Abstract
The Polycomb protein enhancer of zeste homolog 2 (EZH2) is frequently overexpressed in advanced human prostate cancer (PCa), especially in lethal castration-resistant prostate cancer (CRPC). However, the signaling pathways that regulate EZH2 functions in PCa remain incompletely defined. Using EZH2 antibody-based RNA immunoprecipitation-coupled high throughput sequencing (RIP-seq), we demonstrated that EZH2 binds to MALAT1, a long non-coding RNA (lncRNA) that is overexpressed during PCa progression. GST pull-down and RIP assays demonstrated that the 3′ end of MALAT1 interacts with the N-terminal of EZH2. Knockdown of MALAT1 impaired EZH2 recruitment to its target loci and upregulated expression of EZH2 repressed genes. Further studies indicated that MALAT1 plays a vital role in EZH2-enhanced migration and invasion in CRPC cell lines. Meta-analysis and RT-qPCR of patient specimens demonstrated a positive correlation between MALAT1 and EZH2 expression in human CRPC tissues. Finally, we showed that MALAT1 enhances expression of PRC2-independent target genes of EZH2 in CRPC cells in culture and patient-derived xenografts. Together, these data indicate that MALAT1 may be a crucial RNA cofactor of EZH2 and that the EZH2-MALAT1 association may provide a new avenue for development new strategies for treatment of CRPC.
Keywords: MALAT1, EZH2, castration-resistant prostate cancer (CRPC), Polycomb repressive complex 2 (PRC2)
INTRODUCTION
Prostate cancer (PCa) is the most commonly diagnosed malignancy and the second leading cause of cancer death among American men. Androgen deprivation therapy (ADT) is the mainstay of treatment for advanced/metastatic PCa. However, a significant portion of patients experience disease relapse and tumors eventually evolve into castration resistant prostate cancer (CRPC), which is the leading cause of PCa death at present [1].
LncRNAs have been implicated in PCa development and progression [2]. Metastasis-associated lung adenocarcinoma transcript-1 (MALAT1) lncRNA was originally identified as a prognostic factor of tumor development and metastasis in various types of human tumors such as glioma, lung, pancreatic and prostate cancer as well as esophageal squamous cell carcinoma [3–6]. MALAT1 was found positively correlated with Gleason score, the level of prostate specific antigen (PSA), tumor stage and castration resistance in PCa [7]. MALAT1 overexpression appears to be a promising diagnostic urinary biomarker for prostate cancer [8]. Moreover, MALAT1 was identified as the most highly expressed transcript in CRPC by whole transcriptome sequencing in a panel of CRPC bone marrow biopsy specimens [9]. However, the role of MALAT1 in development and progression in PCa, especially CRPC remains elusive.
EZH2, working with EED and SUZ12, the other two essential components of the Polycomb repressive complex-2 (PRC2), functions primarily as a methyltransferase catalyzing histone H3 lysine 27 trimethylation (H3K27me3) and promoting gene silencing [10]. EZH2 has been found frequently overexpressed in variety of human cancers such as prostate and breast cancer [11, 12]. Increasing evidences show that EZH2 levels correlate with increased proliferation rates, invasiveness and metastasis of PCa in patients [13, 14]. Moreover, it has been shown that EZH2 interacts with the HOTAIR lncRNA and facilitates PRC2 targeting in the genome of breast cancer and promotes breast cancer metastasis [15]. Further studies reveal that the EZH2-HOTAIR interaction is regulated by various signaling pathways such as cyclin-dependent kinases (CDKs) and the tumor suppressor protein BRCA1 [16, 17]. PCAT1 was identified as a prostate-specific lncRNA that can bind to EZH2, but the expression of PCAT-1 and EZH2 is nearly mutually exclusive in human PCa [2]. It thus remains unclear which lncRNAs are required for EZH2 functions to facilitate PCa progression.
Despite the fact that MALAT1 is often overexpressed in human cancers including PCa, its functional role in cancer progression is poorly understood. One study demonstrates previously that MALAT1 associates with PRC2 by interacting with SUZ12 but not EZH2 and that inhibition of MALAT1 decreases the binding of SUZ12 to the E-cadherin gene promoter in bladder cancer [18]. In contrast, a recent study reports that MALAT1 can bind to EZH2 and downregulate E-cadherin expression through EZH2-mediated H3K27me3 at the E-cadherin gene promoter in clear renal cancer [19]. Given that the previous findings regarding the mechanism by which MALAT1 affects the function of PRC2 are not consistent, further investigation is warranted. In the present study, we identified MALAT1 as a vital regulator of EZH2 in CRPC cells. We further showed that MALAT1 interacts with and facilitates EZH2 occupancy and the H3K27me3 activity of EZH2 in CRPC cells and that expression of MALAT1 correlates with EZH2 levels in human CRPC specimens.
RESULTS
Identification of MALAT1 as an EZH2-binding lncRNA by RIP-seq in PCa cells
Previous studies show that lncRNAs such as HOTAIR play important roles in facilitating genome-wide occupancy of EZH2 onto chromatin in breast cancer cells [15]. However, HOTAIR is hardly detected in human PCa specimens [2], suggesting that other lncRNAs may be important for EZH2 function in PCa. To identify EZH2 interacting lncRNA(s) in PCa cells, unbiased RNA immunoprecipitation (RIP)-coupled high throughput sequencing (RIP-seq) was used. Given that the crosslink-based RIP is highly susceptible to RNA contamination [20], we performed native (without crosslink) EZH2 RIP-seq in LNCaP-Rf CRPC cells [21]. MALAT1 was identified as one of the lncRNAs that bind to EZH2 (Figure 1A). The binding of MALAT1 with EZH2 was further confirmed by RIP-qPCR in LNCaP-Rf and C4-2, another CRPC cell line (Figure 1B and 1C). No EZH2 binding to other RNA species such as FOXO1 mRNA was detected in both cell lines (Figure 1D). These data suggest that EZH2 specifically binds to MALAT1 in CRPC cells. RIP-qPCR analysis showed that MALAT1 bound to EZH2 in androgen-responsive cell line LNCaP (Figure 1E), suggesting that MALAT1 also impacts EZH2 functions in androgen-sensitive PCa cells.
