Abstract
Background. Multiple host defense mechanisms protect the female genital tract from pathogens, but the impact of sexual intercourse on defense is unknown.
Methods. As part of a hypothesis-generating study, 17 women provided cervicovaginal lavage (CVL) specimens at baseline (all had abstained from sexual intercourse, masturbation, and vaginal product use for 72 hours prior to screening), 2–6 hours and 10–14 hours after vaginal intercourse with a male condom, and 2–6 hours and 10–14 hours after vaginal intercourse without a male condom (5 visits total, including the baseline visit). Vaginal pH, concentrations of immune molecules, and antimicrobial activity at postcoital visits were compared to baseline values.
Results. Vaginal pH and the transforming growth factor β1 level increased, but human beta-defensin 2 (HBD-2), HBD-3, and interleukin 8 levels decreased after unprotected sex. Median Escherichia coli inhibitory activity in CVL specimens decreased significantly from baseline at the visit 2–6 hours after unprotected sex (63% [range, −34% to 99%] vs 5% [range, −51% to 100%]; P = .02) and remained low at the visit 10–14 hours after unprotected sex (6% [range, −19% to 92%]; P = .02). Pooled human seminal plasma enhanced E. coli growth in vitro in a dose-dependent manner and, when added to CVL samples with high anti–E. coli activity, reversed the inhibition.
Conclusions. Unprotected vaginal sex results in a reduction in endogenous anti–E. coli activity, which may reflect, in part, enhancement of bacterial growth by seminal plasma. This finding may contribute to the risk of E. coli vaginal colonization following sexual intercourse.
Keywords: sexual intercourse, semen, female genital tract, mucosal immunity, E. coli, human beta defensins
The female genital tract has multiple host defense mechanisms against bacterial and viral pathogens [1]. Genital tract secretions collected from sexually abstinent women provide variable activity ex vivo against Escherichia coli [2], herpes simplex virus 2 (HSV-2) [3], and human immunodeficiency virus (HIV) [4]. This endogenous activity may be mediated by cytokines, chemokines, antimicrobial peptides, and antibodies, as well as by molecules secreted by vaginal microbiota [5]. Few clinical studies have investigated the impact of semen and sexual intercourse on the genital mucosal immune environment.
In prior in vitro studies, exposure of female genital tract epithelial cells to seminal plasma increased expression of proinflammatory cytokines and chemokines [6, 7]. Furthermore, leukocyte influx has been observed in cervical mucus and smears in response to donor insemination [8, 9]. In one well-conducted clinical study, cervical biopsy specimens were obtained at baseline and after vaginal intercourse with (n = 5 cases) and without (n = 6 cases) a male condom [10]. A significant increase in macrophage, dendritic cell, and CD8+ T cell counts and upregulated expression of CSF2 (which encodes granulocyte macrophage colony-stimulating factor [GM-CSF]) and the genes encoding interleukin 6 (IL-6), interleukin 8 (IL-8), and interleukin 1α (IL-1α) were observed by immunohistochemical and reverse transcription–polymerase chain reaction (RT-PCR) analyses of biopsy tissue specimens obtained following unprotected sex [10]. The high concentration of transforming growth factor β (TGF-β) in seminal plasma is thought to exert a proinflammatory effect on cervical cell signaling pathways [11]. However, the cumulative effect of seminal components on the female genital mucosa is unknown.
The objective of this hypothesis-generating study was to investigate the impact of sexual intercourse and semen on the genital mucosal immune environment by examining antimicrobial activity in genital tract secretions, which may provide a cumulative assessment of mucosal host defense, as well as specific antimicrobial immune mediators, such as defensins. Women were evaluated at multiple time points following unprotected and male condom–protected sexual intercourse. We hypothesized that we would observe a decrease in endogenous antimicrobial activity, a decline in levels of antimicrobial peptides, and an increase in inflammatory mediators following unprotected intercourse but not after condom-protected intercourse.
METHODS
Study Visits
Institutional review board approval was obtained from Albert Einstein College of Medicine. All participants provided written informed consent. Healthy, premenopausal women and their male partners were recruited from the Bronx, New York. Inclusion criteria included being in a monogamous relationship for >6 months and use of contraception (hormonal contraception, intrauterine device, or tubal ligation) for >3 months. Exclusion criteria included chronic medical conditions; pregnancy; breastfeeding; menopause; irregular menses; HIV infection; sexually transmitted infection (STI) in the previous 6 months; recent diagnosis and/or treatment for urinary tract infection (UTI); complaint of dysuria, hematuria, frequency, or vaginal discharge at screening; or abnormal genital examination or urinalysis findings at screening.
Couples were asked to abstain from sexual intercourse, masturbation, and vaginal product use for 72 hours prior to screening. Women underwent urine pregnancy testing, urinalysis, and OraQuick rapid HIV-1/2 antibody testing (OraSure Technologies, Bethlehem, Pennsylvania). A speculum-based examination of the vagina was performed to assess for gross abnormalities (ie, ulcers and abnormal cervical discharge). Testing for other STIs was not performed. Women with sign or symptoms of a UTI or genital tract infection were referred to their physician but were eligible for rescreening 1 month following treatment and symptom resolution. Male partners also underwent HIV testing and a genital examination.
