Abstract
Purpose
To determine the incidence and clinical and biomarker predictors of perioperative thrombosis in children with single ventricle physiology undergoing staged palliation.
Methods
Nineteen patients were enrolled and 16 completed the study. Serial ultrasounds of the central venous system were performed to evaluate for thrombus. Plasma antithrombin III, thrombin–antithrombin complex, protein C, protein S, tissue factor pathway inhibitor, plasminogen activator inhibitor-1, tissue plasminogen activator antigen, D-dimer, soluble CD40 ligand, and urinary thromboxane were measured serially before and after surgery. Cardiopulmonary bypass time, aortic cross clamp time, blood product administration, inotrope score, chest tube output, cardiac function by echocardiography, intensive care unit and hospital lengths of stay, and central venous catheter days were recorded.
Results
The incidence of perioperative thrombus was 31%. Patients who developed a thrombus had poorer preoperative ventricular function (p = 0.03) and longer cardiopulmonary bypass times (p = 0.03) than those who did not develop a thrombus. Preoperative plasma antithrombin III was lower (p = 0.01) and tissue plasminogen activator antigen concentrations were higher (p = 0.02) in patients with a thrombus compared with patients without a thrombus. When measured over time, antithrombin III remained lower (p = 0.002) and tissue plasminogen activator antigen higher (p = 0.005) in those who developed a thrombus compared with those who did not. There were no other statistically significant differences in biomarkers of coagulation between patients with and without thrombosis.
Conclusion
One-third of patients undergoing palliative surgery for single ventricle physiology develop thrombosis. Decreased ventricular function, low antithrombin III, and increased tissue plasminogen activator may predict those most suitable for randomized clinical trials of anticoagulation.
Keywords: Cardiac surgical procedures/adverse effects, Heart defects, congenital/surgery, Thrombosis/epidemiology, Thrombosis/etiology, Postoperative complications/mortality, Blood coagulation tests
Introduction
Single ventricle cardiac lesions account for 1% of congenital cardiac malformations. In most children with single ventricle physiology, a three-stage surgical palliation procedure is required to sustain life. Retrospective and prospective chart review studies report an incidence of thrombus between 1 and 17% after stage I (Norwood) and between 17 and 33% after stage III (Fontan) palliation [1]. Thrombus formation in this population contributes significantly to morbidity and mortality. In a retrospective study of 1,499 patients who underwent cardiac surgery for congenital heart disease, the mortality rate was 40% in neonates with a thrombus versus 15% in those without a thrombus [2]. In a retrospective study of 143 infants the mortality rate was 14% following initial palliation with systemic-to-pulmonary artery shunting. Thrombus formation was judged to be the cause of mortality in a third of those who died [3].
Despite the high long-term incidence of thrombus in children undergoing staged palliation procedures, the incidence of perioperative thrombus in children undergoing palliative surgery for single ventricle physiology has not been defined. Many children require prolonged hospital stays with long courses of total parenteral nutrition, inotropes, and intravenous medications. A thrombus can lead to significant morbidity. Multiple small pulmonary emboli can lead to pulmonary hypertension and compromise the passive pulmonary circulation. Thrombi in the central veins impair placement of central venous catheters making vascular access a challenge. Thrombi in the femoral veins can impede the performance of cardiac catheterization used to define anatomy prior to the Glenn and Fontan surgeries, to measure pulmonary pressures, and to perform interventions such as arch obstruction angioplasty or coiling of collateral vessels that may have formed.
Understanding the incidence of perioperative thrombus formation is necessary to determine the appropriate use of anticoagulation. Current recommendations for thrombus prophylaxis after stage I and stage II procedures are grade 2C. Recommendation for prophylaxis after stage III palliation is more concrete, but the optimal dose and duration is still unknown [4]. Prior studies have collected data beginning at the time of discharge [5–9]. Studies based on chart review may underestimate the true frequency of perioperative thrombus formation [3, 5, 10, 11]. Few studies have evaluated biochemical predictors of thrombus formation in children undergoing palliative surgery for single ventricle physiology and there has been some controversy over the use of appropriate normal ranges for these biomarkers [7, 12]. In one study using transthoracic echocardiographic surveillance at discharge, C-reactive protein concentrations were higher in patients who developed thrombosis [13]. If predictors of thrombus formation can be identified, the children at highest risk of thrombus development may be targeted for more aggressive thrombus prophylaxis.
