Abstract
During pregnancy, iron deficiency anemia is recognized as a specific risk factor for both adverse maternal and perinatal outcome. We decided to test the hypothesis that the daily administration of Lafergin®, a dietary multicomponent based on Ferrazone® (Ferric Sodium EDTA), Lactoferrin, Vitamin C and Vitamin B12, from first trimester of pregnancy until the end of gestation, may significantly reduce, in anemic women, the severity of anemia compared to controls who received ferrous sulfate or liposomal iron.
Keywords: anemia, pregnancy, birth wight, iron supplementation
Introduction
Iron deficiency is universally considered as the most common cause of nutritional deficiency. Children and women of childbearing age are particularly at risk of iron deficiency. During pregnancy, iron deficiency anemia is recognized as a specific risk factor for both adverse maternal and perinatal outcome. In fact, it has been related to an increased risk of low birth weight, premature birth, or altered cognitive development of the child (1).
In 1993, the Institute Of Medicine (IOM) (2) proposed a complex program of iron supplementation during pregnancy, which was based on screening pregnant women in the first and second trimesters of gestation by using hemoglobin and ferritin concentrations. In particular, the IOM recommended that iron supplementation needed to be performed in pregnant women with hemoglobin concentrations < 11 g/dL or with low ferritin levels (< 20 μg/L). Moreover, it has been observed that, if pregnancy begins with low iron levels, the percentage of women with anemia at the end of pregnancy is much greater (3, 4).
Hemoglobin is an ideal parameter to indirectly identify iron levels. This is due to its high correlation with either ferritin serum levels or the presence of genetic alterations of human hemochromatosis protein (HFE) gene (5–7).
Alterations and polymorphisms of HFE gene may be suspected in case of high hemoglobin concentrations, such elevated to discourage iron supplementation.
These clinical parameters are relatively quick and easy to perform and are already systematically used in standard clinical practice, during pregnancy and follow-up (8–11).
Iron salts, such as sulfate or fumarate, have been extensively used in the past, but due to gastrointestinal adverse effects, many patients frequently decide to stop taking them (12).
Recently, a new multicomponent (Lafergin®) based on (NaFe3+-EDTA) (Ferric Sodium EDTA - Ferrazone® a registered trade name of AkzoNobel), which allows a high absorption and high bioavailability, has become available on the market.
The main advantages of iron absorbtion from Ferric Sodium EDTA (Ferrazone®) are (13–14):
- physiologically regulated by the TIBC (Transferrin Iron Binding Capacity) and it is higher in patients with low values of serum ferritin
- from 2.1 to 3.9 higher than ferrous sulfate because the EDTA doesn’t allow the binding of iron to the phytates.
This new formulation appears to be free of the usual iron-related side effects, such as metallic taste and gastrointestinal problems.
Clinical trials involving pregnant women affected by iron deficiency anemia have demonstrated the superiority of Ferric Sodium EDTA (Ferrazone®), compared to ferrous sulfate, in normalizing blood parameters and in improving the quality of life (15–17).
Material and methods
Basing on these premises, we decided to test the hypothesis that the daily administration of a dietary multicomponent (Lafergin®) based on Ferric Sodium EDTA (Ferrazone®), Lactoferrin, Vitamin C and Vitamin B12, from first trimester of pregnancy until the end of gestation, may significantly reduce, in anemic women, the severity of anemia compared to controls who, instead, received ferrous sulfate or liposomal iron.
Lafergin® contains 60 mg of Ferrazone® (Ferric Sodium EDTA) corresponding to 7,8 mg of Fe3+, 25 mg of Lactoferrin, a glycoprotein able to bind the ion Fe3+ (as the one present in Ferrazone®) and to transport it through the blood, with a binding-capacity two times higher than transferrin. In this way Lactoferrin is able to control iron homeostasis and contributes to maintain physiological iron concentrations (17).
Some Authors have already demonstrated the superiority of Lactoferrin, compared to ferrous sulfate, in increasing hemoglobin levels in anemic pregnant women and its capacity in increasing ferritin concentrations (18).
