Abstract
SUMMARY: Ischemic lesion conspicuity on routine diffusion-weighted imaging (DWI, 30 seconds) was compared with an improved sequence (high-resolution DWI [DWI-HR], 256 seconds) having increased spatial resolution and signal to noise and decreased eddy current artifact in 42 patients with acute ischemic stroke. Total lesion volumes were similar; however, twice as many lesions were identified on DWI-HR, predominately in cortical gray matter. Modest improvements to imaging resulted in increased conspicuity, potentially affecting diagnosis, suspected pathogenic mechanism, and therapeutic decision.
Although the sensitivity and specificity of diffusion-weighted imaging (DWI) for the detection of acute ischemic stroke exceeds 90%,1,2 false-negative DWI is not uncommon,3,4 and protocols used in routine clinical practice remain plagued by limited spatial resolution, low signal-to-noise ratio (SNR), and artifacts. We hypothesized that modest improvements in image quality would result in a significant increase in lesion conspicuity.
Description of the Technique
MR imaging studies were performed on a 1.5T clinical MR imaging unit (General Electric Medical Systems, Milwaukee, Wis) using a neurovascular array (In Vivo, Orlando, Fla). DWI was acquired using a spin-echo single-shot echo-planar imaging (SS-EPI) sequence with the following parameters: repetition time (TR)/echo time (TE), 7000/72.8 ms; field of view (FOV), 220 × 220 mm; matrix, 128 × 128; b = (0,1000) along 3 orthogonal directions. Coverage of the brain was accomplished with 20 contiguous sections for a voxel size of 20 mm3 (1.7 × 1.7 × 7 mm), and a total acquisition time of 30 seconds. High-resolution DWI (DWI-HR) was acquired using an SS-EPI sequence with the following parameters; TR/TE, 10250/72.5 ms; FOV, 220 × 220 mm; matrix, 128 × 128. A second refocusing pulse, a pan option in our commercial sequence (release 11.x), was added for reduction of eddy current-induced distortion.5 Acquisition was done with 4 images acquired at b = 0 and 19 images at b = 1000 in 6 gradient directions (acquisition followed a tetrahedronal encoding scheme with the following gradient directions: [0, 1, 1], [−1, 0, 1], [0, 1, 1], [1, 1, 0], [0, 1, −1], and [−1, 1, 0]). Gradient strength (100%) was applied simultaneously along 2 axes affecting a factor of 2 gain in ‖b‖ and allowing for a shorter TE. Total brain coverage was accomplished with 40 sections for a voxel size of 10 mm3 (1.7 × 1.7 × 3.5 mm) and a total acquisition time of 256 seconds.
This was a retrospective study of patients admitted to the National Institutes of Health Suburban Hospital Stroke Service. The inclusion criteria were consent to participate in an institutional review board-approved natural history protocol, have both DWI and DWI-HR in the same imaging examination within the first 24 hours of symptom onset, and have a final clinical diagnosis of acute ischemic stroke. DWI always preceded DWI-HR in the scanning protocol, with a median and average latency time between both sequences of 3 and 4 minutes, respectively. Symptom onset was defined as the time the patient was last seen to be normal.
Lesion segmentation was performed on trace-weighted images using an automated level-set algorithm and a 3D morphometric analysis,6 by 2 experienced readers (research fellow in neurology and clinical trialist) blinded to patient identifiers and to DWI to DWI-HR pairing. Lesions were identified as hyperintense regions by either reader and were verified for acuity using corresponding apparent diffusion coefficient maps and fluid-attenuated inversion recovery imaging. The resulting volumes of interest were saved for each patient and reader then transformed in a common space for analysis. SNR and contrast-to-noise ratio (CNR) were measured for each sequence using method 2 of the National Electrical Manufacturers Association standard.7
SPSS for Windows (version 13; SPSS, Chicago, Ill) was used for statistical analysis. A value of 2-tailed P < .05 was considered significant. Total lesion volume and average volume per lesion were compared between DWI and DWI-HR using a paired Student t test. Lesion count between both techniques was compared using the Wilcoxon rank-paired test. Inter-reader agreement was assessed using the interclass correlation coefficient.
