Abstract
Objectives
To investigate the intrinsic viscoelastic shear properties in porcine TMJ discs.
Materials and Methods
Twelve fresh porcine TMJ discs from young adult pigs (6-8 months) were used. Cylindrical samples (5 mm diameter) with uniform thickness (~1.2 mm) were prepared from five regions of the TMJ disc. Torsional shear tests were performed under 10% compressive strain. Dynamic shear was applied in two methods: (1) a frequency sweep test over the frequency range of 0.01-10 rad/s with a constant shear strain amplitude of 0.025 rad, and (2) a strain sweep test over the range of 0.005-0.05 rad at a constant frequency of 10 rad/s. Transient stress-relaxation tests were also performed to determine the equilibrium shear properties.
Results
As the frequency increased in the frequency sweep test, the dynamic shear complex modulus increased, with values ranging from 7 to 17 kPa. The phase angle, ranging from 11 to 15 degrees, displayed no pattern of regional variation as the frequency increased. The dynamic shear modulus decreased as the shear strain increased. The equilibrium shear modulus had values ranging from 2 to 4.5 kPa. The posterior region had significantly higher values for dynamic shear modulus than those in the anterior region while no significant regional difference was found for equilibrium shear modulus.
Conclusion
Our results suggest that the intrinsic region-dependent viscoelastic shear characteristics of TMJ disc may play a crucial role in determining the local strain of the TMJ disc under mechanical loading.
Keywords: temporomandibular joint disc, dynamic mechanical testing, shear modulus, porcine animal model
Introduction
Temporomandibular disorders (TMD) resulting in pain and disability are the second most common musculoskeletal condition, affecting 5-12% of the population with health care and societal costs of $4 billion in the United States (1). Temporomandibular joint (TMJ) disc dysfunction and associated pain occur in approximately 30% of TMD patients (2,3). The primary function of the TMJ disc is to provide mechanical support and prevent bone to bone contacts that can result in significant damage and loss of joint function. Nickel et al. has suggested that synovial fluid may reduce friction, but abnormal loading as a result of clenching or grinding can reduce the fluid boundary resulting in direct cartilage contacts (4). Sustained mechanical loading can cause decreases in fluid load support and increased friction that may result in excessive tissue shear stress and wear (5). Studies have found that dynamic shear stress or excessive shear strain can result in fatigue and/or failure of the TMJ disc (6-10). Pathological loading may also cause permanent collagen damage and cartilage degradation that can result in the development of osteoarthritis (11,12). However the mechanism through which the mechanical loading initiates pathological events within the TMJ disc is still poorly understood. One requirement for studying TMJ pathology and development of tissue engineered treatments is the characterization of disc mechanical properties and the resulting biological responses. These properties will be crucial in determining a suitable animal model as well as for building a predictive model of TMJ disorders.
Previously, the compression and tensile properties of the TMJ disc were characterized to better understand the complex function and environment in the cartilage tissue (13-18). During jaw motion, the fossa remains stationary while the condyle bone articulates. As a result, the sandwiched TMJ disc is also subjected to the shear force as well as a variety of compressive and tensile forces. Furthermore, due to the incongruities between the bone and cartilage surfaces, non-uniform deformation of the disc will result in the development of local shear stress. Another potential cause of shear stresses is the variation of extracellular matrix (ECM) structure and distribution across the disc. Collagen fibers have a ring-like alignment along the disc periphery and run anteroposteriorly through the central region of the disc (19). Due to the low glycosaminoglycan (GAG) content of TMJ discs, shear properties are largely believed to be associated with the collagen content and orientation. Previous studies have shown that excessive shear stress can result in fatigue and permanent damage of the TMJ disc (6-9). However, the static/equilibrium and dynamic shear properties have not been fully examined in relation to varying frequency, strain, and disc region.
Previous studies have shown that the shear modulus of TMJ discs was largely dependent on frequency and direction of loading which may be a result of region dependent biochemical composition (20). It is generally believed that tensile loads occurring within the cartilage are a result of shear and friction produced during joint motion (21). Studies have found that the viscoelasticity of the disc during tension and shear, unlike compression, is largely flow-independent and primarily supported by the solid phase (22). These findings highlighted the importance of testing within a reasonable frequency (~0.5-2 Hz during chewing) and under sufficient compressive loading (10% strain during clenching) to maintain a physiological basis.
