Abstract
Metabotropic glutamate receptors (mGluRs) are found throughout thalamus and cortex and are clearly important to circuit behavior in both structures, and so considering only participation of ionotropic glutamate receptors (e.g., [R,S]-α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid [AMPA] and N-methyl-d-aspartate receptors [NMDA] receptors) in glutamatergic processing would be an unfortunate oversimplification. These mGluRs are found both postsynaptically, on target cells of glutamatergic afferents, and presynaptically, on various synaptic terminals themselves, and when activated, they produce prolonged effects lasting at least hundreds of msec to several sec and perhaps longer. Two main types exist: activation of group I mGluRs causes postsynaptic depolarization, and group II, hyperpolarization. Both types are implicated in synaptic plasticity, both short term and long term. Their evident importance in functioning of thalamus and cortex makes it critical to develop a better understanding of how these receptors are normally activated, especially because they also seem implicated in a wide range of neurological and cognitive pathologies.
Keywords: metabotropic glutamate receptor, thalamus, cortex, driver, modulator
In thalamus and cortex,1 virtually all information processing is thought to be accomplished by pathways that use glutamate as the neurotransmitter, with other transmitter systems, such as cholinergic, dopaminergic, and even GABAergic, playing modulatory roles that affect how glutamatergic inputs are processed. Such modulation can involve changes in synaptic strength, neuronal responsiveness, and so on, to support various behavioral demands, such as learning and memory, directed attention, and overall vigilance. Until fairly recently, models of such information processing have been based almost exclusively on the activation by these glutamatergic inputs of ionotropic glutamate receptors (iGluRs), mostly (R,S)-α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and N-methyl-d-aspartate (NMDA) receptors. However, it has become increasingly clear that many and probably the majority of these glutamatergic afferents also activate very different receptor types, namely metabotropic glutamate receptors (mGluRs), which are quite abundant in thalamus (Alexander and Godwin 2005, 2006b; Cox and Sherman 1999; Godwin and others 1996a) and cortex (Ohishi and others 1993a, 1993b, 1995; Petralia and others 1997; Reid and others 1995; Shigemoto and others 1993). The purpose of this article is to review our current understanding of the role of mGluRs in thalamic and cortical processing, which in turn suggests a somewhat novel way to look at information processing in these structures.
General Properties of iGluRs and mGluRs
Activation of postsynaptic receptors typically leads to opening or closing of ion channels, allowing charged ions to move into or out of the cell, thereby changing its membrane potential. For example, depolarization (e.g., an excitatory postsynaptic potential [EPSP]) occurs when Na+ or Ca2+ channels open, allowing these ions to enter the cell, or when K+ channels close, blocking these ions from leaving the cell; hyperpolarization (e.g., an inhibitory postsynaptic potential [IPSP]) occurs when Cl− channels open, allowing these ions to enter the cell or when K+ channel open, allowing these ions to leave the cell. Other effects on the cell, beside opening or closing of ion channels, may also occur on activation of postsynaptic receptors, especially for metabotropic receptors, and some examples are given below. For further details of how these channels are controlled, see Catterall (2010).
Figure 1 schematically shows some of the properties of mGluRs by contrasting them to iGluRs. A full description of metabotropic receptors is beyond the scope of this article, but further details can be found elsewhere (for metabotropic receptors generally, Nicoll and others 1990; for mGluRs specifically, Conn and Pin 1997; Pin and Bockaert 1995; Pin and Duvoisin 1995). A typical ionotropic receptor (Fig. 1A) is a complex transmembrane protein composed of several subunits that may combine in different ways to subtly affect receptor functioning; the protein molecule wraps back and forth across the membrane several times (for details of receptor structure and differences among them, see Kandel and others 2000). Importantly, each of these receptor proteins includes an ion channel, but when the receptor is not in contact with the transmitter molecule, the channel is blocked. When the receptor comes into contact with a transmitter, such as glutamate, a conformational change ensues that exposes the ion channel, thereby allowing ions to cross the membrane. In the case of the AMPA receptor, of which several subtly different varieties exist, Na+ and sometimes Ca2+ pass through the channel into the cell, leading to an EPSP. Partly because of the direct linkage of the receptor to the ion channel, the response is fast, with a latency of 1 ms or less and a duration of 10 ms or so.2
Figure 1.
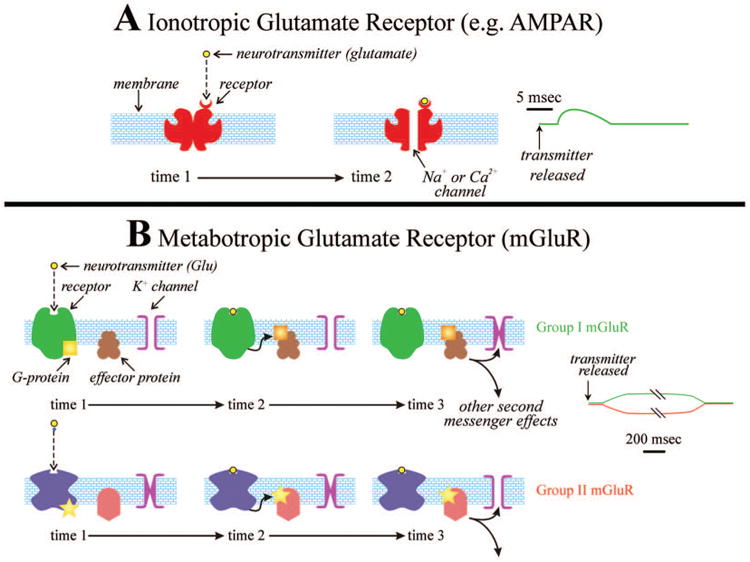
Schematic depiction of iGluR (AMPA receptor [AMPAR], in this example) and mGluRs. Each type is shown repeatedly at different times (time 1 and time 2 for the AMPAR, and time 1, time 2, and time 3 for the mGluRs), and the evoked postsynaptic potentials are shown on the right. For the AMPAR, time 1 represents the period before binding to the transmitter, and time 2 is the period after binding. The binding leads to a conformational change that opens the ion channel, which forms the central core of the receptor complex. For the mGluRs, time 1 is the period before transmitter binding, and just after binding (time 2) a G-protein is released, which reacts with an effector protein to produce a cascade of biochemical reactions eventually resulting in opening or closing of an ion channel, usually a K+ channel (time 3). Not shown is the possibility that for some receptors the G-protein can directly affect the ion channel. Note the much longer time course for the mGluR example than for the iGluR one. Note also that different G-proteins and second messenger systems are involved in the two groups of mGluR: group I mGluRs are associated with Gq-proteins (yellow square), and group II, with Gi/o-proteins (yellow star). Further details in the text. iGluR = ionotropic glutamate receptor; mGluR = metabotropic glutamate receptor.
In addition to AMPA receptors, iGluRs also include NMDA and kainite receptors. Kainate receptors play a rather subtle role in normal adult thalamic and cortical processing, and so they are not considered further here (but see Binns and others 2003; Huettner 2003; Kidd and Isaac 2001; Miyata and Imoto 2009). NMDA receptors are a major component of postsynaptic iGluR responsiveness, and their properties differ in certain ways from the above description (Mayer and Westbrook 1987). One difference is their voltage dependency. At a relatively hyper-polarized level, such as a typical resting potential, Mg2+ ions collect in the channel of this receptor and clog it, thereby blocking passage of other ions (e.g., Na+ and Ca2+), but as the cell becomes increasingly depolarized (by, e.g., activation of AMPA receptors), the Mg2+ ions are increasingly repelled, allowing the ion channel found in the NMDA receptor to pass ions, which includes Ca2+ and Na+ ions (Mayer and Westbrook 1987). This means that EPSPs dependent on activation of NMDA receptors can only be evoked once the cell is already somewhat depolarized. A second difference is that the NMDA EPSP lasts somewhat longer—a few tens of milliseconds—than does the AMPA EPSP. Finally, activation of NMDA EPSPs seems to require the presence of glycine and glutamate (Banke and Traynelis 2003; Gibb, 2004; Hashimoto and Oka 1997; Kawajiri and Dingledine 1993).
Although metabotropic receptors are simpler proteins that usually are made up of a single polypeptide, they represent the start of a much more complicated chain of events that eventually evoke postsynaptic responses. Once the neurotransmitter binds to a metabotropic receptor, a series of actions is triggered (see Fig. 1B). First is a conformational change in the receptor that leads to the release of a G-protein, and this leads to a cascade of biochemical reactions in the postsynaptic cell, a process known as a “second messenger pathway,” and these reactions in turn lead to several changes in the cell. One such change is opening or closing of specific ion channels. In the case of mGluR activation in the thalamus and cortex, the main effect is on K+ channels, although Ca2+ and other channels can also be affected (Anwyl 1999). Postsynaptic potentials produced in this way have a much longer time course than seen with ionotropic receptors, with a latency of 10 ms or more and duration of hundreds of milliseconds to several seconds or more (Govindaiah and Cox 2004). The much longer time course of mGluR activation compared to that of iGluRs is considered further below. However, whereas metabotropic receptors do evoke post-synaptic potentials, second messenger systems can affect other cellular properties besides ion channels, such as internal Ca2+ concentrations, gene expression, and long-term plastic changes in cell responses (Anwyl 2009; Gereau and Conn 1994; Luscher and Huber 2010; Tsanov and Manahan-Vaughan 2009).
