Abstract
The cellular mechanisms regulating branching and growth of the intersegmental vessels (ISVs) are not well understood. We have carried out studies demonstrating that Hedgehog (Hh) signaling is a major regulator of intersomitic vessel growth. Inhibition of Hh activity by cyclopamine completely blocks formation of intersomitic vessels in the avian embryo. Examination of gene expression patterns in Hh deficient embryos shows that components of the VEGF and Notch signaling pathways are down-regulated. However, we find no evidence that Notch signaling plays a significant role in regulation of intersomitic vessel growth. Indeed it appears that Hh modulation of Vascular Endothelial Growth Factor, VEGF, is the primary regulator of growth of intersomitic vessels in the avian embryo. Inhibition of the VEGF pathway results in absence of ISVs, whereas stimulation of VEGF expression leads to precocious branching of ISVs. These results demonstrate that Hh is an essential modulator of VEGF expression during developmental angiogenesis.
Keywords: Hedgehog, SAG, Smoothened agonist, cyclopamine, endothelial development, ISV, DLL4, DAPT
Introduction
The first blood vessels in the embryo are formed by the process of vasculogenesis, which is the assembly of free angioblasts into vascular cords, followed by lumen formation to generate hollow vascular tubes. Subsequent development of the vascular system occurs by angiogenesis. The term, angiogenesis, covers a number of different cellular processes that result in remodeling of the original vascular plexus and includes the formation of branches from primary vessels into avascular tissues. Formation of the intersegmental vessels (ISVs), which branch from the paired dorsal aortae, dorsally between the somites, towards the paired posterior cardinal veins, is amongst the earliest examples of branching angiogenesis in the embryo.
Hedgehog signaling is mediated by three diffusible ligands, Sonic hedgehog, Desert Hedgehog and Indian Hedgehog plus the Hh co-receptors, Smoothened and Patched (Varjosalo and Taipale, 2008). Previous studies have shown that signaling by Hh is essential for correct development of the embryonic vascular system (Brown et al., 2000; Byrd et al., 2002; Vokes et al., 2004; Coultas et al., 2010). The most compelling evidence comes from mouse embryos lacking SMO or PTCH1 function, which results in inhibition or stimulation respectively, of the Hh signaling pathway. Smo knockout embryos die due to severe disruption of vasculogenesis, including lack of endothelial tube formation (Coultas et al., 2010; Vokes et al., 2004). Stimulation of Hh signaling in Ptch1 null embryos also results in early embryonic death due to vascular defects, including formation of dilated dorsal aortae (Coultas et al., 2010). Multiple lines of evidence suggest that Hh does not interact directly with endothelial cells (Byrd and Grabel, 2004; Coultas et al., 2010; Moran et al., 2011). Instead it seems that Hh first signals to non-endothelial tissues, which then express secondary growth regulators to influence growth and patterning of the endothelial network. Several distinct signaling pathways have been shown to lie downstream of Hh signaling in different developmental contexts, including VEGF, (Coultas et al., 2010; Lawson et al., 2002; Pola et al., 2002), Notch (Lawson et al., 2002; Lamont and Childs, 2006) and BMP (Astorga and Carlsson, 2007).
While the importance of Hh signaling for vasculogenesis is evident, much less is known concerning the function of Hh signaling during angiogenic growth and remodeling of the vasculature. This is primarily because mouse embryos lacking Hh signaling die early during development, prior to extensive angiogenesis. Here we report studies using the avian embryo. We find that Hh signaling is essential for the angiogenic growth of ISVs and that stimulation of Hh signaling leads to precocious growth of ISVs. These effects appear to be mediated through the action of VEGF.
Results
Hh is required for growth of intersegmental vessels
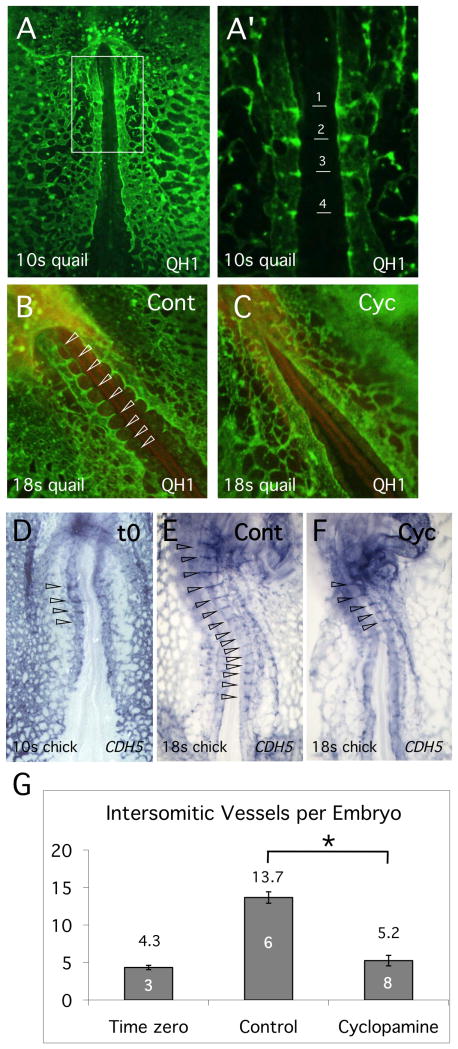
In the avian embryo, the first ISVs are visible at approximately the 8-somite stage, as short protrusions arising from the dorsal aortae, immediately rostral to the first somite. (Coffin and Poole, 1988; Pardanaud et al., 1987). As development proceeds, the first ISVs increase in length as they grows dorsally towards the posterior cardinal vein and additional short angiogenic spikes become visible between more posterior somites. By the 10-somite stage, 4 pairs of ISVs are visible (Fig. 1A, A') and additional ISVs arise in an anterior to posterior direction. To determine a possible function for Hh in regulation of ISV formation, we carried out pilot studies where Hh function was inhibited in quail embryos using the small molecule inhibitor, cyclopamine. Treatment commenced at the 10-somite stage and was continued for 8 hours until the 18-somite stage, when vascular structures were visualized using QH1 antibody. Compared to controls, cyclopamine treated quail embryos showed a dramatic reduction in the growth of ISVs (Fig. 1B,C), suggesting that Hh was important for angiogenic growth of the ISVs.
Fig. 1. Inhibition of Hh signaling blocks growth of ISVs.