Using ultrasound surveillance and serial measurements of biomarkers of coagulation and fibrinolytic pathways, the objectives of this prospective study were (1) to determine the prevalence of thrombus, (2) to describe the time course of biomarkers of coagulation and fibrinolysis, and (3) to test the hypothesis that clinical characteristics and biomarkers differ in those patients who will develop a thrombus compared with those who will not develop a thrombus in children with single ventricle physiology undergoing any one of the three stages of surgical palliation.
Materials and methods
Subjects
This study was approved by the institutional review board of Vanderbilt University and written informed consent was obtained from parents. The parents of all patients who met inclusion criteria during a 1-year period were approached for consent in the study. Patients were eligible for inclusion in the study if they were undergoing any one of the three stages of palliative surgery for single ventricle congenital cardiac disease at Vanderbilt Children’s Hospital and weighed at least 2 kg. Any patient known to have a preexisting thrombus or requiring extracorporeal membranous oxygenation (ECMO) was excluded.
Protocol
All patients underwent serial ultrasounds of the venous system. Venous Doppler was used to examine the upper extremities (including the bilateral jugular veins, subclavian veins, axillary veins, and innominate veins), the superior vena cava, and lower extremities (including the bilateral external iliac veins, common femoral veins, greater saphenous veins, superficial and profunda femoral veins, and popliteal veins). In addition, a limited Doppler examination of the abdomen was performed to evaluate the patency of the intrahepatic inferior vena cava and the infrahepatic inferior vena cava when technically feasible. The examinations included grayscale imaging with appropriately focused linear transducers, and evaluation with both color and spectral analysis, including compression and augmentation. The initial ultrasound was obtained either preoperatively, on the day of surgery, or on postoperative day (POD) 1 because of difficulties in coordinating the extensive ultrasound examination with other preoperative evaluations. Only patients who consented to participate in the study received ultrasound investigation. The second ultrasound was obtained on POD 10 or just prior to discharge if the patient was discharged prior to the tenth POD. A third ultrasound was performed just prior to discharge if more than 10 days had elapsed since the previous ultrasound. Ultrasounds and cardiac catheterizations performed by the patient care team which demonstrated thrombus formation were also used as endpoints; however, echocardiograms and cardiac catheterizations were performed at the discretion of the medical team and were not performed in all study patients. Pre- and postoperative anticoagulation was left to the discretion of the treating physicians. All stage I patients received a heparin infusion at 10 units/kg/h in the immediate postoperative period and were transitioned to aspirin on POD 1 or 2. Partial thromboplastin time was not routinely measured in these patients. Of the four patients undergoing stage II procedures, two were on aspirin before and after surgery, one patient was on low molecular weight heparin (LMWH) before and after surgery with goal anti-factor Xa activity of 0.5–1 U/ml, and one patient was not on any anticoagulation prior to surgery and was placed on aspirin after surgery. Of the seven patients undergoing stage III procedures, five were on aspirin pre- and postoperatively, and two patients were not receiving anticoagulation preoperatively and were placed on aspirin postoperatively.
Clinical parameters including age (days), race, weight, lengths of stay in the intensive care unit (ICU) and hospital, preoperative anticoagulation strategy (if any), postoperative anticoagulation strategy, type of cardiac defect and type of surgery, cardiopulmonary bypass time (min), aortic cross clamp time (min), ventricular function as determined by echocardiography (normal, mildly depressed, moderately depressed, or severely depressed), blood product administration (ml/kg), number of central venous catheter days, inotrope score upon arrival at the critical care unit ([(dopamine + dobutamine + amrinone)] × 1 + (milrinone × 20) + [(epinephrine + nor-epinephrine + isoproterenol) × 100]3; dosages are in μg/kg/min), and chest tube output (ml/kg) were recorded from the patient’s medical record [14]. Central venous line (CVL) catheters were not heparin coated.
Surgical procedure
Operations were performed by two surgeons. All surgeries were performed using cardiopulmonary bypass. Three of the Fontan operations and two of the Glenn operations were done without the use of aortic cross clamp. Three of the Fontan operations were intracardiac Fontan procedures while the other four were extracardiac lateral tunnel (ECLT) Fontan procedures based on surgeon preference.