Lafergin® also contains 0,002 mg of Vitamin B12, which takes part to the development of red blood cells, and 70 mg of Vitamin C that promotes the absorption of iron and protects red blood cells from oxidative stress.
Furthermore, the pharmaceutical formulation of Lafergin ® take advantage from a particular and innovative technology (IOR – Improved Oral Release) which guarantees, after a single daily dose supplemented, the maximum bioavailability of its ingredients directly in the bowel for a prompt use by the organism without any side effect. The primary endpoint of the study was to evaluate the effectiveness of Lafergin® in improving the anemic status, through the evaluation of both hemoglobin and ferritin levels.
Secondary endpoint was to assess birth weights and gestational age at delivery and compare them to controls.
During this observational study we prescribed to a group of pregnant and anemic women a dietary multicomponent (Lafergin®). We longitudinally and prospectively followed our patients from the beginning of pregnancy to childbirth and we compared our results to 2 control “historical” groups, consisting of women longitudinally followed with the same periodicity of the study group (from the beginning of pregnancy to childbirth and precisely at 0, 15, 30, 60, 90 days and at childbirth).
Women from the multicomponent (Lafergin®) (study group) as well as those from the “hystorical” groups (control groups) were also assessed at 120, 150, 180, 210 and 240 and 270 days.
Therefore this study was set up as an observational longitudinal study consisting of a prospective arm (study group) and a retrospective arm (control group).
It is important to underline that the control group is defined as “historical” as it was retrospectively obtained from the database that enclosed all the data of the participating centers, but, actually, it is contemporary to the study group (recruitment period 2014–2015).
Since the assignment to treatment was not randomized, the evaluation of the differences from baseline within the different groups represented a critical point in order to identify selection biases.
The study group consisted of 82 cases, while the “historical” groups were composed by 534 women treated with ferrous sulfate and 527 supplemented with liposomal iron. The comparison between a sample of approximately 530 cases and a sample of 82 cases has an 80% power to detect as statistically significant difference (two tailed, p=0.05) of 33% of the standard deviation.
Considering that the standard deviation of variation from baseline at two particular time points of interest (baseline and 3 months) was equal to 0.53 for hemoglobin and 4.9 for ferritin in control groups, this means that the slightest clinically detectable difference was 0.17 for hemoglobin and 1.6 for ferritin.
Differences below these thresholds were not considered of clinical relevance.
Considering the above cited premises, this study can be considered of adequate sample size, even if the study design was not balanced.
One-way analysis of variance was used to compare the three groups (ferrous sulfate, liposomal iron and Lafergin ®) in terms of age and body mass index, while chi-square test was used to compare dichotomous variables such as smoking habits, diabetes, assisted reproductive technology and multiparity.
The Method of Pairwise Comparisons was used to assess the “Overall” significance within the groups: “ferrous sulfate vs liposomal iron”, “ferrous sulfate vs Lafergin ®” and “liposomal iron vs Lafergin®”. P-value was corrected with the Bonferroni procedure.
The main analysis aimed to detect the differences between the groups in terms of temporal trends of hemoglobin and ferritin. The ANOVA analysis was used to evaluate the differences within the groups (3 levels: ferrous sulfate, liposomal iron and Lafergin®) during follow-up (12 levels, from the beginning of pregnancy to childbirth) in terms of both within-subject and in-subject factors. The Greenhouse-Geisser procedure was used to adjust the degrees of freedom, taking into account the correlations between measures at various time points.
Results
Measurements taken at early pregnancy time point were considered as a reference values. There were not statistically significant differences within the groups in terms of basic clinical characteristics (Table 1).
Table 1.
