Abstract
Background and Purpose
A change in acute-to-chronic lesion volume has been proposed as a biomarker for stroke therapies. The objectives of this study were to determine the magnitude of lesion volume change after standard treatment with tissue plasminogen activator and to determine whether specific volume change thresholds can discriminate clinical responders from nonresponders.
Methods
We measured lesion volume on diffusion weighted at baseline and on 90-day fluid attenuated inversion recovery MRI and scored 3-month modified Rankin Scale in consecutive patients treated with tissue plasminogen activator. We identified variables associated with excellent (modified Rankin Scale 0 to 1) and independent (modified Rankin Scale 0 to 2) outcomes.
Results
We included 53 patients (mean age 69 years, median baseline National Institutes of Health Stroke Scale score 7). The mean acute-to-chronic lesion volume increase was 11.7 (±7.7) cm3. In 23 patients, the chronic lesion was smaller than the baseline lesion. At 3 months, 32 patients had an excellent clinical outcome. Dichotomous volume change variables associated with outcome included decrease in volume ≥30% (P=0.004) and volume increase ≥5 cm3 (P=0.002).
Conclusions
In patients given standard tissue plasminogen activator therapy, changes in lesion volume are associated with clinical outcome, and threshold lesion volumes can differentiate excellent and poor outcome, suggesting these as a potential marker of outcome.
Keywords: acute stroke, brain imaging, brain infarction, MRI, thrombolysis, thrombolytic RX
In humans with acute stroke, abnormalities in water diffusion visualized with MRI may be reversible if blood flow is restored.1–5 Data from recent clinical trials suggest that a reduction in lesion volume is associated with clinical improvement, and volume change has been proposed as a marker of outcome.6–8 Before lesion volume change can be used as an outcome marker in clinical trials, however, it is important to determine whether volume change can discriminate responders from nonresponders when clinically effective therapies are used. Because intravenous tissue plasminogen activator (tPA) used within 3 hours of onset is the only proven effective therapy for acute stroke, the primary question in this analysis is whether volume change can make this discrimination after standard tPA treatment. Our objectives of this study were to determine the frequency and magnitude of the changes in the volume of ischemic lesions after standard treatment with intravenous tPA and to test whether specific volume change thresholds are associated with clinical outcome.
Methods
Patients
This is an analysis of data collected prospectively as part of a natural history study approved by the Institutional Review Board at the National Institutes of Neurological Disorders and Stroke and Suburban Hospital in Bethesda, Md. All patients, or an authorized representative, gave informed consent. For this analysis, we considered consecutive patients with ischemic stroke in any vascular territory treated with intravenous tPA. We treated patients with tPA if they met standard criteria, had a disabling neurological deficit regardless of age and National Institutes of Health Stroke Scale (NIHSS) score and, if they had an MRI, an abnormality on diffusion weighted imaging (DWI), perfusion weighted imaging, or both, consistent with acute stroke.9 For the analysis, we included only those patients who had interpretable pretreatment DWI and follow-up fluid attenuated inversion recovery (FLAIR) obtained at least 3 but no more than 180 days from stroke onset and a modified Rankin Scale (mRS) scored at the time of, or after, the follow-up FLAIR. Because we are trying to replicate clinical trial methodology, we also excluded patients who had a prestroke mRS score >1.
