Abstract
Patient adherence to treatment in chronic obstructive pulmonary disease (COPD) is essential to optimise disease management. As with other chronic diseases, poor adherence is common and results in increased rates of morbidity, healthcare expenditures, hospitalisations and possibly mortality, as well as unnecessary escalation of therapy and reduced quality of life. Examples include overuse, underuse, and alteration of schedule and doses of medication, continued smoking and lack of exercise. Adherence is affected by patients’ perception of their disease, type of treatment or medication, the quality of patient provider communication and the social environment. Patients are more likely to adhere to treatment when they believe it will improve disease management or control, or anticipate serious consequences related to non-adherence. Providers play a critical role in helping patients understand the nature of the disease, potential benefits of treatment, addressing concerns regarding potential adverse effects and events, and encouraging patients to develop self-management skills. For clinicians, it is important to explore patients’ beliefs and concerns about the safety and benefits of the treatment, as many patients harbour unspoken fears. Complex regimens and polytherapy also contribute to suboptimal adherence. This review addresses adherence related issues in COPD, assesses current efforts to improve adherence and highlights opportunities to improve adherence for both providers and patients.
Chronic obstructive pulmonary disease (COPD) is currently the fourth leading cause of death worldwide, with an overall prevalence rate between 4% and 10%.1–3 A recent worldwide study showed higher prevalence and more advanced staging of spirometrically confirmed COPD than had typically been reported.4 Furthermore, the prevalence of COPD is increasing and it is estimated that by the year 2020, COPD will be the third leading cause of death worldwide. The debilitating nature of this highly prevalent disease results in substantial social and economic burdens, including direct and indirect medical as well as other costs.5,6
Although not fully reversible, COPD is treatable. Smoking cessation is the most effective means to slow disease progression; however, long term smoking cessation success rates are low (<20%). Important progress has been achieved in the pharmacological and non-pharmacological treatment of COPD.7–10 While a major goal of therapy is to provide symptom relief, effective management of COPD has been shown to reduce the rate of exacerbations, hospitalisations, possibly mortality and to improve health related quality of life.11 As health professionals, we are responsible for ensuring that our diagnosis is accurate, the prescribed treatment is appropriate and that treatment is likely to do more good than harm. However, the effectiveness of the treatment also relies on patients’ agreement to adhere to the therapeutic regimen.
As with all chronic diseases, non-adherence in patients with COPD is common and contributes to adverse health outcomes, reduced quality of life and increased healthcare expenditures.12 According to the WHO, patient adherence to long term therapy in chronic diseases averages 50% in developed countries.13 Levels of adherence to prescribed treatment in COPD are correspondingly low.14,15 The waste and excess costs associated with non-adherence were estimated to be as high as US$300 billion/year.12 Thus factors that can facilitate patient adherence should be considered when selecting the appropriate treatment.
This review will provide a general overview of adherence related issues in COPD, including the relationship between adherence and the concepts of compliance and concordance. Methods of measuring adherence, factors that influence adherence and specific examples of non-adherence in COPD will be addressed. Finally, we will assess current efforts to improve adherence in patients with COPD and highlight opportunities to improve adherence for both providers and patients.
ADHERENCE: AN OVERVIEW
Compliance, adherence and concordance
Traditionally, compliance has been the term used to assess the extent to which a patient undertakes a treatment regimen and follows medical instructions, such as taking prescribed medication. Inherent to the definition of compliance is the idea that a patient is a passive, acquiescent recipient of expert medical advice with which they should comply. Poor compliance was often viewed as patients choosing to defy their doctors’ orders. However, patients are increasingly viewed as active collaborators in their own health management. A more patient centred perception of medical treatment has emerged, which acknowledges the patient’s role in medical decision making and describes the relationship between a patient and healthcare provider as a partnership.13
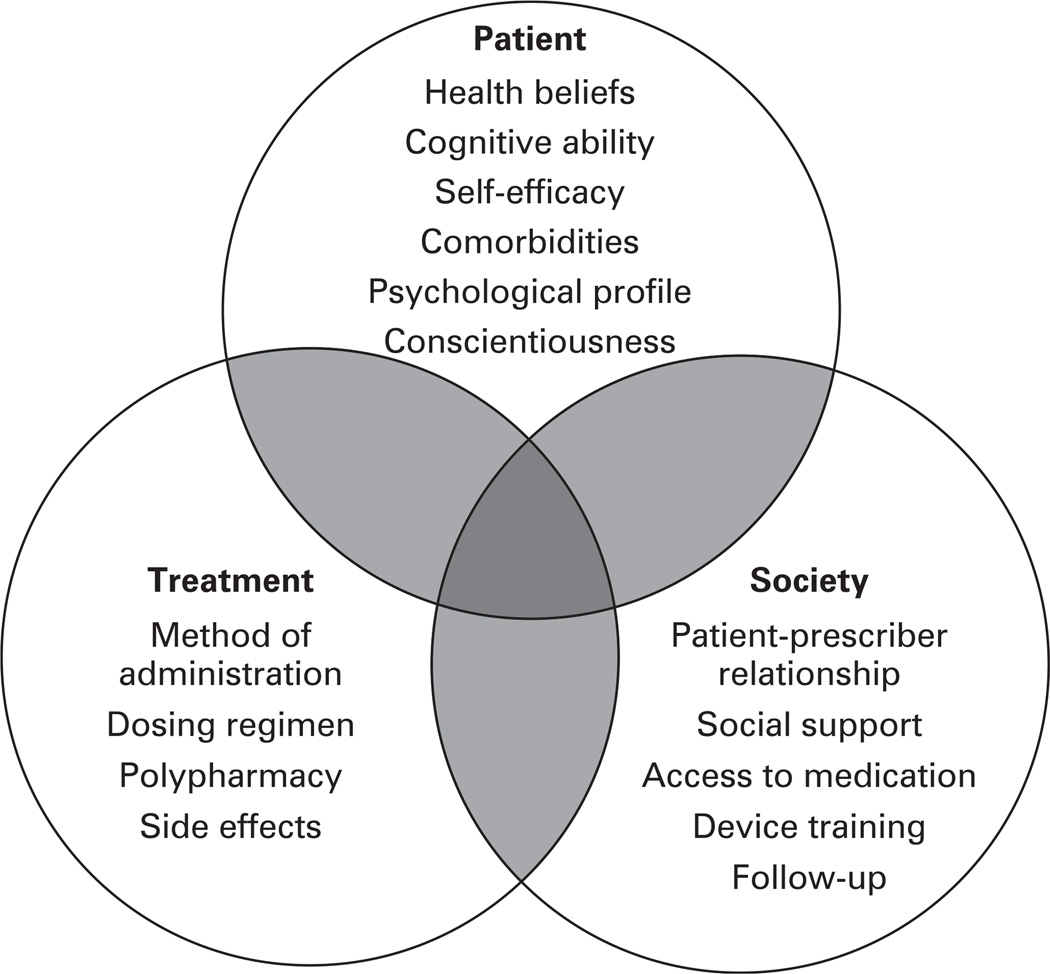
The term adherence, which initially referred solely to adherence to a treatment regimen, has evolved to reflect the changing perceptions of a patient’s role in their own healthcare. The primary difference between “compliance” and the preferred term of “adherence” is that the latter more accurately reflects a patient’s active role in consenting to and following prescribed treatments.13,16 In 2001, the WHO’s Adherence Project extended the definition to include “the extent to which a person’s behaviour taking medication, following a diet, and/or executing lifestyle changes, corresponds with agreed recommendations from a health care provider”.13,17 Adherence is a complex concept, influenced by multiple factors including social/environmental, patient related and treatment related factors (fig 1).