Basic characteristics of the groups.
| Characteristic group | Lafergin® (82) | Liposomal iron (527) | Ferrous sulfate (534) | P |
|---|---|---|---|---|
| Age (y) | 33.8±5.9 | 33.6±6.2 | 34.0±5.8 | 0.587 |
|
| ||||
| Race or ethnicity (%) | ||||
| White | 99.2% | 98.9 | 99.0 | 0.432 |
| Black | 0.2 | 0.2 | 0.1 | 0.09 |
| Hispanic | 0.3 | 0.25 | 0.2 | 0.76 |
| Other | 1 | 1.1 | 1 | 0.89 |
|
| ||||
| Multipara (n, %) | 34 (41%) | 253 (48%) | 278 (52%) | 0.117 |
|
| ||||
| Cigarette smoking (n, %) | 3 (3.7%) | 5 (0.9%) | 3 (0.6%) | 0.095 |
|
| ||||
| BMI (kg/m2) | 25.6±1.7 | 25.6±4.9 | 25.5±4.7 | 0.896 |
|
| ||||
| Gestational age at study entry (wk) | 12.6 (12.1–12.8) | 12.4 (12.0–12.8) | 12.5 (12.1–12.7) | 0.76 |
|
| ||||
| Hemoglobin (g/L) | 11.7±4.4 | 11.7±3.8 | 11.6±4.0 | 0.150 |
|
| ||||
| Ferritin (μg/L) | 13.6±3.3 | 12.5±4.2 | 12.6±4.3 | 0.091 |
Data are median (IQR), number (%), or mean SD
Variations from baseline to childbirth in the group receiving Lafergin® resulted statistically significant compared to the groups treated with liposomal iron and ferrous sulfate (which, instead, resulted almost identical).
Using the baseline values as reference, clinical changes induced by Lafergin® resulted significantly different at each time point compared to the other groups.
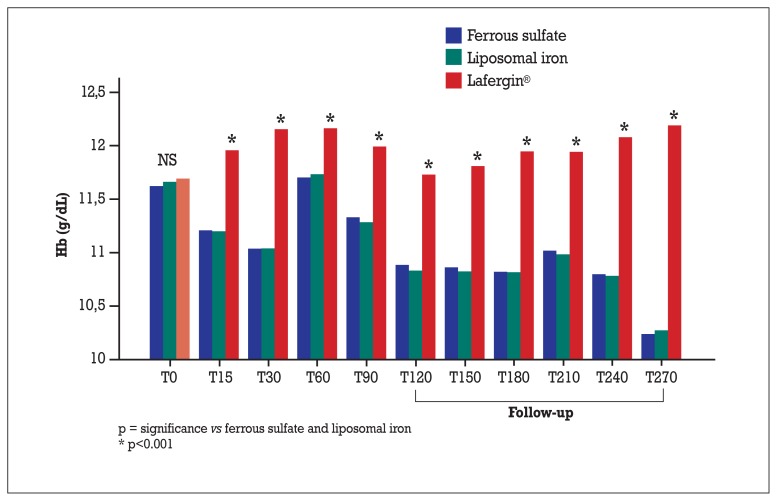
In general, these significant differences were due to an increase in hemoglobin levels registered in the Lafergin ® group and to a decrease of hemoglobin levels in the other two groups. In some cases (such as at 60 days) the increase (vs baseline) in the Lafergin® group corresponded a return to baseline values in the other two groups (Figure 1).
Figure 1.
Absolute values of hemoglobin (g/dL).
On the other hand, at 120 days, the hemoglobin level in the Lafergin® group returned to basal values, however in the other two groups it was observed a significant decrease. Considering this difference, even at this time point, a statistically significant difference, was observed.
The maximum difference registered between the Lafergin ® group and the liposomal iron/ferrous sulfate group manifested itself in the highest degree at the end of follow-up.