(A, A') Quail embryo at approximately the 10-somite stage stained with QH1 antibody to reveal the developing endothelial network. A' is an enlargement of the image in (A), showing outgrowth of the most anterior ISVs. (B,C) Control and cyclopamine treated quail embryos respectively stained with QH1 at the 18-somite stage. Note the absence of ISVs in the cyclopamine treated embryo compared to controls. ISVs are indicated by open arrowheads. (D-F) CDH5 in situ hybridization analysis of chick embryos. The developmental stage is indicated. The endothelial pattern at the onset of treatment (t0) is shown in (D), while (E,F) shows the pattern at the 18-somite stage, after approximately 8 hours of incubation, for control and cyclopamine treatment respectively. Open arrowheads indicate ISVs. (G). Quantitation of ISV growth in control and cyclopamine-treated embryos. Number of embryos assayed is indicated within the columns. Number is ISVs in control embryo is significantly different from both t0 and cyclopamine treated embryos (Student's T-test, P<0.05).
In order to confirm and quantitate these results, additional studies were carried out using chick embryos. Again, cyclopamine treatment was initiated at the 10-somite stage and continued for 8 hours. For chick embryos, vessels were visualized by in situ hybridization analysis using CDH5 (VE-Cadherin) probe. As illustrated in Fig. 1F, inhibition of Hh function effectively blocked growth of new ISVs. Quantitation of the results showed that wild type embryos progressed from about 4 ISVs at the initiation of treatment (time zero) to about 14 ISVs after 8 hours (Fig. 1G). In contrast, the number of ISVs in cyclopamine treated embryos showed little change from the number at time zero (Fig. 1G). Overall development of embryos was not delayed by cyclopamine treatment and neither the structure nor integrity of the DA itself appeared to be altered compared to controls (compare Figs. 1B,C and 1E,F). We conclude from these studies that Hh signaling is essential for ISV formation in the avian embryo.
Analysis of gene expression downstream of Hh signaling
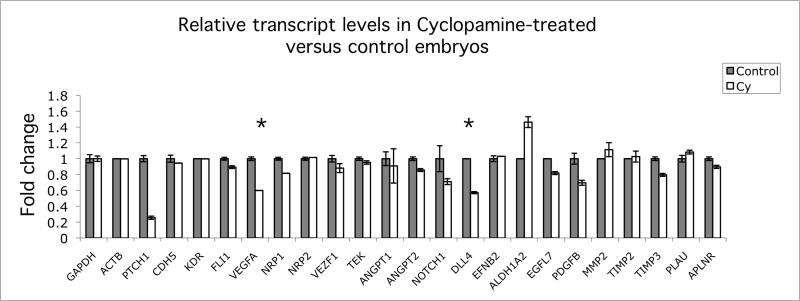
To determine which genes or signaling pathways might be responsible for reduced growth of ISVs following cyclopamine treatment, we used a candidate gene approach (Fig. 2). Briefly, qRT-PCR was used to assay transcript levels for about 20 genes, all of which have previously been implicated in modulation of endothelial cell proliferation or cell behavior. Reduction of expression of the Hh coreceptor, PTCH1, was used as a positive control to confirm that Hh signaling had been successfully inhibited (Pearse et al., 2001). The large majority of transcripts, including the endothelial marker genes CDH5 and KDR (the VEGF receptor), were unchanged in response to inhibition of Hh signaling (Fig. 2). Of the 21 genes examined only two, Vascular Endothelial Growth Factor-A (VEGFA) and Delta-like 4 (DLL4), showed clear reduction in transcript levels following cyclopamine treatment. Both VEGFA and DLL4 transcripts were reduced to approximately half of control levels after cyclopamine treatment (60% and 57% respectively). These genes are of particular interest as possible regulators of endothelial function, since previous studies have placed both VEGF and Notch signaling pathways downstream of Hh regulation (Coultas et al., 2010; Lawson et al., 2002; Pola et al., 2001). The slight differences observed for relative transcript levels of ANGPT1, PDGFB and ALDH1A2 were not reproduced in subsequent experiments (data not shown).
Fig. 2. Quantitative RT-PCR analysis of relative gene expression in control versus Hh inhibited chick embryos.
RNA samples were normalized relative to GAPDH and ACTB transcript levels. Reduction in PTCH1 expression was used as a positive control for inhibition of Hh pathway. Only VEGFA and DLL4 transcript levels (indicated by asterisk) were reproducibly altered when changes were confirmed using additional embryonic samples. Primers used for this study are provided in Table S1 and Experimental Procedures.
Notch signaling is not required for growth of intersomitic vessels in the chick embryo
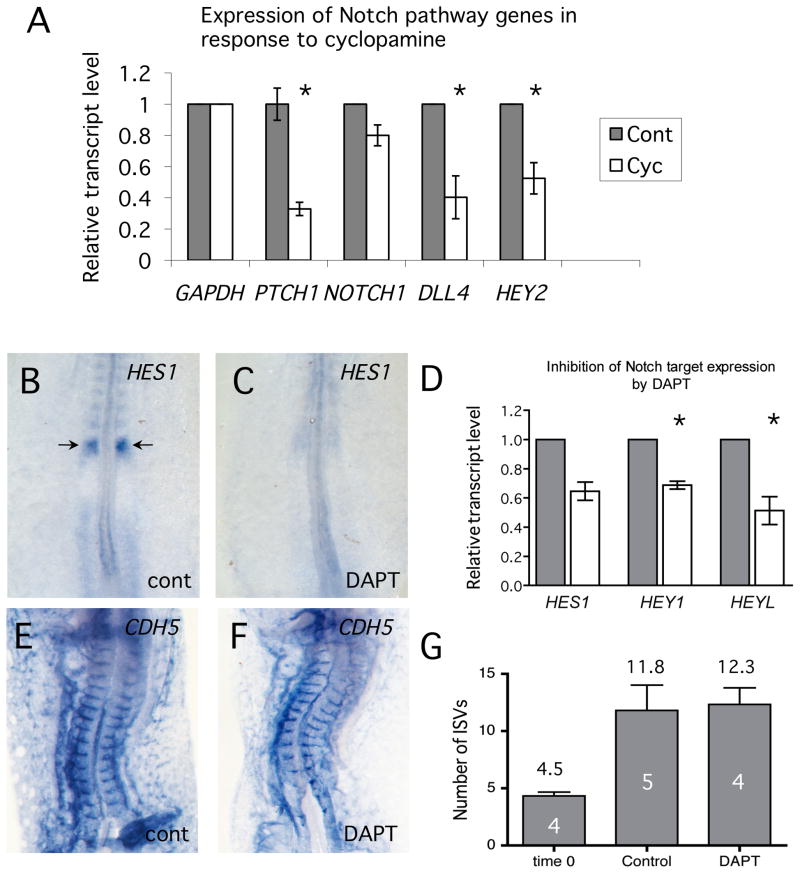
Notch signaling is essential for normal vascular development (Alva and Iruela-Arispe, 2004). Ablation of many individual components of the Notch signaling network result in vascular defects and the problems are often much more severe when redundant activities are depleted. Our qRT-PCR assays (Fig. 2) indicated that expression of at least one component of the Notch signaling pathway, DLL4, was decreased when Hh signaling was inhibited. Down-regulation of DLL4 and to a lesser extent, NOTCH1, in cyclopamine-treated embryos was confirmed using independent samples from 4 different embryos (Fig. 3A). Expression of the endothelial transcription factor, HEY2, which is a direct target of Notch regulation and therefore functions as a marker for active Notch signaling, was also significantly reduced following cyclopamine treatment.
