SUMMARY
Pancreatic β cells are of great interest for the treatment of type 1 diabetes. A number of strategies already exist for the generation of β cells, but a general approach for reprogramming nonendodermal cells into β cells could provide an attractive alternative in a variety of contexts. Here, we describe a stepwise method in which pluripotency reprogramming factors were transiently expressed in fibroblasts in conjunction with a unique combination of soluble molecules to generate definitive endoderm-like cells that did not pass through a pluripotent state. These endoderm-like cells were then directed toward pancreatic lineages using further combinations of small molecules in vitro. The resulting pancreatic progenitor-like cells could mature into cells of all three pancreatic lineages in vivo, including functional, insulin-secreting β-like cells that help to ameliorate hyperglycemia. Our findings may therefore provide a useful approach for generating large numbers of functional β cells for disease modeling and, ultimately, cell-based therapy.
INTRODUCTION
Type 1 diabetes results from autoimmune destruction of the insulin-secreting β cells within pancreatic islets. This disease typically affects children and young adults and requires frequent glucose monitoring and life-long insulin administration for proper management. In conjunction with new strategies to induce immune tolerance, transplantation of healthy islet and β cells to replace lost cells may represent a “cure” for the disease. However, a primary challenge remains in the scarcity of functional, glucose-responsive β cells.
Stem cells may provide an unlimited source of functional β cells and would facilitate biomedical research and drug discovery. Stepwise conditions that recapitulate developmental signaling have been devised to differentiate pluripotent stem cells through a definitive endoderm stage into functional pancreatic β cells (D’Amour et al., 2006, Jiang et al., 2007). Direct reprogramming from non-β cells, such as acinar cells or hepatocytes, has also been used to generate pancreatic β-like cells (Zhou et al., 2008, Ferber et al., 2000).
Conventional direct β-cell reprogramming is faster and more efficient than preparing induced pluripotent stem cells (iPSCs). However, a general approach to converting nonendoderm cells, such as fibroblast cells across the germ-layer boundary toward an endoderm-β cell lineage, has not been developed yet. Cell types derived from the endoderm lineage, such as acinar cells or hepatocytes, may be easier to reprogram into the β cell lineage owing to their similarity. However, applying these methods to cell-based therapy or in vivo therapy may be challenging due to the practicality of starting cells or the virus delivery system. In addition, β-like cells generated by conventional direct reprogramming are postmitotic and have very limited regenerative ability. Thus, pancreatic progenitor cells may be a better cell source for transplantation because of their proliferation and differentiation potential.
Previously, we developed a strategy for direct lineage-specific reprogramming (Efe et al., 2011, Kim et al., 2011; Li et al., 2013). In the current study, we applied this method with a unique combination of soluble molecules to generate definitive endoderm-like cells from mouse fibroblasts. The endoderm-like cells expressed typical endodermal markers and could differentiate into pancreatic lineages that exhibited characteristic properties in vitro and in vivo. Our findings may provide a useful approach for generating large numbers of functional β cells for disease modeling and ultimately cell therapy.
RESULTS
Conversion of Fibroblasts into Definitive Endoderm-like Cells
We used doxycycline (Dox)-inducible secondary mouse embryonic fibroblasts (MEFs) (Wernig et al., 2008) to enable expression of the conventional four iPSC factors (Oct4, Sox2, Klf4, and c-Myc) with precise temporal control. MEFs were prepared using standard procedures and used for reprogramming after three or four passages. Although endoderm cells may exist in starting MEF populations, we did not observe any contamination of these cells in our cultures.
To extend and test the iPSC-factor-based lineage-specific reprogramming paradigm to endoderm, we devised a two-step process to directly reprogram secondary MEF cells into definitive endoderm-like cells (DELCs). The first step (Step I) was culturing secondary MEF cells in media (Med)-I supplemented with 4 μg/ml Dox to initiate epigenetic activation. The second step (Step II) was culturing the epigenetically activated cells in Med-II supplemented with 50 ng/ml Activin A and 1 mM LiCl (Activin/Li) (Li et al., 2011). Sox17 and Foxa2, two relatively specific markers for definitive endoderm, were examined by immunostain at the end of Step II. By testing different durations of Steps I and II, we found that 6 days in Step I followed by 6 days in Step II was an effective condition to generate Sox17+/Foxa2+ cells (hereafter referred to as DELCs) with relatively high efficiency.
We hypothesized that transcriptional activity downstream of lineage-specific signals, in conjunction with iPSC factors that erase the epigenetic identity of the starting cell during early reprogramming, may help set up lineage-specific transcriptional programs. To test this, we compared conditions in which definitive endoderm induction factors (Activin/Li) were added to fibroblasts either after (Approach 1) or during (Approach 2) iPSC factor expression (Figure S1A). Notably, Approach 2 greatly increased the mRNA levels of Sox17 and Foxa2 and the percentage of Sox17+/Foxa2+ colonies (Figures S1B–S1D) over what was observed in Approach 1. With the use of Approach 2, iPSC factor expression in the presence of Activin/Li for 6 days followed by Activin/Li treatment for another 6 days caused high induction of other definitive endoderm marker genes, including Cerberus 1 (Cer) and C-X-C chemokine receptor type 4 (Cxcr4), in addition to Sox17 and Foxa2 (Figures 1B and 1C). These results supported our initial hypothesis.
Figure 1. Initial Approach for Reprogramming MEFs into Pancreatic β-like Cells.
