Abstract
Background
Percutaneous coronary intervention (PCI) for chronic total occlusions (CTO) is challenging and has been associated with low success rates. However, recent advancements in equipment and the flexibility to switch between multiple technical approaches during the same procedure (“hybrid” percutaneous algorithm) have dramatically increased the success of CTO PCI. We sought to compare the contemporary procedural outcomes of “hybrid” CTO with previously published CTO PCI studies.
Methods
The procedural outcomes of 497 consecutive CTO PCIs performed between January 2012 and August 2013 at 5 high-volume centers in the United States were compared with the pooled success and complication rates reported in 39 prior CTO PCI series that included ≥100 patients and were published after 2000.
Results
The baseline clinical and angiographic characteristics of the study patients were comparable to those of previous studies. Technical and procedural success was achieved in 455 (91.5%) and 451 (90.7%) cases, respectively and were significantly higher than the pooled technical and procedural success rates from prior studies (76.5%, p<0.001 and 75.2%, p<0.001 respectively). Major procedural complications occurred in 9/497 (1.8%) patients overall and included death (2 patients), acute myocardial infarction (5 patients) repeat target vessel PCI (1 patient) and tamponade requiring pericardiocentesis (2 patients). The incidence of major complications was similar to that of prior studies (pooled rate 2.0%, p=0.72).
Conclusions
Use of the “hybrid” approach to CTO PCI is associated with higher success and similar complication rates compared to prior studies, supporting its expanded use for treating these challenging lesions.
Introduction
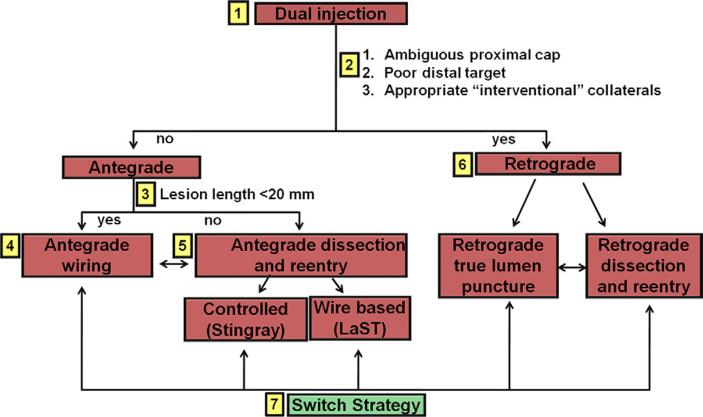
Percutaneous coronary interventions (PCI) of chronic total occlusions (CTO) have traditionally been limited by relatively low success rate,1 mainly due to failure to cross the occlusion with a guidewire.2 In the past decade several techniques have been developed to improve CTO PCI outcomes, such as the retrograde approach 3-5 and antegrade dissection and re-entry.6,7 However, most CTO operators have limited themselves to a single crossing approach per procedure mainly due to contrast and radiation exposure limitations. More recently the “hybrid” approach to CTO PCI (Figure 1) was described, which assesses the angiographic characteristics of the occlusion to provide a standardized and reproducible method for crossing CTOs. 8-12 The “hybrid” algorithm uses all available techniques (antegrade, retrograde, true-to-true lumen crossing or re-entry) tailored to the specific case in the most safe, effective, and efficient way.8-10 As a result, it provides the operator with the flexibility to apply multiple modes of intervention during the same procedure increasing chances of successful revascularization and sparing the need for subsequent hospitalization for a repeat procedure. The goal of the present study was to examine contemporary outcomes with the “hybrid” approach to CTO PCI and compare them to those reported in prior published studies. We hypothesized that the “hybrid” approach CTO PCI would be associated with higher technical and procedural success rates and similar periprocedural major complications.
Figure 1.
Overview of the “hybrid” CTO crossing algorithm. The algorithm starts with dual coronary injection (box 1) to allow assessment of several angiographic parameters (box 2) and allow selection of a primary antegrade (boxes 3 to 5) or primary retrograde (box 6) strategy. Strategy changes are made (box 7), depending on the progress of the case. CTO, chronic total occlusion; LaST, limited antegrade subintimal tracking. Modified with permission from 8.
