A 4-year-old boy developed a headache. Initial evaluation revealed a normal neurologic examination and a right subdural hygroma on CT. Worsening headaches led to hospitalization at an outside institution. MRI showed leptomeningeal enhancement; magnetic resonance angiography (MRA) was normal. Infectious, rheumatologic, hematologic, and CSF studies were unrevealing. He then developed a left-sided hemiparesis. Imaging showed increased leptomeningeal enhancement with punctate infarcts in the right hemisphere. CT angiography demonstrated irregularity involving branches of the circle of Willis suggestive of vasculitis. Methylprednisolone (30 mg·kg−1·d−1 × 5 days) was administered for presumed CNS vasculitis.
Despite continued glucocorticoid therapy, the patient developed increasing left-sided weakness. Repeat imaging showed perivascular inflammation. Headaches continued and examination revealed left-sided hemiparesis, new-onset right leg weakness, bilateral clonus, and extensor plantar responses. IV cyclophosphamide (dosed every 2 weeks for 4 doses; titrated to 1,000 mg/m2) was initiated for presumed CNS vasculitis. Rituximab (750 mg/m2 × 2 doses) and IV immunoglobulin (2 g/kg divided over 4 days) were added for worsening MRI findings. However, he developed new multifocal infarcts. Infliximab (5 mg/kg) was initiated based on use in resistant CNS vasculitis.1 Two months after initial diagnosis, the patient was transferred to our institution for a second opinion.
Upon transfer, examination revealed weakness of the left face, arm, and leg; continued right leg weakness; a left homonymous hemianopsia; and bilateral clonus and extensor plantar responses. Spinal and brain MRI revealed multiple spinal cord lesions and acute infarcts in the anterolateral right pons. The patient developed right cranial nerve III palsy. He developed abdominal pain (attributed to neurogenic bladder and constipation). Abdominal and pelvic MRA and serologies did not demonstrate systemic vasculitis.
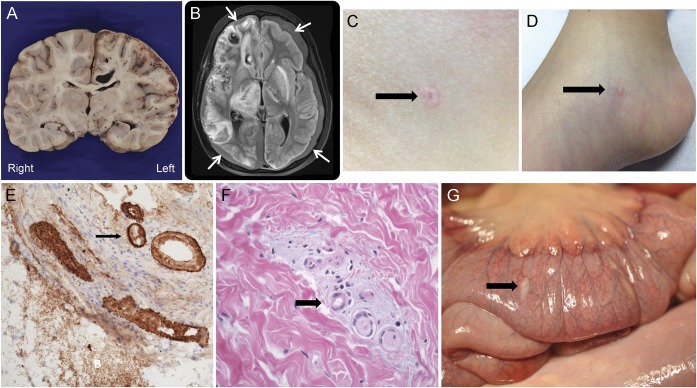
Based on his subdural fluid collections, abdominal pain, and the refractory nature of his vascular process, Degos disease was proposed as a unifying diagnosis.2 Previously rash-free, the patient was now noted to have a 4-mm porcelain white abdominal papule with central atrophy and an erythematous rim (figure, C). A papule with a dull pink-gray center and red telangiectatic rim was present on his right foot (figure, D). Skin biopsy revealed central epidermal atrophy with hyperkeratosis and increased dermal mucin and fibrin thrombi (figure, F). Small dermal vessels demonstrated degeneration of their walls, leading to a smudged appearance (figure, F). Eculizumab3 and treprostinil4 were initiated for presumed Degos.
Figure. Neurologic, gastrointestinal, and dermatologic findings.
(A) Coronal section of the brain. In the right hemisphere, there are multiple hemorrhages in white matter, hippocampus, basal gray matter, and extensive hemorrhagic cortical infarction. (B) MRI fluid-attenuated inversion recovery image shows bilateral subdural collections (arrows) with extensive subacute infarcts and hemorrhage, predominantly on the right. (C) A 4-mm porcelain white papule with central atrophy and an erythematous rim on the patient's left abdomen. (D) Papule with a dull pink gray center and red rim present on the right medial plantar foot. (E) Deposition of membrane attack complex C5b-9 in vessel walls, immunoperoxidase ×10. (F) High-power view of the skin shows increased mucin (arrow) around dermal vessels. The vessel walls have a smudged appearance and 2 are filled with fibrin thrombi. (G) White porcelain lesions (arrow) on the proximal bowel serosa seen at autopsy. Microscopically, these areas show increased mucin, abnormal vessels with degenerating, smudged vessel walls, and thrombi in medium-sized vessels.
The patient developed worsening abdominal pain. He was found to have 3 gastric perforations, which were repaired. He developed right upper extremity myoclonic movements necessitating intubation for sedation. MRI demonstrated worsened right-sided and new left hemispheric leptomeningeal enhancement, as well as a new subacute right thalamic infarct extending to the brainstem (figure, B). Anti-VLA4 therapy (natalizumab; 5 mg/kg) was added to reduce CD4 T-cell access into the CNS. MRI 1 week later revealed increasing bilateral hemispheric disease and progression of the deep gray nuclei and brainstem lesions. Palliative care was provided; the patient died of respiratory failure after elective extubation.
Autopsy findings.