Blood and urine collection
All patients underwent serial evaluation of the coagulation profile. Four milliliters of venous blood was collected preoperatively, on POD 1, POD 3, POD 5, POD 10, and every 10 days thereafter until POD 40, thrombus formation, death, or discharge from the hospital. Blood was collected with the daily blood draw. Blood samples were collected on ice. Three milliliters of blood was placed in a sodium citrate tube and 1 ml was placed in a tube containing EDTA. The blood was immediately centrifuged at 3,720 rpm for 20 min. The plasma was removed and stored at −80°C until analysis. Plasma was collected for determination of activated protein C resistance as well as measurement of protein C, protein S, plasminogen activator inhibitor-1 (PAI-1), antithrombin III (ATIII), D-dimer, tissue plasminogen activator antigen (tPA), tissue factor pathway inhibitor (TFPI), soluble CD40 ligand (sCD40L), and thrombin antithrombin complex (TAT) levels. Urine samples were collected on ice for measurement of thromboxane B2 (TBX) at the same time intervals as blood samples. Urine was obtained via urine collection bag or Foley catheter if one was in place.
Laboratory analysis
ATIII was measured in plasma by use of a chromogenic assay (Chromogenic Assay Kit for Plasma ATIII by Actichrome® American Diagnostica, Stamford, CT, USA). TAT complexes (AssayMax Human TAT Complexes ELISA Kit, Assaypro, St. Charles, MO, USA) and TFPI (Quantikine® Human TFPI Immunoassay, R&D Systems, Minneapolis, MN, USA) levels were measured with a quantitative sandwich enzyme immunoassay technique. sCD40L was measured using an enzyme immunoassay (Quantikine® Human soluble CD40 Ligand Immunoassay, R&D Systems, Minneapolis, MN, USA). tPA plasma antigen levels (TintElize® tPA, Trinity Biotech, Ireland), PAI-1 antigen levels (TriniL-IZE PAI-1 Antigen, Trinity Biotech, Ireland), and D-dimer concentration (TintElize® D-dimer, Trinity Biotech, Ireland) were measured by an enzyme immunoassay utilizing the double antibody principle. Free protein S concentration was measured via two-site immunoassay (Zymutest Free Protein S kit, HYPHEN BioMed, France). Urinary 11-dehydrothromboxane B2 was measured by gas chromatography–mass spectrometry as described elsewhere [15]. Activated protein C resistance caused by the factor V Leiden mutation was measured using a plasma-based functional assay (Pefakit® APC-R factor V Leiden, DSM Nutritional Products Ltd Branch Pentapharm, Switzerland.)
Statistical analysis
Continuous variables measured once in each patient such as cardiopulmonary bypass time, aortic cross clamp time, inotrope score, central venous line days, and blood product administration in relationship to thrombus formation (yes or no) were analyzed using the Mann–Whitney U test. For repeatedly measured biomarkers, mixed-effect models were used to analyze the data with a random subject effect and with the time trend (baseline, POD 1, POD 3, POD 5, POD 10, POD 20, and POD 30) as fixed effects. We explored different plausible covariance matrices such as compound symmetry and a first-order autoregressive process [AR(1)] in the mixed-effect models. Comparisons were made using the data from POD 0, 1, 3, and 5. Data from POD 10, 20, and 30 were excluded from this analysis because most patients had been discharged prior to these dates and, therefore, the data represented a very small number of patients, in some cases only one patient. Hypotheses were tested at the nominal level of α = 0.05. Analysis was performed using SPSS for Windows Version 17.0 (SPSS, Chicago, IL) and SAS® for Windows (Version 9, Cary, NC).
Results
Nineteen patients with a variety of single ventricle congenital cardiac lesions were enrolled. Three patients enrolled in the study were not included in the final analysis. Two of the patients dropped out of the study on POD 1 because of parental concern regarding possible discomfort during the vascular ultrasound. One patient was excluded because of the need for ECMO. The characteristics of the study population appear in Table 1. Five of the 16 patients studied developed a thrombus (31%). Three of the patients who developed a thrombus underwent the Norwood operation at ages 5, 5, and 3 days; one underwent the Glenn operation at age 121 days; and one underwent the Fontan operation at age 850 days. Table 2 provides characteristics of the patients who developed a thrombus, including time to thrombus formation, pre- and postoperative anticoagulation regimens, and thrombus location. Of the patients who developed a thrombus, all except one (patient 1 in Table 2) had normal ultrasound studies prior to the ultrasound study which revealed a thrombus. Patient 1 had no clinical evidence of a thrombus prior to study but for logistical reasons did not undergo the initial ultrasound until POD 1. That study demonstrated a right femoral vein thrombus. Among the patients who developed a thrombus, one required ECMO cannulation as a direct result of cardiovascular collapse secondary to Blalock–Taussig (BT) shunt thrombosis after the Norwood procedure, and one patient had a thromboembolic stroke (Table 2). Two patients died giving a mortality rate of 12.5%. Both deaths were in patients who developed a thrombus, although thrombosis was not deemed to be the direct cause of death in either patient.