Fig. 3. Growth of ISVs in the chick embryo is independent of Notch signaling.
(A) Quantitative RT-PCR analysis of Notch signaling components in control and cyclopamine-treated embryos. Reduction in PTCH1 expression was used as a positive control for inhibition of Hh pathway. RNA from 4 independent samples was assayed. Changes in DLL4 and HEY2 transcript levels (asterisk) were significant compared to controls (Student's T-test, P<0.05), but NOTCH1 levels were not. (B-D) DAPT inhibits Notch signaling in the chick embryo. B,C show in situ analysis of HES1 expression in control and DAPT-treated chick embryos. (D). qRT-PCR analysis of direct targets of Notch regulation. Gray bars are controls and open bars are DAPT-treated. Expression of target genes is inhibited to approximately half of control levels. Significant differences between treatments (Student's T-test, P<0.05) are indicated by asterisks. (E,F) In situ hybridization analysis of CDH5 showed that ISVs formed normally in embryos in which Notch signaling was inhibited. (G) Quantitation of ISV numbers in control and DAPT-treated embryos. Number of embryos assayed is indicated within the columns. There was no significant difference in number of ISVs when Notch signaling was inhibited using DAPT.
To directly test for involvement of the Notch pathway in regulation of ISV branching angiogenesis, we used the gamma-secretase inhibitor, DAPT, to inhibit expression of Notch target genes (Daudet et al., 2007). First, we confirmed that DAPT was effective at inhibiting Notch signaling in the chick embryo. Titration studies showed the treatment of chick embryo with DAPT at 50 μM for 8 hours, strongly inhibited expression of the Notch target HES1 in developing somites compared to controls (Fig. 3B,C). Inhibition of expression of Notch target genes HES1, HEY1 and HEYL, was confirmed by qRT-PCR analysis (Fig. 3D). Treatment with DAPT for 8 hours had no detectable effect on embryonic viability as measured by normal heartbeat and morphology. We then assayed for growth of ISVs in embryos treated with DAPT. Surprisingly, inhibition of Notch signaling produced no detectable effect on growth of the ISVs. Both vascular morphology (Fig. 3E, F) and number of ISVs (Fig. 3G) were apparently identical compared to controls. We conclude from these studies that inhibition of Hh signaling reduced expression of Notch signaling genes in the chick embryo (Figs. 2, 3A) however this is not sufficient to explain the reduction of ISV growth in cyclopamine-treated chick embryos.
BMP signaling is not required for growth in intersomitic vessels in the chick embryo
Previous studies have suggested that Hh regulates BMP4 expression during mouse yolk sac vascular development (Astorga and Carlsson, 2007). In the absence of Hh signaling, Bmp4 transcript levels are decreased and the yolk sac vessels are smaller and thinner. Since both BMP2 and BMP4 are quite strongly expressed in the developing somites and the lateral plate mesoderm of the avian embryo at the time of intersegmental vessels growth (Darnell et al., 2007; Garriock et al., 2010) we determined whether Hh might regulate BMP4 signaling in the embryo proper. First, qRT-PCR studies showed no significant difference in BMP2 and BMP4 transcript levels following cyclopamine treatment, even when PTCH1 transcript levels were reduced to about 38% of control levels (Fig. S1A). In view of the multiple BMP ligands and receptors it is difficult to check for expression levels of all BMP signaling components and so instead, we assayed the level of phosphorylation of SMADs 1 and 5, which functions as a read-out of signaling activity (Tucker et al., 2008). Again, no detectable difference was observed between phospho-SMAD1/5 levels in cyclopamine treated and control embryos (Fig. S1B). We conclude from these experiments that intersegmental vessel growth is unlikely to be mediated by BMP signaling downstream of Hh.
Hh regulates VEGF expression at the time of intersegmental vessel growth
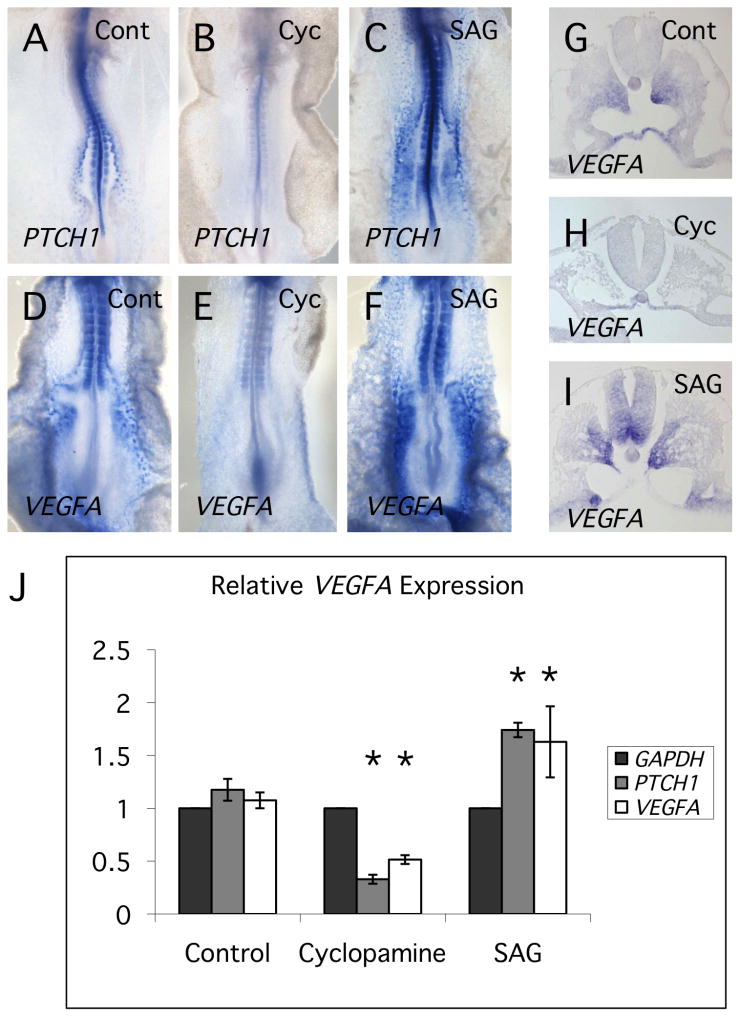
Inhibition of Hh signaling resulted in significant reduction of VEGFA transcript levels (Fig. 2). We have confirmed this result by in situ hybridization and also demonstrated that stimulation of Hh signaling using the agonist, SAG, results in increased VEGFA transcript levels (Fig. 4A-F). Sections through SAG treated embryos show that VEGFA staining is increased in the paraxial regions of the somite, the ventral neural tube and the lateral plate mesoderm (Fig. 4G-I). Each of these tissues is in the immediate vicinity of outgrowing intersegmental vessels. Alterations in VEGFA transcript levels in response to cyclopamine and SAG were confirmed by qRT-PCR (Fig. 4J).
