Abstract
OBJECTIVES
Our aim was to assess the effects of the cholesteryl ester transfer protein (CETP) inhibitor anacetrapib and atorvastatin, both as monotherapy and in combination, on particle concentrations of low-density lipoproteins (LDL), very low-density lipoproteins (VLDL), and intermediate-density lipoproteins in dyslipidemic patients.
BACKGROUND
Although increases in high-density lipoproteins with CETP inhibition are well-documented, effects on atherogenic lipoprotein particle subclasses in dyslipidemic patients have not been extensively characterized.
METHODS
Ion mobility was performed on stored plasma samples collected from patients before and after treatment with anacetrapib alone (150 and 300 mg/d) or in combination with atorvastatin (20 mg/d) in a previously conducted 8-week phase IIb study.
RESULTS
Anacetrapib produced significant placebo-adjusted reductions of total LDL particles and all subfractions except for increases in very small LDL 4a and 4b. Atorvastatin reduced all LDL sub-fractions except LDL 4b. Results were generally additive for anacetrapib + atorvastatin. For patients treated with anacetrapib, the placebo-adjusted reduction in LDL 3a was attenuated and there was an increase in LDL 3b and 4a for those with low vs high triglyceride (TG) levels. For the atorvastatin alone vs placebo treatment comparison, there were small reductions in LDL 3a, 3b, and 4a for those with low vs high TG levels.
CONCLUSIONS
Anacetrapib and atorvastatin produced similar reductions from baseline in total LDL particles, but did not have comparable effects on all LDL particle subfractions, and neither drug reduced the smallest LDL 4b particles. The clinical significance of these changes and the differential effects on very small LDL 4a in patients with higher vs lower TG remain to be determined (clin-icaltrials.gov, NCT00325455).
Keywords: CETP inhibitor, Anacetrapib, Atorvastatin, Dyslipidemia, LDL particle subfractions
Cholesteryl ester transfer protein (CETP) is a hydrophobic plasma protein that promotes the bidirectional transfer of cholesteryl esters (CE) and triglycerides (TG) between high-density lipoprotein (HDL) particles and apolipoprotein (apo) B-containing lipoprotein particles.1 Anacetrapib is an orally active, potent, and selective CETP inhibitor currently in phase III clinical development.2 Krauss et al recently investigated the effects of anacetrapib 150 mg/day vs placebo on plasma lipids, lipoprotein particle concentrations, and lipoprotein composition in 30 healthy, nondyslipidemic subjects after 2 weeks of treatment.3 In that study, treatment with anacetrapib 150 mg/day resulted in significant decreases from baseline in low-density lipoprotein cholesterol (LDL-C; 26%) and apoB (29%) with concomitant increases from baseline in HDL-C (82%) compared with placebo. Plasma concentrations of medium and small very low-density lipoproteins (VLDL), large intermediate-density lipoproteins (IDL), and medium and small LDL (LDL 2a, 2b, and 3a) decreased, whereas levels of very small and dense LDL 4b increased as measured by gas-phase differential electrophoretic ion mobility (IM) methodology. Treatment with anacetrapib 150 mg/day vs placebo also resulted in an enrichment of TG and a reduction of CE in VLDL, IDL, and the smallest LDL fractions, possibly resulting from increased catabolism of large IDL and large/medium LDL particles with an accumulation of very small LDL and large HDL particles.
The effects of anacetrapib, administered either alone or in combination with atorvastatin, on lipoprotein particle concentrations in dyslipidemic patients have not been evaluated to date. Previous studies have not shown an effect of baseline TG on LDL-C reduction after treatment with anacetrapib4; however, it has not been determined whether the effects of anacetrapib on apoB-containing lipoprotein subfractions are influenced by baseline TG levels, which may determine the specific acceptors of HDL-derived CE via CETP.5 This report describes the effects of anacetrapib (150 mg/dL and 300 mg/day), atorvastatin monotherapy (20 mg/day) and anacetrapib coadministered with atorvastatin on particle concentrations of LDL as well as VLDL and IDL in dyslipidemic patients. Given the known relationships of plasma TG with these lipoprotein fractions, the effects of anacetrapib and atorvastatin also were examined in patient subgroups defined by median baseline TG level (ie, ≤ and >154 mg/dL).
Methods
Study design
This was a post-hoc analysis of a phase II (clinicaltrials. gov NCT00325455), randomized, double-blind, placebo-controlled, parallel-group, dose-ranging study, which examined the effects of anacetrapib monotherapy administered at 300-, 150-, 40-, and 10-mg doses, with and without atorvastatin 20 mg.6 Details of the study design and patient selection criteria have been published previously.6 Briefly, patients with primary hypercholesterolemia or mixed hyperlipidemia, 18 to 75 years of age, with baseline LDL-C values ranging from 100 to 190 mg/dL were enrolled. In this study, eligible patients were randomized equally to 1 of 10 groups: 5 groups received background statin therapy of atorvastatin 20 mg and 5 did not, and each of these was randomized to placebo and anacetrapib 10, 40, 150, and 300 mg for 8 weeks. The primary focus of the analysis described here was to examine the effects of anacetrapib (ie, 300 mg, 150 mg), administered both alone and in combination with atorvastatin 20 mg, on LDL, IDL, and VLDL particle concentrations in dyslipidemic patients.