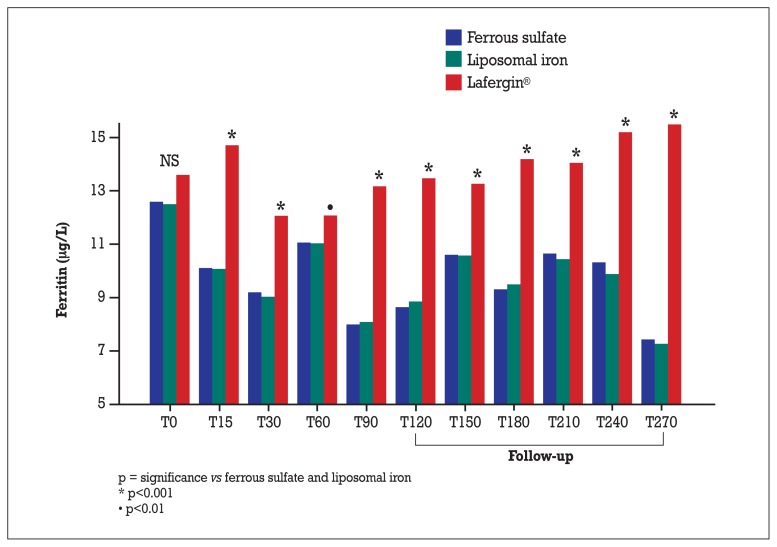
Even as regards ferritin levels, the trend from baseline to childbirth in Lafergin® group was significantly different from the variances observed in the liposomal iron/ferrous sulfate group (which, instead, resulted almost identical).
This overall significance is indicated by time-treatment interaction. Using the baseline value as reference, the changes induced by Lafergin® were significantly different by those induced by liposomal iron/ferrous sulfate at each time point (Figure 2).
Figure 2.
Absolute values of ferritin (μg/L).
Also for ferritin, as for hemoglobin, the significant difference between Lafergin® and the liposomal iron/ferrous sulfate groups manifested itself in the highest degree at the end of follow-up.
As regards the secondary endpoints, we demonstrated a greater effectiveness of Lafergin® compared to controls: in fact newborns had an higher mean birth weight compared to controls and duration of pregnancy resulted statistically longer compared to controls (Table 2).
Table 2.
Gestational age and birth weight according to the treatment received. ANOVA, followed by pairwise comparisons corrected with the Bonferroni procedure, indicates a longer duration of pregnancy and a higher birth weight in the Lafergin® treated group. The effect on weight remains significant even after correcting for gestional age.
| Ferrous sulfate (n=534) | Liposomal iron (n=527) | Lafergin® (n=82) | ANOVA | Post-hoc comparisons (Bonferroni) | |||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | F (2.1140) | p | Lafergin®
vs ferrous sulfate p |
Lafergin®
vs liposomal iron p |
|
| Gestational age (weeks) | 37.5 (1.6) | 37.7 (1.6) | 39.0 (1.6) | 32.129 | <0.001 | <0.001 | <0.001 |
| Birth weight (g) | 3231.4 (462.4) | 3218.6 (491.5) | 3401.2 (120.8) | 5.661 | 0.004 | 0.006 | 0.003 |
Lastly, our data confirmed a good tolerability of Lafergin ® compared to ferrous sulfate; instead, no differences were found comparing Lafergin® to the liposomal iron group.
Discussion
As it is very well known, during pregnancy the need of iron increases from 1–2 mg/day up to more than 6 mg/day. The objective of dietary supplementation of iron in pregnancy is to restore the number of red blood cells, the hemoglobin concentration, the total serum iron and ferritin to physiologic levels. Iron supplementation may be performed by administration of both ferrous and ferric ions. While ferrous ion is directly brought within the enterocyte by a protein (DMT1, Divalent Metals Transporter 1), ferric ion needs to be transformed into ferrous ion first, in order to be transported by the DMT1 within the enterocyte. Therefore, ferrous ion is generally administered in forms of ferrous fumarate, sulfate or gluconate.
In Italy, in case of iron deficiency anemia in pregnancy, 30 mg/day of liposomal iron is used as dietary supplementation while the oral daily treatment is mainly based on 80–100 mg of ferrous sulfate corresponding to about 30 mg of elemental iron, considering the bioavalability data. Moreover it is very well known that doses of ferrous sulfate greater than 60–100 mg/day may increase the incidence of side effects such as gastric (cramps, nausea, vomiting) and/or intestinal disorders (pain, constipation and diarrhea). While the firsts appear to be associated to the irritation of mucous membranes and altered gastric motility (19), depending on the availability of free iron in the gastric lumen, the others could correlate to intestinal flora changes induced by the presence of the iron.