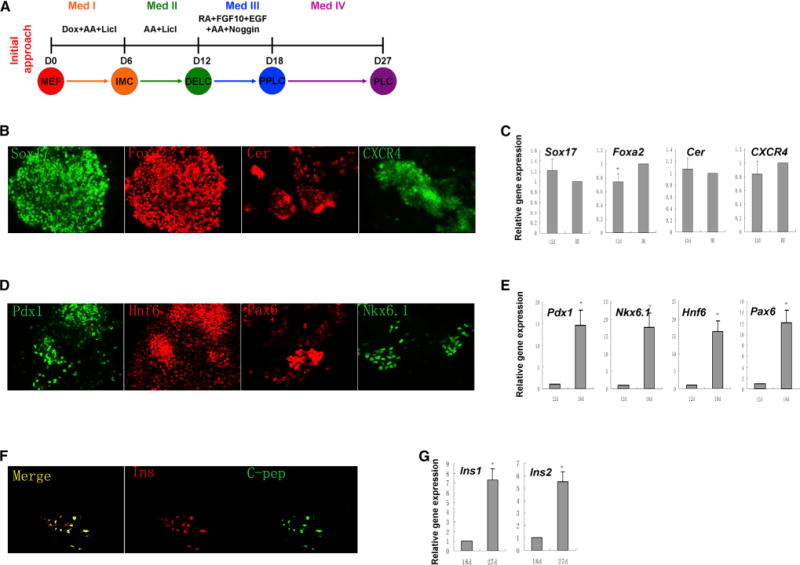
(A) Scheme of the initial approach. Med-IV contains laminin, nicotinamide, and B27 etc., as described (Schroeder et al., 2006). (B) Immunostaining and (C) gene expression of definitive endoderm markers Sox17, CXCR4, Foxa2, and Cerberus 1 on day 12. NGFP1 iPSC-derived definitive endoderm-like cells (DE) were used as a positive control. (D) Immunostaining and (E) gene expression of pancreatic progenitor markers Pdx1, Hnf6, Pax6, and Nkx6.1 on day 18. (F) Immunostaining of pancreatic β cell markers insulin (Ins) and C-peptide (C-pep) on day 27. (G) Gene expression analysis of Ins1 and Ins2 from day 18 to day 27. Results in (C), (E), and (G) are the average of at least three independent experiments. *p < 0.05, **p < 0.01. See also Figure S1.
Next we tested if DELCs possess differentiation potential toward pancreatic lineages. We attempted their differentiation by adopting published pancreatic differentiation conditions (D’Amour et al., 2006, Li et al., 2011). Notably, like definitive endoderm cells derived from mouse embryonic stem cells (mESCs), our DELCs could also give rise to cells expressing specific markers for pancreatic progenitors, including pancreatic and duodenal homeobox gene-1 (Pdx1), hepatocyte nuclear factor-6 (Hnf6), paired-box gene 6 (Pax6), and NK homeobox factor 6.1 (Nkx6.1), as examined by immunostaining and real-time PCR (Figures 1D and 1E). Further differentiation of these pancreatic progenitor-like cells (PPLCs) in vitro gave rise to insulin+/C-peptide+ cells (Figures 1F and 1G). Our results showed that DELCs could differentiate to pancreatic β-like cells in a process similar to what has been previously observed with definitive endoderm cells derived from mESCs.
Despite this success, the efficiency of generating pancreatic-like cells was low, although similar to differentiation of mESCs using the same conditions. For example, only about 5% and 10% of cells were positive for either Nkx6.1 or Pdx1, respectively, and only about 1% were Pdx1+/Nkx6.1+. Further differentiation in vitro gave rise to <0.1% insulin expressing cells that did not respond well to high glucose stimulation. These results indicated that the lineage induction conditions could be further improved, so we then focused on optimizing inductive conditions by small molecules.
Identification of Combinations of Small Molecules that Enhance Generation of PPLCs
β cell formation and maturation are impaired in Nkx6.1 null mutant mice and could be restored upon re-expression of Nkx6.1 in multipotential Pdx1+ pancreatic progenitors (Nelson et al., 2007). In other studies, Pdx1+/Nkx6.1+ pancreatic progenitor cells differentiated from human embryonic stem cells (ESCs) could give rise to all pancreatic cell lineages, including glucose-responsive, insulin-secreting cells, in vivo (Kelly et al., 2011, Rezania et al., 2013). These studies suggest that dual expression of Pdx1 and Nkx6.1 may predict the ability of pancreatic progenitors to give rise to functional β cells. We therefore set out to identify conditions that induce Pdx1+/Nkx6.1+ PPLCs from our reprogrammed DELCs.
We screened a known drug collection of 400 compounds. Med-III (DMEM supplemented with 1xB27, 2 mM Glutamax) was used as a basal condition and double staining of Pdx1 and Nkx6.1 4 days postinduction was used as a readout. This was our third step (Step III) of pancreatic induction. Several primary hits that could increase Pdx1 and/or Nkx6.1 expression were further characterized. Confirmed hits included retinoic acid (RA, a RAR agonist), A83-01 (a TGF-β receptor inhibitor), 2-phospho-L-ascorbic acid (pVc), and LDE225 (a Hedgehog pathway inhibitor) (Figures S2A and S2B). After testing various small molecule combinations, we determined that a much improved pancreatic induction was achieved by treating DELCs with 2 μM RA, 1 μM A83-01, 2 μM LDE225, and 280 μM pVc for 1 day, and then 1 μM A83-01, 2 μM LDE225, and 280 μM pVc for another 3 days. Under these conditions, the pancreatic progenitor markers Pdx1, Nkx6.1, Hnf6, Pax6, and Sox9 were highly expressed and colocalized (Figure 2B). About 35% of the cells expressed Pdx1, and about 30% of the cells expressed Nkx6.1, a 2.5-fold and 5-fold increase, respectively, over the initial approach. More importantly, about 8% of the cells were Pdx1+/Nkx6.1+, a 7-fold increase over the initial approach. qRT-PCR analysis further confirmed expression of Pdx1, Nkx6.1, Hnf6, and Pax6 during the pancreatic induction process (Figure 2C). These results suggest that this combination of small molecules promoted differentiation of DELCs into Pdx1+/Nkx6.1+ PPLCs.