Using rotational shear experiments, the region-dependent equilibrium modulus and dynamic viscoelastic properties of porcine TMJ discs were examined in this study. Due to the inhomogeneous and viscoelastic nature of the disc, the shear properties were examined in relation to frequency and shear strain in five disc regions. It has been showed that the porcine model is an acceptable analog for human samples (23-26). Therefore, the goal of this study was to determine viscoelastic shear properties of the porcine TMJ disc model and how these relate to TMJ disc structure and function. The hypothesis of this study is that mechanical shear properties of TMJ discs are viscoelastic and region-dependent.
Materials and Methods
Sample Preparation
Twelve TMJ discs from the left joint were harvested from pig heads (6-8 month old, Yorkshire, male) obtained from a local slaughterhouse within two hours of sacrifice. Sample size is based on the error analysis of our previous works on viscoelastic properties of human TMJ discs under confined compression (18). The discs were immediately photographed, morphologically examined, and wrapped in gauze soaked in a normal saline solution with protease inhibitors and stored at −80°C until mechanical testing. It has been reported that mechanical properties of porcine discs were retained over five freeze–thaw cycles (27). Discs exhibiting any abnormalities (i.e. fissures or bruising) were discarded.
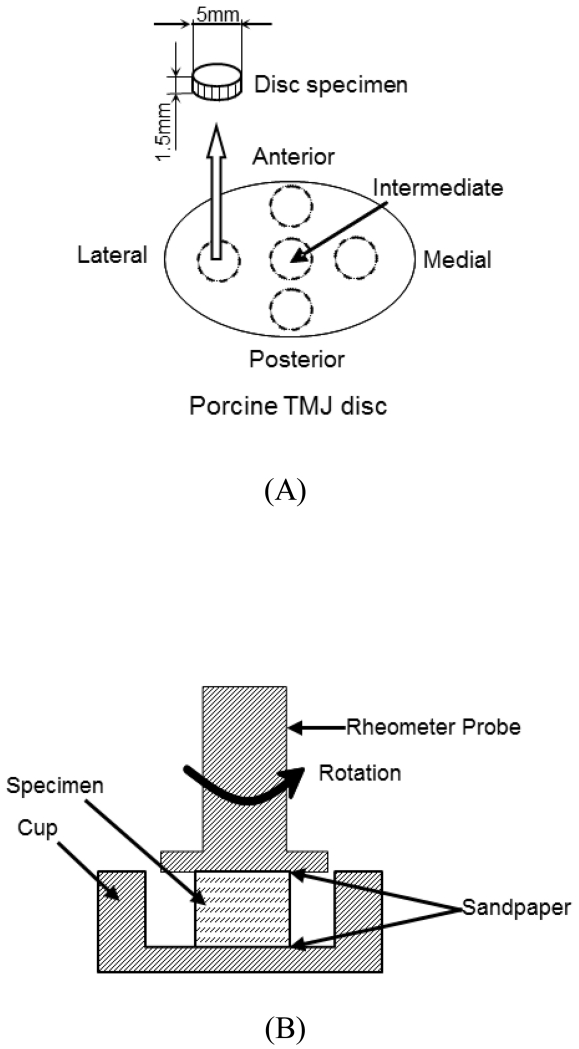
Cylindrical tissue plugs were obtained from the anterior, intermediate, lateral, medial, and posterior regions of the TMJ disc (Figure 1A), using a 5 mm corneal trephine (Biomedical Research Instruments Inc., Silver Spring, MD). Thin layers from the superior and inferior surfaces were removed via a sledge microtome (Model SM2400, Leica Instruments, Nussloch, Germany) with a freezing stage (Model BFS-30, Physitemp Instruments Inc., Clifton, NJ) to eliminate the natural concave shape of the disc and allow for a flat surface during mechanical testing. Shear samples had an average height of 1.0 mm and a diameter of 5 mm.
Figure 1.
A) Schematic of specimen preparation. The region and size of test specimens are shown. Shear samples from the five disc regions had an average height of 1.0mm and a diameter of 5mm. B) Schematic of rotational shear chamber.
