Abstract
Introduction
Cannabis is the most widely used illicit drug. Many individuals are incidentally exposed to secondhand cannabis smoke, but little is known about the effects of this exposure. This report examines the physiological, subjective, and behavioral/cognitive effects of secondhand cannabis exposure, and the influence of room ventilation on these effects.
Methods
Non-cannabis-using individuals were exposed to secondhand cannabis smoke from six individuals smoking cannabis (11.3% THC) ad libitum in a specially constructed chamber for one hour. Chamber ventilation was experimentally manipulated so that participants were exposed under unventilated conditions or with ventilation at a rate of 11 air exchanges/hour. Physiological, subjective and behavioral/cognitive measures of cannabis exposure assessed after exposure sessions were compared to baseline measures.
Results
Exposure to secondhand cannabis smoke under unventilated conditions produced detectable cannabinoid levels in blood and urine, minor increases in heart rate, mild to moderate self-reported sedative drug effects, and impaired performance on the Digit Symbol Substitution Task (DSST). One urine specimen tested positive at using a 50 ng/mL cut-off and several specimens were positive at 20 ng/mL. Exposure under ventilated conditions resulted in much lower blood cannabinoid levels, and did not produce sedative drug effects, impairments in performance, or positive urine screen results.
Conclusions
Room ventilation has a pronounced effect on exposure to secondhand cannabis smoke. Under extreme, unventilated conditions, secondhand cannabis smoke exposure can produce detectable levels of THC in blood and urine, minor physiological and subjective drug effects, and minor impairment on a task requiring psychomotor ability and working memory.
Keywords: Cannabis, Marijuana, THC, Exposure, Secondhand, Cognitive
1. INTRODUCTION
Cannabis is the most widely used illicit drug globally (World Health Organization, 2014). The most popular route of administration is smoking, which often occurs indoors, in automobiles, or in other areas where ventilation is limited or variable. Δ9-tetrahydrocannabinol (THC), the major psychoactive component of cannabis, is present in both mainstream smoke (inhaled by the user) and sidestream smoke (dispersed into environment; Cone et al., 1987; Fehr and Kalant, 1972; Matthais et al., 1997). Metabolism of THC yields the psychoactive metabolite, 11-hydroxy-Δ9-tetrahydrocannabinol (11-OH-THC), which is present in blood after active or passive exposure to cannabis smoke (Huestis et al., 1992; Moore et al., 2011). THC and 11-OH-THC have similar behavioral effects, but 11-OH-THC levels peak later than THC levels after cannabis exposure (Hollister and Gillespie, 1975; Huestis et al., 1992; Järbe et al., 1994; Kosersky et al., 1974; Lemberger et al., 1973). Metabolism of 11-OH-THC yields 11-nor-9-carboxy- Δ9-tetrahydrocannabinol (THC-COOH). THC-COOH is non-psychoactive, but its long half-life (~140 hours) makes it a common biomarker in urine testing for cannabis use.
Many individuals are passively exposed to secondhand cannabis smoke given the current prevalence and patterns of cannabis use, however, there has been little controlled research examining the consequences of secondhand exposure. Most research on secondhand cannabis smoke exposure has focused on detection of cannabinoids (e.g., THC, 11-OH-THC, THC-COOH) in various biological matrices. Cone and Johnson (1986) exposed five healthy men to passive smoke from either 4 or 16 cannabis cigarettes (2.8% THC) in a small, enclosed room for one hour each day on six consecutive days. This secondhand smoke exposure reliably produced detectable levels of cannabinoids in urine and plasma, which varied in an orderly relation to dose (i.e., cannabinoid levels were higher after exposure to smoke from 16 cannabis cigarettes vs. 4 cigarettes). Similar results were obtained from studies using comparable designs (i.e., secondhand cannabis smoke exposure in small closed rooms or motor vehicles; Law et al., 1984; Mason et al., 1983; Mørland et al., 1985; Perez-Reyes et al., 1983).
At least four limitations impede interpretation of these studies with regard to current real-world secondhand cannabis smoke exposure scenarios. First, exposure in these studies always occurred under the “extreme” conditions (i.e., in small spaces without ventilation). Because secondhand cannabis exposure in the real world occurs under conditions with different degrees of air ventilation (e.g., in rooms with air conditioning or open windows, or outdoors), research examining the effects of ventilation on exposure levels is needed. Second, the average potency of “street” cannabis has increased more than 3-fold in the time since these studies were conducted (sinsemilla cannabis seized from 2002–2008 by federal and state law enforcement agencies in the U.S. averaged 11.1% to 11.9% THC; Mehmedic et al., 2010). To our knowledge, only one controlled study has examined passive exposure to higher potency cannabis (10.4% THC) that more closely resembles street cannabis available today (Niedbala et al., 2005). A more thorough examination of secondhand exposure using higher potency cannabis is needed to better model passive exposure to today’s cannabis and its potential effects on drug test results. Third, only one study has reported both physiological and subjective effects of secondhand cannabis exposure (Cone and Johnson, 1986). Cone and Johnson (1986) reported that secondhand cannabis exposure had no systematic effects on heart rate or blood pressure, and that participant’s ratings of subjective drug effects increased significantly after exposure to smoke from 16 cannabis cigarettes relative to placebo ratings. These effects were most pronounced during the first hour after exposure, and resolved within three hours. Fourth, no prior studies have evaluated the effects of secondhand cannabis smoke exposure on behavioral/cognitive performance. Expanded research on the effects of secondhand cannabis smoke exposure in the areas outlined above is timely and warranted.
The present study was conducted to examine the influence of variations in cannabis potency (5.3% THC vs. 11.3% THC) and in room ventilation (unventilated vs. standard residential ventilation) on the effects of secondhand cannabis smoke exposure. The primary objective of the study was to characterize the pharmacokinetic profile of cannabinoids in various biological matrices following secondhand smoke exposure in order to inform federal workplace drug testing standards. A detailed report of the urine cannabinoid concentrations has been previously published (Cone et al., 2015). Here, we report the outcomes of pharmacodynamic assessments, cannabinoid concentrations in whole blood, and provide a brief summary of the urine results. Data analysis was restricted to comparisons of the ventilated vs. unventilated sessions with the same potency of cannabis (11.3% THC) because over 40% more 11.3% THC cannabis was consumed than 5.3% THC cannabis (14.4 grams total vs. 10.2 grams total) in comparable (unventilated) study sessions. The different levels of cannabis consumption between these 2 conditions preclude our ability to validly compare the effects of cannabis potency on study outcomes, particularly pharmacodynamic measures. This study is, to our knowledge, the first to demonstrate the effect of room ventilation on secondhand cannabis smoke exposure and is among the few secondhand cannabis smoke studies to examine pharmacodynamic outcomes or utilize cannabis with a potency that is representative of current “street” cannabis.