Table 1.
Patient characteristics
| Norwood/BT shunt/Sano n = 5 | Glenn n = 4 | Fontan n = 7 | |
|---|---|---|---|
| Age (days)a | 4.6 ± 1.7 | 154.3 ± 26.5 | 864.3 ± 213.4 |
| Weight (kg)a | 3.2 ± 0.29 | 6.1 ± 1.5 | 12.1 ± 21.1 |
| Male | 4 | 3 | 6 |
| Underlying diagnosis | |||
| Hypoplastic left heart syndrome | 4 | 2 | 2 |
| Double outlet right ventricle | 1 | 1 | 1 |
| Tricuspid atresia | 1 | ||
| Double inlet left ventricle | 1 | 2 | |
| Other | 1 | ||
Data are presented as mean ± standard deviation
Table 2.
Characteristics of patients with a thrombus
| Patient | Lesion | Surgery | Thrombus location | Time to thrombus detection (postoperative days) | Complications | Preoperative anticoagulation | Postoperative anticoagulation |
|---|---|---|---|---|---|---|---|
| 1 | HLHS | Norwood | Right femoral vein | 1 | Stroke | None | Heparina followed by aspirin |
| 2 | HLHS | Glenn | Inferior vena cava | 16 | Death | LMWH | LMWH |
| 3 | HLHS | Fontan (ECLT) | Right femoral vein | 8 | None | Aspirin | Aspirin |
| 4 | HLHS | Norwood | BT shunt | 1 | ECMO death | None | Heparina followed by aspirin |
| 5 | HLHS | Norwood | Right atrium | 16 | None | None | Heparina followed by aspirin |
HLHS hypoplastic left heart syndrome, ECMO extracorporeal membranous oxygenation, LMWH low molecular weight heparin, ECLT extracardiac lateral tunnel
Heparin dosing = 10 units/kg/h
Patients with longer cardiopulmonary bypass times (p = 0.03) and poorer cardiac function on preoperative echocardiography (p = 0.03) were more likely to develop a thrombus. Patients who developed a thrombus required longer stays in the ICU (p = 0.03) and the hospital (p = 0.05) compared with those who did not develop a thrombus. There was no statistically significant difference in cross clamp time (p = 0.73), packed red blood cell (PRBC) transfusions (ml/kg) (p = 0.28), fresh frozen plasma (FFP) transfusions (ml/kg) (p = 0.61), cryoprecipitate transfusions (ml/kg) (p = 0.34), platelet transfusions (ml/kg) (p = 0.23), inotrope score (p = 0.53), chest tube output (p = 0.16), or CVL days (p = 0.32) in patients who developed a thrombus compared with those who did not develop a thrombus (Table 3).
Table 3.