There are eight different types of mGluRs recognized in the brain, and these are distributed into three groups. Activation of group I (types 1 and 5) mGluRs leads to prolonged EPSPs, mainly through closing of K+ channels; activation of group II (types 2 and 3) mGluRs leads to prolonged IPSPs, mainly through opening of K+ channels. Group III mGluRs (types 4, 6, and 8) have not been much studied in thalamus or cortex and are not further considered (but see Gu and others 2012; Salt and Eaton 1995). The signaling pathways for groups I and II mGluRs differ, as indicated in Figure 1B, involving different G-proteins (Gq for Group I and Gi/o for Group II) and other features of the second messenger cascade (for further details, see Coutinho and Knopfel 2002; Kim and others 2008).
Metabotropic receptors, in general, and mGluRs specifically, are found both presynaptically, on synaptic terminals, as well as postsynaptically, on target dendrites (see below).3 Regarding postsynaptic distribution, there is evidence that, whereas iGluRs are found within the synaptic zone, mGluRs tend to be located perisynaptically (Kennedy 2000; Lujan and others 1996; Nusser and others 1994). This may explain why mGluRs generally require higher levels of presynaptic activity for their activation than do iGluRs, presumably because more glutamate must be released presynaptically to reach mGluRs; however, mGluRs can nonetheless be activated by fairly low levels of presynaptic activation (Viaene and others 2013). Activation of presynaptic mGluRs requires an even greater movement of glutamate from synapses, because, except for triadic arrangements found in thalamus (see below), the terminals on which the mGluRs are found are not themselves postsynaptic to other inputs. The presynaptic actions of mGluRs are thought to involve the same mechanisms as described above: activating these receptors can affect Ca2+ concentrations inside the terminal, and thus probability of transmitter release, both by affecting membrane potential and by affecting internal Ca2+ stores.
Relationship of Postsynaptic mGluRs to Type of Afferent
There exists a tendency when analyzing any brain circuit to treat all inputs to the circuit as some sort of anatomical democracy, giving equal functional weight to each input. A clear improvement on this is to classify the different inputs and recognize their different functional properties. Often this is limited to transmitter systems for which three general classes are recognized: specific excitatory (i.e., glutamatergic), specific inhibitory (i.e., GABAergic), and less specific modulatory (e.g., cholinergic, noradrenergic, etc.). The implicit assumption in this scheme is that glutamatergic inputs are the dominant ones in thalamus and cortex for transmission and processing of information, whereas the other inputs modify how that information is processed, but as we argue below, this view needs to be reconsidered.
Classes of Glutamatergic Afferent
We have recently made the point that glutamatergic inputs can also be classified into different functional types, and so far two have emerged in the circuitry of thalamus and cortex (Sherman 2012; Sherman and Guillery 2006, 2011). They have been called Class 1 and Class 2 inputs, and a major difference between them involves mGluRs: Class 1 inputs activate only iGluRs on their target cells, whereas Class 2 inputs activate both iGluRs and mGluRs. Table 1 shows the main criteria used to identify each type, and Figure 2 shows some of the properties that distinguish these glutamatergic input types plus the robustness of this classification scheme. That at least two different types of glutamatergic input exist (more may be discovered as thalamic and cortical circuitry are further explored) is clear, and this suggests that they have different functions in circuitry. Thus, identifying the different types of glutamatergic input with an understanding of each function offers new insights into understanding thalamic and cortical circuitry.
Table 1. Class 1 and 2 Properties.
| Class 1 (driver) | Class 2 (modulator) |
|---|---|
| Activates only ionotropic receptors | Activates metabotropic receptors |
| Synapses show paired-pulse depression (high p)a | Synapses show paired-pulse facilitation (low p) |
| Large initial EPSPs | Small initial EPSPs |
| Little or no convergence onto target | Much convergence onto target |
| Thick axons | Thin axons |
| Large terminals on proximal dendrites | Small terminals on distal dendrites |
| Dense, well localized terminal arbors | Delicate terminal arbors |
EPSP = excitatory postsynaptic potential.
For slight variation in this property among thalamocortical inputs, see Viaene and others (2011c).
Figure 2.

Some properties of Class 1 and 2 inputs in thalamus and cortex; data are from slice preparations of mouse brain (reviewed in Sherman 2012; Sherman and Guillery 2006, 2011). (A) Some Class 1 properties. At lower frequency stimulation (15 Hz), the evoked EPSPs show paired-pulse depression (i), and the responses at this frequency are completely abolished with application of iGluR antagonists (ii). High-frequency stimulation (10 shocks at 130 Hz) in the presence of iGluR antagonists evokes no mGluR response (iii). (B) Some Class 2 properties, conventions as in (A). Stimulation at 15 Hz leads to paired-pulse facilitation (i); responses at 15 Hz are blocked by iGluR (ii), but high-frequency stimulation evokes a sustained EPSP (iiia) that is blocked by further addition of a group I (type 1) mGluR antagonist (iiib). (C) More Class 2 properties, which are the same as shown in (B), except that the mGluRs activated are group II and thus produce a sustained IPSP (iii). (C) Three-dimensional scatterplot showing distribution of parameters for Class 1 and 2 inputs. The parameters shown are the ratio of the amplitude of the second EPSP evoked in a train to that of the first (Paired-Pulse Effects), the amplitude of the first EPSP evoked in a train (first EPSP Amplitude), and the maximum voltage change evoked in the 300-ms period after high-frequency stimulation in the presence of jGluR antagonists (Max. mGluR Response). iGluR = ionotropic glutamate receptor; mGluR = metabotropic glutamate receptor; EPSP = excitatory postsynaptic potential; IPSP = inhibitory postsynaptic potential. From Sherman (2012).
Examples of identified Class 1 and Class 2 inputs are shown in Table 2 (details in Sherman and Guillery 2006).
Table 2. Examples of Identified Class 1 and Class 2 Inputs.
| Class 1 inputs | Class 2 inputs |
|---|---|
| Retina to lateral geniculate nucleus | Cortical layer 6 to thalamus |
| Medial lemniscus to ventral posterior (medial and lateral) nucleus | Layer 6 to layer 4 of cortex |
| Inferior colliculus to ventral medial geniculate nucleus | Layers 2/3 to layers 2/3 of cortex |
| Lateral mamillary nucleus to anterodorsal nucleus | Inferior colliculus to dorsal medial geniculate nucleus |
| Cerebellar to ventral lateral nucleus | Most thalamocortical inputs to layers 2/3 |
| Thalamocortical inputs to layers 4-6 and some to layers 2/3 | Various corticocortical pathways |
| Layer 4 to layers 2/3 of cortex | |
| Cortical layer 5 to higher order thalamic nuclei | |
| Various corticocortical pathways |
Hypothesis for Function of Glutamatergic Input Types
We have suggested previously that Class 1 inputs carry the basic information to be processed, and are thus the driver inputs, whereas Class 2 inputs represent another modulatory input, acting much like cholinergic, noradrenergic, and so on, inputs with some specific features noted below (Sherman and Guillery 2006).
There are several examples that offer strong support that Class 1 inputs are the driver inputs, and these involve the elaboration of receptive field properties. The best example is the Class 1 retinal input to the lateral geniculate nucleus. It is clear that the basic center/surround receptive field properties of geniculate relay cells are provided by inputs from one or a small number of retinal afferents (Cleland and others 1971; Cleland and Lee 1985; Mastronarde 1987; Usrey and others 1999). Similarly, lemniscal inputs to the ventral posterior (medial/lateral) and inferior collicular inputs to the ventral medial geniculate nucleus provide relay cells there with their basic receptive field properties (Mountcastle 1980). Another example in the visual system is the geniculocortical input to layer 4 cells, which is Class 1 (Lee and Sherman 2008): The basic receptive field properties of these target cells are established fundamentally from integration of their geniculate inputs (Alonso and others 2001; Ferster 1987; Ferster and others 1996; Hubel and Wiesel 1962).