2. METHODS
2.1. Participants
2.1.1. Recruitment
Frequent cannabis users and cannabis nonusers were recruited from the greater Baltimore, MD area via media advertising and word-of-mouth communication. Interested participants completed a screening session to determine eligibility. Participants provided written informed consent during the screening session. The Institutional Review Board at Johns Hopkins University School of Medicine approved this study.
2.1.2. Smokers
Cannabis users were eligible to participate as smokers if they: (1) were 18–45 years old, (2) reported using cannabis at least two times per week throughout the past 90 days, (3) provided a urine specimen that was positive for THC-COOH and negative for other drugs of abuse at screening and on the day of each study session, (4) had a negative breath alcohol reading at screening and on the day of each study session, (5) had a body mass index of 19–34 kg/m2, (6) were judged to be in good general heath based on physical examination, and (7) were not pregnant or nursing. Cannabis users were encouraged to participate in all three of the exposure sessions as a means of reducing between-session variability in smoke exposure among nonsmokers. Smokers were instructed to remain abstinent from cannabis overnight prior to exposure sessions.
2.1.3. Nonsmokers
Individuals who were not current cannabis users were eligible to participate in the study if they: (1) were 18–45 years old, (2) reported cannabis use at least once in their lifetime, but not within the prior 6 months, (3) provided a urine specimen that was negative for drugs of abuse at screening and on the day of study admission, (4) had a negative breath alcohol reading at screening and on the day of participation, (5) had a BMI ranging from 19–34 kg/m2, (6) were judged to be in good general heath based on a full physical examination, and (7) were not pregnant or nursing. Each nonsmoker participated in only one exposure session due to concern that residual effects of cannabis exposure in one session might influence results of subsequent sessions. Because the primary aim of this study was to examine the pharmacokinetic profile of secondhand cannabis smoke exposure, we restricted the nonsmoker group to individuals who were not current cannabis users so that they would not have residual cannabinoids in biological matrices (e.g., urine, saliva) at baseline.
2.2. Experimental Procedure
Three secondhand cannabis exposure sessions were conducted at the Johns Hopkins University Bayview campus. The first session involved exposure to cannabis containing 5.3% THC in an unventilated environment, the second session involved exposure to cannabis containing 11.3% THC in an unventilated environment, and the third session involved exposure to cannabis containing 11.3% THC in a ventilated environment. We only describe results from the second and third sessions here.
Smokers and non-smokers arrived at approximately 0700 hours on session days. Drug and alcohol testing was conducted upon arrival to confirm that participants still met study eligibility requirements regarding substance use. Smokers were reminded of abstinence requirements the day before each session took place, and study staff met with active smokers when they arrived on session days to check for signs of recent cannabis use (e.g., bloodshot eyes, odor of cannabis smoke) to verify reported abstinence. Nonsmokers were required to urine test negative for cannabis. Nursing staff placed an intravenous catheter in the non-dominant arm of each participant for repeated blood sampling. Tobacco and caffeine use was not permitted during study sessions. One nonsmoker was a daily tobacco user, and was provided with nicotine patches in order to reduce the potential effect of nicotine withdrawal on study outcomes.
After completing baseline assessments, 6 cannabis smokers and 6 nonsmokers entered a specially constructed smoke exposure chamber (approximately 0900 hours). The chamber measured 10 ft. × 13 ft. (3.05 m × 3.96 m) with a 7 ft. (2.13 m) ceiling, was constructed using a combination of Plexiglas and aluminum support beams, and incorporated an adjustable ventilation/exhaust system. Smokers and nonsmokers sat around a table in alternating seats. All participants wore protective clothing over their normal clothes (disposable booties, jumpsuits) during the exposure session, and were provided with swimming goggles to prevent eye irritation from accumulated smoke.
During the unventilated exposure session, ducts at both the intake and exhaust manifolds of the chamber were capped, eliminating the inflow/outflow of air. During the ventilated session a central air conditioning unit fed cool air through a 9.5 in2 (24 cm2) intake register at one end of the chamber. On the opposite end, air was exhausted through a 9.5 in2 (24 cm2) register into a ventilation shaft that contained a motorized fan. The ventilation system was calibrated to achieve an airflow rate of 11 air-exchanges/hour, which is consistent with residential ventilation standards based on the size of the chamber. Other than the differences in ventilation and select study participants, both session protocols were identical.
Each smoker was provided with 10 cannabis cigarettes obtained from the National Institute on Drug Abuse (NIDA) Drug Supply Program and a CReSS Pocket smoking topography device (Borgwaldt KC, Richmond, VA, USA). Each cannabis cigarette contained approximately 1 gram of high potency (11.3% THC) cannabis. The door to the chamber was sealed using magnetic strips around the frame of the door at the start of each 60-minute exposure session. Active smokers were instructed to smoke the provided cannabis cigarettes ad libitum through the CReSS device, which measured the number of puffs taken, puff volume, and other parameters of smoking behavior. Participants were instructed to remain seated for the entirety of the session, but were allowed to engage in leisure activities (e.g., talking, reading, using personal electronic devices, etc.). Research staff monitored the exposure session from outside the chamber to ensure nonsmokers did not actively inhale from the cannabis cigarettes and that smokers only consumed cigarettes from their own supply. Pulse oximeters were used to monitor blood oxygen concentration of study participants every 15 minutes to ensure an adequate oxygen supply was maintained in the chamber.
After 60 minutes the door to the exposure chamber was opened; participants then exited the chamber, immediately discarded protective clothing, and washed their hands and face to minimize contamination of biological samples collected after exposure. Participants then proceeded to a large room where they completed study assessments at regular intervals for 8 hours post-exposure among smokers and 34 hours post-exposure among non-smokers.
2.3. Assessments
2.3.1. Measures of cannabis exposure
The total weight of cannabis cigarettes, including butts and loose plant material, was obtained for each participant prior to and immediately after each session to determine the amount of cannabis combusted. We examined CReSS data on total puff volume to assess temporal characteristics of cannabis consumption. Biological samples (blood, urine, saliva, hair) were obtained for 8-hours post exposure among smokers and for up to 34 hours among nonsmokers. Whole blood specimens (10 mL) were obtained from indwelling venous catheters using grey-top vacutainer blood collection tubes at baseline, immediately after exiting the smoke chamber (Time “0”) and 0.5 (30 minutes), 1, 1.5, 2, 3, 4, 5, 6, 8, 12, 22, 26, 30, and 34 hours after exposure. Spot urine specimens were collected hourly for the first 4 hours and then pooled specimens were obtained for hours 6–8, 8–10, 10–12, 12–22, 22–26, 26–30, and 30–34. Quantitative testing for cannabinoids (THC, 11-OH-THC, THC-COOH) in whole blood was conducted by Immunalysis Corp (Pomona, CA) using LC/MS/MS, with a 0.5 ng/mL limit of quantitation. Urinalysis testing for THC-COOH was performed by Clinical Reference Laboratory (Lenexa, KS) using enzyme-linked immunosorbent assays (ELISA) with cut-offs of both 20 ng/mL and 50 ng/mL and confirmatory GS/MS with a 0.75 ng/mL limit of quantitation (see Cone et al., 2015).