Imaging Technique
Imaging was performed as part of the standard care pathway using a 1.5-Tesla MRI scanner (Twinspeed XL; General Electric). All patients had a pretreatment scan, and follow-up scans were scheduled at 5, 30, and 90 days from stroke onset. Because of clinical care requirements, patient death, or patient requests, follow-up scans were sometimes performed outside these times or not at all. The pretreatment scanning protocol was standardized and, in addition to gradient recalled echo and MR angiography, included DWI, perfusion weighted imaging, and fast spin-echo FLAIR. The last 3 sequences were used for this analysis and had the following typical parameters: 24-cm field of view, 7-mm thick axial oblique slices aligned with the AC–PC, 20 slices contiguous, interleaved, and colocalized. In addition, typical MRI parameters for specific sequences were: for DWI, TR/TE=6000/72 ms, acquisition matrix of 128×128, with both b=0 and b=1000 sec/mm2, isotropically weighted; for FLAIR, TR/TE=9000/85 ms, TI=1750 ms, and a 256×128 matrix. The follow-up FLAIR had higher resolution with 66 slices (2-mm thick contiguous axial–oblique) with TR/TE=9000/92 ms, TI=2200 ms, and a 256×128 matrix. Perfusion weighted images were obtained using the standard bolus passage of contrast method by injecting gadolinium (0.1 mmol/kg through a power injector) with gradient recalled echoplanar imaging, TR/TE of 2000/45 ms, 25 phases, 2 seconds per phase, flip angle of 90°, and a matrix of 64×64. Maps of estimated mean transit time were calculated using concentration-time curves obtained from the perfusion weighted imaging time series. The first moment divided by the zeroth moment of the concentration-time curve was used to generate mean transit time maps by a generic algorithm that is nonproprietary and commercially available.10 This algorithm has been used in multiple clinical trials.6–8,11
Image Analysis
A reader unaware of patient demographics, clinical outcome, lesion side or location, and pretreatment-to-follow-up pairings measured lesion volume on pretreatment DWI and follow-up FLAIR using commercially available image analysis software (Cheshire; Perceptive Informatics). The reader has extensive experience with this method and performed all the measurements.6,8,12,13 We previously showed that quantitative volume measurements, made by this reader and by multiple trained stroke neurologists, are highly consistent and replicable for lesion volume measurements on DWI, mean transit time, and FLAIR.13 To ensure blinding to treatment, images from 9 non-tPA-treated patients were randomly intercalated among the images used for this analysis. A semiautomated technique based on a watershed method was used for lesion volume measurements; the software uses a sampling method to generate regions of interest based on seed points placed by the reader, who then edits the borders using the contralateral hemisphere and adjacent slices for reference. The sum of the area of the region of interest in each slice multiplied by the slice thickness gives the stroke lesion volumes. When evaluating the FLAIR volume, the reader paid particular attention to preexisting chronic lesions present on the acute FLAIR images to avoid replication of these lesion areas in the current FLAIR volume calculations. We subtracted the volume of any lesions seen in the baseline FLAIR so that preexisting chronic lesions did not affect the measurement of chronic lesion volume of the index stroke. We calculated changes in volume as the difference between the chronic and baseline lesion volumes and mismatch volume subtracting DWI lesion volume from mean transit time lesion volume. We used the DWI lesion volume as the denominator calculating percentages.
Clinical Assessments
Neurologists unaware of volume measurements examined all patients at every imaging time point. The primary outcome was functional status at 3 months using 2 cutoffs that are common in clinical trials: (1) excellent (mRS of 0 to 1) and (2) independent (mRS 0 to 2) outcome.
Statistical Analysis
We classified patients into groups based on the 3-month mRS score. To reflect standard clinical trial methodology, when a 3-month mRS was unavailable, we used the last observation (3 to 180 days) carried forward. We assessed for normality with the Kolmogorov-Smirnov test, compared continuous variables with Student t or the Mann–Whitney test, and used χ2 or Fisher exact test for categorical variables. We treated age, pretreatment glucose level, and lesion volumes as continuous variables and divided NIHSS scores into tertiles. Volume change was analyzed as a dichotomous variable; we chose the absolute volume and percentage change thresholds based on prior data that suggest that 30% and 5 cm3 are outside the range of error of measurement.13 We examined 4 dichotomous variables: (1) volume decrease ≥30% or follow-up volume=0, (2) volume increase ≥30% or normal DWI with new lesion on chronic FLAIR, (3) volume decrease ≥5 cm3, and (4) volume increase ≥5 cm3. For the statistical analysis, we used SPSS for Windows, version 12.0.
