tEME-EE’ | 163,13–152,14 | 250 534.946 | 62.9 | 4.83 | † | |||
| tEME-AE | 163,13–152,14 | 250 535.223 | 62.9 | 4.83 | † | |||
| tEME-EA | 163,13–152,14 | 250 537.299 | 62.9 | 4.83 | 250 537.5 | 0.11 | 0.08 | |
| tEME-AA | 163,13–152,14 | 250 537.598 | 62.9 | 4.83 | † | |||
| tEME-EE’ | 173,15–162,14 | 252 188.288 | 69.5 | 5.19 | 252 188.5 | 0.59 | 0.11 | CH3CH2CN v13/v21 |
| tEME-EE | 173,15–162,14 | 252 188.318 | 69.5 | 5.19 | † | |||
| tEME-AE | 173,15–162,14 | 252 188.592 | 69.5 | 5.19 | † | |||
| tEME-EA | 173,15–162,14 | 252 190.846 | 69.5 | 5.19 | … | … | 0.08 | CH3CH2CN v13/v21 |
| tEME-AA | 173,15–162,14 | 252 191.135 | 69.5 | 5.19 | † | |||
| tEME-EE’ | 321,32–310,31 | 252 818.715 | 200.8 | 26.74 | … | … | 0.29 | CH3OH |
| tEME-EE | 321,32–310,31 | 252 818.715 | 200.8 | 26.74 | † | |||
| tEME-AE | 321,32–310,31 | 252 818.724 | 200.8 | 26.74 | † | |||
| tEME-EA | 321,32–310,31 | 252 818.826 | 200.8 | 26.74 | † | |||
| tEME-AA | 321,32–310,31 | 252 818.835 | 200.8 | 26.74 | † | |||
| tEME-EE’ | 282,27–271,26 | 253 307.713 | 161.0 | 12.42 | … | … | 0.12 | 13CH3OH |
| tEME-EE | 282,27–271,26 | 253 307.713 | 161.0 | 12.42 | † | |||
| tEME-AE | 282,27–271,26 | 253 307.800 | 161.0 | 12.42 | † | |||
| tEME-EA | 282,27–271,26 | 253 308.856 | 161.0 | 12.42 | † | |||
| tEME-AA | 282,27–271,26 | 253 308.943 | 161.0 | 12.42 | † | |||
| tEME-EE | 55,0–44,0 | 255 923.090 | 34.6 | 4.50 | 255 923.1 | 0.42 | 0.29 | CH3CH2CN |
| tEME-EE’ | 55,1–44,1 | 255 923.151 | 34.6 | 4.50 | † | |||
| tEME-EE’ | 55,0–44,0 | 255 923.293 | 34.6 | 4.50 | † | |||
| tEME-EE | 55,1–44,1 | 255 923.354 | 34.6 | 4.50 | † | |||
| tEME-AE | 55,0–44,0 | 255 923.648 | 34.6 | 4.50 | † | |||
| tEME-AE | 55,1–44,1 | 255 923.709 | 34.6 | 4.50 | † | |||
| tEME-EA | 55,1–44,1 | 255 926.362 | 34.6 | 4.50 | 255 926.5 | 0.37 | 0.20 | SO2 |
| tEME-EA | 55,0–44,0 | 255 926.565 | 34.6 | 4.50 | † | |||
| tEME-AA | 55,1–44,0 | 255 926.920 | 34.6 | 4.50 | † | |||
| tEME-AA | 55,0–44,1 | 255 926.920 | 34.6 | 4.50 | † | |||
| tEME-AA | 341,33–332,32 | 256 180.110 | 232.7 | 17.54 | 256 180.5 | 0.28 | 0.11 | CH3OCOH vt = 1 |
| tEME-EA | 341,33–332,32 | 256 180.131 | 232.7 | 17.54 | † | |||
| tEME-AE | 341,33–332,32 | 256 180.771 | 232.7 | 17.54 | † | |||
| tEME-EE | 341,33–332,32 | 256 180.792 | 232.7 | 17.54 | † | |||
| tEME-EE’ | 341,33–332,32 | 256 180.792 | 232.7 | 17.54 | † | |||
| tEME-EA | 114,8–103,7 | 256 265.991 | 43.9 | 2.59 | … | … | 0.10 | SO2 |
| tEME-AA | 114,8–103,7 | 256 267.169 | 43.9 | 4.68 | † | |||
| tEME-EE | 114,7–103,7 | 256 267.504 | 43.9 | 3.09 | † | |||
| tEME-EA | 114,7–103,7 | 256 267.843 | 43.9 | 2.09 | † | |||
| tEME-AE | 114,8–103,8 | 256 280.321 | 43.9 | 3.24 | … | … | 0.07 | CH3OCOH vt = 1 |
| tEME-EE | 114,8–103,8 | 256 280.554 | 43.9 | 3.09 | † | |||
| tEME-AE | 114,7–103,8 | 256 290.759 | 43.9 | 1.44 | … | … | 0.07 | CH3CCH |
| tEME-EE’ | 114,7–103,8 | 256 291.611 | 43.9 | 1.30 | † | |||
| tEME-EA | 330,33–321,32 | 258 126.741 | 213.2 | 27.74 | … | … | 0.29 | CH3CN v8 = 1,CH3OCOH |
| tEME-AA | 330,33–321,32 | 258 126.744 | 213.2 | 27.74 | † | |||
| tEME-EE | 330,33–321,32 | 258 126.784 | 213.2 | 27.74 | † | |||
| tEME-EE’ | 330,33–321,32 | 258 126.784 | 213.2 | 27.74 | † | |||
| tEME-AE | 330,33–321,32 | 258 126.787 | 213.2 | 27.74 | † | |||
| tEME-EE’ | 292,28–281,27 | 258 936.835 | 172.1 | 13.24 | … | … | 0.12 | SO2 |
| tEME-EE | 292,28–281,27 | 258 936.835 | 172.1 | 13.24 | † | |||
| tEME-AE | 292,28–281,27 | 258 936.915 | 172.1 | 13.24 | † | |||
| tEME-EA | 292,28–281,27 | 258 937.931 | 172.1 | 13.24 | † | |||
| tEME-AA | 292,28–281,27 | 258 938.012 | 172.1 | 13.24 | † | |||
| tEME-EE’ | 183,16–172,15 | 259 109.696 | 76.5 | 5.39 | … | … | 0.11 | CH3OCOH |
| tEME-EE | 183,16–172,15 | 259 109.717 | 76.5 | 5.39 | † | |||
| tEME-AE | 183,16–172,15 | 259 109.992 | 76.5 | 5.39 | † | |||
| tEME-EA | 183,16–172,15 | 259 112.236 | 76.5 | 5.39 | … | … | 0.08 | CH3OCOH |
| tEME-AA | 183,16–172,15 | 259 112.522 | 76.5 | 5.39 | † | |||
| tEME-EE | 173,14–162,15 | 259 135.164 | 69.5 | 4.97 | … | … | 0.11 | CH3OCOH |
| tEME-EE’ | 173,14–162,15 | 259 135.193 | 69.5 | 4.97 | † | |||
| tEME-AE | 173,14–162,15 | 259 135.477 | 69.5 | 4.97 | † | |||
| tEME-EA | 173,14–162,15 | 259 137.561 | 69.5 | 4.97 | … | … | 0.08 | CH3OCOH |
| tEME-AA | 173,14–162,15 | 259 137.860 | 69.5 | 4.97 | † | |||
| tEME-EE | 192,17–181,18 | 260 106.865 | 78.5 | 2.13 | … | … | 0.05 | CH3CH2OH |
| tEME-EE’ | 192,17–181,18 | 260 106.865 | 78.5 | 2.13 | † | |||
| tEME-AE | 192,17–181,18 | 260 107.129 | 78.5 | 2.13 | † | |||
| tEME-EA | 192,17–181,18 | 260 108.541 | 78.5 | 2.13 | … | … | 0.05 | CH3CH2OH |
| tEME-AA | 192,17–181,18 | 260 108.805 | 78.5 | 2.13 | † | |||
| tEME-EE’ | 331,33–320,32 | 260 393.451 | 213.3 | 27.76 | … | … | 0.29 | CH3OCOH |
| tEME-EE | 331,33–320,32 | 260 393.451 | 213.3 | 27.76 | † | |||
| tEME-AE | 331,33–320,32 | 260 393.460 | 213.3 | 27.76 | † | |||
| tEME-EA | 331,33–320,32 | 260 393.552 | 213.3 | 27.76 | † | |||
| tEME-AA | 331,33–320,32 | 260 393.560 | 213.3 | 27.76 | † | |||
| tEME-EE’ | 406,35–405,36 | 260 504.644 | 358.5 | 20.96 | … | … | SiO | |
| tEME-EE | 406,35–405,36 | 260 505.033 | 358.5 | 21.22 | † | |||
| tEME-AE | 406,35–405,36 | 260 505.210 | 358.5 | 21.10 | † | |||
| tEME-EA | 406,35–405,36 | 260 507.959 | 358.5 | 21.43 | † | |||
| tEME-AA | 406,35–405,36 | 260 508.333 | 358.5 | 21.45 | † | |||
| tEME-EE | 386,32–385,33 | 260 681.821 | 327.9 | 19.53 | … | … | 0.03 | CH3CH2CN |
| tEME-AE | 386,32–385,33 | 260 682.506 | 327.9 | 19.20 | † | |||
| tEME-EE’ | 386,32–385,33 | 260 682.515 | 327.9 | 18.84 | † | |||
| tEME-EA | 386,32–385,33 | 260 683.965 | 327.9 | 20.17 | † | |||
| tEME-AA | 386,32–385,33 | 260 684.291 | 327.9 | 20.22 | † | |||
| tEME-EE | 376,31–375,32 | 261 001.915 | 313.2 | 18.45 | … | … | 0.03 | OC34S, CH3OCOH |
| tEME-AE | 376,31–375,32 | 261 002.704 | 313.2 | 17.96 | † | |||
| tEME-EE’ | 376,31–375,32 | 261 002.807 | 313.2 | 17.46 | † | |||
| tEME-EE’ | 386,33–385,34 | 261 029.561 | 327.9 | 18.84 | 261 030.4 | 0.27 | 0.03 | CH3OCOH vt = 1 |
| tEME-EE | 386,33–385,34 | 261 030.255 | 327.9 | 19.53 | † | |||
| tEME-AE | 386,33–385,34 | 261 030.302 | 327.9 | 19.20 | † | |||
| tEME-EA | 386,33–385,34 | 261 033.470 | 327.9 | 20.16 | … | … | 0.03 | CH3OCOH |
| tEME-AA | 386,33–385,34 | 261 033.876 | 327.9 | 20.22 | † | |||
| tEME-EE’ | 376,32–375,33 | 261 270.008 | 313.2 | 17.46 | … | … | 0.03 | CH3CH2CN v13/v21 |
| tEME-AE | 376,32–375,33 | 261 270.856 | 313.2 | 17.95 | † | |||
| tEME-EE | 376,32–375,33 | 261 270.899 | 313.2 | 18.45 | † | |||
| tEME-AE | 366,30–365,31 | 261 291.812 | 298.9 | 16.50 | … | … | 0.04 | CH3CH2OH |
| tEME-EE’ | 366,30–365,31 | 261 292.008 | 298.9 | 15.92 | † | |||
| tEME-EA | 366,30–365,31 | 261 292.672 | 298.9 | 18.82 | † | |||
| tEME-AA | 366,30–365,31 | 261 292.970 | 298.9 | 19.00 | † | |||
| tEME-AE | 366,31–365,32 | 261 496.448 | 298.9 | 16.50 | … | … | 0.03 | CH2CN |
| tEME-EE | 366,31–365,32 | 261 496.589 | 298.9 | 17.15 | † | |||
| tEME-EA | 366,31–365,32 | 261 500.259 | 298.9 | 18.82 | … | … | 0.03 | CH3OCOH, CH2CN |
| tEME-AA | 366,31–365,32 | 261 500.719 | 298.9 | 19.00 | † | |||
| tEME-EE’ | 356,29–355,30 | 261 553.036 | 285.0 | 14.38 | 261 553.2 | 0.15 | 0.06 | CH3O13COH vt = 1 |
| tEME-EA | 356,29–355,30 | 261 553.277 | 285.0 | 18.06 | † | |||
| tEME-AA | 356,29–355,30 | 261 553.546 | 285.0 | 18.40 | † | |||
| tEME-AE | 356,30–355,31 | 261 707.095 | 285.0 | 14.94 | … | … | 0.03 | CH3OH |
| tEME-EE | 356,30–355,31 | 261 707.325 | 285.0 | 15.65 | † | |||
| tEME-EA | 356,30–355,31 | 261 711.277 | 285.0 | 18.06 | … | … | 0.03 | CH3OCOH |
| tEME-AA | 356,30–355,31 | 261 711.778 | 285.0 | 18.40 | † | |||
| tEME-EE | 346,28–345,29 | 261 787.091 | 271.4 | 14.12 | … | … | 0.07 | CH3OCOH, CH3OH, SO |
| tEME-AE | 346,28–345,29 | 261 788.168 | 271.4 | 13.47 | † | |||
| tEME-EA | 346,28–345,29 | 261 788.351 | 271.4 | 17.16 | † | |||
| tEME-EE’ | 346,28–345,29 | 261 788.486 | 271.4 | 13.02 | † | |||
| tEME-AA | 346,28–345,29 | 261 788.577 | 271.4 | 17.80 | † | |||
| tEME-EE’ | 346,29–345,30 | 261 901.878 | 271.4 | 13.02 | … | … | 0.03 | CH3OCH3,CH3COCH3 |
| tEME-AE | 346,29–345,30 | 261 902.977 | 271.4 | 13.47 | † | |||
| tEME-EE | 346,29–345,30 | 261 903.273 | 271.4 | 14.12 | † | |||
| tEME-EA | 346,29–345,30 | 261 907.508 | 271.4 | 17.16 | … | … | 0.03 | CH3OCH3,CH3COCH3 |
| tEME-AA | 346,29–345,30 | 261 908.064 | 271.4 | 17.80 | † | |||
| tEME-EA | 336,27–335,29 | 262 000.261 | 258.3 | 16.05 | … | … | 0.08 | CCH |
| tEME-AE | 336,27–335,29 | 262 000.337 | 258.3 | 12.24 | † | |||
| tEME-AA | 336,27–335,29 | 262 000.424 | 258.3 | 17.21 | † | |||
| tEME-EE’ | 336,27–335,29 | 262 000.673 | 258.3 | 11.95 | † | |||
| tEME-AE | 336,27–335,29 | 262 084.400 | 258.3 | 12.24 | … | … | 0.03 | CH3OCOH |
| tEME-EE | 336,27–335,29 | 262 084.731 | 258.3 | 12.72 | † | |||
| tEME-EA | 336,27–335,29 | 262 089.211 | 258.3 | 16.05 | … | … | 0.03 | CH3OCOH |
| tEME-AA | 336,27–335,29 | 262 089.841 | 258.3 | 17.21 | † | |||
| tEME-EA | 326,27–325,28 | 262 191.119 | 245.5 | 14.67 | … | … | 0.08 | CH3CH2CN |
| tEME-AA | 326,26–325,28 | 262 191.200 | 245.5 | 16.62 | † | |||
| tEME-AE | 326,26–325,28 | 262 191.354 | 245.5 | 11.34 | † | |||
| tEME-EE’ | 326,26–325,28 | 262 191.682 | 245.5 | 11.22 | † | |||