Methods
“Hybrid” CTO PCI patients
We collected the clinical and angiographic characteristics and procedural outcomes of patients undergoing hybrid CTO PCI between January 2012 and August 2013 at 5 high-volume CTO PCI centers in the United States: Appleton Cardiology, Appleton Wisconsin; Piedmont Heart Institute, Atlanta Georgia; St. Joseph Medical Center, Bellingham Washington; St. Luke's Health System's Mid-America Heart Institute, Kansas City, Missouri; and VA North Texas Healthcare System, Dallas, Texas. A single operator performed all CTO procedures in 2 centers (Appleton Cardiology, St. Joseph Medical Center), whereas in the other centers CTO procedures were performed by high volume operators or operators who worked with a high volume operator. Data from 497 CTO procedures were collected both prospectively and retrospectively using a dedicated centralized database (PROGRESS CTO, Clinicaltrials.gov Identifier: NCT02061436). The study was approved by each center's Institutional Review Board.
All procedures were performed by operators with significant expertise in CTO PCI using the “hybrid” approach. The first step in the “hybrid” algorithm is the performance of dual injection that is used to assess 4 key angiographic characteristics: (1) proximal cap ambiguity; (2) quality of the vessel distal to the occlusion; (3) lesion length; and (4) presence of adequate collateral vessels allowing adequate crossing strategy selection. Initial antegrade wire escalation is favored for <20 mm long lesions, whereas antegrade dissection and re-entry is favored for ≥20 mm long lesions. An initial retrograde (primary retrograde) approach is favored for lesions with ambiguous proximal cap, diffuse distal disease and bifurcation at the distal cap, when appropriate collateral vessels are present. Early change of crossing strategy is recommended if the initially selected crossing strategy is unsuccessful or if no significant progress is achieved within a short period of time.8-12
Literature review
We performed a comprehensive search of the Pubmed and Cochrane Library databases for manuscripts on CTO PCI. Bibliographies of the retrieved studies were searched by hand for other relevant studies. Human studies in English published between January 2000 and August 2013 were included if they reported technical or procedural success and complication rates from ≥100 consecutive CTO PCI cases. Series that included non-consecutive CTO PCI cases based on the use of a specialized technique (such as retrograde or dissection/reentry only) were excluded. Review articles, letters to the editor, case reports, and studies in which procedural complications could not be accurately assessed from the published manuscript were also excluded. A list of the included studies is shown in Supplemental Table 1. The pooled technical and procedural success and complication rates were calculated from the above studies in accordance to the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines.13 Baseline clinical and angiographic characteristics as well as CTO PCI efficiency data (fluoroscopy times, radiation exposure and contrast administration) could not be pooled due to the lack of raw data. The authors of the present manuscript developed the “hybrid approach” and have published all related literature to date, hence we are fairly certain that the “hybrid approach” was not used in prior reports. Moreover, each published study was carefully evaluated to ascertain that the hybrid approach was not used.
Definitions
CTOs were defined as coronary obstructions with Thrombolysis in Myocardial Infarction (TIMI) flow grade 0 of at least 3 months’ duration. Estimation of the occlusion duration was based on first onset of anginal symptoms, prior history of myocardial infarction in the target vessel territory, or comparison with a prior angiogram. Technical success was defined as angiographic evidence of <30% residual stenosis with restoration of TIMI 3 antegrade flow in the CTO target vessel.14 Procedural success was defined as technical success with no procedural major adverse cardiac effects (MACE), including death, Q-wave myocardial infarction (troponin or creatine kinase leak was not classified as a major complication as it often occurs transiently post CTO PCI and resolves spontaneously), recurrent cardiac symptoms requiring repeat target vessel PCI or coronary artery bypass surgery (CABG), cardiac tamponade requiring pericardiocentesis or surgery and stroke before hospital discharge. Major bleeding was defined as bleeding causing hemoglobin drop ≥ 3g/dL or bleeding requiring transfusion or surgical intervention. Vascular access complications included major bleeding from the access site (see above) or other complication requiring surgical intervention.