Figure 1.
Patient adherence in chronic obstructive pulmonary disease is multifactorial and is influenced by the patient, the physician and society.
Most recently, the term concordance has been used (largely in the UK) to describe the “therapeutic alliance” that exists between patients and healthcare professionals.18 Concordance is achieved when the patient and provider discuss and agree on a therapeutic course of action; it therefore encompasses the responsibility and decision making contribution of both parties and empowers the patient to play an active role in disease management. Use of the term “concordance” signifies an important shift in conceptualisation for those who suggest that respect for the patient’s agenda is a fundamental aspect of health care.
Defining adherence
There are several definitions of adherence. Examples of primary non-adherence in COPD include patients never filling prescriptions or failing to begin a prescribed exercise programme. Blais et al evaluated prescription fill rates in 3768 elderly Canadian patients with COPD and found that in 1995 slightly more than half (53.1%) filled at least one prescription for prescribed daily inhaled corticosteroids.18 Secondary forms of non-adherence include overuse, underuse, forgetfulness and alteration of schedules and doses. Ineffective inhaler technique resulting in suboptimal drug delivery also can be considered a form of non-adherence. Patients may also be non-adherent or partially adherent to healthcare behaviours such as attending follow-up visits with providers or failing to maintain health related behaviour changes over time (eg, smoking cessation, pulmonary rehabilitation). Hughes19 notes that adherence is not a dichotomous variable (ie, adherent/non-adherent). For example, a patient may take, on average, 50% of their inhaled steroids, relying instead on overuse of fast acting bronchodilators (130% adherence), while attending all scheduled clinic visits (100% adherence) but no pulmonary rehabilitation sessions (0% adherence). Furthermore, there is no universal definition of adherence. The level of adherence to therapy needed to achieve adequate health and health related quality of life outcomes remains unclear at this time. While some studies use average adherence over time, and others utilise a cut-point (commonly ≥50% or 80%) to classify patients as being adherent, it is usually not known whether this level of adherence is adequate for disease control.19,20 In addition, definitions of adherence can vary depending on whether they are outcome oriented (eg, exacerbations, hospitalisations, etc) or process oriented (eg, medication or supplemental oxygen use, number of exercise sessions attended). Rather than simply mandating compliance to physician recommendations, the goal of improving adherence in COPD should centre on maintaining clinically meaningful improvements in patient health status and reducing healthcare costs associated with loss of disease control.
Methods of assessing adherence
There are a number of ways to measure adherence, and each method has strengths and limitations. Most studies of COPD have focused on assessing medication adherence (table 1). Biochemical evaluation of drug levels can confirm ingestion of many medications. However, it is an expensive and invasive process and often reflects other factors resulting in pharmacokinetic variations.16 Other limitations include the availability of assays, limited monitoring intervals and insensitivity to inhaled medication. Electronic devices can provide objective evidence of the temporal history of medication access. For instance, patients’ pill bottles can be equipped with caps that provide a date and time record of each time the cap was removed. Cap removal suggests (but does not confirm) medication ingestion. Chronologs can be attached to metered dose inhalers to provide a record of dosing and, in some cases, even give an indication of the quality of inhaling technique. Similar devices are also available for use with nebulisers and supplemental oxygen. Electronic monitors are increasingly used in clinical trials to measure adherence. They provide accurate and reliable records of dosing21 but are expensive to use, subject to malfunction and cannot usually confirm ingestion.
Table 1.
Methods of assessing adherence
| Advantage | Disadvantage | |
|---|---|---|
| Measured directly | Confirm drug use and dose | Misleading if drug taken prior to testing |
| Biochemical* | Less subject to bias | Limited intervals that can be monitored |
| Little information regarding use over time | ||
| Insensitive to inhaled drug | ||
| Limited availability and costly | ||
| Measured indirectly | ||
| Physician’s estimate and patient self-report | Easy to obtain | Unreliable |
| Pharmacy records filling prescription | Confirm filling prescription | Need centralised pharmacy records |
| Pharmacy database can be incomplete | ||
| Pill count or inhaler weighing† | Easy to obtain | Overestimate compliance |
| Correlate well with other methods of measurement | ||
| Therapeutic outcome | Easy to obtain | Clinical outcomes can depend on other factors |
| Electronic monitoring† | Helps explain how medications were taken | Expensive and not adapted to all inhalation devices |
Measurement includes blood levels or urinary excretion of the medication, a metabolite or a marker.
It does not verify that the medicine was ingested.
In clinical settings, the easiest approach to assessing adherence is to simply query the patient. However, when compared with data obtained through electronic monitoring, studies have consistently demonstrated that self-reports are inaccurate as patients generally over report medication use.12,22 One exception is that the minority of patients who admit to being non-adherent are usually correct.23 Unfortunately, physician assessments of their patients’ adherence is similarly unreliable.24–26 Other common methods of assessment include patient diaries, pill counts, canister weighing and analysis of computerised pharmacy records. There are important limitations to each of these approaches. Pill counts are largely limited to oral medications and assess only whether the correct number of pills have been removed from the bottle with no indication of ingestion, dose or dose frequency. Likewise, canister weighing often used to monitor inhaled bronchodilator or corticosteroid use can also be deceiving. Rand and colleagues26–28 showed that dumping of ICS medication (activation of canisters or removal of pills immediately prior to a clinic visit presumably to make oneself appear adherent) occurred in as many as 30% of patients with COPD who were participating in a large multicentre clinical trial over the course of 1 year. Others have reported similar findings.29–32 Analysis of pharmacy records provide evidence of drug refill patterns but cannot assess ingestion or patterns of use. Studies comparing patient diaries, pill counts, canister weighting and pharmacy records with data obtained from electronic monitors confirm that each of these methods tends to significantly overestimate adherence and that actual adherence declines over time.33 Hence, in clinical settings, reliance on any single method of assessment can be deceitful.
