Abstract
Background. A linked donor-recipient study was conducted during epidemics in 2 cities in Brazil to investigate transfusion-transmitted (TT) dengue virus (DENV) by DENV RNA–positive donations.
Methods. During February–June 2012, samples were collected from donors and recipients and retrospectively tested for DENV RNA by transcription-mediated amplification. Recipient chart review, using a case (DENV positive)–control (DENV negative and not known to be exposed) design, was conducted to assess symptoms.
Results. Of 39 134 recruited blood donors, DENV-4 viremia was confirmed in 0.51% of donations from subjects in Rio de Janeiro and 0.80% of subjects in Recife. Overall, 42 DENV RNA–positive units were transfused into 35 recipients. Of these, 16 RNA-positive units transfused into 16 susceptible recipients were identified as informative: 5 cases were considered probable TT cases, 1 possible TT case, and 10 nontransmissions. The TT rate was 37.5% (95% confidence interval [CI], 15.2%–64.6%), significantly higher than the viremia rate of 0.93% (95% CI, .11%–3.34%) in nonexposed recipients (P < .0001). Chart review did not find significant differences between cases and controls in symptoms or mortality.
Conclusions. During a large epidemic of DENV-4 infection in Brazil, >0.5% of donations were RNA positive, and approximately one third of components resulted in TT. However, no significant clinical differences were evident between RNA-positive and RNA-negative recipients.
Keywords: dengue, transfusion-transmission, NAT, clinical symptoms
(See the editorial commentary by Levi on pages 689–90.)
Although dengue has afflicted humans for centuries, there has been a marked global reemergence in the last 25 years [1–5]. The 4 antigenically related dengue viruses (DENV-1–4) that cause similar disease manifestations—asymptomatic infection; mild febrile illness; dengue fever (DF), consisting of fever plus at least 2 other concurrent symptoms; severe dengue hemorrhagic fever (DHF); and dengue shock syndrome (DSS) [6, 7]—are now present in >100 countries [4, 5]. Although there is extensive cross-reactivity in serological tests, there is no long-term cross-protective immunity, and hence humans can have as many as 4 DENV infections during their life [8, 9].
Concern over potential transmission of dengue and other arboviruses by transfusion increased following documentation of transfusion-transmitted (TT) West Nile virus in US epidemics, beginning in 2003 [1, 10]. In 2002, the first cases of DENV TT were documented in 3 recipients in Hong Kong [2, 11], and in 2008, transmission to 2 recipients was reported in Singapore [2, 11]; a case of DHF in a recipient of a DENV RNA–positive transfusion was identified through a look-back study in Puerto Rico [12]. More recently, 2 cases of TT DENV infection in blood recipients were described, one in Brazil and the other in Singapore [13, 14]. Studies of blood donor samples from multiple dengue-endemic countries, using nucleic acid amplification technology assays, have documented rates of viremia as high as 0.4% during outbreaks [12, 15–17].
Reasons why TT of such a common blood-borne infection like dengue would go essentially unrecognized include a lack of active surveillance in the context of very large mosquito-borne epidemics, inhibition of infection or amelioration of disease manifestations by preexisting or cotransfused DENV antibodies, reduced infection rates following parenteral exposure to a human-derived relative to a cutaneous mosquito-derived inoculum, and reduced clinical symptoms in transfusion recipients due to immune suppression of underlying disease [10, 18, 19].
Brazil is the country with the largest number of dengue cases reported annually in the Americas [20–22]. We conducted a study of linked blood donors and transfusion recipients during large epidemics in 2012 in 2 cities in Brazil (Rio de Janeiro and Recife) to investigate rates of transmission by DENV RNA–positive donors and clinical symptoms of DENV infection in the transfused population during the first large-scale DENV-4 outbreak since its reintroduction to Brazil in 2010.
METHODS
Ethics committees in Brazil and institutional review boards in the United States approved this study. On the basis of 2011 data from the Brazil Ministry of Health that suggested the potential for DENV-4 outbreaks in 2012, 2 blood centers were chosen to participate in the study: Hemorio (Rio de Janeiro) and Hemope (Recife). These blood centers are linked to hospitals that treat and provide transfusions to patients with hematological disease. We also included 2 other hospitals in Recife: Hospital Procape, specializing in cardiovascular disease, and Hospital Oswaldo Cruz, a general hospital.
From February through June 2012, all donors from both centers were asked to participate in the study, and an extra blood specimen was collected from each consenting donor into a plasma preparation tube (PPT; Becton Dickinson, Franklin Lakes, New Jersey). The PPTs were centrifuged within 6 hours of collection and frozen at −20°C for subsequent aliquoting of plasma in Brazil and for batch testing for detection of DENV RNA in the United States.
Our focus was on recipients who received components from a consented donor; these recipients were identified daily and invited to participate on the day following transfusion. If the recipient consented, a blood sample collected into a PPT was obtained 3–21 days after the transfusion, and plasma from the routine pretransfusion sample used for cross-match compatibility testing was retrieved and frozen.
All samples collected before and after transfusion from enrolled recipients (n = 1144), all donor samples collected from individuals in Rio de Janeiro (n = 15 866), and the samples obtained from donors Recife that were linked to enrolled recipients (n = 4051) were sent to Sao Paulo after the end of enrollment, aliquoted using a Starlet Aliquoting System (Hamilton, Reno, Nevada) pipetting robot, and frozen at −80°C. Two aliquots of plasma from each sample were sent on dry ice to the United States for testing.