Results
Between March 1, 2000, and December 31, 2004, we treated 134 patients with tPA. Although 105 patients had an MRI before treatment, 4 refused consent and thus we considered only 101 patients for this analysis. We excluded 48 patients for several reasons: refusal of follow-up MRI,7 death before follow-up MRI,17 loss to follow-up,3 incomplete data set,12 and preexisting disability (mRS ≥2; 7). The final sample includes 53 patients. Characteristics of the included and excluded patients are shown in Table 1. Patients who were excluded because they died before the follow-up MRI had larger DWI lesions and larger areas of perfusion–diffusion mismatch than included patients, but the differences were not significant (23.6 cm3 versus 19.0 cm3 [P=0.08] and 79.5 cm3 versus 48.6 cm3 [P=0.164], respectively).
Table 1.
Demographics of Included and Excluded Patients
| Included (N = 53) |
Excluded (N = 48) |
|
|---|---|---|
| Age, mean (SD) | 69 (14.7) | 77 (15.4) |
| Female, % | 42% | 56% |
| Prestroke mRS, median (IQR) | 0 (0–0) | 0 (0–3) |
| Pre-tPA glucose, mean (SD) | 125.3 (50.4) | 132.5 (47.2) |
| Baseline NIHSS, median (IQR) | 7 (3–16) | 11 (4–20) |
| Onset to treatment, minutes’ mean (SD) | 140 (30.7) | 143 (28.2) |
IQR indicates interquartile range (25% to 75%).
Baseline Clinical and Imaging Characteristics
The final sample comprised 22 women and 31 men. The median age was 73 years (mean±SEM, 69.1±2.0 years; range, 25 to 91 years). The median NIHSS score at presentation was 7 (range, 0 to 37). Patients were divided into tertiles based on pretreatment NIHSS score; because of the distribution of these scores, the groups are not of equal size. In 19 patients, the score was 0 to 4; in 18 patients, it was 5 to 11; and in 16, 12 to 37. The median time from onset to MRI was 83 minutes (range, 35 to 165 minutes) and from onset to treatment, 135 minutes (range, 82 to 192 minutes). The median volume of the DWI abnormality was 3.0 cm3 (mean±SEM, 19.0±4.5 cm3; range, 0 to 140 cm3). In eight patients, a DWI lesion was not discernable to the blinded reader doing the volume measurements (but may have been seen by the clinician managing the patient). However, in these 8 patients, symptoms persisted at least until the tPA infusion was started, and additional studies confirmed the diagnosis of stroke. Supplemental Table I, available online at http://stroke.ahajournals.org, shows the individual lesion volume data.
Follow-Up Imaging and Magnitude of Lesion Volume Change
The median time to follow-up FLAIR was 90 days (range, 3 to 145 days). The median volume of the stroke on FLAIR was 6.0 cm3 (mean±SEM, 30.7±9.3 cm3, range, 0 to 368 cm3), and the median acute-to-chronic volume change was 0.01 cm3 (mean±SEM, −11.7±7.7 cm3, range, −60 to 275 cm3). In 27 patients (50.9%), the lesion grew and in 3 (5.7%), the lesion volume did not change. In 23 patients (43.4%), the chronic lesion was smaller than the baseline DWI lesion; in this group, the median volume decrease was 7.6 cm3 (mean±SEM, 13.8±3.4 cm3, range, −0.1 to −60.2 cm3). The Figure shows an example of a patient whose lesion decreased in size after treatment with tPA. In 13 patients (24.5%), the chronic lesion volume on FLAIR was at least 5 cm3 smaller than the DWI lesion, whereas in 20 patients (37.7%), it was at least 30% smaller. Compared with the acute DWI lesion, the chronic FLAIR abnormality was at least 5 cm3 larger in 17 patients (32.1%) and it was at least 30% larger in 28 patients (52.8%).
Figure.