Determinants of adherence
Despite years of research, few factors have been reliably associated with treatment adherence. In a meta-analysis of 50 years of adherence research in chronic diseases,12 it was found that higher levels of adherence were associated with more circumscribed regimens (ie, medication use versus health behaviour change) and with greater patient resources (social and emotional support). Factors generally not believed to influence adherence include gender, age, educational level and socioeconomic status.34 Below, we outline factors related to medications and regimens, patients and providers that may influence adherence—many of which are potentially modifiable (see table 2).
Table 2.
Factors that influence treatment adherence in chronic obstructive pulmonary disease (COPD)
| Factor | Influence on adherence |
Reference |
|---|---|---|
| Medication | ||
| Regimen | ↓ ↓ | Farmer 1999,21Sacket 1976,36Rand 2005,35Rapoff 200616 |
| Lengthier and more complicated | ||
| Polypharmacy* | ||
| Duration | ↓ | |
| Long term | ||
| Type | ↓ | |
| Not have medication direct effect | ||
| Administration | ↓ | Garcia-Aymerich 2000,40James 1985,38Tashkin 199534 |
| Inhaler | ||
| Cost | ↓ | Hughes 2004,19Soumerai 199143 |
| Inability to pay or reduce access | ||
| Adverse effects | ↓ | Col 199042 |
| Patient | ||
| Old age | ↑ | Rand 199528 |
| Comorbidity including anxiety and depression | ↓ | Cully 2006,46Hughes 2004,19Morris 199286 |
| Cognitive defect | ↓ | Incalzi 199744 |
| Difficulty reading | ↓ | Zuccollo 198545 |
| Perception of the disease | ↓ ↑ | DiMatteo,12Rapoff 200616 |
| Lack of regular symptom | ||
| Affect daily activities and anticipate problem | ||
| Perception or social support | ↑ | Tashkin 199534 |
| Stable family life and caregiver | ||
| Health care provider | ||
| Type of physician | ↑ | Lau 199648 |
| Specialist | ||
| Type of care and service | ↑ ↑ | Tashkin 199534 |
| Continuity of care follow-up and written supervision | ||
| Written instruction | ||
| Self-management with positive reinforcement | ↑ | Turner 1995,15Dunbar 197952 |
Polypharmacy has not always proven to be associated with decreased adherence.
Older age may be associated with greater number of comorbid conditions, cognitive defect and difficulty reading, which reduces adherence.
Medication and regimen factors
Characteristics of COPD medications and regimens can contribute to non-adherence. In general, the lengthier and more complicated the regimen, the lower the adherence.16,35,36 Frequency of dosing has been identified as one of the most important factors inversely affecting adherence.13 Patients with COPD are generally older and require medication for other medical conditions. Polypharmacy is another common and important contributor to poor adherence. A nationally representative sample of adults aged 65 years and older in the US37 found that 41% reported taking five or more prescription medications, and more than half had two or more prescribing providers. Patients taking multiple medications, each with a different dosing pattern, are often frustrated by complicated dosing regimens, which may lead to missed doses.
The route of administration can also influence adherence. Adherence is higher to oral forms of medication than to inhaled drugs of the same class (eg, β2 agonists or corticosteroids).34,38 This is an issue particularly for patients with asthma or COPD where use of inhaled medications is desirable. Errors in inhaler technique are common and interfere with adequate drug administration.39 For example, in one Spanish study of patients with COPD admitted to hospital as a result of an exacerbation, 43% of patients had inadequate inhaler technique.40
Other medication and regimen factors are also important. Adherence is lower for medications that do not have an immediate or direct effect on symptoms. Additional factors can include drug side effects.41,42 Patients may choose to discontinue a treatment to avoid unpleasant side effects, even if it provides clinically meaningful improvements in their condition. In some healthcare systems, inability to pay or reduced access through government programmes may contribute to both primary and secondary non-adherence.19,43
Patient factors
Across chronic diseases, adherence is often better in older persons, including patients with COPD.28 However, increasing age is also associated with a greater number of comorbid conditions and polypharmacy.13,22 Furthermore, both age and COPD duration are associated with cognitive decline; in turn, cognitive impairment, especially memory problems, are associated with non-adherence.44 Other problems in the elderly may include difficulty reading the small print on medication labels45 and difficulty opening containers fitted with safety devices.
Adherence is also influenced by a patient’s perception of the disease. Lack of clinical symptoms is often interpreted to mean that the disease can be treated episodically, and daily medications are discontinued.12 Adherence to treatment regimens is higher when patients believe that their daily health and activities are affected by their disease, or if they anticipate potentially serious consequences resulting from non-adherence.16 Patients often alter the prescribed dosage of a medication if they perceive it to be excessive, inconvenient or too expensive.13
Psychiatric comorbidity is also higher in patients with COPD. Up to 40% are clinically depressed; a similar number also experience moderate to high levels of anxiety.46 Both depression and anxiety result in lower health status and greater functional impairments. Depression has been independently linked with lower adherence.47 Perceived and actual social support are also important factors. A stable family life with caregivers who provide support and encouragement is associated with improved adherence.34
Healthcare provider and caregiver factors
Healthcare providers and caregivers contribute to patients’ perception of their disease, healthcare and medication and, ultimately, their adherence. For instance, adherence to medication has been reported to increase if the prescribing physician is a specialist compared with a general practitioner.48 Continuity of care, explanation of the rationale underlying prescribed treatments, written instructions on medication dosing regimens, follow-up supervision and participation in self-management programmes have all been shown to improve patient adherence.34
However, there is often a lack of communication between providers and patients. The quality of communication between patients and providers also influences adherence to therapy. Adherence improves when patients report better overall communication with their providers.18 Specific information about each medication prescribed is also essential to enhancing secondary non-adherence related to misunderstandings. In the study of non-adherence in older adults previously mentioned, nearly one-third reported not having talked to their doctor about all of their different medicines in the past 12 months.37 Other studies suggest that 29–39% of patients report receiving no instruction from their providers on how to take new medications while up to 62% receive only dosing directions.49,50
While these studies relied on patient recall, there is also objective evidence that patients receive suboptimal counselling, especially when starting new medications. Tarn et al51 recorded and reviewed 185 outpatient encounters between 44 family and specialty physicians who were prescribing new medications for their patients. Communication was rated using a five point index that gave points for providing medication name, purpose, duration, adverse effects, dose and frequency. On average, physicians addressed 3.1 of the 5 key elements. Directions for dosing were provided less than 60% of the time and patients were informed of the duration of use, adverse effects or adverse events only one-third of the time. Providing more information (eg, purpose of medication, how and when it should be taken, potential side effects) about medications improves adherence,19,20 partly because it reduces the likelihood that patients will not understand how to take their medications. Setting appropriate expectations for medication effects such as time to action (immediate versus delayed), effect on symptoms such as dyspnoea and how you will judge if it is working as planned can also improve adherence.