Testing Configuration
Cylindrical tissue samples were placed in custom designed shear chamber that allowed for complete immersion in PBS to prevent dehydration (Figure 1B). Prior to testing, 200 grit sandpaper was affixed to both surfaces of the stainless steel 8 mm parallel plate geometry with cyancrylate glue. This was necessary to prevent sample slipping during high frequency rotations. The sandpaper was allowed to soak in PBS prior to zeroing of the gap to prevent errors in the measurement of sample height.
Rotational shear experiments were performed with a TA Instruments AR G2 (New Castle, DE) at 37°C maintained by a water cooled Peltier plate. The instrument has a displacement resolution of 25 nrad and a torque resolution of 0.1 nN/m. Initial height was measured by lowering the probe onto the sample and recording the height occurring at 5 mN of force. Samples were then compressed 10% of the initial measured height to ensure full contact of the surface and prevent slipping during rotation. Studies have suggested that this amount of strain corresponds to a loaded joint (28).
Loading Protocol
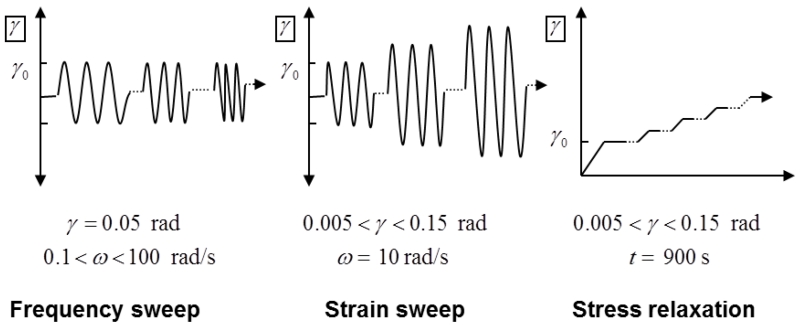
Three testing protocols were used to measure the static and dynamic viscoelastic properties of the disc. These included a frequency sweep, strain sweep, and a stress relaxation test (Figure 2). The frequency sweep was over a range of 0.1-10 radians/second (0.016-1.59 Hz) at a constant angular strain of 0.05 radians (~2.86°; ). The strain sweep was over a strain range of 0.005 to 0.15 radians (~0.29°-8.6°) at an angular frequency of 10 radians/second. The stress relaxation occurred in strain steps of 0.05 radian increases from 0.005-0.15 radians with each level maintained for 900 seconds to measure the equilibrium shear modulus. In this study, small shear strains were used in order to measure the flow-independent material properties. Complex modulus and phase angle were recorded for dynamic experiments. Equilibrium shear modulus was calculated from the slope of the equilibrium stress versus strain curve generated from all five stress relaxation steps.
Figure 2.
Loading protocols of frequency sweep, strain sweep, and stress relaxation experiments. γ0 represents initial amplitude of the shear strain.