Statistical analysis
Continuous data were summarized as mean ± standard deviation (normally distributed data) or median and interquartile range (non-normally distributed data) and compared using t-test or Wilcoxon rank-sum test, as appropriate. Categorical data were presented as frequencies or percentages and compared using chi square or Fisher's exact test, as appropriate. A two sided p value of <0.05 was considered statistically significant. Analyses were performed using JMP version 9.0 (SAS Institute, Cary, North Carolina).
Results
Clinical and angiographic characteristics
Between January 2012 and August 2013 497 patients underwent hybrid CTO PCI at the 5 participating centers. The clinical and baseline angiographic characteristics are presented in Table 1. Mean age was 64.7 ± 9.9 years and most (87%) of patients were men with high frequency of diabetes mellitus (42%), prior myocardial infarction (37%) and prior CABG (36%). All CTO target vessels (except one in a saphenous vein graft) were in a native coronary vessel. A prior CTO PCI attempt was performed in 18% of cases. The target CTO vessel was the right coronary artery in 61% of patients, followed by the left descending (21%) and left circumflex artery (13%). The median visually estimated CTO occlusion length was 30 (interquartile range 22 to 55) mm and CTO reference vessel diameter was 2.8 ± 0.5 mm. Median occlusion duration was 21 (interquartile range 3 to 72) months.
Table 1.
Clinical and Angiographic Characteristics of the study patients
| Age (yrs) | 64.7 ± 9.9 |
| >75 (%) | 14 |
| Men (%) | 87 |
| CTO duration | |
| Known by prior angiography (%) | 31 |
| Estimated based on clinical history (%) | 69 |
| CTO lesion age based on angiography (months) | 21 (3-72) |
| Diabetes mellitus (%) | 42 |
| Dyslipidemia (%) | 95 |
| Hypertension (%) | 91 |
| Current or recent (within 1 year) smoking (%) | 40 |
| Prior myocardial infarction (%) | 37 |
| Prior PCI (%) | 61 |
| Prior CABG (%) | 36 |
| Prior valve surgery (%) | 3 |
| LVEF (%) | 55 (45-60) |
| <40 (%) | 19 |
| CTO vessel | |
| LAD (%) | 21 |
| LCX (%) | 13 |
| RCA (%) | 61 |
| Others (%) | 6 |
| Severe calcification (≥50% reference lesion diameter) (%) | 15 |
| Severe proximal tortuosity (2 bends >90 deg or 1 bend>120 deg) (%) | 6 |
| CTO occlusion length (mm) | 30 (22-55) |
| CTO reference vessel diameter (mm) | 2.8 ± 0.5 |
| Prior attempt to open CTO (%) | 18 |
| In-stent CTO (%) | 12 |
| J-CTO score | 2.7 ± 1.2 |
Values are mean ± standard deviation or median (interquartile range)
LAD, left anterior descending artery; LCX, left circumflex artery; RCA, right coronary artery.
Procedural techniques and outcomes
Technical success and procedural success were 455/497 (91.5%) and 451/497 (90.7%), respectively (Table 2). The final successful CTO crossing strategy was antegrade in 41%, retrograde in 32% and antegrade dissection/re-entry in 28%. The retrograde approach was used in 218 patients (44%) with 86% technical and 85% procedural success and 3.2% incidence of MACE. Stents were implanted in 98% of successful cases, mainly drug eluting stents (DES, 99.8%). Median contrast volume and fluoroscopy time were 260 (195-375) ml and 41 (26-67) min, respectively. Radial access (unilateral or bilateral) was used in 18% of the CTO PCI cases.
Table 2.