Clinical characteristics
| Thrombus (n = 5) | No thrombus (n = 11) | p value | |
|---|---|---|---|
| Age (days) | 196.8 ± 368.7 | 518.7 ± 436.2 | 0.09 |
| Weight (kg) | 5.18 ± 4.3 | 9 ± 4 | 0.08 |
| CPB time (min) | 150.2 ± 30 | 106 ± 29.4 | 0.03 |
| Cross clamp time (min) | 28.4 ± 24.3 | 23.6 ± 23.2 | 0.73 |
| PRBC transfusions (ml/kg) | 108.6 ± 106 | 42.2 ± 32.5 | 0.28 |
| FFP transfusions (ml/kg) | 38.6 ± 44.8 | 16.5 ± 13.3 | 0.61 |
| Cryoprecipitate transfusions (ml/kg) | 9.4 ± 9.1 | 4.1 ± 5.7 | 0.34 |
| Platelet transfusions (ml/kg) | 40.2 ± 42.8 | 17.18 ± 13.9 | 0.23 |
| Inotrope score on arrival to ICU | 14.2 ± 6.6 | 16.5 ± 5.2 | 0.53 |
| Chest tube output (ml/kg) | 254 ± 223.4 | 100.6 ± 85.1 | 0.16 |
| CVL days | 11.4 ± 9.3 | 8.7 ± 10.5 | 0.32 |
| ICU length of stay (days) | 22.4 ± 14.8 | 6.3 ± 4 | 0.03 |
| Hospital length of stay (days) | 35.6 ± 23.2 | 14.6 ± 10.7 | 0.05 |
Data are presented as means ± standard deviations
CPB cardiopulmonary bypass, PRBC packed redblood cells, FFP fresh frozen plasma, ICU intensive care unit, CVL central venous line
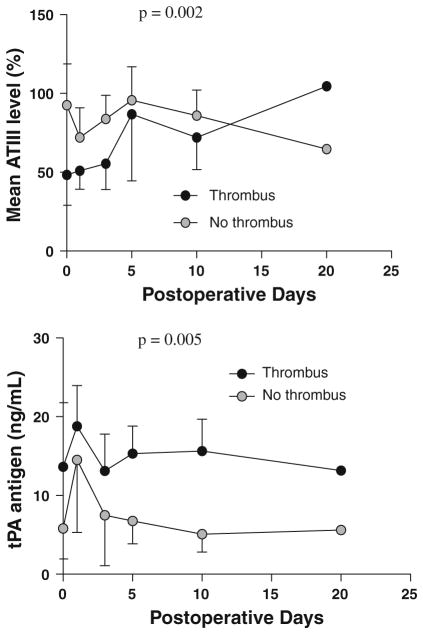
Patients who developed a thrombus had significantly higher preoperative levels of tPA antigen (p = 0.02) and significantly lower preoperative ATIII levels (p = 0.01) compared with those who did not develop a thrombus (Table 4). Analysis of the data over time revealed that levels of tPA antigen and ATIII remained significantly different between the two groups (Fig. 1). There were no statistically significant differences in levels of PAI-1 (p = 0.11), D-dimer (p = 0.18), protein C activity (p = 0.32), sCD40L (p = 0.57), thrombin antithrombin complex (p = 0.71), tissue factor pathway inhibitor (p = 0.07), urinary TBX (p = 0.07), or protein S activity (p = 0.22) when analyzed over time. None of the patients demonstrated activated protein C resistance as caused by the factor V Leiden mutation.
Table 4.
Preoperative biomarker levels in patients with and without a thrombus
| Thrombus | No thrombus | p value | |
|---|---|---|---|
| ATIII (%) | 48.3 ± 19.2 | 92.5 ± 26.4 | 0.01 |
| D-dimer (ng/ml) | 443.8 ± 542 | 194.9 ± 113.2 | 0.61 |
| Protein C (%) | 35.8 ± 24.1 | 45.8 ± 21 | 0.38 |
| Protein S (%) | 33.9 ± 8.8 | 41.9 ± 12 | 0.17 |
| TFPI (pg/ml) | 16,544.4 ± 8,425.9 | 10,408.9 ± 2,369.7 | 0.09 |
| TAT (ng/ml) | 19.5 ± 8.3 | 22.8 ± 8.2 | 0.52 |
| PAI-1 (ng/ml) | 21.1 ± 23.8 | 8.7 ± 6.2 | 0.22 |
| tPA (ng/ml) | 13.6 ± 8.2 | 5.8 ± 3.9 | 0.02 |
| sCD40L (ng/ml) | 69.3 ± 24.4 | 74.9 ± 23 | 0.44 |
| Urinary TBX (ng/mg creatinine) | 7.9 ± 7.1 | 5.8 ± 7.7 | 0.77 |
Data are presented as means ± standard deviations
ATIII antithrombin III, TFPI tissue factor pathway inhibitor, TAT thrombin antithrombin complex, PAI-1 plasminogen activator inhibitor-1, tPA tissue plasminogen activator, sCD40L soluble CD 40 ligand, TBX thromboxane B2
Fig. 1.
Mean ATIII and tPA concentrations in patients with and without a thrombus over time
Discussion
In this prospective study, the prevalence of thrombus formation was 31%. The consequences of a thrombus were significant, including stroke in one patient and BT shunt thrombosis requiring ECMO in another. Both of the deaths in this study population occurred in patients who developed a thrombus. Clinical predictors of thrombus formation were poorer cardiac function on preoperative echocardiography and longer cardiopulmonary bypass times in patients who developed a thrombus. Notably, three of the patients who developed a thrombus underwent the Norwood operation which is technically more challenging and requires longer cardiopulmonary bypass times. The biomarker predictors of thrombus development were significantly lower ATIII levels and significantly higher tPA antigen levels in patients who developed a thrombus compared with patients who did not develop a thrombus. Throughout the postoperative course, the levels of tPA and ATIII remained significantly different between the two groups.
