Abstract
Myocardial injury because of oxidative stress manifesting through reductions in left ventricular ejection fraction (LVEF) may occur after the administration of anthracycline-based chemotherapy (A-bC). We hypothesized that bilirubin, an effective endogenous antioxidant, may attenuate the reduction in LVEF that sometimes occurs after receipt of A-bC. We identified 751 consecutively treated patients with cancer who underwent a pre-A-bC LVEF measurement, exhibited a serum total bilirubin level <2 mg/dl, and then received a post-A-bC LVEF assessment because of symptomatology associated with heart failure. Analysis of variance, Tukey’s Studentized range test, and chi-square tests were used to evaluate an association between bilirubin and LVEF changes. The LVEF decreased by 10.7 ± 13.7%, 8.9 ± 11.8%, and 7.7 ± 11.5% in group 1 (bilirubin at baseline ≤0.5 mg/dl), group 2 (bilirubin 0.6 to 0.8 mg/dl), and group 3 (bilirubin 0.9 to 1.9 mg/dl), respectively. More group 1 patients experienced >15% decrease in LVEF compared with those in group 3 (p = 0.039). After adjusting for age, coronary artery disease/myocardial infarction, diabetes mellitus, hematocrit, and the use of cardioactive medications, higher precancer treatment bilirubin levels and lesser total anthracycline doses were associated with LVEF preservation (p =0.047 and 0.011, respectively). In patients treated with anthracyclines who subsequently develop symptoms associated with heart failure, pre-anthracycline treatment serum bilirubin levels inversely correlate with subsequent deterioration in post-cancer treatment LVEF. In conclusion, these results suggest that increased levels of circulating serum total bilirubin, an intrinsic antioxidant, may facilitate preservation of LVEF in patients receiving A-bC for cancer.
Leukemia, lymphoma, and tumors of the breast and skeletal muscle are frequently treated with anthracycline-based chemotherapy (A-bC)1–4; however, its use is limited by dose-dependent cardiotoxic effects including heart failure (HF). The mechanism for A-bC–mediated cardiac damage and HF is not fully elucidated. Cellular injury by increased oxidative stress within the cardiac myocytes is thought to play a central role in left ventricular ejection fraction (LVEF) reductions.2,5 For many years, bilirubin was considered a toxic by-product of heme metabolism due to the jaundice and brain damage it caused in newborns with severe hyperbilirubinemia.6 However, recent studies have demonstrated that higher levels of total serum bilirubin are associated with reduced risk of cardiovascular (CV) disease.7,8 Bilirubin’s cardioprotective effects are thought to be mediated through its endogenous antioxidant properties.9 Because bilirubin exhibits antioxidant properties, we sought to determine if an association was present between serum bilirubin levels and change in LVEF in those receiving A-bC. To address this question, we compared precancer treatment serum bilirubin levels to subsequent changes in LVEF that occurred in individuals who developed HF symptoms after receipt of A-bC.
Methods
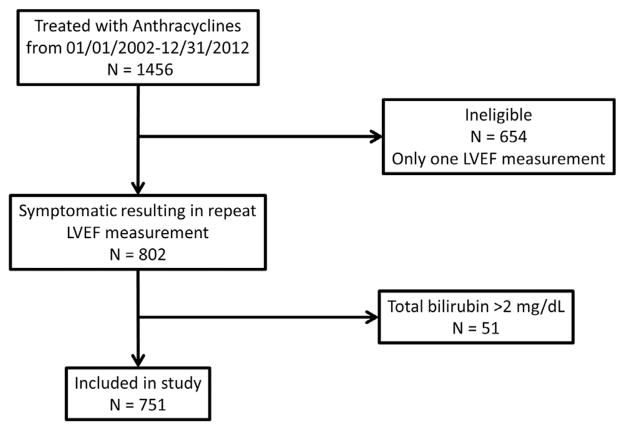
This retrospective cohort study was approved by the Institutional Review Board at Wake Forest Health Sciences. Because the study posed minimum risk to the participants, informed consent was waived to gather data from previous health records. We identified all patients with cancer who received A-bC at Wake Forest Baptist Medical Center from January 2002 to January 2012 and underwent 2 measurements of LVEF, the first before receipt of treatment for their cancer and the second after experiencing symptoms suggestive of HF. We excluded those subjects with a diagnosis of congestive HF (International Classification of Diseases, Ninth Revision, code 428.x) before receipt of A-bC or serum total bilirubin levels ≥2 mg/dl due to intrinsic liver disease before A-bC administration. We identified 751 patients who met the inclusion/exclusion criteria (Figure 1).