Results
A total of 42 patients [20 men (mean age, 75; range, 42–94)] were included. The median time between time of symptom onset and first scan was 5 hours and 21 minutes (mean, 8:06; range, 0:47–23:29). No patients were excluded for inability to complete the imaging examination, and image quality was adequate in all.
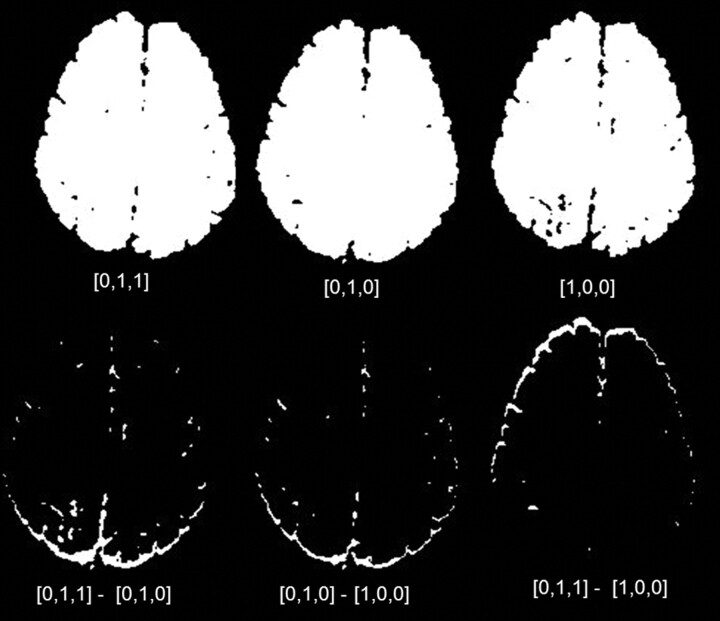
The anteroposterior translational shift, a typical effect of residual eddy currents occurring between the different diffusion gradient directions, was significantly decreased by the addition of a second refocusing pulse as shown in Fig 2. This resulted in a decrease in the blurring of small lesions particularly those located on the cortex. The average SNR in conventional DWI was 29.7 (range, 23.9–37) compared with 35.6 (range, 27.7–43.2) on DWI-HR (P < .01) representing an increase of SNR by 20%. The average CNR was 10.7 (range, 8–17) on DWI compared with an average of 14.8 (range, 12.6–20.6) on DWI-HR (P < .01), increasing the CNR by 42%.
Fig 2.
Thresholded binary images resulting from source diffusion-weighted images (DWI) in 2 gradient directions at the top left and center, and from source high-resolution diffusion-weighted images (DWI-HR) at the bottom left and center. Note the greater anteroposterior shift on the image resulting from the DWI source images subtraction at the top right compared with the one resulting from the dual-echo corrected DWI-HR source images at the bottom left.
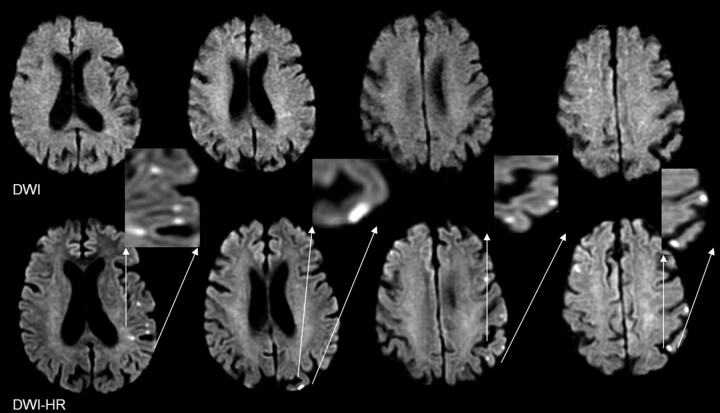
A total of 129 discrete lesions was identified on DWI and 253 lesions on DWI-HR for an average of 3.07 lesions per patient on DWI and 6.04 on DWI-HR (P < .001) (Table 1). Seven lesions unique to DWI were identified in 5 patients, whereas 134 lesions unique to DWI-HR were identified in 23 patients; these lesions were nearly 3 times more prevalent in the cortex than in other brain structures, as illustrated in Fig 1.