| tEME-AE | 326,27–325,28 | 262 251.766 | 245.5 | 11.34 | … | … | 0.04 | SO2 |
| tEME-EE | 326,27–325,28 | 262 252.104 | 245.5 | 11.61 | † | |||
| tEME-EA | 326,27–325,28 | 262 256.756 | 245.5 | 14.67 | … | … | 0.04 | SO2 |
| tEME-AA | 326,27–325,28 | 262 257.479 | 245.5 | 16.62 | † | |||
| tEME-AE | 326,26–325,28 | 262 260.269 | 245.5 | 5.28 | † | |||
| tEME-EE’ | 326,26–325,28 | 262 260.849 | 245.5 | 5.41 | † | |||
| tEME-AA | 316,25–315,26 | 262 362.797 | 233.1 | 16.04 | … | … | 0.08 | CH313CH2CN |
| tEME-EA | 316,25–315,26 | 262 362.814 | 233.1 | 13.11 | † | |||
| tEME-AE | 316,25–315,26 | 262 363.092 | 233.1 | 10.77 | † | |||
| tEME-EE’ | 316,25–315,26 | 262 363.388 | 233.1 | 10.80 | † | |||
| tEME-EE’ | 316,26–315,27 | 262 404.436 | 233.1 | 10.80 | … | … | 0.04 | 13CH3OCOH vt = 1 |
| tEME-AE | 316,26–315,27 | 262 405.547 | 233.1 | 10.77 | † | |||
| tEME-EE | 316,25–315,27 | 262 405.867 | 233.1 | 10.84 | † | |||
| tEME-EA | 316,26–315,27 | 262 410.601 | 233.1 | 13.11 | … | … | 0.04 | 13CH3OCOH vt = 1 |
| tEME-AA | 316,26–315,27 | 262 411.432 | 233.1 | 16.04 | † | |||
| tEME-EA | 306,25–305,25 | 262 515.014 | 221.2 | 3.88 | … | … | 0.09 | U-line |
| tEME-EE | 306,24–305,25 | 262 516.140 | 221.2 | 10.39 | † | |||
| tEME-AA | 306,24–305,25 | 262 516.918 | 221.2 | 15.45 | † | |||
| tEME-EA | 306,24–305,25 | 262 517.034 | 221.2 | 11.57 | † | |||
| tEME-AE | 306,24–305,25 | 262 517.242 | 221.2 | 10.51 | † | |||
| tEME-EE’ | 306,24–305,25 | 262 517.483 | 221.2 | 10.68 | † | |||
| tEME-EE’ | 306,25–305,26 | 262 545.215 | 221.2 | 10.68 | … | … | 0.04 | H2CCO |
| tEME-AE | 306,25–305,26 | 262 546.281 | 221.2 | 10.51 | † | |||
| tEME-EE | 306,25–305,26 | 262 546.558 | 221.2 | 10.39 | † | |||
| tEME-EA | 306,25–305,26 | 262 551.283 | 221.2 | 11.57 | … | … | 0.04 | H2CCO |
| tEME-AA | 306,25–305,26 | 262 552.224 | 221.2 | 15.45 | † | |||
| tEME-EE | 306,24–305,26 | 262 553.207 | 221.2 | 5.06 | † | |||
| tEME-EA | 306,24–305,26 | 262 553.303 | 221.2 | 3.88 | † | |||
| tEME-EE | 296,23–295,24 | 262 654.283 | 209.6 | 10.25 | … | … | 0.10 | CH3COCH3 |
| tEME-AA | 296,23–295,24 | 262 655.095 | 209.6 | 14.87 | † | |||
| tEME-EA | 296,23–295,24 | 262 655.300 | 209.6 | 10.29 | † | |||
| tEME-AE | 296,23–295,24 | 262 655.326 | 209.6 | 10.53 | † | |||
| tEME-EE’ | 296,23–295,24 | 262 655.485 | 209.6 | 10.83 | † | |||
| tEME-EE’ | 296,24–295,25 | 262 673.561 | 209.6 | 10.83 | … | … | 0.05 | HC13CCN |
| tEME-AE | 296,24–295,25 | 262 674.556 | 209.6 | 10.53 | † | |||
| tEME-EE | 296,24–295,25 | 262 674.763 | 209.6 | 10.25 | † | |||
| tEME-EA | 296,24–295,25 | 262 679.394 | 209.6 | 10.29 | … | … | 0.04 | HC13CCN |
| tEME-AA | 296,24–295,25 | 262 680.434 | 209.6 | 14.87 | † | |||
| tEME-EA | 296,23–295,25 | 262 681.315 | 209.6 | 4.58 | † | |||
| tEME-EE | 296,23–295,25 | 262 681.410 | 209.6 | 4.63 | † | |||
| tEME-EA | 286,23–285,23 | 262 777.103 | 198.3 | 4.94 | … | … | 0.10 | CH3OCH3 |
| tEME-EE | 286,22–285,23 | 262 777.763 | 198.3 | 10.37 | † | |||
| tEME-AA | 286,22–285,23 | 262 778.714 | 198.3 | 14.30 | † | |||
| tEME-AE | 286,22–285,23 | 262 778.719 | 198.3 | 10.78 | † | |||
| tEME-EE’ | 286,22–285,23 | 262 778.771 | 198.3 | 11.17 | † | |||
| tEME-EA | 286,22–285,23 | 262 778.988 | 198.3 | 9.35 | † | |||
| tEME-EE’ | 286,23–285,24 | 262 790.103 | 198.3 | 11.17 | 262 791.0 | 0.25 | 0.05 | U-line |
| tEME-AE | 286,23–285,24 | 262 791.000 | 198.3 | 10.78 | † | |||
| tEME-EE | 286,23–285,24 | 262 791.111 | 198.3 | 10.37 | † | |||
| tEME-EA | 286,23–285,24 | 262 795.561 | 198.3 | 9.35 | … | … | 0.04 | CH2OHCHO |
| tEME-AA | 286,23–285,24 | 262 796.681 | 198.3 | 14.30 | † | |||
| tEME-EA | 286,22–285,24 | 262 797.446 | 198.3 | 4.94 | † | |||
| tEME-EE | 286,22–285,24 | 262 797.772 | 198.3 | 3.92 | † | |||
| tEME-EA | 276,22–275,22 | 262 887.474 | 187.5 | 4.97 | … | … | 0.10 | CH3COOH, CH3OCH3 |
| tEME-EE | 276,21–275,22 | 262 887.831 | 187.5 | 10.70 | † | |||
| tEME-EE’ | 276,21–275,22 | 262 888.603 | 187.5 | 11.58 | † | |||
| tEME-AE | 276,21–275,22 | 262 888.676 | 187.5 | 11.18 | † | |||
| tEME-AA | 276,21–275,22 | 262 889.035 | 187.5 | 13.72 | † | |||
| tEME-EA | 276,21–275,22 | 262 889.353 | 187.5 | 8.75 | † | |||
| tEME-EE’ | 276,22–275,23 | 262 895.483 | 187.5 | 11.58 | … | … | 0.07 | CH3OCH3 |
| tEME-EE | 276,22–275,23 | 262 896.256 | 187.5 | 10.70 | † | |||
| tEME-AE | 276,22–275,23 | 262 896.265 | 187.5 | 11.18 | † | |||
| tEME-EA | 276,22–275,23 | 262 900.436 | 187.5 | 8.75 | … | … | 0.05 | CH3COOH |
| tEME-AA | 276,22–275,23 | 262 901.609 | 187.5 | 13.72 | † | |||
| tEME-EA | 276,21–275,23 | 262 902.316 | 187.5 | 4.97 | † | |||
| tEME-EE | 276,21–275,23 | 262 902.939 | 187.5 | 3.02 | † | |||
| tEME-EE | 266,20–265,21 | 262 985.638 | 177.1 | 11.11 | … | … | 0.10 | CH318OH |
| tEME-EA | 266,21–265,21 | 262 985.653 | 177.1 | 4.68 | † | |||
| tEME-EE’ | 266,20–265,21 | 262 986.169 | 177.1 | 11.87 | † | |||
| tEME-AE | 266,20–265,21 | 262 986.363 | 177.1 | 11.54 | † | |||
| tEME-AA | 266,20–265,21 | 262 987.205 | 177.1 | 13.15 | † | |||
| tEME-EA | 266,20–265,21 | 262 987.541 | 177.1 | 8.47 | † | |||
| tEME-EE’ | 266,21–265,22 | 262 990.328 | 177.1 | 11.87 | … | … | 0.09 | CH318OH |
| tEME-EE | 266,21–265,22 | 262 990.859 | 177.1 | 11.11 | † | |||
| tEME-AE | 266,21–265,22 | 262 990.997 | 177.1 | 11.54 | † | |||
| tEME-EA | 266,21–265,22 | 262 994.684 | 177.1 | 8.47 | … | … | 0.05 | SO2 v2 = 1 |
| tEME-AA | 266,21–265,22 | 262 995.883 | 177.1 | 13.15 | † | |||
| tEME-EA | 266,20–265,22 | 262 996.572 | 177.1 | 4.68 | † | |||
| tEME-EE | 266,20–265,22 | 262 997.567 | 177.1 | 2.04 | † | |||
| tEME-EE | 256,19–255,20 | 263 072.263 | 167.0 | 11.39 | … | … | 0.11 | U-line |
| tEME-EE’ | 256,19–255,20 | 263 072.594 | 167.0 | 11.92 | † | |||
| tEME-EA | 256,20–255,20 | 263 072.699 | 167.0 | 4.12 | † | |||
| tEME-AE | 256,19–255,20 | 263 072.885 | 167.0 | 11.70 | † | |||
| tEME-AA | 256,19–255,20 | 263 074.277 | 167.0 | 12.58 | … | … | 0.11 | U-line |
| tEME-EA | 256,19–255,20 | 263 074.602 | 167.0 | 8.46 | † | |||
| tEME-EE’ | 256,20–255,21 | 263 075.234 | 167.0 | 11.92 | † | |||
| tEME-EE | 256,20–255,21 | 263 075.564 | 167.0 | 11.39 | † | |||
| tEME-AE | 256,20–255,21 | 263 075.815 | 167.0 | 11.70 | † | |||
| tEME-EA | 256,20–255,21 | 263 078.980 | 167.0 | 8.46 | … | … | 0.05 | U-line |
| tEME-AA | 256,20–255,21 | 263 080.176 | 167.0 | 12.58 | † | |||
| tEME-EE | 246,19–245,19 | 263 141.974 | 157.4 | 0.60 | 263 149.1 | 0.44 | 0.11 | CH3O13COH,CH3OCOD |
| tEME-EE | 246,18–245,19 | 263 148.731 | 157.4 | 11.41 | † | |||
| tEME-EE’ | 246,18–245,19 | 263 148.928 | 157.4 | 11.70 | † | |||
| tEME-AE | 246,18–245,19 | 263 149.281 | 157.4 | 11.59 | † | |||
| tEME-EA | 246,19–245,19 | 263 149.579 | 157.4 | 3.30 | † | |||
| tEME-EE’ | 246,19–245,20 | 263 150.791 | 157.4 | 11.70 | 263 151.0 | 0.58 | 0.20 | CH3O13COH,CH3OCOD |
| tEME-EE | 246,19–245,20 | 263 150.988 | 157.4 | 11.41 | † | |||
| tEME-AA | 246,18–245,19 | 263 151.216 | 157.4 | 12.01 | † | |||
| tEME-AE | 246,19–245,20 | 263 151.318 | 157.4 | 11.59 | † | |||
| tEME-EA | 246,18–245,19 | 263 151.498 | 157.4 | 8.71 | † | |||
| tEME-EA | 246,19–245,20 | 263 153.999 | 157.4 | 8.71 | † | |||
| tEME-AA | 246,19–245,20 | 263 155.161 | 157.4 | 12.01 | 263 155.2 | 0.25 | 0.05 | CH3OCOD |
| tEME-EA | 246,18–245,20 | 263 155.918 | 157.4 | 3.30 | † | |||
| tEME-EE | 236,17–235,18 | 263 215.996 | 148.1 | 11.17 | 263 216.3 | 0.58 | 0.14 | SO2 |
| tEME-EE’ | 236,17–235,18 | 263 216.118 | 148.1 | 11.31 | † | |||
| tEME-AE | 236,17–235,18 | 263 216.506 | 148.1 | 11.26 | † | |||
| tEME-EA | 236,18–235,18 | 263 217.183 | 148.1 | 2.31 | 263 217.8 | 0.59 | 0.14 | SO2 |
| tEME-EE’ | 236,18–235,19 | 263 217.615 | 148.1 | 11.31 | † | |||
| tEME-EE | 236,18–235,19 | 263 217.736 | 148.1 | 11.17 | † | |||
| tEME-AE | 236,18–235,19 | 263 218.114 | 148.1 | 11.26 | † | |||
| tEME-AA | 236,17–235,18 | 263 218.914 | 148.1 | 11.44 | † | |||
| tEME-EA | 236,17–235,18 | 263 219.118 | 148.1 | 9.14 | † | |||
| tEME-EA | 236,18–235,19 | 263 220.415 | 148.1 | 9.14 | … | … | 0.05 | SO2 |
| tEME-AA | 236,18–235,19 | 263 221.507 | 148.1 | 11.44 | † | |||
| tEME-EA | 236,17–235,19 | 263 222.351 | 148.1 | 2.31 | † | |||
| tEME-EE | 226,16–225,17 | 263 274.922 | 139.2 | 10.76 | … | … | 0.14 | CH3OCH3 |
| tEME-EE’ | 226,16–225,17 | 263 275.007 | 139.2 | 10.82 | † | |||
| tEME-AE | 226,16–225,17 | 263 275.415 | 139.2 | 10.80 | † | |||
| tEME-EA | 226,17–225,17 | 263 276.341 | 139.2 | 1.34 | † | |||
| tEME-EE’ | 226,17–225,18 | 263 276.343 | 139.2 | 10.82 | † | |||
| tEME-EE | 226,17–225,18 | 263 276.429 | 139.2 | 10.76 | † | |||
| tEME-AE | 226,17–225,18 | 263 276.831 | 139.2 | 10.80 | † | |||
| tEME-AA | 226,16–225,17 | 263 278.199 | 139.2 | 10.88 | … | … | 0.11 | CH3OCH3 |
| tEME-EA | 226,16–225,17 | 263 278.292 | 139.2 | 9.53 | † | |||
| tEME-EA | 226,17–225,18 | 263 278.882 | 139.2 | 9.53 | † | |||
| tEME-AA | 226,17–225,18 | 263 279.871 | 139.2 | 10.88 | † | |||
| tEME-EE | 216,15–215,16 | 263 326.288 | 130.7 | 10.27 | 263 326.7 | 0.42 | 0.14 | HC3N v7 = 1,CH3COCH3 |
| tEME-EE’ | 216,15–215,16 | 263 326.357 | 130.7 | 10.29 | † | |||
| tEME-AE | 216,15–215,16 | 263 326.775 | 130.7 | 10.28 | † | |||
| tEME-EE’ | 216,16–215,17 | 263 327.628 | 130.7 | 10.29 | 263 327.4 | 0.51 | 0.14 | HC3N v7 = 1,CH3COCH3 |