One plasma aliquot from each selected donor and all posttransfusion recipient samples were tested by a transcription-mediated amplification assay (TMA; Hologic/Grifols) with a 50% detection limit of approximately 5 RNA copies/mL for each of the 4 DENVs [23]. For samples with repeat reactivity detected by the TMA, the second plasma aliquot was tested at a separate laboratory by real-time polymerase chain reaction (PCR) assays to confirm infection, define DENV type, and estimate virus load [16]. Virus load was estimated using a standard curve derived from serially diluted cultured DENV 1-4 provided by the US Centers for Disease Control and Prevention and quantified in plaque forming units (PFU), assuming 100 RNA copies per PFU. Plasma samples from DENV-confirmed RNA-positive donors and pretransfusion and posttransfusion plasma samples from recipients of RNA-positive donations were tested for DENV immunoglobulin M (IgM) and immunoglobulin G (IgG) antibodies in Sao Paulo (Focus Diagnostics, Cypress, California). Table 1 provides the definitions used to define probable, possible, inconclusive, and nontransmission categories of TT based on results of testing.
Table 1.
Transfusion-Based Transmission Cluster Categories and Definitions for Recipients of a Dengue Virus (DENV) RNA–Positive Donation
| Transmission Category |
Recipient Status |
|
|---|---|---|
| Before Transfusion | After Transfusion | |
| Probable | DENV RNA negative and IgM negative, or data not available | DENV RNA positive |
| Possible | DENV RNA negative and IgM negative | DENV RNA negative and IgM positive |
| Nontransmission | DENV RNA negative and IgM negative, or data not available | DENV RNA negative and IgM negative |
| Inconclusive | Data not available | DENV RNA negative and IgM positive |
| Recently exposed not susceptible | DENV RNA positive or IgM positive | IgM positivea |
Abbreviation: IgM, immunoglobulin M.
a Immediately after transfusion.
Once DENV testing had been completed, blinded chart review of recipients who tested positive for DENV RNA and/or who received blood from at least 1 RNA-positive component (cases) and a subset of RNA-negative transfusion recipients who had not received known RNA-positive components (controls) was conducted to assess symptoms and measures of disease severity (Supplementary Figure 1). Chart review occurred at the hospitals between January and March 2014. The chart for each patient was assessed for a 45-day peritransfusion period.
To select the interval during which symptoms were assessed, the cases were divided into 2 subgroups. Case group 1 comprised transfusion recipients who tested positive for DENV RNA and for whom at least 1 component received was from a donor who was positive for DENV RNA (donor positive/recipient positive [D+/R+]). The interval for chart review was from 15 days before to 30 days after transfusion of the RNA-positive unit. Case group 2 comprised transfusion recipients who tested positive for DENV RNA but for whom none of the tested components from linked donors was positive for DENV RNA (donor negative/recipient positive [D−/R+]). The interval for chart review was from 15 days before to 30 days after the patient tested positive for RNA. If a patient had >1 RNA-positive test result, the date of the first positive test result was used to calculate the medical chart review start date, and the date of the last positive test result was used to calculate the end date.
Controls were selected from participating patients in the same hospitals during the period of study who were negative for DENV RNA after transfusion and were not known to have been exposed to DENV RNA–positive components (donor negative/recipient negative [D−/R−]). The interval for chart review was from 15 days before to 30 days after a randomly selected transfusion date for each control. Controls were frequency matched to cases by age (<50 and ≥50 years), hospital, admission ward, number of transfusions received, proportion of transfusions that were red blood cell (RBC) units, and proportion of units transfused into the patient that were tested for DENV RNA. For each case, 2 controls were selected. In strata with the exact number of matching controls, all controls were selected for chart abstraction, and for strata with excess numbers of controls, controls were randomly selected from among all potential control recipients in that strata to ensure equal probability of selection.
Abstracted data from all controls were included in statistical analyses. Because recipients who were exposed to dengue in blood components but tested negative (D+/R−) are not informative to the outcomes of infection following TT, they were excluded from statistical analysis.
Statistical Analysis
Rates of TT were calculated from the linked donor and recipient clusters. The 95% confidence interval (CI) for the rate of TT was calculated using the Clopper–Pearson (exact) method. Factors potentially related to transmission were evaluated using the Fisher exact test, for binary risk factors, and the Wilcoxon test, for continuous risk factors. The Fisher exact test was also used to compare rates of transmission between exposed and unexposed subjects.
We compared several clinical outcomes between cases and controls: fever alone, fever plus 2 other symptoms (DF), hemorrhagic manifestations (blood in vomit, tar-like stool or blood in stool, easy bruising or hematomas, nose bleed, other mild bleeding or petechiae, blood in urine, and unusual vaginal bleeding in females), 30-day posttransfusion mortality, and mortality recorded in the medical record at any time. Logistic regression, Kaplan–Meier, and Cox proportional hazard model analyses were used to compare the occurrence of symptoms and survival between cases and controls.