Figure 2. Identification of Small Molecule Conditions that Enhance Generation of PPLCs.
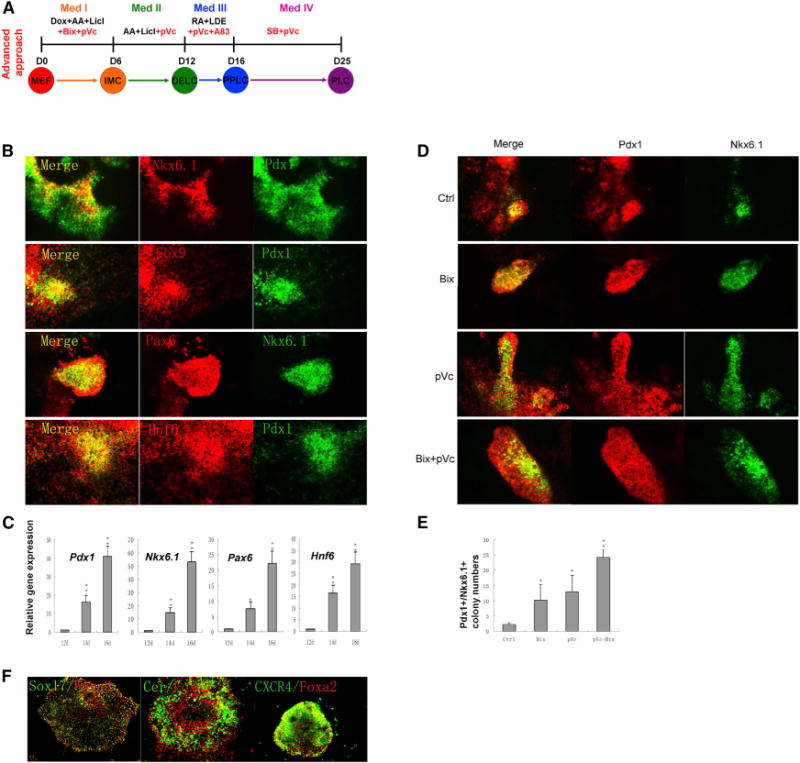
(A) Scheme of the advanced approach. Red indicates small molecules that significantly improved induction efficiencies.
(B) Immunostaining of pancreatic progenitor markers Pdx1, Hnf6, Sox9, Pax6, and Nkx6.1 on day 16. Cells were treated with the combination of four small molecules (RA, A83-01, LDE225, and pVc) from day 12 to day 16.
(C) Gene expression analysis of markers during the pancreatic progenitor induction process by qPCR. Results are the average of three independent experiments. *p < 0.05, **p < 0.01.
(D) Immunostaining of Pdx1 and Nkx6.1 on day 16.
(E) Pdx1+/Nkx6.1+ colony numbers under indicated conditions on day 16. Four thousand cells were seeded into each well of 24-well plate on day 0. Results are the average of three independent experiments. *p < 0.05.
(F) Immunostaining of Sox17, CXCR4, Foxa2, and Cerberus 1 on day 12.
In (D), (E), and (F), Bix (Bix-01294) at 1 μM was added from day 0 to day 6 and pVc (2-Phospho-L-ascorbic acid trisodium salt) at 280 μM was added from day 0 to day 12. See also Figures S2, S3, and S4.
We next hypothesized that overlapping lineage-specific patterning conditions with early reprogramming based on small molecules would enhance pancreatic induction. We further screened our known drug collection during the Step I and II inductions, followed by double staining for Pdx1 and Nkx6.1 at the end of Step III (day 16) as a readout. Remarkably, several small molecules, when applied during Step I and/or Step II, greatly enhanced pancreatic induction. Particularly, 1 μM Bix-01294 (a G9a inhibitor), when added from day 0 to day 6, and 280 μM pVc, when added from day 0 to day 12, increased the number of Pdx1+/Nkx6.1+ colonies to about 3-fold and 4-fold, respectively (Figures 2D and 2E). Further testing revealed that the combination of 1 μM Bix-01294 (added from day 0 to day 6) and 280 μM pVc (added from day 0 to day 12) generated about an 8-fold increase in Pdx1+/Nkx6.1+ colonies (Figures 2D and 2E). Interestingly, treatment with Bix and/or pVc did not alter the numbers of Sox17 and Foxa2 single-positive and double-positive cells.