Procedural characteristics and outcomes of the study patients
| Technical success (%) | 91.5 |
| Procedural success (%) | 90.7 |
| Radial Access (unilateral or bilateral) (%) | 18 |
| Successful crossing strategy (%) | |
| Antegrade wiring | 41 |
| Antegrade dissection and re-entry | 28 |
| Retrograde | 32 |
| Stenting in successful cases (%) | 98 |
| Total stents implanted (N) | 1,117 |
| DES (%) | 99.8 |
| BMS (%) | 0.2 |
| Stents per patient (N) (mean ± standard deviation) | 2.6 ± 1.1 |
| Fluoroscopic time (min) | 41 (26-67) |
| Contrast (ml) | 260 (195-375) |
| Air Kerma Radiation dose (Gray) | 3.8 (2.2-5.9) |
| Dose Area Product Fluoroscopy dose (Gray-cm2) | 267 (152-413) |
| Total procedural time (min) | 108 (75-158) |
| MACE (%) | 1.8 |
| Death (%) | 0.4 |
| Acute myocardial infarction (%) | 1.0 |
| Urgent repeat PCI on target vessel (%) | 0.2 |
| Cardiac Tamponade, requiring pericardiocentesis (%) | 0.4 |
Values are mean ± standard deviation or median (interquartile range)
DES, drug-eluting stents; BMS: bare metal stent; MACE, major adverse cardiac effects; PCI, percutaneous coronary intervention.
MACE occurred in 8 patients (1.8%), as follows: death (2 patients), acute myocardial infarction (5 patients), urgent target vessel revascularization with PCI (1 patient) and cardiac tamponade requiring pericardiocentesis (2 patients). No patient experienced a stroke and no patient required emergency CABG. Perforation (not resulting in MACE) was the most common procedural adverse effect (3.2%), followed by dissection (2.4%) and vascular access complications (1.6%).
Comparison with prior studies
Database and bibliography search retrieved 448 publications, of which 409 were excluded because they did not directly report success and complication rates or only studied a specific interventional approach (such as retrograde), thus leaving 39 studies that were included in the present analysis. Overall success ranged between 54.3% and 88.9% (Supplemental Table 1).
The technical and procedural success rate in our study were significantly higher than the pooled success rates reported in prior studies (technical success 91.5% vs. 76.5%, p<0.001 and procedural success 90.7% vs 75.2%, p<0.001). Technical success continued to be higher, even when analyses were limited to the most recent studies published between 2010 and 2013 (91.5% vs. 76.4%, p<0.001).
Complication rates from previous studies were similar to our study (Table 3): MACE occurred in 2.0% of patients, 0.6% of patients had Q-wave myocardial infarction, 0.3% required emergent CABG and 1.2% had a stroke. Perforations occurred in 2.7% of the lesions and were the most common procedural adverse effect.
Table 3.
Comparison of procedural complications between the present study and previously published CTO PCI cases.
| Variable | Present study n/N (%) | Other studies n/N (%) | P |
|---|---|---|---|
| Death | 2/497 (0.4%) | 77/18,536 (0.4%) | 0.96 |
| MACE | 9/497 (1.8%) | 216/10,555 (2.0%) | 0.72 |
| Q-wave MI | 5/497 (1.0%) | 91/14,772 (0.6%) | 0.28 |
| Emergency CABG | 0 | 45/17,003 (0.3%) | 0.25 |
| Cerebrovascular accident | 0 | 21/18,364 (0.1%) | 0.45 |
| Perforation, per lesion | 16/497 (3.2%) | 382/14,097 (2.7%) | 0.49 |
| Cardiac tamponade | 2/497 (0.4%) | 65/12,955 (0.5%) | 0.76 |
| Bleeding | 3/497 (0.6%) | 28/3,735 (0.7%) | 0.72 |
MACE, major adverse cardiac events; MI, myocardial infarction; CABG, coronary artery bypass graft surgery.
Discussion
The main finding of our multicenter registry are that the “hybrid” approach to CTO PCI is associated with significantly higher success and similar complication rates compared to published CTO PCI series.
The “hybrid” approach was developed through the combined experiences of high volume North American CTO PCI operators aiming to open the occluded vessel, using all feasible techniques (antegrade, retrograde, true-to-true lumen crossing or re-entry) in the most safe, effective, and efficient way.8 The basic underlying principle of the “hybrid” approach is that no single procedural crossing strategy should be pursued to exhaustion, but an alternative strategy should be attempted if a given crossing strategy does not progress.10 Hence, the “hybrid” CTO PCI strategy places emphasis not only on procedural success, but also on procedural efficiency and forms the basis of contemporary CTO program development.15 The optimal techniques and technologies are applied during the specific time of the procedure when they are most likely to be effective. The practical ramifications of this method are that changes of strategy should occur very early, and often cycle rapidly, to maximize the likelihood of early successful crossing.