2.3.2. Physiological Assessments
Heart rate (BPM) and systolic and diastolic blood pressure (mm/hg) were measured using automated monitors while participants were in the seated position at baseline, immediately after exiting the smoke exposure chamber (Time “0”), and at 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 12, 22, 26, 30, and 34 hours post-exposure.
2.3.3. Self-Report Assessments
Participants completed a 15-item Drug Effect Questionnaire (DEQ) to assess subjective ratings of drug effects. Individual items on the DEQ included three ratings of drug effects (“do you feel a drug effect?,” “do you feel a pleasant drug effect?,” “do you feel an unpleasant drug effect?”) and twelve ratings of behavioral/mood states often associated with cannabis intoxication (“sick”, “heart racing”, “anxious”, “relaxed”, “paranoid”, “tired”, “alert”, “irritable”, “vigorous”, “restless”, “hungry/have munchies”, “craving cannabis”). Participants rated each item using a 100 mm visual analog scale (VAS) anchored with “not at all” on one end and “extremely” on the other. The DEQ was administered at baseline and at 0, 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 12, 22, 26, 30, and 34 hours post-exposure.
2.3.4. Behavioral/Cognitive Assessments
Participants completed three different computerized behavioral tasks to assess aspects of psychomotor/cognitive performance known to be sensitive to the acute effects of smoked cannabis and relevant to functioning in the workplace and/or operating a motor vehicle or heavy machinery (Hooker and Jones, 1987; Wilson et al., 1994). Study participants completed each task three times during the screening visit while under the supervision of study staff in order to ensure proper understanding and minimize the influence of practice effects on task performance during the study, and were administered at baseline and at 0, 0.5, 1, 1.5, 2, 3, 4, 5, 6, and 8 hours post-exposure.
2.3.4.1. Divided Attention Task (DAT; Casswell and Marks, 1973)
On this task, participants simultaneously tracked a central stimulus and monitored peripheral stimuli. The central stimulus was a diamond that moved back and forth horizontally across the computer screen. Participants were instructed to track this stimulus using the computer mouse while monitoring a target digit at the lower center of the computer screen. Peripheral stimuli were digits (1–9) that appeared in each of the four corners of the screen. Participants were instructed to click the computer mouse once each time one of the digits in the corners of the screen matched the target digit. Task duration was 6 minutes. Primary outcomes on the DAT are the number of correct peripheral targets identified, reaction time (milliseconds) on correct responses, and mean distance (number of pixels) of cursor from the central stimulus.
2.3.4.2. Digit Symbol Substitution Task (DSST; McLeod et al., 1982)
On this task, participants viewed a series of nine geometric patterns. Each pattern was numbered (1–9) and consisted of three highlighted squares on a 3×3 grid. When the number associated with a particular pattern appeared on the center of the screen, participants were instructed to replicate the shape of that pattern using the an assigned 3×3 section of the computer keyboard. Task duration was 90 seconds. Primary outcomes of the DSST were total number of trials attempted, total number correct, and percentage of attempted trials completed correctly.
2.3.4.3. Paced Auditory Serial Addition Task (PASAT; Gronwall et al., 1977)
On this task, participants were presented with a string of single digit integers on the computer screen. Participants were instructed to calculate the sum of each successive pair of integers presented and select the correct answer from a list of choices displayed on the screen using the computer mouse. These integers appeared on the screen every 2.8 seconds during the initial battery of 30 trials. Following a 30 second break, integers appeared every 2.4 seconds during the second battery of 60 additional trials. Task duration was 5 minutes. Primary outcomes on the PASAT were total number of trials correct, and reaction time (milliseconds) on correct items.
2.4. Data Analysis
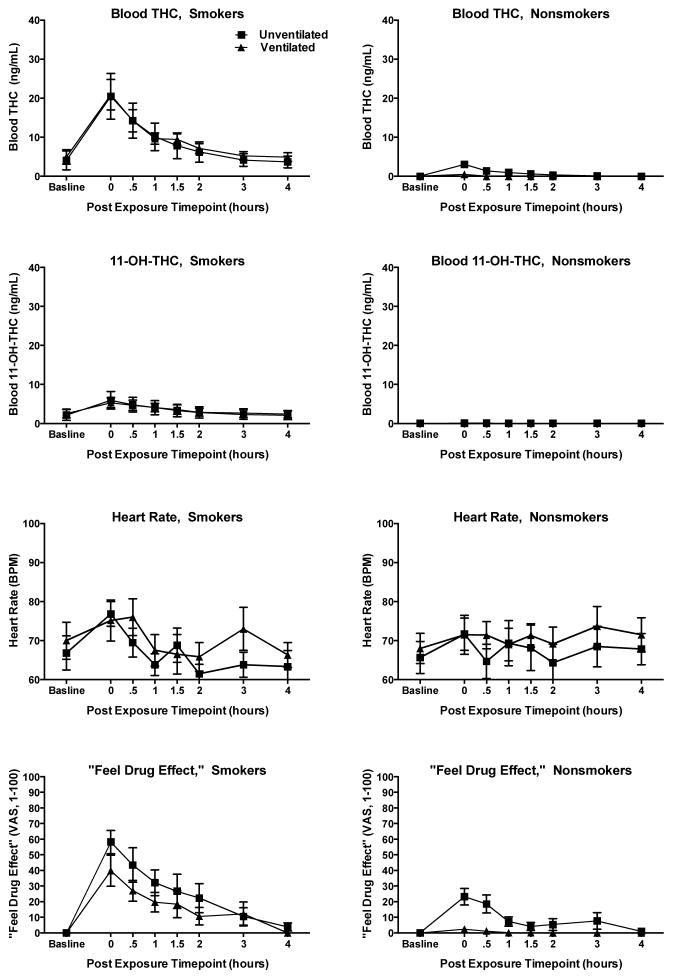
Demographic variables were compared between smokers and nonsmokers, and between nonsmokers in unventilated and ventilated sessions using independent-samples t-tests for continuous variables and chi-square tests for categorical variables. We limited analyses to the time window when subjective and behavioral/cognitive effects were likely to be observed because of the abundance of zero or near-zero blood cannabinoids levels and subjective drug effect ratings at later post-exposure time points. To select this window, we plotted levels of THC and of 11-OH-THC in whole blood at baseline and during the first four hours post-exposure to examine total intoxicant exposure during the post-exposure timeframe when effects are typically present. Blood cannabinoid levels peaked immediately after exposure and declined to below the level of quantitation by 90 minutes post-exposure among nonsmokers. DEQ ratings of “feel drug effect” appeared to parallel changes in blood cannabinoid levels among both smokers and nonsmokers (see Figure 1). Consequently, we limited outcome analyses of physiological, subjective, and behavioral/cognitive effects to the first hour after cannabis exposure.