Figure 1. MALAT1 binds to EZH2 in PCa cells.
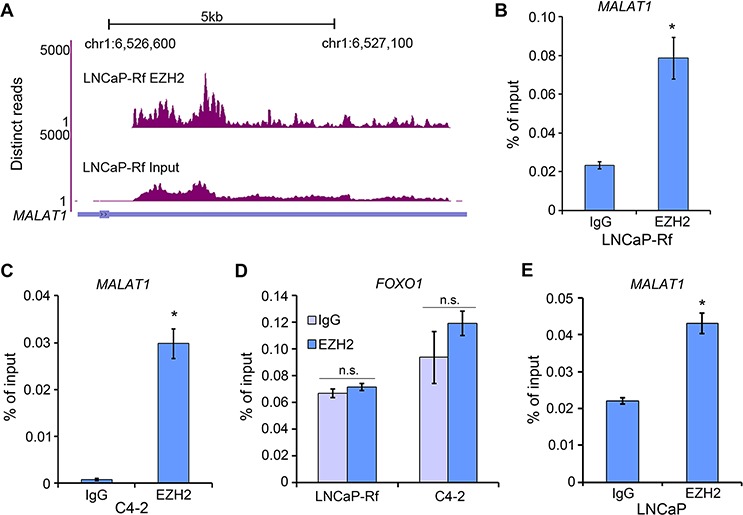
A. RIP-seq profile showing the pulldown of MALAT1 by EZH2. Screen shots of the UCSC genome browser showing coverage profiles of MALAT1 in EZH2 RIP-seq and input samples. Both RIP-seq and input samples were normalized into the same sequencing depth. B–D. LNCaP-Rf and C4-2 CRPC cells were harvested for RIP with anti-EZH2 antibody or control IgG. Bound MALAT1 (B, C) and FOXO1 mRNA (negative control) (D) were analyzed by RT-qPCR (n = 3). Data are means ± S.D. from experiments with three replicates. *P < 0.01 comparing to the IgG control group. n.s., not statistically significant. E. RT-qPCR analysis of MALAT1 in RIP samples pulled down by anti-EZH2 antibody in LNCaP androgen-sensitive cells. *P < 0.01 comparing to the IgG control group.
Identification of region(s) in MALAT1 and EZH2 responsible for their interaction
To determine which region(s) of EZH2 protein mediate its interaction with MALAT1, we performed glutathione S-transferase (GST) pulldown assays. GST-EZH2 recombinant proteins were purified as we described previously [17] and GST pull-down assays were performed using C4-2 cell lysates (Figure 2A and 2B). We found that MALAT1 preferentially bound to EZH2 in two regions (amino acids 1–173 and 336–554), which are known to bind to EED and SUZ12, respectively (Figure 2B and 2C). It is worth noting that knockdown of MALAT1 had no effect on EZH2 binding with EED and SUZ12 in C4-2 cells (Figure 2D). Additionally, we performed reciprocal in vitro RNA binding assays. In vitro transcribed MALAT1 fragments (M1-M6) were incubated with GST-EZH2-N (amino acids 1–554), the region that strongly binds to MALAT1, and RT-qPCR was used to detect the RNAs pulled down by GST-EZH2-N. We demonstrated that the region containing the nucleotides 7501–8708 at the 3′ end of MALAT1 interacted strongly with EZH2-N (Figure 2E–2G). These data indicate that MALAT1 directly interacts with the N-terminal of EZH2 and EZH2 interacts with the 3′ end of MALAT1, at least under in vitro conditions.
Figure 2. Analysis of the regions in MALAT1 and EZH2 responsible for their interaction.
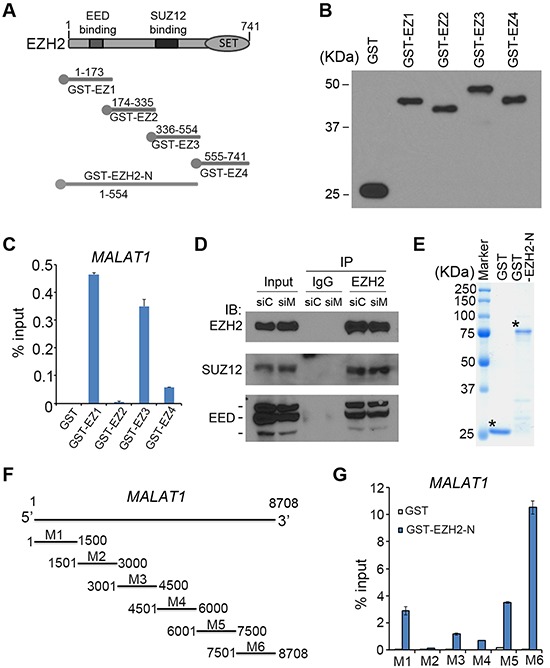
A, B. Schematic diagram and western blot detection of GST and GST-EZH2 recombinant proteins indicated. C. RT-qPCR analysis of MALAT1 lncRNA pulled down by GST or GST-EZH2 recombinant proteins. Data are means ± S.D. from experiments with three replicates. D. C4-2 cells were transfected with non-specific control (siC) or a pool of MALAT1-specific siRNAs (siM) for 48 h and cells were harvested for immunoprecipitation using control IgG or EZH2 antibody and western blot using indicated antibodies. E. Coomassie blue staining of GST and GST-EZH2-N (amino acids 1–554) fusion protein. Asterisks indicate the proteins with correct molecular mass. F. Schematic diagram of MALAT1 regions M1-M6 used for GST pull down assays. G. RT-qPCR analysis of the in vitro transcribed different regions of MALAT1 lncRNA pulled down by GST or GST-EZH2-N (1–554) recombinant proteins. Data are means ± S.D. from experiments with three replicates.