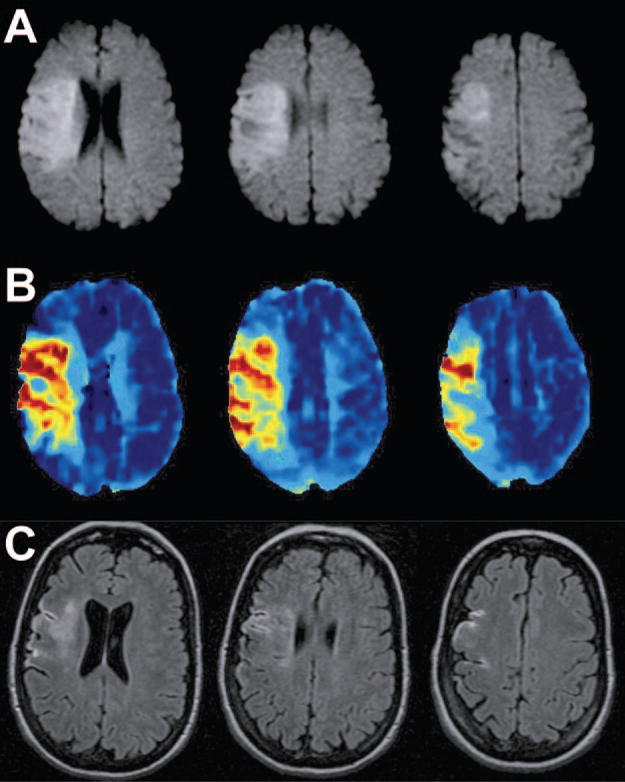
A 50-year-old woman presented with dysarthria, left hemiplegia, neglect, and right gaze deviation (NIHSS=19). Intravenous tPA was administered 93 minutes after onset. A, Pretreatment DWI obtained 70 minutes after symptom onset; B, pretreatment mean transit time; C, 90-day follow-up FLAIR. The initial DWI lesion volume was 72 cm3 and chronic lesion volume 12 cm3 (83% reduction). Three months later, she had mild facial weakness and sensory loss; her mRS=1.
Clinical Outcome and Imaging Discriminators of Outcome
The mRS was scored a median of 92 days (range, 5 to 151 days) after stoke. Thirty-two patients had excellent neurological outcome (mRS 0 or 1), and the mRS was 2 in another 5 patients. Three patients died after the follow-up MRI but before 3 months from the stroke had elapsed. As detailed in Table 2, variables associated with excellent neurological outcome at 3 months were younger age (P=0.008), lower initial NIHSS scores (P=0.002), smaller baseline DWI lesions (P=0.033), smaller chronic FLAIR lesions (P<0.0001), and decrease in lesion volume between the acute and chronic stages (P=0.001). Dichotomous volume change variables associated with outcome include decrease in volume ≥30% (P=0.004) and volume increase ≥5 cm3 (P=0.002). The same variables associated with mRS 0 to 1 were also associated with independent clinical recovery (Table 2).
Table 2.
Association of Clinical and Imaging Variables and Outcome
| Variable | Excellent Outcome
|
Independent Outcome
|
||||
|---|---|---|---|---|---|---|
| mRS 0–1 (N=32) |
mRS 2–6 (N=21) |
P Value | mRS 0–2 (N=37) |
mRS 3–6 (N = 16) |
P Value | |
| Age, mean±SEM | 65.1±2.8 | 75.1±2.3 | 0.008 | 65.5±2.5 | 77.3±2.4 | 0.007 |
| NIHSS | 0.002 | <0.001 | ||||
| 0–4 | 15 | 4 | 17 | 2 | ||
| 5–11 | 13 | 5 | 16 | 2 | ||
| >11 | 4 | 12 | 4 | 12 | ||
| Pre-tPA glucose, mg/dL; mean±SEM | 119.2±4.8 | 134.6±15.9 | 0.841 | 119.1±4.3 | 139.7±20.7 | 0.985 |
| Onset to treatment, minutes; mean±SEM | 139.6±5.6 | 140.4±6.5 | 0.93 | 140.5±5.1 | 138.6±7.6 | 0.84 |
| DWI volume, cm3; mean±SEM | 11.4±3.4 | 30.6±9.8 | 0.033 | 11.9±3.4 | 32.3±12.1 | 0.037 |
| FLAIR volume, cm3; mean±SEM | 6.1±2.1 | 68.2±21.0 | <0.001 | 8.6±2.6 | 81.7±26.6 | <0.001 |
| Volume change, cm3; mean±SEM | −5.3±3.4 | 37.6±17.5 | 0.001 | −3.3±3.2 | 46.4±22.6 | 0.003 |
| Is mismatch present?* | 1.0 | 1.0 | ||||
| Yes | 25 | 12 | 29 | 8 | ||
| No | 6 | 2 | 6 | 2 | ||
| Is mismatch present?† | 0.179 | 0.178 | ||||
| Yes | 24 | 21 | 27 | 18 | ||
| No | 5 | 10 | 6 | 9 | ||
| Volume decrease ≥30% | 0.004 | 0.013 | ||||
| Yes | 17 | 3 | 18 | 2 | ||
| No | 15 | 18 | 19 | 14 | ||
| Volume increase ≥30% | 0.102 | 0.127 | ||||
| Yes | 14 | 14 | 17 | 11 | ||
| No | 18 | 7 | 20 | 5 | ||
| Volume decrease ≥5 cm3 | 0.160 | 0.299 | ||||
| Yes | 10 | 3 | 11 | 2 | ||
| No | 22 | 18 | 26 | 14 | ||
| Volume increase ≥5 cm3 | 0.002 | 0.002 | ||||
| Yes | 5 | 12 | 7 | 10 | ||
| No | 27 | 9 | 30 | 6 | ||
N=45; mismatch is defined as volume of mean transit time delay 20% larger than volume of DWI abnormality.