The problem of poor tolerance to oral iron therapy is, as previously said, very common. Side effects, in case of therapy with ferrous sulfate, occur in about 32% of patients and from the literature it appears quite clearly that this type of therapy still has some important issues (20).
To obtain clinically acceptable results in the treatment of iron deficiency anemia, in terms of increase of red blood cells and hemoglobin concentrations, it is usually necessary to administer high daily doses, which usually results in an accumulation of iron at enteric level and consequently lead to an increase of gastrointestinal side effects, such as constipation, diarrhea, nausea and vomiting (21).
Ferrazone® (part of the Lafergin® composition) as ferric sodium has the advantage over ferrous salts, to be directly absorbed by the enterocyte thanks to its bond to EDTA. It acts like haem-iron and its absorption is about 25% compared to the 2% of the non-haem iron (ferrous ions). Furthermore, the particular and innovative technology used in the pharmaceutical formulation of Lafergin®, guarantees the maximum bioavailability of ingredients.
In this study we demonstrated the effectiveness, both in terms of maternal and neonatal outcomes, of a dietary supplementation with Lafergin® in the treatment of pregnant women affected by sideropenic anemia (Tables 2, 3).
Table 3.
Hemoglobin and ferritin values: statistical analysis between study and control groups.
| Hemoglobin (g/dL) | Basal | T15 | T30 | T60 | T90 | At term of pregnancy |
|---|---|---|---|---|---|---|
| Liposomal iron | 11.6±0.4 | 11.2±0.3 | 11.0±0.3 | 11.7±0.4 | 11.3±0.4 | 10.2±0.8 |
| Ferrous sulfate | 11.7±0.4 | 11.2±0.3 | 11.0±0.3 | 11.7±0.4 | 11.3 ±0.4 | 10.2±0.7 |
| Lafergin® | 11.7±0.4 | 12.0±0.2 | 12.2±0.3 | 12.2±0.2 | 12.0±0.2 | 12.3±0.2 |
| Lafergin® vs liposomal iron (Bonferroni p-value) | 0.451 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Lafergin® vs ferrous solfate (Bonferroni p-value) | 0.99 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
|
| ||||||
| Ferritin (μg/L) | Basal | T15 | T30 | T60 | T90 | At term of pregnancy |
|
| ||||||
| Liposomal iron | 12.6±4.3 | 10.1±1.9 | 9.2±2.5 | 11.0±2.6 | 8.0±2.6 | 8.5±3 |
| Ferrous sulfate | 12.5±4.2 | 10.1±1.9 | 9.0±2.4 | 11.0±2.6 | 8.1±2.5 | 8.6±2.9 |
| Lafergin® | 13.6±3.3 | 14.7±1.7 | 12.1±1.3 | 12.1±0.8 | 13.1±1.4 | 16.5±1.7 |
| Lafergin® vs liposomal iron (Bonferroni p-value) | 0.132 | <0.001 | <0.001 | 0.001 | <0.001 | <0.001 |
| Lafergin® vs ferrous solfate (Bonferroni p-value) | 0.09 | <0.001 | <0.001 | 0.002 | <0.001 | <0.001 |
In particular, this supplementation is particularly effective in obtaining good control of maternal levels of hemoglobin and ferritin during the entire duration of pregnancy. Furthermore, supplementation with Lafergin ® has proved to be more effective than ferrous sulfate and liposomal iron in terms of average duration of pregnancy and infant weight at birth. Finally, we demonstrated a better tolerability of Lafergin® compared to iron salts in terms of lower incidence of gastrointestinal side effects (abdominal cramps, constipation, diarrhea).
According to ANOVA for Repeated Measures, both hemoglobin and ferritin trends during pregnancy were statistically different among treatments. Precisely, considering the time points 0, 15, 30, 60, 90 days and at term of pregnancy, such diversity of trends was confirmed by the significant interaction “Time X Treatment” (hemoglobin: F(6.9, 3926.4)=86.9; p<0.001; ferritin F(7.9, 4554.1)=34.7; p<0.001). This interaction was only due to the Lafergin® supplementation, since the trends observed in liposomal iron and ferrous solfate supplementations were closely parallel (p>0.80 for both variables).