MALAT1 is important for EZH2-mediated silencing of Polycomb-dependent target genes
EZH2 is a core subunit of PRC2 that promotes gene silencing via its histone methyltransferase activity. To investigate the role of MALAT1 on EZH2-mediated Polycomb-dependent gene silencing, we employed two independent MALAT1 siRNA (siM-1 and siM-2) to knock down MALAT1 in PC-3 and C4-2 CRPC cell lines. We demonstrated that expression of MALAT1 was effectively knocked down by MALAT1 siRNAs in both cell lines (Figure 3A and 3B). Importantly, MALAT1 knockdown by both siRNAs invariably resulted in derepression of EZH2 repressed genes examined including DAB2IP and BRACHYURY (or called T gene) (Figure 3C and 3D). Western blot analysis showed that MALAT1 knockdown also increased DAB2IP protein levels in both PC-3 and C4-2 cell lines (Figure 3E). These results suggest that MALAT1 enhances EZH2-mediated repression of Polycomb-dependent target genes in CRPC cells.
Figure 3. The effect of MALAT1 on expression of Polycomb-dependent target genes of EZH2.
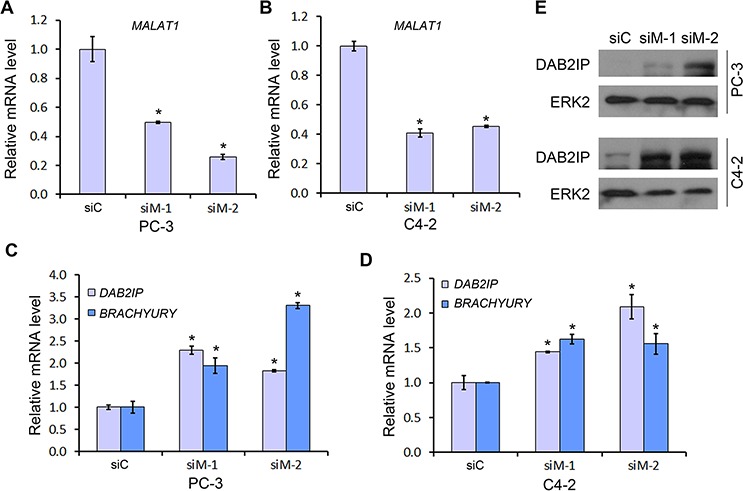
A–D. PC-3 (A, C) and C4-2 (B, D) cells were transfected with non-specific control (siC) or two individual MALAT1-specific siRNAs (siM-1 and siM-2) for 48 h and then cells were harvested for RT-qPCR analysis of MALAT1 (A, B) and EZH2 Polycomb-dependent target genes DAB2IP and BRACHYURY (C, D). Data are means ± S.D. from experiments with three replicates. *P < 0.01 comparing to the siC group. E. PC-3 and C4-2 cells were transfected as in (A) and expression of DAB2IP proteins were analyzed by western blot. ERK2 was used as a loading control.
MALAT1 is important for EZH2 occupancy and H3K27me3 levels at Polycomb target loci
Since our data suggest that EZH2 binds directly to MALAT1 and enhances EZH2-mediated gene repression, we sought to determine whether MALAT1 promotes EZH2 occupancy and increases H3K27me3 level at EZH2 target loci. Chromatin immunoprecipitation (ChIP) assay using EZH2 and H3K27me3 antibodies demonstrated that MALAT1 knockdown not only decreased EZH2 recruitment to the promoters of its target genes DAB2IP and BRACHYURY in PC-3 and C4-2 cells (Figure 4A and 4B), but also decreases H3K27me3 levels at these gene promoters in both cell lines (Figure 4C and 4D). These results indicate that MALAT1 facilitates EZH2 targeting and enhances the H3K27me3 activity of EZH2 at its target gene loci in CRPC cells.
Figure 4. The effect of MALAT1 on EZH2 recruitment and H3K27me3 levels at EZH2 Polycomb-dependent target gene loci.
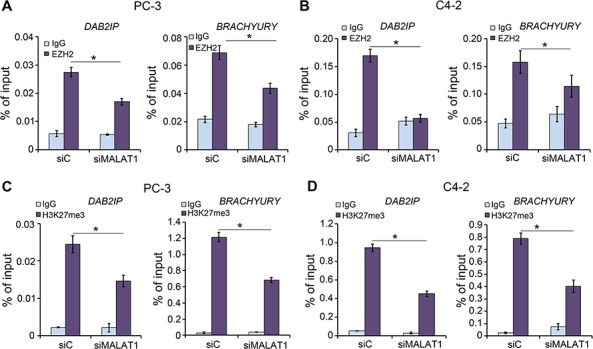
A, B. ChIP-qPCR analysis of EZH2 occupancy at its target loci DAB2IP and BRACHYURY. PC-3 (A) and C4-2 (B) cells were transfected with non-specific control (siC) or a pool of MALAT1-specific siRNAs (siMALAT1). 48 h after transfection, cells were harvested for ChIP assay with non-specific IgG or EZH2 antibody. Data are means ± S.D. from experiments with three replicates. *P < 0.01. C, D. ChIP-qPCR analysis of H3K27me3 levels at the promoters of EZH2 target loci DAB2IP and BRACHYURY. PC-3 (C) and C4-2 (D) cells were transfected with non-specific control (siC) or a pool of MALAT1-specific siRNAs. 48 h after transfection, cells were harvested for ChIP assay with non-specific IgG or H3K27me3 antibody. Data are means ± S.D. from experiments with three replicates. *P < 0.01.