Likewise, in select cases, mostly involving layer 6 corticothalamic inputs, which are Class 2 (Sherman 2012; Sherman and Guillery 2006, 2011), it is clear that these serve as modulatory, rather than driving inputs. For example, the receptive fields of corticogeniculate cells are elongated, show orientation and direction selectivity, and are usually binocularly driven (Gilbert and Wiesel 1979), properties not present for receptive fields of geniculate relay cells. Instead, this input provides more subtle effects on geniculate relay cells that can be classified as modulatory, such as minor adjustments to receptive field properties (Andolina and others, 2007; Baker and Malpeli 1977; Geisert and others 1981; Kalil and Chase 1970; McClurkin and Marrocco 1984; McClurkin and others 1994; Schmielau and Singer 1977) and controlling the burst/tonic firing mode transition (Andolina and others 2012; Godwin and others 1996b).
However, for the most part, direct evidence identifying Class 1 inputs as drivers and Class 2 as modulators is presently unavailable. Nonetheless, the properties of each glutamatergic input type are consistent with this functional assignment, and the difference in activation of mGluRs is central to this hypothesis. Class 1 properties are consistent with those expected of a main information source (see Table 1): Large EPSPs are clearly useful for processing of information; paired-pulse depression is usually associated with high probability of transmitter release (Dobrunz and Stevens 1997) and may serve to dynamically regulate neuronal sensitivity (Chung and others 2002); and lack of an mGluR response ensures relatively brief EPSPs, allowing a more faithful relay of temporal information. Some Class 2 properties plausibly serve a modulatory function: these weaker and more convergent inputs can combine in many different ways to provide a variety of modulatory functions; the prolonged mGluR response provides an effective control for various time- and voltage-dependent conductances with long time constants for inactivation kinetics (e.g., IT, Ih, and IA; reviewed in Sherman and Guillery 2006); furthermore, the response outlasts activity in the input, often by seconds (Govindaiah and Cox 2004), which may be useful for modulation but distorts the transmission of temporal information.
Different Effects of Activating Group I and Group II mGluRs
Because of the variety of mGluR subtypes, Class 2 inputs can in theory have a wide range of modulatory effects. Whereas activation of group I mGluRs inputs provide prolonged EPSPs, certain Class 2 inputs in cortex activate group II mGluRs, which leads to prolonged IPSPs and thus inhibition, thereby establishing these Class 2 inputs as potentially inhibitory (Covic and Sherman 2011; DePasquale and Sherman 2011; Lee and Sherman 2009a). It follows that not all inhibition in cortex is because of GABAergic inputs but may also include some glutamatergic ones. Furthermore, mGluR activation triggers intracellular second messenger pathways that can affect many neuronal functions other than the state of K+ or Ca2+ channels, such as synaptic plasticity (see below) and gene expression (Anwyl 2009; Bellone and others 2008; Jia and others 2001; Kullmann and Lamsa 2008; Mao and others 2008; Tsanov and Manahan-Vaughan 2009). Indeed, postsynaptic mGluR activation can strongly affect the strength of a Class 1 input (see below). Moreover, mGluR activation in a postsynaptic cell can modulate its synaptic inputs through the release of endocannabinoids that act retrogradely on afferent synaptic terminals (Brown and others 2003; Zhang and Alger 2010; Zhang and others 2009). Finally, it is worth reemphasizing the long time course of mGluR effects, implying that their role in modulation is relatively prolonged and delayed compared with iGluR activation. Thus, for example, the Class 2 input of the layer 6 corticothalamic projection has an early, fast iGluR effect followed by a prolonged mGluR one.
Technical Issues
Some technical limitations to our knowledge of effects of mGluR activation in thalamus and cortex must be noted. Virtually all of the studies described below involved in vitro recording from brain slice preparations, usually from rats or mice, and the usual issues with this approach must be considered, such as circuitry absent or cut, general lack of background activity, general lack of classic modulatory actions (e.g., from cholinergic or noradrenergic inputs), and so forth. Most of these studies have described effects on recorded cells of applying various agonists or antagonists to mGluRs. Such an approach does not identify possible glutamatergic inputs that under physiological conditions would be expected to activate the mGluRs in question. Finally, whereas a limited subset of studies does identify glutamatergic afferents (i.e., Class 2 inputs) that activate mGluRs, these have involved activation of populations of afferent axons, so it remains unclear the extent to which individual axons contribute to the mGluR activation described in such cases.
Effects in Thalamus
Both groups I and II mGluRs are found in thalamus and serve to modulate the thalamic relay to cortex. Group I mGluRs are found in several locations (Alexander and Godwin 2005, 2006b; Cox and Sherman 1999; Godwin and others 1996a). One location is relay cell dendrites postsynaptic to layer 6 inputs, and here, increased firing in the layer 6 input evokes a prolonged EPSP in the target relay cell. This, among other things, promotes tonic as opposed to burst firing in the relay cell by inactivating the T-type Ca2+ channels that underlie bursting (Godwin and others 1996b).
Another location for these group I mGluRs relates to triadic circuitry, which is shown schematically in Figure 3 (reviewed in Sherman 2004; Sherman and Guillery 2006). In the lateral geniculate nucleus, this involves a synaptic terminal from a retinal axon that synapses onto both a relay cell dendritic appendage4 and a synaptic terminal from a dendrite of a GABAergic interneuron, and the GABAergic terminal synapses onto the same relay cell dendrite. Therefore, three synapses are involved (and thus “triad”): from the retinal terminal to the relay cell dendrite, from the retinal terminal to the GABAergic terminal, and from the GABAergic terminal to the same relay cell dendrite. Note also that the GABAergic terminal is both presynaptic and postsynaptic. Triadic circuitry exists throughout thalamus in complex synaptic zones known as glomeruli, and the main information to be relayed (i.e., the Class 1 or driver input) is organized in these triads as is the retinal input to the lateral geniculate nucleus; thus, for instance, this would be the lemniscal input to the ventral posterior nucleus, and so on. Note that not all relay cells receive their driver input via triads and instead receive simple synapses from Class 1 terminals onto their dendrites (Sherman and Guillery 2006; Wilson and others 1984).
Figure 3.

Schematic view of triad in lateral geniculate nucleus. Shown are the various synaptic contacts (arrows), whether they are inhibitory or excitatory, and the related postsynaptic receptors. The triad includes terminals from a retinal axon (green) and interneuron dendrite (black) and involves three synapses: from the retinal terminal to the interneuron terminal, from the retinal terminal to an appendage of the relay cell dendrite (orange), and from the interneuron terminal to the same appendage.
One important feature of the triadic circuit is the nature of glutamate receptors activated by the retinal terminal: on the relay cell dendrite, these are strictly iGluRs, whereas on the GABAergic terminals, these are both iGluRs and mGluRs, the latter being group I (Cox and others 1998; Govindaiah and Cox 2004) (Figure 3). Whereas the functioning of the triad and role of mGluRs present is far from clear, one suggestion is that it provides for a gain control mechanism that, in the visual relay of the lateral geniculate nucleus, could serve as a partial basis for contrast gain control (Sherman 2004). The reasoning for this is as follows. At very low firing rates, relatively large monosynaptic EPSPs are seen in the relay cell, and disynaptic IPSPs through the GABAergic terminal are relatively small, because mGluR activation generally requires higher afferent firing rates (Batchelor and Garthwaite 1997; Brasnjo and Otis 2001; Huang and others 2004); but see Viaene and others 2013). Thus, at low retinal firing rates, the post-synaptic relay cell response shows a relatively large advantage of EPSPs versus IPSPs through the triad. Increasing contrast evokes higher firing levels in the retinal afferent, and this will lead to relatively more mGluR activation on the GABAergic terminal, and thus relatively larger IPSPs in the relay cell. Therefore, as contrast, and thus retinal firing increases, the gain through the triad onto the relay cells is reduced, and this would serve to increase the input/output effective range before postsynaptic response saturation is reached. Furthermore, the prolonged response of the mGluR activation means that, for a period after an elevated retinal firing rate drops to baseline levels, IPSPs would still be generated through the triad, and the gain would be lower; this period for the triad in the lateral geniculate nucleus has been measured to be several seconds (Govindaiah and Cox 2004), which is roughly the time constant for contrast adaptation effects seen psychophysically.
Also, group I mGluRs are found presynaptically on retinal terminals in the lateral geniculate nucleus, and activation of these mGluRs reduces transmitter release and thus evoked EPSPs in relay cells (Govindaiah and others 2012).
Both groups I and II mGluRs are found on thalamic reticular nucleus (TRN) cells, meaning the glutamatergic inputs can either depolarize (group I) or hyperpolarize (group II) these cells (Cox and Sherman 1999). The main glutamatergic inputs to these TRN cells are branches of axons from layer 6 corticothalamic cells and from thalamic relay cells, and it is not known whether one or both of these inputs activate these mGluRs. Also, activation of mGluRs (group unspecified) produces a long-term reduction in electric coupling via gap junctions in TRN cells (Landisman and Connors 2005).