Efficacy measurements
This study used an IM methodology that enabled the simultaneous measurement of lipoprotein particle concentrations (nmol/L) and their distributions (ie, sizes; Å).7 In addition, standard lipid/apo measurements of whole plasma included LDL-C (primary efficacy endpoint), total cholesterol (TC), HDL-C, TG, non–HDL-C, apoB, apoAI, and lipoprotein a [Lp(a)].
Analytical methodology
Whole plasma lipid/lipoprotein measurements
Lipid and apo determinations were performed by a centralized laboratory certified by the Centers for Disease Control and Prevention (Pharmaceutical Product Development, Inc., Highland Heights, KY). TC and TG were quantified using a standardized enzymatic assay. LDL-C was calculated using Friedewald’s equation: LDL-C = TC– HDL-C – TG/5, although a previous study has indicated that this method underestimates the LDL-C after treatment with anacetrapib.3 HDL-C was measured by separating HDL from LDL and VLDL using heparin-manganese Cl precipitation. Lp(a) was measured using the Poly-Chem chemistry analyzer (Polymedco, Cortland Manor, NY). ApoAI and apoB were measured using the BN nephelometer (Siemens Healthcare Diagnostics, Washington, DC).
Lipoprotein particle analysis by ion mobility
Lipoprotein particle concentrations were measured using IM, which uniquely allows for direct particle quantification.7 The IM instrument uses an electrospray to create an aerosol of particles, which then passes through a Differential Mobility Analyzer coupled to a particle counter. Particle diameter and quantity are determined and converted to lipoprotein particle concentration and LDL peak particle size. Interassay variability is minimized by including 2 in-house controls in each analytic run. For the analyses in the present report, the previously published procedure6 was modified by excluding the ultracentrifugal removal of albumin and other plasma proteins. Because the size distribution of these proteins overlaps HDL, but not LDL, IDL, or VLDL, the present report does not include HDL measurements. As reported previously,3 particle concentrations were measured in 7 LDL size intervals defining large (LDL 1), medium (LDL 2a and 2b), small (LDL 3a and 3b), and very small (LDL 4a and 4b) LDL particles; 2 IDL size intervals (large and small); and 3 VLDL size intervals (large, medium, and small).
Statistical methodology
For assessing changes from baseline, data normality was first assessed. For normally distributed data, analysis of covariance models including modeling terms for treatment group and baseline lipid level were used to assess change from baseline to week 8. Treatment differences in least squares mean change from baseline and corresponding 2-sided 95% confidence intervals were determined. For nonnormally distributed data, Hodges-Lehman estimates of the differences in median change from baseline and distribution-free confidence intervals were calculated. The interaction of treatment effect by TG subgroup, defined by median levels (≤ and >154 mg/dL), was explored using analysis of variance with terms for treatment, TG subgroup, and treatment-by-subgroup interaction.
Results of fasting whole plasma lipid/apo and IM measurements, demographic and other baseline characteristics at baseline and after treatment were summarized using means, standard errors, medians, and interquartile ranges as appropriate. The appropriateness of pooling the anacetrapib 300- and 150-mg doses (with, and separately, without atorvastatin 20 mg) to increase the statistical precision of the point estimate was tested with a pairwise comparison of doses tested at 0.05 level. Dose response across all anacetrapib doses available was also assessed for each anacetrapib comparison, with and without atorvastatin 20 mg.
For the primary analysis comparing the effects of combined high dose (300 mg and 150 mg) anacetrapib monotherapy with placebo on particle concentrations, the method of Benjamini and Hochberg8 for controlling the false discovery rate (FDR) was applied to limit the proportion of false positives across testing of VLDL, IDL, and LDL subfractions. For all other comparisons, the 2-stage FDR approach (“double FDR”) of Mehrotra and Adewale9 was applied separately for each analysis of lipoproteins and lipoprotein subfractions, including the analyses of interaction by median TG subgroup. Each method controlled the FDR at 5%, except for TG interaction, which was tested at a 10% level.
Results
Preliminary analyses demonstrated similar effects of anacetrapib 150 mg and 300 mg (with, and separately, without atorvastatin 20 mg coadministration) on whole plasma lipid/apo and IM particle concentration measurements with no significant between-group differences observed across any of the VLDL, IDL, or LDL subfractions tested (P ≥ .05). For each subsequent analysis, the results of the anacetrapib 300- and 150-mg doses (with and, separately, without atorvastatin 20 mg coadministration) were combined.