Fig. 4. Hh signaling regulates levels of VEGFA transcript in the chick embryo.
(A-C) In situ hybridization analysis using PTCH1 probe, showing decrease and increase in staining in response to cyclopamine and SAG treatment respectively. SAG is a small molecule activator of the Hh signaling pathway. (D-F) In situ hybridization analysis using VEGFA probe, showing decreased and increased staining in response to cyclopamine and SAG treatments respectively. (G-I) Sections through in situ stained embryos, showing pattern of VEGFA expression in somites and ventral neural tube in response to altered Hh signaling. (J) Quantitative RT-PCR analysis of PTCH1 and VEGFA transcript levels in cyclopamine and SAG-treated embryos. Plotted values are relative to GAPDH transcript levels for each treatment. Significant differences between treatment and control values (Student's T-test, P<0.05) are indicated by asterisks.
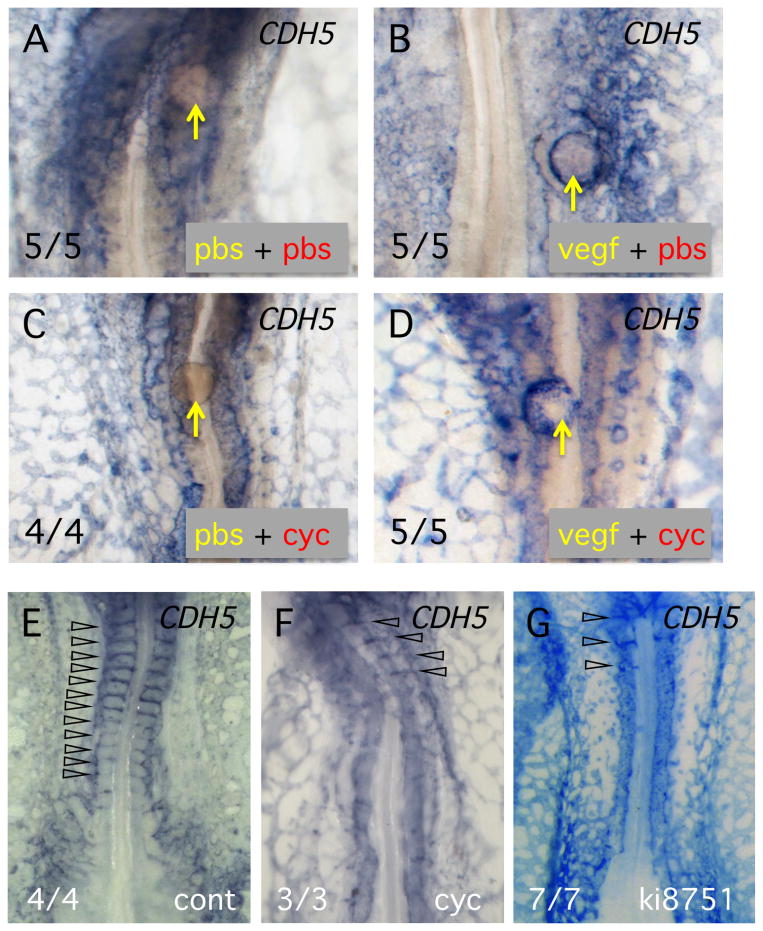
We next carried out a series of experiments to determine whether VEGF might be the critical growth factor regulating ISV growth, downstream of Hh signaling. First, experiments were performed to determine whether VEGF signaling could rescue angiogenic growth in cyclopamine-treated embryos. In this study, agarose beads were loaded with a very low concentration of recombinant VEGFA protein (2 ng/ml) or loaded with BSA as a control. Beads were placed adjacent to the DA in control or cyclopamine-treated chick embryos and after 8 hours incubation, vascular structures were assayed by in situ hybridization using CDH5 probe (Fig. 5). As expected, placing a VEGF bead in control embryos resulted is recruitment of endothelial cells, often completely surrounding the bead (5 of 5 embryos, Fig. 5B). When PBS beads were placed in embryos treated with cyclopamine, no recruitment of endothelial cells was observed (0 of 5 embryos – Fig. 5C). On the other hand, VEGF beads in cyclopamine treated embryos strongly recruited endothelial cells to the vicinity of the bead (Fig. 5D). As expected from previous studies, (Moran et al., 2011), the structure of the surrounding vascular plexus was strongly disrupted in cyclopamine-treated embryos (Fig. 5C,D). These studies did not attempt to restore normal growth of ISVs in the cyclopamine treated embryos, but do serve to demonstrate that VEGF signaling can rescue the inhibition of angiogenesis caused by inhibition of Hh signaling. Overall, these results are consistent with a role for VEGF downstream of Hh signaling.
Fig. 5. VEGF is sufficient to restore angiogenic branching in the presence of cyclopamine.
(A-D) In situ hybridization analysis using CDH5 probe to detect vascular structures. Yellow lettering indicates the solution on the bead and red lettering the incubation medium. Number of embryos showing the response is indicated at lower left. (E-G) Inhibition of VEGF signaling mimics the effect of reduced Hh signaling. In situ hybridization analysis using CDH5 probe. Treatment is indicated at lower right. Note that incubation of embryos with cyclopamine, or the VEGF signaling inhibitor, ki8751, eliminated ISV growth. ISVs are indicated by open arrowheads. Number of embryos showing the response is indicated at lower left.