To the best of our knowledge this is the first published series of the “hybrid” approach to CTO PCI. A “hybrid registry” of 144 cases performed at CTO PCI workshops between January 2011 and October 2012 was presented at the 2013 CTO Summit (New York, New York) demonstrating 94% procedural success, although lesion complexity was high (average J-CTO score was 2.3 and 46% of lesions had a J-CTO score >3). Similar to that registry our technical and procedural success rates were 91.5% and 90.7%, respectively.
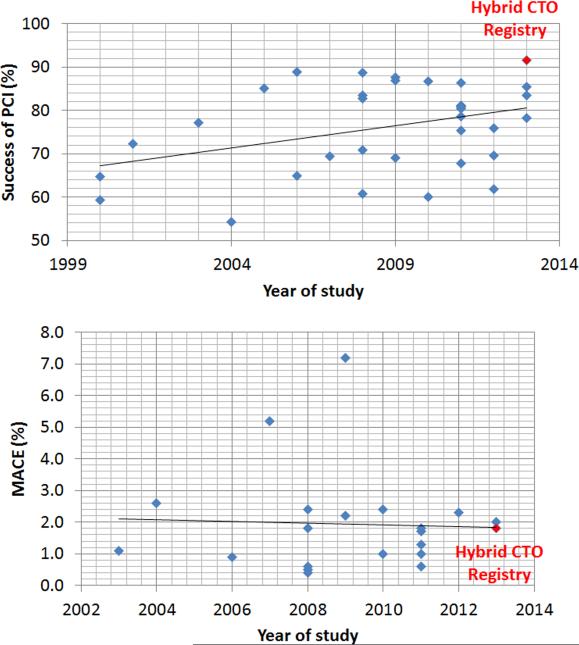
This is the first all comer publication reporting >90% success rates in CTO PCI (Supplemental Table 1 and Figure 1). Such high success rates have thus far only been reported for retrograde CTO PCI series in Japan. Kimura et al reported technical and procedural success of 92.4% and 90.6%, respectively among 224 patients treated with the retrograde approach in 43 centers.16 Tsuchikane et al reported 98.9% success (in 92 of 93 cases) in a two-operator series.17 Rathore et al reported 100% success among 31 patients treated with intravascular ultrasound-guided reverse controlled antegrade and retrograde tracking and dissection (CART).18 Our results extend the high success rates to an unselected, highly complex CTO PCI population with very high frequency of prior coronary artery bypass graft surgery (35%), which has been associated with lower CTO PCI success rates.19
The prior collective experience of many of the centers participating in the current registry was recently published, showing 85.4% technical success among 1,361 patients that were treated before the routine application of the “hybrid” approach.20 Hence, although overall CTO PCI success rates have been modestly increasing over time (Figure 1), use of the “hybrid” approach to CTO PCI has resulted in a significant and clinically important increase in procedural success. Importantly, this was achieved without incurring a penalty in terms of procedural complications.
Our study has important limitations. It was observational without independent review of the coronary angiograms by an angiographic core laboratory and without adjudication of the clinical outcomes by a clinical events committee. However, adjudication would be unlikely to affect reporting of death, urgent repeat revascularization and the need for pericardiocentesis. Serial cardiac biomarker measurements were not performed and only Q wave myocardial infarction was recorded as part of the study. All participating centers have established CTO PCI programs15 with expertise in all crossing techniques that are part of the “hybrid” algorithm, hence the outcomes observed in the study may not be achievable by less experienced centers. Furthermore, operator experience can significantly increase success rates of CTO PCI and this relation has been previously described.4 Finally, long-term clinical or angiographic follow-up was not performed.
Our findings have important implications for everyday clinical practice. First, they suggest that the efficacy of CTO PCI is has significantly improved, hence the presence of a CTO may not necessarily need to be linked with lower level recommendation for PCI in the appropriateness use criteria for coronary revascularization.21 Second they suggest that such results can be achieved across various hospitals and operators. Third, they demonstrate that high success can be achieved without incurring more complications,1 which is important for an elective procedure, such as CTO PCI.