Includes data from 15 patients who died before follow-up MRI was done but who had baseline mean transit time images.
Discussion
Ischemic brain injury seen on the acute MRI is reversible and, in patients given standard tPA therapy, changes in lesion volume can discriminate responders from nonresponders. In our study, almost 40% of patients had a chronic lesion that was at least 30% smaller than the baseline DWI abnormality, and this change was associated with a better outcome. Conversely, an increase ≥5 cm3 was associated with worse outcomes. Reversing even small areas of ischemia may have positive effects, which would explain why percentage rather than absolute decrease in volume was a more powerful predictor of outcome. An increase ≥5 cm3 is a more powerful predictor of a bad outcome than percentage increase, presumably because there is a lesion volume beyond which outcome is invariably poor. If the association between these volume change thresholds and outcome are confirmed in other cohorts, these could be used as a dichotomous marker of outcome in future clinical trials. In addition, the possibility that the combination of volume thresholds and stroke location data can lead to even more precise predictive models will have to be tested in future studies.14 The absolute volume and percentage change thresholds were chosen based on prior data that suggest that 30% and 5 cm3 are outside the range of error of measurement.13
This is the first study to show an association between volume change and clinical outcome in patients treated with intravenous tPA within 3 hours of symptom onset. Our group previously showed that mean transit time lesion volume 2 hours after tPA may be an early marker of long-term clinical benefit of thrombolytics therapy.12 Perfusion imaging before and shortly after tPA treatment is not always feasible, but baseline DWI and chronic FLAIR are frequently done, making volume change an attractive outcome measure for clinical trials. Other investigators have reported instances of DWI lesion reversal with thrombolytics up to 6 hours after onset, and this phenomenon has been seen after treatment with neuroprotective agents.2,4,6,8 In both the citicoline MRI study and the MRI Substudy of the GAIN trial, there was an association between lesion volume decrease and NIHSS improvement ≥7 points, irrespective of treatment assignment.6,8 In the GAIN MRI Substudy, lesion volume change was also associated with mRS score and Barthel Index at 3 months.8 Volume changes with tPA treatment have been studied by others.2,4,15,16 Not all investigators, however, found volume decrease after tPA treatment. For example, Röther et al found that median 7-day T2 volumes were larger than the baseline DWI (35 mL versus 22 mL) among 76 patients treated with intravenous tPA within 6 hours of onset of symptoms.17 The difference with this study may be explained by the choice of imaging time points; in the first week after stroke, vasogenic edema may lead to spuriously high lesion volumes.