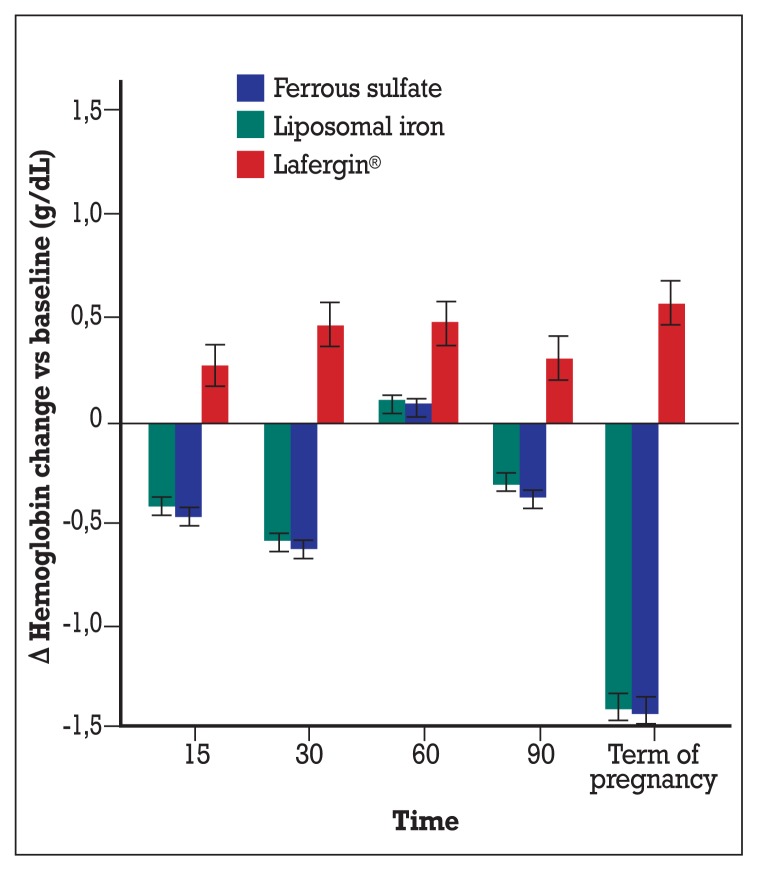
Looking at Figure 3 (where the mean differences between each time point and basal were plotted, with corresponding 95% confidence intervals), significant hemoglobin reductions were observed, in both liposomal iron and ferrous solfate supplementations at 15 days, 30 days, 90 days and especially at term of pregnancy, when the mean reduction was −1.39 g/dL (95% CI: −1.32; −1.46) for liposomal iron group and −1.42 (95% CI: −1.35; −1.49) for ferrous solfate group. On the other hand a quite stable increase was found in the group supplemented with Lafergin®, ranging from +0.26 (95% CU: +0.16; +0.37) at 15 days to +0.58 (95% CI: +0.47; +0.69) at term of pregnancy.
Figure 3.
Changes vs baseline of hemoglobin depending on administered treatments. Means and 95% confidence intervals are represented. Only in Lafergin® group a consistent and significant increase of hemoglobin was found; in the other groups at each follow-up time (but at 60 days), significant hemoglobin decreases were observed, particulary at term of pregnancy.