Positive reinforcement of the importance of therapeutic regimens was found to improve or correlate with adherence.15,52 For example, patients with COPD requiring supplemental oxygen therapy are frequently embarrassed to be seen in public with an oxygen cylinder, leading to an insufficient number of hours on therapy.53,54 In these patients, support and positive reinforcement of adherence from the therapeutic team is particularly important.
PATIENT ADHERENCE IN THE TREATMENT OF COPD
Poor adherence in patients with COPD is a significant concern. Medication adherence by patients with COPD is generally poor, with reports citing adherence rates to various treatment regimens of approximately 50%.39 In a study of adherence in patients with COPD, 31% of patients consciously decided to forego administration of their medication if they were “feeling good.”55 In this study, forgetting or deciding not to take a dose was reported as the most frequent cause of non-adherence.29 Conversely, these patients reported overusing medication during periods of respiratory distress. Additional factors contributing to non-adherence included interruptions or changes in normal routines, adverse side effects, running out of medication and polypharmacy with complex dosing regimens.
Patients with COPD often have comorbidities and are faced with complex therapeutic regimens—all factors associated with lower adherence. They are often prescribed a combination of medications and oxygen therapy in addition to other medications that might be required to treat comorbidities. For example, Dolce et al55 reported that patients with COPD were prescribed an average of 6.3 medications. The polypharmacy and the need for multiple daily doses involved in treating COPD alone lead to complex dosing regimens. Furthermore, because COPD is commonly seen in older patient populations, previously described issues of adherence in the elderly are also relevant to many in COPD.56
Non-adherence to medication in COPD
Several studies were written at a time when most of the drugs currently used for maintenance therapy in COPD were not available. An early study of compliance to a prophylactic short acting inhaled bronchodilator compared with placebo in 95 patients with COPD used an electronic monitoring device to objectively measure drug use patterns.27 Only 15% of patients used their bronchodilator according to the prescribed dosing frequency and number of puffs. Although adherence rates in clinical trials may be as high as 70–90%,28,57,58 in clinical practice it is in the range of 10–40%.59–61 Simplification of dosing regimens by reducing dose frequencies has been associated with an increase in medication adherence in chronic disease.62 Recently, this has been confirmed with the use of a long acting anticholinergic once a day in patients with COPD.63
Turner et al15 examined sociodemographic, physiological and health status in patients with COPD with prebronchodilator FEV1 <60% predicted to identify characteristics associated with adherence to long term home nebuliser therapy. Overall, 49.4% of 985 patients were determined to be non-adherent to home nebuliser/intermittent positive pressure breathing therapy (<25 min/day).15 Adherent and non-adherent patients reported 3.5 (9.9) min and 11.6 (10.3) min more of therapy than was recorded by the meter, respectively (p<0.0001). In addition, non-adherent patients were more likely to drop out of the study, miss home and clinic visits, and adhere less to prescribed oral theophylline regimens.15
In this same study, compared with non-adherent patients, those who used their nebuliser as prescribed were more likely to report that treatment helped them substantially (73% vs 61%; p=0.012) and view their physician as being supportive (62% vs 52%; p=0.047).15 Overall, adherent patients were older, better educated and had a stable lifestyle. Significant predictors of adherence included white race (deemed reflective of stability/healthcare beliefs), married, abstinent from cigarettes and alcohol, serum theophylline levels ≥9 µg/ml, severe dyspnoea and lower FEV1 (p<0.05).10 Also, adherent patients had lower pre- and post-bronchodilator FEV1 and dyspnoea, suggesting that perception of their disease may influence adherence.
Another study by Corden et al14 in patients with COPD reported nebuliser compliance rates of 44%, based on taking >60% of prescribed treatment. At the end of 4 weeks, compliance was negatively correlated with health status total score, suggesting that poor compliance leads to greater impairment in health status.14 Furthermore, compliance was not related to demographic characteristics (age, sex, socioeconomic status, marital or employment status), disease duration or dosing frequency. Differences in adherence related characteristics reported by the two studies are likely affected by the use of different cut-points for adherence (median cut off versus >60% of prescribed treatment) as well as the fact that patients in the Turner et al study were aware that their adherence was being monitored while those in the Corden et al study were not.14,15 Although these two study results are important, conclusions should be drawn with caution as regular nebulised therapy is a cumbersome and expensive treatment, not advocated in current COPD guidelines.
Another treatment that could be of importance for some patients with COPD is long term oxygen therapy. In a study of patients with COPD with documented hypoxia who were prescribed oxygen therapy for an average of 16 h/day, less than half (45%) used their oxygen ≥15 h/day and 30% used oxygen for <80% of the prescribed duration.53 In a recent randomised double blind, crossover trial64 with a comparison of ambulatory oxygen versus ambulatory compressed air, patients used very little ambulatory oxygen. They went out of their home three times more often without cylinders than with oxygen or air cylinders. Liquid oxygen could be a more efficient way to deliver oxygen in the context of ambulation. In another study of patients who had been prescribed liquid oxygen, only 77% reported using liquid oxygen outdoors, and 23% refused to use liquid oxygen away from home.54 Patients reported feeling ashamed to use oxygen in public, and had a significantly lower life satisfaction according to the Freiburg Personality (FPI-R) questionnaire (p<0.05).
Suboptimal adherence to non-drug therapy in COPD
Adherence to therapeutic interventions, including a healthy lifestyle and regular exercise programme, are crucial health behaviour in the management of chronic respiratory disease. Most of the studies have focused on the short term benefits of pulmonary rehabilitation10,65,66 without addressing the challenges of long term adherence to exercise. Limited studies available suggest that the benefits of pulmonary rehabilitation regress towards baseline values after 6–12 months.67–69 However, in some studies benefits appear to be sustained in the absence of any specific maintenance therapy, suggesting that the desired change in lifestyle probably became relatively entrenched.