Figure 1.
Selection of patients with cancer for retrospective assessment of bilirubin and anthracycline-induced LV systolic dysfunction. Data from 751 participants were analyzed after excluding 654 patients with cancer because they did not have a post-anthracycline LV systolic function assessment and 51 patients with cancer for total bilirubin >2 mg/dl.
Baseline demographic data, such as age, gender, race, weight, height, CV co-morbidities, cancer type, anthracycline dose, and CV medications, were obtained from medical records. The anthracycline dose was determined by reviewing all the medications that were dispensed from the hospital pharmacy for each participant. After summing the A-bC doses, they were converted to doxorubicin isotoxic equivalents as previously described (total doxorubicin dose × 1, total daunorubicin dose × 0.833, total epirubicin dose × 0.67, total idarubicin dose × 5, total mitoxantrone dose × 4).10
The LVEF was measured by transthoracic echocardiography, multigated acquisition scan, or cardiac magnetic resonance scan as determined from the medical records. The change in LVEF was calculated as the difference between the pre-anthracycline LVEF and the lowest post-anthracycline LVEF during the follow-up period. Systemic arterial hypertension, coronary artery disease/myocardial infarction (CAD/ MI), and diabetes mellitus were considered present if their respective International Classification of Diseases, Ninth Revision, codes were listed in the participants’ charts at the time of the first anthracycline dose. Serum total bilirubin, aspartate aminotransferase, alanine aminotransferase, albumin, and hematocrit levels from assessments obtained before initiation of their A-bC were recorded. Also, potentially CV-active concurrent medications, such as β blockers, angiotensin-converting enzyme inhibitors (ACEi), angiotensin receptor blockers (ARB), and statins, administered with cancer treatment were recorded. We documented whether participants received mediastinal, chest, or whole-body ionizing radiation as part of their cancer treatment regimen.
The data were analyzed using the SAS statistical software system (Cary, North Carolina). Participants were assigned to 1 of 3 groups according to their precancer treatment serum bilirubin levels (group 1 [bilirubin ≤0.5 mg/dl], group 2 [bilirubin 0.6 to 0.8 mg/dl], and group 3 [bilirubin 0.9 to 1.9 mg/dl]). The total bilirubin limits for each group were chosen to have similar numbers in each group. Participants with ≥2.0 mg/dl were excluded to avoid inclusion of those with dramatic elevations in serum bilirubin because of severe liver disease. Continuous data were presented as mean ± SD, whereas categorical data were presented as number (percent). Analysis of variance was used to test for the difference in the mean LVEF change across these groups. Tukey’s Studentized range and Bonferroni (Dunn) t tests were used in pairwise comparisons. The chi-square test was used for differences in categorical variables across the 3 groups. Cochran-Armitage trend test was used to assess for trends across the 3 groups.
Because the distribution of bilirubin levels was highly skewed toward higher values, logarithmically transformed values of bilirubin were used in the quantitative models of analysis. The correlation between the precancer treatment serum bilirubin levels and the serial pre- to post-chemotherapy changes in LVEF was analyzed using linear regression models. Adjustments for age, gender, potentially CV-active medications, thoracic radiation, and CV co-morbidities (hypertension, CAD/MI, diabetes) were performed to account for their potential influence on change in LVEF after receipt of A-bC. A p value <0.05 was considered significant in all analyses.
Results
The demographic data for the study participants are listed in Table 1. Participants averaged 54 ± 16 years in age, 52% were men, 85% were Caucasian, and 13% were African-American. The most common locations of the cancers were the blood and bone marrow, lymph nodes, and breast in 80%, 10%, and 5% of the cases, respectively. There were no differences in the pre-anthracycline measures of LVEF between the groups (Table 1).
Table 1.