| tEME-EE | 216,16–215,17 | 263 327.697 | 130.7 | 10.27 | † | |||
| tEME-EA | 216,16–215,16 | 263 327.838 | 130.7 | .64 | † | |||
| tEME-AE | 216,16–215,17 | 263 328.113 | 130.7 | 10.28 | † | |||
| tEME-EA | 216,15–215,16 | 263 329.805 | 130.7 | 9.67 | 263 329.9 | 0.74 | 0.30 | HC3N v7 = 1,CH3COCH3 |
| tEME-AA | 216,15–215,16 | 263 329.839 | 130.7 | 10.31 | † | |||
| tEME-EA | 216,16–215,17 | 263 330.025 | 130.7 | 9.67 | † | |||
| tEME-AA | 216,16–215,17 | 263 330.894 | 130.7 | 10.31 | † | |||
| tEME-EE | 206,14–205,15 | 263 370.802 | 122.6 | 9.73 | 263 370.7 | 0.51 | 0.16 | SO18O, CH3OCOH |
| tEME-EE’ | 206,14–205,15 | 263 370.865 | 122.6 | 9.74 | † | |||
| tEME-AE | 206,14–205,15 | 263 371.289 | 122.6 | 9.73 | † | |||
| tEME-EE’ | 206,15–205,16 | 263 372.112 | 122.6 | 9.74 | 263 372.2 | 0.37 | 0.16 | SO18O, CH3OCOH |
| tEME-EE | 206,15–205,16 | 263 372.174 | 122.6 | 9.73 | † | |||
| tEME-AE | 206,15–205,16 | 263 372.598 | 122.6 | 9.73 | † | |||
| tEME-EA | 206,14–205,15 | 263 374.395 | 122.6 | 9.49 | … | … | 0.17 | SO18O, CH3OCOH |
| tEME-EA | 206,15–205,16 | 263 374.447 | 122.6 | 9.49 | † | |||
| tEME-AA | 206,14–205,15 | 263 374.550 | 122.6 | 9.74 | † | |||
| tEME-AA | 206,15–205,16 | 263 375.201 | 122.6 | 9.74 | † | |||
| tEME-EE | 196,13–205,14 | 263 409.117 | 114.8 | 9.17 | … | … | 0.17 | CH3OCH3 |
| tEME-EE’ | 196,13–205,14 | 263 409.177 | 114.8 | 9.17 | † | |||
| tEME-AE | 196,13–205,14 | 263 409.605 | 114.8 | 9.17 | † | |||
| tEME-EE’ | 196,14–205,15 | 263 410.419 | 114.8 | 9.17 | † | |||
| tEME-EE | 196,14–205,15 | 263 410.479 | 114.8 | 9.17 | † | |||
| tEME-AE | 196,14–205,15 | 263 410.907 | 114.8 | 9.17 | † | |||
| tEME-EA | 196,14–205,15 | 263 412.731 | 114.8 | 9.09 | … | … | 0.19 | CH3OCH3 |
| tEME-EA | 196,13–205,14 | 263 412.749 | 114.8 | 9.09 | † | |||
| tEME-AA | 196,13–205,14 | 263 413.003 | 114.8 | 9.18 | † | |||
| tEME-EE | 186,12–185,13 | 263 441.843 | 107.5 | 8.61 | … | … | 0.17 | 34SO2,33SO2 |
| tEME-EE’ | 186,12–185,13 | 263 441.902 | 107.5 | 8.61 | † | |||
| tEME-AE | 186,12–185,13 | 263 442.334 | 107.5 | 8.61 | † | |||
| tEME-EE’ | 186,13–185,14 | 263 443.145 | 107.5 | 8.61 | † | |||
| tEME-EE | 186,13–185,14 | 263 443.204 | 107.5 | 8.61 | † | |||
| tEME-AE | 186,13–185,14 | 263 443.636 | 107.5 | 8.61 | † | |||
| tEME-EA | 186,13–185,14 | 263 445.453 | 107.5 | 8.58 | … | … | 0.19 | 34SO2,33SO2 |
| tEME-EA | 186,12–185,13 | 263 445.498 | 107.5 | 8.58 | † | |||
| tEME-AA | 186,12–185,13 | 263 445.822 | 107.5 | 8.61 | † | |||
| tEME-AA | 186,13–185,14 | 263 446.051 | 107.5 | 8.61 | † | |||
| tEME-EE | 176,11–175,12 | 263 469.553 | 100.5 | 8.04 | 263 469.7 | 0.43 | 0.17 | CH3CH2OH |
| tEME-EE’ | 176,11–175,12 | 263 469.612 | 100.5 | 8.04 | † | |||
| tEME-AE | 176,11–175,12 | 263 470.047 | 100.5 | 8.04 | † | |||
| tEME-EE’ | 176,12–175,13 | 263 470.859 | 100.5 | 8.04 | † | |||
| tEME-EE | 176,12–175,13 | 263 470.917 | 100.5 | 8.04 | † | |||
| tEME-AE | 176,12–175,13 | 263 471.352 | 100.5 | 8.04 | † | |||
| tEME-EA | 176,12–175,13 | 263 473.169 | 100.5 | 8.03 | 263 473.9 | 0.41 | 0.19 | CH3CH2OH |
| tEME-EA | 176,11–175,12 | 263 473.223 | 100.5 | 8.03 | † | |||
| tEME-AA | 176,11–175,12 | 263 473.595 | 100.5 | 8.04 | † | |||
| tEME-AA | 176,12–175,13 | 263 473.725 | 100.5 | 8.04 | † | |||
| tEME-EE | 166,10–165,11 | 263 492.786 | 94.0 | 7.46 | 263 492.9 | 0.26 | 0.17 | CH3OCH3 |
| tEME-EE’ | 166,10–165,11 | 263 492.845 | 94.0 | 7.46 | † | |||
| tEME-AE | 166,10–165,11 | 263 493.283 | 94.0 | 7.46 | † | |||
| tEME-EE’ | 166,11–165,12 | 263 494.096 | 94.0 | 7.46 | 263 494.2 | 0.33 | 0.17 | CH3OCH3 |
| tEME-EE | 166,11–165,12 | 263 494.155 | 94.0 | 7.46 | † | |||
| tEME-AE | 166,11–165,12 | 263 494.592 | 94.0 | 7.46 | † | |||
| tEME-EA | 166,11–165,12 | 263 496.411 | 94.0 | 7.46 | 263 496.6 | 0.55 | 0.19 | CH3OCH3 |
| tEME-EA | 166,10–165,11 | 263 496.468 | 94.0 | 7.46 | † | |||
| tEME-AA | 166,10–165,11 | 263 496.871 | 94.0 | 7.46 | † | |||
| tEME-AA | 166,11–165,12 | 263 496.942 | 94.0 | 7.46 | † | |||
| tEME-EE | 156,9–155,10 | 263 512.049 | 87.8 | 6.89 | … | … | 0.17 | CH3OCH3 |
| tEME-EE’ | 156,9–155,10 | 263 512.108 | 87.8 | 6.89 | † | |||
| tEME-AE | 156,9–155,10 | 263 512.548 | 87.8 | 6.89 | † | |||
| tEME-EE’ | 156,10–155,11 | 263 513.363 | 87.8 | 6.89 | … | … | 0.17 | CH3OCH3 |
| tEME-EE | 156,10–155,11 | 263 513.422 | 87.8 | 6.89 | † | |||
| tEME-AE | 156,10–155,10 | 263 513.862 | 87.8 | 6.89 | † | |||
| tEME-EA | 156,10–155,11 | 263 515.683 | 87.8 | 6.88 | … | … | 0.19 | CH3CH2CN |
| tEME-EA | 156,9–155,10 | 263 515.742 | 87.8 | 6.88 | † | |||
| tEME-AA | 156,9–155,10 | 263 516.163 | 87.8 | 6.89 | † | |||
| tEME-AA | 156,10–155,11 | 263 516.200 | 87.8 | 6.89 | † | |||
| tEME-EE | 146,8–145,9 | 263 527.818 | 82.0 | 6.30 | … | … | 0.16 | CH3OCH3, CH3CH2CN |
| tEME-EE’ | 146,8–145,9 | 263 527.877 | 82.0 | 6.30 | † | |||
| tEME-AE | 146,8–145,9 | 263 528.319 | 82.0 | 6.30 | † | |||
| tEME-EE’ | 146,9–145,10 | 263 529.136 | 82.0 | 6.30 | † | |||
| tEME-EE | 146,9–145,10 | 263 529.195 | 82.0 | 6.30 | † | |||
| tEME-AE | 146,9–145,10 | 263 529.637 | 82.0 | 6.30 | † | |||
| tEME-EA | 146,9–145,10 | 263 531.461 | 82.0 | 6.30 | … | … | 0.18 | CH3OCH3 |
| tEME-EA | 146,8–145,9 | 263 531.520 | 82.0 | 6.30 | † | |||
| tEME-AA | 146,8–145,9 | 263 531.953 | 82.0 | 6.30 | † | |||
| tEME-AA | 146,9–145,10 | 263 531.971 | 82.0 | 6.30 | † | |||
| tEME-EE | 136,7–135,8 | 263 540.536 | 76.6 | 5.71 | … | … | 0.15 | NH2CHO, SO2 |
| tEME-EE’ | 136,7–135,8 | 263 540.595 | 76.6 | 5.71 | † | |||
| tEME-AE | 136,7–135,8 | 263 541.040 | 76.6 | 5.71 | † | |||
| tEME-EE’ | 136,8–135,9 | 263 541.858 | 76.6 | 5.71 | † | |||
| tEME-EE | 136,8–135,9 | 263 541.917 | 76.6 | 5.71 | † | |||
| tEME-AE | 136,8–135,9 | 263 542.362 | 76.6 | 5.71 | † | |||
| tEME-EA | 136,8–135,9 | 263 544.188 | 76.6 | 5.71 | … | … | 0.18 | NH2CHO, SO2 |
| tEME-EA | 136,7–135,8 | 263 544.247 | 76.6 | 5.71 | † | |||
| tEME-AA | 136,7–135,8 | 263 544.687 | 76.6 | 5.71 | † | |||
| tEME-AA | 136,8–135,9 | 263 544.696 | 76.6 | 5.71 | † | |||
| tEME-EE | 126,6–125,7 | 263 550.619 | 71.5 | 5.11 | … | … | 0.14 | SO2 |
| tEME-EE’ | 126,6–125,7 | 263 550.679 | 71.5 | 5.11 | † | |||
| tEME-AE | 126,6–125,7 | 263 551.125 | 71.5 | 5.11 | † | |||
| tEME-EE’ | 126,7–125,8 | 263 551.945 | 71.5 | 5.11 | † | |||
| tEME-EE | 126,7–125,8 | 263 552.004 | 71.5 | 5.11 | † | |||
| tEME-AE | 126,7–125,8 | 263 552.451 | 71.5 | 5.11 | † | |||
| tEME-EA | 126,7–125,8 | 263 554.279 | 71.5 | 5.11 | … | … | 0.16 | SO2 |
| tEME-EA | 126,6–125,7 | 263 554.338 | 71.5 | 5.11 | † | |||
| tEME-AA | 126,6–125,7 | 263 554.783 | 71.5 | 5.11 | † | |||
| tEME-AA | 126,7–125,8 | 263 554.787 | 71.5 | 5.11 | † | |||
| tEME-EE | 116,5–115,6 | 263 558.451 | 66.9 | 4.49 | … | … | 0.13 | SO2 |
| tEME-EE’ | 116,5–115,6 | 263 558.511 | 66.9 | 4.49 | † | |||
| tEME-AE | 116,5–115,6 | 263 558.959 | 66.9 | 4.49 | † | |||
| tEME-EE’ | 116,6–115,7 | 263 559.780 | 66.9 | 4.49 | … | … | 0.13 | SO2 |
| tEME-EE | 116,6–115,7 | 263 559.839 | 66.9 | 4.49 | † | |||
| tEME-AE | 116,6–115,7 | 263 560.288 | 66.9 | 4.49 | † | |||
| tEME-EA | 116,6–115,7 | 263 562.118 | 66.9 | 4.49 | … | … | 0.15 | SO2 |
| tEME-EA | 116,5–H5,6 | 263 562.178 | 66.9 | 4.49 | † | |||
| tEME-AA | 116,5–115,6 | 263 562.626 | 66.9 | 4.49 | † | |||
| tEME-AA | 116,6–115,7 | 263 562.627 | 66.9 | 4.49 | † | |||
| tEME-EE | 106,4–105,5 | 263 564.386 | 62.7 | 3.85 | … | … | 0.11 | SO2 |
| tEME-EE’ | 106,4–105,5 | 263 564.446 | 62.7 | 3.85 | † | |||
| tEME-AE | 106,4–105,5 | 263 564.897 | 62.7 | 3.85 | † | |||
| tEME-EE’ | 106,5–105,6 | 263 565.718 | 62.7 | 3.85 | … | … | 0.11 | SO2 |
| tEME-EE | 106,5–105,6 | 263 565.777 | 62.7 | 3.85 | † | |||
| tEME-AE | 106,5–105,6 | 263 566.228 | 62.7 | 3.85 | † | |||
| tEME-EA | 106,5–105,6 | 263 568.061 | 62.7 | 3.85 | … | … | 0.21 | SO2 |
| tEME-EA | 106,4–105,5 | 263 568.120 | 62.7 | 3.85 | † | |||
| tEME-AA | 106,4–105,5 | 263 568.570 | 62.7 | 3.85 | † | |||
| tEME-AA | 106,5–105,6 | 263 568.571 | 62.7 | 3.85 | † | |||
| tEME-EE | 96,3–95,4 | 263 568.750 | 58.8 | 3.19 | † | |||
| tEME-EE’ | 96,3–95,4 | 263 568.810 | 58.8 | 3.19 | † | |||
| tEME-AE | 96,3–95,4 | 263 569.262 | 58.8 | 3.19 | † | |||
| tEME-EE’ | 96,4–95,5 | 263 570.083 | 58.8 | 3.19 | † | |||
| tEME-EE | 96,4–95,5 | 263 570.143 | 58.8 | 3.19 | † | |||
| tEME-AE | 96,4–95,5 | 263 570.595 | 58.8 | 3.19 | † | |||
| tEME-AE | 86,2–85,3 | 263 572.350 | 55.3 | 2.50 | 263 572.9 | 0.47 | 0.20 | CH3OCH3, HNCO |
| tEME-EA | 96,4–95,5 | 263 572.430 | 58.8 | 3.19 | † | |||
| tEME-EA | 96,3–95,4 | 263 572.490 | 58.8 | 3.19 | † | |||
| tEME-AA | 96,3–95,4 | 263 572.942 | 58.8 | 3.19 | † | |||
| tEME-AA | 96,4–95,5 | 263 572.942 | 58.8 | 3.19 | † | |||
| tEME-EE’ | 86,3–85,4 | 263 573.172 | 55.3 | 2.50 | † | |||
| tEME-EE | 86,3–85,4 | 263 573.232 | 55.3 | 2.50 | † | |||
| tEME-AE | 86,3–85,4 | 263 573.685 | 55.3 | 2.50 | † | |||
| tEME-EE | 76,1–75,2 | 263 573.911 | 52.2 | 1.75 | † | |||
| tEME-EE’ | 76,1–75,2 | 263 573.972 | 52.2 | 1.75 | † | |||
| tEME-AE | 76,1–75,2 | 263 574.426 | 52.2 | 1.75 | † | |||
| tEME-EE | 66,0–65,1 | 263 575.211 | 49.5 | 0.93 | … | … | 0.17 | CH3OCH3, HNCO |
| tEME-EE’ | 76,2–75,3 | 263 575.249 | 52.2 | 1.75 | † | |||
| tEME-EE’ | 66,0–65,1 | 263 575.271 | 49.5 | 0.93 | † | |||
| tEME-EE | 76,2–75,3 | 263 575.309 | 52.2 | 1.75 | † | |||