Although not specific to patients with chronic respiratory disease, valuable knowledge comes from studies of the elderly.70,71 Exercise self-efficacy and expected benefits from regular exercise have been shown to be predictors of exercise adherence while depression is associated with poor adherence. In a review of 27 cross sectional and 14 longitudinal studies of individuals 65 years or older, educational level and past exercise behaviour were positively associated with regular exercise.71 Conversely, perceiving one’s health as “poor” was the biggest barrier to exercise adoption and maintenance. Similar results were reported in a qualitative study of patients with COPD72 where barriers to lifestyle change included progression of COPD and associated comorbid conditions. A recent study of patients with COPD following pulmonary rehabilitation reported the most consistent barriers to adherence were chest infection and disease exacerbation.73
Strategies to enhance adherence
Although relatively few studies have evaluated interventions to target adherence to COPD therapy, several important behavioural findings have emerged from the literature. Since dose frequency is inversely related to adherence, once or twice daily dosing should be the goal. Synchronising doses for patients on multiple drug therapy is also important. Whenever possible, medication regimens should be coordinated so that doses are only taken at one or two points during the day. Helping patients identify other lifestyle activities they regularly practice which can cue them to take their medications (eg, brushing teeth morning and night, breakfast and dinner, etc) can also increase adherence.
Providers have an important opportunity to influence adherence through improved communication. Providers must help patients understand the chronic nature of their disease and the rationale for comprehensive treatment, including medication, smoking cessation and exercise. Patients often have little or no understanding of their symptoms, warning signs when they have exacerbations and specific action to be taken.74 However, neither the physician nor nurse alone is responsible for improving communication: assessment of adherence and strategies to optimise enhanced discussions of relevant issues should be woven into routine clinical encounters by each member of the treatment team to ensure repetition and reinforcement of key messages. Providing information in both written and verbal forms, linking it directly to patient symptoms and laboratory results and allowing an opportunity for discussion can increase knowledge and adherence.75,76 However, patient education alone is rarely successful.77 Providers should provide opportunities for patients to talk about their perceptions of the benefits and costs of therapy. When patients are given an opportunity to identify and discuss their concerns about medications and/or exercise, fears can be allayed and barriers can be addressed.
It is also important to clarify with patients the critical role they have in managing their disease. Important lessons have been learned from COPD and other chronic disease self-management training programmes. For example, counselling is most effective when information about how to self-manage is presented in a structured manner over a period of at least 15 min. Prescribing behavioural components that are tailored to the barriers of individual patients (eg, keeping medications in one place, self-monitoring of symptoms and medication use, etc) is more effective in changing patient behaviour than offering general suggestions. Patients with COPD may be especially vulnerable to non-adherence as most individuals have more difficulty adhering to preventive or chronic medications that do not have an immediate impact on symptoms.16 For example, in patients with asthma, rescue medication use is generally higher than adherence to inhaled steroids.61 Patients also benefit from regular opportunities to discuss use of self-management strategies and problem solving around barriers with different members of the treatment team. Self-management groups led by professional staff or trained lay persons can provide a cost effective75–80 and enjoyable way for patients to learn about managing their disease and rehearse new behaviours81,82 while receiving support from other families and patients with COPD. Recognising the partnership between patient and physician and actively involving patients in establishing a treatment plan that is specifically tailored to meet their needs have been shown to improve adherence. Gallefoss et al83,84 offered a self-management programme which included educational brochures, two 2 hour education sessions with a nurse or physiotherapist, and a personalised treatment plan for each patient. One year follow-up showed that patients used less rescue medication (eg, β2 agonists), had fewer absences from work and fewer physician visits, leading to reduced healthcare costs.
Recently, a Cochrane review specifically reviewed the use of action plans for COPD.85 All interventions involved the use of a written action plan, an information booklet and self-management education. While patients who were provided with an action plan had a better knowledge of the importance of early intervention and how to implement appropriate treatment for an exacerbation, no overall therapeutic benefit was evident. However, the small sample size limited the power of these studies and more research is needed to clarify the benefits of using action plans in COPD.
CONCLUSIONS
Suboptimal adherence is associated with a significant health and economic burden in patients with COPD. As with other chronic diseases, suboptimal adherence is common and results from many factors related to patients, providers, their interactions and treatment components. Adherence to all components of comprehensive treatments should be viewed as an important goal for both providers and patients to work towards in treatments to control COPD. Providers play a critical role in helping patients to understand the nature of the disease, the potential benefits of treatment, addressing concerns about potential adverse effects and in encouraging patients to develop the skills necessary for optimal self-management. Active involvement of patients in designing a health management plan appears to improve adherence. There is a need to develop effective treatments for COPD with simplified treatment regimens (infrequent dosing, simple delivery), rapid onset of action and durable effect, which would increase the probability of patient adherence. In addition, patient self-management education, as part of an ongoing communication between patients and providers, needs to be properly tested. Enhancing patient self-efficacy as part of self-management education is also important to promote long term adherence. Shared decision making during the initial and regular follow-up visits helps to solidify the partnership between patient and physician which leads to improved adherence.
Further research is needed to gain insight into health behaviour change interventions in COPD in order to design and implement more effective self-management programmes. Such programmes offer the potential to confer clinically and cost effective strategies for long term maintenance of pharmacological and non-pharmacological treatment. Long term studies are needed to assess how successfully patients can sustain behaviour changes over time. Thus the identification and management of adherence related factors in COPD will improve not only patient health outcomes but also help improve the health status of patients and reduce the economic and societal burden associated with COPD. Trials are needed to document effects on clinically important patient outcomes, feasibility in usual practice settings and durability.
Acknowledgements
The authors would like to thank Ms Louise Auclair for her secretarial assistance.
Funding: JB is a recipient of the Fraser, Monat and McPherson Award, Faculty de Medicine, McGill University.
Footnotes
Competing interests: None.
REFERENCES
- 1.Halbert RJ, Isonaka S, George D, et al. Interpreting COPD prevalence estimates: what is the true burden of disease? Chest. 2003;123:1684–1692. doi: 10.1378/chest.123.5.1684. [DOI] [PubMed] [Google Scholar]
- 2.Murray C, Lopez A. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet. 1997;349:1498–1504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 3.US Department of Health and Human Services NIoHNHLaBI. Chronic obstructive pulmonary disease. [accessed 10 July 2008]; Available at: http://www.nhlbi.nih.gov/health/public/lung/other/copd_fact.htm.