Supplementary Material
Figure 2.
CTO PCI technical success and complication rates among studies published between 2000 and 2013.
MACE: Major Adverse Cardiac Effects
Acknowledgement
Data collection was performed using REDCap, which is supported by CTSA NIH Grant UL1-RR024982.
Footnotes
Conflict of Interest:
Dr. Christopoulos: none
Mr Menon: none
Dr. Karmpaliotis: speaker bureau, Abbott Vascular and Medtronic; consultant, Bridgepoint Medical.
Dr. Alaswad: consulting fees from Terumo and Boston Scientific; consultant, no financial, Abbott Laboratories.
Dr. Lombardi: equity with Bridgepoint Medical
Dr. Grantham: Speaking fees, consulting, and honoraria from Boston Scientific, Asahi Intecc. Research grants from Boston Scientific, Asahi Intecc, Abbott Vascular, Medtronic.
Dr. Patel: none
Dr. Rangan: none
Dr. Kotsia: none
Dr. Lembo: speaker bureau: Medtronic; advisory board Abbott Vascular and Medtronic.
Dr. Kandzari: research/grant support and consulting honoraria from Boston Scientific and Medtronic Cardiovascular, and research/grant support from Abbott.
Dr. Lee: none.
Dr. Kalynych: none.
Dr. Carlson: none.
Dr. Garcia: consulting fees from Medtronic
Dr. Banerjee: research grants from Gilead and the Medicines Company; consultant/speaker honoraria from Covidien and Medtronic; ownership in MDCARE Global (spouse); intellectual property in HygeiaTel.
Dr. Thompson: consultant for Abbott Vascular, Bridgepoint, Terumo, Volcano; equity Bridgepoint Medical
Dr. Brilakis: consulting honoraria/speaker fees from Sanofi, Janssen, St Jude Medical, Terumo, Asahi, Abbott Vascular, and Boston Scientific; research grant from Guerbet; spouse is an employee of Medtronic.
References
- 1.Patel VG, Brayton KM, Tamayo A, et al. Angiographic success and procedural complications in patients undergoing percutaneous coronary chronic total occlusion interventions: a weighted meta-analysis of 18,061 patients from 65 studies. JACC Cardiovasc Interv. 2013;6:128–36. doi: 10.1016/j.jcin.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 2.Garcia S, Abdullah S, Banerjee S, Brilakis ES. Chronic total occlusions: patient selection and overview of advanced techniques. Curr Cardiol Rep. 2013;15:334. doi: 10.1007/s11886-012-0334-2. [DOI] [PubMed] [Google Scholar]
- 3.Brilakis ES, Grantham JA, Thompson CA, et al. The retrograde approach to coronary artery chronic total occlusions: a practical approach. Catheter Cardiovasc Interv. 2012;79:3–19. doi: 10.1002/ccd.23004. [DOI] [PubMed] [Google Scholar]
- 4.Thompson CA, Jayne JE, Robb JF, et al. Retrograde techniques and the impact of operator volume on percutaneous intervention for coronary chronic total occlusions an early U.S. experience. JACC Cardiovasc Interv. 2009;2:834–42. doi: 10.1016/j.jcin.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 5.Karmpaliotis D, Michael TT, Brilakis ES, et al. Retrograde coronary chronic total occlusion revascularization: procedural and in-hospital outcomes from a multicenter registry in the United States. JACC Cardiovasc Interv. 2012;5:1273–9. doi: 10.1016/j.jcin.2012.06.025. [DOI] [PubMed] [Google Scholar]
- 6.Michael TT, Papayannis AC, Banerjee S, Brilakis ES. Subintimal dissection/reentry strategies in coronary chronic total occlusion interventions. Circ Cardiovasc Interv. 2012;5:729–38. doi: 10.1161/CIRCINTERVENTIONS.112.969808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whitlow PL, Burke MN, Lombardi WL, et al. Use of a novel crossing and re-entry system in coronary chronic total occlusions that have failed standard crossing techniques: results of the FAST-CTOs (Facilitated Antegrade Steering Technique in Chronic Total Occlusions) trial. JACC Cardiovasc Interv. 2012;5:393–401. doi: 10.1016/j.jcin.