Employing these advanced conditions (Figure 2A), we analyzed the cells on day 12 (DELC stage) and day 16 (PPLC stage) in greater detail. On day 0, 2 × 104 MEF cells were seeded in a well of a 24-well plate. On day 12, the cell number in the well was about 9 × 105. Immunostaining showed that definitive endoderm marker genes, Sox17, Foxa2, Cer, and Cxcr4, were highly expressed (Figure 2F). FACS analysis revealed that the percentage of Sox17+/Foxa2+ cells was about 54%. Importantly, we found that DELCs could be isolated and expanded in Activin/Li for over 1 month in serial multiple passages (Figures S2C and S2D) without losing their potential to differentiate into pancreatic progenitors (Figure S2E). This expansion translates into an approximately 65,000-fold increase in cell number. On day 12, we also examined some nonendodermal markers. About 0.46% of cells were Brachyury/T+ (which indicates the presence of an early mesoderm marker gene), while Sox1+ cells (which indicate the presence of an early neuroectoderm marker gene) were not detected (Figure S4C). On day 16, the cell number increased to about 1.1 × 106 per well. FACS analysis showed about 30% of the cells are PDX1+/NKX6.1+ Some endocrine progenitor marker genes, namely Ngn3, Neurod1, and Nkx2.2, were also detected at low levels (Figures S2F and S2G). However, we have not yet found an appropriate condition to expand PPLCs in a prolonged manner. On day 16, we also examined some nonpancreatic markers. About 0.66% of cells were CDX2+ (indicative of an intestinal marker gene) and about 0.87% of cells were positive for GATA4 (a midstage mesoderm marker gene), while those positive for Tuj1 (a neuronal marker gene) and MAP2 (another neuronal marker gene) were not detected (Figures S4D and S4E).
PPLCs Can Be Further Differentiated into Mature Pancreatic-like Cells
To determine whether PPLCs generated by the above conditions can be further differentiated into mature pancreatic-like cells (PLCs), we treated PPLCs with Med-IV containing laminin, nicotinamide, B27, etc. as previously reported (Schroeder et al., 2006). After 9 days of culture in Med-IV, a small population (about 0.5%) of insulin and C-peptide double-positive cells was detected. To improve efficiency of pancreatic maturation, we screened our known drug collection in this fourth step (Step IV) with double staining of insulin and Pdx1 on day 25 as a readout. We found that 5 μM SB203580 and 280 μM pVc significantly increased the number of insulin+/Pdx1+ cells when used individually and further enhanced the insulin+/Pdx1+ cell population (about 2%) when combined (Figure 3A). Insulin and C-peptide double staining confirmed that the insulin was produced by the cells and not by absorption from Med-IV (Figure 3A). Gene expression analysis of insulin, Pdx1, and Nkx6.1 showed consistent results (Figure 3B). Glucagon-producing pancreatic α-like cells, somatostatin-producing pancreatic δ-like cells, and amylase-producing pancreatic acinar-like cells were also detected on day 25 (Figure 3C). Importantly, endocrine-like cells produced only one hormone, a defining characteristic of mature pancreatic endocrine cells. Insulin-positive and C-peptide-positive β-like cells were also Nkx6.1-positive and Pdx1-positive (Figure 3C). Functional analysis showed that β-like cells could release insulin and C-peptide in response to glucose, tolbutamide, and IBMX stimulation. However, their response to glucose stimulation was impaired when compared to that of native mouse islets (Figure 3D). Thus, these differentiated PPLCs gave rise to pancreatic endocrine and exocrine-like cells in vitro, including functional insulin-secreting β-like cells.
Figure 3. PPLCs Can Be Further Induced into PLCs.
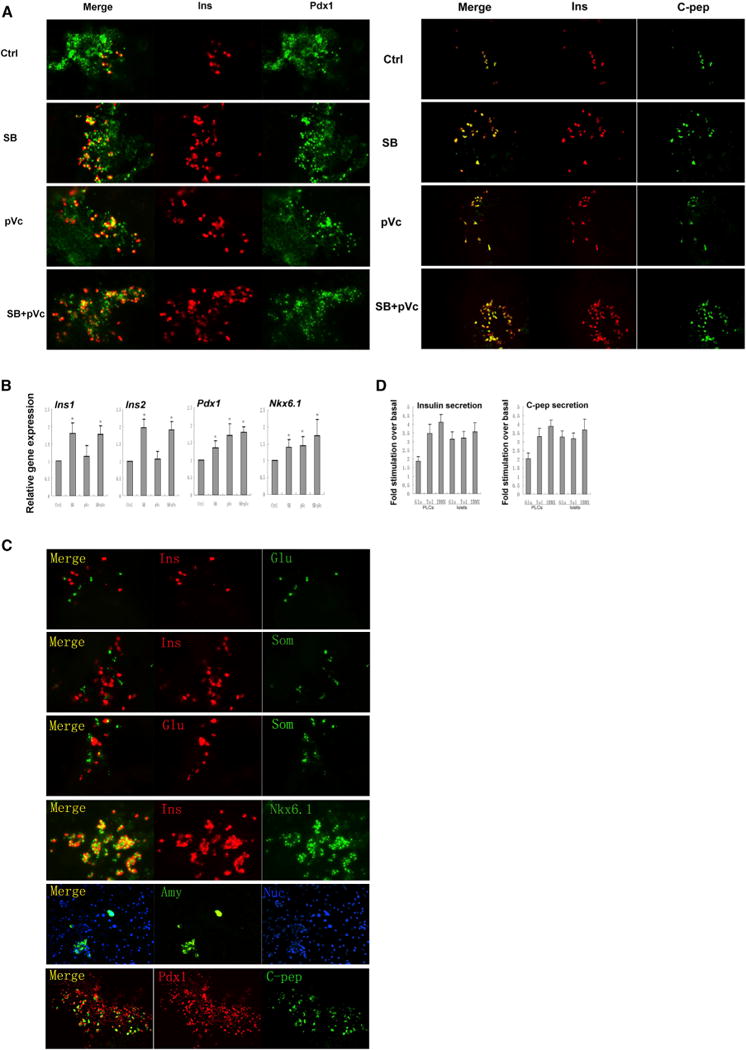
(A) Immunostaining of Pdx1/insulin and insulin/C-peptide on day 25 after treatment with SB (SB203580) at 5 μM and pVc (2-Phospho-L-ascorbic acid trisodium salt) at 280 μM from day 16 to day 25.