MALAT1 enhances EZH2-mediated PCa cell invasion and migration
It has been reported that EZH2 promotes invasion and migration in PCa cells [13, 22, 23]. Since MALAT1 enhances EZH2-mediated repression of the EZH2 target gene DAB2IP, an inhibitor of cell invasion and migration [22, 24], we sought to determine whether MALAT1 enhances EZH2-promoted invasion and migration in CRPC cells. We demonstrated that knockdown of either MALAT1 or EZH2 decreased invasion in both PC-3 and C4-2 cells (Figure 5A–5D and Supplementary Figure S1A and S1B). Concomitant knockdown of EZH2 did not further inhibit cell invasion compared to the effect of MALAT1 or EZH2 knockdown alone (Figure 5A–5D and Supplementary Figure S1A and S1B). Importantly, we demonstrated that EZH2 knockdown-impaired invasion of PC-3 and C4-2 cells was largely reversed by restored expression of siRNA-resistant EZH2 (Figure 5A–5D and Supplementary Figure S1B). In contrast, ectopic expression of EZH2 protein in MALAT1 knockdown cells had only very minimal rescue effect on cell invasion although the EZH2 protein levels were comparable under these two conditions (Figure 5A–5D and Supplementary Figure S1A and S1B). Similar results were obtained from migration assays in PC-3 and C4-2 cells (Figure 5E–5G and Supplementary Figure S1C). These findings suggest that the effects of MALAT1 on PCa cell invasion and migration are mediated, at least in part through EZH2. In agreement with these observations, knockdown of MALAT1 caused derepression of the EZH2 target DAB2IP, an inhibitor of migration and invasion (Figure 5A), but little or no further increase in DAB2IP expression in MALAT1 and EZH2 co-knockdown cells (Figure 5A). Taking together, we demonstrated a role of MALAT1 in promoting CRPC cell migration and invasion and this effect appears to be mediated, at least partially through EZH2.
Figure 5. MALAT1 facilitates EZH2-mediated PCa cell migration and invasion.
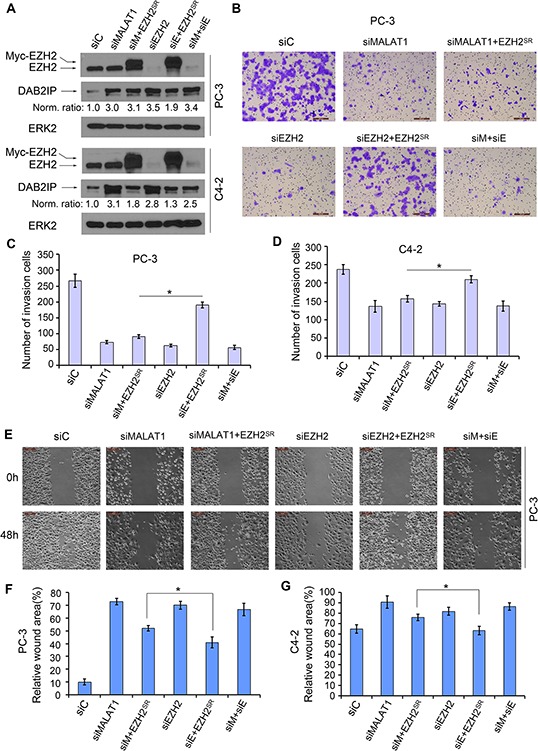
A. PC-3 and C4-2 cells were transfected with non-specific control (siC), MALAT1- and/or EZH2-specific siRNAs (siM and siE, respectively) in combination with siRNA-resistant EZH2 expression vector (EZH2SR). At 48 h after transfection, cells were harvested for western blot analysis using the indicated antibodies. ERK2 was used as a loading control. The western blot density of DAB2IP was first normalized to the density of ERK2 in each lane and then normalized further to the value in cells transfected with siC. B–D. Cells were transfected as in (A). At 48 h after transfection, cells were used for Matrigel invasion assays. Representative images of invasion assay performed in PC-3 cells are shown in (B) and the quantification results from PC-3 and C4-2 cells are shown in (C) and (D), respectively. Scale bar, 200 μm. Data are means ± S.D. from experiments with three replicates. *P < 0.01. E–G. PC-3 and C4-2 cells were transfected with indicated siRNAs and plasmids as in (A). At 24 h after transfection, artificial wounds were created on cells grown in confluence. Images were taken at 0, 48 h after wound. Representative images from PC-3 cells are shown in (E) and the quantification results are shown in (F) and (G). Scale bar, 50 μm. Data are shown as means ± S.D. from three individual experiments. *P < 0.01.
MALAT1 promotes expression of PRC2-independent targets of EZH2 in CRPC cells
Both EZH2 and MALAT1 are highly expressed in human prostate cancers, especially metastatic CRPC [7, 9, 14, 25]. Based on our findings that MALAT1 binds to EZH2 and enhances EZH2 functions in CRPC cells, it would be clinically significant to determine the correlation between MALAT1 and EZH2 expression in human CRPC samples. Meta-analysis of a gene expression dataset in public domain [26] revealed that there exists a positive correlation (r = 0.5, P = 4.3E-7) between MALAT1 and EZH2 mRNA expression in human primary and castration-resistant PCa specimens (Figure 6A). The positive correlation between MALAT1 and EZH2 mRNA expression was further confirmed by RT-qPCR in an independent cohort of human CRPC samples (r = 0.57, P = 0.026) (Figure 6B). It has been reported previously that Polycomb (or PRC2)-independent functions of EZH2 are important for castration-resistant progression of PCa [27]. Thus, we sought to determine the role of MALAT1 in EZH2-mediated expression of Polycomb-independent genes in CRPC cells. To this end, we knocked down MALAT1 with two individual siRNAs in C4-2 cells and examined expression of CKS2 and TMEM48, two Polycomb-independent genes of EZH2 identified recently [27]. Both CKS2 and TMEM48 were significantly downregulated in MALAT1 knockdown cells (Figure 6C). As expected, knockdown of EZH2 also decreased expression of TMEM48 (Figure 6D), but concomitant knockdown of EZH2 and MALAT1 did not further decrease the expression of this gene in C4-2 cells (Figure 6D), suggesting that MALAT1-induced expression of Polycomb-independent EZH2 target genes is mediated through EZH2. Moreover, in line with the findings in human CRPC tissues and CRPC cells in culture, we demonstrated that both MALAT1 and EZH2 mRNAs were expressed at much higher levels in castration-resistant patient-derived xenografts (23.1AI) than in androgen-dependent counterparts (23.1) (Figure 6E). Accordingly, expression of Polycomb-dependent EZH2-repressed gene DAB2IP was significantly lower whereas expression of Polycomb-independent EZH2-activated target genes TMEM48 and KIAA0101 was significantly higher in 23.1AI xenografts in comparison to 23.1 androgen-dependent xenografts (Figure 6E and 6F). These results indicate that MALAT1 regulates expression of both Polycomb-dependent and -independent EZH2 target genes and that co-expression of MALAT1 and EZH2 associates with CRPC progression.