While the elevation in tPA antigen in patients who developed a thrombus seems counterintuitive, it supports results found in a study by Nowak-Gottl et al. [16] of children with congenital heart disease undergoing cardiac catheterization. In that study, infants at risk of early thromboembolism during cardiac catheterization had elevated tPA antigen levels. Adults at risk of coronary thrombosis also have higher levels of endogenous tPA antigen [17, 18]. The apparently paradoxical association between elevated tPA antigen and thrombus formation likely reflects the fact that most tPA circulates in tPA/PAI-1 complexes. tPA/PAI-1 complexes are cleared more slowly than free tPA, such that tPA antigen increases when PAI-1 antigen is increased [19]. Although not statistically significant, PAI-1 concentrations tended to be higher in patients who developed a thrombus compared with those who did not.
ATIII was significantly lower in children who developed a thrombus. Low levels of ATIII impair the body’s natural mechanism for overcoming clot formation. Interestingly, Odegard et al. [20] found lower levels of ATIII in patients with HLHS compared with controls prior to all three palliative surgery stages.
All patients who developed a thrombus in this study had HLHS as their underlying diagnosis. Perhaps these patients have a lower flow state than patients with anatomical single left ventricles, for example, and are thus at increased risk of thrombus formation. This is an interesting observation and further study to determine whether HLHS alone predisposes patients to thrombus formation is warranted. Interestingly, the only patient with a Fontan operation to develop a thrombus in this study had undergone an ECLT Fontan. In a recent study by Hasaniya et al. [21] the thrombosis rate was 1.25% in a series of 160 patients after ECLT Fontan, lower than most previously published rates of thrombosis after Fontan (17–33%) [1].
Many studies have demonstrated that there are differences between the coagulation profiles of children with single ventricle physiology and those of healthy children [5–9, 20, 22–26]. In 2007, Cholette et al. [13] reported the first and only prior study to assess infants undergoing stage I palliation for thrombosis using echocardiography. The investigators evaluated the coagulation profile at baseline and on POD 3 and performed active surveillance of the venous system for a thrombus at discharge in each patient. The prior study was limited to the stage I population and did not report clinical parameters associated with thrombus formation. Our study builds on their design by measuring coagulation parameters serially and evaluating the vasculature at least twice. To the best of our knowledge, this is the only study to date to report clinical parameters associated with thrombus formation in children undergoing palliative surgery.
The major limitations of this study are its small sample size, the inability to obtain preoperative vascular ultrasound on all patients, and the unequal distribution of patients among surgical intervention groups. Nevertheless, the high prevalence of thrombus formation in this study and the Cholette study, as well as the significant consequences of thrombosis in this fragile group of children, suggest that multicenter randomized clinical trials of perioperative anticoagulation are needed. The use of clinical and biomarker predictors such as poor cardiac function, decreased ATIII, and increased tPA may help to identify those children at highest risk and most likely to benefit from anticoagulation.
Acknowledgments
Vanderbilt Clinical and Translational Science Award Grant UL1 RR024975 from NCRR/NIH. National Institutes of Health Grant HL060906. National Institutes of Health Grant HL 077389.
Contributor Information
Deanna R. Todd Tzanetos, Email: drtodd0@uky.edu, Department of Pediatrics, Division of Critical Care, University of Kentucky School of Medicine, Lexington, KY, USA, Tel.: +1-859-3231496 Fax: +1-859-2576066
Chang Yu, Department of Biostatistics, Vanderbilt University School of Medicine, Nashville, TN, USA.
Marta Hernanz-Schulman, Department of Radiology, Vanderbilt University School of Medicine, Nashville, TN, USA.