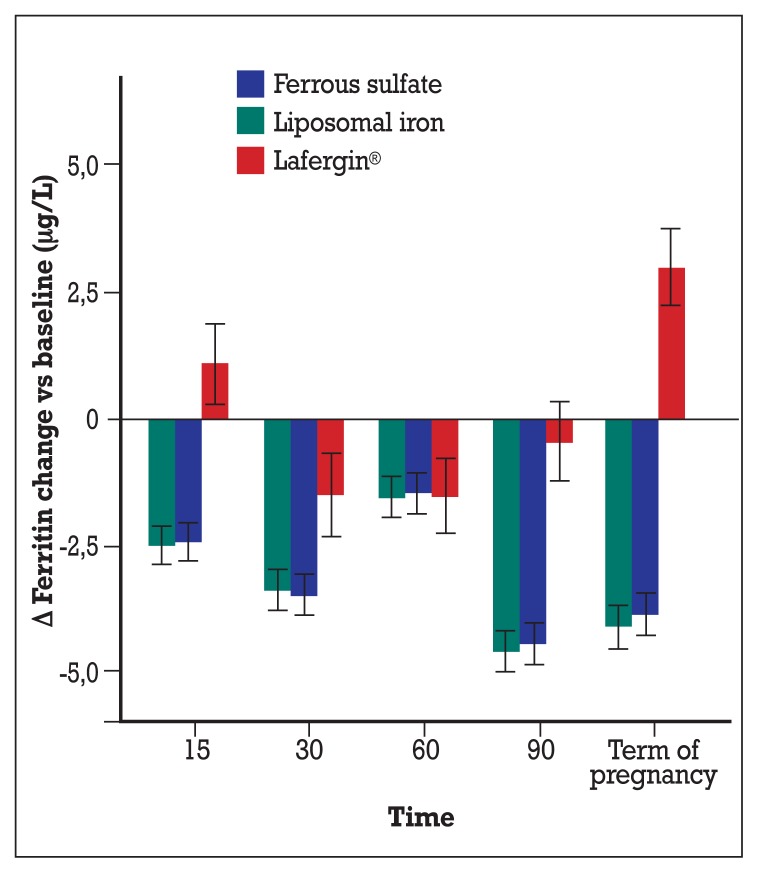
In Figure 4, the ferritin changes over basal level (and 95% CI) were plotted. Similarly to hemoglobin, significant reductions were observed at each time point in the group supplemented with ferrous sulfate and liposomal iron, ranging from −1.54 (95% CI: −1.11; −1.97) for liposomal iron group and −1.45 (95% CI: −1.03; −1.88) for ferrous solfate group at 60 days and −4.60 (95% CI: −4.18; −5.02) for liposomial iron group and −4.43 (95% CI: −4.01; −5.02) for ferrous solfate group at term of pregnancy. In the Lafergin ® group, after an increase of ferritin at 15 days (+1.10; 95% CI: +0.30;+1.90) a decrease was found at 30 and 60 days, followed by a return to basal value at 90 days and finally to a significant increase at term of pregnancy (+2.98; 95% CI: +2.22; +3.73).
Figure 4.
Changes vs baseline of ferritin depending on administered treatments. Means and 95% confidence intervals are represented. In Lafergin® group, after an up-and-down trend, a consistent and significant increase was found at term of pregnancy; in the other groups a quite stable and significant decrease was observed during the follow-up until term of pregnancy.
The best tolerability of Lafergin® probably led to better compliance and adherence of patients to the study. No difference, in terms of tolerability, was observed when we compared Lafergin® to liposomal iron.
In conclusion, dietary supplementation with Lafergin® may be considered a valid and well tolerated option in pregnant women, since the first trimester, for the control of iron deficiency anemia and for a good neonatal outcome.