This study has some limitations. Because tPA is standard of care for eligible patients with stroke, there is no control group. Although the images were read without access to clinical information to reduce bias, this may lead to decreased accuracy, because clinical information may be of help when interpreting stoke MRI. Patients in our series had relatively mild strokes. Because we included consecutive patients treated with tPA enrolled in a natural history study, and we did not systematically exclude patients with severe strokes, other than those that died before a follow-up scan could be done, the distribution of stroke severity represents the distribution of severity commonly seen in a primary stroke center. In our series, some of the patients had the last follow-up scan as early as 3 days after the acute stroke. This may have led us to underestimate the acute-to-chronic volume change. However, because we were interested in evaluating the role of volume change in acute clinical trials, when later observations were not available, we used the last observation carried forward as is commonly done in the intent-to-treat analyses of imaging-based clinical trials.
In conclusion, among patients given standard tPA therapy, changes in lesion volume are associated with clinical outcome, and threshold lesion volumes can be used to differentiate clinical responders from nonresponders, suggesting these volume measures as potential markers of outcome in clinical trials.
Acknowledgments
We thank the members of the NIH Stroke Program at Suburban Hospital who assisted with data collection and patient care.
Source of Funding
This research was supported by the Division of Intramural Research of the National Institute of Neurological Disorders and Stroke, National Institutes of Health.
Footnotes
Disclosures
None.
References
- 1.Schlaug G, Siewert B, Benfield A, Edelman RR, Warach S. Time course of the apparent diffusion coefficient (ADC) abnormality in human stroke. Neurology. 1997;49:113–119. doi: 10.1212/wnl.49.1.113. [DOI] [PubMed] [Google Scholar]
- 2.Kidwell CS, Saver JL, Mattiello J, Starkman S, Vinuela F, Duckwiler G, Gobin YP, Jahan R, Vespa P, Kalafut M, Alger JR. Thrombolytic reversal of acute human cerebral ischemic injury shown by diffusion/perfusion magnetic resonance imaging. Ann Neurol. 2000;47:462–469. [PubMed] [Google Scholar]
- 3.Lansberg MG, O’Brien MW, Tong DC, Moseley ME, Albers GW. Evolution of cerebral infarct volume assessed by diffusion-weighted magnetic resonance imaging. Arch Neurol. 2001;58:613–617. doi: 10.1001/archneur.58.4.613. [DOI] [PubMed] [Google Scholar]
- 4.Parsons MW, Barber PA, Chalk J, Darby DG, Rose S, Desmond PM, Gerraty RP, Tress BM, Wright PM, Donnan GA, Davis SM. Diffusion-and perfusion-weighted MRI response to thrombolysis in stroke. Ann Neurol. 2002;51:28–37. doi: 10.1002/ana.10067. [DOI] [PubMed] [Google Scholar]
- 5.Fiehler J, Foth M, Kucinski T, Knab R, von Bezold M, Weiller C, Zeumer H, Rother J. Severe ADC decreases do not predict irreversible tissue damage in humans. Stroke. 2002;33:79–86. doi: 10.1161/hs0102.100884. [DOI] [PubMed] [Google Scholar]
- 6.Warach S, Pettigrew LC, Dashe JF, Pullicino P, Lefkowitz DM, Sabounjian L, Harnett K, Schwiderski U, Gammans R. Effect of citicoline on ischemic lesions as measured by diffusion-weighted magnetic resonance imaging. Citicoline 010 investigators. Ann Neurol. 2000;48:713–722. [PubMed] [Google Scholar]