RESULTS
Dengue RNA Prevalence and Rates of Transmission
During the study, 48 139 individuals donated blood in Rio de Janeiro and Recife, of whom 39 134 (81%) consented to participate and had a specimen collected in a PPT for DENV testing (Figure 1). A total of 15 866 donor samples (3425 linked and 12 441 unlinked to enrolled recipients) from Rio de Janeiro and 4051 linked donor samples from Recife were identified for testing for DENV RNA by TMA, with confirmation by PCR. Not all linked donors and units could be located or amplified during testing; 7238 linked donors (95%) with 7871 linked units from Rio de Janeiro and Recife were successfully tested. Rates of confirmed infection with DENV, all of which typed as DENV-4, were 0.51% in Rio de Janeiro and 0.80% in Recife. The peak of the epidemic occurred in week 19 (early May) of 2012 in Rio de Janeiro and week 14 (early April) in Recife, with rates of confirmed DENV RNA detection of >1% and >2%, respectively.
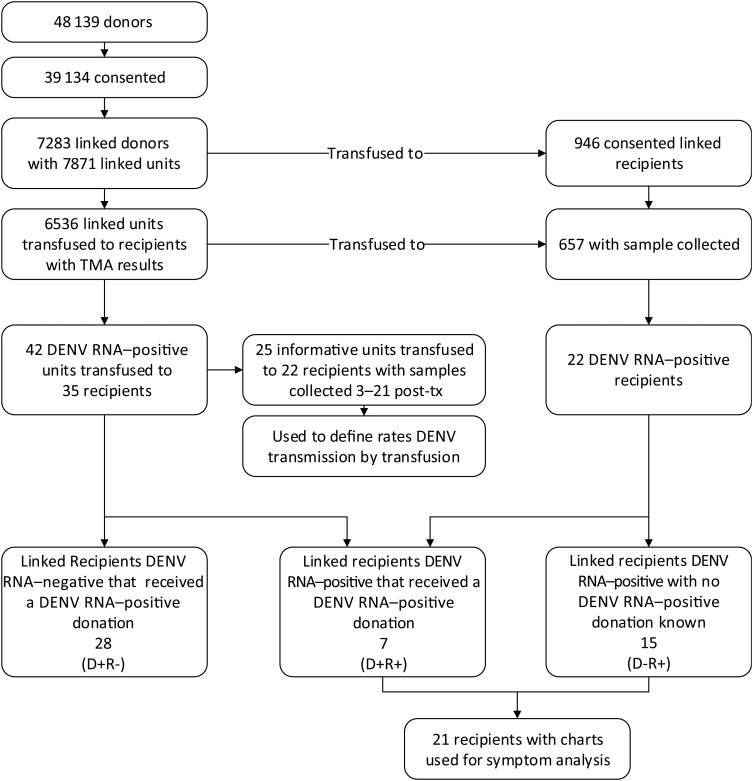
Figure 1.
Flow chart defining blood donor and recipient dengue virus (DENV) RNA testing results and selection of subjects for analysis of clinical manifestations. Abbreviations: −, negative; +, positive; D, donor; R, recipient; TMA, transcription-mediated amplification assay.
We enrolled 946 recipients who received at least 1 unit from enrolled donors, and posttransfusion samples were obtained from 657 enrolled recipients (69%). Of the 6536 blood components collected from enrolled/tested donors that were transfused into these 657 recipients, 42 (0.64%) were confirmed to be positive for DENV RNA, and these units were transfused into 35 recipients. In total, 73% of the components given to these 657 linked recipients with posttransfusion samples were tested; of those not tested, 91% came from donors who did not give consent for DENV testing.
After reviewing the dates of sample collection in relation to the dates of transfusion of RNA-positive units, we identified 25 units transfused into 22 recipients who had a sample collected 3–21 days following transfusion of a confirmed DENV-4 RNA–positive blood component (see Supplementary Table 1 for comparisons of recipients with evidence of transmission and nontransmission). These cases were considered potentially informative to estimation of the rate of TT dengue by DENV RNA–positive components. Table 2 summarizes findings for these cases. DENV RNA–positive units were transfused into 5 recipients (cases 1–5) who became RNA positive on days 5 or 6 after the transfusion episode. For 3 of these recipients, pretransfusion samples were available for testing, and all tested negative for DENV RNA; 2 also tested negative for IgM before transfusion. These were considered cases of probable TT. One additional recipient (case 6) received 2 RNA-positive units; the posttransfusion sample from this patient was collected on day 15 and was negative for DENV RNA, but the recipient seroconverted to DENV IgM. This case was classified as a possible transmission case with 1 exposure, because both RNA-positive units were transfused the same day.
Table 2.