Frederick E. Barr, Department of Pediatrics, Division of Critical Care, University of Mississippi School of Medicine, Jackson, MS, USA
Nancy J. Brown, Department of Medicine, Division of Clinical Pharmacology, Vanderbilt University School of Medicine, Nashville, TN, USA
References
- 1.Monagle P. Thrombosis in children with BT shunts, Glenns, and Fontans. Prog Pediatr Cardiol. 2005;21:17–21. [Google Scholar]
- 2.Petaja J, Lundstrom U, Sairanen H, Marttinen E, Griffin J. Central venous thrombosis after cardiac operations in children. J Thorac Cardiovasc Surg. 1996;112:883–889. doi: 10.1016/S0022-5223(96)70087-9. [DOI] [PubMed] [Google Scholar]
- 3.Fenton K, Siewers R, Rebovich B, Pigula F. Interim mortality in infants with systemic-to-pulmonary artery shunts. Ann Thorac Surg. 2003;76:152–156. doi: 10.1016/s0003-4975(03)00168-1. [DOI] [PubMed] [Google Scholar]
- 4.Monagle P, Chalmers E, Chan A, DeVeber G, Kirkham F, Massicotte P, et al. Antithrombotic therapy in neonates and children: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition) Chest. 2008;133:887S–968S. doi: 10.1378/chest.08-0762. [DOI] [PubMed] [Google Scholar]
- 5.Ravn HB, Hjortdal VE, Stenbog EV, Emmertsen K, Kromann O, Pedersen J, et al. Increased platelet reactivity and significant changes in coagulation markers after cavopulmonary connection. Heart. 2001;85:61–65. doi: 10.1136/heart.85.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cromme-Dijkhuis AH, Henkens CMA, Bijleveld CMA, Hillege HL, Bom VJJ, Van der Meer J. Coagulation factor abnormalities as possible thrombotic risk factors after Fontan operations. Lancet. 2001;336:1087–1090. doi: 10.1016/0140-6736(90)92568-3. [DOI] [PubMed] [Google Scholar]
- 7.Jahangiri M, Shore D, Kakkar V, Lincoln C, Shinebourne E. Coagulation factor abnormalities after the Fontan procedure and its modifications. J Thorac Cardiovasc Surg. 1997;113:989–992. doi: 10.1016/S0022-5223(97)70283-6. [DOI] [PubMed] [Google Scholar]
- 8.Odegard KC, McGowan FX, Jr, Zurakowski D, DiNardo JA, Castro RA, del Nido PJ, et al. Coagulation factor abnormalities in patients with single-ventricle physiology immediately prior to the Fontan procedure. Ann Thorac Surg. 2002;73:1770–1777. doi: 10.1016/s0003-4975(02)03580-4. [DOI] [PubMed] [Google Scholar]
- 9.Odegard KC, McGowan FX, Jr, Zurakowski D, DiNardo JA, Castro RA, del Nido PJ. Procoagulant and anticoagulant factor abnormalities following the Fontan procedure: increased factor VIII may predispose to thrombosis. J Thorac Cardiovasc Surg. 2002;125:1260–1267. doi: 10.1016/s0022-5223(02)73605-2. [DOI] [PubMed] [Google Scholar]
- 10.Rosenthal DN, Friedman AH, Kleinman CS, Kopf GS, Rosenfeld LE, Hellenbrand WE. Thromboembolic complications after Fontan operations. Circulation. 1995;92:287–293. doi: 10.1161/01.cir.92.9.287. [DOI] [PubMed] [Google Scholar]
- 11.Coon PD, Rychik J, Novello RT, Ro PS, Gaynor JW, Spray TL. Thrombus formation after the Fontan operation. Ann Thorac Surg. 2001;71:1990–1994. doi: 10.1016/s0003-4975(01)02472-9. [DOI] [PubMed] [Google Scholar]
- 12.Monagle P, Andrew M. Coagulation abnormalities after Fontan. J Thorac Cardiovasc Surg. 1998;115:732–733. [PubMed] [Google Scholar]
- 13.Cholette JM, Rubenstein JS, Alfieris GM, McDermott MP, Harmon WG, Vermilion R, et al. Elevated risk of thrombosis in neonates undergoing initial palliative cardiac surgery. Ann Thorac Surg. 2007;84:1320–1325. doi: 10.1016/j.athoracsur.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 14.Agarwal HS, Churchwell KB, Doyle TP, Christian KG, Drinkwater DC, Jr, Byrne DW, et al. Inhaled nitric oxide use in bidirectional Glenn anastomosis for elevated Glenn pressures. Ann Thorac Surg. 2006;81:1429–1434. doi: 10.1016/j.athoracsur.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Morrow JD, Minton TA. Improved assay for the quantification of 11-dehydrothromboxane B2 by gas chromatography-mass spectrometry. J Chromatogr. 1993;612:79–85. doi: 10.1016/0378-4347(93)80161-v. [DOI] [PubMed] [Google Scholar]