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 8.Brilakis ES, Grantham JA, Rinfret S, et al. A percutaneous treatment algorithm for crossing coronary chronic total occlusions. JACC Cardiovasc Interv. 2012;5:367–79. doi: 10.1016/j.jcin.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Thompson CA. The Hybrid Approach for Percutaneous Revascularization of Coronary Chronic Total Occlusions. Interventional Cardiology Clinics. 2012;1:349–53. doi: 10.1016/j.iccl.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 10.Sabbagh AE, Banerjee S, Brilakis ES. Illustration of the ‘hybrid’ approach to chronic total occlusion crossing. Interventional Cardiology. 2012;4:639–43. [Google Scholar]
- 11.Michael TT, Mogabgab O, Fuh E, et al. Application of the “hybrid approach” to chronic total occlusion interventions: a detailed procedural analysis. J Interv Cardiol. 2014;27:36–43. doi: 10.1111/joic.12083. [DOI] [PubMed] [Google Scholar]
- 12.Brilakis ES, editor. Manual of Coronary Chronic Total Occlusion Interventions. A Step-By-Step Approach. Elsevier; Waltham, MA: 2013. [Google Scholar]
- 13.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 14.Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: Executive Summary A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Journal of the American College of Cardiology. 2011;58:2550–83. doi: 10.1016/j.jacc.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 15.Karmpaliotis D, Lembo N, Kalynych A, et al. Development of a high-volume, multiple-operator program for percutaneous chronic total coronary occlusion revascularization: procedural, clinical, and cost-utilization outcomes. Catheter Cardiovasc Interv. 2013;82:1–8. doi: 10.1002/ccd.24387. [DOI] [PubMed] [Google Scholar]
- 16.Kimura M, Katoh O, Tsuchikane E, et al. The efficacy of a bilateral approach for treating lesions with chronic total occlusions the CART (controlled antegrade and retrograde subintimal tracking) registry. JACC Cardiovasc Interv. 2009;2:1135–41. doi: 10.1016/j.jcin.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 17.Tsuchikane E, Katoh O, Kimura M, Nasu K, Kinoshita Y, Suzuki T. The first clinical experience with a novel catheter for collateral channel tracking in retrograde approach for chronic coronary total occlusions. JACC Cardiovasc Interv. 2010;3:165–71. doi: 10.1016/j.jcin.2009.10.026. [DOI] [PubMed] [Google Scholar]
- 18.Rathore S, Katoh O, Tuschikane E, Oida A, Suzuki T, Takase S. A novel modification of the retrograde approach for the recanalization of chronic total occlusion of the coronary arteries intravascular ultrasound-guided reverse controlled antegrade and retrograde tracking. JACC Cardiovasc Interv. 2010;3:155–64. doi: 10.1016/j.jcin.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 19.Michael TT, Karmpaliotis D, Brilakis ES, et al. Impact of prior coronary artery bypass graft surgery on chronic total occlusion revascularisation: insights from a multicentre US registry. Heart. 2013;99:1515–8. doi: 10.1136/heartjnl-2013-303763. [DOI] [PubMed] [Google Scholar]
- 20.Michael TT, Karmpaliotis D, Brilakis ES, et al. Procedural Outcomes of Revascularization of Chronic Total Occlusion of Native Coronary Arteries (from a Multicenter United States Registry). Am J Cardiol. 2013;112:488–92. doi: 10.1016/j.amjcard.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Patel MR, Dehmer GJ, Hirshfeld JW, Smith PK, Spertus JA. ACCF/SCAI/STS/AATS/AHA/ASNC/HFSA/SCCT 2012 Appropriate use criteria for coronary revascularization focused update: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons, American Association for Thoracic Surgery, American Heart Association, American Society of Nuclear Cardiology, and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol. 2012;59:857–81. doi: 10.1016/j.jacc.2011.12.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.