(B) Gene expression analysis of Ins1, Ins2, Pdx1, and Nkx6.1 by qPCR. Cells treated with dissolvent were used as control. Results are the average of three independent experiments. *p < 0.05, **p < 0.01.
(C) Immunostaining of pancreatic cell markers insulin, C-peptide, Glucagon, Somatostatin, Amylase, Pdx1, and Nkx6.1 on day 25.
(D) Insulin and C-peptide release on day 25. Fold stimulation of insulin and C-peptide release over the respective basal condition for glucose and other insulin secretion agonists. Native mouse islets served as a control. Glu, 16.7mM D-glucose; TOL, 100 μM tolbutamide; IBMX, 0.5 mM 3 isobutil-1-methylxanthine. Results are the average of four independent experiments.
See also Figures S3 and S4.
iPSCs Were Not Generated During the Reprogramming of Fibroblasts to PLCs
Generating iPSCs with the secondary MEFs requires at least 9 days of Dox treatment with LIF, followed by an additional 7–10 days of culture, as confirmed by expression of a knockin GFP reporter for Nanog, a pluripotency gene (Wernig et al., 2008). Using our method, we did not detect any Nanog-GFP-positive cells during the whole process from MEFs to PLCs, which we assessed via time-lapse imaging (Figures S3B–S3D). This finding is consistent with our previous studies on direct cardiac, neural, and endothelial cell reprogramming (Efe et al., 2011; Kim et al., 2011; Li et al., 2013) and confirmed that our approach could directly reprogram MEFs to DELCs and eventually PLCs that bypassed the iPSC stage.
In Vivo Characterizations of PPLCs
To assess PPLC function in vivo, we transplanted PPLCs under the kidney capsule in immunodeficient NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice induced to become hyperglycemic by intraperitoneal injection of Streptozocin (STZ). Each recipient received a renal subcapsular transplant of 300 native mouse islets, 3 × 106 PPLCs, or 3 × 106 MEF cells. Untreated normal and STZ-only-treated NSG mice were used as controls.
In the untreated group, blood glucose levels were unchanged, as expected (Figure 4A). In the STZ-only group, glucose levels increased gradually and peaked by the end of the fourth week. In the group transplanted with mouse native islets, diabetic mice almost achieved normal glycemia 1 week after transplantation, which served as positive control. In the group transplanted with PPLCs derived from MEFs, diabetic mice exhibited a transient increase in glucose levels within the first week, which then decreased gradually, approaching levels in normal mice. Importantly, these mice returned to diabetic glucose levels after nephrectomies of kidneys transplanted with PPLCs reprogrammed from MEFs, confirming a direct link between transplantation of the PPLCs and amelioration of hyperglycemia. In contrast, in the groups transplanted with PPLCs derived from mouse iPSCs, either by our own optimized differentiation conditions or those previously published (D’Amour et al., 2006; Li et al., 2011; Nostro et al., 2011), significant amelioration of hyperglycemia was not observed. In the group transplanted with MEFs, glucose levels increased similarly to the STZ-only group. Eight weeks posttransplantation, we analyzed glucose-stimulated insulin secretion (GSIS) and conducted a glucose tolerance test (GTT). The PPLCs that were reprogrammed from MEFs gave rise to functional glucose-responsive, insulin-secreting cells in vivo, as measured by levels of serum insulin and blood glucose before and after glucose stimulation (Figures 4B and 4C).
Figure 4. In Vivo Characterizations of PPLCs.