- 16.Nowak-Gottl U, Kotthoff S, Hagemeyer E, Junker R, Kehl HG, Vielhaber H, Kececioglu D. Interaction of fibrinolysis and prothrombotic risk factors in neonates, infants and children with and without thromboembolism and underlying cardiac disease. A prospective study. Thromb Res. 2001;103:93–101. doi: 10.1016/s0049-3848(01)00281-x. [DOI] [PubMed] [Google Scholar]
- 17.Wiman B, Andersson T, Hallqvist J, Reuterwall C, Ahlbom A, de Faire U. Plasma levels of tissue plasminogen activator/plasminogen activator inhibitor-1 complex and von Willebrand factor are significant risk markers for recurrent myocardial infarction in the Stockholm Heart Epidemiology Program (SHEEP) study. Arterioscler Thromb Vasc Biol. 2000;20:2019–2023. doi: 10.1161/01.atv.20.8.2019. [DOI] [PubMed] [Google Scholar]
- 18.Geppert A, Graf S, Beckmann R, Hornykewycz S, Schuster E, Binder B, et al. Concentration of endogenous tPA antigen in coronary artery disease: relation to thrombotic events, aspirin treatment, hyperlipidemia, and multivessel disease. Arterioscler Thromb Vasc Biol. 1998;18:1634–1642. doi: 10.1161/01.atv.18.10.1634. [DOI] [PubMed] [Google Scholar]
- 19.Chandler WL, Alessi MC, Aillaud MF, Henderson P, Vague P, Juhan-Vague I. Clearance of tissue plasminogen activator (TPA) and TPA/plasminogen activator inhibitor type 1 (PAI-1) complex: relationship to elevated TPA antigen in patients with high PAI-1 activity levels. Circulation. 1997;96:761–768. doi: 10.1161/01.cir.96.3.761. [DOI] [PubMed] [Google Scholar]
- 20.Odegard KC, Zurakowski D, DiNardo JA, Castro RA, McGowan FX, Neufeld EJ, et al. Prospective longitudinal study of coagulation profiles in children with hypoplastic left heart syndrome from stage I through Fontan completion. J Thorac Cardiovasc Surg. 2009;137:934–941. doi: 10.1016/j.jtcvs.2008.09.031. [DOI] [PubMed] [Google Scholar]
- 21.Hasaniya NW, Razzouk AJ, Mulla NF, Larsen RL, Bailey LL. In situ pericardial extracardiac lateral tunnel Fontan operation: fifteen-year experience. J Thorac Cardiovasc Surg. 2010;140:1076–1083. doi: 10.1016/j.jtcvs.2010.07.068. [DOI] [PubMed] [Google Scholar]
- 22.Jahangiri M, Kreutzer J, Zurakowski D, Bacha E, Jonas RA. Evaluation of hemostatic and coagulation factor abnormalities in patients undergoing the Fontan operation. J Thorac Cardiovasc Surg. 2000;120:778–782. doi: 10.1067/mtc.2000.108903. [DOI] [PubMed] [Google Scholar]
- 23.Cheung EWY, Chay GW, Ma ESK, Cheung YF. Systemic oxygen saturation and coagulation factor abnormalities before and after the Fontan procedure. Am J Cardiol. 2005;96:1571–1575. doi: 10.1016/j.amjcard.2005.07.074. [DOI] [PubMed] [Google Scholar]
- 24.van Nieuwenhuizen RC, Peters M, Lubbers LJ, Trip MD, Tijssen JGP, Mulder BJM. Abnormalities in liver function and coagulation profile following the Fontan procedure. Heart. 1999;82:40–46. doi: 10.1136/hrt.82.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Procelewska M, Kolcz J, Januszewska K, Mroczek T, Malec E. Coagulation abnormalities and liver function after hemi-Fontan and Fontan procedures—the importance of hemodynamics in the early postoperative period. Eur J Cardiothorac Surg. 2007;31:866–872. doi: 10.1016/j.ejcts.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 26.Heying R, van Oeveren W, Wilhelm S, Schumacher K, Grabitz RG, Messmer BJ, et al. Children undergoing cardiac surgery for complex cardiac defects show imbalance between pro-and anti-thrombotic activity. Crit Care. 2006;10:R165. doi: 10.1186/cc5108. [DOI] [PMC free article] [PubMed] [Google Scholar]