Baseline characteristics
This post-hoc analysis included data for 464 patients with paired IM baseline and week 8 measurements, which represented approximately 80% of the initial randomized clinical trial sample. A total of 282 patients assigned to anacetrapib 150/300 mg/day (anacetrapib alone), anacetrapib 150/300 mg/day + atorvastatin 20 mg/day (anacetrapib + atorvastatin), atorvastatin 20 mg/day (atorvastatin alone), and placebo with baseline and week 8 IM measurements were included in the primary analysis. Baseline demographic and laboratory characteristics for these 282 patients are presented in Table 1 (overall) and Table S1 (by TG subgroup). In general, there were no meaningful differences in patient characteristics observed between the treatment groups. The mean patient age was 56 years, and patients were predominantly female (57%) and white (83%) (Table 1). The average LDL-C and HDL-C levels were 140 mg/dL and 50 mg/dL, respectively. On average, patients in the higher baseline TG subgroup had higher baseline TC, non–HDL-C, and apoB levels and lower HDL-C and apoAI levels than those with baseline TG below the median (Table S1). Additionally, patients with higher baseline TG levels had, as expected, higher baseline VLDL and small LDL particle concentrations, and lower levels of IDL and large LDL.
Table 1.
Baseline demographic characteristics
| Parameter | Placebo (N = 41) | Anacetrapib alone (N = 95) |
Atorvastatin alone (N = 44) |
Anacetrapib + atorvastatin (N = 102) |
|---|---|---|---|---|
| Age, mean (SD) | 53 (8) | 58 (9) | 56 (9) | 56 (11) |
| Male gender, % | 44 | 47 | 39 | 40 |
| Race, % white | 78 | 90 | 80 | 85 |
| BMI, mean (SD) | 32 (7) | 30 (6) | 29 (5) | 31 (7) |
| % ≤TG median | 56 | 57 | 39 | 51 |
BMI, body mass index; SD, standard deviation, TG, triglycerides.
Anacetrapib, combined anacetrapib 150- and 300-mg treatment groups; atorvastatin, atorvastatin 20 mg.
Whole plasma lipid and apolipoprotein measurements
In general, there were no meaningful differences in lipid and apo concentrations between the treatment groups at baseline (Table 2). Treatment with anacetrapib alone for 8 weeks resulted in significant placebo-adjusted decreases from baseline in LDL-C (43%), non–HDL-C (36%), apoB (32%), and Lp(a) (43%) as well as significant placebo-adjusted increases in HDL-C (134%) and apoAI (41%) (Table 3). No significant between-group effects on TC and TG were seen with anacetrapib alone vs placebo. Significant placebo-adjusted decreases from baseline in TC (31%), LDL-C (46%), TG (21%), non–HDL-C (41%), and apo B (36%) were seen with atorvastatin alone. No significant between-group effects on HDL-C, apoAI, and Lp(a) were seen with atorvastatin alone vs placebo. Compared with atorvastatin alone, coadministration of anacetrapib + atorvastatin resulted in incremental between-group reductions in LDL-C (25%), non–HDL-C (21%), apoB (14%), and Lp(a) (38%) compared with atorvastatin alone. Significant between-group increases in TC (9%), HDL-C (121%), and apoAI (39%) also were observed with anacetrapib + atorvastatin vs atorvastatin alone. No significant between-group effect on TG was seen with anacetrapib + atorvastatin vs atorvastatin alone.
Table 2.
Baseline and week 8 whole plasma lipids
| Placebo (N = 41) |
Anacetrapib alone (N = 95) |
Atorvastatin alone (N = 44) |
Anacetrapib + atorvastatin (N = 102) |
|||||
|---|---|---|---|---|---|---|---|---|
| Mean (SE) |
Mean (SE) |
Mean (SE) |
Mean (SE) |
|||||
| Parameter (mg/dL) | Baseline | Week 8 | Baseline | Week 8 | Baseline | Week 8 | Baseline | Week 8 |
| TC | 224 (5) | 228 (4) | 225 (3) | 234 (4) | 228 (4) | 161 (4) | 223 (3) | 179 (3) |
| LDL-C | 138 (4) | 141 (3) | 142 (2) | 84 (3) | 142 (3) | 81 (3) | 138 (2) | 45 (2) |
| HDL-C | 53 (2) | 55 (2) | 50 (1) | 118 (3) | 49 (2) | 52 (2) | 49 (1) | 109 (3) |
| TG | 145 (78) | 104 (40) | 147 (94) | 140 (84) | 162 (110) | 138 (79) | 152 (119) | 103 (62) |
| Non–HDL-C | 171 (4) | 173 (4) | 175 (3) | 115 (4) | 178 (4) | 109 (4) | 174 (3) | 70 (2) |
| Apo B | 140 (3) | 142 (3) | 141 (2) | 98 (2) | 146 (3) | 95 (3) | 141 (2) | 72 (2) |
| Apo AI | 172 (4) | 177 (5) | 169 (2) | 243 (3) | 166 (5) | 166 (4) | 164 (3) | 228 (4) |
| Lp(a) | 10 (19) | 12 (21) | 10 (25) | 5 (17) | 10 (23) | 10 (25) | 11 (28) | 5 (22) |
apoAI, apolipoprotein AI; apoB, apolipoprotein B; atorvastatin, atorvastatin 20 mg; CI, confidence interval; HDL, high-density lipoprotein; IQR, interquartile range; LDL, low-density lipoprotein; Lp(a), lipoprotein(a); SE, standard error; TC, total cholesterol; TG, triglyceride.
LDL-C estimated using Friedewald equation.