Figure 1.
Whole blood THC levels, whole blood 11-OH-THC levels, heart rate (BPM) and DEQ visual analog scale ratings (VAS, 0–100) of “feel drug effect” among smokers and nonsmokers in unventilated and ventilated study conditions. Error bars are standard error of the mean.
We fit mixed effects regression models (Gueorguieva and Krystal, 2004) to assess differences between baseline and the first hour post-exposure for each measure. This model allowed us to compare the mean difference between baseline values and post-exposure values while accounting for (1) within-subject variability among post-exposure time points collected during the first hour after exposure within each session, and (2) between-session variability among baseline measures and post-exposure measures for the five active smokers who completed both exposure sessions. Data from active smokers who participated in multiple sessions were analyzed together and are presented together because their levels of cannabinoid exposure did not significantly differ as a function of room ventilation (see Figure 1). The absence of significant effects of cannabis exposure on heart rate among active smokers using mixed effects regression models that included data from the first hour post exposure prompted follow up comparisons between heart rate at baseline and heart rate at Time 0 using a second set of mixed effects regression models in order to examine if any transient effects on heart rate were present in smokers and in nonsmokers. Smokers and nonsmokers were not compared. Data on smokers are presented to serve as a reference for evaluating the levels of biological exposure and corresponding drug effects among nonsmokers. All statistical tests were two-tailed, and significance was determined at p < 0.05.
3. RESULTS
3.1. Participants
Table 1 shows demographics of the nineteen participants (7 smokers and 12 nonsmokers) who completed the high potency (11.3% THC) unventilated and ventilated sessions of the study. Seven smokers were included in analyses because one of the original six participants who completed the unventilated session withdrew from the study and was replaced. There were no significant differences between these groups on any of the variables examined.
Table 1.
Demographic characteristics among smokers, nonsmokers in the unventilated session, nonsmokers in the ventilated session. Average grams of cannabis consumed per study session among smokers.
| Characteristic | Smokers (n=7)* | Nonsmokers Unventilated (n=6) | Nonsmokers Ventilated (n=6) |
|---|---|---|---|
| Age (mean, SD) | 29.4 (5.8) | 28.7 (8.8) | 30.2 (8.2) |
| Gender (% male) | 57 | 50 | 50 |
| Race (%) | |||
| Caucasian | 71 | 83 | 100 |
| African American | 29 | 17 | 0 |
| Ethnicity (% Hispanic/Latino) | 14 | 0 | 33 |
| Body Mass Index (kg/M2) | 25.6 (5.5) | 25.3 (4.5) | 23.7(1.9) |
| Average grams of cannabis consumed per study session (mean, SD, range) | 2.6 (0.5, 1.6–3.3) | N/A | N/A |
Note. SD = standard deviation, kg/M2 = weight in kilograms/height in meters squared,
One of the original six smokers who participated in the unventilated session withdrew from the study, and was replaced for the ventilated session, resulting in n=7 included in analyses.
3.2. Exposure Session Conditions
Smokers consumed a considerable amount of cannabis in both the unventilated (14.4 grams total) and ventilated (16.5 grams total) exposure sessions. The chamber was visibly very smoky during the unventilated session (became difficult to see through to the opposing wall clearly; see Figure 2 in Cone et al., 2015). Cannabis smoke was visible during the ventilated session, but it did not obstruct chamber transparency. CReSS data were available for 10/12 smoking bouts (one CReSS device malfunctioned in the unventilated session and the battery died for one unit during the ventilated session). Data on puff volume indicated that smokers consumed 34% less cannabis during the second 30 minutes of the exposure period than during the first 30 minutes. Participants tolerated both study sessions well, with some complaints of mild eye irritation during the unventilated session, mainly among participants who did not wear or removed their protective eye goggles.
3.3. Effects of Cannabis Exposure on Cannabinoid Levels in Blood and Urine
3.3.1. Smokers
Figure 1 shows mean blood THC levels, blood 11-OH-THC levels, heart rate, and ratings of “feel drug effect” among smokers and nonsmokers at baseline and during the first four hours after exposure. All smokers had detectible levels of THC (mean 4.5 ng/ml) and 11-OH-THC (mean 2.4 ng/ml) at baseline, though all reported overnight abstinence and did not show any evidence of recent cannabis use (e.g., cannabis odor, bloodshot eyes). Blood levels of THC and 11-OH-THC peaked immediately following exposure in both sessions (means of 20.7 ng/ml and 5.6 ng/ml, respectively), and returned to baseline within 4 hours.
3.3.2. Nonsmokers
3.3.2.1. Unventilated Session
None of the nonsmokers in the unventilated session had detectable blood cannabinoids at baseline, but all six had detectible levels of THC following secondhand smoke exposure. Levels peaked immediately following exposure (Time 0 mean = 3.2 ng/ml), and remained detectible (>0.5 ng/mL) for 1–3 hours post-exposure. Only one participant had detectible levels of 11-OH-THC following exposure. One urine specimen tested positive in accordance with federal drug testing guidelines (screen with ELISA >50 ng/ml THC-COOH and confirmed with GC/MS >15 ng/ml THC-COOH) 4 hours post-exposure. Using a lower cut-off that is common in some commercial drug testing programs (ELISA >20 ng/ml THC-COOH; GC/MS > 15 ng/mL), 4 of 6 nonsmokers produced urine samples that screened and confirmed positive with a window of detection that ranged from 2 to 22 hours post-exposure.
3.3.2.2. Ventilated Session
None of the nonsmokers in the ventilated session had detectable blood cannabinoids at baseline. Four of the six nonsmokers had detectible levels of blood cannabinoids immediately following exposure. These levels were lower than those observed in the unventilated session (Time 0 mean = 0.7 ng/ml), and were not detectable after Time 0. None of these participants had detectible levels of 11-OH-THC following exposure. No urine samples collected from nonsmokers in the ventilated session screened positive, even using the lower (ELISA >20 ng/ml) cut-off level.