Donor and Recipient Transmission Classification Based on Dengue Virus (DENV) Test Results
| Transmission Category, Case No. |
Component | Donor |
Blood Recipient |
|||||
|---|---|---|---|---|---|---|---|---|
| IgM Result | IgG Result | VL (copies/mL) | Before Transfusion |
After Transfusion |
||||
| NAT Result | IgM Result | IgM Result; Day of Sample Collection |
NAT-Based VL (copies/mL); Day(s) of Sample Collection | |||||
| Probable | ||||||||
| 1 | PLT | − | + | 200 | NR | − | −; 5 | 48; 5 |
| 2 | Plasma | − | + | 2720 | NR | − | −; 6 | 200; 6 |
| 3 | RBC | − | + | 1546 | NR | NA | −; 5 | 100; 5 |
| 4 | RBC | − | − | 84 400 | NA | NA | +; 5 | 100; 5 |
| 5 | PLT | + | + | 36 | NA | NA | −; 6 | 64 700; 6 |
| Possible | ||||||||
| 6* | ||||||||
| Unit 1 | Plasma | + | + | 48 | NR | − | +; 15 | −; 15 |
| Unit 2 | Plasma | − | + | 200 | NR | − | +; 15 | −; 15 |
| Nontransmission casesa | ||||||||
| 7 | RBC | − | + | 4010 | + (200) | − | +; 4 | −; 4, 11 |
| 8 | PLT | NA | NA | NA | NR | + | −; 9 | −; 9, 14, 19 |
| 9 | RBC | − | + | 200 | NR | + | −; 7 | −; 7 |
| 10 | ||||||||
| Unit 1 | Plasma | − | + | 12 | NR | − | +; 4 | −; 4, 11, 18 |
| Unit 2 | Plasma | − | + | 339 | NR | + | +; 6 | −; 6, 13 |
| Unit 3 | Plasma | − | + | 48 | NR | + | +; 7 | −; 7 |
| 11 | RBC | − | + | 48 | NR | NA | +; 5 | −; 5, 11 |
| Nontransmission | ||||||||
| 12 | PLT | + | − | 852 | NR | NA | −; 3 | −; 3 |
| 13 | RBC | + | + | 49 500 | NR | NA | −; 6 | −; 6 |
| 14 | Plasma | + | + | 24 | NR | NA | −; 4 | −; 4 |
| 15 | RBC | + | + | 100 | NR | − | −; 8 | −; 8 |
| 16 | PLT | − | + | 1200 | NR | − | −; 6 | −; 6, 20 |
| 17 | RBC | − | + | 15 210 000 | NR | − | −; 9 | −; 9 |
| 18 | PLT | − | + | 1987 | NR | − | −; 9 | −; 9 |
| 19 | PLT | − | + | 200 | NR | NA | −; 6 | −; 6, 13 |
| 20 | RBC | − | + | 200 | NR | NA | −; 7 | −; 7, 12 |
| 21 | RBC | − | + | 200 | NA | NA | −; 7 | −; 7 |
| Inconclusive | ||||||||
| 22 | Plasma | − | + | 27 | NA | NA | +; 17 | NR; 17 |
Abbreviations: −, negative; +, positive; IgG, immunoglobulin G; IgM, immunoglobulin M; NA, not available; NAT, nucleic acid test; NR, nonreactive; PLT, platelets; RBC, red blood cells; VL, virus load.
a All had signs of infection before transfusion of the DENV RNA–positive unit.
* Two DENV RNA-positive units were transfused on the same day.
Five other recipients (cases 7–11) received 7 RNA-positive units, but all posttransfusion samples tested negative for DENV RNA, suggesting nontransmission. In case 7, DENV RNA was detected in the pretransfusion sample, and in 3 other cases (cases 8–10), DENV IgM was detected in the pretransfusion samples. Case 11 did not have a pretransfusion sample, but IgM was detected on day 5 after transfusion. These 5 cases were considered previously exposed/probably immune to DENV-4 infection before transfusion of the RNA-positive unit and, hence, were not considered susceptible to TT dengue.
Ten additional recipients (cases 12–21), who did not have any evidence of recent DENV infection (tests for IgM and RNA were negative prior to transfusion, if samples were available), received 10 RNA-positive units but tested negative for DENV RNA and IgM on all follow-up samples. In all 10 cases, recipient samples were collected on days 3–9 after transfusion, when RNA would likely have been detectable had infection occurred. These were considered nontransmission cases.
Last, case 22 was considered inconclusive because we could not determine whether the recipient was infected. A sample collected on day 17 following receipt of an RNA-positive transfusion tested negative for DENV RNA but positive for IgM; no IgM results were available before transfusion.
Considering the 5 cases of probable transmission, 1 case of possible transmission, and 10 susceptible recipients who did not become infected by DENV, the TT rate was estimated to be 37.5% (95% CI, 15.2%–64.6%). No association between donation viral load and transmission to recipients was evident; the median virus load was 200 copies/mL (interquartile range [IQR], 48–2720) and 200 copies/mL (IQR, 100–1987) for the probable/possible transmission and nontransmission groups, respectively (P = 1.0), and blood component types were similar between possible transmission and nontransmission cases (2 platelet components, 2 RBC components, and 2 plasma components vs 4 platelet components, 5 RBC components, and 1 plasma component, respectively). The mean and median postdonation storage periods of the DENV RNA–positive components were similar for each group (6.4 [5], and 12.7 [5] days, respectively; P = .75).
In 214 of 657 linked recipients with samples, all transfused blood components were tested for DENV RNA and found to be negative. Among these recipients, 2 were positive for DENV RNA; these were considered community-acquired infections, giving a rate of acquisition of 0.93% (95% CI, .11%–3.34%) among nonexposed recipients, which was significantly lower than the 37.5% rate of DENV RNA detected among susceptible recipients who received positive units (P < .0001, by the Fisher exact test).
Medical Chart Review
In no case or control did the physician record notes indicating suspected dengue (see Supplementary Table 2 for patient information on TT). Table 3 shows that the frequencies of symptoms consistent with DF, hemorrhagic manifestations, and survival were similar between cases and controls. The adjusted odds ratio for cases versus controls was highest for 30-day mortality following transfusion (3.1; 95% CI, 0.6–17.3), but this was not significant.
Table 3.