Anacetrapib, combined anacetrapib 150- and 300-mg treatment groups.
Table 3.
Percent change from baseline to week 8 in whole plasma lipid concentrations between treatment groups
| Difference in LS mean percent change from baseline (95% CI)* |
|||
|---|---|---|---|
| Parameter (mg/dL) | Placebo vs anacetrapib alone | Placebo vs atorvastatin alone |
Atorvastatin alone vs anacetrapib + atorvastatin |
| TC | 2.0 (−2.4, 6.4) | −31.0 (−36.1, −26.0)† | 9.3 (5.1, 13.5)†,‡ |
| LDL-C | −43.3 (−49.9, −36.7)†,‡ | −45.7 (−53.3, −38.0)† | −24.5 (−30.8, −18.1)†,‡ |
| HDL-C | 134.4 (120.9, 147.9)†,‡ | 1.28 (−14.4, 16.9) | 121.4 (108.4, 134.4)†,‡ |
| TG§ | 26.4 (−16.8, 3.5) | −21.2 (−33.0, −10.5)† | −5.12 (−13.2, 1.5) |
| Non–HDL-C | −36.0 (−41.6, −30.4)†,‡ | −40.7 (−47.2, −34.2)† | −20.7 (−26.2, −15.3)†,‡ |
| Apo B | −32.1 (−37.0, −27.2)†,‡ | −36.3 (−41.9, −30.6)† | −14.4 (−19.1, −9.7)†,‡ |
| Apo AI | 41.3 (35.4, 47.1)†,‡ | −2.9 (−9.7, 4.0) | 39.1 (33.5, 44.8)†,‡ |
| Lp(a)§ | −42.9 (−57.6, −31.3)†,‡ | 0.00 (−7.6, 13.0) | −38.08 (−50.0, −27.0)†,‡ |
apoAI, apolipoprotein AI; apoB, apolipoprotein B; atorvastatin, atorvastatin 20 mg; CI, confidence interval; HDL, high-density lipoprotein; IQR, interquartile range; LDL, low-density lipoprotein; Lp(a), lipoprotein(a); TC, total cholesterol; TG, triglyceride.
LDL-C estimated using Friedewald equation.
Anacetrapib, combined anacetrapib 150- and 300-mg treatment groups.
Least squares mean and 95% CI from analysis of covariance model with treatment as a factor and baseline lipid level as a covariate.
Significant between-group difference with 2-stage false discovery rate approach, which ensures the overall false discovery rate to be less than 5%.
Significant dose-response including placebo and all anacetrapib doses (ie, 10 mg, 40 mg, 150 mg/300 mg).
Hodges-Lehman estimate of median and distribution-free CI presented for change from baseline results; median ± IQR presented at baseline and week 8.
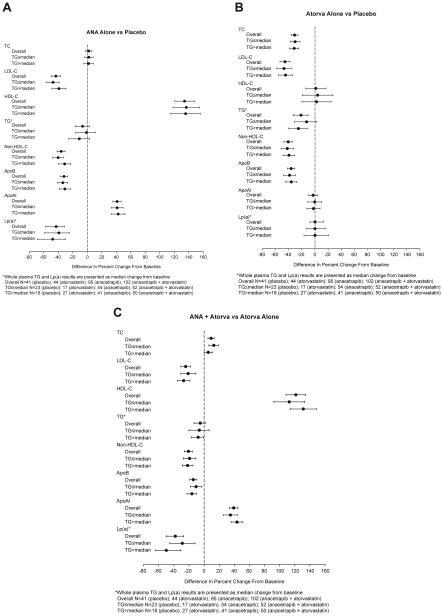
Figure 1 shows the between-group differences in the least squares mean percentage changes from baseline in whole plasma lipid and apo measurements for the overall population and within the subgroups defined by baseline TG level. No significant treatment-by-subgroup interactions were observed for any of the whole plasma lipid or apo parameters (Fig. 1A–C).
Figure 1.
Between-group differences in mean percent change from baseline for whole plasma lipid/apolipoprotein (apo) concentrations as assayed by standard techniques in the overall analysis population and subgroups defined by median baseline triglyceride (TG) value (≤ and >154 mg/dL). (A–C) Between-group differences for the placebo vs anacetrapib alone, placebo vs atorvastatin alone, and atorvastatin alone vs atorvastatin + anacetrapib treatment comparisons, respectively. Negative values favor anacetrapib alone or atorvastatin alone vs placebo or atorvastatin + anacetrapib vs atorvastatin alone except for high-density lipoprotein cholesterol (HDL-C) and apolipoprotein AI (apoAI). HDL-C, high-density lipoprotein cholesterol; Lp(a), lipoprotein a; TC, total cholesterol.
Particle concentrations
There were no notable between-group differences in any particle concentrations at baseline (Table 4). When examined in the overall population (ie, pooled across baseline TG subgroups), treatment with anacetrapib alone for 8 weeks resulted in significant placebo-adjusted reductions from baseline in large, medium and small VLDL (35%, 38%, and 28%, respectively), large IDL (37%), and large, medium, and small LDL (range 43 to 72%) along with significant elevations in very small LDL 4a and 4b (63% and 66%, respectively) (Table 5). In general, treatment with anacetrapib alone produced larger placebo-adjusted changes in the LDL fractions compared with the VLDL and IDL fractions. No significant effects on small IDL and LDL 3b were seen with anacetrapib alone relative to placebo.