If VEGF normally mediates the effects of Hh signaling during growth of intersomitic vessels, then specific inhibition of VEGF signaling should prevent growth of the vessels. To inhibit VEGF signaling we used the small molecule inhibitor, ki8751, which is highly specific for the VEGFA receptor, KDR (Kubo et al., 2005). A range of doses of inhibitor from 0.01 to 1.0 μM was applied to chick embryos at the 10-somite stage and after 8 hours the embryos were assayed for vascular structures by in situ hybridization. At the lowest dose of ki8751 used (0.01 μM) vascular structures were indistinguishable from controls, while at 0.5 μM, the dorsal aortae and the vascular plexus disintegrated (data not shown). However, at the intermediate dose of 0.1 μM, the effect on vascular patterning was broadly equivalent to cyclopamine inhibition (compare Fig. 5F,G). At this dose the vascular plexus was less contiguous and there was a complete absence of growth of new intersegmental vessels. This result is consistent with the hypothesis that VEGF signaling, mediated via KDR, is required for normal outgrowth of the intersomitic vessels.
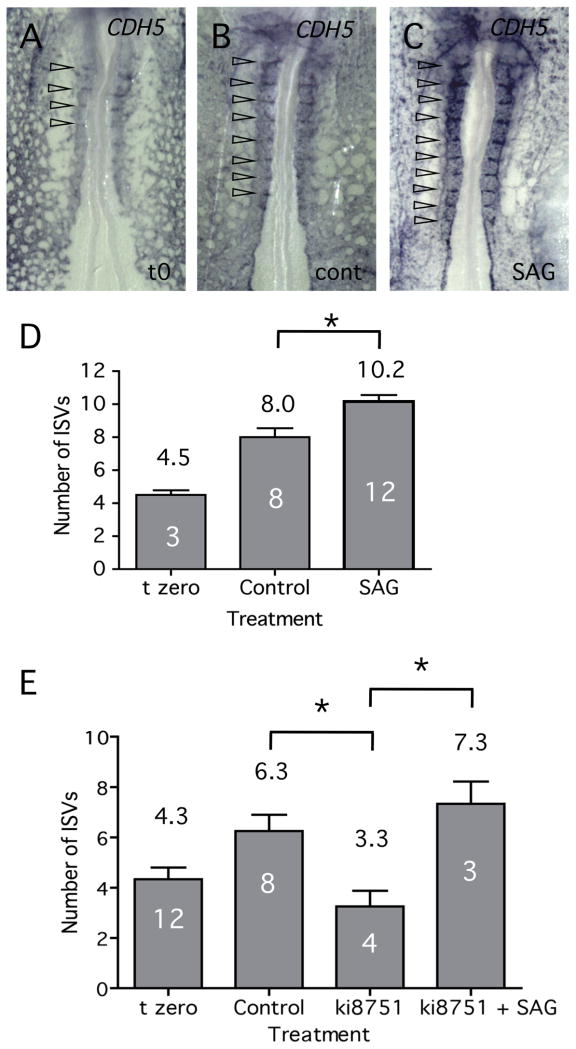
In situ hybridization and qRT-PCR assays demonstrated that treatment of embryos with the Hh agonist, SAG, resulted in up-regulation of VEGFA transcript levels (Fig. 4D-F, J). If VEGF is indeed mediating the effects of Hh on vascular patterning, then it is possible that SAG treatment might lead to increased or precocious branching of intersegmental vessels in the chick embryo. To test this model, embryos were treated with either SAG or carrier alone for 4 hours, starting at the 10-somite stage, and then assayed for vascular structures (Fig. 6). After 4 hours, control embryos increased the number of intersegmental vessels from 4.5 at time zero, to approximately 8 (Figs. 6A, B, D). However, treatment with SAG further increased the number of intersegmental vessels to approximately 10 (Figs. 6B, D), which is significantly greater than observed for control embryos (P<0.05, Student's T-test). Previous studies have shown that VEGF signaling activates expression of Notch signaling components, including DLL4 (Lobov et al., 2007). We have used qRT-PCR to demonstrate that levels of DLL4 transcript do indeed change in response to both activation and inhibition of VEGF activity (Fig. S2). Therefore, alteration of VEGF levels in response to Hh signals could explain the changes to Notch signaling components noted previously (Fig. 2 and Fig. 3A). We next determined whether modulation of VEGF activity could explain the alterations in growth of ISVs. First we confirmed that inhibition of VEGF signaling using low levels of ki8751 resulted in a block to growth of new ISVs (Fig. 6E). Second, we tested whether up-regulation of VEGF expression by SAG might rescue ISV growth when the VEGF pathway was partially blocked. As shown in Fig. 6E, simultaneous treatment of embryos with ki8751 and SAG resulted in normal ISV growth. While other explanations may be possible, this result is consistent with restoration of normal VEGF signaling levels through a balance of ki8751-mediated inhibition and SAG induced stimulation.
Fig. 6. Stimulation of Hh signaling results in precocious growth of ISVs.
(A-C) SAG treatment of chick embryos for 4 hours resulted in increased number of ISVs relative to controls. In situ hybridization analysis using CDH5 probe. (D) Quantitation of the experiment illustrated in (A-C) above. Increased Hh signaling, coupled with increased VEGF signaling, resulted in a significant increase in the number of ISVs. (E) Stimulation of VEGF expression using SAG can counteract the partial inhibition of VEGF signaling achieved through treatment with ki8751. Significant differences between treatments (Student's T-test, P<0.05) are indicated by asterisks. Number of embryos assayed is shown within the columns.
Discussion
Hh signaling is essential for formation of ISVs
Either inhibition or overactivation of Hh signaling in the mouse knockout model results in embryonic death early in development, due to failure to assemble a functional vascular endothelial system (Byrd and Grabel, 2004; Coultas et al., 2010; Ellis et al., 2003; Vokes et al., 2004). Because of this early embryonic lethality, the role of Hh signaling in regulation of later events in the formation of the embryonic vasculature, particularly regulation of branching angiogenesis, remains largely unexplored. To examine this question, we have carried out studies in the avian embryo, using cyclopamine to inhibit Hh signaling activity after the initial endothelial network had already assembled. Our results demonstrate that Hh signaling is essential for the formation of the intersegmental vessels, ISVs, which are amongst the first blood vessels to form through branching angiogenesis (Fig. 1). These are the first studies to show an essential role for Hh signaling in regulation of angiogenic growth during embryonic development.