| tEME-EA | 86,3–85,4 | 263 575.522 | 55.3 | 2.50 | † | |||
| tEME-EA | 86,2–85,3 | 263 575.582 | 55.3 | 2.50 | † | |||
| tEME-AE | 66,0–65,1 | 263 575.727 | 49.5 | 0.93 | † | |||
| tEME-AE | 76,2–75,3 | 263 575.764 | 52.2 | 1.75 | † | |||
| tEME-AE | 66,1–65,2 | 263 577.066 | 49.5 | 0.93 | … | … | 0.08 | CH3OCH3, HNCO |
| tEME-EA | 76,2–75,3 | 263 577.602 | 52.2 | 1.75 | † | |||
| tEME-EA | 76,1–75,2 | 263 577.662 | 52.2 | 1.75 | † | |||
| tEME-AA | 76,1–75,2 | 263 578.117 | 52.2 | 1.75 | † | |||
| tEME-AA | 76,2–75,3 | 263 578.117 | 52.2 | 1.75 | † | |||
| tEME-EA | 66,1–65,2 | 263 578.906 | 49.5 | 0.93 | † | |||
| tEME-EA | 66,0–65,1 | 263 578.966 | 49.5 | 0.93 | † | |||
| tEME-AA | 66,0–65,1 | 263 579.422 | 49.5 | 0.93 | † | |||
| tEME-AA | 66,1–65,2 | 263 579.422 | 49.5 | 0.93 | † | |||
| tEME-EE | 65,1–54,1 | 263 974.290 | 36.9 | 4.57 | 263 974.6 | 0.84 | 0.32 | CH3CH2CN v20 = 1, CH313CH2CN |
| tEME-EE’ | 65,2–54,2 | 263 974.352 | 36.9 | 4.57 | † | |||
| tEME-EE’ | 65,1–54,1 | 263 974.491 | 36.9 | 4.57 | † | |||
| tEME-EE | 65,2–54,2 | 263 974.554 | 36.9 | 4.57 | † | |||
| tEME-AE | 65,1–54,1 | 263 974.847 | 36.9 | 4.57 | † | |||
| tEME-AE | 65,2–54,2 | 263 974.909 | 36.9 | 4.57 | † | |||
| tEME-EA | 65,2–54,2 | 263 977.560 | 36.9 | 4.57 | 263 977.9 | 1.19 | 0.21 | CH3CH2CN v20 = 1,CH313CH2CN, CH3OCOH vt = 1 |
| tEME-EA | 65,1–54,1 | 263 977.761 | 36.9 | 4.57 | † | |||
| tEME-AA | 65,2–54,1 | 263 978.116 | 36.9 | 4.57 | † | |||
| tEME-AA | 65,1–54,2 | 263 978.117 | 36.9 | 4.57 | † | |||
| tEME-EE’ | 124,9–113,8 | 264 279.974 | 48.6 | 1.86 | … | … | 0.04 | CH3CH2CN v13/v21 |
| tEME-AE | 124,9–113,8 | 264 281.470 | 48.6 | 1.98 | † | |||
| tEME-EE | 124,8–113,8 | 264 282.160 | 48.6 | 2.11 | † | |||
| tEME-EA | 124,9–113,8 | 264 288.936 | 48.6 | 3.18 | 264 290.1 | 0.32 | 0.08 | CH3CH2CN v13/v21 |
| tEME-AA | 124,9–113,8 | 264 289.975 | 48.6 | 4.89 | † | |||
| tEME-EE | 124,8–113,8 | 264 290.781 | 48.6 | 2.78 | † | |||
| tEME-EA | 124,8–113,8 | 264 290.868 | 48.6 | 1.70 | † | |||
| tEME-AE | 124,8–113,8 | 264 291.901 | 48.6 | 2.91 | 264 292.2 | 0.33 | 0.07 | CH3CH2CN v13/v21 |
| tEME-EE’ | 124,8–113,8 | 264 292.217 | 48.6 | 3.02 | † | |||
| tEME-EE’ | 124,9–113,9 | 264 316.948 | 48.6 | 3.02 | … | … | 0.06 | CH3OH |
| tEME-AE | 124,9–113,9 | 264 318.041 | 48.6 | 2.91 | † | |||
| tEME-EE | 124,9–113,9 | 264 318.385 | 48.6 | 2.78 | † | |||
| tEME-EA | 124,9–113,9 | 264 324.363 | 48.6 | 1.70 | … | … | 0.10 | CH3OH |
| tEME-AA | 124,8–113,9 | 264 326.032 | 48.6 | 4.89 | † | |||
| tEME-EA | 124,8–113,9 | 264 326.294 | 48.6 | 3.18 | † | |||
| tEME-EE | 124,8–113,9 | 264 327.005 | 48.6 | 2.11 | † | |||
| tEME-AE | 124,8–113,9 | 264 328.472 | 48.6 | 1.98 | … | … | 0.04 | CH3OH |
| tEME-EE’ | 124,8–113,9 | 264 329.191 | 48.6 | 1.86 | † | |||
| tEME-EE’ | 302,29–291,28 | 264 623.050 | 183.6 | 14.10 | … | … | 0.14 | CH3CH2CN v13/v21 |
| tEME-EE | 302,29–291,28 | 264 623.050 | 183.6 | 14.10 | † | |||
| tEME-AE | 302,29–291,28 | 264 623.125 | 183.6 | 14.10 | † | |||
| tEME-EA | 302,29–291,28 | 264 624.097 | 183.6 | 14.10 | † | |||
| tEME-AA | 302,29–291,28 | 264 624.172 | 183.6 | 14.10 | † | |||
| tEME-AA | 351,34–342,33 | 265 627.614 | 246.2 | 18.59 | … | … | 0.13 | CH3COOCH3 |
| tEME-EA | 351,34–342,33 | 265 627.630 | 246.2 | 18.59 | † | |||
| tEME-AE | 351,34–342,33 | 265 628.218 | 246.2 | 18.59 | † | |||
| tEME-EE | 351,34–342,33 | 265 628.234 | 246.2 | 18.59 | † | |||
| tEME-EE’ | 351,34–342,33 | 265 628.234 | 246.2 | 18.59 | † | |||
| tEME-EE’ | 193,17–182,16 | 265 859.733 | 83.8 | 5.59 | … | … | 0.13 | HCN |
| tEME-EE | 193,17–182,16 | 265 859.748 | 83.8 | 5.59 | † | |||
| tEME-AE | 193,17–182,16 | 265 860.022 | 83.8 | 5.59 | † | |||
| tEME-EA | 193,17–182,16 | 265 862.261 | 83.8 | 5.59 | … | … | 0.09 | HCN |
| tEME-AA | 193,17–182,16 | 265 862.543 | 83.8 | 5.59 | † | |||
| tEME-EA | 340,34–331,33 | 266 065.053 | 226.0 | 28.77 | … | … | 0.28 | CH3COCH3 |
| tEME-AA | 340,34–331,33 | 266 065.056 | 226.0 | 28.77 | † | |||
| tEME-EE | 340,34–331,33 | 266 065.087 | 226.0 | 28.77 | † | |||
| tEME-EE’ | 340,34–331,33 | 266 065.087 | 226.0 | 28.77 | † | |||
| tEME-AE | 340,34–331,33 | 266 065.091 | 226.0 | 28.77 | † | |||
| tEME-EE | 183,15–172,16 | 267 851.372 | 76.5 | 5.09 | 267 851.6 | 0.71 | 0.13 | CH3OCOH vt = 2 |
| tEME-EE’ | 183,15–172,16 | 267 851.394 | 76.5 | 5.09 | † | |||
| tEME-AE | 183,15–172,16 | 267 851.682 | 76.5 | 5.09 | † | |||
| tEME-EA | 183,15–172,16 | 267 853.764 | 76.5 | 5.09 | … | … | 0.09 | CH3OCOH vt = 2 |
| tEME-AA | 183,15–172,16 | 267 854.063 | 76.5 | 5.09 | † | |||
| tEME-EE’ | 341,34–330,33 | 267 994.638 | 226.1 | 28.78 | 267 994.7 | 0.95 | 0.28 | CH3OCOH vt = 2, CH3CH2CN |
| tEME-EE | 341,34–330,33 | 267 994.638 | 226.1 | 28.78 | † | |||
| tEME-AE | 341,34–330,33 | 267 994.646 | 226.1 | 28.78 | † | |||
| tEME-EA | 341,34–330,33 | 267 994.729 | 226.1 | 28.78 | † | |||
| tEME-AA | 341,34–330,33 | 267 994.737 | 226.1 | 28.78 | † | |||
| tEME-EE’ | 312,30–301,29 | 270 379.929 | 195.5 | 14.99 | 270 380.3 | 0.21 | 0.15 | |
| tEME-EE | 312,30–301,29 | 270 379.929 | 195.5 | 14.99 | † | |||
| tEME-AE | 312,30–301,29 | 270 379.998 | 195.5 | 14.99 | † | |||
| tEME-EA | 312,30–301,29 | 270 380.924 | 195.5 | 14.99 | † | |||
| tEME-AA | 312,30–301,29 | 270 380.993 | 195.5 | 14.99 | † | |||
| tEME-EE | 75,2–64,2 | 272 025.103 | 39.6 | 4.70 | 272 025.5 | 1.10 | 0.35 | CH3CH2CN v13/v21 |
| tEME-EE’ | 75,3–64,3 | 272 025.167 | 39.6 | 4.70 | † | |||
| tEME-EE’ | 75,2–64,2 | 272 025.304 | 39.6 | 4.70 | † | |||
| tEME-EE | 75,3–64,3 | 272 025.368 | 39.6 | 4.70 | † | |||
| tEME-AE | 75,2–64,2 | 272 025.659 | 39.6 | 4.70 | † | |||
| tEME-AE | 75,3–64,3 | 272 025.723 | 39.6 | 4.70 | † | |||
| tEME-EA | 75,3–64,3 | 272 028.370 | 39.6 | 4.70 | 272 028.6 | 0.59 | 0.23 | CH3CH2CN v13/v21 |
| tEME-EA | 75,2–64,2 | 272 028.571 | 39.6 | 4.70 | † | |||
| tEME-AA | 75,3–64,2 | 272 028.925 | 39.6 | 4.70 | † | |||
| tEME-AA | 75,2–64,3 | 272 028.927 | 39.6 | 4.70 | † | |||
| tEME-EE’ | 134,10–123,9 | 272 291.842 | 53.6 | 2.35 | 272 292.1 | 0.21 | 0.03 | U-line |
| tEME-AE | 134,10–123,9 | 272 293.250 | 53.6 | 2.46 | 272 293.6 | 0.26 | 0.05 | U-line |
| tEME-EE | 134,10–123,9 | 272 293.863 | 53.6 | 2.58 | † | |||
| tEME-EA | 134,10–123,9 | 272 300.368 | 53.6 | 3.94 | 272 301.0 | 0.18 | 0.08 | CH3OCOH |
| tEME-AA | 134,10–123,9 | 272 301.238 | 53.6 | 5.09 | † | |||
| tEME-EE | 134,9–123,9 | 272 302.533 | 53.6 | 2.51 | † | |||
| tEME-EA | 134,9–123,9 | 272 302.564 | 53.6 | 1.15 | † | |||
| tEME-AE | 134,9–123,9 | 272 303.710 | 53.6 | 2.64 | 272 303.9 | 0.24 | 0.07 | CH3CH2CN v20 = 1 |
| tEME-EE’ | 134,9–123,9 | 272 304.098 | 53.6 | 2.74 | † | |||
| tEME-EE’ | 134,10–123,10 | 272 351.722 | 53.6 | 2.74 | … | … | 0.06 | CH3OH, CH3OD |
| tEME-AE | 134,10–123,10 | 272 352.884 | 53.6 | 2.63 | † | |||
| tEME-EE | 134,10–123,10 | 272 353.288 | 53.6 | 2.51 | † | |||
| tEME-EA | 134,10–123,10 | 272 359.310 | 53.6 | 1.15 | … | … | 0.13 | CH3OH, CH3OD |
| tEME-AA | 134,9–123,10 | 272 361.410 | 53.6 | 5.09 | † | |||
| tEME-EA | 134,9–123,10 | 272 361.506 | 53.6 | 3.94 | † | |||
| tEME-EE | 134,9–123,10 | 272 361.957 | 53.6 | 2.58 | † | |||
| tEME-AE | 134,9–123,10 | 272 363.344 | 53.6 | 2.46 | … | … | 0.05 | CH3OH, CH3OD |
| tEME-EE’ | 134,9–123,10 | 272 363.978 | 53.6 | 2.35 | † | |||
| tEME-EE’ | 203,18–192,17 | 272 432.754 | 91.6 | 5.79 | 272 433.0 | 0.33 | 0.14 | U-line |
| tEME-EE | 203,18–192,17 | 272 432.765 | 91.6 | 5.79 | † | |||
| tEME-AE | 203,18–192,17 | 272 433.037 | 91.6 | 5.79 | † | |||
| tEME-EA | 203,18–192,17 | 272 435.273 | 91.6 | 5.79 | 272 435.7 | 0.35 | 0.09 | U-line |
| tEME-AA | 203,18–192,17 | 272 435.550 | 91.6 | 5.79 | † | |||
| tEME-AA | 402,38–393,37 | 272 596.983 | 325.0 | 12.66 | … | … | 0.03 | U-line |
| tEME-EA | 402,38–393,37 | 272 597.055 | 325.0 | 12.66 | † | |||
| tEME-AE | 402,38–393,37 | 272 598.688 | 325.0 | 12.66 | † | |||
| tEME-EE | 402,38–393,37 | 272 598.760 | 325.0 | 12.66 | † | |||
| tEME-EE’ | 402,38–393,37 | 272 598.760 | 325.0 | 12.66 | † | |||
| tEME-EE | 202,18–191,19 | 273 141.137 | 86.3 | 2.00 | … | … | 0.05 | CH3OCOH |
| tEME-EE’ | 202,18–191,19 | 273 141.138 | 86.3 | 2.00 | † | |||
| tEME-AE | 202,18–191,19 | 273 141.411 | 86.3 | 2.00 | † | |||
| tEME-EA | 202,18–191,19 | 273 142.818 | 86.3 | 2.00 | … | … | 0.03 | CH3OCOH |
| tEME-AA | 202,18–191,19 | 273 143.092 | 86.3 | 2.00 | † | |||
| tEME-EA | 350,35–341,34 | 273 979.063 | 239.2 | 29.79 | 273 979.1 | 0.73 | 0.28 | CH3CH2CN, SO2 |
| tEME-AA | 350,35–341,34 | 273 979.067 | 239.2 | 29.79 | † | |||
| tEME-EE | 350,35–341,34 | 273 979.089 | 239.2 | 29.79 | † | |||
| tEME-EE’ | 350,35–341,34 | 273 979.089 | 239.2 | 29.79 | † | |||
| tEME-AE | 350,35–341,34 | 273 979.093 | 239.2 | 29.79 | † | |||
| tEME-AA | 361,35–352,34 | 274 946.543 | 260.0 | 19.65 | 274 946.9 | 0.57 | 0.14 | CH3OCOH vt = 1, H2CS |
| tEME-EA | 361,35–352,34 | 274 946.555 | 260.0 | 19.65 | † | |||
| tEME-AE | 361,35–352,34 | 274 947.090 | 260.0 | 19.65 | † | |||
| tEME-EE | 361,35–352,34 | 274 947.102 | 260.0 | 19.65 | † | |||
| tEME-EE’ | 361,35–352,34 | 274 947.102 | 260.0 | 19.65 | † | |||
| tEME-EE’ | 351,35–340,34 | 275 618.927 | 239.3 | 29.80 | 275 618.9 | 0.22 | 0.28 | |
| tEME-EE | 351,35–340,34 | 275 618.927 | 239.3 | 29.80 | † | |||