Finally, group II mGluRs are found presynaptically on terminals from layer 6 axons in the thalamus, and activation of these mGluRs reduces the amplitude of cortico-thalamic EPSPs evoked in the target thalamic cells (Alexander and Godwin 2005, 2006b; Hermes and Renaud 2011).
Effects in Cortex
Evidence for the widespread and heterogeneous effects of mGluR activation in cortex is clear. Both groups I and II mGluRs are present there (Ohishi and others 1993a, 1993b, 1995; Petralia and others 1997; Reid and others 1995; Shigemoto and others 1993), and whereas both groups are found postsynaptically, presynaptic presence is mostly limited to group II mGluRs. The effects vary and include the following: relatively prolonged changes in membrane potential (i.e., a prolonged EPSP or IPSP), which also affects voltage-gated properties of the cell; changes in short term plasticity, such as affecting the size of evoked PSPs for a time period roughly equivalent to mGluR-evoked PSPs; a different plasticity effect involving a role in endocannabinoid retrograde control of synaptic strength (e.g., Hashimotodani and others 2007; Pattij and others 2008); and longer term plastic changes, such as long-term potentiation or depression (LTP or LTD). Examples of these are described below.
Postsynaptic PSPs
Many reports exist of cortical cells showing changes in membrane potential in response to application of mGluR agonists, and both depolarization (group I mGluRs) as well as hyperpolarization (group II mGluRs) have been noted, although group I responses are more common. In slices of mouse cortex, some afferent pathways have been shown to evoke responses in certain of these mGluRs, and in each case this is because of a Class 2 input that has been activated. Examples are shown schematically in Figure 4 and include the following:
Primary or first-order thalamic input to layers 2/3 of primary cortical areas (i.e., the ventral posterior medial nucleus to primary somatosensory cortex or the ventral medial geniculate nucleus to primary auditory cortex) activates group I mGluRs in roughly three fourths of the target cells, whereas the remaining input thalamic input to these layers and all to layers 4 to 6 are Class 1 and thus do not activate mGluRs (Viaene and others 2011b, 2011c).
In the case of activating the higher order posterior medial nucleus input to primary somatosensory cortex, target cells in all layers receive input that activates group I mGluR responses (Viaene and others 2011a).
The input from layer 6 to layer 4 activates both group I and group II mGluRs (Lee and Sherman 2009a, 2009b). As noted above, it is not clear if this pattern is because of some individual layer 6 axons activating group I mGluRs, and others, group II, or whether many or all activate both groups.
The lateral input from layers 2/3 to other cells in those layers activates group II mGluRs (DePasquale and Sherman 2012).
Finally, the projections between primary and secondary visual and auditory cortices in both directions evokes both groups of mGluR with a complicated laminar relationship, the details of which can be found elsewhere (Covic and Sherman 2011; DePasquale and Sherman 2011) but with the following generalizations. There is little difference in the laminar patterns either between sensory modalities or between the feedback and feedforward direction of the projection. All layers give rise to Class 2 projections that evoke mGluRs, but, with few exceptions, cells in layer 5b receive only Class 1 inputs through these corticocortical routes and thus show no mGluR responses, all cells in layers 5a and 6 receive only Class 2 inputs and do show mGluR responses, whereas some cells in layers 2 to 4 receive Class 1 inputs, and the others, Class 2 inputs. Group II mGluR responses are found for cells only in target layers 4 and 5a, whereas group I responses are found for cells in layers 2 to 5a and 6.
Figure 4.
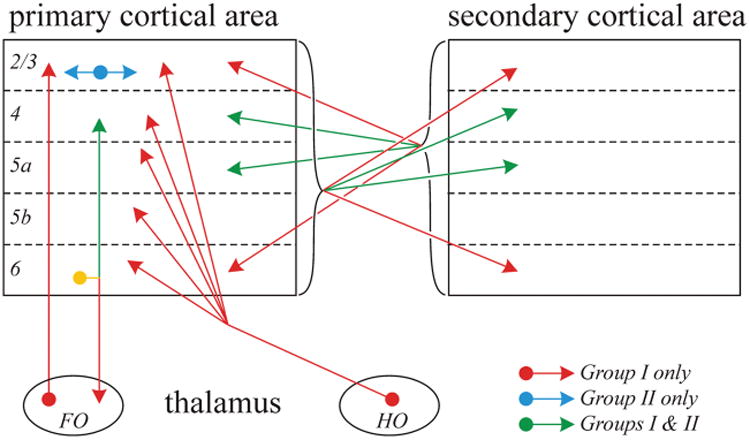
Pattern of Class 2 afferents that activate postsynaptic metabotropic glutamate receptors (mGluRs) at various targets as shown. FO = first-order thalamic relay; HO = higher order thalamic relay. See text for further details.
Short-term plasticity
Numerous reports exist regarding the effect of mGluR agonists on synaptic processing in cortex. Most studies have investigated the effects of agonist or antagonist application on evoked potentials with extracellular recording or on PSPs with intracellular recording. For example, recordings of pyramidal cells in the prefrontal cortex have shown that application of group II mGluR agonists enhances NMDA currents (Tyszkiewicz and others 2004), and application of group I mGluR agonists enhances feedforward inhibition (Sun and Neugebauer 2011).
There is also evidence of specific Class 2 inputs that lead to similar effects on EPSPs. For instance, layer 6 input to layer 4 thalamorecipient cells activates presynaptic group II mGluRs on thalamocortical terminals to reduce the amplitude of their EPSPs (Lee and Sherman 2012; Mateo and Porter, 2007). Likewise, Class 2 input from nearby cells in layers 2/3 causes a reduction of EPSPs from layer 4 input to these cells by activating postsynaptic group II mGluRs (DePasquale and Sherman 2012).
A final form of short-term plasticity seen in many parts of the brain is based on the involvement of group I mGluRs in endocannabinoid retrograde signaling, generally to reduce the amplitude of evoked EPSPs (Hashimotodani and others 2007; Safo and others 2006). There is evidence for such plasticity in cortex as well (Hashimotodani and others 2007; Kiritoshi and others 2013; Marinelli and others 2008; Trettel and others 2004).
LTP and LTD
The role played by mGluRs in the production of long term potentiation (LTP) and long term depression (LTD) in cortex is varied and complex but also widespread (Anwyl, 2009). In different studies, both groups I and II mGluRs have been implicated and these are involved in different ways in the production of both LTD and LTP, although examples of involvement in LTD are more common. The descriptions of effects are so varied that it is not clear so far if any common features can be described. In these experiments, agonists or antagonists were applied to determine effects of mGluR activation on LTP and LTD, so there has as yet been no identification of any afferent glutamatergic pathway involved in evoking these mGluR responses.
In prefrontal cortex, both groups I and II mGluRs are required for LTD (Gerber and others 2000; Huang and others 2007; Otani and others 1999, 2002; Zhong and others 2008), and group II mGluR involvement in retrograde endocannabinoid signaling described above is required for LTD (Barbara and others 2003). In visual cortex, group I mGluR activation is needed for LTD in one study (Tsanov and Manahan-Vaughan 2009), and mGluR activation unspecified as to group is required for LTP in another study (Huemmeke and others 2002). Presynaptic group I mGluR activation in thalamic inputs to auditory cortex are needed to express LTD (Blundon and others 2011). Finally, in somatosensory cortex, two examples of mGluR activation, unspecified as to group, are required for LTD: One involves retrograde endocannabinoid signaling (Nevian and Sakmann 2006), and the other apparently does not (Stiefel and others 2005).
Cortical and Thalamic mGluRs and Disease
Because of the multiple roles mGluRs play in cortical and thus cognitive functioning, a great deal of attention has been directed at the application of mGluR antagonists and agonists for potential therapeutic use (Doherty and Dingledine 2003; Niswender and Conn 2010; Ritzen and others 2005). The implication here is that abnormal processing involving mGluRs can lead to various pathological states. In some cases, specific roles for mGluRs have been suggested, such as abnormal LTD production in fragile X syndrome (Bear and others 2004; Goebel-Goody and Lombroso 2012; Zhang and Alger 2010), and group I mGluR involvement in endocannabinoid control of synaptic processing in schizophrenia and addiction (Melis and others 2004), but for the most part, the actual role of mGluRs in various diseases remains to be elucidated or even suggested. Cognitive diseases that have been so far implicated with regard to mGluR functioning include schizophrenia, anxiety disorders, mental retardation, fragile X syndrome and autism, Alzheimer's, Parkinson's, Huntingdon's, anxiety, depression, addiction, and epilepsy (Alexander and Godwin 2006a; Harrison and others 2008; Huang and others 2007; Luscher and Huber 2010; Marek 2010; Ribeiro and others 2011; Tamminga 2006; Ure and others 2006; Wong and others 1999).