Demographics and results
| Bilirubin (mg/dl)
| ||||
|---|---|---|---|---|
| Measure | ≤0.5 (n=186) | 0.6–0.8 (n=287) | 0.9– 1.9 (n=281) | p |
| Age (years) | 54 ± 16 | 55±17 | 54±15 | 0.1 |
| Female | 46% | 49% | 59% | 0.0071 |
| White | 84% | 90% | 82% | 0.18 |
| Black | 15% | 9% | 16% | |
| Body mass index (kg/m2) | 28 ± 8 | 29 ±4 | 30 ±7 | 0.011 |
| Albumin (g/dl) | 3.5 ± 0.6 | 3.4 ±0.6 | 3.2 ±0.6 | 0.15 |
| Hematocrit (%) | 29 ± 6 | 28 ±5 | 27 ±5 | 0.0062 |
| Prior coronary artery disease/myocardial infarction | 11% | 12% | 13% | 0.92 |
| Prior diabetes mellitus | 15% | 18% | 14% | 0.37 |
| Prior hypertension | 35% | 38% | 48% | 0.009 |
| On angiotensin converting enzyme inhibitor | 9% | 9% | 6% | 0.23 |
| On angiotensin receptor blocker | 2% | 4% | 4% | 0.47 |
| On β-blocker | 9% | 6% | 7% | 0.52 |
| On statin | 15% | 13% | 11% | 0.51 |
| Pre-anthracycline-based chemotherapy left ventricular ejection fraction (%) | 59.5 ± 7.0 | 58.9 ±6.7 | 58.1 ±7.2 | 0.10 |
| Doxorubicin equivalent (mg/m2) | 392 ± 180 | 408 ±230 | 406 ±165 | 0.70 |
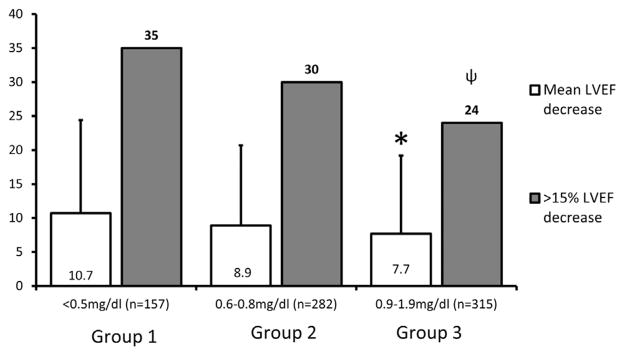
The time between the LVEF measurements averaged 496 ± 625 days (median: 285 days, minimum: 14 days, and maximum: 3,502 days). The participants with the lowest precancer treatment serum bilirubin levels (group 1) sustained the largest reduction in LVEF when presenting with symptoms warranting a repeat LVEF assessment (Figure 2). Conversely, the lowest LVEF reductions observed between the precancer and post-cancer treatment assessments were observed in group 3 (p = 0.041, Figure 2). Using pairwise comparisons, there was a significant difference between the average reduction in LVEF when groups 1 and 3 were compared (difference between means of 2.9%, 95% confidence limits 0.2 to 5.6; p <0.05). The LVEF change was related to the bilirubin groups when the Cochran-Armitage trend test was applied (p = 0.011).
Figure 2.
Change in LVEF in each bilirubin group. The y axis is a percentage scale; open bars reflect the mean decrease in LVEF (in percent), and gray bars reflect the percentage of patients with cancer in each group who sustained >15% decrease in their LVEF. Patients with bilirubin levels at the upper end of the normal range are less likely to have >15% loss of LV ejection fraction after A-bC compared with those at the lower end of the spectrum. “*” and “ψ,” p <0.05 when group 1 is compared with group 3.
After adjusting for age, gender, and the use of potentially CV-active medications, the pre-A-bC serum bilirubin level and the cumulative doxorubicin equivalent dose of anthracycline administered were the only variables independently associated with a reduction in LVEF (p = 0.017 and 0.0085, respectively; Table 2). Total bilirubin and cumulative doxorubicin equivalent dose remained associated with LVEF reduction (p = 0.047 and 0.011, respectively, Table 3) after adjusting for age, gender, hypertension, CAD, diabetes, and thoracic radiation.
Table 2.