Molecular response to inhibition of Hh signaling
Two pieces of evidence strongly indicate that Hh does not signal directly to endothelial cells to modulate their behavior. First, although global knockout of Smoothened function causes early embryonic death due to vascular defects (Coultas et al., 2010; Vokes et al., 2004), mice lacking Smoothened function specifically in endothelial cells are viable (White et al., 2007). Second, activation of PTCH1 expression, which serves as an indicator of active Hh signaling (Pearse et al., 2001), was not detected in chick endothelial cells (Moran et al., 2011). Together these data strongly suggest that the effects of Hh on development of the endothelium are mediated by other regulatory factors.
In order to identify the molecular pathways influenced by inhibition of Hh signaling we examined expression levels of 21 candidate genes previously implicated in regulation of endothelial growth or function. Our results show that only two, DLL4 and VEGFA, were substantially reduced when Hh signaling was impaired (Fig. 2). Precise regulation of expression levels of both of these genes is known to be crucial for vascular development, since loss of a single copy is embryonic lethal (Carmeliet et al., 1996; Ferrara et al, 1996; Duarte et al., 2004; Gale et al., 2004). On the other hand, the observation that transcript levels of numerous endothelial-restricted or specific markers was unaltered shows that cyclopamine treatment had little or no effect on the overall number of endothelial cells or their gene expression profile.
Neither Notch nor BMP signaling is required for growth of ISVs
Because DLL4 expression was reduced in Hh inhibited chick embryos, we examined the role of Notch signaling in ISV growth in more detail. While we were able to demonstrate that transcript levels for a number of Notch pathway components was indeed depressed when Hh signaling is inhibited (Fig. 3A), use of the Notch inhibitor, DAPT, failed to cause any detectable alteration in ISV formation. This experiment was repeated many different times and with higher doses of DAPT (data not shown), but no effects on vascular patterning were observed, up to the doses where embryonic viability was compromised. These results are surprising, since previous studies have shown that disruption to Notch signaling in the mouse embryo disrupts vascular patterning. For example, when NOTCH4 function is ablated the initial vascular network forms normally but remodeling and branching of the vessels is severely compromised (Krebs et al., 2000). Similarly, mouse embryos heterozygous deficient for the Notch ligand, DLL4, exhibit vascular branching and remodeling defects (Duarte et al., 2004; Gale et al., 2004; Krebs et al., 2004). On the other hand, Notch signaling does not appear to be essential for growth in ISVs in zebrafish embryos. Knockdown of Dll4 using morpholinos, or treatment of embryos with DAPT, resulted in overall normal formation of ISVs (Leslie et al., 2007). Studies of zebrafish embryos with mutations in several other Notch signaling genes (deltaC, deltaD and notch1a) showed fairly subtle abnormalities in ISV growth but only in posterior regions of the embryo (Therapontus and Vargesson, 2010). Overall, our data suggests that Notch signaling does not play a central role in regulation of ISV growth in the chick embryo, a situation that more closely resembles vascular patterning in zebrafish than mouse.
Hh has been shown to regulate BMP4 expression during development of the murine yolk sac vasculature (Astorga and Carlsson, 2007) and so we carried out a series of experiments to determine whether BMP signaling might be involved in regulation of ISV outgrowth. However, examination of BMP2 and BMP4 transcript levels by qRT-PCR showed no change when Hh signaling was inhibited and we observed no discernible alteration in the amount of phosphorylated SMAD1/5 (Fig. S1). It seems very likely therefore, that BMP signaling does not regulate ISV growth downstream of Hh.
VEGF signaling is regulated by Hh and is required for ISV formation
Previous studies have demonstrated that VEGF expression is regulated by Hh (Coultas et al., 2010; Pola et al., 2001; White et al., 2007) and our survey of endothelial regulatory and marker genes showed VEGFA to be one of the only genes whose transcript levels were reduced when Hh signaling was inhibited (Fig. 2). Experimentally, addition of VEGF on beads was able to restore angiogenic branching in the presence of the Hh inhibitor cyclopamine (Fig. 5A-D), while specific inhibition of the VEGF signaling pathway resulted in a complete absence of new ISVs, apparently identical to the Hh inhibition phenotype (Fig. 5E-F, Fig. 6E). In agreement with previous studies (Lobov et al., 2007), we have shown that VEGF activates DLL4 expression (Fig. S2). Therefore, the observed reduction in DLL4 expression in cyclopamine treated embryos (Fig. 2) may be a downstream effect of reduction in VEGF activity.
Stimulation of Hh signaling results in upregulation of VEGFA expression levels, assayed by both in situ hybridization and qRT-PCR (Fig. 4), and concomitant increase in the number of ISVs (Fig. 6D). This last observation is in contrast to results from previous studies examining stimulation of Hh signaling in the mouse embryo by ablation of PTCH1 function (Coultas et al., 2010). In Ptch1 null animals, altered vascular structures were observed, including increased diameter of the DA, but no increase in Vegfa expression was detected. There are at least two reasons for this apparent discrepancy between our studies and the mouse work. First, the chick studies examined a later stage of vascular development after the initial vascular plexus was established and second, the mouse embryos were already severely developmentally compromised by the time the assays were carried out and may not have reflected a normal signaling response. Nevertheless, our observations of increased VEGFA expression in response to enhanced Hh signaling provide a plausible mechanism for the increase in ISV formation, while no alternative to VEGF was advanced in the mouse studies.
Taken together, our studies point to a continuing role for Hh in regulation of vascular patterning throughout embryonic development, including regulation of branching angiogenesis. Rather than function as an on-off switch for VEGF expression, it appears that Hh functions to maintain VEGF expression levels within the very tight boundaries required for normal development of the endothelium.
Experimental Procedures
Treatment of embryos
Fertilized chick eggs were obtained from Hy-Line Inc. and quail eggs from Strickland Game and Bird Farm. Embryos at approximately the 10-somite stage were removed from the egg and maintained in New culture. Cyclopamine (Calbiochem) was dissolved in DMSO and mixed with the carrier, 5- hydroxy-beta-cyclodextran (HBC). Cyclopamine in HBC or HBC alone, as a control, was applied to the top of the embryo in a final volume of 150 μl. After 4 hours in culture the overlying fluid was removed and another 150 μl of cyclopamine solution was applied. Total incubation in cyclopamine was 8 hours at which time embryos were harvested for analysis. SAG (EMD) was dissolved in DMSO and applied to the embryos exactly as described for cyclopamine. After characterization of dose curves, all further studies were carried out using cyclopamine at 100 μM and SAG at 20 μM. Dose curve analysis was carried out with DAPT (Tocris) from 20 μM to 200 μM in DMSO. High doses of DAPT (100 and 200 μM) sometimes caused developmental delay or death and so all experiments were carried out at a final concentration of 50 μM. The VEGFR2 specific inhibitor Ki8751 (Calbiochem) was dissolved in DMSO and applied at final concentrations from 0.01- 1.0 μM using the procedure described for cyclopamine.