Concluding Remarks
The plethora of mGluRs in thalamus and cortex absolutely requires that we consider their functions as part of glutamatergic circuitry there. This requirement is underscored by the potential of therapeutic advances based on further knowledge of mGluR functioning as described briefly above. In particular, the observation that one type (Class 2) of glutamatergic afferent in these structures activates postsynaptic mGluRs and the other (Class 1) does not also points to the need to parse glutamatergic afferent types in order to understand these complex circuits. Available evidence suggests that Class 2 inputs act like modulators, much like classical modulatory inputs (e.g., cholinergic and noradrenergic), and what these all have in common as a key part of their modulatory actions is the ability to activate metabotropic receptors. Perhaps one thing that sets off Class 2 inputs from these other modulatory inputs is that these glutamatergic inputs are generally highly topographical in organization, whereas most other modulatory inputs are not, suggesting that Class 2 inputs can uniquely bring highly localized modulatory influences to thalamic and cortical circuits.
Activation of mGluRs, much like activation of metabotropic receptors related to other modulatory inputs (e.g., muscarinic), leads to effects with a longer time constant than those generally associated with activation of iGluRs. These include two major categories. One includes shorter term effects that last roughly as long as the evoked mGluR-associated PSPs, that is, hundreds of milliseconds to several seconds. Many of these effects reduce synaptic transmission, either by postsynaptic hyperpolarizing actions of group II mGluRs or presynaptic effects that reduce transmitter release. Among other possibilities, these reductions in circuit activity might be a general substrate for gain control: that is, as activity in a circuit increases, including that of Class 2 inputs that can activate the relevant mGluRs, such a process would serve to dampen such increases; a special case of such gain control suggested above is the action of the triadic synaptic arrangements in thalamus (Sherman 2004). It should also be reiterated that postsynaptic activation of group II mGluRs, especially in cortex, provides a basis for glutamatergic inhibition, suggesting that the great importance often associated with inhibitory inputs in cortical circuits and assumed to be dependent solely on GABAergic inputs (Anderson and others 2000; Ferster and Miller 2000; Hirsch and Martinez, 2006; Monier and others 2003) may also include glutamatergic inputs. Less clear is the function of the prolonged depolarization cause by activation of group I mGluRs, except that this will likely play a role at least in controlling voltage-gated events in target cells, such as activation of NMDA receptors and various voltage-gated conductances. Finally, their participation in LTP and LTD indicates a clear role of mGluRs in longer term plasticity underlying such processes as learning and memory.
One feature generally missing from the description of mGluRs in this review is an understanding of which glutamatergic afferents activate them, and under what conditions. Limited data cited above suggest that Class 2 glutamatergic inputs are the chief player here, at least for activating postsynaptic mGluRs. However, some examples exist of Class 1 inputs activating mGluRs, apparently as a gain control mechanism: the retinal input to the triad activates mGluRs on the inhibitory terminal (see Figure 3), and both retinogeniculate and thalamocortical terminals have presynaptic mGluRs that can be activated by high firing rates in these inputs (Govindaiah and others 2012; Lee and Sherman 2012). However, it is clear that we need more information about mGluRs and especially how they are activated under physiological conditions.
Acknowledgments
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The author's laboratory is supported by Grants DC008794 and EY022338 from the National Institutes of Health.
Footnotes
Unless otherwise noted, by “thalamus,” we mean dorsal thalamus (i.e., the thalamic nuclei that project to neocortex), and by “cortex,” we mean neocortex.
The underlying synaptic conductance changes lead to current flow across the membrane. Because of the resistive– capacitive properties of the membrane, the voltage changes seen across the membrane last longer than the current flow, and this relates to the membrane time constant. Thus measures of synaptic events recorded with the technique of voltage clamp, which measure postsynaptic currents (e.g., EPSCs), produce briefer events than the same events measured during current clamp, which measure postsynaptic voltages (e.g., EPSPs).
For the purposes of this article, a “postsynaptic” site for a receptor is just adjacent to a synaptic terminal and is usually found on dendrites of the postsynaptic target, whereas a “presynaptic” site is on a synaptic terminal, which, with one exception, is not itself clearly postsynaptic to any other terminal. The exception is found in complex thalamic circuits known as triads, in which a GABAergic terminal from an interneuron is both presynaptic to a relay cell dendrite and postsynaptic to a glutamatergic terminal (Sherman 2004; Sherman and Guillery 2006). Further details of triads are presented subsequently.
These appendages are not spines, as seen, for instance, in cortical cells, because they lack features such as the spine apparatus; they appear instead to be small protrusions of dendritic shafts.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Alexander GM, Godwin DW. Presynaptic inhibition of corticothalamic feedback by metabotropic glutamate receptors. J Neurophysiol. 2005;94:163–75. doi: 10.1152/jn.01198.2004. [DOI] [PubMed] [Google Scholar]
- Alexander GM, Godwin DW. Metabotropic glutamate receptors as a strategic target for the treatment of epilepsy. Epilepsy Res. 2006a;71:1–22. doi: 10.1016/j.eplepsyres.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Alexander GM, Godwin DW. Unique presynaptic and postsynaptic roles of group II metabotropic glutamate receptors in the modulation of thalamic network activity. Neuroscience. 2006b;141:501–13. doi: 10.1016/j.neuroscience.2006.03.060. [DOI] [PubMed] [Google Scholar]
- Alonso JM, Usrey WM, Reid RC. Rules of connectivity between geniculate cells and simple cells in cat primary visual cortex. J Neurosci. 2001;21:4002–15. doi: 10.1523/JNEUROSCI.21-11-04002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JS, Carandini M, Ferster D. Orientation tuning of input conductance, excitation, and inhibition in cat primary visual cortex. J Neurophysiol. 2000;84:909–26. doi: 10.1152/jn.2000.84.2.909. [DOI] [PubMed] [Google Scholar]
- Andolina IM, Jones HE, Sillito AM. The effects of cortical feedback on the spatial properties of relay cells in the lateral geniculate nucleus. J Neurophysiol. 2012 Oct 24; doi: 10.1152/jn.00194.2012. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andolina IM, Jones HE, Wang W, Sillito AM. Corticothalamic feedback enhances stimulus response precision in the visual system. Proc Nat Acad Sci U S A. 2007;104:1685–90. doi: 10.1073/pnas.0609318104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwyl R. Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Res Brain Res Rev. 1999;29:83–120. doi: 10.1016/s0165-0173(98)00050-2. [DOI] [PubMed] [Google Scholar]
- Anwyl R. Metabotropic glutamate receptor-dependent long-term potentiation. Neuropharmacology. 2009;56:735–40. doi: 10.1016/j.neuropharm.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Baker FH, Malpeli JG. Effects of cryogenic blockade of visual cortex on the responses of lateral geniculate neurons in the monkey. Exp Brain Res. 1977;29:433–44. doi: 10.1007/BF00236182. [DOI] [PubMed] [Google Scholar]
- Banke TG, Traynelis SF. Activation of NR1/NR2B NMDA receptors. Nat Neurosci. 2003;6:144–52. doi: 10.1038/nn1000. [DOI] [PubMed] [Google Scholar]
- Barbara JG, Auclair N, Roisin MP, Otani S, Valjent E, Caboche J, et al. Direct and indirect interactions between cannabinoid CB1 receptor and group II metabotropic glutamate receptor signalling in layer V pyramidal neurons from the rat prefrontal cortex. Eur J Neurosci. 2003;17:981–90. doi: 10.1046/j.1460-9568.2003.02533.x. [DOI] [PubMed] [Google Scholar]