Discrete lesions identified on diffusion-weighted MR imaging (DWI) and high-resolution DWI (DWI-HR)
| DWI | DWI-HR | P Value | |
|---|---|---|---|
| Signal-to-noise ratio, mean (range) | 29.7 (23.9–37) | 35.6 (27.7–43.2) | <.01 |
| Contrast-to-noise ratio, mean (range) | 10.7 (8–17) | 14.8 (12.6–20.6) | <.01 |
| Total no. of lesions | 129 | 253 | <.001 |
| Average no. of lesions | 3.07 | 6.04 | <.001 |
| No. of unique lesions | 7 | 123 | <.001 |
| Total lesion volume (mm3) | 7040 ± 2010 | 7052 ± 1950 | .95 |
| Average volume per lesion (mm3) | 3600 ± 1300 | 2800 ± 1200 | <.001 |
| Average volume per lesion of unique lesions (mm3) | 134.41 ± 88.23 | 70.50 ± 16.90 | .65 |
| No. of patients with a single lesions, n (%) | 16 (37%) | 12 (28%) | n/a |
| Number of patients with greater than 5 lesions, n (%) | 8 (19%) | 19 (42%) | n/a |
Note:—DWI-HR was acquired following a tetrahedronal encoding scheme with the following gradient directions: (0, 1, 1), (−1, 0, 1), (0, 1, 1), (1, 1, 0), (0, 1, −1), (−1, 1, 0). 100% gradient strength was applied simultaneously along 2 axes affecting a factor of 2 gain in ‖b‖, and allowing for a shorter echo time.
Fig 1.
Cortical ischemic lesions identified by high resolution diffusion-weighted imaging (bottom), missed by conventional DWI (top). Note in the magnifications, the location of the lesions mainly along the cortical gray ribbon.
Total lesion volume per patient was 7040 ± 2010 mm3 on DWI and 7052 ± 1950 mm3 on DWI-HR (P = .95), for an average of 3600 ± 1300 mm3 and 2800 ± 1200 mm3 on DWI and DWI-HR, respectively (P < .001). The average volume per lesion of lesions unique to DWI was 134.41 ± 88.23 mm3 and 70.50 ± 16.90 mm3 of those unique to DWI-HR (P = .65).
A total of 16 (37%) patients had a single lesion, and 8 (19%) patients had more than 5 lesions on DWI, whereas 12 (28%) patients had a single lesion, and more than 19 (42%) patients had more than 5 lesions on DWI-HR. Eight (19%) patients with a single lesion on DWI had multiple lesions on DWI-HR.
Inter-reader reliability was excellent for lesion volumes in both DWI-HR and DWI (0.98 [range, 0.92–0.99]) and 0.96 [range, 0.93, −0.98]), respectively. Interclass correlation for lesion count was higher for DWI-HR 0.92 (range, 0.85–0.96) compared with DWI of 0.77 (range, 0.59–0.87).
Discussion
Time constraints on imaging in critically ill stroke patients have limited the DWI used in routine practice to minimum trace-weighted images calculated from 3 orthogonal directions. Although clinicians are reluctant to extend the protocol without clear benefit, our results indicate that modest improvements to the present DWI protocol can provide substantially more information, which may alter patient management.
DWI-HR resulted not only in increased lesion conspicuity but also in a dramatic increase in the number of cortical ischemic lesions as shown in Fig 1. Such lesions were present on DWI-HR and absent on DWI in more than half of the patients studied. Moreover, a change in lesion pattern was observed, with more patients having multiple lesions on DWI-HR. Lesion pattern has been shown to be linked to clinical outcome8 as well as to lesion recurrence, potentially pointing to a stroke prone state,8,9 which could prompt more vigorous preventive measures.