Multiple skin lesions were histologically similar to the initial skin biopsy. Scattered porcelain plaques were identified on the serosal surface of the small bowel corresponding to areas of mucin deposition in the serosa and thrombosis of small to medium-sized vessels on histology (figure, G). There was evidence of impending distal small bowel perforation. The areas of prior repair in the stomach showed thrombosis of small to medium-sized vessels with necrosis. Neuropathology (brain and spinal cord) revealed extensive CNS vasculopathy, with multiple foci of hemorrhage and infarction (figure, F). There were fibrin plugs in capillaries, venules, and arterioles in both the CNS parenchyma and leptomeninges. Deposition of membrane attack complex (C5b-9) was demonstrated in the cortical vessel walls (figure, E).
Discussion.
Degos disease (malignant atrophic papulosis) is a rare multiorgan thrombo-obliterative disorder. With systemic disease, median survival time is approximately 2 years, with a 3-year survival rate of less than 50%.5,6 Pathognomonic skin lesions facilitate a diagnosis. These small (0.5–1 cm) skin lesions are usually located on the trunk and extremities and exhibit central porcelain white atrophy with a surrounding telangiectatic rim.7 There are fewer than 200 cases of Degos reported in the literature, and it is exceedingly uncommon in children.7
This case demonstrates the challenges of diagnosing and treating rare inflammatory CNS vasculopathies in children. As in our case, Degos disease may present with isolated neurologic manifestations prior to the development of its cutaneous and gastrointestinal manifestations. The presence of subtle yet unique skin lesions in combination with neurologic complications, including subdural fluid collections, provide critical clues to the diagnosis of this rare disease.
Footnotes
Author contributions: Dr. Gmuca is the corresponding author and drafted the original manuscript and helped finalize the manuscript prior to submission. Dr. Boos revised the manuscript and added additional information and images with regards to the dermatologic aspects of the case. Drs. Treece and Harding revised the manuscript and drafted the information relevant to the pathologic findings and also contributed images for the manuscript. Drs. Perman, Laje, Burnham, Narula, and Billinghurst edited and revised the final manuscript. Dr. Vossough corrected and added information with regards to the radiology findings and contributed images to the manuscript. Dr. Banwell made multiple revisions to the work from the drafting stages through the final revisions of the manuscript and provided intellectual oversight.
Study funding: No targeted funding.
Disclosure: S. Gmuca, M.D. Boos, and A. Treece report no disclosures. S. Narula received research support from the National Multiple Sclerosis Society. L. Billinghurst, T. Bhatti, and P. Laje report no disclosures. M.J. Perman's spouse holds a patent for compositions and methods for treatment of HSCT-associated thrombotic microangiopathy. A. Vossough is on the editorial board of the American Journal of Neuroradiology, receives royalties from Oxford University Press, has consulted for Banyan, and received research support from NIH. B. Harding reports no disclosures. J. Burnham received research support from NIH, Children's Hospital of Philadelphia, and Lupus Foundation of America; and was an expert witness for Smith, Hulsey & Busey. B. Banwell is on the scientific advisory board for Biogen-Idec, Sanofi, Eli Lilly, and Novartis; receives speaker honoraria and/or travel support from Biogen-Idec, Merck-Serono, Teva Neuroscience, and Bayer; is on the editorial boards of Neurology® and Multiple Sclerosis and Related Disorders; has consulted for Biogen-Idec, Eli Lilly, and Sanofi; has spoken an event supported by the Consortium of MS Centers; and received research support from Multiple Sclerosis Society of Canada, Multiple Sclerosis Scientific Research Foundation, and National Multiple Sclerosis Society. Go to Neurology.org/nn for full disclosure forms. The Article Processing Charge was paid by the authors.
References
- 1.Batthish M, Banwell B, Laughlin S, et al. Refractory primary central nervous system vasculitis of childhood: successful treatment with infliximab. J Rheumatol 2012;39:2227–2229. [DOI] [PubMed] [Google Scholar]
- 2.Karaoğlu P, Topçu Y, Bayram E, et al. Severe neurologic Involvement of Degos disease in a pediatric patient. J Child Neurol 2014;29:550–554. [DOI] [PubMed] [Google Scholar]
- 3.Magro CM, Wang X, Garrett-Bakelman F, Laurence J, Shapiro LS, DeSancho MT. The effects of eculizumab on the pathology of malignant atrophic papulosis. Orphanet J Rare Dis 2013;8:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shapiro LS, Toledo-Garcia AE, Farrell JF. Effective treatment of malignant atrophic papulosis (Kohlmeier-Degos disease) with treprostinil: early experience. Orphanet J Rare Dis 2013;8:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernández-Pérez ER, Grabscheid E, Scheinfeld NS. A case of systemic malignant atrophic papulosis (Köhlmeier-Degos' disease). J Natl Med Assoc 2005;97:421–425. [PMC free article] [PubMed] [Google Scholar]
- 6.Scheinfeld N. Malignant atrophic papulosis. J Natl Med Assoc 2007;32:483–487. [DOI] [PubMed] [Google Scholar]
- 7.Theodoridis A, Makrantonaki E, Zouboulis CC. Malignant atrophic papulosis (Kohlmeier-Degos disease): a review. Orphanet J Rare Dis 2013;8:10. [DOI] [PMC free article] [PubMed] [Google Scholar]