Symptoms Consistent With Dengue in Cases and Controls, Based on Medical Chart Review, According to Presence of Dengue Virus (DENV) RNA in 21 DENV RNA–Positive Recipients and 92 DENV RNA–Negative Controls
| Condition or Outcome | Cases, No. (%) | Controls, No. (%) | Odds Ratio (95% CI)a | P Value |
|---|---|---|---|---|
| Dengue suspicion in record | 0 | 0 | NA | NA |
| Fever | 7 (33) | 34 (37) | 1.32 (.4–4.5) | .66 |
| Fever plus 2 other symptoms | 6 (29) | 30 (33) | 1.41 (.4–5.4) | .62 |
| Hemorrhagic manifestations | 8 (38) | 39 (42) | 1.09 (.38–3.18) | .87 |
| Mortality within 30 days of transfusion | 3 (14) | 9 (10) | 3.0 (.52–17.0) | .22 |
| Mortality in medical record at any point | 6 (29) | 18 (20) | 2.44 (.70–8.45) | .16 |
Abbreviations: CI, confidence interval; NA, not applicable.
a By logistic regression models adjusted for age, sex, number of transfused components received, proportion of components that were red cells, and proportion of components tested for DENV RNA.
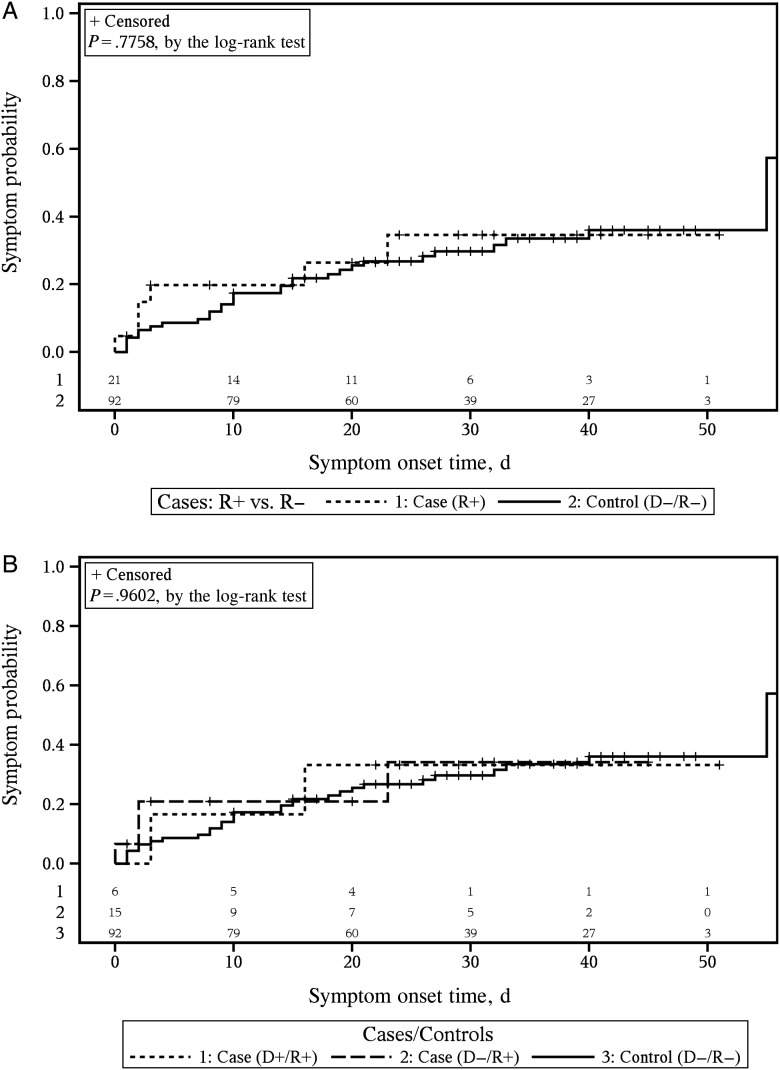
The time to occurrence of symptoms consistent with DF was not significantly different between cases and controls, nor was it significant when the case groups were stratified into D+/R+ and D−/R+ groups (Figure 2A and 2B; see Supplementary Table 3 for comparisons of the case group 1 members).
Figure 2.
Time to occurrence of symptoms consistent with dengue fever. Symptoms status for case and control patients during the 45-day chart review period. A, Comparison of all cases, defined as persons receiving a dengue virus (DENV) RNA–positive unit and/or who tested positive for DENV RNA, to controls. B, Comparison of cases, separated into 3 groups based on whether a patient tested positive for DENV RNA and/or was exposed to a DENV RNA–positive unit, to controls. The number of subjects at risk for each group and at each time point of interest are listed at the bottom of the graphs. The number at risk at each specific time point is the number of remaining subjects after all of the events and censoring (time of loss-to-follow-up in the medical chart and indicated with by + sign in each plot) have been accounted for. Abbreviations: −, negative; +, positive; D, donor; R, recipient.