Total bilirubin and anthracycline dose are independent predictors of left ventricular ejection fraction change after adjusting for age, gender, thoracic radiation and cardioprotective medication use
| Covariate | β estimate (95% CI) | p |
|---|---|---|
| Age (years) | −0.02 (−0.08 to 0.04) | 0.57 |
| Gender | −0.71 (−2.60 to 1.27) | 0.48 |
| Angiotensin converting enzyme inhibitor use | 0.38 (−3.43 to 4.18) | 0.85 |
| Statin use | 0.39 (−2.77 to 3.55) | 0.81 |
| β-blocker use | 0.32 (−2.84 to 3.47) | 0.84 |
| Angiotensin receptor blocker use | −2.63 (−8.11 to 2.85) | 0.34 |
| Thoracic radiation | −0.36 (−6.12 to 5.41) | 0.90 |
| Log total bilirubin (mg/dL) | 2.69 (0.48 to 4.89) | 0.017 |
| Doxorubicin equivalents (mg/m2) | −0.0067 (−0.011 to −0.0017) | 0.0085 |
Table 3.
Total bilirubin and anthracycline dose are independent predictors of left ventricular ejection fraction change after adjusting for age, gender, hypertension, coronary artery disease, diabetes mellitus and thoracic radiation
| Covariate | β estimate (95% CI) | p |
|---|---|---|
| Age (years) | −0.02 (−0.08 to 0.04) | 0.51 |
| Gender | −0.88 (−2.87 to 1.11) | 0.38 |
| Hypertension | 1.81 (−0.36 to 3.97) | 0.099 |
| Coronary artery disease | −0.26 (−3.48 to 2.96) | 0.87 |
| Diabetes mellitus | −1.30 (−4.18 to 1.58) | 0.37 |
| Thoracic radiation | 0.14 (−5.62 to 5.90) | 0.96 |
| Log total bilirubin (mg/dL) | 2.26 (0.026 to 4.49) | 0.047 |
| Doxorubicin equivalents (mg/m2) | −0.0065 (−0.011 to −0.0015) | 0.011 |
Discussion
There are several important findings in this study. First, mild elevations of precancer treatment serum total bilirubin levels are associated with less severe reductions in LVEF after receipt of A-bC (Figure 2). Second, this association remains after accounting for age, gender, cumulative anthracycline dose, the use of potentially cardioprotective medications (e.g., β blockers, ACEi, ARB, or statins), or thoracic radiation (Table 2). Third, this association also remains after accounting for the presence of CV co-morbidities, such as hypertension, CAD/MI, or diabetes mellitus (Table 3). These findings provide further data to investigate whether reduction of oxidative stress during treatment for cancer could modify A-bC–associated reduction in LVEF.
Numerous in vitro and in vivo studies indicate that exposure to anthracyclines increases reactive oxygen species (ROS).11,12 These ROS can induce apoptosis and necrosis in cardiac myocytes resulting in LV systolic dysfunction.13 Several preclinical studies have reported beneficial effects of antioxidants in mitigating anthracycline-induced oxidative stress and LV systolic dysfunction.13–15 Bilirubin is one of the most effective, naturally occurring antioxidants in mammals.9 This study suggests that people with higher bilirubin levels might be protected from A-bC–induced reductions in LVEF. We speculate that the mechanism of protection is related to bilirubin’s antioxidant properties; however, further studies are needed to delineate the mechanism associated with our finding.
Anthracyclines induce LV systolic dysfunction in a dose-dependent manner.2 The reported rate of anthracycline-induced cardiomyopathy is 3% to 5% of individuals receiving <400 mg/m2 doxorubicin.16 We may have observed higher rates of LV systolic dysfunction in our cohort because the participants in this study were selected for a repeat LVEF assessment because of symptomatology associated with HF (e.g., shortness of breath or lower extremity edema).
Statin, β blocker, ACEi, and ARB medications have been shown beneficial in improving LVEF measures after receipt of A-bC.17 After adjusting for the use of these cardioprotective medications, precancer treatment serum bilirubin levels were independently predictive of preservation of LVEF after A-bC administration (Table 2). The significant association of precancer treatment serum bilirubin levels and pre- to post-A-bC LVEF remained after adjustment for hypertension, CAD, and diabetes (Table 3). These findings suggest that the bilirubin effect is independent of these co-morbidities and concurrent cardioprotective medication use.