| tEME-AE | 351,35–340,34 | 275 618.934 | 239.3 | 29.80 | † | |||
| tEME-EA | 351,35–340,34 | 275 619.009 | 239.3 | 29.80 | † | |||
| tEME-AA | 351,35–340,34 | 275 619.016 | 239.3 | 29.80 | † | |||
| tEME-EE’ | 322,31–311,30 | 276 219.631 | 207.8 | 15.92 | 276 219.6 | 0.34 | 0.15 | 13CH3OCOH |
| tEME-EE | 322,31–311,30 | 276 219.631 | 207.8 | 15.92 | † | |||
| tEME-AE | 322,31–311,30 | 276 219.695 | 207.8 | 15.92 | † | |||
| tEME-EA | 322,31–311,30 | 276 220.573 | 207.8 | 15.92 | † | |||
| tEME-AA | 322,31–311,30 | 276 220.637 | 207.8 | 15.92 | † | |||
| tEME-EE | 193,16–182,17 | 276 700.152 | 83.9 | 5.20 | 276 700.3 | 0.29 | 0.13 | CH3CN v8 = 1, CH3OH |
| tEME-EE’ | 193,16–182,17 | 276 700.167 | 83.9 | 5.20 | † | |||
| tEME-AE | 193,16–182,17 | 276 700.458 | 83.9 | 5.20 | † | |||
| tEME-EA | 193,16–182,17 | 276 702.533 | 83.9 | 5.20 | 276 702.5 | 0.34 | 0.08 | CH3CN v8 = 1, CH3OH |
| tEME-AA | 193,16–182,17 | 276 702.832 | 83.9 | 5.20 | † | |||
| tEME-EE’ | 213,19–202,18 | 278 825.580 | 99.7 | 6.00 | 278 825.7 | 0.39 | 0.14 | CH3CH2CN, CH3CH2CN v20 = 1, CH313CH2CN |
| tEME-EE | 213,19–202,18 | 278 825.588 | 99.7 | 6.00 | † | |||
| tEME-AE | 213,19–202,18 | 278 825.856 | 99.7 | 6.00 | † | |||
| tEME-EA | 213,19–202,18 | 278 828.090 | 99.7 | 6.00 | 278 828.4 | 0.33 | 0.09 | CH3CH2CN, CH3CH2CN v20 = 1, CH313CH2CN |
| tEME-AA | 213,19–202,18 | 278 828.362 | 99.7 | 6.00 | † | |||
| tEME-EE | 85,3–74,3 | 280 075.262 | 42.7 | 4.85 | 280 075.6 | 0.40 | 0.38 | |
| tEME-EE’ | 85,4–74,4 | 280 075.328 | 42.7 | 4.85 | † | |||
| tEME-EE’ | 85,3–74,3 | 280 075.462 | 42.7 | 4.85 | † | |||
| tEME-EE | 85,4–74,4 | 280 075.527 | 42.7 | 4.85 | † | |||
| tEME-AE | 85,3–74,3 | 280 075.816 | 42.7 | 4.85 | † | |||
| tEME-AE | 85,4–74,4 | 280 075.882 | 42.7 | 4.85 | † | |||
| tEME-EA | 85,4–74,4 | 280 078.526 | 42.7 | 4.85 | 280 078.5 | 0.31 | 0.25 | |
| tEME-EA | 85,3–74,3 | 280 078.726 | 42.7 | 4.85 | † | |||
| tEME-AA | 85,4–74,3 | 280 079.076 | 42.7 | 4.85 | † | |||
| tEME-AA | 85,3–74,4 | 280 079.084 | 42.7 | 4.85 | † | |||
| tEME-EE’ | 144,11–133,10 | 280 288.955 | 59.0 | 2.84 | … | … | 0.07 | CH3COOH vt = 1 |
| tEME-AE | 144,11–133,10 | 280 290.299 | 59.0 | 2.96 | † | |||
| tEME-EE | 144,11–133,10 | 280 290.853 | 59.0 | 3.11 | † | |||
| tEME-EA | 144,11–133,10 | 280 297.013 | 59.0 | 4.69 | 280 297.7 | 0.12 | 0.10 | |
| tEME-AA | 144,11–133,10 | 280 297.720 | 59.0 | 5.30 | † | |||
| tEME-EE | 144,10–133,10 | 280 299.704 | 59.0 | 2.19 | 280 301.1 | 0.10 | 0.06 | U-line |
| tEME-EA | 144,10–133,10 | 280 299.889 | 59.0 | 0.61 | † | |||
| tEME-AE | 144,10–133,10 | 280 300.898 | 59.0 | 2.34 | † | |||
| tEME-EE’ | 144,10–133,10 | 280 301.316 | 59.0 | 2.46 | † | |||
| tEME-EE’ | 144,11–133,11 | 280 383.694 | 59.0 | 2.46 | 280 385.0 | 0.11 | 0.06 | CH3OCOH vt = 1 |
| tEME-AE | 144,11–133,11 | 280 384.884 | 59.0 | 2.34 | † | |||
| tEME-EE | 144,11–133,11 | 280 385.307 | 59.0 | 2.19 | † | |||
| tEME-EA | 144,10–133,11 | 280 394.040 | 59.0 | 4.69 | … | … | 0.16 | CH3OCOH |
| tEME-AA | 144,10–133,11 | 280 394.104 | 59.0 | 5.30 | † | |||
| tEME-EE | 144,10–133,11 | 280 394.157 | 59.0 | 3.11 | † | |||
| tEME-AE | 144,10–133,11 | 280 395.482 | 59.0 | 2.96 | † | |||
| tEME-EE’ | 144,10–133,11 | 280 396.055 | 59.0 | 2.83 | † | |||
| tEME-EA | 360,36–351,35 | 281 871.802 | 252.8 | 30.81 | 281 871.7 | 0.25 | 0.27 | U-line |
| tEME-AA | 360,36–351,35 | 281 871.806 | 252.8 | 30.81 | † | |||
| tEME-EE | 360,36–351,35 | 281 871.822 | 252.8 | 30.81 | † | |||
| tEME-EE’ | 360,36–351,35 | 281 871.822 | 252.8 | 30.81 | † | |||
| tEME-AE | 360,36–351,35 | 281 871.826 | 252.8 | 30.81 | † | |||
| tEME-EE’ | 332,32–321,31 | 282 152.573 | 220.4 | 16.88 | 282 153.2 | 0.56 | 0.16 | HC3N v5 = 1 |
| tEME-EE | 332,32–321,31 | 282 152.573 | 220.4 | 16.88 | † | |||
| tEME-AE | 332,32–321,31 | 282 152.631 | 220.4 | 16.88 | † | |||
| tEME-EA | 332,32–321,31 | 282 153.461 | 220.4 | 16.88 | † | |||
| tEME-AA | 332,32–321,31 | 282 153.519 | 220.4 | 16.88 | † | |||
| tEME-EE’ | 361,36–350,35 | 283 263.283 | 252.8 | 30.82 | 283 263.4 | 0.44 | 0.27 | 13CH3OH |
| tEME-EE | 361,36–350,35 | 283 263.283 | 252.8 | 30.82 | † | |||
| tEME-AE | 361,36–350,35 | 283 263.290 | 252.8 | 30.82 | † | |||
| tEME-EA | 361,36–350,35 | 283 263.357 | 252.8 | 30.82 | † | |||
| tEME-AA | 361,36–350,35 | 283 263.364 | 252.8 | 30.82 | † | |||
| tEME-AE | 412,39–403,38 | 283 951.422 | 341.0 | 13.47 | … | … | 0.03 | CH3OCH3 |
| tEME-EE | 412,39–403,38 | 283 951.485 | 341.0 | 13.47 | † | |||
| tEME-EE’ | 412,39–403,38 | 283 951.485 | 341.0 | 13.47 | † | |||
| tEME-AA | 371,36–362,35 | 284 137.669 | 274.2 | 20.72 | 288 138.3 | 0.34 | 0.14 | CH3OCOH vt = 1 |
| tEME-EA | 371,36–362,35 | 284 137.677 | 274.2 | 20.72 | † | |||
| tEME-AE | 371,36–362,35 | 284 138.161 | 274.2 | 20.72 | † | |||
| tEME-EE | 371,36–362,35 | 284 138.169 | 274.2 | 20.72 | † | |||
| tEME-EE’ | 371,36–362,35 | 284 138.169 | 274.2 | 20.72 | † | |||
| tEME-EE’ | 223,20–212,19 | 285 037.438 | 108.2 | 6.21 | … | … | 0.14 | CH3CH2CN v13/v21 |
| tEME-EE | 223,20–212,19 | 285 037.444 | 108.2 | 6.21 | † | |||
| tEME-AE | 223,20–212,19 | 285 037.708 | 108.2 | 6.21 | † | |||
| tEME-EA | 223,20–212,19 | 285 039.939 | 108.2 | 6.21 | … | … | 0.09 | CH3CH2CN v13/v21 |
| tEME-AA | 223,20–212,19 | 285 040.206 | 108.2 | 6.21 | † | |||
| tEME-EE | 203,17–192,18 | 285 699.473 | 91.6 | 5.28 | … | … | 0.14 | SO2 |
| tEME-EE’ | 203,17–192,18 | 285 699.484 | 91.6 | 5.28 | † | |||
| tEME-AE | 203,17–192,18 | 285 699.777 | 91.6 | 5.28 | † | |||
| tEME-EA | 203,17–192,18 | 285 701.840 | 91.6 | 5.28 | … | … | 0.10 | SO2 |
| tEME-AA | 203,17–192,18 | 285 702.139 | 91.6 | 5.28 | † | |||
| tEME-EE | 212,19–201,20 | 286 585.825 | 94.5 | 1.86 | … | … | 0.05 | CH3COOH vt = 1 |
| tEME-EE’ | 212,19–201,20 | 286 585.825 | 94.5 | 1.86 | † | |||
| tEME-AE | 212,19–201,20 | 286 586.110 | 94.5 | 1.86 | † | |||
| tEME-EA | 212,19–201,20 | 286 587.511 | 94.5 | 1.86 | … | … | 0.03 | U-line |
| tEME-AA | 212,19–201,20 | 286 587.796 | 94.5 | 1.86 | † | |||
| tEME-EE | 95,4–84,4 | 288 124.443 | 46.1 | 5.02 | 288 124.8 | 0.59 | 0.41 | |
| tEME-EE’ | 95,5–84,5 | 288 124.510 | 46.1 | 5.02 | † | |||
| tEME-EE’ | 95,4–84,4 | 288 124.641 | 46.1 | 5.02 | † | |||
| tEME-EE | 95,5–84,5 | 288 124.708 | 46.1 | 5.02 | † | |||
| tEME-AE | 95,4–84,4 | 288 124.995 | 46.1 | 5.02 | † | |||
| tEME-AE | 95,5–84,5 | 288 125.062 | 46.1 | 5.02 | † | |||
| tEME-EA | 95,5–84,5 | 288 127.703 | 46.1 | 5.02 | … | … | 0.27 | CH3OCOH |
| tEME-EA | 95,4–84,4 | 288 127.901 | 46.1 | 5.02 | † | |||
| tEME-AA | 95,5–84,4 | 288 128.242 | 46.1 | 5.02 | † | |||
| tEME-AA | 95,4–84,5 | 288 128.268 | 46.1 | 5.02 | † | |||
| tEME-EE’ | 342,33–331,32 | 288 187.160 | 233.4 | 17.86 | 288 187.5 | 0.54 | 0.17 | CH3OCOH |
| tEME-EE | 342,33–331,32 | 288 187.160 | 233.4 | 17.86 | † | |||
| tEME-AE | 342,33–331,32 | 288 187.214 | 233.4 | 17.86 | † | |||
| tEME-EA | 342,33–331,32 | 288 187.993 | 233.4 | 17.86 | † | |||
| tEME-AA | 342,33–331,32 | 288 188.047 | 233.4 | 17.86 | † | |||
| tEME-EE’ | 154,12–143,11 | 288 267.539 | 64.8 | 3.40 | … | … | 0.09 | SO18O, CH3COCH3 |
| tEME-AE | 154,12–143,11 | 288 268.817 | 64.8 | 3.55 | † | |||
| tEME-EE | 154,12–143,11 | 288 269.302 | 64.8 | 3.75 | † | |||
| tEME-EA | 154,12–143,11 | 288 274.971 | 64.8 | 5.24 | 288 275.12 | 0.14 | 0.12 | SO18O |
| tEME-AA | 154,12–143,11 | 288 275.555 | 64.8 | 5.50 | † | |||
| tEME-EE | 154,11–143,11 | 288 278.681 | 64.8 | 1.75 | … | … | 0.05 | U-line |
| tEME-AE | 154,11–143,11 | 288 279.849 | 64.8 | 1.95 | † | |||
| tEME-EE’ | 154,11–143,11 | 288 280.260 | 64.8 | 2.11 | † | |||
| tEME-EE’ | 154,12–143,12 | 288 413.150 | 64.8 | 2.11 | … | … | 0.05 | U-line |
| tEME-AE | 154,12–143,12 | 288 414.329 | 64.8 | 1.95 | † | |||
| tEME-EE | 154,12–143,12 | 288 414.729 | 64.8 | 1.75 | † | |||
| tEME-EE | 154,11–143,12 | 288 424.108 | 64.8 | 3.75 | 288 424.7 | 0.50 | 0.20 | CH3OC18OH |
| tEME-EA | 154,11–143,12 | 288 424.469 | 64.8 | 5.24 | † | |||
| tEME-AA | 154,11–143,12 | 288 424.653 | 64.8 | 5.50 | † | |||
| tEME-AE | 154,11–143,12 | 288 425.361 | 64.8 | 3.55 | † | |||
| tEME-EE’ | 154,11–143,12 | 288 425.871 | 64.8 | 3.40 | † | |||
| tEME-EA | 370,37–361,36 | 289 745.973 | 266.7 | 31.83 | 289 745.8 | 0.49 | 0.26 | U-line |
| tEME-AA | 370,37–361,36 | 289 745.977 | 266.7 | 31.83 | † | |||
| tEME-EE | 370,37–361,36 | 289 745.986 | 266.7 | 31.83 | † | |||
| tEME-EE’ | 370,37–361,36 | 289 745.986 | 266.7 | 31.83 | † | |||
| tEME-AE | 370,37–361,36 | 289 745.991 | 266.7 | 31.83 | † | |||
| tEME-EE’ | 371,37–360,36 | 290 924.982 | 266.8 | 31.83 | 290 925.0 | 0.71 | 0.27 | 33SO2 |
| tEME-EE | 371,37–360,36 | 290 924.982 | 266.8 | 31.83 | † | |||
| tEME-AE | 371,37–360,36 | 290 924.989 | 266.8 | 31.83 | † | |||
| tEME-EA | 371,37–360,36 | 290 925.049 | 266.8 | 31.83 | † | |||
| tEME-AA | 371,37–360,36 | 290 925.056 | 266.8 | 31.83 | † | |||
| tEME-AA | 381,37–372,36 | 293 203.620 | 288.8 | 21.79 | 293 203.7 | 0.28 | 0.14 | |
| tEME-EA | 381,37–372,36 | 293 203.624 | 288.8 | 21.79 | † | |||
| tEME-AE | 381,37–372,36 | 293 204.059 | 288.8 | 21.79 | † | |||
| tEME-EE | 381,37–372,36 | 293 204.064 | 288.8 | 21.79 | † | |||
| tEME-EE’ | 381,37–372,36 | 293 204.064 | 288.8 | 21.79 | † | |||
| tEME-EE’ | 352,34–341,33 | 294 329.625 | 246.8 | 18.86 | 294 330.0 | 0.28 | 0.17 | |