- 4.Buist AS, McBurnie MA, Vollmer WM, et al. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet. 2007;370:741–750. doi: 10.1016/S0140-6736(07)61377-4. [DOI] [PubMed] [Google Scholar]
- 5.Chapman KR, Mannino DM, Soriano JB, et al. Epidemiology and costs of chronic obstructive pulmonary disease. Eur Respir J. 2006;27:188–207. doi: 10.1183/09031936.06.00024505. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. The World Health Report 1997. [accessed 10 July 2008];Conquering suffering, enriching humanity. Available at: http://www.who.int/whr/1997/en/whr97_en.pdf. [PubMed]
- 7.BTS guidelines for the management of chronic obstructive pulmonary disease. The COPD Guidelines Group of the Standards of Care Committee of the BTS. Thorax. 1997;52(Suppl 5):S1–S28. [PMC free article] [PubMed] [Google Scholar]
- 8.Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. [accessed 10 July 2008]; Updated 2004. Available at: http://www.goldcopd.com. [Google Scholar]
- 9.O’Donnell DE, Aaron S, Bourbeau J, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease—2007 update. Can Respir J. 2007;14(Suppl B):5B–32B. doi: 10.1155/2007/830570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nici L, Donner C, Wouters E, et al. American Thoracic Society/European Respiratory Society statement on pulmonary rehabilitation. Am J Respir Crit Care Med. 2006;173:1390–1413. doi: 10.1164/rccm.200508-1211ST. [DOI] [PubMed] [Google Scholar]
- 11.Sin DD, McAlister FA, Man SF, et al. Contemporary management of chronic obstructive pulmonary disease: scientific review. JAMA. 2003;290:2301–2312. doi: 10.1001/jama.290.17.2301. [DOI] [PubMed] [Google Scholar]
- 12.DiMatteo MR. Variations in patients’ adherence to medical recommendations: a quantitative review of 50 years of research. Med Care. 2004;42:200–209. doi: 10.1097/01.mlr.0000114908.90348.f9. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. Adherence to long-term therapies: policy for action. [accessed 10 July 2008];Meeting report. 2001 Jun 4–5; Available at: http://www.who.int/chronic_conditions/adherence/en/
- 14.Corden ZM, Bosley CM, Rees PJ, et al. Home nebulized therapy for patients with COPD: patient compliance with treatment and its relation to quality of life. Chest. 1997;112:1278–1282. doi: 10.1378/chest.112.5.1278. [DOI] [PubMed] [Google Scholar]
- 15.Turner J, Wright E, Mendella L, et al. Predictors of patient adherence to long-term home nebulizer therapy for COPD. The IPPB Study Group. Intermittent Positive Pressure Breathing. Chest. 1995;108:394–400. doi: 10.1378/chest.108.2.394. [DOI] [PubMed] [Google Scholar]
- 16.Rapoff MA, Bartlett SJ. Adherence in children and adults. In: Bartlett SJ, editor. Clinical care in the rheumatic diseases. Atlanta: American College of Rheumatology; 2006. pp. 279–284. [Google Scholar]
- 17.World Health Organization. Adherence to long-term therapies: Evidence for action. [accessed 22 July, 2008];2003 Available at: www.who.int/chp/knowledge/publications/adherence_report/en/index.html.
- 18.Blais L, Bourbeau J, Sheehy O, et al. Inhaled corticosteroids in COPD. Trends in patient’s persistence on treatment and determinants of use. Can Respir J. 2003;11:27–32. doi: 10.1155/2004/289420. [DOI] [PubMed] [Google Scholar]
- 19.Hughes CM. Medication non-adherence in the elderly: how big is the problem? Drugs Aging. 2004;21:793–811. doi: 10.2165/00002512-200421120-00004. [DOI] [PubMed] [Google Scholar]
- 20.Raynor D. Patient compliance: the pharmacist’s role. Int J Pharm Prac. 1992;1:126–135. [Google Scholar]
- 21.Farmer KC. Methods for measuring and monitoring medication regimen adherence in clinical trials and clinical practice. Clin Ther. 1999;21:1074–1090. doi: 10.1016/S0149-2918(99)80026-5. [DOI] [PubMed] [Google Scholar]
- 22.Rand CS. I took the medicine like you told me, doctor: Self-report of adherence with medical regimens. In: Stone A, editor. The science of self-report: implications for research and practice. Mahway, NJ: Lawrence Erlbaum Associates; 2000. pp. 257–276. [Google Scholar]
- 23.Haynes RB, Sackett DL, Taylor DW. How to detect and manage low patient compliance in chronic illness. Geriatrics. 1980;35:91–97. [PubMed] [Google Scholar]
- 24.Caron HS, Roth HP. Objective assessment of cooperation with an ulcer diet: relation to antacid intake and to assigned physician. Am J Med Sci. 1971;261:61–66. doi: 10.1097/00000441-197102000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Mushlin AI, Appel FA. Diagnosing potential noncompliance. Physicians’ ability in a behavioral dimension of medical care. Arch Intern Med. 1977;137:318–321. doi: 10.1001/archinte.137.3.318. [DOI] [PubMed] [Google Scholar]
- 26.Simmons MS, Nides MA, Rand CS, et al. Unpredictability of deception in compliance with physician-prescribed bronchodilator inhaler use in a clinical trial. Chest. 2000;118:290–295. doi: 10.1378/chest.118.2.290. [DOI] [PubMed] [Google Scholar]
- 27.Rand CS, Wise RA, Nides M, et al. Metered-dose inhaler adherence in a clinical trial. Am Rev Respir Dis. 1992;146:1559–1564. doi: 10.1164/ajrccm/146.6.1559. [DOI] [PubMed] [Google Scholar]
- 28.Rand CS, Nides M, Cowles MK, et al. Long-term metered-dose inhaler adherence in a clinical trial. The Lung Health Study Research Group. Am J Respir Crit Care Med. 1995;152:580–588. doi: 10.1164/ajrccm.152.2.7633711. [DOI] [PubMed] [Google Scholar]
- 29.Coutts JA, Gibson NA, Paton JY. Measuring compliance with inhaled medication in asthma. Arch Dis Child. 1992;67:332–333. doi: 10.1136/adc.67.3.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mawhinney H, Spector SL, Kinsman RA, et al. Compliance in clinical trials of two nonbronchodilator, antiasthma medications. Ann Allergy. 1991;66:294–299. [PubMed] [Google Scholar]
- 31.Nides MA, Tashkin DP, Simmons MS, et al. Improving inhaler adherence in a clinical trial through the use of the nebulizer chronolog. Chest. 1993;104:501–507. doi: 10.1378/chest.104.2.501. [DOI] [PubMed] [Google Scholar]