- Batchelor AM, Garthwaite J. Frequency detection and temporally dispersed synaptic signal association through a metabotropic glutamate receptor pathway. Nature. 1997;385:74–7. doi: 10.1038/385074a0. [DOI] [PubMed] [Google Scholar]
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–7. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Bellone C, Luscher C, Mameli M. Mechanisms of synaptic depression triggered by metabotropic glutamate receptors. Cell Mol Life Sci. 2008;65:2913–23. doi: 10.1007/s00018-008-8263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binns KE, Turner JP, Salt TE. Kainate receptor (GluR5)-mediated disinhibition of responses in rat ventrobasal thalamus allows a novel sensory processing mechanism. J Physiol. 2003;551(Pt 2):525–37. doi: 10.1113/jphysiol.2003.045096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundon JA, Bayazitov IT, Zakharenko SS. Presynaptic gating of postsynaptically expressed plasticity at mature thalamocortical synapses. J Neurosci. 2011;31:16012–25. doi: 10.1523/JNEUROSCI.3281-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasnjo G, Otis TS. Neuronal glutamate transporters control activation of postsynaptic metabotropic glutamate receptors and influence cerebellar long-term depression. Neuron. 2001;31:607–16. doi: 10.1016/s0896-6273(01)00377-4. [DOI] [PubMed] [Google Scholar]
- Brown SP, Brenowitz SD, Regehr WG. Brief presynaptic bursts evoke synapse-specific retrograde inhibition mediated by endogenous cannabinoids. Nat Neurosci. 2003;6:1048–57. doi: 10.1038/nn1126. [DOI] [PubMed] [Google Scholar]
- Catterall WA. Ion channel voltage sensors: structure, function, and pathophysiology. Neuron. 2010;67:915–28. doi: 10.1016/j.neuron.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S, Li X, Nelson SB. Short-term depression at thalamocortical synapses contributes to rapid adaptation of cortical sensory responses in vivo. Neuron. 2002;34:437–46. doi: 10.1016/s0896-6273(02)00659-1. [DOI] [PubMed] [Google Scholar]
- Cleland BG, Dubin MW, Levick WR. Sustained and transient neurones in the cat's retina and lateral geniculate nucleus. J Physiol. 1971;217:473–96. doi: 10.1113/jphysiol.1971.sp009581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland BG, Lee BB. A comparison of visual responses of cat lateral geniculate nucleus neurones with those of ganglion cells afferent to them. J Physiol. 1985;369:249–68. doi: 10.1113/jphysiol.1985.sp015899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–37. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Coutinho V, Knopfel T. Metabotropic glutamate receptors: electrical and chemical signaling properties. Neuroscientist. 2002;8:551–61. doi: 10.1177/1073858402238514. [DOI] [PubMed] [Google Scholar]
- Covic EN, Sherman SM. Synaptic properties of connections between the primary and secondary auditory cortices in mice. Cereb Cortex. 2011;21:2425–41. doi: 10.1093/cercor/bhr029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox CL, Sherman SM. Glutamate inhibits thalamic reticular neurons. J Neurosci. 1999;19:6694–9. doi: 10.1523/JNEUROSCI.19-15-06694.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox CL, Zhou Q, Sherman SM. Glutamate locally activates dendritic outputs of thalamic interneurons. Nature. 1998;394:478–82. doi: 10.1038/28855. [DOI] [PubMed] [Google Scholar]
- DePasquale R, Sherman SM. Synaptic properties of corticocortical connections between the primary and secondary visual cortical areas in the mouse. J Neurosci. 2011;31:16494–506. doi: 10.1523/JNEUROSCI.3664-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePasquale R, Sherman SM. Modulatory effects of metabotropic glutamate receptors on local cortical circuits. J Neurosci. 2012;32:7364–72. doi: 10.1523/JNEUROSCI.0090-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrunz LE, Stevens CF. Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron. 1997;18:995–1008. doi: 10.1016/s0896-6273(00)80338-4. [DOI] [PubMed] [Google Scholar]
- Doherty J, Dingledine R. Functional interactions between cannabinoid and metabotropic glutamate receptors in the central nervous system. Curr Opin Pharmacol. 2003;3:46–53. doi: 10.1016/s1471-4892(02)00014-0. [DOI] [PubMed] [Google Scholar]
- Ferster D. Origin of orientation-selective EPSPs in simple cells of cat visual cortex. J Neurosci. 1987;7:1780–91. doi: 10.1523/JNEUROSCI.07-06-01780.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferster D, Chung S, Wheat H. Orientation selectivity of thalamic input to simple cells of cat visual cortex. Nature. 1996;380:249–52. doi: 10.1038/380249a0. [DOI] [PubMed] [Google Scholar]
- Ferster D, Miller KD. Neural mechanisms of orientation selectivity in the visual cortex. Annu Rev Neurosci. 2000;23:441–71. doi: 10.1146/annurev.neuro.23.1.441. [DOI] [PubMed] [Google Scholar]
- Geisert EE, Langsetmo A, Spear PD. Influence of the corticogeniculate pathway on reponse properties of cat lateral geniculate neurons. Brain Res. 1981;208:409–15. doi: 10.1016/0006-8993(81)90568-0. [DOI] [PubMed] [Google Scholar]
- Gerber G, Zhong J, Youn D, Randic M. Group II and group III metabotropic glutamate receptor agonists depress synaptic transmission in the rat spinal cord dorsal horn. Neuroscience. 2000;100:393–406. doi: 10.1016/s0306-4522(00)00269-4. [DOI] [PubMed] [Google Scholar]
- Gereau RW, Conn PJ. Potentiation of cAMP responses by metabotropic glutamate receptors depresses excitatory synaptic transmission by a kinase-independent mechanism. Neuron. 1994;12:1121–9. doi: 10.1016/0896-6273(94)90319-0. [DOI] [PubMed] [Google Scholar]
- Gibb AJ. NMDA receptor subunit gating—uncovered. Trends Neurosci. 2004;27:7–10. doi: 10.1016/j.tins.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Gilbert CD, Wiesel TN. Morphology and intracortical projections of functionally characterised neurones in the cat visual cortex. Nature. 1979;280:120–5. doi: 10.1038/280120a0. [DOI] [PubMed] [Google Scholar]
- Godwin DW, Van Horn SC, Eriir A, Sesma M, Romano C, Sherman SM. Ultrastructural localization suggests that retinal and cortical inputs access different metabotropic glutamate receptors in the lateral geniculate nucleus. J Neurosci. 1996a;16:8181–92. doi: 10.1523/JNEUROSCI.16-24-08181.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godwin DW, Vaughan JW, Sherman SM. Metabotropic glutamate receptors switch visual response mode of lateral geniculate nucleus cells from burst to tonic. J Neurophysiol. 1996b;76:1800–16. doi: 10.1152/jn.1996.76.3.1800. [DOI] [PubMed] [Google Scholar]
- Goebel-Goody SM, Lombroso PJ. Taking STEPs forward to understand fragile X syndrome. Results Probl Cell Differ. 2012;54:223–41. doi: 10.1007/978-3-642-21649-7_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindaiah G, Cox CL. Synaptic activation of metabotropic glutamate receptors regulates dendritic outputs of thalamic interneurons. Neuron. 2004;41:611–23. doi: 10.1016/s0896-6273(04)00013-3. [DOI] [PubMed] [Google Scholar]
- Govindaiah G, Wang T, Gillette MU, Cox CL. Activity-dependent regulation of retinogeniculate signaling by metabotropic glutamate receptors. J Neurosci. 2012;32:12820–31. doi: 10.1523/JNEUROSCI.0687-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z, Liu W, Wei J, Yan Z. Regulation of N-methyl-d-aspartic acid (NMDA) receptors by metabotropic glutamate receptor 7. J Biol Chem. 2012;287:10265–75. doi: 10.1074/jbc.M111.325175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PJ, Lyon L, Sartorius LJ, Burnet PW, Lane TA. The group II metabotropic glutamate receptor 3 (mGluR3, mGlu3, GRM3): expression, function and involvement in schizophrenia. J Psychopharmacol. 2008;22:308–22. doi: 10.1177/0269881108089818. [DOI] [PubMed] [Google Scholar]
- Hashimoto A, Oka T. Free d-aspartate and d-serine in the mammalian brain and periphery. Prog Neurobiol. 1997;52:325–53. doi: 10.1016/s0301-0082(97)00019-1. [DOI] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Kano M. Ca(2+)-assisted receptor-driven endocannabinoid release: mechanisms that associate presynaptic and postsynaptic activities. Curr Opin Neurobiol. 2007;17:360–5. doi: 10.1016/j.conb.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Hermes ML, Renaud LP. Postsynaptic and presynaptic group II metabotropic glutamate receptor activation reduces neuronal excitability in rat midline paraventricular thalamic nucleus. J Pharmacol Exp Ther. 2011;336:840–9. doi: 10.1124/jpet.110.176149. [DOI] [PubMed] [Google Scholar]