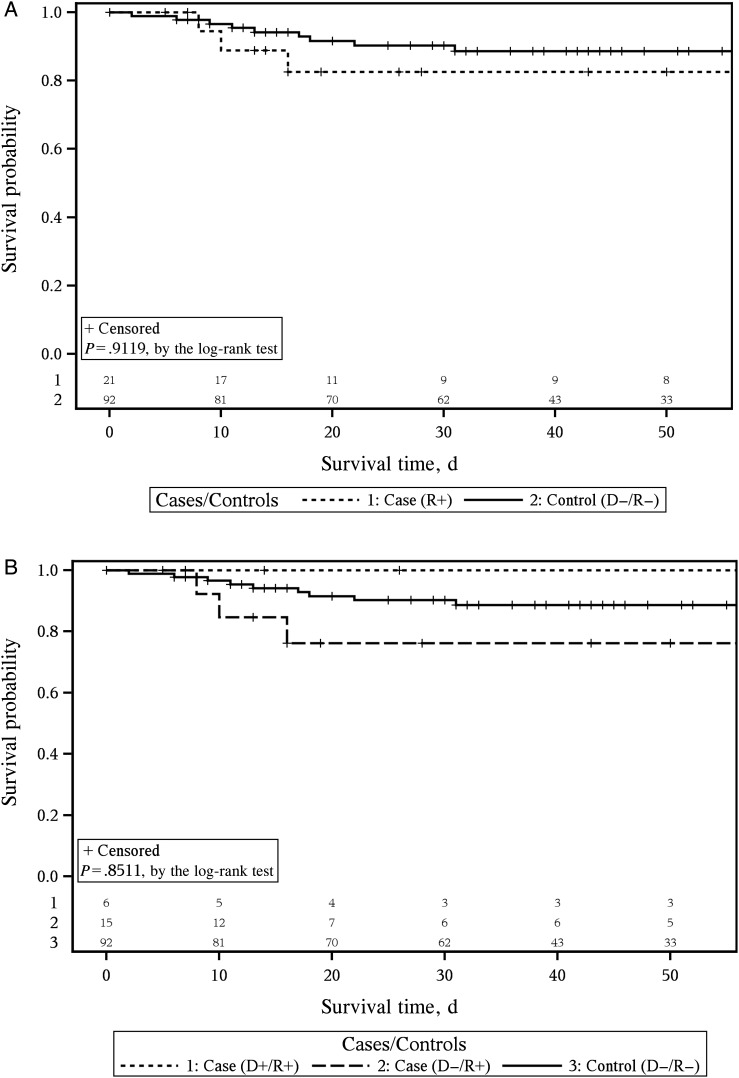
Survival time was not different for cases, compared with controls (Figure 3A). When cases were stratified into D+/R+, and D−/R+, and D−/R− groups, there was evidence suggesting a different pattern of survival, with D+/R+ cases having lower mortality than controls. No known fatalities occurred in the 5 probable or 1 possible TT DENV cases (D+/R+) within 30 days after transfusion, whereas D−/R+ cases had increased mortality, compared with controls (Figure 3B). Because of small case numbers, these difference were not statistically significant. Using an alternate method of Cox proportional hazards modeling to adjust for survival according to the number of components transfused, we found that survival in the D+/R+, D−/R+, and D−/R− groups was not significantly different (P = .34).
Figure 3.
Survival status for case and control patients during the 45-day chart review period. A, Comparison of all cases, defined as persons receiving a dengue virus (DENV) RNA–positive unit and/or who tested positive for DENV RNA, to controls. B, Comparison of cases, separated into 3 groups based on whether a patient tested positive for DENV RNA and/or was exposed to a DENV RNA–positive unit, to controls. The number of subjects at risk for each group and at each time point of interest are listed at the bottom of the graphs. The number at risk at each specific time point is the number of remaining subjects after all of the events and censoring (time of loss-to-follow-up in the medical chart and indicated with by + sign in each plot) have been accounted for. Abbreviations: −, negative; +, positive; D, donor; R, recipient.
DISCUSSION
This is the largest study of TT dengue to date. Our findings confirm that, during seasonal epidemics, substantial proportions of asymptomatic donors with infection are donating blood and that recipients are receiving RNA-positive blood components. The overall rates of viremia in our study, 0.51% in Rio de Janeiro and 0.80% in Recife, are higher than rates reported using the same TMA assay in previous studies from Brazil, Puerto Rico, and Honduras, reflecting the very large size of the 2012 epidemic in Brazil. Our study documented 6 cases of TT DENV, substantially adding to the total number of transmission cases reported to date [2, 11–14].
Approximately one third of components from RNA-positive donations transmitted infection during the 2012 DENV-4 outbreak in Brazil. This infection rate is vastly higher than the 0.93% rate of DENV RNA detection in samples collected near the time of transfusion from enrolled recipients who were exclusively transfused with DENV RNA-negative components. To estimate the rate of TT dengue, we restricted the analysis to 16 cases who had optimal posttransfusion sampling intervals to document transmission and excluded 5 recipients who had evidence of recent infection before receipt of the RNA-positive study unit; had we included these 5 recipients, the rate of TT dengue would have been 29% instead of 37%.
Our findings failed to show significant associations between transmission and viral load in the transfused RNA-positive units, recipient demographic characteristics, component type, or duration of storage prior to transfusion. In terms of inhibition of infection by preexisting or cotransfused DENV antibodies, approximately 90% of donor and recipient samples had DENV IgG, reflecting past infection(s) with 1 or more DENV types, and of these nearly 7% had IgM, reflecting recent infection, perhaps with DENV-4 (data not shown). Further work on the capacity of heterotypic and homotypic antibodies to prevent TT dengue is in progress, using samples from this study and animal models of DENV infection. Evidence suggests that reduced infection rates may occur following parenteral exposure to a human-derived versus a cutaneous mosquito-derived inoculum, attributable to different glycosylation patterns of viruses replicating in mosquitos than humans and to injection of mosquito saliva, which triggers local inflammation and other potentiating factors that may increase local viral replication and systemic infection [24–26].