Figure 6. MALAT1 expression correlates with EZH2 mRNA levels and MALAT1 enhances expression of PRC2-independent target genes of EZH2 in CRPC cells.
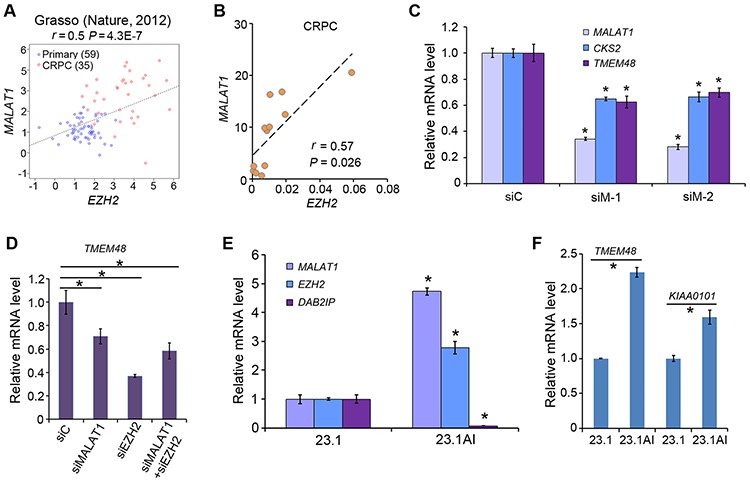
A. Meta-analysis of correlation between MALAT1 and EZH2 mRNA expression in human primary and castration-resistant PCa specimens. Pearson correlation test r = 0.5, P = 4.3E-7. B. RT-qPCR analysis of expression of MALAT1 and EZH2 mRNA in 12 cases of human CRPC specimens. Pearson correlation test r = 0.57, P = 0.026. C. RT-qPCR analysis of MALAT1, CKS2 and TMEM48 in C4-2 cells at 48 h after transfected with control siRNAs (siC) or MALAT1-specific siRNAs (siM-1 and siM-2). Data are means ± S.D. from experiments with three replicates. *P < 0.01 comparing to the siC group. D. The effect of MALAT1 on TMEM48 expression is mediated through EZH2. C4-2 cells were transfected with non-specific control (siC) or MALAT1- and/or EZH2-specific siRNA and at 48 h after transfection cells were harvested for RNA isolation and RT-qPCR analysis of expression of TMEM48. Data are means ± S.D. from experiments with three replicates. *P < 0.01. E. mRNA expression of MALAT1, EZH2 and DAB2IP in androgen-dependent (LuCaP 23.1) and castration-resistant (LuCaP 23.1AI) xenograft tumors analyzed by RT-qPCR. Data are means ± S.D. from experiments with three replicates. *P < 0.01 comparing the data in 23.1AI with those in 23.1 tumors. F. RT-qPCR analysis of TMEM48 and KIAA0101 expression in LuCaP 23.1 and LuCaP 23.1AI xenograft tumors. Data are means ± S.D. from experiments with three replicates. *P < 0.01.
DISCUSSION
LncRNAs are implicated in regulation of development and tumorigenesis. MALAT1 is one of the highly cancer-relevant lncRNAs, expression of which is associated with progression of lung, pancreatic, prostate cancer and glioma and therefore, it has been proposed that MALAT1 could be a biomarker of several cancer types [3–5, 7]. To date, however, the precise mechanism of action of MALAT1 in oncogenesis is largely unclear. Recent studies indicate that MALAT1 enhances the activity of the PRC2 complex in bladder cancer by interacting with the subunit SUZ12 but not the subunit EZH2 in PRC2 [18]. Surprisingly, a most recent report shows that MALAT1 promotes the activation of PRC2 by binding to EZH2 and enhances EZH2-mediated repression of Polycomb-dependent target gene E-Cadherin in clear renal cancer [19]. At present, however, the molecular basis of the interaction between MALAT1 and EZH2 is unclear. By performing high throughput EZH2 RIP-seq in LNCaP-Rf CRPC cells, we identified MALAT1 as one of the lncRNAs that binds to EZH2. We not only confirmed their interaction in different CRPC cell lines, but also provided evidence that MALAT1 interacts directly with EZH2. Importantly, we demonstrated for the first time that the N-terminal of EZH2 and the 3′ end of MALAT1 are responsible for their interaction. Thus, using various means we have demonstrated the interaction between MALAT1 and EZH2 in CRPC cells and unravel the mechanism underlying their interaction.
Highly conserved triple helical structures at the 3′end of MALAT1 was reported to be responsible for the stability and high level expression of MALAT1 in cells [28]. Moreover, the 3′-end portion of MALAT1 is not only found highly mutated in colorectal cancer cells, but has also been shown to be essential in various biological processes such as cell proliferation, migration and invasion [29]. Our study demonstrates that the 3′ end of MALAT1 (nucleotides 7501–8708) preferentially binds to EZH2. These data imply that the structure of the 3′ end of MALAT1 may also be vital in regulating the oncogenic activity of EZH2 and EZH2-mediated progression of CRPC cells.
EZH2 is the catalytic component of PRC2 complex that is responsible for H3K27me3 and repression of Polycomb-dependent target genes. A recent study identifies a Polycomb-independent oncogenic function of EZH2 in CRPC cells [27]. In the current study we found that MALAT1 binds to EZH2 and enhances EZH2-mediated repression of Polycomb-dependent target genes such as DAB2IP and BRACHYURY. Furthermore, we provided evidence that MALAT1 also enhances Polycomb-independent function of EZH2 and is involved in regulation of expression of EZH2-activated genes such as CKS2, TMEM48 and KIAA0101 in CRPC cells. This data indicates that MALAT1 represents a viable target to inhibit the Polycomb-dependent and -independent oncogenic functions of EZH2 in CRPC. Thus, MALAT1 regulation of EZH2 may provide new directions for development strategies for treatment of CRPC.