3.4. Physiological Effects
3.4.1. Smokers
Table 2 shows detailed outcomes and results from analyses comparing baseline values to values from the first hour post-exposure for physiological, subjective, and behavioral/cognitive study measures. No significant effects on heart rate were observed using the mixed effects regression model that compared baseline to the first hour post exposure (see Table 2). Follow-up analyses that compared baseline heart rate to heart rate at Time 0 indicated no main effect of ventilation condition [beta = 1.21 (SEM = 3.33), p = 0.72], a significant main effect of time, [beta = 10.00 (SEM = 3.20) p = .002), and no ventilation condition by time interaction [beta = −4.83 (SEM = 4.53), p = 0 .29]. These results indicate that heart rate was significantly higher at Time 0 compared with baseline (heart rate increased from 66.8 BPM vs. 76.8 BPM in the unventilated session, and 70.0 BPM vs. 75.2 BPM in the ventilated session following cannabis exposure) and that there was no statistically significantly difference in the effect of time on heart rate between the unventilated and ventilated conditions. A modest but statistically significant reduction in systolic blood pressure was observed after cannabis use among smokers (see Table 2). No effect on diastolic blood pressure was observed.
Table 2.
Physiological, subjective, and behavioral/cognitive measures among smokers in both sessions, nonsmokers in the unventilated session, and nonsmokers in the ventilated session at the baseline assessment (pre) and the average of assessments during the first hour post-exposure (post). Significance levels were obtained using ANOVA, with significant differences (p < .05) between pre vs. post-exposure assessments are shown in bold.
| Smokers (n=7) | Nonsmokers, Unventilated (n=6) | Nonsmokers, Ventilated (n=6) | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Pre | Post | p-value | Pre | Post | p-value | Pre | Post | p-value | |
| Physiological | |||||||||
| Heart rate (BPM) | 69 | 72 | .21 | 66 | 69 | .41 | 68 | 70 | .23 |
| Systolic blood pressure (mm Hg) | 132 | 122 | .001 | 127 | 132 | .06 | 126 | 126 | .87 |
| Diastolic blood pressure (mm Hg) | 73 | 69 | .06 | 75 | 76 | .67 | 72 | 73 | .35 |
| Subjective (DEQ - VAS, 0–100) | |||||||||
| feel drug effect | 0 | 37 | <.001 | 0 | 16 | <.001 | 0 | 1 | .26 |
| unpleasant drug effect | 0 | 1 | .11 | 0 | 3 | .12 | 0 | 1 | .32 |
| good drug effect | 0 | 44 | <.001 | 0 | 20 | <.001 | 0 | 1 | .51 |
| sick | 0 | 0 | .24 | 1 | 1 | .73 | 1 | 1 | .88 |
| heart racing | 0 | 1 | .10 | 0 | 3 | .48 | 0 | 1 | .64 |
| anxious | 0 | 1 | .12 | 2 | 4 | .68 | 5 | 2 | .10 |
| relaxed | 35 | 49 | .04 | 58 | 47 | .31 | 54 | 59 | .41 |
| paranoid | 0 | 1 | .20 | 2 | 1 | .28 | 1 | 1 | .79 |
| tired | 26 | 25 | .88 | 21 | 45 | .009 | 41 | 39 | .85 |
| alert | 32 | 37 | .48 | 67 | 41 | .02 | 61 | 60 | .88 |
| irritable | 1 | 3 | .43 | 9 | 7 | .84 | 14 | 16 | .23 |
| vigorous | 19 | 30 | .049 | 41 | 21 | <.001 | 34 | 29 | .42 |
| restless | 2 | 6 | .25 | 15 | 13 | .81 | 5 | 5 | .96 |
| hungry/munchies | 5 | 34 | <.001 | 14 | 42 | .001 | 3 | 22 | .02 |
| craving cannabis | 41 | 26 | .004 | 0 | 2 | .19 | 0 | 0 | 1.00 |
| Behavioral/Cognitive | |||||||||
| Divided Attention Task | |||||||||
| average distance from target | 16.4 | 21.3 | .03 | 21.6 | 23.0 | .59 | 17.5 | 18.9 | .31 |
| mean overall response time (miliseconds) | 1105 | 1287 | .11 | 1228 | 1213 | .84 | 1252.0 | 1205.0 | .57 |
| total number correct | 21.3 | 21.0 | .71 | 22.5 | 21.9 | .31 | 22.0 | 22.2 | .67 |
| Digit Symbol Substitution Test | |||||||||
| total attempted | 51.1 | 50.2 | .44 | 44.0 | 47.7 | .01 | 43.3 | 46.8 | .009 |
| total correct | 48.5 | 47.7 | .56 | 42.3 | 44.4 | .26 | 41.8 | 45.3 | .03 |
| percent correct | 95 | 95 | .93 | 97.0 | 93.0 | .02 | 96.0 | 97.0 | .73 |
| Paced Auditory Serial Addition Test | |||||||||
| total correct | 70.8 | 69.9 | .68 | 73.0 | 70.4 | .23 | 76.5 | 81.9 | .04 |
| mean response time on correct items (miliseconds) | 1207 | 1257 | .51 | 1434.0 | 1147.0 | .12 | 1204.0 | 1066.0 | .47 |
Note. BPM = beats per minute, mm Hg = millimeters of mercury, DEQ = drug effect questionnaire, VAS = visual analog scale
3.4.2. Nonsmokers
3.4.2.1. Unventilated Session
No significant effect on heart rate was observed with the initial fixed effect regression model comparing baseline heart rate to heart rate during the first hour post exposure (see Table 2). Follow up analyses comparing baseline heart rate to heart rate at Time 0 indicated a small, but statistically significant increase in heart rate [65.7 BPM vs. 71.7 BPM, beta = 6.00 (SEM = 2.78) p = 0.03]. There was a non-significant trend toward increased systolic blood pressure following exposure and no effect on diastolic blood pressure (see Table 2).
3.4.2.2. Ventilated Session
No significant effects on heart rate or blood pressure were observed (see Table 2).
3.5. Subjective Effects
3.5.1. Smokers
Ratings of “drug effect,” “pleasant drug effect,” “relaxed,” “vigorous,” and “hungry/have munchies” were significantly higher than baseline during the hour following cannabis exposure. Craving for cannabis was significantly lower than baseline during this period. All of these effects resolved (i.e., returned to baseline) within 4 hours.
3.5.2. Nonsmokers
3.5.2.1. Unventilated Session
Ratings of “drug effect,” “pleasant drug effect,” “tired,” and “hungry/have munchies” were significantly higher than baseline during the hour post exposure. Ratings of “alert” and “vigorous” were significantly lower. These effects resolved within 1.5 hours post exposure.
3.5.2.2. Ventilated session
Ratings of “hungry/have munchies” increased significantly from baseline during the first hour post exposure. No other subjective ratings showed significant changes.
3.6. Behavioral/Cognitive Effects
3.6.1. Smokers
Average cursor distance from target increased significantly from baseline during the first hour post exposure on the DAT, indicating decreased tracking accuracy. Performance did not differ from baseline on any other measures examined.