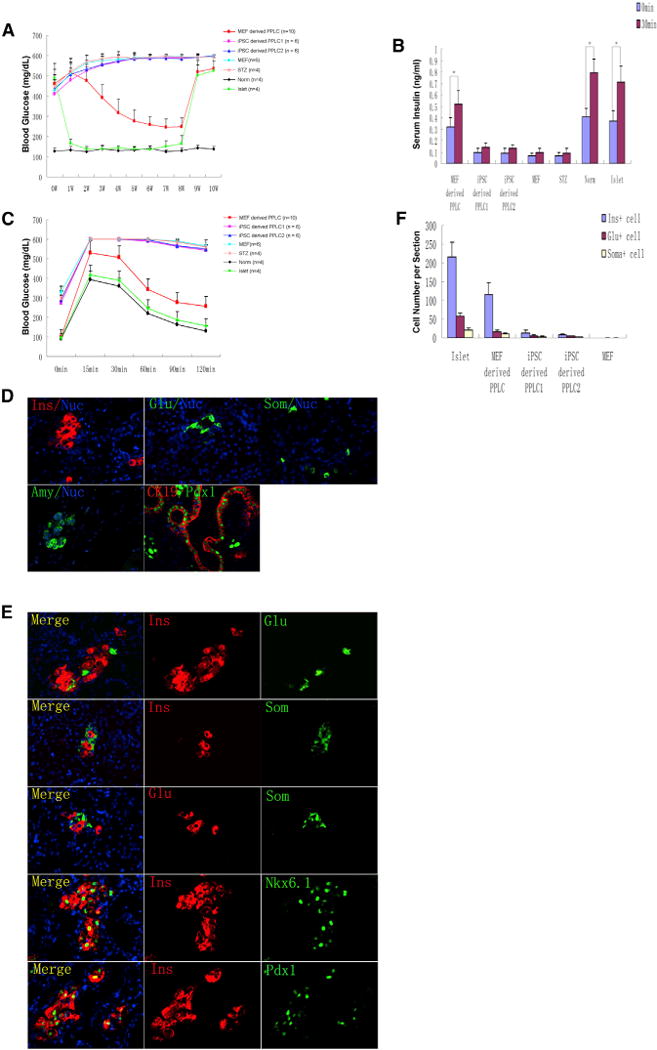
(A) Blood glucose level of normal and STZ-induced diabetic mice without grafts or transplanted with PPLCs, MEFs, or islets. Under anesthesia, the left kidney of diabetic mice received a renal subcap-sular transplant of 300 native mouse islets in 30 μl Matrigel (Islet, n = 4), 3 × 106 PPLCs reprogrammed from MEFs (MEF-derived PPLC, n = 10), 3 × 106 NGFP1 mouse iPSC-derived PPLCs by our own optimized differentiation conditions (iPSC-derived PPLC1, n = 6), 3 × 106 NGFP1 mouse iPSC-derived PPLCs by published differentiation conditions (iPSC-derived PPLC2, n = 6), or 3 × 106 secondary MEF cells (MEF, n = 6). Untreated normal mice (Norm, n = 4) and mice treated with STZ only (STZ, n = 4) were controls. Eight weeks after transplantation, the left kidneys were surgically removed.
(B) Glucose-stimulated insulin secretion (GSIS). Mice were fasted overnight, D-glucose (3 g/kg body weight) was injected intraperitoneally, and serum insulin levels of each group were measured before and 30 min after glucose stimulation.
(C) Intraperitoneal glucose tolerance tests (IPGTTs). Mice were treated as in (B) and blood glucose levels were measured at 0, 15, 30, 60, 90, and 120 min after glucose stimulation.
(D and E) Immunofluorescence staining of kidneys engrafted with MEF-derived PPLCs.
(F) Quantification of endocrine cell numbers of mice from MEF, PPLC, and islet grafts. For each experimental group, endocrine cells of four mice were counted and averaged as described in the Experimental Procedures.
Subsequently, the left kidneys were surgically removed for immunohistochemistry analysis. In kidney capsules engrafted with PPLCs reprogrammed from MEFs, we identified glucagon-producing pancreatic α-like cells, insulin-producing pancreatic β-like cells, somatostatin-producing pancreatic δ-like cells, amylase-producing pancreatic acinar-like cells, and Pdx1+/Ck19+ pancreatic ductal-like cells (Figure 4D). The insulin-positive cells were Pdx1-positive and Nkx6.1-positive (Figure 4E). Importantly, these endocrine-like cells were nearly all singly hormonal, and bihormonal cells were rarely detected, consistent with our in vitro findings (Figure 4E). In contrast, we detected much fewer PLCs in the groups transplanted with PPLCs derived from mouse iPSCs (by either our or published differentiation conditions) and no PLCs in the MEF group. As shown in Figure 4F, endocrine cell numbers in histological sections of each group were quantified. The β-like cells in the group transplanted with PPLCs reprogrammed from MEFs are about 50% of islet group.
These results showed that PPLCs could mature into cells of all three pancreatic lineages, including functional, insulin-secreting β-like cells that help to ameliorate hyperglycemia in vivo.
DISCUSSION
Our results demonstrate that functional DELCs and PPLCs can be generated from fibroblasts without going through a pluripotent state. Our approach is faster and potentially safer than methods that have iPSCs as an intermediate step and has the potential to provide sufficient numbers of functional progenitor cells for transplantation and other applications. In addition, we provide findings related to how lineage specification may be achieved and facilitated.
First, we found that providing the lineage specification signal at the beginning of iPSC-factor-mediated epigenetic activation improved the efficiency of direct definitive endoderm reprogramming. This observation suggests that transcriptional programs downstream of lineage specification signals specifically redirect iPSC-factor-mediated epigenome remodeling before an intermediate stage is established. Furthermore, this suggests that providing early lineage specification signals ensures that pluripotent cells are not generated.
We also found that, while Pdx1 or Nkx6.1 single-positive cells could be readily generated from DELCs by known conditions, they rarely coexpressed the other master pancreatic specification gene and consequently failed to mature into terminally differentiated, authentic pancreatic endocrine cells. To overcome this challenge, we devised a screening strategy to uncover combinations of small molecules that could generate Pdx1+/Nkx6.1+ cells. Remarkably, we found that a combination of four small molecules (RA, A83-01, LDE225, and pVc), in the absence of growth factors used in other protocols (such as FGF10 and EGF), could effectively induce Pdx1+/Nkx6.1+ cells from DELCs, increasing the production of Pdx1+/Nkx6.1+ cells about 7-fold. While RA and Hedgehog pathway inhibitor are known to induce pancreatic differentiation, the other two small molecules—and their synergistic ability to induce Pdx1+/Nkx6.1+ cells when combined—have not, to our knowledge, been reported before. Thus, dissection of how inhibition of the TGF-β/Activin-A pathway by A83-01, and the pleiotropic signaling pathways triggered by pVc exposure, contributes to pancreatic specification is worthwhile.