After reacting with ROS, bilirubin is oxidized to biliverdin, and the biliverdin is recycled back to bilirubin by biliverdin reductase, an ubiquitous enzyme.18 The cycle is fueled by reduced nicotinamide adenine dinucleotide phosphate and is repeated perpetually in the presence of sustained nicotinamide adenine dinucleotide phosphate levels. This recycling allows a single bilirubin molecule to catalyze the neutralization up to 10,000-fold ROS molecules19; hence, at safe levels, bilirubin can be an effective antioxidant. We have presented evidence in this human study that moderately elevated bilirubin levels could be beneficial in mitigating anthracycline-induced LV systolic dysfunction. Although preclinical studies have suggested a protective role for exogenous antioxidants, such as vitamin C and E against anthracycline-induced oxidative stress damage, this result has not been replicated in human studies. One potential reason is the inability to achieve similar intracellular antioxidant concentrations in human as achieved in the preclinical studies. Given that bilirubin is an effective antioxidant at lower concentrations and the recycling mechanism is ubiquitous in all mammalian cells,19,20 the results of this study suggest further studies are warranted to evaluate the relation between plasma and intracardiac myocyte bilirubin levels and the presence of intramyocellular free radicals in subjects exposed to anthracycline agents.
Our study exhibits the following limitations. First, there was no opportunity to measure either cardiac or systemic oxidative stress during anthracycline treatment; this could have improved the study by demonstrating reduced oxidative stress in participants with elevated bilirubin levels. Second, we relied on LV systolic function assessments that were performed at the discretion of physicians’ perceptions of HF; therefore, the results of this study can only be applied to those subjects who develop symptoms that necessitate evaluation of LV systolic function after anthracycline treatment. Third, the study uses the pre-A-bC serum bilirubin levels, rather than assessment of bilirubin levels during and after A-bC therapy. Further studies are required to determine if serum bilirubin levels during treatment are associated with attenuation of anthracycline-induced cardiomyopathy.
Acknowledgments
This work was supported by grants R01HL076438, P30AG21332, R01CA167821, R01HL118740, and T32HL091824 from the National Institutes of Health, Bethesda, Maryland.
Footnotes
Disclosures
None of the authors have conflicts of interest to present.
References
- 1.Peng X, Chen B, Lim CC, Sawyer DB. The cardiotoxicology of anthracycline chemotherapeutics: translating molecular mechanism into preventative medicine. Mol Interv. 2005;5:163–171. doi: 10.1124/mi.5.3.6. [DOI] [PubMed] [Google Scholar]
- 2.Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97:2869–2879. doi: 10.1002/cncr.11407. [DOI] [PubMed] [Google Scholar]
- 3.Ewer MS, Ali MK, Mackay B, Wallace S, Valdivieso M, Legha SS, Benjamin RS, Haynie TP. A comparison of cardiac biopsy grades and ejection fraction estimations in patients receiving adriamycin. J Clin Oncol. 1984;2:112–117. doi: 10.1200/JCO.1984.2.2.112. [DOI] [PubMed] [Google Scholar]
- 4.Cardinale D, Sandri MT, Colombo A, Colombo N, Boeri M, Lamantia G, Civelli M, Peccatori F, Martinelli G, Fiorentini C, Cipolla CM. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation. 2004;109:2749–2754. doi: 10.1161/01.CIR.0000130926.51766.CC. [DOI] [PubMed] [Google Scholar]
- 5.Tsai H-T, Marshall JL, Weiss SR, Huang C-Y, Warren JL, Freedman AN, Fu AZ, Sansbury LB, Potosky AL. Bevacizumab use and risk of cardiovascular adverse events among elderly patients with colorectal cancer receiving chemotherapy: a population-based study. Ann Oncol. 2013;24:1574–1579. doi: 10.1093/annonc/mdt019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shapiro SM. Definition of the clinical spectrum of kernicterus and bilirubin-induced neurologic dysfunction (BIND) J Perinatol. 2005;25:54–59. doi: 10.1038/sj.jp.7211157. [DOI] [PubMed] [Google Scholar]