Statistical analysis
The shear properties were examined for significant differences between disc regions using SPSS statistics software (SPSS 16.0, IBM, NY). One-way ANOVA and Tukey’s post hoc tests were performed to determine if significant differences existed. Statistical differences were reported at p-values <0.05.
Results
Frequency Sweep
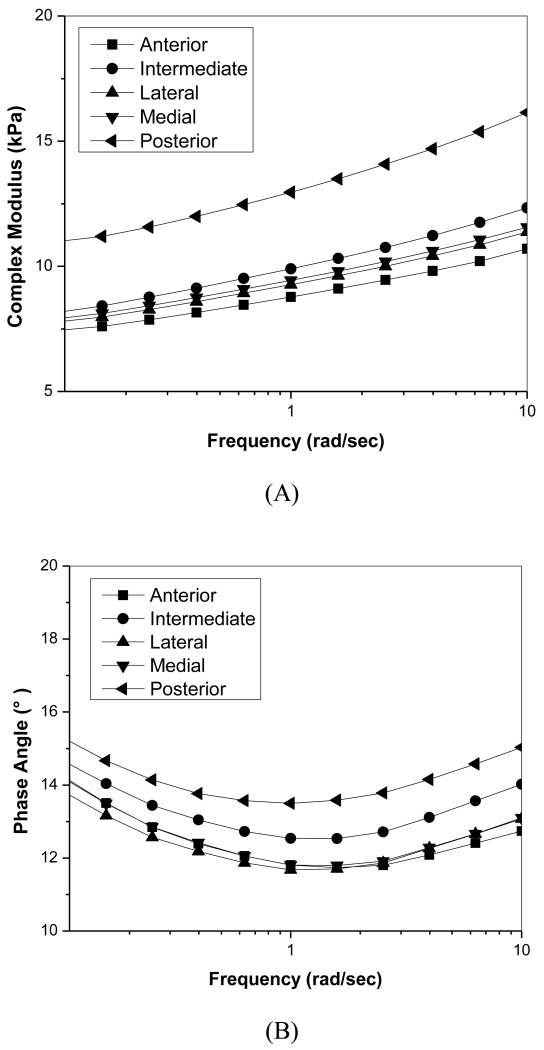
In the shear frequency sweep experiments, the magnitude of the complex modulus |G*| and phase angle were significantly frequency dependent (Figure 3). Complex modulus values for the posterior region were significantly higher than those in the anterior region over the entire frequency range (p=0.025). Average standard deviations for complex moduli were approximately 45% of average moduli values (not shown in the figure). At a frequency of 6.28 radians/second (~1Hz), the average |G*| of the central region was 11.22 ± 5.2 kPa, 10.20 ± 4.9 kPa for the anterior, and 15.38 ± 6.07 kPa for the posterior region. Phase angle decreased with increasing frequency until 1 radian/second was reached then began to increase with increasing frequency. Phase angle values only varied between 11° - 15° and showed no regional variation. Average phase angle standard deviations were approximately 15% of measured phase angles (not shown in figure).
Figure 3.
Shear frequency sweep of porcine TMJ discs. A) Relationship of complex modulus with frequency. B) Relationship of phase angle with frequency.
Strain Sweep
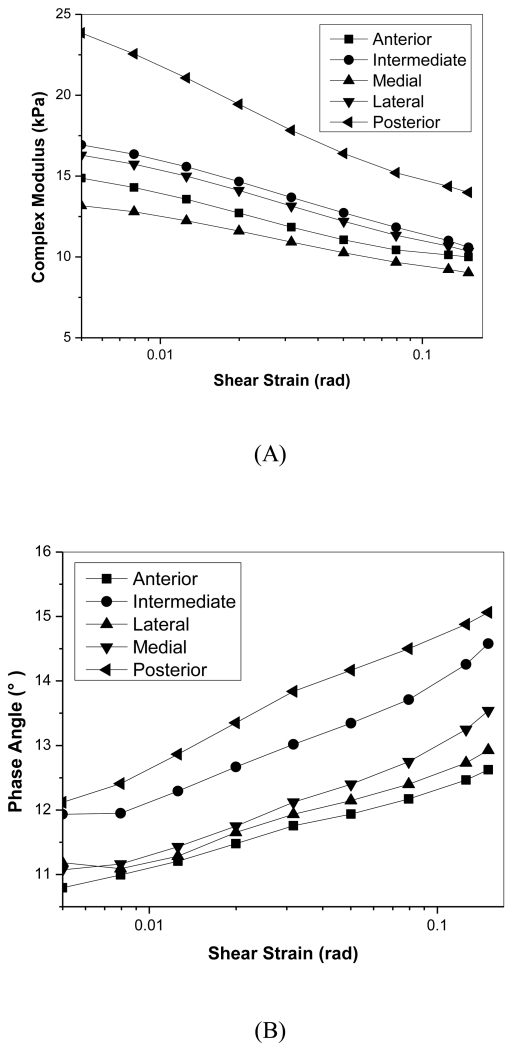
In the strain sweep experiments, the complex modulus significantly decreased with increasing rotational strains (Figure 4). The posterior region once again was significantly higher than anterior and medial regions for |G*| over all strains tested (p=0.018). Average standard deviations were 40% of the magnitude of complex modulus values (not shown in the figure). Although phase angle increased with increasing strain for all regions, it only varied between 11° - 15° and showed no significant regional variation. Average phase angle standard deviations were 15% of measured values (not shown in figure).
Figure 4.
Shear strain sweep of porcine TMJ discs. A) Relationship of complex modulus with shear strain. B) Relationship of phase angle with shear strain.