Additionally, we explored whether influencing early steps in lineage induction might significantly impact later specification steps. To date, efforts to influence lineage induction to a particular stage of development or differentiation have mostly focused on the step immediately preceding the particular stage and overlooked earlier steps. To further improve pancreatic cell induction (the third step in our reprogramming), we screened for new small molecules during the first two steps of reprogramming toward definitive endoderm cells. Here we used double staining of Pdx1/Nkx6.1 as a final readout after the third step. Two small molecules, Bix-01294 and pVc, were found to further increase the number of Pdx1+/Nkx6.1+ cells when used at the first two steps. Notably, treatment with Bix-01294 and pVc during the first two steps did not affect the numbers of Sox17/Foxa2 single- and double-positive cells on day 12. This suggests that until we better understand the steps and identification of more predictive markers in an early stage, using general markers of the early developmental stage may have limitations.
Importantly, we found that Bix-01294 and pVc could promote human ESC differentiation into Pdx1+/Nkx6.1+ pancreatic progenitors. As shown in Figures S2H and S2I, both Bix-01294 and pVc increased the number of Pdx1+/Nkx6.1+ colonies, respectively, and their combination had a synergistic effect. This result indicated that our findings in mouse cells might translate to similar mechanisms in human cells.
We also carried out screens for small molecules that may promote pancreatic β-cell maturation. SB203580 and pVc strongly and synergistically increased the number of insulin+/Pdx1+ cells. These small molecules facilitated reprogramming and also provided chemical tools for further mechanistic studies.
Finally, we tested the developmental potential of our PPLCs in vivo. PPLCs generated from MEFs by our advanced approach efficiently generated all three pancreatic lineages, including functional insulin secreting β-like cells, and ameliorated hyperglycemia in vivo. The PPLCs reprogrammed from MEFs appeared to be more functional than the PPLCs derived from mouse iPSCs. These results highlight the important roles of these small molecules and the therapeutic potential of our approach.
EXPERIMENTAL PROCEDURES
Direct Reprogramming of Fibroblasts into DELCs
Dox-inducible secondary MEFs were plated at 1 × 104 cells/cm2 and cultured in MEF medium for an additional day. The medium was then changed to Med-I supplemented with 4 μg/ml Dox, 50 ng/ml Activin A, and 1 mM LiCl for the indicated time. Thereafter, the medium was changed to Med-II supplemented with 50 ng/ml Activin A and 1 mM LiCl for the indicated time. Detailed protocols can be found in Supplemental Experimental Procedures.
Differentiation of DELCs to PPLCs
DELCs were further cultured in Med-III and treated with 2 μM RA, 1 μM A83-01, 2 μM LDE225, and 280 μM pVc for 1 day, and then treated with 1 μM A83-01, 2 μM LDE225, and 280 μM pVc for another 3 days. Detailed protocols can be found in Supplemental Experimental Procedures.
miPSC Culture and Differentiation
NGFP1 mouse iPSCs were cultured on MEF feeder cells in iPSC medium. Pancreatic differentiation was performed with a published differentiation protocol (D’Amour et al., 2006; Li et al., 2011; Nostro et al., 2011) with minor modifications or with our own optimized differentiation protocol. Briefly, our differentiation protocol involved seeding trypsinized NGFP1 mouse iPSCs (20,000 cells/ml) in Med-II in Petri dishes for 2 days. Then EBs were collected by brief centrifugation and resuspended in Med-II supplemented with 50 ng/ml Activin A, 2 mM LiCl, and 280 μM pVc for another 2 days. After that, the cells were trypsinized and replated (50,000 cells/cm2) on Matrigel-coated dishes in Med-III and treated with 2 μM RA, 1 μM A83-01, 2 μM LDE225, and 280 μM pVc for 1 day, and then treated with 1 μM A83-01, 2 μM LDE225, and 280 μM pVc for another 3 days. Detailed protocols can be found in Supplemental Experimental Procedures.
Transplantation Assay
Male, 8- to 10-week-old NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice were injected i.p. with 35 mg/kg STZ daily for 4 days. Mice were considered to be diabetic if blood glucose measurements were > 300 mg/dl for 4 consecutive days, after which they were used as transplant recipients. Under anesthesia, the left kidneys of diabetic mice received a renal subcapsular transplant of 300 native mouse islets in 30 μl Matrigel (Islet, n = 4), 3 × 106 PPLCs reprogrammed from MEFs (MEF-derived PPLC, n = 10), 3 × 106 NGFP1 mouse iPSCs derived PPLCs by our own optimized differentiation conditions (iPSC-derived PPLC1, n = 6), 3 × 106 NGFP1 mouse iPSC-derived PPLCs by the published differentiation conditions (iPSC derived PPLC2, n = 6), or 3 × 106 secondary MEF cells (MEF, n = 6). Untreated normal mice (Norm, n = 4) and mice treated with STZ only (STZ, n = 4) were used as controls. Nonfasting blood glucose levels were measured weekly after transplantation for 10 weeks. At the end of 8 weeks, glucose-stimulated insulin secretion assay and intraperitoneal glucose tolerance tests were carried out. Right after that, the left kidneys were surgically removed for immunohistochemistry analysis. Detailed protocols can be found in the Supplemental Experimental Procedures. All animal work was approved by the institutional IACUC committee.