Table 4.
Baseline and week 8 lipoprotein particle concentrations (nmol/L) as measured by IM methodology
| Placebo (N = 41) |
Anacetrapib alone (N = 95) |
Atorvastatin alone (N = 44) |
Anacetrapib + atorvastatin (N = 102) |
|||||
|---|---|---|---|---|---|---|---|---|
| Mean (SE) |
Mean (SE) |
Mean (SE) |
Mean (SE) |
|||||
| Parameter (mg/dL) | Baseline | Week 8 | Baseline | Week 8 | Baseline | Week 8 | Baseline | Week 8 |
| Total VLDL | 119.9 (36.3) | 129.8 (59.8) | 126.5 (43.8) | 97.2 (43.5) | 128.2 (37.6) | 98.9 (43.1) | 119.9 (50.7) | 67.5 (33.9) |
| VLDL Large | 13.8 (8.4) | 17.2 (13.7) | 14.6 (8.4) | 14.3 (12.4) | 17.2 (9.3) | 12.7 (9.1) | 13.9 (8.9) | 9.7 (7.2) |
| VLDL Medium | 39.4 (15.8) | 46.6 (29.1) | 41.4 (18.1) | 33.1 (18.5) | 45.5 (18.1) | 38.0 (18.0) | 41.0 (22.9) | 24.2 (14.3) |
| VLDL Small | 67.5 (23.8) | 62.8 (22.2) | 68.3 (23.0) | 48.3 (21.3) | 72.3 (22.6) | 49.4 (18.3) | 63.7 (27.0) | 34.1 (14.1) |
| Total IDL | 356.7 (132.1) | 471.8 (157.0) | 388.5 (108.8) | 427.5 (181.9) | 401.0 (114.2) | 313.1 (97.1) | 368.6 (125.1) | 265.6 (99.6) |
| IDL Large | 176.8 (46.9) | 191.4 (62.5) | 193.0 (56.5) | 137.7 (61.0) | 192.5 (59.7) | 139.5 (54.2) | 184.5 (58.0) | 96.7 (35.1) |
| IDL Small | 184.7 (102.7) | 265.0 (132.8) | 195.9 (68.4) | 283.0 (126.2) | 178.4 (91.5) | 170.0 (74.9) | 172.2 (88.9) | 165.1 (72.6) |
| Total LDL | 1291.2 (339.0) | 1648.2 (418.8) | 1287.9 (333.4) | 993.9 (309.9) | 1364.2 (411.0) | 1077.5 (345.0) | 1261.8 (451.7) | 812.9 (241.5) |
| LDL Large 1 | 323.1 (195.1) | 420.7 (258.7) | 295.1 (182.1) | 185.5 (99.5) | 264.4 (176.3) | 245.5 (96.2) | 273.3 (183.6) | 131.9 (56.8) |
| LDL Medium 2a | 224.4 (126.5) | 296.7 (157.0) | 221.2 (124.4) | 122.3 (54.4) | 211.8 (110.3) | 183.0 (87.4) | 214.9 (113.6) | 85.8 (34.8) |
| LDL Medium 2b | 296.6 (161.7) | 346.2 (240.6) | 303.1 (150.1) | 164.1 (58.7) | 310.3 (178.2) | 246.7 (150.9) | 288.1 (155.9) | 125.1 (45.5) |
| LDL Small 3a | 164.1 (192.1) | 237.9 (210.5) | 163.5 (213.0) | 143.8 (52.1) | 279.8 (247.3) | 167.0 (106.2) | 215.9 (239.5) | 125.4 (42.4) |
| LDL Small 3b | 51.1 (47.8) | 61.7 (40.5) | 49.4 (68.9) | 72.1 (28.8) | 66.2 (85.8) | 50.7 (22.5) | 55.2 (74.3) | 65.8 (21.1) |
| LDL Very small 4a | 64.8 (24.0) | 72.2 (35.8) | 57.9 (40.3) | 118.4 (43.7) | 70.5 (42.3) | 64.1 (18.2) | 61.4 (39.3) | 113.5 (43.0) |
| LDL Very small 4b | 74.1 (31.3) | 91.2 (30.1) | 78.2 (38.1) | 150.8 (64.6) | 78.8 (32.9) | 92.4 (27.6) | 80.7 (37.3) | 155.6 (56.9) |
IDL, intermediate-density lipoprotein; IM, ion mobility; SE, standard error; LDL, low-density lipoprotein; VLDL, very low-density lipoprotein.
Anacetrapib, combined anacetrapib 150- and 300-mg treatment groups; atorvastatin, atorvastatin 20 mg.
Table 5.