Equilibrium Shear Modulus
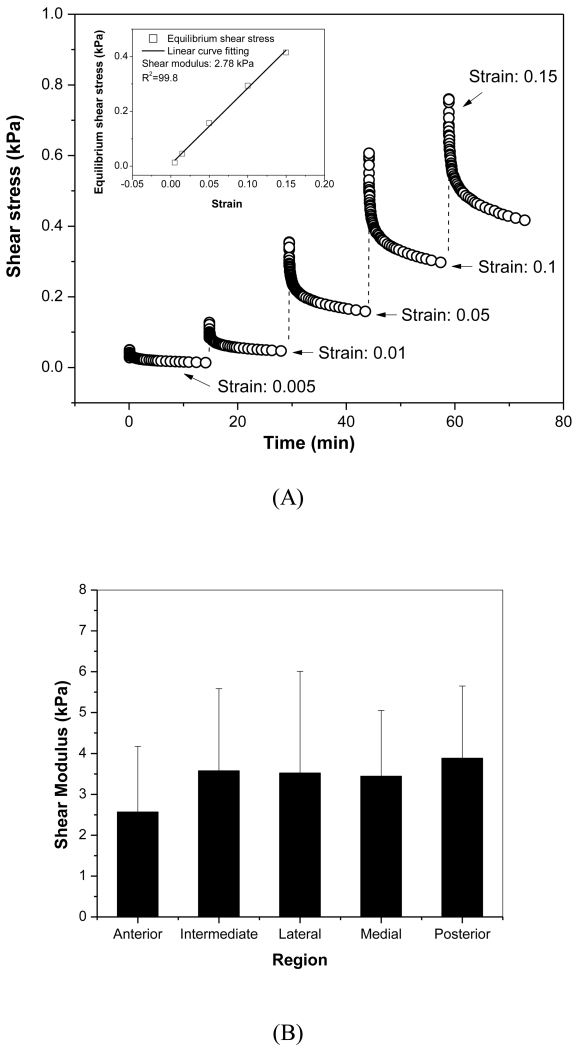
The rotational shear equilibrium modulus was measured by performing stepwise stress relaxations at increasing strain steps (Figure 5). The slope of the resulting stress versus strain curve at equilibrium was used to calculate the modulus. Although no significant regional variation was detected, the posterior region had the highest average modulus value and the anterior had lowest average modulus value (Posterior: 3.88±1.76 kPa; Anterior: 2.57±1.61 kPa). The overall average equilibrium modulus across the disc was 3.53±1.61 kPa.
Figure 5.
A) Typical stress relaxation curve for disc sample from anterior region. The multi-strain levels included five steps (0.005, 0.01, 0.05, 0.1, 0.15). As shown in the inset plot, the shear equilibrium modulus was calculated from the slope of equilibrium stress versus strain curve generated from all five stress relaxation steps. B) Average ± standard deviation of regional shear equilibrium modulus.
Discussion
The objective of this study was to determine the viscoelastic shear properties of the porcine TMJ disc model and how these relate to TMJ disc structure and function. The results demonstrated that the viscoelastic shear properties are dependent on region, loading frequency, and strain. Lai et al. investigated the static shear properties of human TMJ discs and found the shear moduli of the peripheral regions to be significantly higher than those in the central portions. In our study, the posterior region exhibited significantly higher complex moduli for frequency and strain sweeps as well as a relatively higher average equilibrium modulus. By contrast, the anterior region showed relatively lower moduli for all of the experiments. This unique regional difference could be explained by the inhomogeneous collagen fiber structure and biochemical components in the porcine TMJ disc which were shown in our previous conductivity and fluorescence recovery after photobleaching (FRAP) studies (29,30). The scanning electron microscope (SEM) study also further confirmed that collagen fiber structure in the anterior region is more isotropic with a significantly lower coherency coefficient than the other regions in the disc (30).
The relationship of dynamic shear properties with the frequency and strain in the TMJ disc is consistent with results found in bovine meniscus and human annulus fibrosus (AF) (7,31). Previous studies also found the tensile and shear properties of TMJ discs to be largely fluid flow independent suggesting much greater load support from the solid phase of the tissue (22). These findings were further supported with the results of our dynamic frequency and strain sweeps. Phase angles only varied between 11°-15° which was a significantly smaller range than observed during dynamic compression experiments in our previous study (~15°-30°). By contrast, dynamic shear modulus values were found to be significantly lower than confined compression dynamic modulus values (18). Furthermore, the phase angle was found to be increased while the complex modulus was decreased under larger shear strain levels. All of these findings suggest that during small rotational strain experiments, the solid phase of the tissue behaves elastically and the flow dependent effect was isolated from the flow independent viscoelasticity (31). Therefore, the shear modulus measured in this study represents the intrinsic viscoelasticity in the TMJ disc.