In situ hybridization and antibody analysis
The chick PTCH1 probe construction has been described previously (Pearse et al., 2001). In situ hybridization probes for chick VEGFA (ChEST820k21) HEY2 (ChEST191k22), HES1 (ChEST900h16 5), CDH5 (ChEST59g22) and DLL4 (ChEST714c11) were prepared by linearization of templates and use of the appropriate RNA polymerase. Whole-mount in situ hybridization was carried out using digoxigenin-labeled probes and standard conditions. QH1 antibody was obtained from the Developmental Studies Hybridoma Bank and staining was carried out as described previously (Vokes et al., 2004).
qRT-PCR analysis
For quantitative RT-PCR analysis of chick transcripts, primers were as follows: PTCH1 F - GTGTCAGGCATCAGTGAGGA; PTCH1 R - TGCAATCTGGGACTTGACTG; GAPDH F - TCTGGAGAAACCAGCCAAGT; GAPDH R – CGAATCAAAGGTGGAAGAAT; ACTB F - TGGTACCACAATGTACCCTGGCAT; ACTB R – ACTCCTGCTTGCTGATCCACATCT; VEGFA F – CCCTGTGGATGTGTACAACG; VEGFA R – CTTTTGACCCTTCCCCTTTC; DLL4 F - AGTAGATGGACGTTCCTGCTGCAT; DLL4 R - ACAGTCTGTCCAGTTCACCCAGTT; HEY2 F - TGAAGCGACCTTGCGAGGA; HEY2 R - TTAGAAGGCTCCGACCTCCGT. Real time PCR was performed in a Rotor-Gene Q using two standard curve quantitation. A list of additional primers used for qRT-PCR is provided in Table S1.
Supplementary Material
Fig. S1. BMP signaling is not regulated by Hh during ISV formation. (A). Quantitative RT-PCR analysis of BMP2 and BMP4 transcript levels in control and cyclopamine treated embryos (triplicate samples). Reduction in PTCH1 levels was used as the positive control. (B). Protein blot analysis of pSMAD1/5 activity as a measure of active BMP signaling. Two different cyclopamine-treated samples (each from 3 embryos) were compared to control. Total SMAD1 levels were assayed in identical samples (lower panel). No difference was detected in pSMAD1/5 levels between control and cyclopamine-treated embryos.
Fig. S2. Expression of DLL4 correlates to VEGF signaling activity. qRT-PCR analysis of DLL4 transcript levels in response to stimulation (SAG) and inhibition (cyclopamine and ki8751) of VEGF signaling. Alteration in VEGF activity correlates with differences in DLL4 expression observed following up or down-regulation of Hh signaling. Significant differences between treatments (Student's T-test, P<0.05) are indicated by asterisks.
Acknowledgments
Thanks to Tanya Yatskievych for assistance with bead implant experiments and to Verena Koenniger and the Gregorio laboratory for help with protein blots. CTM is supported by a Molecular Cardiovascular Research Fellowship generously provided by the Bellows Foundation and NIH Training Grant (# T32 GM08659). PAK is the Allan C. Hudson and Helen Lovaas Endowed Professor of the Sarver Heart Center at the University of Arizona College of Medicine. This work was supported by the Sarver Heart Center and by the NHLBI of the NIH, grant #HL093694.
References
- Alva JA, Iruela-Arispe ML. Notch signaling in vascular morphogenesis. Curr Opin Hematol. 2004;11:278–283. doi: 10.1097/01.moh.0000130309.44976.ad. [DOI] [PubMed] [Google Scholar]
- Astorga J, Carlsson P. Hedgehog induction of murine vasculogenesis is mediated by Foxf1 and Bmp4. Development. 2007;134:3753–3761. doi: 10.1242/dev.004432. [DOI] [PubMed] [Google Scholar]
- Brown LA, Rodaway AR, Schilling TF, Jowett T, Ingham PW, Patient RK, Sharrocks AD. Insights into early vasculogenesis revealed by expression of the ETS-domain transcription factor Fli-1 in wild-type and mutant zebrafish embryos. Mech Dev. 2000;90:237–252. doi: 10.1016/s0925-4773(99)00256-7. [DOI] [PubMed] [Google Scholar]
- Byrd N, Becker S, Maye P, Narasimhaiah R, St-Jacques B, Zhang X, McMahon J, McMahon A, Grabel L. Hedgehog is required for murine yolk sac angiogenesis. Development. 2002;129:361–372. doi: 10.1242/dev.129.2.361. [DOI] [PubMed] [Google Scholar]
- Byrd N, Grabel L. Hedgehog signaling in murine vasculogenesis and angiogenesis. Trends Cardiovasc Med. 2004;14:308–313. doi: 10.1016/j.tcm.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- Coffin JD, Poole TJ. Embryonic vascular development: immunohistochemical identification of the origin and subsequent morphogenesis of the major vessel primordia in quail embryos. Development. 1988;102:735–748. doi: 10.1242/dev.102.4.735. [DOI] [PubMed] [Google Scholar]
- Coultas L, Nieuwenhuis E, Anderson GA, Cabezas J, Nagy A, Henkelman RM, Hui CC, Rossant J. Hedgehog regulates distinct vascular patterning events through VEGF-dependent and -independent mechanisms. Blood. 2010;116:653–660. doi: 10.1182/blood-2009-12-256644. [DOI] [PubMed] [Google Scholar]
- Darnell DK, Kaur S, Stanislaw S, Davey S, Konieczka JH, Yatskievych TA, Antin PB. GEISHA: an in situ hybridization gene expression resource for the chicken embryo. Cytogenet Genome Res. 2007;117:30–35. doi: 10.1159/000103162. [DOI] [PubMed] [Google Scholar]
- Daudet N, Ariza-McNaughton L, Lewis J. Notch signalling is needed to maintain, but not to initiate, the formation of prosensory patches in the chick inner ear. Development. 2007;134:2369–2378. doi: 10.1242/dev.001842. [DOI] [PubMed] [Google Scholar]