MATERIALS AND METHODS
Cell lines and cell culture
C4-2 cells were purchased from UroCorporation. PC-3 and LNCaP cells were purchased from ATCC. LNCaP-Rf, a castration-resistant subline of LNCaP was described previously [21]. LNCaP-Rf and C4-2 cells were cultured in RPMI 1640 medium supplemented with 10% charcoal-stripped fetal bovine serum (FBS) (Life Technologies) (or called androgen-depleted medium) and 100 μg/ml penicillin-streptomycin-glutamine (Life Technologies) at 37°C with 5% CO2. PC-3 and LNCaP cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) (Life Technologies).
Plasmids and antibodies
EZH2 fragments (1–173, 174–335, 336–554, 555–746) and EZH2-N (1–554) were amplified by PCR and subcloned into pGEX-4T-1 vector (GE Healthcare Life Sciences) to generate recombinant GST-EZH2 fusion proteins. Different regions of MALAT1 (M1-M6) were amplified from purified cDNA of C4-2 cells and subcloned into the backbone vector pcDNA3.1 (Life Technologies). Antibodies used are as follows: anti-EZH2 (XP, Cell Signaling Technology); anti-EED (Millipore); anti-DAB2IP (Abcam); anti-SUZ12 and anti-ERK2 (Santa Cruz Biotechnology); and anti-GST (GE Healthcare Life Sciences).
Prostate cancer xenografts
The androgen dependent (AD) (LuCaP23.1) and castration-resistant (or androgen independent, AI) (LuCaP23.1AI) xenografts were originally kindly provided by Dr. Robert L. Vessella (Department of Urology, University of Washington Medical Center, Seattle, WA). AD LuCaP xenografts were propagated in BALB/c nu/nu mice and AI xenografts were propagated in SCID mice as described previously [30].
Native RNA immunoprecipitation-coupled high throughput sequencing (RIP-Seq)
LNCaP-Rf cells grown to ~ 70–80% confluence were washed twice with 1 × PBS and trypsinized. After washing with ice-cold 1 × PBS, cell lysate was obtained by centrifugation (12,000 rpm for 10 min at 4°C) after incubation with RIP buffer [50 mM Tris-HCl (pH 7.9), 0.25 M NaCl, 1% Nonidet P-40 (NP-40) 10 mM EDTA and RNase inhibitor (Promega)] for 30 min. Cell lysate was incubated with antibody/beads for 18 h at 4°C. Protein-RNA complexes bound to beads were washed three times in NT2 buffer [50 mM Tris-HCl (pH 7.4), 300 mM NaCl, 1 mM MgCl2, 0.05% Nonidet P-40 (NP40),1 × PIC, RNase inhibitor], followed by treated with DNase I for 15 min at 37°C and further washed twice with NT2 buffer. Co-purified RNA was extracted by RNA purification kit (RNAeasy Mini Elute kit, QIAGEN) and cDNA was synthesized using the SuperScript kit from Life Technologies and the quality of cDNA was analyzed by real-time polymerase chain reaction (PCR). Double-strained cDNA was synthesized and fragmented for next generation sequencing. RIP-seq raw reads were mapped to human reference genome (hg19/GRCh37) using Tophat (v1.4.0) [31]. Raw count mapped to each Refseq gene was calculated using RSeQC package [32]. Hypergeometric test was applied to evaluate the significance of enrichment of RIP-seq reads at each gene.
RNA interference
Smart pool of siRNAs targeting EZH2 and MALAT1 and nonspecific control siRNAs were purchased from Dharmacon. Two individual siRNA targeting human MALAT1 (siM-1 and siM-2) were synthesized by Dharmacon. siRNA transfection of cells was performed following the manufacturer's instruction.
RT-qPCR
Total RNA was isolated from cells with TRIzol and cDNA was synthesized using the RT-PCR kit from Promega. Three-step real-time polymerase chain reaction (PCR) was performed using the SYBR Green Mix (BioRad) and an iCycler Iqtm system (BioRad). The primer sequences used for PCR are described in Supplementary Table S1.
Chromatin immunoprecipitation (ChIP) assay
ChIP assay was performed as described previously [33]. Briefly, cells were crosslinked with 1% formaldehyde at room temperature. After washing with 1 × PBS, cell nuclei were extracted with lysis buffer 1 (50 mM HEPES, pH 7.5, 140 mM NaCl, 1 mM EDTA, 10% glycerol, 0.5% Nonidet P-40, 0.25% Triton X-100) and washed with lysis buffer 2 (10 mM Tris-HCl, pH 8.0, 200 mM NaCl, 1 mM EDTA). Cell nuclei was resuspended in lysis buffer 3 (10 mM Tris-HCl, pH 8.0, 100 mM NaCl, 1 mM EDTA, 0.1% sodium deoxycholate) and sonicated. Cell lysis was incubated overnight at 4°C with 10 μg antibody bound-protein G beads (Life Technologies). After several time washing, DNA-protein complex was elucidated, reverse crosslinked and purified. Real-time PCR was used to detect the amount of DNA immunoprecipitated by antibody.
Wound healing assay
C4-2 cells were transfected with indicated siRNA and seeded into six-well plates. Artificial wounds were created on the cell monolayer using culture-inserts for live cells analysis. Migrated cells and wound healing were visualized at 0 and 48 h. For each group, at least 3 artificial wounds were photographed immediately and at the time points indicated after the wound formation. Cell migration was evaluated by measuring the difference of wound areas.
Invasion assay
In vitro invasion assay was performed using BioCoat Matrigel invasion chamber (BD Biosciences) according to the manufacturer's protocol. PC-3 and C4-2 cells were transfected with indicated siRNAs for 24 h and cultured in the insert for 24 h. Cells were fixed in methanol for 15 min and then stained with 1 mg/ml crystal violet staining for 20 min. At least 5 fields for each group were photographed after staining. Invasion was evaluated by counting the number of the invaded cells.