Very few studies have characterized the viscoelastic properties of the human TMJ disc which may be due to difficulty in obtaining samples (32,33). Many studies have characterized the compressive, tensile, and shear mechanical properties of a variety of animal species. Several groups concluded that the pig is the best experimental model of the TMJ after comparison to sheep, cows, dogs, cats, rabbits, rats, and goats (24,26). In particular, selection of the pig was attributed to the similar size of TMJ structures, shape of the disc, and omnivorous diet. In addition, the pig and human TMJs have been shown to have similar gross morphology and structure, including the disc and its attachments. Moreover, both pig and human TMJs have a similar range of motion (23,25). While all these reasons served as the rationale for using porcine TMJ discs in this study, it is necessary to measure the viscoelastic shear properties of human TMJ discs in a future study.
Lai’s study also found significant increases in shear moduli with increasing age suggesting changes in morphology over time. The average shear modulus measured in the Lai study ranged between 1-1.75 MPa which was several hundred times greater than the values measured in this study (~3 kPa). These significant differences are likely a result of testing configuration differences. In the Lai study, samples were loaded with parallel shear plates and tested to failure which suggests forces much higher than those experienced in vivo(34). Tissue samples were attached to the testing apparatus with adhesive which could infiltrate the tissue and alter the viscoelastic properties measured. Furthermore, compared with the human AF, which was measured with a similar torsional shear testing configuration (31), the shear modulus in this study are relatively smaller but still in the same kPa magnitude (this study: 5-25 kPa; human AF: 20-150 kPa). Larger values in human AF tissue could be due to the difference of normal stresses applied to the samples (TMJ discs in this study: 2.5-7.4 kPa for 10% strain compression; human AF: 17.5-35 kPa due to directly compressive loading). Previous studies also indicated that the dynamic properties of the TMJ disc are affected by the temperature sensitive collagen and proteoglycan components. A higher temperature (body temperature instead of room temperature) may reduce the stiffness and strength of the disc (8,14,35). In addition, the sample preparation method used in this study also has its limitations. Bone trephine punching and microtome cutting could alter the ECM collagen structure by reducing the intrinsic tension of the collagen fiber and weaken its sustainability against shear force.
Conclusions
In conclusion, this study shows that the porcine TMJ disc has frequency and strain dependent inhomogeneous shear properties which are similar to that in the human TMJ disc. It helps to further understand the role of mechanical loading on TMJ daily function and the pathological mechanism of TMJ disorders. Furthermore, it also provides essential mechanical parameters for further finite element analysis on TMJ kinematics and mechanics for early diagnosis or function assessment.
Clinical Relevance.
The National Institute of Dental and Craniofacial Research (NIDCR) reports that TMJ disorders resulting in pain and disability are the second most common musculoskeletal condition, affecting 5-12% of the population with annual health care costs of $4 billion. Mechanical dysfunction of the TMJ disc, especially displacement due to tissue degeneration, is central to many TMJ disorders. It is generally believed that pathological mechanical loadings, e.g. sustained jaw clenching or malocclusion, trigger a cascade of molecular events leading to TMJ disc degeneration. Therefore, characterization of disc mechanical properties is essential to help understand the pathological mechanism and provide guidelines for disc tissue regeneration.
Acknowledgements
This project was supported by NIH grants DE018741, DE021134, and AR055775, a NIH F31 training grant DE020230 to JK, and a NIH F31 training grant DE023482 to GJW.