Percent change from baseline to week 8 in lipoprotein particle concentrations (nmol/L) as measured by IM methodology
| Difference in median percent change from baseline (95% CI)* | |||
|---|---|---|---|
| Parameter (mg/dL) | Placebo vs anacetrapib alone | Placebo vs atorvastatin alone | Atorvastatin alone vs anacetrapib + atorvastatin |
| Total VLDL | −32.5 (−45.4, −20.3)†,‡ | −38.3 (−54.4, −25.2)† | −14.2 (−22.9, −4.9)†,‡ |
| VLDL large | −35.2 (−56.5, −16.2)†,‡ | −53.1 (−77.7, −31.4)† | −10.3 (−22.7, 1.4) |
| VLDL medium | −37.9 (−54.7, −23.2)†,‡ | −40.7 (−60.2, −22.5)† | −15.1 (−25.3, −5.1)†,‡ |
| VLDL small | −28.4 (−39.2, 219.1)†,‡ | −34.6 (−47.2, −22.5)† | −15.2 (−23.0, −7.5)†,‡ |
| Total IDL | −17.2 (−29.2, −5.1)†,‡ | −40.9 (−55.1, −30.4)† | −13.1 (−21.0, 24.5)†,‡ |
| IDL large | −36.7 (−46.8, −27.7)†,‡ | −39.6 (−49.4, −27.1)† | −19.3 (−25.6, −13.1)†,‡ |
| IDL small | 4.0 (−14.3, −2.6) | −43.1 (−57.9, −27.3)† | −5.3 (−17.5, 7.6) |
| Total LDL | −51.0 (−60.5, −41.3)†,‡ | −49.6 (−59.2, −39.9)† | −15.4 (−22.9, −7.9)†,‡ |
| LDL large 1 | −65.9 (−79.3, −52.6)†,‡ | −45.7 (−61.5, −28.9)† | −37.1 (−48.1, −26.9)†,‡ |
| LDL medium 2a | −71.8 (284.9, −58.7)†,‡ | −43.9 (−58.3, −28.4)† | −39.1 (−48.5, −31.4)†,‡ |
| LDL medium 2b | −66.9 (−80.1, −53.6)†,‡ | −48.0 (−63.3, −33.4) | −33.6 (−43.2, −23.4)†,‡ |
| LDL small 3a | −43.1 (−60.5, −25.3)†,‡ | −50.5 (−66.7, −35.4)† | −6.6 (−22.1, 10.5)† |
| LDL small 3b | 8.0 (−18.5, 39.3) | −47.8 (−65.6, −28.6)† | 40.3 (16.1, 64.9)†,‡ |
| LDL very small 4a | 62.6 (35.0, 89.4)†,‡ | −30.1 (−46.1, −15.1)† | 83.6 (62.6, 108.7)†,‡ |
| LDL very small 4b | 65.8 (48.5, 86.2)†,‡ | −7.4 (−21.4, 7.0) | 76.2 (54.9, 98.2)†,‡ |
CI, confidence interval; HDL, high-density lipoprotein; IDL, intermediate-density lipoprotein; IM, ion mobility; IQR, interquartile range; LDL, low-density lipoprotein; VLDL, very low-density lipoprotein.
Anacetrapib, combined anacetrapib 150- and 300-mg treatment groups; atorvastatin, atorvastatin 20 mg.
Change from baseline data not normally distributed; Hodges-Lehman estimate of median and distribution-free CI presented; median (IQR) presented at baseline and week 8.
Significant between-group difference with false discovery rate less than 5%.
Significant dose-response including placebo and all anacetrapib doses (ie, 10 mg, 40 mg, 150 mg/300 mg).
Treatment with atorvastatin alone vs placebo produced significant placebo-adjusted reductions from baseline in all VLDL and IDL subfractions (range, 35 to 53%) and large, medium, and small LDL subfractions (range, 44 to 51%) (Table 5). There was a smaller, though significant, reduction in very small LDL 4a (30%), and no significant change in LDL 4b.
In general, the between-group changes from baseline in particle concentrations observed after treatment with anacetrapib + atorvastatin vs atorvastatin alone were similar in pattern to those seen with anacetrapib alone vs placebo when examined in the overall population (Table 5). Relative to atorvastatin alone, anacetrapib + atorvastatin resulted in significant but modest between-group reductions in all VLDL and large IDL subfractions (10 to 15%) as well as greater reductions in large and medium LDL particles (34 to 39%), with a smaller but significantly greater reduction for small LDL 3a (7%). In contrast, coadministration of anacetrapib + atorvastatin vs atorvastatin alone resulted in significant increases in LDL 3b (40%) and very small LDL 4a and 4b (84% and 76%, respectively).