3.6.2. Nonsmokers
3.6.2.1. Unventilated session
The number of DSST patterns attempted increased significantly from baseline during the hour post-exposure, but the percentage of attempted patterns that were completed correctly decreased significantly. There were no significant changes in total number correct on the DSST or performance on the DAT or PASAT.
3.6.2.2. Ventilated session
The number of DSST patterns attempted and the number completed correctly increased significantly from baseline during the hour post exposure without a change in percent correct. Total number correct on the PASAT also increased significantly from baseline. Reaction time on the PASAT did not change, nor did performance on the DAT.
4. DISCUSSION
This report describes the first study of which we are aware to examine the influence of room ventilation on secondhand exposure to cannabis smoke, and the first study to examine the effects of secondhand cannabis smoke exposure on behavioral/cognitive performance. Exposure to secondhand cannabis smoke in an unventilated chamber the size of a small room produced minor increases in heart rate, mild to moderate subjective drug effects, and minor, but detectible, levels of performance impairment on some behavioral/cognitive assessments. The time course of these effects paralleled the levels of psychoactive cannabinoids in blood and was followed by detectable levels of THC-COOH in urine. The THC-COOH concentration in one specimen was sufficient to trigger a positive urine drug screen at a cut-off of >50 ng/ml (i.e., the cut-off recommended for use by the SAMHSA mandatory guidelines for federal workplace drug testing programs), and multiple positive urine results were observed for four participants using a more stringent cut-off of >20 ng/ml, which is used by some commercial/private workplace drug testing programs, within a day of exposure. Ventilating the exposure chamber in a manner that approximated standard residential heating or air conditioning units dramatically reduced levels of exposure among nonsmokers, evidenced by much lower (in some cases undetectable) levels of cannabinoids in blood and urine and the absence of subjective and behavioral/cognitive effects.
We observed some notable patterns in subjective drug effects. First, the effects reported by nonsmokers in the unventilated condition were small in magnitude and predominantly sedative in nature (increased ratings of “tired” decreased ratings of “alert” and “vigorous”). In contrast, the subjective effects reported by active smokers were larger in magnitude and not exclusively sedative (increases in both “relaxed” and “vigorous”). Differential effects after smoking high-potency cannabis (9.75% and 23.12% THC) have been reported elsewhere in the literature (e.g., decreases in both “alertness” and in “calmness”; Hunault et al., 2014). Second, although absolute levels were low, the rise and fall of blood cannabinoid levels observed in nonsmokers following exposure in the unventilated condition paralleled the time course of subjective effects. Because nonsmokers in the ventilated condition did not report subjective drug effects, it is unlikely that the subjective intoxication reported by nonsmokers after the unventilated session was the result of social contagion, since nonsmoking participants were around equivalently-intoxicated active smokers during both study sessions while assessments were being completed. Furthermore, in a prior study of controlled smoked cannabis in our laboratory, subjective drug effects remained elevated from baseline an hour after exposure, while mean plasma THC levels had returned to baseline (about 7 ng/mL) (Milman et al., 2014; Vandrey et al., 2013). Indeed, blood cannabinoid concentrations typically peak immediately after inhalation and degrade rapidly, and do not necessarily reflect levels of intoxication (see Hollister et al., 1981). However, the influence of expectancy effects resulting from the smokiness of the chamber in the unventilated session was not controlled for, may have affected results, and should be considered when interpreting these outcomes.
Nonsmokers in the ventilated session did not have detectable levels of cannabinoids in blood beyond the initial 30 minutes following the exposure period, did not screen positive on urine tests, and did not report significant increases in subjective drug effects except for “hungry/have munchies.” Because this increase did not correspond with any other ratings of drug effect, we interpret the increase in hunger as unrelated to cannabis exposure (lunch hour was approaching). These findings suggest that ventilation dramatically reduces secondhand smoke exposure, even when large quantities of cannabis (~16.5 grams) are smoked in relatively small spaces in a short amount of time. Although room ventilation reduced levels of exposure among nonsmokers, ventilation appeared to have very little effect on levels of cannabinoids in blood or subjective effects among smokers. Smokers consumed an average of 2.6 grams of cannabis each through active smoking, thus, the comparatively small amounts of THC absorbed passively by smokers in the unventilated condition were likely not sufficient to produce additional effects.
The finding that exposure to secondhand cannabis smoke under extreme unventilated conditions can produce minor, but detectible impairments in performance on a task that measures psychomotor ability and working memory is novel. Passive exposure increased speed of responding but decreased accuracy, reflecting speed-accuracy trade-offs that are consistent with the effects of THC on behavioral/cognitive performance observed among infrequent cannabis users (e.g., Curran et al., 2002). In addition, individuals in the ventilated session showed post-exposure increases in both speed and accuracy on the DSST, and increases in accuracy on the PASAT. The lack of such practice effects among individuals in the unventilated condition may be further evidence of impaired performance, but that cannot be determined with confidence based on the design and small sample size of the present study.
Interestingly, although smokers consumed large quantities of cannabis, had increased blood cannabinoid levels, and reported moderate to high levels of subjective effects, the expected increase in heart rate commonly associated with cannabis use was relatively small and only very minor signs of decreased performance on one measure of behavioral/cognitive performance (i.e., the DAT) were observed. This may reflect pharmacodynamic tolerance, as all of the active smokers were chronic heavy cannabis users. It is also possible that larger effects on heart rate and performance were missed. Smokers consumed cannabis over a 60-minute time period, and consumed 34% less cannabis during the second 30 minutes of the exposure period than during the first 30 minutes. Thus, it is possible that more robust physiological changes occurred and smokers were impaired during the exposure period, but this impairment resolved prior to post-exposure testing. The effects of cannabis smoking on heart rate may resolve within 45 minutes of smoking (Vandrey et al., 2013; Cooper and Haney, 2014), and heart rate was assessed 30, 60, and 90 minutes after many of the active smokers had ceased intensive smoking. The fact that significant differences in heart rate were observed among smokers when comparing baseline to Time 0, but not when comparing baseline to the first hour post exposure suggests that any effects on heart rate were transient. Although acute THC has been shown to reliably produce cognitive/working memory impairment (see Ranganathan and D’Souza, 2006 for review), many of these studies recruited infrequent or moderate cannabis users. Heavier users may show little if any impairment (Ramaekers et al., 2009). Alternatively, the absence of observed cognitive effects may be the result of acute tolerance within study sessions. Additional research evaluating the effects of cannabis on working memory with infrequent users as smokers utilizing the current study procedure would help with interpretation of these results.