Reference
- 1.Stowell AW, Gatchel RJ, Wildenstein L. Cost-effectiveness of treatments for temporomandibular disorders: biopsychosocial intervention versus treatment as usual. J Am Dent Assoc. 2007;138:202–208. doi: 10.14219/jada.archive.2007.0137. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad M, Hollender L, Anderson Q, Kartha K, Ohrbach R, Truelove EL, et al. Research diagnostic criteria for temporomandibular disorders (RDC/TMD): development of image analysis criteria and examiner reliability for image analysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:844–860. doi: 10.1016/j.tripleo.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schiffman EL, Truelove EL, Ohrbach R, Anderson GC, John MT, List T, et al. The Research Diagnostic Criteria for Temporomandibular Disorders. I: overview and methodology for assessment of validity. J Orofac Pain. 2010;24:7–24. [PMC free article] [PubMed] [Google Scholar]
- 4.Nickel JC, McLachlan KR. In vitro measurement of the stress-distribution properties of the pig temporomandibular joint disc. Arch Oral Biol. 1994;39:439–448. doi: 10.1016/0003-9969(94)90175-9. [DOI] [PubMed] [Google Scholar]
- 5.Park S, Krishnan R, Nicoll SB, Ateshian GA. Cartilage interstitial fluid load support in unconfined compression. J Biomech. 2003;36:1785–1796. doi: 10.1016/s0021-9290(03)00231-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu W, Mow VC, Koob TJ, Eyre DR. Viscoelastic shear properties of articular cartilage and the effects of glycosidase treatments. J Orthop Res. 1993;11:771–781. doi: 10.1002/jor.1100110602. [DOI] [PubMed] [Google Scholar]
- 7.Zhu W, Chern KY, Mow VC. Anisotropic viscoelastic shear properties of bovine meniscus. Clin Orthop Relat Res. 1994:34–45. [PubMed] [Google Scholar]
- 8.Tanaka E, van Eijden T. Biomechanical behavior of the temporomandibular joint disc. Crit Rev Oral Biol M. 2003;14:138–150. doi: 10.1177/154411130301400207. [DOI] [PubMed] [Google Scholar]
- 9.Spirt AA, Mak AF, Wassell RP. Nonlinear viscoelastic properties of articular cartilage in shear. J Orthop Res. 1989;7:43–49. doi: 10.1002/jor.1100070107. [DOI] [PubMed] [Google Scholar]
- 10.Gallo LM, Nickel JC, Iwasaki LR, Palla S. Stress-field translation in the healthy human temporomandibular joint. J Dent Res. 2000;79:1740–1746. doi: 10.1177/00220345000790100201. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka E, Rego EB, Iwabuchi Y, Inubushi T, Koolstra JH, van Eijden TM, et al. Biomechanical response of condylar cartilage-on-bone to dynamic shear. J Biomed Mater Res. 2008;85:127–132. doi: 10.1002/jbm.a.31500. [DOI] [PubMed] [Google Scholar]
- 12.Zarb GA, Carlsson GE. Temporomandibular disorders: osteoarthritis. J Orofac Pain. 1999;13:295–306. [PubMed] [Google Scholar]
- 13.Beatty MW, Nickel JC, Iwasaki LR, Leiker M. Mechanical response of the porcine temporomandibular joint disc to an impact event and repeated tensile loading. J Orofac Pain. 2003;17:160–166. [PubMed] [Google Scholar]
- 14.Detamore MS, Athanasiou KA. Tensile properties of the porcine temporomandibular joint disc. J Biomech Eng. 2003;125:558–565. doi: 10.1115/1.1589778. [DOI] [PubMed] [Google Scholar]
- 15.Lomakin J, Sprouse PA, Detamore MS, Gehrke SH. Effect of pre-stress on the dynamic tensile behavior of the TMJ disc. J Biomech Eng. 2014;136:011001. doi: 10.1115/1.4025775. [DOI] [PubMed] [Google Scholar]
- 16.Snider GR, Lomakin J, Singh M, Gehrke SH, Detamore MS. Regional dynamic tensile properties of the TMJ disc. J Dent Res. 2008;87:1053–1057. doi: 10.1177/154405910808701112. [DOI] [PubMed] [Google Scholar]