Prostate cancer patient samples for RT-qPCR analysis
RT-qPCR analyses of MALAT1 and EZH2 mRNA expression in CRPC patient samples were approved by the Mayo Clinic Institutional Review Board (IRB). CRPC patient samples were selected randomly from patients who have been treated at Mayo Clinic between January 1995 and January 2014. The age of the patients ranged from 45 to 78 years. Total RNA was isolated from 10 micron section of FFPE samples using the Ambio RNA Isolation kit (Thermo Fisher Scientific) with DNase treatment and RT-qPCR was performed to detect MALAT1 and EZH2 expression.
Statistics
Experiments were carried out with three or more replicates. Statistical analyses were performed by two-tailed Student's t test. P < 0.05 is considered statistically significant.
SUPPLEMENTARY FIGURE AND TABLE
ACKNOWLEDGMENTS AND FUNDING
We would like to thank Drs. Robert L. Vessella and Donald J. Tindall for providing androgen-dependent and castration-resistant prostate cancer xenograft samples. This work was supported in part by grants from the National Institutes of Health (CA134514 and CA130908 to H.H.), the Department of Defense (W81XWH-09-1-622 and W81XWH-14-1-0486 to H.H.) and the Mayo Clinic Center for Individualized Medicine (to H.H.).
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
REFERENCES
- 1.Cai C, Chen S, Ng P, Bubley GJ, Nelson PS, Mostaghel EA, Marck B, Matsumoto AM, Simon NI, Wang H, Balk SP. Intratumoral de novo steroid synthesis activates androgen receptor in castration-resistant prostate cancer and is upregulated by treatment with CYP17A1 inhibitors. Cancer Res. 2011;71:6503–6513. doi: 10.1158/0008-5472.CAN-11-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prensner JR, Iyer MK, Balbin OA, Dhanasekaran SM, Cao Q, Brenner JC, Laxman B, Asangani IA, Grasso CS, Kominsky HD, Cao X, Jing X, Wang X, Siddiqui J, Wei JT, Robinson D, et al. Transcriptome sequencing across a prostate cancer cohort identifies PCAT-1, an unannotated lincRNA implicated in disease progression. Nature Biotechnology. 2011;29:742–749. doi: 10.1038/nbt.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ma KX, Wang HJ, Li XR, Li T, Su G, Yang P, Wu JW. Long noncoding RNA MALAT1 associates with the malignant status and poor prognosis in glioma. Tumour Biol. 2015;36:3355–3359. doi: 10.1007/s13277-014-2969-7. [DOI] [PubMed] [Google Scholar]
- 4.Pang EJ, Yang R, Fu XB, Liu YF. Overexpression of long non-coding RNA MALAT1 is correlated with clinical progression and unfavorable prognosis in pancreatic cancer. Tumour Biol. 2015;36:2403–2407. doi: 10.1007/s13277-014-2850-8. [DOI] [PubMed] [Google Scholar]
- 5.Shen L, Chen L, Wang Y, Jiang X, Xia H, Zhuang Z. Long noncoding RNA MALAT1 promotes brain metastasis by inducing epithelial-mesenchymal transition in lung cancer. J Neurooncol. 2015;121:101–108. doi: 10.1007/s11060-014-1613-0. [DOI] [PubMed] [Google Scholar]
- 6.Ren S, Wang F, Shen J, Sun Y, Xu W, Lu J, Wei M, Xu C, Wu C, Zhang Z, Gao X, Liu Z, Hou J, Huang J. Long non-coding RNA metastasis associated in lung adenocarcinoma transcript 1 derived miniRNA as a novel plasma-based biomarker for diagnosing prostate cancer. Eur J Cancer. 2013;49:2949–2959. doi: 10.1016/j.ejca.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 7.Ren S, Liu Y, Xu W, Sun Y, Lu J, Wang F, Wei M, Shen J, Hou J, Gao X, Xu C, Huang J, Zhao Y. Long noncoding RNA MALAT-1 is a new potential therapeutic target for castration resistant prostate cancer. J Urol. 2013;190:2278–2287. doi: 10.1016/j.juro.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Wang F, Ren S, Chen R, Lu J, Shi X, Zhu Y, Zhang W, Jing T, Zhang C, Shen J, Xu C, Wang H, Wang Y, Liu B, Li Y, Fang Z, et al. Development and prospective multicenter evaluation of the long noncoding RNA MALAT-1 as a diagnostic urinary biomarker for prostate cancer. Oncotarget. 2014;5:11091–11102. doi: 10.18632/oncotarget.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sowalsky AG, Xia Z, Wang L, Zhao H, Chen S, Bubley GJ, Balk SP, Li W. Whole transcriptome sequencing reveals extensive unspliced mRNA in metastatic castration-resistant prostate cancer. Mol Cancer Res. 2015;13:98–106. doi: 10.1158/1541-7786.MCR-14-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao Q, Yu J, Dhanasekaran SM, Kim JH, Mani RS, Tomlins SA, Mehra R, Laxman B, Cao X, Yu J, Kleer CG, Varambally S, Chinnaiyan AM. Repression of E-cadherin by the polycomb group protein EZH2 in cancer. Oncogene. 2008;27:7274–7284. doi: 10.1038/onc.2008.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simon JA, Lange CA. Roles of the EZH2 histone methyltransferase in cancer epigenetics. Mutation research. 2008;647:21–29. doi: 10.1016/j.mrfmmm.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Bachmann IM, Halvorsen OJ, Collett K, Stefansson IM, Straume O, Haukaas SA, Salvesen HB, Otte AP, Akslen LA. EZH2 expression is associated with high proliferation rate and aggressive tumor subgroups in cutaneous melanoma and cancers of the endometrium, prostate, and breast. J Clin Oncol. 2006;24:268–273. doi: 10.1200/JCO.2005.01.5180. [DOI] [PubMed] [Google Scholar]
- 13.Bryant RJ, Cross NA, Eaton CL, Hamdy FC, Cunliffe VT. EZH2 promotes proliferation and invasiveness of prostate cancer cells. Prostate. 2007;67:547–556. doi: 10.1002/pros.20550. [DOI] [PubMed] [Google Scholar]