| tEME-EE | 352,34–341,33 | 294 329.625 | 246.8 | 18.86 | † | |||
| tEME-AE | 352,34–341,33 | 294 329.673 | 246.8 | 18.86 | † | |||
| tEME-EA | 352,34–341,33 | 294 330.403 | 246.8 | 18.86 | † | |||
| tEME-AA | 352,34–341,33 | 294 330.451 | 246.8 | 18.86 | † | |||
| tEME-EE | 213,18–202,19 | 294 868.623 | 99.8 | 5.34 | … | … | 0.15 | CH3OCOH |
| tEME-EE’ | 213,18–202,19 | 294 868.631 | 99.8 | 5.34 | † | |||
| tEME-AE | 213,18–202,19 | 294 868.926 | 99.8 | 5.34 | † | |||
| tEME-EE | 372,36–362,35 | 294 869.441 | 274.8 | 36.86 | † | |||
| tEME-EE’ | 372,36–362,35 | 294 869.441 | 274.8 | 36.86 | † | |||
| tEME-AE | 372,36–362,35 | 294 869.456 | 274.8 | 36.86 | † | |||
| tEME-EA | 372,36–362,35 | 294 869.504 | 274.8 | 36.86 | † | |||
| tEME-AA | 372,36–362,35 | 294 869.519 | 274.8 | 36.86 | † | |||
| tEME-EA | 213,18–202,19 | 294 870.971 | 99.8 | 5.34 | … | … | 0.10 | CH3OCOH |
| tEME-AA | 213,18–202,19 | 294 871.271 | 99.8 | 5.34 | † | |||
| tEME-AA | 422,40–413,39 | 295 213.922 | 357.4 | 14.32 | … | … | 0.03 | CH3CN v8 = 1 |
| tEME-EA | 422,40–413,39 | 295 213.976 | 357.4 | 14.32 | † | |||
| tEME-AE | 422,40–413,39 | 295 215.469 | 357.4 | 14.32 | † | |||
| tEME-EE | 422,40–413,39 | 295 215.522 | 357.4 | 14.32 | † | |||
| tEME-EE’ | 422,40–413,39 | 295 215.522 | 357.4 | 14.32 | † | |||
| tEME-EE | 105,5–94,5 | 296 172.265 | 50.0 | 5.20 | … | … | 0.45 | SO2 |
| tEME-EE’ | 105,6–94,6 | 296 172.333 | 50.0 | 5.20 | † | |||
| tEME-EE’ | 105,5–94,5 | 296 172.462 | 50.0 | 5.20 | † | |||
| tEME-EE | 105,6–94,6 | 296 172.530 | 50.0 | 5.20 | † | |||
| tEME-AE | 105,5–94,5 | 296 172.815 | 50.0 | 5.20 | † | |||
| tEME-AE | 105,6–94,6 | 296 172.884 | 50.0 | 5.20 | † | |||
| tEME-EA | 105,6–94,6 | 296 175.521 | 50.0 | 5.20 | … | … | 0.30 | SO2 |
| tEME-EA | 105,5–94,5 | 296 175.717 | 50.0 | 5.20 | † | |||
| tEME-AA | 105,6–94,5 | 296 176.037 | 50.0 | 5.20 | † | |||
| tEME-AA | 105,5–94,6 | 296 176.104 | 50.0 | 5.20 | † | |||
| tEME-EE’ | 164,13–153,12 | 296 223.035 | 71.0 | 4.07 | 296 224.2 | 0.49 | 0.11 | CH3OCOH |
| tEME-AE | 164,13–153,12 | 296 224.220 | 71.0 | 4.26 | † | |||
| tEME-EE | 164,13–153,12 | 296 224.598 | 71.0 | 4.49 | † | |||
| tEME-EA | 164,13–153,12 | 296 229.670 | 71.0 | 5.60 | 296 230.0 | 0.30 | 0.13 | CH3OCOH |
| tEME-AA | 164,13–153,12 | 296 230.175 | 71.0 | 5.70 | † | |||
| tEME-EE | 164,12–153,12 | 296 235.284 | 71.0 | 1.21 | … | … | 0.04 | CH3CH2CN v12 = 1 |
| tEME-AE | 164,12–153,12 | 296 236.371 | 71.0 | 1.44 | † | |||
| tEME-EE’ | 164,12–153,12 | 296 236.725 | 71.0 | 1.63 | † | |||
| tEME-EE’ | 164,13–153,13 | 296 440.645 | 71.0 | 1.63 | … | … | 0.04 | CH3CH2CN |
| tEME-AE | 164,13–153,13 | 296 441.764 | 71.0 | 1.44 | † | |||
| tEME-EE | 164,13–153,13 | 296 442.086 | 71.0 | 1.21 | † | |||
| tEME-EE | 164,12–153,13 | 296 452.772 | 71.0 | 4.49 | … | … | 0.24 | CH3CH2CN |
| tEME-EA | 164,12–153,13 | 296 453.716 | 71.0 | 5.59 | † | |||
| tEME-AE | 164,12–153,13 | 296 453.915 | 71.0 | 4.26 | † | |||
| tEME-AA | 164,12–153,13 | 296 453.976 | 71.0 | 5.70 | † | |||
| tEME-EE’ | 164,12–153,13 | 296 454.335 | 71.0 | 4.07 | † | |||
| tEME-EE’ | 243,22–232,21 | 296 926.193 | 126.4 | 6.66 | 296 926.5 | 0.17 | 0.14 | |
| tEME-EE | 243,22–232,21 | 296 926.197 | 126.4 | 6.66 | † | |||
| tEME-AE | 243,22–232,21 | 296 926.450 | 126.4 | 6.66 | † | |||
| tEME-EA | 243,22–232,21 | 296 928.675 | 126.4 | 6.66 | 296 928.7 | 0.42 | 0.09 | CH3OCOH vt = 2 |
| tEME-AA | 243,22–232,21 | 296 928.931 | 126.4 | 6.66 | † | |||
| tEME-EA | 380,38–371,37 | 297 603.969 | 281.1 | 32.84 | … | … | 0.25 | CH3OCOH vt = 2 |
| tEME-AA | 380,38–371,37 | 297 603.974 | 281.1 | 32.84 | † | |||
| tEME-EE | 380,38–371,37 | 297 603.978 | 281.1 | 32.84 | † | |||
| tEME-EE’ | 380,38–371,37 | 297 603.978 | 281.1 | 32.84 | † | |||
| tEME-AE | 380,38–371,37 | 297 603.982 | 281.1 | 32.84 | † | |||
| tEME-EE’ | 381,38–370,37 | 298 601.597 | 281.1 | 32.85 | … | … | 0.26 | SO2 |
| tEME-EE | 381,38–370,37 | 298 601.597 | 281.1 | 32.85 | † | |||
| tEME-AE | 381,38–370,37 | 298 601.604 | 281.1 | 32.85 | † | |||
| tEME-EA | 381,38–370,37 | 298 601.658 | 281.1 | 32.85 | † | |||
| tEME-AA | 381,38–370,37 | 298 601.665 | 281.1 | 32.85 | † | |||
| tEME-EE | 222,20–211,21 | 300 442.750 | 103.1 | 1.73 | … | … | 0.05 | H13CCCN v7 = 1, CH3OCOH |
| tEME-EE’ | 222,20–211,21 | 300 442.750 | 103.1 | 1.73 | † | |||
| tEME-AE | 222,20–211,21 | 300 443.046 | 103.1 | 1.73 | † | |||
| tEME-EA | 222,20–211,21 | 300 444.445 | 103.1 | 1.73 | … | … | 0.03 | H13CCCN v7 = 1, CH3OCOH |
| tEME-AA | 222,20–211,21 | 300 444.741 | 103.1 | 1.73 | † | |||
| tEME-EE’ | 362,35–351,34 | 300 583.963 | 260.6 | 19.88 | 300 584.4 | 0.25 | 0.17 | |
| tEME-EE | 362,35–351,34 | 300 583.963 | 260.6 | 19.88 | † | |||
| tEME-AE | 362,35–351,34 | 300 584.008 | 260.6 | 19.88 | † | |||
| tEME-EA | 362,35–351,34 | 300 584.687 | 260.6 | 19.88 | † | |||
| tEME-AA | 362,35–351,34 | 300 584.731 | 260.6 | 19.88 | † | |||
| tEME-AA | 391,38–382,37 | 302 148.596 | 303.8 | 22.87 | … | … | 0.14 | SO2 |
| tEME-EA | 391,38–382,37 | 302 148.597 | 303.8 | 22.87 | † | |||
| tEME-AE | 391,38–382,37 | 302 148.985 | 303.8 | 22.87 | † | |||
| tEME-EE | 391,38–382,37 | 302 148.986 | 303.8 | 22.87 | † | |||
| tEME-EE’ | 391,38–382,37 | 302 148.986 | 303.8 | 22.87 | † | |||
| tEME-EE’ | 253,23–242,22 | 302 611.864 | 136.1 | 6.91 | … | … | 0.14 | CH3COCH3 |
| tEME-EE | 253,23–242,22 | 302 611.867 | 136.1 | 6.91 | † | |||
| tEME-AE | 253,23–242,22 | 302 612.114 | 136.1 | 6.91 | † | |||
| tEME-EA | 253,23–242,22 | 302 614.334 | 136.1 | 6.91 | … | … | 0.09 | CH3COCH3 |
| tEME-AA | 253,23–242,22 | 302 614.582 | 136.0 | 6.91 | † | |||
| tEME-EE’ | 174,14–163,13 | 304 150.005 | 77.5 | 4.81 | … | … | 0.14 | CH2CHCN |
| tEME-AE | 174,14–163,13 | 304 151.052 | 77.5 | 4.99 | † | |||
| tEME-EE | 174,14–163,13 | 304 151.281 | 77.5 | 5.19 | † | |||
| tEME-EA | 174,14–163,13 | 304 155.769 | 77.5 | 5.86 | … | … | 0.14 | CH2CHCN |
| tEME-AA | 174,14–163,13 | 304 156.225 | 77.5 | 5.90 | † | |||
| tEME-EE | 115,6–104,6 | 304 218.289 | 54.3 | 5.39 | … | … | 0.48 | CH3OH |
| tEME-EE’ | 115,7–104,7 | 304 218.360 | 54.3 | 5.39 | † | |||
| tEME-EE’ | 115,6–104,6 | 304 218.485 | 54.3 | 5.39 | † | |||
| tEME-EE | 115,7–104,7 | 304 218.556 | 54.3 | 5.39 | † | |||
| tEME-AE | 115,6–104,6 | 304 218.838 | 54.3 | 5.39 | † | |||
| tEME-AE | 115,7–104,7 | 304 218.909 | 54.3 | 5.39 | † | |||
| tEME-EA | 115,7–104,7 | 304 221.544 | 54.3 | 5.38 | … | … | 0.32 | CH3OH |
| tEME-EA | 115,6–104,6 | 304 221.733 | 54.3 | 5.38 | † | |||
| tEME-AA | 115,7–104,6 | 304 222.011 | 54.3 | 5.39 | † | |||
| tEME-AA | 115,6–104,7 | 304 222.167 | 54.3 | 5.39 | † | |||
| tEME-EE | 223,19–212,20 | 304 228.127 | 108.3 | 5.38 | … | … | 0.14 | CH3COCH3 |
| tEME-EE’ | 223,19–212,20 | 304 228.133 | 108.3 | 5.38 | † | |||
| tEME-AE | 223,19–212,20 | 304 228.430 | 108.3 | 5.38 | † | |||
| tEME-EA | 223,19–212,20 | 304 230.454 | 108.3 | 5.38 | … | … | 0.10 | CH3COCH3 |
| tEME-AA | 223,19–212,20 | 304 230.754 | 108.3 | 5.38 | † | |||
| tEME-EE | 174,13–163,14 | 304 481.623 | 77.5 | 5.19 | … | … | 0.25 | CH3OCOH vt = 1 |
| tEME-AE | 174,13–163,14 | 304 482.613 | 77.5 | 4.99 | † | |||
| tEME-EE’ | 174,13–163,14 | 304 482.900 | 77.5 | 4.81 | † | |||
| tEME-EA | 174,13–163,14 | 304 483.137 | 77.5 | 5.85 | † | |||
| tEME-AA | 174,13–163,14 | 304 483.441 | 77.5 | 5.90 | † | |||
| tEME-EA | 390,39–381,38 | 305 447.899 | 295.7 | 33.86 | 305 448.1 | 0.96 | 0.24 | CH3CH2CN v13/v21 |
| tEME-EE | 390,39–381,38 | 305 447.903 | 295.7 | 33.86 | † | |||
| tEME-EE’ | 390,39–381,38 | 305 447.903 | 295.7 | 33.86 | † | |||
| tEME-AA | 390,39–381,38 | 305 447.904 | 295.7 | 33.86 | † | |||
| tEME-AE | 390,39–381,38 | 305 447.908 | 295.7 | 33.86 | † | |||
| tEME-EE | 391,39–380,38 | 306 290.978 | 295.7 | 33.86 | … | … | 0.24 | CH3OH |
| tEME-EE’ | 391,39–380,38 | 306 290.978 | 295.7 | 33.86 | † | |||
| tEME-AE | 391,39–380,38 | 306 290.985 | 295.7 | 33.86 | † | |||
| tEME-EA | 391,39–380,38 | 306 291.035 | 295.7 | 33.86 | † | |||
| tEME-AA | 391,39–380,38 | 306 291.041 | 295.7 | 33.86 | † | |||
| tEME-AA | 432,41–423,40 | 306 373.059 | 374.1 | 15.22 | … | … | 0.03 | CH3OCH3 |
| tEME-EA | 432,41–423,40 | 306 373.104 | 374.1 | 15.22 | † | |||
| tEME-AE | 432,41–423,40 | 306 374.522 | 374.1 | 15.22 | † | |||
| tEME-EE | 432,41–423,40 | 306 374.567 | 374.1 | 15.22 | † | |||
| tEME-EE’ | 432,41–23,40 | 306 374.567 | 374.1 | 15.22 | † |
Notes. Lines of trans-CH3CH2OCH3 (tEME) ground state present in the spectral scan of Orion KL from the 30 m telescope. Column 1 indicates the species, Col. 2 gives the transition, Col. 3 the predicted frequency, Col. 4 upper level energy, Col. 5 the line strength, Col. 6 observed frequency at the peak Channel of the line (relative to a vLSR of +7.5 km s−1), Col. 7 main beam temperature at the peak Channel of the line, and Col. 8 shows blends with other molecular species.
Observed frequencies and intensities are not provided for features that appear totally blended with lines from other species.
We address all features provided by our model with TMB > 0.01 K, TMB > 0.02 K, and TMB > 0.03 K in the frequency ranges between 80.7–116, 122.7–150, and 150–306.7 GHz, respectively.
Blended with previous line.
Table A.3.
Detected lines of gauche-trans-n-CH3CH2CH2OH.