Our study showed no patients with a negative DWI and positive corresponding DWI-HR; a follow-up study, however, is under way, including both patients with ischemic stroke and patients with transient ischemic attack to explore a potential increased sensitivity for negative DWI. Moreover, understanding the relationship of these cortical lesions to the vascular territories affected, and thus potentially to the stroke etiology, was beyond the scope of this study; however, it is being addressed in a follow-up study.
Both signal intensity averaging to increase SNR and decreased section thickness to decrease partial volume averaging, improved CNR, and contributed to the increased conspicuity of small lesions; however, the most dramatic improvement was probably realized through use of a dual-echo technique.5 Alternative techniques to the EPI readout may provide a similar advantage, yet remain esoteric and have not received widespread clinical use.10,11
In conclusion, modest improvements in spatial resolution, increased signal intensity-to-noise ratio, and eddy current distortion reduction, resulted in significant improvement in lesion conspicuity. Lesions revealed by the improved imaging could result in a change in observed lesion pattern, presumed underlying pathogenic mechanisms,12 diagnosis, and treatment strategy.
References
- 1.Gonzalez RG, Schaefer PW, Buonanno FS, et al. Diffusion-weighted MR imaging: diagnostic accuracy in patients imaged within 6 hours of stroke symptom onset. Radiology 1999;210:155–62 [DOI] [PubMed] [Google Scholar]
- 2.Warach S, Dashe JF, Edelman RR. Clinical outcome in ischemic stroke predicted by early diffusion-weighted and perfusion magnetic resonance imaging: a preliminary analysis. J Cereb Blood Flow Metab 1996;16:53–59 [DOI] [PubMed] [Google Scholar]
- 3.Oppenheim C, Logak M, Dormont D, et al. Diagnosis of acute ischaemic stroke with fluid-attenuated inversion recovery and diffusion-weighted sequences. Neuroradiology 2000;42:602–07 [DOI] [PubMed] [Google Scholar]
- 4.Warach S, Gaa J, Siewert B, et al. Acute human stroke studied by whole brain echo planar diffusion-weighted magnetic resonance imaging. Ann Neurol 1995;37:231–41 [DOI] [PubMed] [Google Scholar]
- 5.Reese TG, Heid O, Weisskoff RM, et al. Reduction of eddy-current-induced distortion in diffusion MRI using a twice-refocused spin echo. Magn Reson Med 2003;49:177–82 [DOI] [PubMed] [Google Scholar]
- 6.McAuliffe MJ, Lalonde F, McGarry DP. Medical image processing, analysis & visualization in clinical research. 14th IEEE Symposium on Computer-Based Medical Systems (CBMS 2001). Los Alamitos, Calif: IEEE Computer Society;2001. :381–386. Available at: http://doi.ieeecomputersociety.org/10.1109/CBMS.2001.941749
- 7.National Electrical Manufacturers Association. NEMA Standards Publication MS 1–2001: Determination of Signal-to-Noise Ratio (SNR) in Diagnostic Magnetic Resonance Imaging. Rosslyn, Va: National Electrical Manufacturers Association,2001. .
- 8.Bang OY, Lee PH, Heo KG, et al. Specific DWI lesion patterns predict prognosis after acute ischaemic stroke within the MCA territory. J Neurol Neurosurg Psychiatry 2005;76:1222–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang DW, Latour LL, Chalela JA, et al. Early and late recurrence of ischemic lesion on MRI: evidence for a prolonged stroke-prone state? Neurology 2004;63:2261–65 [DOI] [PubMed] [Google Scholar]
- 10.Forbes KP, Pipe JG, Karis JP, et al. Improved image quality and detection of acute cerebral infarction with PROPELLER diffusion-weighted MR imaging. Radiology 2002;225:551–55 [DOI] [PubMed] [Google Scholar]
- 11.Pipe JG, Farthing VG, Forbes KP. Multishot diffusion-weighted FSE using PROPELLER MRI. Magn Reson Med 2002;47:42–52 [DOI] [PubMed] [Google Scholar]
- 12.Kang DW, Chu K, Ko SB, et al. Lesion patterns and mechanism of ischemia in internal carotid artery disease: a diffusion-weighted imaging study. Arch Neurol 2002;59:1577–82 [DOI] [PubMed] [Google Scholar]