The results of this study must be considered in light of its limitations. First, we did not conduct a placebo exposure session (i.e., session where cannabis devoid of THC was smoked), making it impossible to determine whether the subjective and behavioral/cognitive effects observed among active smokers and among nonsmokers in the unventilated session were the result of THC exposure or of expectancy. The design of this study would make a placebo session difficult, as active smokers would likely recognize 0% THC cannabis as placebo during the study session. Thus, it would be unlikely they would smoke sufficient quantities, and likely that they would be vocal about the absence of drug effects during the study session, thereby unblinding nonsmokers. There were a relatively small number of participants in each study session, and all participants were exposed to cannabis smoke and completed assessments simultaneously in the same laboratory room. As a result, it is possible that subjective and behavioral/cognitive effects reported by non-smokers may have been influenced by suggestibility or study demand characteristics. However, the finding that nonsmokers in the ventilated session did not show any drug effects, and that the effects observed among nonsmokers in the unventilated session closely paralleled levels of cannabinoids in blood indicate these effects may be the result of passively-absorbed THC. In addition, the experimental conditions created here do not reflect the full spectrum of secondhand cannabis smoke exposure scenarios that occur outside of the laboratory, and should not be interpreted to reflect normative conditions. This study models two scenarios of acute exposure in an enclosed room. The size of room, amount of cannabis consumed, duration of exposure, and frequency of such exposure are all variables that likely would influence outcomes in the real world. That said, several study participants reported previous secondhand exposure experiences that resembled the unventilated study condition, which indicates our exposure model has some degree of ecological validity. Furthermore, testing the effects of passive exposure among a balanced sample of women and men should be regarded as a strength because it speaks to the generality of our results.
In conclusion, this study indicates that absorption of cannabinoids can result from secondhand exposure to cannabis smoke. Room ventilation had a significant impact on the degree of cannabinoid absorption and on resultant pharmacodynamic effects. Nonsmokers exposed under unventilated conditions reported low to moderate levels of sedative drug effects that corresponded with minor impairment in cognitive performance, while nonsmokers exposed under ventilated conditions reported no significant subjective effects and did not have impairment in cognitive performance. These results suggest that extreme conditions like those examined in this study may result in biological exposure sufficient to produce measureable subjective effects, decreases in behavioral/cognitive performance, and could produce a positive drug test result within a short window of time following exposure.
Highlights.
Exposure to secondhand cannabis smoke results in absorption of cannabinoids.
Secondhand exposure can produce mild subjective and behavioral/cognitive effects
Room ventilation ameliorates the effects of secondhand cannabis smoke exposure.
Acknowledgments
Role of Funding Source
Funding for this project was provided by SAMHSA. Additional resources were provided by: (1) the Johns Hopkins Clinical Research Unit, which is funded by Grant UL1 RR025005 from the National Center for Research Resources (NCRR), a component of the NIH, and NIH Roadmap for Medical Research, (2) NIDA training grant T32-DA07209, which supported Dr. Herrmann, and (3) The NIDA Drug Supply Program for providing cannabis.
The authors thank Jeannie M. Leoutsakos for statistical support, the outstanding support of the research, nursing, and pharmacy staff of the Johns Hopkins Behavioral Pharmacology Research Unit (BPRU), the Johns Hopkins Bayview Facilities group, Johns Hopkins Bayview Clinical Research Unit, Support staff at RTI International, and the U.S. Substance Abuse and Mental Health Services Administration (SAMHSA). Without the coordinated effort of all these people, this study would not have been possible.
Footnotes
Contributors
Authors Edward Cone, John Mitchell, George Bigelow, Charles LoDico, Ron Flegel, and Ryan Vandrey designed the study and developed the protocol. Evan Herrmann, Edward Cone, and Ryan Vandrey managed literature searches and summaries of previous work. Evan Herrmann and Ryan Vandrey undertook the statistical analysis. Evan Herrmann, Edward Cone, John Mitchell, George Bigelow, Charles LoDico, Ron Flegel, and Ryan Vandrey wrote the final draft of the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of Interest
All authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Casswell S, Marks D. Cannabis induced impairment of performance of a divided attention task. Nature. 1973;241:60–61. doi: 10.1038/241060b0. [DOI] [PubMed] [Google Scholar]
- Cone EJ, Bigelow GE, Herrmann ES, Mitchell JM, LoDico C, Flegel R, Vandrey R. Non-smoker exposure to secondhand cannabis smoke. I Urine screening and confirmation results. J Anal Toxicol. 2015;39:1–12. doi: 10.1093/jat/bku116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone EJ, Johnson RE. Contact highs and urinary cannabinoid excretion after passive exposure to marijuana smoke. Clin Pharmacol Ther. 1986;40:247–256. doi: 10.1038/clpt.1986.171. [DOI] [PubMed] [Google Scholar]
- Cone EJ, Johnson RE, Darwin WD, Yousefnejad D, Mell LD, Paul BD, Mitchell J. Passive inhalation of marijuana smoke: urinalysis and room air levels of delta-9-tetrahydrocannabinol. J Anal Toxicol. 1987;11:89–96. doi: 10.1093/jat/11.3.89. [DOI] [PubMed] [Google Scholar]
- Cooper ZD, Haney M. Investigation of sex-dependent effects of cannabis in daily cannabis smokers. Drug Alcohol Depend. 2014;136:85–91. doi: 10.1016/j.drugalcdep.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran VH, Brignell C, Fletcher S, Middleton P, Henry J. Cognitive and subjective dose-response effects of acute oral Δ9-tetrahydrocannabinol (THC) in infrequent cannabis users. Psychopharmacology. 2002;164:61–70. doi: 10.1007/s00213-002-1169-0. [DOI] [PubMed] [Google Scholar]