By record review, we found evidence of symptomatic disease consistent with dengue in the transfused DENV RNA–positive population. Patients in the chart review substudy were matched with respect to clinically relevant factors. Results do not suggest higher rates of symptomatic infection in RNA-positive recipients, but the study has low power to detect these outcomes, owing to the relatively small number of DENV RNA–positive recipients identified. Two recent publications have reported on 2 cases of febrile illness in blood recipients in dengue-endemic countries [13, 14]. While TT dengue was subsequently demonstrated, neither of these patients progressed to DHF or DSS. These case reports show that symptomatic infection in blood recipients does occur, albeit rarely. Outside of these reports, when it does it occur, the clinical manifestations may be attributed to other causes. Even so, in our study, in which medical charts were reviewed for 21 DENV RNA–positive recipients, including 6 cases of probable and possible TT dengue, no recipient developed highly symptomatic infection, such as DHF or DSS, and symptoms consistent with DF were not in excess of those for similar RNA-negative patients who received a transfusion.
This study has limitations. Although the study was conducted during large epidemics and identified RNA-positive donations and documents recipient exposures to these units, only 16 DENV RNA–positive units were transfused into susceptible recipients for whom informative posttransfusion samples were available to establish transmission. We assessed clinical outcomes in recipients, using retrospective chart reviews, but high background rates of nonspecific symptoms in controls confounded assessment of clinical dengue in exposed cases.
This study was conducted in a DENV-hyperendemic setting with high rates of past exposure resulting in partial immunity and large numbers of community-acquired DENV infections. Consequently, we recommend caution in extrapolation of our findings to settings of nonendemicity or low endemicity where returning travelers could be infected with DENV, which may result in local autochthonous transmissions, including by blood transfusion, to DENV-naive patients who could manifest more-severe disease. Temporary (eg, 1-month) deferral from donation from persons who have traveled to DENV-epidemic regions and inactivation of pathogens in platelets and plasma components are under consideration and may be warranted to address this concern.
In conclusion, during epidemics of dengue in dengue-endemic countries, TT is occurring, with more than one third of components from RNA-positive donations transmitting infection. During peak epidemic periods, 1%–2% of donations may be RNA positive and 0.3%–0.6% of all transfusions may transmit DENV. However, against the background of the large burden of mosquito-transmitted infection (eg, during the 2012 study period, 113 000 cases of clinical dengue [1.8% of the population] were reported in Rio de Janeiro) and because clinical disease progression may be obscured by the underlying reason why the patient required transfusion, interventions to reduce TT dengue may not impact the burden of clinically apparent disease.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. The Recipient Epidemiology and Donor Evaluation Study-III, International Component Brazil, is the responsibility of the following persons: Cesar de Almeida Neto and Alfredo Mendrone-Junior (Fundação Pró-Sangue Hemocentro de São Paulo, Brazil); Anna Bárbara Carneiro-Proietti (Fundação Hemominas, Belo Horizonte, Brazil); Divaldo de Almeida Sampaio and Paula Loureiro (Fundação Hemope, Recife); Clarisse Lobo and Maria Esther Lopes (Hemorio, Rio de Janeiro); Ester Cerdeira Sabino, Ligia Capuani, João Eduardo Ferreira, Marcio Oikawa, and Pedro Losco Takecian (University of São Paulo); Cláudia Di Lorenzo Oliveira (University of São João de Rei, Brazil); Brian Custer, Michael P. Busch, Shannon Kelly, and Thelma T. Gonçalez (Blood Systems Research Institute and University of California–San Francisco, San Francisco, California); Donald Brambilla and Christopher McClure (RTI international, Rockville, Maryland); and Simone A. Glynn (National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health (NIH), Bethesda, Maryland).
Each author attests to the following: substantial contributions to the conception or design of the work or to the acquisition, analysis, or interpretation of data for the work; drafting the work or revising it critically for important intellectual content; final approval of the version to be published; and agreement to be accountable for all aspects of the work by ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Financial support. This work was supported by the NHLBI, NIH (contract HHSN268201100007I to the Recipient Epidemiology and Donor Evaluation Study-III); and the Fundação de Amparo à Pesquisa do Estado de São Paulo (grant 2011/18955-1).