- 32.Tashkin DP, Rand C, Nides M, et al. A nebulizer chronolog to monitor compliance with inhaler use. Am J Med. 1991;91(Suppl 4A):33S–36S. doi: 10.1016/0002-9343(91)90260-5. [DOI] [PubMed] [Google Scholar]
- 33.Bender B, Milgrom H, Apter A. Adherence intervention research: what have we learned and what do we do next? J Allergy Clin Immunol. 2003;112:489–494. doi: 10.1016/s0091-6749(03)01718-4. [DOI] [PubMed] [Google Scholar]
- 34.Tashkin DP. Multiple dose regimens. Impact on compliance. Chest. 1995;107(5 Suppl):176S–182S. doi: 10.1378/chest.107.5_supplement.176s. [DOI] [PubMed] [Google Scholar]
- 35.Rand CS. Non-adherence with asthma therapy: more than just forgetting. J Pediatr. 2005;146:157–159. doi: 10.1016/j.jpeds.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 36.Sackett DL, Haynes RB. Compliance with therapeutic regimens. Baltimore: John Hopkins University Press; 1976. [Google Scholar]
- 37.Wilson IB, Schoen C, Neuman P, et al. Physician–patient communication about prescription medication nonadherence: a 50-state study of America’s seniors. J Gen Intern Med. 2007;22:6–12. doi: 10.1007/s11606-006-0093-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.James PN, Anderson JB, Prior JG, et al. Patterns of drug taking in patients with chronic airflow obstruction. Postgrad Med J. 1985;61:7–10. doi: 10.1136/pgmj.61.711.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Make BJ. Chronic obstructive pulmonary disease: developing comprehensive management. Respir Care. 2003;48:1225–1234. [PubMed] [Google Scholar]
- 40.Garcia-Aymerich J, Barreiro E, Farrero E, et al. Patients hospitalized for COPD have a high prevalence of modifiable risk factors for exacerbation (EFRAM study) Eur Respir J. 2000;16:1037–1042. doi: 10.1034/j.1399-3003.2000.16f03.x. [DOI] [PubMed] [Google Scholar]
- 41.Cohen R, Marzouk K, Berkoski P, et al. Body composition and resting energy expenditure in clinically stable, non-weight-losing patients with severe emphysema. Chest. 2003;124:1365–1372. doi: 10.1378/chest.124.4.1365. [DOI] [PubMed] [Google Scholar]
- 42.Col N, Fanale JE, Kronholm P. The role of medication noncompliance and adverse drug reactions in hospitalizations of the elderly. Arch Intern Med. 1990;150:841–845. [PubMed] [Google Scholar]
- 43.Soumerai SB, Ross-Degnan D, Avorn J, et al. Effects of Medicaid drug-payment limits on admission to hospitals and nursing homes. N Engl J Med. 1991;325:1072–1077. doi: 10.1056/NEJM199110103251505. [DOI] [PubMed] [Google Scholar]
- 44.Incalzi RA, Capparella O, Gemma A, et al. The interaction between age and comorbidity contributes to predicting the mortality of geriatric patients in the acute-care hospital. J Intern Med. 1997;242:291–298. doi: 10.1046/j.1365-2796.1997.00132.x. [DOI] [PubMed] [Google Scholar]
- 45.Zuccollo G, Liddell H. The elderly and the medication label: doing it better. Age Ageing. 1985;14:371–376. doi: 10.1093/ageing/14.6.371. [DOI] [PubMed] [Google Scholar]
- 46.Cully JA, Graham DP, Stanley MA, et al. Quality of life in patients with chronic obstructive pulmonary disease and comorbid anxiety or depression. Psychosomatics. 2006;47:312–319. doi: 10.1176/appi.psy.47.4.312. [DOI] [PubMed] [Google Scholar]
- 47.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160:2101–2107. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- 48.Lau HS, Beuning KS, Postma-Lim E, et al. Non-compliance in elderly people: evaluation of risk factors by longitudinal data analysis. Pharm World Sci. 1996;18:63–68. doi: 10.1007/BF00579707. [DOI] [PubMed] [Google Scholar]
- 49.Morris LA, Tabak ER, Gondek K. Counseling patients about prescribed medication: 12-year trends. Med Care. 1997;35:996–1007. doi: 10.1097/00005650-199710000-00002. [DOI] [PubMed] [Google Scholar]
- 50.Stewart JE, Martin JL. Correlates of patients’ perceived and real knowledge of prescription directions. Contemp Pharm Pract. 1979;2:144–148. [PubMed] [Google Scholar]
- 51.Tarn DM, Heritage J, Paterniti DA, et al. Physician communication when prescribing new medications. Arch Intern Med. 2006;166:1855–1862. doi: 10.1001/archinte.166.17.1855. [DOI] [PubMed] [Google Scholar]
- 52.Dunbar J, Marshall G, Hovel M. Behavioral strategies for improving compliance. In: Haynes R, editor. Compliance in health care. Baltimore: Johns Hopkins University Press; 1979. pp. 174–190. [Google Scholar]
- 53.Pepin JL, Barjhoux CE, Deschaux C, et al. Long-term oxygen therapy at home. Compliance with medical prescription and effective use of therapy. ANTADIR Working Group on Oxygen Therapy. Association Nationale de Traitement a Domicile des Insuffisants Respiratories. Chest. 1996;109:1144–1150. doi: 10.1378/chest.109.5.1144. [DOI] [PubMed] [Google Scholar]
- 54.Wurtemberger G, Hutter BO. Health-related quality of life, psychological adjustment and compliance to treatment in patients on domiciliary liquid oxygen. Monaldi Arch Chest Dis. 2000;55:216–224. [PubMed] [Google Scholar]
- 55.Dolce JJ, Crisp C, Manzella B, et al. Medication adherence patterns in chronic obstructive pulmonary disease. Chest. 1991;99:837–841. doi: 10.1378/chest.99.4.837. [DOI] [PubMed] [Google Scholar]
- 56.Salzman C. Medication compliance in the elderly. J Clin Psychiatry. 1995;56(Suppl 1):18–22. [PubMed] [Google Scholar]
- 57.Kesten S, Flanders J, Serby C. Compliance with tiotropium, a once daily dry powder inhaled bronchodilator, in one year COPD trials. Chest. 2000;118:191S–192S. [Google Scholar]
- 58.van Grunsven PM, van Schayck CP, van Deuveren M, et al. Compliance during long-term treatment with fluticasone propionate in subjects with early signs of asthma or chronic obstructive pulmonary disease (COPD): results of the Detection, Intervention, and Monitoring Program of COPD and Asthma (DIMCA) Study. J Asthma. 2000;37:225–234. doi: 10.3109/02770900009055445. [DOI] [PubMed] [Google Scholar]