Immunohistochemistry
Kidneys were fixed in 4% paraformaldehyde and used for paraffin section and cryosection. Kidneys were transversally sectioned and stained with primary antibodies. Then, the antigen-primary antibody immune complex was visualized with fluorescent secondary antibodies. Cell nuclei were counterstained with DAPI. To quantify endocrine cell numbers of mice from MEF, PPLC, and islet grafts, the endocrine cells were counted in four sections of a mouse and these were averaged to obtain the endocrine cell numbers of the mouse. For each group, four mice were averaged.
Supplementary Material
Acknowledgments
We thank Dr. Shuai Han and Dr. Chen Yu for scientific discussion. K.L. is supported by a Roddenberry fellowship. S.D. is supported by funding from NICHD, NHLBI, NEI, and NIMH/NIH; California Institute for Regenerative Medicine; DoD; Roddenberry Foundation; William K. Bowes, Jr. Foundation; and Gladstone Institutes. H.A.R is supported by a Richard G. Klein fellowship and a JDRF fellowship (3-2012-266). Research in M.H.’s laboratory is supported by funds from the Leona M. & Harry B. Helmsley Charitable Trust.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information for this article includes Supplemental Experimental Procedures and four figures and can be found with this article online at http://dx.doi.org/10.1016/j.stem.2014.01.006.
References
- D’Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392–1401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- Efe JA, Hilcove S, Kim J, Zhou H, Ouyang K, Wang G, Chen J, Ding S. Conversion of mouse fibroblasts into cardiomyocytes using a direct reprogramming strategy. Nat Cell Biol. 2011;13:215–222. doi: 10.1038/ncb2164. [DOI] [PubMed] [Google Scholar]
- Ferber S, Halkin A, Cohen H, Ber I, Einav Y, Goldberg I, Barshack I, Seijffers R, Kopolovic J, Kaiser N, Karasik A. Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia. Nat Med. 2000;6:568–572. doi: 10.1038/75050. [DOI] [PubMed] [Google Scholar]
- Jiang W, Shi Y, Zhao D, Chen S, Yong J, Zhang J, Qing T, Sun X, Zhang P, Ding M, et al. In vitro derivation of functional insulin-producing cells from human embryonic stem cells. Cell Res. 2007;17:333–344. doi: 10.1038/cr.2007.28. [DOI] [PubMed] [Google Scholar]
- Kelly OG, Chan MY, Martinson LA, Kadoya K, Ostertag TM, Ross KG, Richardson M, Carpenter MK, D’Amour KA, Kroon E, et al. Cell-surface markers for the isolation of pancreatic cell types derived from human embryonic stem cells. Nat Biotechnol. 2011;29:750–756. doi: 10.1038/nbt.1931. [DOI] [PubMed] [Google Scholar]
- Kim J, Efe JA, Zhu S, Talantova M, Yuan X, Wang S, Lipton SA, Zhang K, Ding S. Direct reprogramming of mouse fibroblasts to neural progenitors. Proc Natl Acad Sci USA. 2011;108:7838–7843. doi: 10.1073/pnas.1103113108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, He Z, Li Y, Liu P, Chen F, Wang M, Zhu H, Ding X, Wangensteen KJ, Hu Y, Wang X. Combined activin A/LiCl/Noggin treatment improves production of mouse embryonic stem cell-derived definitive endoderm cells. J Cell Biochem. 2011;112:1022–1034. doi: 10.1002/jcb.22962. [DOI] [PubMed] [Google Scholar]
- Li J, Huang NF, Zou J, Laurent TJ, Lee JC, Okogbaa J, Cooke JP, Ding S. Conversion of human fibroblasts to functional endothelial cells by defined factors. Arterioscler Thromb Vasc Biol. 2013;33:1366–1375. doi: 10.1161/ATVBAHA.112.301167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SB, Schaffer AE, Sander M. The transcription factors Nkx6.1 and Nkx6.2 possess equivalent activities in promoting beta-cell fate specification in Pdx1+ pancreatic progenitor cells. Development. 2007;134:2491–2500. doi: 10.1242/dev.002691. [DOI] [PubMed] [Google Scholar]
- Nostro MC, Sarangi F, Ogawa S, Holtzinger A, Corneo B, Li X, Micallef SJ, Park IH, Basford C, Wheeler MB, et al. Stage-specific signaling through TGFβ family members and WNT regulates patterning and pancreatic specification of human pluripotent stem cells. Development. 2011;138:861–871. doi: 10.1242/dev.055236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezania A, Bruin JE, Xu J, Narayan K, Fox JK, O’Neil JJ, Kieffer TJ. Enrichment of human embryonic stem cell-derived NKX6.1-expressing pancreatic progenitor cells accelerates the maturation of insulin-secreting cells in vivo. Stem Cells. 2013;31:2432–2442. doi: 10.1002/stem.1489. [DOI] [PubMed] [Google Scholar]
- Schroeder IS, Rolletschek A, Blyszczuk P, Kania G, Wobus AM. Differentiation of mouse embryonic stem cells to insulin-producing cells. Nat Protoc. 2006;1:495–507. doi: 10.1038/nprot.2006.71. [DOI] [PubMed] [Google Scholar]
- Wernig M, Lengner CJ, Hanna J, Lodato MA, Steine E, Foreman R, Staerk J, Markoulaki S, Jaenisch R. A drug-inducible transgenic system for direct reprogramming of multiple somatic cell types. Nat Biotechnol. 2008;26:916–924. doi: 10.1038/nbt1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