| Species | Transition |
Predicted frequency (MHz) |
Eupp (K) |
Sij | Observed frequency (MHz) |
T (K) |
Blends/ Comments |
|---|---|---|---|---|---|---|---|
| G-n-propanol | 127,6–126,6 | 124 417.241 | 58.0 | 4.25 | 124 417.1 | 0.04 | 30 m; U-line |
| G-n-propanol | 127,5–126,6 | 124 417.256 | 58.0 | 4.89 | † | ||
| G-n-propanol | 127,6–126,7 | 124 418.125 | 58.0 | 4.89 | 124 418.2† | 0.06 | 30 m; U-line |
| G-n-propanol | 127,5–126,7 | 124 418.139 | 58.0 | 4.25 | † | ||
| G-n-propanol | 152,14–141,13 | 141 219.413 | 55.4 | 9.14 | 141 219.8 | 0.04 | 30 m |
| Gt-n-propanol | 65,2–54,1 | 143 143.854 | 21.1 | 4.51 | 143 144.6 | 0.10 | 30 m; CH3CH2CN v12 = 1 |
| Gt-n-propanol | 65,1–54,1 | 143 143.873 | 21.1 | 4.65 | † | ||
| Gt-n-propanol | 65,2–54,2 | 143 144.406 | 21.1 | 4.65 | † | ||
| Gt-n-propanol | 65,1–54,2 | 143 144.426 | 21.1 | 4.51 | † | ||
| Gt-n-propanol | 87,2–76,1 | 200 433.919 | 38.9 | 6.49 | 200 434.4 | 0.13 | 30 m |
| Gt-n-propanol | 87,1–76,1 | 200 433.919 | 38.9 | 6.63 | † | ||
| Gt-n-propanol | 87,2–76,2 | 200 433.920 | 38.9 | 6.63 | † | ||
| Gt-n-propanol | 87,1–76,2 | 200 433.920 | 38.9 | 6.49 | † | ||
| Gt-n-propanol | 2311,13–2310,13 | 200 508.301 | 181.2 | 8.64 | 200 508.8 | 0.07 | 30 m |
| Gt-n-propanol | 2311,12–2310,13 | 200 508.302 | 181.2 | 10.20 | † | ||
| Gt-n-propanol | 2311,13–2310,14 | 200 508.311 | 181.2 | 10.20 | † | ||
| Gt-n-propanol | 2311,12–2310,14 | 200 508.312 | 181.2 | 8.64 | † | ||
| Gt-n-propanol | 97,3–86,2 | 209 892.537 | 43.0 | 6.54 | 209 892.5 | 0.19 | 30 m; CH2CHCN v11 = 1 |
| Gt-n-propanol | 97,2–86,2 | 209 892.538 | 43.0 | 6.78 | † | ||
| Gt-n-propanol | 97,3–86,3 | 209 892.542 | 43.0 | 6.78 | † | ||
| Gt-n-propanol | 97,2–86,3 | 209 892.542 | 43.0 | 6.54 | † | ||
| Gt-n-propanol | 240,24–231,23 | 210 248.928 | 127.7 | 22.15 | 210 249.1 | 0.17 | 30 m |
| Gt-n-propanol | 241,24–231,23 | 210 250.060 | 127.7 | 23.86 | † | ||
| Gt-n-propanol | 240,24–230,23 | 210 250.810 | 127.7 | 23.86 | 210 252.8 | 0.16 | 30 m |
| Gt-n-propanol | 241,24–230,23 | 210 251.942 | 127.7 | 22.15 | † | ||
| Gt-n-propanol | 4412,32–4411,34 | 210 252.224 | 517.3 | 16.71 | † | ||
| Gt-n-propanol | 3712,26–3711,26 | 215 663.302 | 386.2 | 14.57 | … | … | ALMA; CH3CH2CN v13/v21 |
| Gt-n-propanol | 3712,25–3711,26 | 215 663.793 | 386.2 | 19.40 | … | … | ” |
| Gt-n-propanol | 3712,26–3711,27 | 215 663.793 | 386.2 | 19.40 | … | … | ” |
| Gt-n-propanol | 3712,25–3711,27 | 215 663.793 | 386.2 | 19.40 | … | … | ” |
| Gt-n-propanol | 3712,26–3711,27 | 215 672.076 | 386.2 | 19.40 | … | … | ALMA; CH3COOH vt = 1 |
| Gt-n-propanol | 3712,25–3711,27 | 215 672.568 | 386.2 | 14.57 | … | … | ” |
| Gt-n-propanol | 243,21–234,20 | 216 493.373 | 143.6 | 9.36 | … | … | ALMA; CH3OCOH |
| Gt-n-propanol | 145,10–134,9 | 217 132.672 | 59.3 | 5.22 | … | … | ALMA; CH3O13COH vt = 1, CH3OD |
| Gt-n-propanol | 3412,23–3411,23 | 217 158.546 | 337.0 | 13.43 | 217 159.0 | 0.48 | ALMA; CH3COOCH3 |
| Gt-n-propanol | 3412,22–3411,23 | 217 158.612 | 337.0 | 17.25 | † | ||
| Gt-n-propanol | 3412,23–3411,24 | 217 159.977 | 337.0 | 17.25 | † | ||
| Gt-n-propanol | 3412,22–3411,24 | 217 160.043 | 337.0 | 13.43 | † | ||
| Gt-n-propanol | 3212,21–3211,21 | 217 935.916 | 306.4 | 12.61 | … | … | ALMA;CH3OCH3, CH3O13COH vt = 1 |
| Gt-n-propanol | 3212,20–3211,21 | 217 935.931 | 306.4 | 15.85 | … | … | ” |
| Gt-n-propanol | 3212,21–3211,22 | 217 936.298 | 306.4 | 15.85 | … | … | ” |
| Gt-n-propanol | 3212,20–3211,22 | 217 936.314 | 306.4 | 12.61 | … | … | ” |
| Gt-n-propanol | 3112,20–3111,20 | 218 268.548 | 291.9 | 12.18 | 218 269.0 | 0.84 | ALMA; U-line |
| Gt-n-propanol | 3112,19–3111,20 | 218 268.555 | 291.9 | 12.15 | † | ||
| Gt-n-propanol | 3112,20–3111,21 | 218 268.739 | 291.9 | 12.15 | † | ||
| Gt-n-propanol | 3112,19–3111,21 | 218 268.746 | 291.9 | 12.18 | † | ||
| Gt-n-propanol | 3012,19–3011,19 | 218 567.524 | 277.7 | 11.74 | … | … | ALMA; CH3O13COH vt = 1 |
| Gt-n-propanol | 3012,18–3011,19 | 218 567.527 | 277.7 | 14.45 | … | … | ” |
| Gt-n-propanol | 3012,19–3011,20 | 218 567.616 | 277.7 | 14.45 | … | … | ” |
| Gt-n-propanol | 3012,18–3011,20 | 218 567.619 | 277.7 | 11.74 | … | … | ” |
| Gt-n-propanol | 2912,18–2911,18 | 218 835.376 | 264.1 | 11.28 | 218 835.5 | 1.05 | ALMA; |
| Gt-n-propanol | 2912,17–2911,18 | 218 835.377 | 264.1 | 13.76 | † | ||
| Gt-n-propanol | 2912,18–2911,19 | 218 835.419 | 264.1 | 13.76 | † | ||
| Gt-n-propanol | 2912,17–2911,19 | 218 835.421 | 264.1 | 11.28 | † | ||
| Gt-n-propanol | 250,25–241,24 | 218 880.233 | 138.2 | 23.15 | 218 881.2 | 0.80 | ALMA |
| Gt-n-propanol | 251,25–241,24 | 218 880.912 | 138.2 | 24.86 | † | ||
| Gt-n-propanol | 250,25–240,24 | 218 881.365 | 138.2 | 24.86 | † | ||
| Gt-n-propanol | 251,25–240,24 | 218 882.044 | 138.2 | 23.15 | † | ||
| Gt-n-propanol | 126,7–115,6 | 218 945.655 | 52.0 | 5.95 | 218 946.4 | 0.73 | ALMA |
| Gt-n-propanol | 126,6–115,6 | 218 946.539 | 52.0 | 6.77 | † | ||
| Gt-n-propanol | 126,7–115,7 | 218 959.585 | 52.0 | 6.77 | 218 960.4 | 0.76 | ALMA |
| Gt-n-propanol | 163,14–152,14 | 218 960.392 | 65.9 | 5.29 | † | ||
| Gt-n-propanol | 126,6–115,7 | 218 960.468 | 52.0 | 5.95 | † | ||
| Gt-n-propanol | 264,22–255,21 | 219 065.066 | 170.6 | 6.54 | … | … | ALMA; CH3OCOH vt = 1 |
| Gt-n-propanol | 2812,17–2811,17 | 219 074.469 | 250.9 | 10.82 | … | … | ALMA; CH3OCOH vt = 1 |
| Gt-n-propanol | 2812,16–2811,17 | 219 074.470 | 250.9 | 13.07 | … | … | ” |
| Gt-n-propanol | 2812,17–2811,18 | 219 074.489 | 250.9 | 13.07 | … | … | ” |
| Gt-n-propanol | 2812,16–2811,18 | 219 074.490 | 250.9 | 10.82 | … | … | ” |
| Gt-n-propanol | 2712,16–2711,16 | 219 287.021 | 238.1 | 10.34 | … | … | ALMA; CH3OCOH vt = 2 |
| Gt-n-propanol | 2712,15–2711,16 | 219 287.021 | 238.1 | 12.38 | … | … | ” |
| Gt-n-propanol | 2712,16–2711,17 | 219 287.030 | 238.1 | 12.38 | … | … | ” |
| Gt-n-propanol | 2712,15–2711,17 | 219 287.030 | 238.1 | 10.34 | … | … | ” |
| Gt-n-propanol | 107,4–96,3 | 219 345.954 | 47.6 | 6.62 | … | … | ALMA; CH3O13COH |
| Gt-n-propanol | 107,3–96,3 | 219 345.955 | 47.6 | 6.97 | … | … | ” |
| Gt-n-propanol | 107,4–96,4 | 219 345.976 | 47.6 | 6.97 | … | … | ” |
| Gt-n-propanol | 107,3–96,4 | 219 345.977 | 47.6 | 6.62 | … | … | ” |
| Gt-n-propanol | 88,1–77,0 | 219 604.840 | 45.8 | 7.48 | 219 604.8 | 1.39 | ALMA |
| Gt-n-propanol | 88,1–77,0 | 219 604.840 | 45.8 | 7.52 | † | ||
| Gt-n-propanol | 88,1–77,0 | 219 604.840 | 45.8 | 7.52 | † | ||
| Gt-n-propanol | 88,1–77,0 | 219 604.840 | 45.8 | 7.48 | † | ||
| Gt-n-propanol | 2512,14–2511,14 | 219 640.715 | 214.0 | 9.34 | … | … | ALMA; CH3OCOH vt = 1 |
| Gt-n-propanol | 2512,13–2511,14 | 219 640.715 | 214.0 | 11.00 | … | … | ” |
| Gt-n-propanol | 2512,14–2511,15 | 219 640.717 | 214.0 | 11.00 | … | … | ” |
| Gt-n-propanol | 2512,13–2511,15 | 219 640.717 | 214.0 | 9.34 | … | … | ” |
| Gt-n-propanol | 2212,11–2211,11 | 220 020.607 | 181.3 | 7.74 | … | … | ALMA; U-line |
| Gt-n-propanol | 2212,10–2211,11 | 220 020.607 | 181.3 | 8.91 | … | … | ” |
| Gt-n-propanol | 2212,11–2211,12 | 220 020.607 | 181.3 | 8.91 | … | … | ” |
| Gt-n-propanol | 2212,10–2211,12 | 220 020.607 | 181.3 | 7.74 | … | … | ” |
| Gt-n-propanol | 2112,10–2111,10 | 220 113.805 | 171.3 | 7.17 | 220 113.2 | 0.81 | ALMA; U-line, CH2CHCN v11 = 1 |
| Gt-n-propanol | 2112,9–2111,10 | 220 113.805 | 171.3 | 7.17 | † | ||
| Gt-n-propanol | 2112,10–2111,11 | 220 113.805 | 171.3 | 7.17 | † | ||
| Gt-n-propanol | 2112,9–2111,11 | 220 113.805 | 171.3 | 7.17 | † | ||
| Gt-n-propanol | 1812,7–1811,7 | 220 313.948 | 144.0 | 5.36 | 220 314.2 | 0.76 | ALMA |
| Gt-n-propanol | 1812,6–1811,7 | 220 313.948 | 144.0 | 6.01 | † | ||
| Gt-n-propanol | 1812,7–1811,8 | 220 313.948 | 144.0 | 6.01 | † | ||
| Gt-n-propanol | 1812,6–1811,8 | 220 313.948 | 144.0 | 5.36 | † | ||
| Gt-n-propanol | 1512,4–1511,4 | 220 422.125 | 120.8 | 3.32 | 220 422.3 | 0.65 | ALMA; 13CH3OCOH, CH3OC18OH |
| Gt-n-propanol | 1512,3–1511,4 | 220 422.125 | 120.8 | 3.66 | † | ||
| Gt-n-propanol | 1512,4–1511,5 | 220 422.125 | 120.8 | 3.66 | † | ||
| Gt-n-propanol | 1512,3–1511,5 | 220 422.125 | 120.8 | 3.32 | † | ||
| Gt-n-propanol | 1412,2–1411,3 | 220 443.126 | 114.0 | 2.82 | … | … | ALMA; CH3CH2OH |
| Gt-n-propanol | 1412,3–1411,3 | 220 443.126 | 114.0 | 2.57 | … | … | ” |
| Gt-n-propanol | 1412,2–1411,4 | 220 443.126 | 114.0 | 2.57 | … | … | ” |
| Gt-n-propanol | 1412,3–1411,4 | 220 443.126 | 114.0 | 2.82 | … | … | ” |
| Gt-n-propanol | 242,22–233,21 | 220 450.169 | 139.1 | 14.32 | ALMA; CH3CH2OCOH | ||
| Gt-n-propanol | 1312,1–1311,3 | 220 458.292 | 107.6 | 1.77 | 220 458.3 | 0.36 | ALMA |
| Gt-n-propanol | 1312,1–1311,2 | 220 458.292 | 107.6 | 1.94 | † | ||
| Gt-n-propanol | 1312,2–1311,3 | 220 458.292 | 107.6 | 1.94 | † | ||
| Gt-n-propanol | 1312,2–1311,2 | 220 458.292 | 107.6 | 1.77 | † | ||
| Gt-n-propanol | 260,26–251,25 | 227 509.942 | 149.1 | 24.15 | 227 510.7 | 1.03 | ALMA |
| Gt-n-propanol | 261,26–251,25 | 227 510.348 | 149.1 | 25.86 | † | ||
| Gt-n-propanol | 260,26–250,25 | 227 510.621 | 149.1 | 25.86 | † | ||
| Gt-n-propanol | 261,26–250,25 | 227 511.028 | 149.1 | 24.15 | † | ||
| Gt-n-propanol | 117,5–106,4 | 228 791.566 | 52.6 | 6.71 | 228 791.6 | 1.08 | ALMA |
| Gt-n-propanol | 117,4–106,4 | 228 791.570 | 52.6 | 7.18 | † | ||
| Gt-n-propanol | 117,5–106,5 | 228 791.564 | 52.6 | 7.18 | † | ||
| Gt-n-propanol | 117,4–106,5 | 228 791.658 | 52.6 | 6.71 | † | ||
| Gt-n-propanol | 98,2–87,1 | 229 067.766 | 49.9 | 7.49 | 229 066.5 | 2.40 | ALMA; U-line |
| ” | ” | ” | ” | ” | 229 068.4 | 0.30 | 30 m |
| Gt-n-propanol | 98,1–87,1 | 229 067.766 | 49.9 | 7.62 | † | ||
| Gt-n-propanol | 98,2–87,2 | 229 067.766 | 49.9 | 7.62 | † | ||
| Gt-n-propanol | 98,1–87,2 | 229 067.766 | 49.9 | 7.49 | † | ||
| Gt-n-propanol | 243,21–233,20 | 229 460.087 | 143.6 | 23.32 | 229 462.0 | 0.55 | ALMA |
| Gt-n-propanol | 252,23–243,22 | 229 461.862 | 150.2 | 15.38 | † | ||
| Gt-n-propanol | 234,20–223,19 | 234 033.112 | 133.2 | 8.73 | 234 033.2 | 0.74 | ALMA; U-line |
| Gt-n-propanol | 3913,27–3912,27 | 235 206.448 | 432.7 | 15.38 | … | … | ALMA; CH3OCOH vt = 1 |
| Gt-n-propanol | 3913,26–3912,27 | 235 206.535 | 432.7 | 20.18 | … | … | ” |
| Gt-n-propanol | 3913,27–3912,28 | 235 208.140 | 432.7 | 20.18 | … | … | ” |
| Gt-n-propanol | 3913,26–3912,28 | 235 208.227 | 432.7 | 15.38 | … | … | ” |
| Gt-n-propanol | 3813,26–3812,26 | 235 684.048 | 414.9 | 15.00 | … | … | ALMA; DCOOH |
| Gt-n-propanol | 3813,25–3812,26 | 235 684.093 | 414.9 | 19.46 | … | … | ” |
| Gt-n-propanol | 3813,26–3812,27 | 235 684.969 | 414.9 | 19.46 | … | … | ” |
| Gt-n-propanol | 3813,25–3812,27 | 235 685.014 | 414.9 | 15.00 | … | … | ” |
| Gt-n-propanol | 193,16–182,16 | 236 101.301 | 92.6 | 8.67 | … | … | ALMA; D2CO, CH38OCOH |
| Gt-n-propanol | 3713,25–3712,25 | 236 120.704 | 397.6 | 14.60 | … | … | ALMA; CH3O13COH, CH3OCOH vt = 2 |
| Gt-n-propanol | 3713,24–3712,25 | 236 120.726 | 397.6 | 18.75 | … | … | ” |
| Gt-n-propanol | 3713,25–3712,26 | 236 121.195 | 397.6 | 18.75 | … | … | ” |
| Gt-n-propanol | 3713,24–3712,26 | 236 121.218 | 397.6 | 14.60 | … | … | ” |
| Gt-n-propanol | 270,27–261,26 | 236 138.084 | 160.4 | 25.15 | 236 138.5 | 0.74 | ALMA |
| Gt-n-propanol | 271,27–261,26 | 236 138.327 | 160.4 | 26.86 | † | ||
| Gt-n-propanol | 270,27–260,26 | 236 138.490 | 160.4 | 26.86 | † | ||
| Gt-n-propanol | 271,27–260,26 | 236 138.733 | 160.4 | 25.15 | † | ||
| Gt-n-propanol | 172,16–161,16 | 236 879.561 | 69.9 | 2.82 | … | … | ALMA; CH3OCOH |
| Gt-n-propanol | 3513,23–3512,23 | 236 882.213 | 364.3 | 13.78 | … | … | ” |