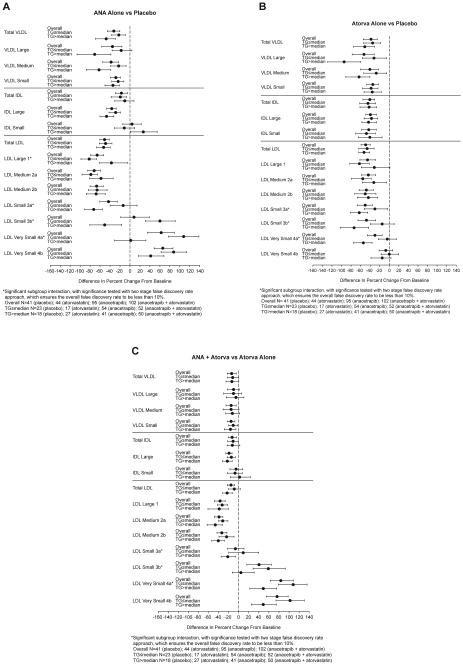
As compared with the findings for the full study population, the same general pattern of reductions in particle concentrations of VLDL, large IDL, and large and medium LDL, and increases in concentrations of very small LDL, was observed in the low- and high-TG subgroups, for both the anacetrapib alone vs placebo and anacetrapib + atorvastatin vs atorvastatin alone treatment comparisons (Fig. 2A and C). However, there was an increase in LDL 3b and 4a in the low vs high baseline TG subgroup for mono- and combination therapy (FDR <10%). For LDL 3a, there was also a significant subgroup interaction with an attenuated placebo adjusted reduction for monotherapy and an increase for combination therapy for the low vs high TG subgroups. For the atorvastatin alone vs placebo treatment comparison (Fig. 2B), there were smaller reductions in LDL 3a, 3b, and LDL 4a particles for the low vs high TG subgroups.
Figure 2.
Between-group differences in median percent change from baseline for particle concentrations as analyzed by ion mobility in the overall analysis population and subgroups defined by median baseline triglyceride (TG) value (≤ and >154 mg/dL). (A–C) Between-group differences for placebo vs anacetrapib alone, placebo vs atorvastatin alone, and atorvastatin alone vs atorvastatin + anacetrapib, respectively. Negative values favor anacetrapib alone or atorvastatin alone vs placebo or atorvastatin + anacetrapib vs atorvastatin alone. IDL, intermediate-density lipoprotein; LDL, low-density lipoprotein; VLDL, very low-density lipoprotein.
Discussion
The data from the present study in patients with dyslipidemia confirm our earlier findings in normolipidemic individuals3 that treatment with anacetrapib results in discordant changes in concentrations of LDL particles across the particle size spectrum. Specifically, there were substantial reductions in large- and medium-sized LDL species and a lesser reduction in small LDL 3a, and significant increases in the less abundant small LDL 3b and very small LDL 4a and 4b. Interestingly, although there were no significant changes in plasma TG, levels of TG-rich lipoprotein particles (VLDL and large IDL) were reduced. These results are consistent with further TG enrichment of these particles as a result of reduced CETP-mediated TG transfer to higher density lipoproteins.3 Given the evidence that small, lipid-depleted LDL arise from intravascular TG lipolysis and progressive remodeling of lipolytic remnants of VLDL and IDL,10 it is possible that TG enrichment of these particles and their LDL products leads to an increase in their lipolytic conversion to small and very small LDL. This process may also contribute to the observed reductions in levels of particles in the size range of IDL and medium and small LDL, although recent findings indicate that increased fractional plasma clearance also plays a significant role.11 In this regard, the accumulation of smaller LDL in plasma with anacetrapib treatment may result at least in part from relatively reduced LDL receptor-mediated uptake of these particles.12 In our previous study in normolipidemic individuals,3 we found that treatment with anacetrapib vs placebo resulted in relative enrichment of apoE, and to a lesser extent, apoC-III, in very small, dense LDL fractions. We speculated that these changes may have resulted from selective retention of these particles in the course of their generation from catabolism of their TG-rich precursors and/or from reduced transfer of these apolipoproteins to HDL in conjunction with reduced TG-CE exchange. The potential clinical significance of the observed changes in levels and composition of very small LDL particles with anacetrapib treatment remains unknown.
The metabolic consequences of TG enrichment of VLDL and IDL particles may also be responsible for the differential drug effects on levels of small and very small LDL observed in patients with lower vs higher baseline plasma TG levels. For patients with TG values above the median of 154 mg/dL, anacetrapib-induced reductions in small LDL3a were greater and increases in smaller LDL 3b, 4a, and 4b were lower than for patients with TG below the median. It is possible that a greater proportion larger, more TG-rich VLDL particles in patients with higher TG13 generates a sufficiently high production of small and very small LDL such that the incremental effect of TG enrichment by CETP inhibition is blunted.
Treatment with atorvastatin resulted in a similar pattern of changes in apoB-containing lipoprotein subfractions as was seen with anacetrapib, except that levels of LDL 3b, 4a, and 4b were not increased. Rather, there was an attenuated reduction in levels of these particles compared with larger LDL. This differential effect may result at least in part from reduced LDL receptor-mediated clearance of smaller LDL as noted previously.12
The combination of atorvastatin 20 mg/d with anacetrapib 150/300 mg/d generally resulted in additive changes in levels of lipoprotein fractions. Notably, atorvastatin did not modify the increases in very small LDL particles observed with anacetrapib treatment in patients with higher TG levels, consistent with a mechanism for this effect that is independent of LDL receptor upregulation.