- Duarte A, Hirashima M, Benedito R, Trindade A, Diniz P, Bekman E, Costa L, Henrique D, Rossant J. Dosage-sensitive requirement for mouse Dll4 in artery development. Genes Dev. 2004;18:2474–2478. doi: 10.1101/gad.1239004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis T, Smyth I, Riley E, Graham S, Elliot K, Narang M, Kay GF, Wicking C, Wainwright B. Patched 1 conditional null allele in mice. Genesis. 2003;36:158–161. doi: 10.1002/gene.10208. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O'Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- Gale NW, Dominguez MG, Noguera I, Pan L, Hughes V, Valenzuela DM, Murphy AJ, Adams NC, Lin HC, Holash J, Thurston G, Yancopoulos GD. Haploinsufficiency of delta-like 4 ligand results in embryonic lethality due to major defects in arterial and vascular development. Proc Natl Acad Sci U S A. 2004;101:15949–15954. doi: 10.1073/pnas.0407290101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garriock RJ, Czeisler C, Ishii Y, Navetta AM, Mikawa T. An anteroposterior wave of vascular inhibitor downregulation signals aortae fusion along the embryonic midline axis. Development. 2010;137:3697–3706. doi: 10.1242/dev.051664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs LT, Xue Y, Norton CR, Shutter JR, Maguire M, Sundberg JP, Gallahan D, Closson V, Kitajewski J, Callahan R, Smith GH, Stark KL, Gridley T. Notch signaling is essential for vascular morphogenesis in mice. Genes Dev. 2000;14:1343–1352. [PMC free article] [PubMed] [Google Scholar]
- Krebs LT, Shutter JR, Tanigaki K, Honjo T, Stark KL, Gridley T. Haploinsufficient lethality and formation of arteriovenous malformations in Notch pathway mutants. Genes Dev. 2004;18:2469–2473. doi: 10.1101/gad.1239204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo K, Shimizu T, Ohyama S, Murooka H, Iwai A, Nakamura K, Hasegawa K, Kobayashi Y, Takahashi N, Takahashi K, Kato S, Izawa T, Isoe T. Novel potent orally active selective VEGFR-2 tyrosine kinase inhibitors: synthesis, structure-activity relationships, and antitumor activities of N-phenyl-N'-{4-(4-quinolyloxy)phenyl}ureas. J Med Chem. 2005;48:1359–1366. doi: 10.1021/jm030427r. [DOI] [PubMed] [Google Scholar]
- Lamont RE, Childs S. MAPping out arteries and veins. Sci STKE. 2006:39. doi: 10.1126/stke.3552006pe39. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Vogel AM, Weinstein BM. sonic hedgehog and vascular endothelial growth factor act upstream of the Notch pathway during arterial endothelial differentiation. Dev Cell. 2002;3:127–136. doi: 10.1016/s1534-5807(02)00198-3. [DOI] [PubMed] [Google Scholar]
- Leslie JD, Ariza-McNaughton L, Bermange AL, McAdow R, Johnson SL, Lewis J. Endothelial signalling by the Notch ligand Delta-like 4 restricts angiogenesis. Development. 2007;134:839–844. doi: 10.1242/dev.003244. [DOI] [PubMed] [Google Scholar]
- Lobov IB, Renard RA, Papadopoulos N, Gale NW, Thurston G, Yancopoulos GD, Wiegand SJ. Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc Natl Acad Sci U S A. 2007;104:3219–3224. doi: 10.1073/pnas.0611206104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran CM, Salanga MC, Krieg PA. Hedgehog signaling regulates size of the dorsal aortae and density of the plexus during avian vascular development. Dev Dyn. 2011;240:1354–1364. doi: 10.1002/dvdy.22600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardanaud L, Altmann C, Kitos P, Dieterlen-Lievre F, Buck CA. Vasculogenesis in the early quail blastodisc as studied with a monoclonal antibody recognizing endothelial cells. Development. 1987;100:339–349. doi: 10.1242/dev.100.2.339. [DOI] [PubMed] [Google Scholar]
- Pearse RV, 2nd, Vogan KJ, Tabin CJ. Ptc1 and Ptc2 transcripts provide distinct readouts of Hedgehog signaling activity during chick embryogenesis. Dev Biol. 2001;239:15–29. doi: 10.1006/dbio.2001.0430. [DOI] [PubMed] [Google Scholar]
- Pola R, Ling LE, Silver M, Corbley MJ, Kearney M, Blake Pepinsky R, Shapiro R, Taylor FR, Baker DP, Asahara T, Isner JM. The morphogen Sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nat Med. 2001;7:706–711. doi: 10.1038/89083. [DOI] [PubMed] [Google Scholar]
- Therapontos C, Vargesson N. Zebrafish notch signalling pathway mutants exhibit trunk vessel patterning anomalies that are secondary to somite misregulation. Dev Dyn. 2010;239:2761–2768. doi: 10.1002/dvdy.22410. [DOI] [PubMed] [Google Scholar]
- Tucker JA, Mintzer KA, Mullins MC. The BMP signaling gradient patterns dorsoventral tissues in a temporally progressive manner along the anteroposterior axis. Dev Cell. 2008;14:108–119. doi: 10.1016/j.devcel.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varjosalo M, Taipale J. Hedgehog: functions and mechanisms. Genes Dev. 2008;22:2454–2472. doi: 10.1101/gad.1693608. [DOI] [PubMed] [Google Scholar]
- Vokes SA, Yatskievych TA, Heimark RL, McMahon J, McMahon AP, Antin PB, Krieg PA. Hedgehog signaling is essential for endothelial tube formation during vasculogenesis. Development. 2004;131:4371–4380. doi: 10.1242/dev.01304. [DOI] [PubMed] [Google Scholar]
- White AC, Lavine KJ, Ornitz DM. FGF9 and SHH regulate mesenchymal Vegfa expression and development of the pulmonary capillary network. Development. 2007;134:3743–3752. doi: 10.1242/dev.004879. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. BMP signaling is not regulated by Hh during ISV formation. (A). Quantitative RT-PCR analysis of BMP2 and BMP4 transcript levels in control and cyclopamine treated embryos (triplicate samples). Reduction in PTCH1 levels was used as the positive control. (B). Protein blot analysis of pSMAD1/5 activity as a measure of active BMP signaling. Two different cyclopamine-treated samples (each from 3 embryos) were compared to control. Total SMAD1 levels were assayed in identical samples (lower panel). No difference was detected in pSMAD1/5 levels between control and cyclopamine-treated embryos.
Fig. S2. Expression of DLL4 correlates to VEGF signaling activity. qRT-PCR analysis of DLL4 transcript levels in response to stimulation (SAG) and inhibition (cyclopamine and ki8751) of VEGF signaling. Alteration in VEGF activity correlates with differences in DLL4 expression observed following up or down-regulation of Hh signaling. Significant differences between treatments (Student's T-test, P<0.05) are indicated by asterisks.