- 14.Saramaki OR, Tammela TL, Martikainen PM, Vessella RL, Visakorpi T. The gene for polycomb group protein enhancer of zeste homolog 2 (EZH2) is amplified in late-stage prostate cancer. Genes Chromosomes Cancer. 2006;45:639–645. doi: 10.1002/gcc.20327. [DOI] [PubMed] [Google Scholar]
- 15.Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, Wang Y, Brzoska P, Kong B, Li R, West RB, van de Vijver MJ, et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–1076. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaneko S, Li G, Son J, Xu CF, Margueron R, Neubert TA, Reinberg D. Phosphorylation of the PRC2 component Ezh2 is cell cycle-regulated and up-regulates its binding to ncRNA. Genes Dev. 2010;24:2615–2620. doi: 10.1101/gad.1983810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L, Zeng X, Chen S, Ding L, Zhong J, Zhao JC, Sarver A, Koller A, Zhi J, Ma Y, Yu J, Chen J, Huang H. BRCA1 is a negative modulator of the PRC2 complex. Embo J. 2013;32:1584–1597. doi: 10.1038/emboj.2013.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fan Y, Shen B, Tan M, Mu X, Qin Y, Zhang F, Liu Y. TGF-beta-induced upregulation of malat1 promotes bladder cancer metastasis by associating with suz12. Clin Cancer Res. 2014;20:1531–1541. doi: 10.1158/1078-0432.CCR-13-1455. [DOI] [PubMed] [Google Scholar]
- 19.Hirata H, Hinoda Y, Shahryari V, Deng G, Nakajima K, Tabatabai ZL, Ishii N, Dahiya R. Long noncoding RNA MALAT1 promotes aggressive renal cell carcinoma through Ezh2 and interacts with miR-205. Cancer Res. 2015 doi: 10.1158/0008-5472.CAN-14-2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaneko S, Son J, Shen SS, Reinberg D, Bonasio R. PRC2 binds active promoters and contacts nascent RNAs in embryonic stem cells. Nat Struct Mol Biol. 2013;20:1258–1264. doi: 10.1038/nsmb.2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murillo H, Huang H, Schmidt LJ, Smith DI, Tindall DJ. Role of PI3K signaling in survival and progression of LNCaP prostate cancer cells to the androgen refractory state. Endocrinology. 2001;142:4795–4805. doi: 10.1210/endo.142.11.8467. [DOI] [PubMed] [Google Scholar]
- 22.Chen S, Bohrer LR, Rai AN, Pan Y, Gan L, Zhou X, Bagchi A, Simon JA, Huang H. Cyclin-dependent kinases regulate epigenetic gene silencing through phosphorylation of EZH2. Nat Cell Biol. 2010;12:1108–1114. doi: 10.1038/ncb2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berezovska OP, Glinskii AB, Yang Z, Li XM, Hoffman RM, Glinsky GV. Essential role for activation of the Polycomb group (PcG) protein chromatin silencing pathway in metastatic prostate cancer. Cell Cycle. 2006;5:1886–1901. doi: 10.4161/cc.5.16.3222. [DOI] [PubMed] [Google Scholar]
- 24.Xie D, Gore C, Liu J, Pong RC, Mason R, Hao G, Long M, Kabbani W, Yu L, Zhang H, Chen H, Sun X, Boothman DA, Min W, Hsieh JT. Role of DAB2IP in modulating epithelial-to-mesenchymal transition and prostate cancer metastasis. Proc Natl Acad Sci U S A. 2010;107:2485–2490. doi: 10.1073/pnas.0908133107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varambally S, Dhanasekaran SM, Zhou M, Barrette TR, Kumar-Sinha C, Sanda MG, Ghosh D, Pienta KJ, Sewalt RG, Otte AP, Rubin MA, Chinnaiyan AM. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature. 2002;419:624–629. doi: 10.1038/nature01075. [DOI] [PubMed] [Google Scholar]
- 26.Grasso CS, Wu YM, Robinson DR, Cao X, Dhanasekaran SM, Khan AP, Quist MJ, Jing X, Lonigro RJ, Brenner JC, Asangani IA, Ateeq B, Chun SY, Siddiqui J, Sam L, Anstett M, et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature. 2012;487:239–243. doi: 10.1038/nature11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu K, Wu ZJ, Groner AC, He HH, Cai C, Lis RT, Wu X, Stack EC, Loda M, Liu T, Xu H, Cato L, Thornton JE, Gregory RI, Morrissey C, Vessella RL, et al. EZH2 oncogenic activity in castration-resistant prostate cancer cells is Polycomb-independent. Science. 2012;338:1465–1469. doi: 10.1126/science.1227604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilusz JE, Jnbaptiste CK, Lu LY, Kuhn CD, Joshua-Tor L, Sharp PA. A triple helix stabilizes the 3′ ends of long noncoding RNAs that lack poly(A) tails. Genes Dev. 2012;26:2392–2407. doi: 10.1101/gad.204438.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu C, Yang M, Tian J, Wang X, Li Z. MALAT-1: a long non-coding RNA and its important 3′ end functional motif in colorectal cancer metastasis. Int J Oncol. 2011;39:169–175. doi: 10.3892/ijo.2011.1007. [DOI] [PubMed] [Google Scholar]
- 30.Dehm SM, Schmidt LJ, Heemers HV, Vessella RL, Tindall DJ. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res. 2008;68:5469–5477. doi: 10.1158/0008-5472.CAN-08-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25:1105–1111. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang L, Wang S, Li W. RSeQC: quality control of RNA-seq experiments. Bioinformatics. 2012;28:2184–2185. doi: 10.1093/bioinformatics/bts356. [DOI] [PubMed] [Google Scholar]
- 33.Ding L, Chen S, Liu P, Pan Y, Zhong J, Regan KM, Wang L, Yu C, Rizzardi A, Cheng L, Zhang J, Schmechel SC, Cheville JC, Van Deursen J, Tindall DJ, Huang H. CBP Loss Cooperates with PTEN Haploinsufficiency to Drive Prostate Cancer: Implications for Epigenetic Therapy. Cancer Res. 2014;74:2050–2061. doi: 10.1158/0008-5472.CAN-13-1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