Despite the differing changes in LDL subfraction profiles induced by anacetrapib and atorvastatin, at the doses used here they resulted in comparable reductions in plasma concentrations of total LDL particles and apoB irrespective of TG subgroup. This suggests that, particularly for anacetrapib, estimates of cardiovascular risk reduction based on lowering of total LDL may not reflect the impact of changes in LDL particle subclasses, and that these changes may be more favorable in individuals with higher plasma TG levels. The effect of anacetrapib on cardiovascular outcomes is currently being evaluated in the Rapid Evaluation of Vessel Healing After Angioplasty study (ClinicalTrials.gov NCT01252953).
Supplementary Material
Acknowledgment
Editorial support was provided by Kathleen Newcomb and Jennifer Rotonda, Merck & Co., Inc., Whitehouse Station, NJ, USA. Financial support for the conduct of this study was provided by Merck & Co., Inc., Whitehouse Station, NJ, USA.
Footnotes
Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jacl.2014.09.013.
Disclosures: Ronald M. Krauss has been a member of the Global Atherosclerosis Advisory Board of Merck and has received grant support from them. Dr. Krauss is also co-inventor on a patent for ion mobility analysis of lipoproteins. Cathy Anne Pinto, Yang Liu, Amy O. Johnson-Levonas, and Hayes M. Dansky are employees of Merck & Co., Inc., Whitehouse Station, NJ, USA and may own stock or stock options in the company. All authors are responsible for the work in this paper and were involved in at least one of the following: (conception, design, acquisition, analysis, statistical analysis, interpretation of data) and (drafting the manuscript and/or revising the manuscript for important intellectual content). All authors provided final approval of the version to be published. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- 1.Tall AR. Plasma cholesteryl ester transfer protein. J Lipid Res. 1993;34:1255–1274. [PubMed] [Google Scholar]
- 2.Gutstein DE, Krishna R, Johns D, et al. Anacetrapib, a novel CETP inhibitor: pursuing a new approach to cardiovascular risk reduction. Clin Pharmacol Ther. 2012;91:109–122. doi: 10.1038/clpt.2011.271. [DOI] [PubMed] [Google Scholar]
- 3.Krauss RM, Wojnooski K, Orr J, et al. Changes in lipoprotein subfraction concentration and composition in healthy individuals treated with the CETP inhibitor anacetrapib. J Lipid Res. 2012;53:540–547. doi: 10.1194/jlr.M018010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brinton E, Stepanavage M, Cannon CP, et al. Lipid-modifying effects of anacetrapib in patients with lower versus higher baseline levels of HDL-C, LDL-C, and TG: Pre-specified subgroup analyses of the DEFINE (Determining the efficacy and tolerability of CETP inhibition with anacetrapib) trial (abstr) Circulation. 2011;124:A9649. [Google Scholar]
- 5.Guerin M, Dolphin PJ, Chapman MJ. A new in vitro method for the simultaneous evaluation of cholesteryl ester exchange and mass transfer between HDL and apoB-containing lipoprotein subspecies. Identification of preferential cholesteryl ester acceptors in human plasma. Arterioscler Thromb. 1994;14:199–206. doi: 10.1161/01.atv.14.2.199. [DOI] [PubMed] [Google Scholar]
- 6.Bloomfield D, Carlson GL, Sapre A, et al. Efficacy and safety of the cholesteryl ester transfer protein inhibitor anacetrapib as monotherapy and coadministered with atorvastatin in dyslipidemic patients. Am Heart J. 2009;157:352–360. doi: 10.1016/j.ahj.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 7.Caulfield MP, Li S, Lee G, et al. Direct determination of lipoprotein particle sizes and concentrations by ion mobility analysis. Clin Chem. 2008;54:1307–1316. doi: 10.1373/clinchem.2007.100586. [DOI] [PubMed] [Google Scholar]
- 8.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc Series B. 1995;57:289–300. [Google Scholar]
- 9.Mehrotra DV, Heyse JF. Use of the false discovery rate for evaluating clinical safety data. Stat Methods Med Res. 2004;13:227–238. doi: 10.1191/0962280204sm363ra. [DOI] [PubMed] [Google Scholar]
- 10.Berneis KK, Krauss RM. Metabolic origins and clinical significance of LDL heterogeneity. J Lipid Res. 2002;43:1363–1379. doi: 10.1194/jlr.r200004-jlr200. [DOI] [PubMed] [Google Scholar]
- 11.Millar JS, Brousseau ME, Diffenderfer MR, et al. Effects of the cholesteryl ester transfer protein inhibitor torcetrapib on apolipoprotein B100 metabolism in humans. Arterioscler Thromb Vasc Biol. 2006;26:1350–1356. doi: 10.1161/01.ATV.0000219695.84644.56. [DOI] [PubMed] [Google Scholar]
- 12.Campos H, Arnold KS, Balestra ME, Innerarity TL, Krauss RM. Differences in receptor binding of LDL subfractions. Arterioscler Thromb Vasc Biol. 1996;16:794–801. doi: 10.1161/01.atv.16.6.794. [DOI] [PubMed] [Google Scholar]
- 13.Brunzell JD, Albers JJ, Chait A, Grundy SM, Groszek E, McDonald GB. Plasma lipoproteins in familial combined hyperlipidemia and monogenic familial hypertriglyceridemia. J Lipid Res. 1983;24:147–155. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.