- 7.Hacke W, Albers G, Al-Rawi Y, Bogousslavsky J, Davalos A, Eliasziw M, Fischer M, Furlan A, Kaste M, Lees KR, Soehngen M, Warach S. The desmoteplase in acute ischemic stroke trial (DIAS): a phase II MRI-based 9-hour window acute stroke thrombolysis trial with intravenous desmoteplase. Stroke. 2005;36:66–73. doi: 10.1161/01.STR.0000149938.08731.2c. [DOI] [PubMed] [Google Scholar]
- 8.Warach S, Kaufman D, Chiu D, Devlin T, Luby M, Rashid A, Clayton L, Kaste M, Lees KR, Sacco R, Fisher M. Effect of the glycine antagonist gavestinel on cerebral infarcts in acute stroke patients, a randomized placebo-controlled trial: the GAIN MRI substudy. Cerebrovasc Dis. 2006;21:106–111. doi: 10.1159/000090208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang DW, Chalela JA, Dunn W, Warach S. MRI screening before standard tissue plasminogen activator therapy is feasible and safe. Stroke. 2005;36:1939–1943. doi: 10.1161/01.STR.0000177539.72071.f0. [DOI] [PubMed] [Google Scholar]
- 10.Weisskoff RM, Chesler D, Boxerman JL, Rosen BR. Pitfalls in MR measurement of tissue blood flow with intravascular tracers: which mean transit time? Magn Reson Med. 1993;29:553–558. doi: 10.1002/mrm.1910290420. [DOI] [PubMed] [Google Scholar]
- 11.Furlan AJ, Eyding D, Albers GW, Al-Rawi Y, Lees KR, Rowley HA, Sachara C, Soehngen M, Warach S, Hacke W. Dose Escalation of Desmoteplase for Acute ischemic Stroke (DEDAS): evidence of safety and efficacy 3 to 9 hours after stroke onset. Stroke. 2006;37:1227–1231. doi: 10.1161/01.STR.0000217403.66996.6d. [DOI] [PubMed] [Google Scholar]
- 12.Chalela JA, Kang DW, Luby M, Ezzeddine M, Latour LL, Todd JW, Dunn B, Warach S. Early magnetic resonance imaging findings in patients receiving tissue plasminogen activator predict outcome: insights into the pathophysiology of acute stroke in the thrombolysis era. Ann Neurol. 2004;55:105–112. doi: 10.1002/ana.10781. [DOI] [PubMed] [Google Scholar]
- 13.Luby M, Bykowski JL, Schellinger PD, Merino JG, Warach S. Intra- and inter-rater reliability of ischemic lesion volumes measurements on diffusion-weighted, mean transit time, and fluid-attenuated inversion recovery MRI. Stroke. 2006;37:2951–2956. doi: 10.1161/01.STR.0000249416.77132.1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Menezes NM, Ay H, Zhu MW, Lopez CJ, Singhal AB, Karonen JO, Aronen HJ, Liu Y, Nuutinen J, Koroshetz WJ, Sorensen AG. The real estate factor: quantifying the impact of infarct location on stroke severity. Stroke. 2007;38:194–197. doi: 10.1161/01.STR.0000251792.76080.45. [DOI] [PubMed] [Google Scholar]
- 15.Beaulieu C, de Crespigny A, Tong DC, Moseley ME, Albers GW, Marks MP. Longitudinal magnetic resonance imaging study of perfusion and diffusion in stroke: evolution of lesion volume and correlation with clinical outcome. Ann Neurol. 1999;46:568–578. doi: 10.1002/1531-8249(199910)46:4<568::aid-ana4>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 16.Pialat JB, Wiart M, Nighoghossian N, Adeleine P, Derex L, Hermier M, Froment JC, Berthezene Y. Evolution of lesion volume in acute stroke treated by intravenous tPA. J Magn Reson Imaging. 2005;22:23–28. doi: 10.1002/jmri.20363. [DOI] [PubMed] [Google Scholar]
- 17.Rother J, Schellinger PD, Gass A, Siebler M, Villringer A, Fiebach JB, Fiehler J, Jansen O, Kucinski T, Schoder V, Szabo K, Junge-Hulsing GJ, Hennerici M, Zeumer H, Sartor K, Weiller C, Hacke W. Effect of intravenous thrombolysis on MRI parameters and functional outcome in acute stroke <6 hours. Stroke. 2002;33:2438–2445. doi: 10.1161/01.str.0000030109.12281.23. [DOI] [PubMed] [Google Scholar]