- 7.Djoussé L, Levy D, Cupples LA, Evans JC, D’Agostino RB, Ellison RC. Total serum bilirubin and risk of cardiovascular disease in the Framingham offspring study. Am J Cardiol. 2001;87:1196–1200. doi: 10.1016/s0002-9149(01)01494-1. [DOI] [PubMed] [Google Scholar]
- 8.Kang SJ, Kim D, Park HE, Chung GE, Choi SH, Choi SY, Lee W, Kim JS, Cho S-H. Elevated serum bilirubin levels are inversely associated with coronary artery atherosclerosis. Atherosclerosis. 2013;230:242–248. doi: 10.1016/j.atherosclerosis.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 9.Stocker R, Glazer AN, Ames BN. Antioxidant activity of albumin-bound bilirubin. Proc Natl Acad Sci U S A. 1987;84:5918–5922. doi: 10.1073/pnas.84.16.5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christiansen JR, Hamre H, Massey R, Dalen H, Beitnes JO, Fosså SD, Kiserud CE, Aakhus S. Left ventricular function in long-term survivors of childhood lymphoma. Am J Cardiol. 2014;114:483–490. doi: 10.1016/j.amjcard.2014.04.055. [DOI] [PubMed] [Google Scholar]
- 11.Soucek P, Kondrova E, Hermanek J, Stopka P, Boumendjel A, Ueng Y-F, Gut I. New model system for testing effects of flavonoids on doxorubicin-related formation of hydroxyl radicals. Anticancer Drugs. 2011;22:176–184. doi: 10.1097/cad.0b013e328341a17b. [DOI] [PubMed] [Google Scholar]
- 12.Rohde LE, Belló-Klein A, Pereira RP, Mazzotti NG, Geib G, Weber C, Silva LF, Clausell N. Superoxide dismutase activity in adriamycin-induced cardiotoxicity in humans: a potential novel tool for risk stratification. J Card Fail. 2005;11:220–226. doi: 10.1016/j.cardfail.2004.08.161. [DOI] [PubMed] [Google Scholar]
- 13.Šimůnek T, Štěrba M, Popelová O, Adamcová M, Hrdina R, Geršl V. Anthracycline-induced cardiotoxicity: overview of studies examining the roles of oxidative stress and free cellular iron. Pharmacol Rep. 2009;61:154–171. doi: 10.1016/s1734-1140(09)70018-0. [DOI] [PubMed] [Google Scholar]
- 14.Wouters KA, Kremer LC, Miller TL, Herman EH, Lipshultz SE. Protecting against anthracycline-induced myocardial damage: a review of the most promising strategies. Br J Haematol. 2005;131:561–578. doi: 10.1111/j.1365-2141.2005.05759.x. [DOI] [PubMed] [Google Scholar]
- 15.Tatlidede E, Sehirli O, Velioğlu-Oğünc A, Cetinel S, Yeğen BC, Yarat A, Süleymanoğlu S, Sener G. Resveratrol treatment protects against doxorubicin-induced cardiotoxicity by alleviating oxidative damage. Free Radic Res. 2009;43:195–205. doi: 10.1080/10715760802673008. [DOI] [PubMed] [Google Scholar]
- 16.Wouters KA, Kremer LCM, Miller TL, Herman EH, Lipshultz SE. Protecting against anthracycline-induced myocardial damage: a review of the most promising strategies. Br J Haematol. 2005;131:561–578. doi: 10.1111/j.1365-2141.2005.05759.x. [DOI] [PubMed] [Google Scholar]
- 17.Kalam K, Marwick TH. Role of cardioprotective therapy for prevention of cardiotoxicity with chemotherapy: a systematic review and meta-analysis. Eur J Cancer. 2013;49:2900–2909. doi: 10.1016/j.ejca.2013.04.030. [DOI] [PubMed] [Google Scholar]
- 18.Sedlak TW, Snyder SH. Bilirubin benefits: cellular protection by a biliverdin reductase antioxidant cycle. Pediatrics. 2004;113:1776–1782. doi: 10.1542/peds.113.6.1776. [DOI] [PubMed] [Google Scholar]
- 19.Baranano DE, Rao M, Ferris CD, Snyder SH. Biliverdin reductase: a major physiologic cytoprotectant. Proc Natl Acad Sci U S A. 2002;99:16093–16098. doi: 10.1073/pnas.252626999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonkovsky HL, Guo JT, Hou W, Li T, Narang T, Thapar M. Porphyrin and heme metabolism and the porphyrias. Compr Physiol. 2013;3:365–401. doi: 10.1002/cphy.c120006. [DOI] [PubMed] [Google Scholar]