- Hirsch JA, Martinez LM. Circuits that build visual cortical receptive fields. Trends Neurosci. 2006;29:30–9. doi: 10.1016/j.tins.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Huang CC, Yang PC, Lin HJ, Hsu KS. Repeated cocaine administration impairs group II metabotropic glutamate receptor-mediated long-term depression in rat medial prefrontal cortex. J Neurosci. 2007;27:2958–68. doi: 10.1523/JNEUROSCI.4247-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YH, Sinha SR, Tanaka K, Rothstein JD, Bergles DE. Astrocyte glutamate transporters regulate metabotropic glutamate receptor-mediated excitation of hippocampal interneurons. J Neurosci. 2004;24:4551–9. doi: 10.1523/JNEUROSCI.5217-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962;160:106–54. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huemmeke M, Eysel UT, Mittmann T. Metabotropic glutamate receptors mediate expression of LTP in slices of rat visual cortex. Eur J Neurosci. 2002;15:1641–5. doi: 10.1046/j.1460-9568.2002.02002.x. [DOI] [PubMed] [Google Scholar]
- Huettner JE. Kainate receptors and synaptic transmission. Prog Neurobiol. 2003;70:387–407. doi: 10.1016/s0301-0082(03)00122-9. [DOI] [PubMed] [Google Scholar]
- Jia Z, Lu YM, Agopyan N, Roder J. Gene targeting reveals a role for the glutamate receptors mGluR5 and GluR2 in learning and memory. Physiol Behav. 2001;73:793–802. doi: 10.1016/s0031-9384(01)00516-9. [DOI] [PubMed] [Google Scholar]
- Kalil RE, Chase R. Corticofugal influence on activity of lateral geniculate neurons in the cat. J Neurophysiol. 1970;33:459–74. doi: 10.1152/jn.1970.33.3.459. [DOI] [PubMed] [Google Scholar]
- Kandel ER, Schwartz JH, Jessell TM. Principles of neural science. New York: McGraw-Hill; 2000. [Google Scholar]
- Kawajiri S, Dingledine R. Multiple structural determinants of voltage-dependent magnesium block in recombinant NMDA receptors. Neuropharmacology. 1993;32:1203–11. doi: 10.1016/0028-3908(93)90014-t. [DOI] [PubMed] [Google Scholar]
- Kennedy MB. Signal-processing machines at the postsynaptic density. Science. 2000;290:750–4. doi: 10.1126/science.290.5492.750. [DOI] [PubMed] [Google Scholar]
- Kidd FL, Isaac JT. Kinetics and activation of postsynaptic kainate receptors at thalamocortical synapses: role of glutamate clearance. J Neurophysiol. 2001;86:1139–48. doi: 10.1152/jn.2001.86.3.1139. [DOI] [PubMed] [Google Scholar]
- Kim CH, Lee J, Lee JY, Roche KW. Metabotropic glutamate receptors: phosphorylation and receptor signaling. J Neurosci Res. 2008;86:1–10. doi: 10.1002/jnr.21437. [DOI] [PubMed] [Google Scholar]
- Kiritoshi T, Sun H, Ren W, Stauffer SR, Lindsley CW, Conn PJ, et al. Modulation of pyramidal cell output in the medial prefrontal cortex by mGluR5 interacting with CB1. Neuropharmacol. 2013;66:170–8. doi: 10.1016/j.neuropharm.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann DM, Lamsa K. Roles of distinct glutamate receptors in induction of anti-Hebbian long-term potentiation. J Physiol. 2008;586:1481–6. doi: 10.1113/jphysiol.2007.148064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landisman CE, Connors BW. Long-term modulation of electrical synapses in the mammalian thalamus. Science. 2005;310:1809–13. doi: 10.1126/science.1114655. [DOI] [PubMed] [Google Scholar]
- Lee CC, Sherman SM. Synaptic properties of thalamic and intracortical inputs to layer 4 of the first- and higher-order cortical areas in the auditory and somatosensory systems. J Neurophysiol. 2008;100:317–26. doi: 10.1152/jn.90391.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Sherman SM. Glutamatergic inhibition in sensory neocortex. Cereb Cortex. 2009a;19:2281–9. doi: 10.1093/cercor/bhn246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Sherman SM. Modulator property of the intrinsic cortical projection from layer 6 to layer 4. Front Syst Neurosci. 2009b;3:1–5. doi: 10.3389/neuro.06.003.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Sherman SM. Intrinsic modulators of auditory thalamocortical transmission. Hear Res. 2012;287:43–50. doi: 10.1016/j.heares.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan R, Nusser Z, Roberts JD, Shigemoto R, Somogyi P. Perisynaptic location of metabotropic glutamate receptors mGluR1 and mGluR5 on dendrites and dendritic spines in the rat hippocampus. Eur J Neurosci. 1996;8:1488–500. doi: 10.1111/j.1460-9568.1996.tb01611.x. [DOI] [PubMed] [Google Scholar]
- Luscher C, Huber KM. Group 1 mGluR-dependent synaptic long-term depression: mechanisms and implications for circuitry and disease. Neuron. 2010;65:445–59. doi: 10.1016/j.neuron.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao LM, Zhang GC, Liu XY, Fibuch EE, Wang JQ. Group I metabotropic glutamate receptor-mediated gene expression in striatal neurons. Neurochem Res. 2008;33:1920–4. doi: 10.1007/s11064-008-9654-4. [DOI] [PubMed] [Google Scholar]
- Marek GJ. Metabotropic glutamate2/3 (mGlu2/3) receptors, schizophrenia and cognition. Eur J Pharmacol. 2010;639:81–90. doi: 10.1016/j.ejphar.2010.02.058. [DOI] [PubMed] [Google Scholar]
- Marinelli S, Pacioni S, Bisogno T, Di Marzo V, Prince DA, Huguenard JR, et al. The endocannabinoid 2-arachidonoylglycerol is responsible for the slow self-inhibition in neocortical interneurons. J Neurosci. 2008;28:13532–41. doi: 10.1523/JNEUROSCI.0847-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastronarde DN. Two classes of single-input X-cells in cat lateral geniculate nucleus. I. Receptive field properties and classification of cells. J Neurophysiol. 1987;57:357–80. doi: 10.1152/jn.1987.57.2.357. [DOI] [PubMed] [Google Scholar]
- Mateo Z, Porter JT. Group II metabotropic glutamate receptors inhibit glutamate release at thalamocortical synapses in the developing somatosensory cortex. Neuroscience. 2007;146:1062–72. doi: 10.1016/j.neuroscience.2007.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL. The physiology of excitatory amino acids in the vertebrate central nervous system. Prog Neurobiol. 1987;28:197–276. doi: 10.1016/0301-0082(87)90011-6. [DOI] [PubMed] [Google Scholar]
- McClurkin JW, Marrocco RT. Visual cortical input alters spatial tuning in monkey lateral geniculate nucleus cells. J Physiol. 1984;348:135–52. doi: 10.1113/jphysiol.1984.sp015103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClurkin JW, Optican LM, Richmond BJ. Cortical feedback increases visual information transmitted by monkey parvocellular lateral geniculate nucleus neurons. Vis Neurosci. 1994;11:601–17. doi: 10.1017/s0952523800002492. [DOI] [PubMed] [Google Scholar]
- Melis M, Perra S, Muntoni AL, Pillolla G, Lutz B, Marsicano G, et al. Prefrontal cortex stimulation induces 2-arachidonoyl-glycerol-mediated suppression of excitation in dopamine neurons. J Neurosci. 2004;24:10707–15. doi: 10.1523/JNEUROSCI.3502-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata M, Imoto K. Contrary roles of kainate receptors in transmitter release at corticothalamic synapses onto thalamic relay and reticular neurons. J Physiol. 2009;587:999–1012. doi: 10.1113/jphysiol.2008.164996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monier C, Chavane F, Baudot P, Graham LJ, Fregnac Y. Orientation and direction selectivity of synaptic inputs in visual cortical neurons: a diversity of combinations produces spike tuning. Neuron. 2003;37:663–80. doi: 10.1016/s0896-6273(03)00064-3. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB. Neural mechanismsm in somesthesis. In: Mountcastle VB, editor. Medical physiology. St. Louis, MO: Mosby; 1980. pp. 348–90. [Google Scholar]
- Nevian T, Sakmann B. Spine Ca2+ signaling in spike-timing-dependent plasticity. J Neurosci. 2006;26:11001–13. doi: 10.1523/JNEUROSCI.1749-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll RA, Malenka RC, Kauer JA. Functional comparison of neurotransmitter receptor subtypes in mammalian central nervous system. Physiol Rev. 1990;70:513–65. doi: 10.1152/physrev.1990.70.2.513. [DOI] [PubMed] [Google Scholar]
- Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Mulvihill E, Streit P, Somogyi P. Subsynaptic segregation of metabotropic and ionotropic glutamate receptors as revealed by immunogold localization. Neuroscience. 1994;61:421–7. doi: 10.1016/0306-4522(94)90421-9. [DOI] [PubMed] [Google Scholar]
- Ohishi H, Akazawa C, Shigemoto R, Nakanishi S, Mizuno N. Distributions of the mRNAs for L-2-amino-4-phosphonobutyrate- sensitive metabotropic glutamate receptors, mGluR4 and mGluR7, in the rat brain. J Comp Neurol. 1995;360:555–70. doi: 10.1002/cne.903600402. [DOI] [PubMed] [Google Scholar]