Potential conflicts of interest. J. M. L. is an employee of Hologic, the manufacturer of one of the dengue virus RNA tests used in this study. M. P. B. has served as a scientific advisor to Hologic. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Collaborators: for the International Component of the NHLBI Recipient Epidemiology and Donor Evaluation Study-III, Cesar de Almeida Neto, Alfredo Mendrone-Junior, Anna Bárbara Carneiro-Proietti, Divaldo de Almeida Sampaio, Paula Loureiro, Clarisse Lobo, Maria Esther Lopes, Ester Cerdeira Sabino, Ligia Capuani, João Eduardo Ferreira, Marcio Oikawa, Pedro Losco Takecian, Cláudia Di Lorenzo Oliveira, Brian Custer, Michael P. Busch, Shannon Kelly, Thelma T. Gonçalez, Donald Brambilla, Christopher McClure, and Simone A. Glynn
References
- 1.Bianco C. Dengue and Chikungunya viruses in blood donations: risks to the blood supply? Transfusion 2008; 48:1279–81. [DOI] [PubMed] [Google Scholar]
- 2.Chuang V, Wong TY, Leung YH et al. Review of dengue fever cases in Hong Kong during 1998 to 2005. Hong Kong Med J 2008; 14:170–7. [PubMed] [Google Scholar]
- 3.Holmes EC, Twiddy SS. The origin, emergence and evolutionary genetics of dengue virus. Infect Genet Evol 2003; 3:19–28. [DOI] [PubMed] [Google Scholar]
- 4.Laughlin CA, Morens DM, Cassetti MC et al. Dengue research opportunities in the Americas. J Infect Dis 2012; 206:1121–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mackenzie JS, Gubler DJ, Petersen LR. Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat Med 2004; 10:S98–109. [DOI] [PubMed] [Google Scholar]
- 6.Gubler DJ. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev 1998; 11:480–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO. Dengue haemorrhagic fever, diagnosis, treatment, prevention and control. 2nd ed Geneva: World Health Organization, 1997. [Google Scholar]
- 8.Dejnirattisai W, Jumnainsong A, Onsirisakul N et al. Cross-reacting antibodies enhance dengue virus infection in humans. Science 2010; 328:745–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.OhAinle M, Balmaseda A, Macalalad AR et al. Dynamics of dengue disease severity determined by the interplay between viral genetics and serotype-specific immunity. Sci Transl Med 2011; 3:114ra28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petersen LR, Busch MP. Transfusion-transmitted arboviruses. Vox Sang; 2010; 98:495–503. [DOI] [PubMed] [Google Scholar]
- 11.Tambyah PA, Koay ES, Poon ML, Lin RV, Ong BK. Dengue hemorrhagic fever transmitted by blood transfusion. N Engl J Med 2008; 359:1526–7. [DOI] [PubMed] [Google Scholar]
- 12.Stramer SL, Linnen JM, Carrick JM et al. Dengue viremia in blood donors identified by RNA and detection of dengue transfusion transmission during the 2007 dengue outbreak in Puerto Rico. Transfusion 2012; 52:1657–66. [DOI] [PubMed] [Google Scholar]
- 13.Levi JE, Nishiya A, Felix AC et al. Real-time symptomatic case of transfusion-transmitted dengue. Transfusion 2015; 55:961–4. [DOI] [PubMed] [Google Scholar]
- 14.Oh HB, Muthu V, Daruwalla ZJ, Lee SY, Koay ES, Tambyah PA. Bitten by a bug or a bag? Transfusion-transmitted dengue: a rare complication in the bleeding surgical patient. Transfusion 2015; doi:10.1111/trf.13054. [DOI] [PubMed] [Google Scholar]
- 15.Mohammed H, Linnen JM, Munoz-Jordan JL et al. Dengue virus in blood donations, Puerto Rico, 2005. Transfusion 2008; 48:1348–54. [DOI] [PubMed] [Google Scholar]
- 16.Linnen JM, Vinelli E, Sabino EC et al. Dengue viremia in blood donors from Honduras, Brazil, and Australia. Transfusion 2008; 48:1355–62. [DOI] [PubMed] [Google Scholar]
- 17.Dias LL, Amarilla AA, Poloni TR, Covas DT, Aquino VH, Figueiredo LT. Detection of dengue virus in sera of Brazilian blood donors. Transfusion 2012; 52:1667–71. [DOI] [PubMed] [Google Scholar]
- 18.Teo D, Ng LC, Lam S. Is dengue a threat to the blood supply? Transfus Med 2009; 19:66–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilder-Smith A, Chen LH, Massad E, Wilson ME. Threat of dengue to blood safety in dengue-endemic countries. Emerg Infect Dis 2009; 15:8–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Honorio NA, Nogueira RM, Codeco CT et al. Spatial evaluation and modeling of Dengue seroprevalence and vector density in Rio de Janeiro, Brazil. PLoS Negl Trop Dis 2009; 3:e545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teixeira M, Costa M, Barreto M, Mota E. Dengue and dengue hemorrhagic fever epidemics in Brazil: what research is needed based on trends, surveillance, and control experiences? Cad Saude Publica 2005; 21:1307–15. [DOI] [PubMed] [Google Scholar]
- 22.Vargas J, Ferreira O, Corgozinho P. Tratamento Logístico das Ocorrências Anuais de Dengue no Rio de Janeiro (1985–2008). Economia Energia 2009; 12:71. [Google Scholar]
- 23.Munoz-Jordan JL, Collins CS, Vergne E et al. Highly sensitive detection of dengue virus nucleic acid in samples from clinically ill patients. J Clin Microbiol 2009; 47:927–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dejnirattisai W, Webb AI, Chan V et al. Lectin switching during dengue virus infection. J Infect Dis 2011; 203:1775–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conway MJ, Watson AM, Colpitts TM et al. Mosquito saliva serine protease enhances dissemination of dengue virus into the mammalian host. J Virol 2014; 88:164–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Surasombatpattana P, Ekchariyawat P, Hamel R et al. Aedes aegypti saliva contains a prominent 34-kDa protein that strongly enhances dengue virus replication in human keratinocytes. J Invest Dermatol 2014; 134:281–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.