- Fehr KOB, Kalant H. Analysis of cannabis smoke obtained under different combustion conditions. Can J Physiol Pharmacol. 1972;50:761–767. doi: 10.1139/y72-111. [DOI] [PubMed] [Google Scholar]
- Gronwall DM. Paced auditory serial-addition task: a measure of recovery from concussion. Percept Mot Skills. 1977;44:367–373. doi: 10.2466/pms.1977.44.2.367. [DOI] [PubMed] [Google Scholar]
- Gueorguieva R, Krystal JH. Move over anova: Progress in analyzing repeated-measures data andits reflection in papers published in the Archives of General Psychiatry. Arch Gen Psychiatry. 2004;61:310–317. doi: 10.1001/archpsyc.61.3.310. [DOI] [PubMed] [Google Scholar]
- Hollister LE, Gillespie HK. Action of delta-9-tetrahydrocannabinol. An approach to the active metabolite hypothesis. Clin Pharmacol Ther. 1975;18:714–719. doi: 10.1002/cpt1975186714. [DOI] [PubMed] [Google Scholar]
- Hollister LE, Gillespie HK, Ohlsson A, Lindgren JE, Wahlen A, Agurell S. Do plasma concentrations of δ9-tetrahydrocannabinol reflect the degree of intoxication? J Clin Pharmacol. 1981;21:171S–177S. doi: 10.1002/j.1552-4604.1981.tb02593.x. [DOI] [PubMed] [Google Scholar]
- Hooker WD, Jones RT. Increased susceptibility to memory intrusions and the Stroop interference effect during acute marijuana intoxication. Psychopharmacology. 1987;91:20–24. doi: 10.1007/BF00690920. [DOI] [PubMed] [Google Scholar]
- Hunault CC, Böcker KB, Stellato RK, Kenemans JL, de Vries I, Meulenbelt J. Acute subjective effects after smoking joints containing up to 69 mg Δ9-tetrahydrocannabinol in recreational users: a randomized, crossover clinical trial. Psychopharmacology. 2014 doi: 10.1007/s00213-014-3630-2. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Huestis MA, Henningfield JE, Cone EJ. Blood cannabinoids. I Absorption of THC and formation of 11-OH-THC and THCCOOH during and after smoking marijuana. J Anal Toxicol. 1992;16:276–282. doi: 10.1093/jat/16.5.276. [DOI] [PubMed] [Google Scholar]
- Järbe TU, Mechoulam R, Zahalka J. Discriminative stimulus-and open-field effects of the enantiomers of 11-hydroxy-delta-8-tetrahydrocannabinol in pigeons and gerbils. Pharmacol Biochem Behav. 1994;47:113–119. doi: 10.1016/0091-3057(94)90119-8. [DOI] [PubMed] [Google Scholar]
- Kosersky DS, McMillan DE, Harris LS. Δ9-Tetrahydrocannabinol and 11-hydroxy-Δ9-tetrahydrocannabinol: behavioral effects and tolerance development. J Pharmacol Exp Ther. 1974;189:61–65. [PubMed] [Google Scholar]
- Law B, Mason PA, Moffat AC, King LJ, Marks V. Passive inhalation of cannabis smoke. J Pharm Pharmacol. 1984;36:578–581. doi: 10.1111/j.2042-7158.1984.tb04901.x. [DOI] [PubMed] [Google Scholar]
- Lemberger L, Martz R, Rodda B, Forney R, Rowe H. Comparative pharmacology of Δ9-tetrahydrocannabinol and its metabolite, 11-OH-Δ9-tetrahydrocannabinol. J Clin Invest. 1973;52:2411–2417. doi: 10.1172/JCI107431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason AP, Perez-Reyes M, McBay AJ, Foltz RL. Cannabinoid concentrations in plasma after passive inhalation of marijuana smoke. J Anal Toxicol. 1983;7:172–174. doi: 10.1093/jat/7.4.172. [DOI] [PubMed] [Google Scholar]
- Matthias P, Tashkin DP, Marques-Magallanes JA, Wilkins JN, Simmons MS. Effects of varying marijuana potency on deposition of tar and δ9 thc in the lung during smoking. Pharmacol Biochem Behav. 1997;58:1145–1150. doi: 10.1016/s0091-3057(97)00328-6. [DOI] [PubMed] [Google Scholar]
- McLeod DR, Griffiths RR, Bigelow GE, Yingling J. An automated version of the digit symbol substitution test (DSST) Behav Res Methods. 1982;14:463–466. [Google Scholar]
- Mehmedic Z, Chandra S, Slade D, Denham H, Foster S, Patel AS, Ross SA, Khan IA, ElSohly MA. Potency trends of Δ9-THC and other cannabinoids in confiscated cannabis preparations from 1993 to 2008. J Forensic Sci. 2010;55:1209–1217. doi: 10.1111/j.1556-4029.2010.01441.x. [DOI] [PubMed] [Google Scholar]
- Milman G, Bergamaschi MM, Lee D, Mendu DR, Barnes AJ, Vandrey R, Huestis MA. Plasma cannabinoid concentrations during dronabinol pharmacotherapy for cannabis dependence. Ther Drug Monit. 2014;36:218–224. doi: 10.1097/FTD.0b013e3182a5c446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore C, Coulter C, Uges D, Tuyay J, Van der Linde S, Van Leeuwen A, Garnier M, Orbita J., Jr Cannabinoids in oral fluid following passive exposure to marijuana smoke. Forensic Sci Int. 2011;212:227–230. doi: 10.1016/j.forsciint.2011.06.019. [DOI] [PubMed] [Google Scholar]
- Mørland J, Bugge A, Skuterud B, Steen A, Wethe GH, Kjeldsen T. Cannabinoids in blood and urine after passive inhalation of Cannabis smoke. J Forensic Sci. 1985;30:997–1002. [PubMed] [Google Scholar]
- Niedbala RS, Kardos KW, Fritch DF, Kunsman KP, Blum KA, Newland GA, Waga J, Kurtz L, Bronsgeest M, Cone EJ. Passive cannabis smoke exposure and oral fluid testing. II Two studies of extreme cannabis smoke exposure in a motor vehicle. J Anal Toxicol. 2005;29:607–615. doi: 10.1093/jat/29.7.607. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes M, Davis KH, Di Guiseppi S. Passive inhalation of marijuana smoke and urinary excretion of cannabinoids. JAMA. 1983;249:475–475. doi: 10.1001/jama.1983.03330280025021. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes M, Timmons MC, Ipton MA, Davis KH, Wall ME. Intravenous injection in man of Δ9-tetrahydrocannabinol and 11-OH-Δ9-tetrahydrocannabinol. Science. 1972;177:633–635. doi: 10.1126/science.177.4049.633. [DOI] [PubMed] [Google Scholar]
- Ramaekers JG, Kauert G, Theunissen EL, Toennes SW, Moeller MR. Neurocognitive performance during acute THC intoxication in heavy and occasional cannabis users. J Psychopharmacology. 2009;23:266–277. doi: 10.1177/0269881108092393. [DOI] [PubMed] [Google Scholar]
- Ranganathan M, D’souza DC. The acute effects of cannabinoids on memory in humans: a review. Psychopharmacology. 2006;188:425–444. doi: 10.1007/s00213-006-0508-y. [DOI] [PubMed] [Google Scholar]
- Vandrey R, Stitzer ML, Mintzer MZ, Huestis MA, Murray JA, Lee D. The dose effects of short-term dronabinol (oral thc) maintenance in daily cannabis users. Drug Alcohol Depend. 2013;128:64–70. doi: 10.1016/j.drugalcdep.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson WH, Ellinwood EH, Mathew RJ, Johnson K. Effects of marijuana on performance of a computerized cognitive-neuromotor test battery. Psychiatry Res. 1994;51:115–125. doi: 10.1016/0165-1781(94)90031-0. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Facts and figures, cannabis. 2014 http://www.who.int/substance_abuse/facts/cannabis/en/