References
- 1.Hernández-Martínez C, Canals J, Aranda N, Ribot B, Escribano J, Arija V. Effects of iron deficiency on neonatal behavior at different stages of pregnancy. Early Hum Dev. 2011;87:165–169. doi: 10.1016/j.earlhumdev.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 2.Institute of Medicine. Iron deficiency anemia: recommended guidelines for the prevention, detection, and management among US children and women of childbearing age. Washington, DC: National Academy Press; 1993. [PubMed] [Google Scholar]
- 3.Bothwell TH. Iron requirements in pregnancy and strategies to meet them. Am J Clin Nutr. 2000;72(suppl):257–64. doi: 10.1093/ajcn/72.1.257S. [DOI] [PubMed] [Google Scholar]
- 4.Ribot B, Aranda N, Viteri F, Hernández-Martínez C, Canals J, Arija V. Depleted iron stores without anaemia early in pregnancy carries increased risk of lower birth weight even when supplemented daily with moderate iron. Hum Reprod. 2012;27:1260–6. doi: 10.1093/humrep/des026. [DOI] [PubMed] [Google Scholar]
- 5.Allen LH. Anemia and iron deficiency: effects on pregnancy outcome. Am J Clin Nutr. 2000;71(5 Suppl):1280S–4S. doi: 10.1093/ajcn/71.5.1280s. [DOI] [PubMed] [Google Scholar]
- 6.Provan D. Mechanisms and management of iron deficiency anaemia. Br J Haematol. 1999;105(suppl 1):19–26. [PubMed] [Google Scholar]
- 7.School TO. Iron status during pregnancy: setting the stage for mother and infant. Am J Clin Nutr. 2005;81:1218–2. doi: 10.1093/ajcn/81.5.1218. [DOI] [PubMed] [Google Scholar]
- 8.Brabin JB, Hakimi M, Pelletier D. Iron-Deficiency Anemia: reexamining the nature and magnitude of the public health problem. J Nutr. 2001;5:604–15. [Google Scholar]
- 9.Meier PR, Nickerson HJ, Olson KA, et al. Prevention of iron deficiency anemia in adolescent and adult pregnancies. Clin Med Res. 2003;1(1):29–36. doi: 10.3121/cmr.1.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Recommendations to prevent and control iron deficiency in the United States. MMWR Recomm Rep. 1998;47:1–29. [PubMed] [Google Scholar]
- 11.American Academy of Pediatrics, American College of Obstetricians and Gynecologists. Guidelines for perinatal care. 4th ed. Elk Grove Village, IL: The Academy; 1997. [Google Scholar]
- 12.Harvey RS, Reffitt DM, Doig LA, Meenan J, Ellis RD, Thompson RP, Powell JJ. Ferric trimaltol corrects iron deficiency anaemia in patients intolerant of iron. Aliment Pharmacol Ther. 1998;12(9):845–8. doi: 10.1046/j.1365-2036.1998.00380.x. [DOI] [PubMed] [Google Scholar]
- 13.Candela E, Camacho MV, Martínez-Torres C, Perdomo J, Mazzarri G, Acurero G, Layrisse M. Iron absorption by humans and swine from Fe(III)-EDTA. Further studies. J Nutr. 1984;114:2204–11. doi: 10.1093/jn/114.12.2204. [DOI] [PubMed] [Google Scholar]
- 14.Hallberg L, Hulthén L, Gramatkovski E. Iron absorption from the whole diet in men: How effective is the regulation of iron absorption? Am J Clin Nutr. 1997;66:347–356. doi: 10.1093/ajcn/66.2.347. [DOI] [PubMed] [Google Scholar]
- 15.Joint FAO/WHO, Expert Committee on Food Additives (JECFA) Summary Fifty-third meeting; Rome. June 1ȓ10, 1999. [Google Scholar]
- 16.Sarkate P, Patil A, Parulekar S, Rege NN, Samant BD, Lokhande J, Gupta A, Kulkarni K. A randomised double-blind study comparing sodium feredetate with ferrous fumarate in anaemia in pregnancy. J Indian Med Assoc. 2007;105(5):278, 280–1, 284. [PubMed] [Google Scholar]
- 17.Han XX, Sun YY, Ma AG, Yang F, Zhang FZ, Jiang DC, Li Y. Moderate NaFeEDTA and ferrous sulfate supplementation can improve both hematologic status and oxidative stress in anemic pregnant women. Asia Pac J Clin Nutr. 2011;20(4):514–20. [PubMed] [Google Scholar]
- 18.Paesano R, Torcia F, Berlutti F, et al. Oral administration of lactoferrin increases hemoglobin and total serum iron in pregnant women. Biochem Cell Biol. 2006;84:377–80. doi: 10.1139/o06-040. [DOI] [PubMed] [Google Scholar]
- 19.www.rowett.ac.uk/femmes.
- 20.Schümann K, Ettle T, Szegner B, et al. On risks and benefits of iron supplementation recommendations for iron intake revisited. J Trace Elem Med Biol. 2007;21(3):147–68. doi: 10.1016/j.jtemb.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 21.Masotti A. Rivista della SIMG n°4. 2013. [Google Scholar]