| Gt-n-propanol | 3513,22–3512,23 | 236 882.218 | 364.3 | 17.34 | … | … | ” |
| Gt-n-propanol | 3513,23–3512,24 | 236 882.345 | 364.3 | 17.34 | … | … | ” |
| Gt-n-propanol | 3513,22–3512,24 | 236 882.350 | 364.3 | 13.78 | … | … | ” |
| Gt-n-propanol | 3413,22–3412,22 | 237 212.075 | 348.4 | 13.36 | … | … | ALMA; CH3OCOH vt = 2 |
| Gt-n-propanol | 3413,21–3412,22 | 237 212.077 | 348.4 | 16.65 | … | … | ” |
| Gt-n-propanol | 3413,22–3412,23 | 237 212.141 | 348.4 | 16.65 | … | … | ” |
| Gt-n-propanol | 3413,21–3412,23 | 237 212.143 | 348.4 | 13.36 | … | … | ” |
| Gt-n-propanol | 146,9–135,9 | 237 691.949 | 64.3 | 7.37 | 237 692.7 | 0.62 | ALMA; 13CH3OCOH |
| Gt-n-propanol | 146,8–135,9 | 237 697.842 | 64.3 | 6.15 | 237 697.2 | 1.20 | ALMA; 13CH3OCOH vt = 1 |
| Gt-n-propanol | 253,22–243,21 | 237 779.216 | 155.0 | 24.29 | … | … | ALMA; 13CH3OCOH vt = 1 |
| Gt-n-propanol | 3213,20–3212,20 | 237 781.304 | 317.9 | 12.47 | … | … | ” |
| Gt-n-propanol | 3213,19–3212,20 | 237 781.304 | 317.9 | 15.26 | … | … | ” |
| Gt-n-propanol | 3213,20–3212,21 | 237 781.319 | 317.9 | 15.26 | … | … | ” |
| Gt-n-propanol | 3213,19–3212,21 | 237 781.319 | 317.9 | 12.47 | … | … | ” |
| Gt-n-propanol | 3113,19–3112,19 | 238 024.778 | 303.3 | 12.01 | … | … | ALMA; CH3OCOH vt = 1 |
| Gt-n-propanol | 3113,18–3112,19 | 238 024.778 | 303.3 | 14.57 | … | … | ” |
| Gt-n-propanol | 3113,19–3112,20 | 238 024.785 | 303.3 | 14.57 | … | … | ” |
| Gt-n-propanol | 3113,18–3112,20 | 238 024.785 | 303.3 | 12.01 | … | … | ” |
| Gt-n-propanol | 165,11–154,12 | 238051.137 | 73.5 | 5.21 | 238 051.4 | 0.64 | ALMA |
| Gt-n-propanol | 376,31–375,33 | 238 051.929 | 342.5 | 1.83 | † | ” | ” |
| Gt-n-propanol | 127,6–116,5 | 238 226.322 | 58.0 | 6.81 | … | … | ALMA; CH3OCOH vt = 1 |
| ” | ” | ” | ” | ” | 238 226.8 | 0.25 | 30 m; CH3OCOH vt = 1 |
| Gt-n-propanol | 127,5–116,5 | 238 226.337 | 58.0 | 7.42 | † | ||
| Gt-n-propanol | 127,6–116,6 | 238226.619 | 58.0 | 7.42 | † | ||
| Gt-n-propanol | 127,5–116,6 | 238 226.633 | 58.0 | 6.81 | † | ||
| Gt-n-propanol | 3013,18–3012,18 | 238 243.369 | 289.2 | 11.53 | … | … | ALMA; U-line |
| Gt-n-propanol | 3013,17–3012,18 | 238 243.369 | 289.2 | 13.88 | … | … | ” |
| Gt-n-propanol | 3013,18–3012,19 | 238 243.372 | 289.2 | 13.88 | … | … | ” |
| Gt-n-propanol | 3013,17–3012,19 | 238 243.372 | 289.2 | 11.53 | … | … | ” |
| Gt-n-propanol | 266,24–253,23 | 238 348.325 | 161.7 | 16.44 | … | … | ALMA; CH3OCOH |
| Gt-n-propanol | 2913,17–2912,17 | 238 438.858 | 275.5 | 11.05 | … | … | ALMA; CH3OH vt = 1 |
| Gt-n-propanol | 2913,16–2912,17 | 238 438.858 | 275.5 | 13.19 | … | … | ” |
| Gt-n-propanol | 2913,17–2912,18 | 238 438.859 | 275.5 | 13.19 | … | … | ” |
| Gt-n-propanol | 2913,16–2912,18 | 238 438.859 | 275.5 | 11.05 | … | … | ” |
| Gt-n-propanol | 108,3–97,2 | 238 528.368 | 54.5 | 7.53 | 238528.6 | 1.01 | ALMA |
| ” | ” | ” | ” | ” | 238528.8 | 0.18 | 30 m |
| Gt-n-propanol | 108,2–97,2 | 238 528.368 | 54.5 | 7.76 | † | ||
| Gt-n-propanol | 108,3–97,3 | 238 528.368 | 54.5 | 7.76 | † | ||
| Gt-n-propanol | 108,2–97,3 | 238 528.368 | 54.5 | 7.53 | † | ||
| Gt-n-propanol | 2313,11–2312,11 | 239 215.407 | 203.2 | 7.87 | … | … | ALMA; CH318OCOH |
| Gt-n-propanol | 2313,10–2312,11 | 239 215.407 | 203.2 | 9.01 | … | … | ” |
| Gt-n-propanol | 2313,11–2312,12 | 239 215.407 | 203.2 | 9.01 | … | … | ” |
| Gt-n-propanol | 2313,10–2312,12 | 239 215.407 | 203.2 | 7.87 | … | … | ” |
| Gt-n-propanol | 2213,10–2212,10 | 239 291.987 | 192.7 | 7.29 | … | … | ALMA; CH3OCOH vt = 1 |
| Gt-n-propanol | 2213,9–2212,10 | 239 291.987 | 192.7 | 8.29 | … | … | ” |
| Gt-n-propanol | 2213,10–2212,11 | 239 291.987 | 192.7 | 8.29 | … | … | ” |
| Gt-n-propanol | 2213,9–2212,11 | 239 291.987 | 192.7 | 7.29 | … | … | ” |
| Gt-n-propanol | 244,21–233,20 | 239 313.697 | 144.1 | 9.74 | 239 313.9 | 0.68 | ALMA; CH3OCOH vt = 2 |
| Gt-n-propanol | 2113,9–2112,9 | 239 356.844 | 182.7 | 6.69 | 239 357.4 | 0.26 | ALMA |
| Gt-n-propanol | 2113,8–2112,9 | 239 356.844 | 182.7 | 7.56 | † | ||
| Gt-n-propanol | 2113,9–2112,10 | 239 356.844 | 182.7 | 7.56 | † | ||
| Gt-n-propanol | 2113,8–2112,10 | 239 356.844 | 182.7 | 6.69 | † | ||
| Gt-n-propanol | 2013,8–2012,8 | 239 411.108 | 173.2 | 6.07 | 239 411.6 | 0.65 | ALMA; U-line |
| Gt-n-propanol | 2013,7–2012,8 | 239 411.108 | 173.2 | 6.83 | † | ||
| Gt-n-propanol | 2013,8–2012,9 | 239 411.108 | 173.2 | 6.83 | † | ||
| Gt-n-propanol | 2013,7–2012,9 | 239 411.108 | 173.2 | 6.07 | † | ||
| Gt-n-propanol | 1913,6–1912,7 | 239 455.850 | 164.1 | 6.07 | … | … | ALMA; U-line |
| Gt-n-propanol | 1913,7–1912,7 | 239 455.850 | 164.1 | 5.43 | … | … | ” |
| Gt-n-propanol | 1913,6–1912,8 | 239 455.850 | 164.1 | 5.43 | … | … | ” |
| Gt-n-propanol | 1913,7–1912,8 | 239 455.850 | 164.1 | 6.07 | … | … | ” |
| Gt-n-propanol | 1813,5–1812,6 | 239 492.090 | 155.5 | 5.30 | … | … | ALMA; CH3OCOH |
| Gt-n-propanol | 1813,6–1812,6 | 239 492.090 | 155.5 | 4.77 | … | … | ” |
| Gt-n-propanol | 1813,5–1812,7 | 239 492.090 | 155.5 | 4.77 | … | … | ” |
| Gt-n-propanol | 1813,6–1812,7 | 239 492.090 | 155.5 | 5.30 | … | … | ” |
| Gt-n-propanol | 1713,4–1712,5 | 239 520.792 | 147.3 | 4.51 | 239 520.8 | 0.37 | ALMA |
| Gt-n-propanol | 1713,4–1712,6 | 239 520.792 | 147.3 | 4.07 | † | ||
| Gt-n-propanol | 1713,5–1712,5 | 239 520.792 | 147.3 | 4.07 | † | ||
| Gt-n-propanol | 17l3,5–17l2,6 | 239 520.792 | 147.3 | 4.51 | † | ||
| Gt-n-propanol | 263,24–252,23 | 239 523.360 | 161.7 | 16.45 | 239 523.8 | 0.44 | ALMA |
| Gt-n-propanol | 1613,4–1612,5 | 239 542.869 | 139.6 | 3.69 | … | … | ALMA; CH3OCOH |
| Gt-n-propanol | 1613,3–1612,5 | 239 542.869 | 139.6 | 3.35 | … | … | ” |
| Gt-n-propanol | 1613,3–1612,4 | 239 542.869 | 139.6 | 3.69 | … | … | ” |
| Gt-n-propanol | 1613,4–1612,4 | 239 542.869 | 139.6 | 3.35 | … | … | ” |
| Gt-n-propanol | 280,28–271,27 | 244 764.654 | 172.2 | 26.16 | 244 765.0 | 0.80 | ALMA |
| ” | ” | ” | ” | ” | 244 765.0 | 0.08 | 30 m |
| Gt-n-propanol | 281,28–271,27 | 244 764.799 | 172.2 | 27.86 | † | ||
| Gt-n-propanol | 280,28–270,27 | 244 764.897 | 172.2 | 27.86 | † | ||
| Gt-n-propanol | 281,28–270,27 | 244 765.042 | 172.2 | 26.16 | † | ||
| Gt-n-propanol | 254,22–243,21 | 245 104.539 | 155.3 | 10.81 | 245 104.4 | 0.44 | ALMA |
| Gt-n-propanol | 99,1–88,0 | 248 228.722 | 57.7 | 8.48 | 248 228.5 | 0.14 | 30 m |
| Gt-n-propanol | 99,0–88,0 | 248 228.722 | 57.7 | 8.52 | † | ||
| Gt-n-propanol | 99,1–88,1 | 248 228.722 | 57.7 | 8.52 | † | ||
| Gt-n-propanol | 99,0–88,1 | 248 228.722 | 57.7 | 8.48 | † | ||
| Gt-n-propanol | 148,7–137,6 | 276 312.369 | 77.2 | 7.88 | 276 312.8 | 0.15 | 30 m |
| Gt-n-propanol | 148,6–137,6 | 276 312.370 | 77.2 | 8.60 | † | ||
| Gt-n-propanol | 148,7–137,7 | 276 312.417 | 77.2 | 8.60 | † | ||
| Gt-n-propanol | 148,6–137,7 | 276 312.419 | 77.2 | 7.88 | † | ||
| Gt-n-propanol | 1010,1–99,0 | 276 842.794 | 71.0 | 9.48 | 276 842.8 | 0.10 | 30 m |
| Gt-n-propanol | 1010,0–99,0 | 276 842.794 | 71.0 | 9.52 | † | ||
| Gt-n-propanol | 1010,1–99,1 | 276 842.794 | 71.0 | 9.52 | † | ||
| Gt-n-propanol | 1010,0–99,1 | 276 842.794 | 71.0 | 9.48 | † | ||
| Gt-n-propanol | 139,5–128,4 | 286 069.306 | 78.6 | 8.66 | 286 069.5 | 0.10 | 30 m |
| Gt-n-propanol | 139,4–128,4 | 286 069.306 | 78.6 | 9.10 | † | ||
| Gt-n-propanol | 139,5–128,5 | 286 069.306 | 78.6 | 9.10 | † | ||
| Gt-n-propanol | 139(4–128(5 | 286 069.306 | 78.6 | 8.66 | † |
Notes. Lines of gauche-trans-n-CH3CH2CH2OH (Gt–n–propanol) ground state present in the spectral scan of Orion KL from the IRAM-30 m telescope and the ALMA interferometer. Col. 1 indicates the species, Col. 2 gives the transition, Col. 3 the predicted frequency, Col. 4 upper level energy, Col. 5 the line strength, Col. 6 observed frequency at the peak channel of the line (relative to a vLSR of +8.0 km s−1), Col. 7 temperature at the peak channel of the line (main beam temperature for the IRAM data), and Col. 8 shows blends with other molecular species and comments.
Blended with previous line.
Footnotes
This paper makes use of the following ALMA data: ADS/JAO.ALMA#2011.0.00009.SV. ALMA is a partnership of ESO (representing its member states), NSF (USA), and NINS (Japan) with NRC (Canada), NSC, and ASIAA (Taiwan), and KASI (Republic of Korea), in cooperation with the Republic of Chile. The Joint ALMA Observatory is operated by ESO, AUI/NRAO, and NAOJ. This work was also based on observations carried out with the IRAM 30-m telescope. IRAM is supported by INSU/CNRS (France), MPG (Germany), and IGN (Spain).
Appendix A is available in electronic form at http://www.aanda.org
References
- Abdurakhmanov AA, Ragimova RA, Imanov LM. Opt. Spektrosk. 1969;26:135. (English transl. in 1969, Opt. Spectrosc., 25, 75) [Google Scholar]
- Balucani N, Ceccarelli C, Taquet V. MNRAS. 2015;449:L16. [Google Scholar]
- Brouillet N, Despois D, Baudry A, et al. A&A. 2013;550:A46. [Google Scholar]
- Brouillet N, Despois D, Lu X-H, et al. A&A. 2015 accepted. [Google Scholar]
- Carroll PB, McGuire BA, Blake GA, et al. ApJ. 2015;799:15. [Google Scholar]
- Carvajal M, Margulès L, Tercero B, et al. A&A. 2009;500:1109. [Google Scholar]
- Cernicharo J. Internal IRAM Report. IRAM; Granada: 1985. [Google Scholar]
- Cernicharo J. In: Stehl C, Joblin C, d’Hendecourt L, editors. ECLA-2011: Proc. Europ. Conf. on Laboratory Astrophysics; Cambridge: Cambridge Univ. Press. 2012. p. 251. EAS PS. [Google Scholar]
- Cernicharo J, Marcelino N, Roueff E, et al. ApJ. 2012;759:L43. [Google Scholar]
- Cernicharo J, Tercero B, Fuente A, et al. ApJ. 2013;771:L10. [Google Scholar]
- Coudert LH, Drouin BJ, Tercero B, et al. ApJ. 2013;779:119. [Google Scholar]
- Daly AM, Bermúdez C, López A, et al. ApJ. 2013;768:81. [Google Scholar]
- Demyk K, Mäder H, Tercero B, et al. A&A. 2007;466:255. [Google Scholar]
- Favre C, Despois D, Brouillet N, et al. A&A. 2011;532:A32. [Google Scholar]
- Feng SY, Beuther H, Henning T. A&A. 2015 accepted. [Google Scholar]
- Fuchs U, Winnewisser G, Groner P, De Lucia FC, Herbst E. ApJS. 2003;144:277. [Google Scholar]
- Fuchs GW, Fuchs U, Giesen TF, Wyrowski F. A&A. 2005;444:521. [Google Scholar]
- Hayashi M, Kuwada K. J. Mol. Struct. 1975;28:147. [Google Scholar]
- Haykal I, Carvajal M, Tercero B, et al. A&A. 2014;568:A58. [Google Scholar]
- Högbom JA. A&AS. 1974;15:417. [Google Scholar]
- Kolesniková L, Tercero B, Cernicharo J, et al. ApJ. 2014;784:L7. [Google Scholar]
- López A, Tercero B, Kisiel Z, et al. A&A. 2014;572:A44. [Google Scholar]
- Maeda A, De Lucia F,C, Herbst E, et al. ApJS. 2006;162:428. [Google Scholar]
- Margulès L, Motiyenko RA, Demyk K, et al. A&A. 2009;493:565. [Google Scholar]
- Margulès L, Huet TR, Demaison J, et al. ApJ. 2010;714:1120. [Google Scholar]
- Motiyenko RA, Tercero B, Cernicharo J, Margulès L. A&A. 2012;548:A71. [Google Scholar]
- Neill JL, Steber AL, Muckle MT, et al. J. Phys. Chem. A. 2011;115:6472. doi: 10.1021/jp200539b. [DOI] [PubMed] [Google Scholar]
- Pardo JR, Cernicharo J, Serabyn E. IEEE Trans. Antennas and Propagation. 2001;49:12. [Google Scholar]
- Peng T-C, Despois D, Brouillet N, Parise B, Baudry A. A&A. 2012;543:A152. [Google Scholar]
- Peng T-C, Despois D, Brouillet N, et al. A&A. 2013;554:A78. [Google Scholar]
- Tercero B. Univ. Complutense de Madrid; 2012. Ph.D. Thesis. [Google Scholar]
- Tercero B, Cernicharo J, Pardo JR, Goicoechea JR. A&A. 2010;517:A96. [Google Scholar]
- Tercero B, Margulès L, Carvajal M, et al. A&A. 2012;538:A119. [Google Scholar]
- Tercero B, Kleiner I, Cernicharo J, et al. ApJ. 2013;770:L13. [Google Scholar]