- 59.Bender BG, Pedan A, Varasteh LT. Adherence and persistence with fluticasone propionate/salmeterol combination therapy. J Allergy Clin Immunol. 2006;118:899–904. doi: 10.1016/j.jaci.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 60.Breekveldt-Postma NS, Gerrits CM, Lammers JW, et al. Persistence with inhaled corticosteroid therapy in daily practice. Respir Med. 2004;98:752–759. doi: 10.1016/j.rmed.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 61.Krigsman K, Nilsson JL, Ring L. Refill adherence for patients with asthma and COPD: comparison of a pharmacy record database with manually collected repeat prescriptions. Pharmacoepidemiol Drug Saf. 2007;16:441–448. doi: 10.1002/pds.1321. [DOI] [PubMed] [Google Scholar]
- 62.Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23:1296–1310. doi: 10.1016/s0149-2918(01)80109-0. [DOI] [PubMed] [Google Scholar]
- 63.Breekveldt-Postma NS, Koerselman J, Erkens JA, et al. Enhanced persistence with tiotropium compared with other respiratory drugs in COPD. Respir Med. 2007;101:1398–1405. doi: 10.1016/j.rmed.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 64.Lacasse Y, Lecours R, Pelletier C, et al. Randomised trial of ambulatory oxygen in oxygen-dependent COPD. Eur Respir J. 2005;25:1032–1038. doi: 10.1183/09031936.05.00113504. [DOI] [PubMed] [Google Scholar]
- 65.Lacasse Y, Wong E, Guyatt GH, et al. Meta-analysis of respiratory rehabilitation in chronic obstructive pulmonary disease. Lancet. 1996;348:1115–1119. doi: 10.1016/S0140-6736(96)04201-8. [DOI] [PubMed] [Google Scholar]
- 66.Maspero JF, Duenas-Meza E, Volovitz B, et al. Oral montelukast versus inhaled beclomethasone in 6- to 11-year-old children with asthma: results of an open-label extension study evaluating long-term safety, satisfaction, and adherence with therapy. Curr Med Res Opin. 2001;17:96–104. [PubMed] [Google Scholar]
- 67.Foglio K, Bianchi L, Bruletti G, et al. Long-term effectiveness of pulmonary rehabilitation in patients with chronic airway obstruction. Eur Respir J. 1999;13:125–132. doi: 10.1183/09031936.99.13112599. [DOI] [PubMed] [Google Scholar]
- 68.Guell R, Casan P, Belda J, et al. Long-term effects of outpatient rehabilitation of COPD: A randomized trial. Chest. 2000;117:976–983. doi: 10.1378/chest.117.4.976. [DOI] [PubMed] [Google Scholar]
- 69.Ries AL, Kaplan RM, Limberg TM, et al. Effects of pulmonary rehabilitation on physiologic and psychosocial outcomes in patients with chronic obstructive pulmonary disease. Ann Intern Med. 1995;122:823–832. doi: 10.7326/0003-4819-122-11-199506010-00003. [DOI] [PubMed] [Google Scholar]
- 70.Brassington GS, Atienza AA, Perczek RE, et al. Intervention-related cognitive versus social mediators of exercise adherence in the elderly. Am J Prev Med. 2002;23(2 Suppl):80–86. doi: 10.1016/s0749-3797(02)00477-4. [DOI] [PubMed] [Google Scholar]
- 71.McAuley E, Lox C, Duncan TE. Long-term maintenance of exercise, self-efficacy, and physiological change in older adults. J Gerontol. 1993;48:218–224. doi: 10.1093/geronj/48.4.p218. [DOI] [PubMed] [Google Scholar]
- 72.Nault D, Dagenais J, Perreault V, et al. Qualitative evaluation of a disease specific self-management program “Living well with COPD”. Eur Respir J. 2000;16:317S. [Google Scholar]
- 73.Brooks D, Krip B, Mangovski-Alzamora S, et al. The effect of postrehabilitation programmes among individuals with chronic obstructive pulmonary disease. Eur Respir J. 2002;20:20–29. doi: 10.1183/09031936.02.01852001. [DOI] [PubMed] [Google Scholar]
- 74.Kessler R, Stahl E, Vogelmeier C, et al. Patient understanding, detection, and experience of COPD exacerbations: an observational, interview-based study. Chest. 2006;130:133–142. doi: 10.1378/chest.130.1.133. [DOI] [PubMed] [Google Scholar]
- 75.Ley P. Communicating with the patient. London: Croom Helm; 1988. [Google Scholar]
- 76.Morris LA, Halperin JA. Effects of written drug information on patient knowledge and compliance: a literature review. Am J Public Health. 1979;69:47–52. doi: 10.2105/ajph.69.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bourbeau J, Maltais F, Julien M, et al. Predictors of high utilization of health care services in patients with chronic obstructive pulmonary disease. Can Respir J. 2003;10:207. [Google Scholar]
- 78.Bourbeau J, Collet JP, Schwartzman K, et al. Economic benefits of self-management education in COPD. Chest. 2006;130:1704–1711. doi: 10.1378/chest.130.6.1704. [DOI] [PubMed] [Google Scholar]
- 79.Bourbeau J. Disease-specific self-management programs in patients with advanced chronic obstructive pulmonary disease. a comprehensive and critical evaluation. Dis Manage Health. 2003;11:311–319. [Google Scholar]
- 80.Gadoury MA, Schwartzman K, Rouleau M, et al. Self-management reduces both short- and long-term hospitalisation in COPD. Eur Respir J. 2005;26:853–857. doi: 10.1183/09031936.05.00093204. [DOI] [PubMed] [Google Scholar]
- 81.Bourbeau J, Nault D, Dang-Tan T. Self-management and behaviour modification in COPD. Patient Educ Couns. 2004;52:271–277. doi: 10.1016/S0738-3991(03)00102-2. [DOI] [PubMed] [Google Scholar]
- 82.Gallefoss F, Bakke PS, Rsgaard PK. Quality of life assessment after patient education in a randomized controlled study on asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;159:812–817. doi: 10.1164/ajrccm.159.3.9804047. [DOI] [PubMed] [Google Scholar]
- 83.Gallefoss F, Bakke P. Cost-benefit and cost-effectiveness analysis of self-management in patients with COPD—a 1-year follow-up randomized, controlled trial. Respir Med. 2002;96:424–431. doi: 10.1053/rmed.2002.1293. [DOI] [PubMed] [Google Scholar]
- 84.Gallefoss F, Bakke PS. Impact of patient education and self-management on morbidity in asthmatics and patients with chronic obstructive pulmonary disease. Respir Med. 2000;94:279–287. doi: 10.1053/rmed.1999.0749. [DOI] [PubMed] [Google Scholar]
- 85.Turnock AC, Walters EH, Walters JA, et al. Action plans for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2005;4:CD005074. doi: 10.1002/14651858.CD005074.pub2. [DOI] [PubMed] [Google Scholar]
- 86.Morris LS, Schulz RM. Patient compliance—an overview. J Clin Pharm Ther. 1992;17:283–295. doi: 10.1111/j.1365-2710.1992.tb01306.x. [DOI] [PubMed] [Google Scholar]