- 17.Willard VP, Kalpakci KN, Reimer AJ, Athanasiou KA. The regional contribution of glycosaminoglycans to temporomandibular joint disc compressive properties. J Biomech Eng. 2012;134:011011. doi: 10.1115/1.4005763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuo J, Zhang L, Bacro T, Yao H. The region-dependent biphasic viscoelastic properties of human temporomandibular joint discs under confined compression. J Biomech. 2010;43:1316–1321. doi: 10.1016/j.jbiomech.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allen KD, Athanasiou KA. Tissue Engineering of the TMJ Disc: A Review. Tissue Eng. 2006;12:1183–1196. doi: 10.1089/ten.2006.12.1183. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka E, Hanaoka K, van Eijden T, Tanaka M, Watanabe M, Nishi M, et al. Dynamic shear properties of the temporomandibular joint disc. J Dent Res. 2003;82:228–231. doi: 10.1177/154405910308200315. [DOI] [PubMed] [Google Scholar]
- 21.Singh M, Detamore MS. Biomechanical properties of the mandibular condylar cartilage and their relevance to the TMJ disc. J Biomech. 2009;42:405–417. doi: 10.1016/j.jbiomech.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 22.Huang CY, Mow VC, Ateshian GA. The role of flow-independent viscoelasticity in the biphasic tensile and compressive responses of articular cartilage. J Biomech Eng. 2001;123:410–417. doi: 10.1115/1.1392316. [DOI] [PubMed] [Google Scholar]
- 23.Herring SW, Decker JD, Liu ZJ, Ma T. Temporomandibular joint in miniature pigs: anatomy, cell replication, and relation to loading. Anat Rec. 2002;266:152–166. doi: 10.1002/ar.10049. [DOI] [PubMed] [Google Scholar]
- 24.Kalpakci KN, Willard VP, Wong ME, Athanasiou KA. An interspecies comparison of the temporomandibular joint disc. J Dent Res. 2011;90:193–198. doi: 10.1177/0022034510381501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mills DK, Daniel JC, Herzog S, Scapino RP. An animal model for studying mechanisms in human temporomandibular joint disc derangement. J Oral Maxillofac Surg. 1994;52:1279–1292. doi: 10.1016/0278-2391(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 26.Stembirek J, Kyllar M, Putnova I, Stehlik L, Buchtova M. The pig as an experimental model for clinical craniofacial research. Lab Anim. 2012;46:269–279. doi: 10.1258/la.2012.012062. [DOI] [PubMed] [Google Scholar]
- 27.Allen KD, Athanasiou KA. A surface-regional and freeze-thaw characterization of the porcine temporomandibular joint disc. Ann Biomed Eng. 2005;33:951–962. doi: 10.1007/s10439-005-3872-6. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka E, Iwabuchi Y, Rego EB, Koolstra JH, Yamano E, Hasegawa T, et al. Dynamic shear behavior of mandibular condylar cartilage is dependent on testing direction. J Biomech. 2008;41:1119–1123. doi: 10.1016/j.jbiomech.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 29.Kuo J, Wright GJ, Bach DE, Slate EH, Yao H. Effect of mechanical loading on electrical conductivity in porcine TMJ discs. J Dent Res. 2011;90:1216–1220. doi: 10.1177/0022034511415275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi C, Wright GJ, Ex-Lubeskie CL, Bradshaw AD, Yao H. Relationship between anisotropic diffusion properties and tissue morphology in porcine TMJ disc. Osteoarthr Cartilage. 2013;21:625–633. doi: 10.1016/j.joca.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iatridis JC, Kumar S, Foster RJ, Weidenbaum M, Mow VC. Shear mechanical properties of human lumbar annulus fibrosus. J Orthop Res. 1999;17:732–737. doi: 10.1002/jor.1100170517. [DOI] [PubMed] [Google Scholar]
- 32.Beek M, Aarnts MP, Koolstra JH, Feilzer AJ, van Eijden TM. Dynamic properties of the human temporomandibular joint disc. J Dent Res. 2001;80:876–880. doi: 10.1177/00220345010800030601. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka E, Shibaguchi T, Tanaka M, Tanne K. Viscoelastic properties of the human temporomandibular joint disc in patients with internal derangement. J Oral Maxillofac Surg. 2000;58:997–1002. doi: 10.1053/joms.2000.8743. [DOI] [PubMed] [Google Scholar]
- 34.Lai WF, Bowley J, Burch JG. Evaluation of shear stress of the human temporomandibular joint disc. J Orofac Pain. 1998;12:153–159. [PubMed] [Google Scholar]
- 35.Tanaka E, Aoyama J, Tanaka M, Van Eijden T, Sugiyama M, Hanaoka K, et al. The proteoglycan contents of the temporomandibular joint disc influence its dynamic viscoelastic properties. J Biomed Mater Res. 2003;65:386–392. doi: 10.1002/jbm.a.10496. [DOI] [PubMed] [Google Scholar]