- Ohishi H, Shigemoto R, Nakanishi S, Mizuno N. Distribution of the messenger RNA for a metabotropic glutamate receptor, mGluR2, in the central nervous system of the rat. Neuroscience. 1993a;53:1009–18. doi: 10.1016/0306-4522(93)90485-x. [DOI] [PubMed] [Google Scholar]
- Ohishi H, Shigemoto R, Nakanishi S, Mizuno N. Distribution of the mRNA for a metabotropic glutamate receptor (mGluR3) in the rat brain: an in situ hybridization study. J Comp Neurol. 1993b;335:252–66. doi: 10.1002/cne.903350209. [DOI] [PubMed] [Google Scholar]
- Otani S, Auclair N, Desce JM, Roisin MP, Crepel F. Dopamine receptors and groups I and II mGluRs cooperate for long-term depression induction in rat prefrontal cortex through converging postsynaptic activation of MAP kinases. J Neurosci. 1999;19:9788–802. doi: 10.1523/JNEUROSCI.19-22-09788.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani S, Daniel H, Takita M, Crepel F. Long-term depression induced by postsynaptic group II metabotropic glutamate receptors linked to phospholipase C and intracellular calcium rises in rat prefrontal cortex. J Neurosci. 2002;22:3434–44. doi: 10.1523/JNEUROSCI.22-09-03434.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattij T, Wiskerke J, Schoffelmeer AN. Cannabinoid modulation of executive functions. Eur J Pharmacol. 2008;585:458–63. doi: 10.1016/j.ejphar.2008.02.099. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Singh S, Wu C, Shi L, Wei J, et al. A monoclonal antibody shows discrete cellular and subcellular localizations of mGluR1 alpha metabotropic glutamate receptors. J Chem Neuroanat. 1997;13:77–93. doi: 10.1016/s0891-0618(97)00023-9. [DOI] [PubMed] [Google Scholar]
- Pin JP, Bockaert J. Get receptive to metabotropic glutamate receptors. Curr Opin Neurobiol. 1995;5:342–9. doi: 10.1016/0959-4388(95)80047-6. [DOI] [PubMed] [Google Scholar]
- Pin JP, Duvoisin R. The metabotropic glutamate receptors: structure and functions. Neuropharmacology. 1995;34:1–26. doi: 10.1016/0028-3908(94)00129-g. [DOI] [PubMed] [Google Scholar]
- Reid SN, Romano C, Hughes T, Daw NW. Immunohistochemical study of two phosphoinositide-linked metabotropic glutamate receptors (mGluR1 alpha and mGluR5) in the cat visual cortex before, during, and after the peak of the critical period for eye-specific connections. J Comp Neurol. 1995;355:470–7. doi: 10.1002/cne.903550311. [DOI] [PubMed] [Google Scholar]
- Ribeiro FM, Pires RG, Ferguson SS. Huntington's disease and group I metabotropic glutamate receptors. Mol Neurobiol. 2011;43:1–11. doi: 10.1007/s12035-010-8153-1. [DOI] [PubMed] [Google Scholar]
- Ritzen A, Mathiesen JM, Thomsen C. Molecular pharmacology and therapeutic prospects of metabotropic glutamate receptor allosteric modulators. Basic Clin Pharmacol Toxicol. 2005;97:202–13. doi: 10.1111/j.1742-7843.2005.pto_156.x. [DOI] [PubMed] [Google Scholar]
- Safo PK, Cravatt BF, Regehr WG. Retrograde endocannabinoid signaling in the cerebellar cortex. Cerebellum. 2006;5:134–45. doi: 10.1080/14734220600791477. [DOI] [PubMed] [Google Scholar]
- Salt TE, Eaton SA. Modulation of sensory neurone excitatory and inhibitory responses in the ventrobasal thalamus by activation of metabotropic excitatory amino acid receptors. Neuropharmacology. 1995;34:1043–51. doi: 10.1016/0028-3908(95)00052-8. [DOI] [PubMed] [Google Scholar]
- Schmielau F, Singer W. The role of visual cortex for binocular interactions in the cat lateral geniculate nucleus. Brain Res. 1977;120:354–61. doi: 10.1016/0006-8993(77)90914-3. [DOI] [PubMed] [Google Scholar]
- Sherman SM. Interneurons and triadic circuitry of the thalamus. Trends Neurosci. 2004;27:670–5. doi: 10.1016/j.tins.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Sherman SM. Thalamocortical interactions. Curr Opin Neurobiol. 2012;17:417–22. doi: 10.1016/j.conb.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. Exploring the thalamus and its role in cortical function. Cambridge, MA: MIT Press; 2006. [Google Scholar]
- Sherman SM, Guillery RW. Distinct functions for direct and transthalamic corticocortical connections. J Neurophysiol. 2011;106:1068–77. doi: 10.1152/jn.00429.2011. [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Nomura S, Ohishi H, Sugihara H, Nakanishi S, Mizuno N. Immunohistochemical localization of a metabotropic glutamate receptor, mGluR5, in the rat brain. Neurosci Lett. 1993;163:53–7. doi: 10.1016/0304-3940(93)90227-c. [DOI] [PubMed] [Google Scholar]
- Stiefel KM, Tennigkeit F, Singer W. Synaptic plasticity in the absence of backpropagating spikes of layer II inputs to layer V pyramidal cells in rat visual cortex. Eur J Neurosci. 2005;21:2605–10. doi: 10.1111/j.1460-9568.2005.04094.x. [DOI] [PubMed] [Google Scholar]
- Sun H, Neugebauer V. mGluR1, but not mGluR5, activates feed-forward inhibition in the medial prefrontal cortex to impair decision making. J Neurophysiol. 2011;106:960–73. doi: 10.1152/jn.00762.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamminga CA. The neurobiology of cognition in schizophrenia. J Clin Psychiatry. 2006;67:e11. [PubMed] [Google Scholar]
- Trettel J, Fortin DA, Levine ES. Endocannabinoid signalling selectively targets perisomatic inhibitory inputs to pyramidal neurones in juvenile mouse neocortex. J Physiol. 2004;556:95–107. doi: 10.1113/jphysiol.2003.058198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsanov M, Manahan-Vaughan D. Synaptic plasticity in the adult visual cortex is regulated by the metabotropic glutamate receptor, mGLUR5. Exp Brain Res. 2009;199:391–9. doi: 10.1007/s00221-009-1965-4. [DOI] [PubMed] [Google Scholar]
- Tyszkiewicz JP, Gu Z, Wang X, Cai X, Yan Z. Group II metabotropic glutamate receptors enhance NMDA receptor currents via a protein kinase C-dependent mechanism in pyramidal neurones of rat prefrontal cortex. J Physiol. 2004;554:765–77. doi: 10.1113/jphysiol.2003.056812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ure J, Baudry M, Perassolo M. Metabotropic glutamate receptors and epilepsy. J Neurol Sci. 2006;247:1–9. doi: 10.1016/j.jns.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Usrey WM, Reppas JB, Reid RC. Specificity and strength of retinogeniculate connections. J Neurophysiol. 1999;82:3527–40. doi: 10.1152/jn.1999.82.6.3527. [DOI] [PubMed] [Google Scholar]
- Viaene AN, Petrof I, Sherman SM. Properties of the thalamic projection from the posterior medial nucleus to primary and secondary somatosensory cortices in the mouse. Proc Nat Acad Sci U S A. 2011a;108:18156–61. doi: 10.1073/pnas.1114828108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viaene AN, Petrof I, Sherman SM. Synaptic properties of thalamic input to layers 2/3 in primary somatosensory and auditory cortices. J Neurophysiol. 2011b;105:279–92. doi: 10.1152/jn.00747.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viaene AN, Petrof I, Sherman SM. Synaptic properties of thalamic input to the subgranular layers of primary somatosensory and auditory cortices in the mouse. J Neurosci. 2011c;31:12738–47. doi: 10.1523/JNEUROSCI.1565-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viaene AN, Petrof I, Sherman SM. Activation requirements for metabotropic glutamate receptors. Neurosci Lett. 2013 doi: 10.1016/j.neulet.2013.02.004. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JR, Friedlander MJ, Sherman SM. Fine structural morphology of identified X- and Y-cells in the cat's lateral geniculate nucleus. Proc Roy Soc Lond B. 1984;221:411–36. doi: 10.1098/rspb.1984.0042. [DOI] [PubMed] [Google Scholar]
- Wong RK, Bianchi R, Taylor GW, Merlin LR. Role of metabotropic glutamate receptors in epilepsy. Adv Neurol. 1999;79:685–98. [PubMed] [Google Scholar]
- Zhang L, Alger BE. Enhanced endocannabinoid signaling elevates neuronal excitability in fragile X syndrome. J Neurosci. 2010;30:5724–9. doi: 10.1523/JNEUROSCI.0795-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SY, Xu M, Miao QL, Poo MM, Zhang XH. Endocannabinoid-dependent homeostatic regulation of inhibitory synapses by miniature excitatory synaptic activities. J Neurosci. 2009;29:13222–31. doi: 10.1523/JNEUROSCI.1710-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong P, Liu W, Gu Z, Yan Z. Serotonin facilitates long-term depression induction in prefrontal cortex via p38 MAPK/Rab5-mediated enhancement of AMPA receptor internalization. J Physiol. 2008;586:4465–79. doi: 10.1113/jphysiol.2008.155143. [DOI] [PMC free article] [PubMed] [Google Scholar]