Female participants underwent baseline sample collection (detailed below) during the screening study visit. They returned for sample collection following 4 episodes of vaginal intercourse: 2–6 hours after unprotected sex, 10–14 hours after unprotected sex, 2–6 hours after male condom-protected sex, and 10–14 hours after condom-protected sex. Participants were instructed to complete either the condom-associated visits or the non–condom-associated visits first. Study visits were scheduled to not coincide with menses and were separated by 2–5 days, with a minimum of 48 hours of sexual abstinence prior to planned intercourse. Male condoms that did not contain nonoxynol-9 (N-9) were provided. Participants were instructed not to use any vaginal products for the study duration.
Specimen Collection and Processing
At each study visit, pH of the lateral vaginal wall was measured (Whatman pH paper, pH 3.8–5.5 and pH 6.0–8.1) followed by cervicovaginal lavage (CVL) of the cervical os and vaginal walls with 10 mL of normal saline. A vaginal swab specimen was collected for Nugent scoring [12]. All specimens were transported to the laboratory on ice and processed within 4–16 hours. The CVL specimen was clarified by low-speed centrifugation at 800g for 10 minutes at 4°C. CVL supernatant aliquots, cell pellets, and vaginal swab specimens were stored at −80°C.
Measurement of Protein and Immune Mediators
Total protein concentrations in CVL specimens were quantified by the microBCA assay (ThermoScientific, Rockford, Illinois). Enzyme-linked immunosorbent assay kits were used to quantify secretory leukocyte protease inhibitor, TGF-β1, and human macrophage inflammatory protein 3α (MIP-3α; R&D Systems, Minneapolis, Minnesota); human neutrophil peptides 1–3 (Hycult Biotech, Plymouth Meeting, Pennsylvania); and human beta defensin 1 (HBD-1), HBD-2, and HBD-3 (Alpha Diagnostic International, San Antonio, Texas). CVL specimens were diluted to obtain a value within the standardized curve. Concentrations below the lower limit of detection (LLOD) were set to the midpoint between 0 and the LLOD, multiplied by the sample dilution factor. Other CVL cytokines and chemokines (GM-CSF, IL-1α, interleukin 1β, IL-6, IL-8, macrophage inflammatory protein 1α [MIP-1α], MIP-1β, monocyte chemotactic protein 1, RANTES [regulated on activation, normal T-cell expressed], tumor necrosis factor α, interferon γ, and interleukin 17) were assayed using the Fluorokine MultiAnalyte profiling system (R&D Systems), measured with the Bioluminex 100 system (Bioluminex, Austin, Texas), and analyzed with StarStation, version 2.3 (Applied Cytometry Systems, Dinnington, South Yorkshire, United Kingdom).
Assays for Semen Detection
The presence of semen in CVL specimens was determined by an immunochromatographic rapid test for prostate-specific antigen (PSA) (SERATEC PSA Semiquant, Goettingen, Germany). CVL pellets were also subjected to Y chromosome (Yc) DNA PCR to quantify semen exposure. Nucleic acid was extracted by incubating the cell pellet in 2 mg/mL proteinase K solution (Life Technologies) for 30 minute at 55°C. Guanidinium isothiocyanate (Sigma-Aldrich, St. Louis, Missouri) and glycogen (Roche, Indianapolis, Indiana) were added to concentrations of 4.58 M and 0.47 mg/mL, respectively, and incubated at room temperature for 30 minutes. Nucleic acids were precipitated by centrifugation at 15 000g in the presence of a nearly equal volume of isopropanol. Nucleic acid pellets were washed repetitively with 70% ethanol and air dried before suspension in 5 mM Tris-buffered saline (pH 8).
Yc and total human DNA were detected using the commercially available Quantifiler Duo kit (Applied Biosystems) as previously described [13]. Approximate total cell numbers were calculated in each swab cell pellet and quantified according to kit manufacturer's instructions. Normalized Yc DNA copy number was calculated by dividing the number of Yc DNA copies by the number of cells per sample.
Nugent Scoring
Frozen (−80°C) vaginal swab specimens were thawed on ice and wrung out, and 50 µL of the liquid was transferred to a slide, heat fixed, and Gram stained. Gram-positive, gram-negative, or gram-variable bacterial morphotypes were quantified. A Nugent score of 0–3 was considered normal, 4–6 was considered intermediate, and 7–10 was considered consistent with bacterial vaginosis [12]. To ensure that freezing did not affect scores, Nugent scoring was also performed on parallel samples from fresh and frozen swab specimens from a separate study; no significant differences between frozen versus fresh swab specimens were observed.
Antimicrobial Activity Assays
Anti–HIV-1 activity
TZM-bl cells were plated at 3 × 105 cells/well in 96-well plates and then infected with HIV-1BAL mixed 1:1 with CVL or control buffer (normal saline containing 0.2 mg/mL bovine serum albumin [BSA]). After incubation for 48 hours at 37°C, the inoculum was removed, cells were lysed, and luciferase activity was measured in relative light units (RLU) and compared to values for control wells [14]. All samples were tested in triplicate.
Anti–HSV-2 activity
Vero (monkey kidney epithelial) cells were plated in 24-well plates overnight and then infected with approximately 50–200 plaque-forming units of HSV-2(G) mixed 1:1 with CVL or control buffer (normal saline containing 0.2 mg/mL BSA) in triplicate wells. After 1 hour of incubation, cells were washed and overlaid with fresh medium. Plaques were counted after 48 hours of incubation at 37°C, and the percentage inhibition was determined relative to values for control wells [3].
Anti–E. coli activity
E. coli (ATCC strain 43 827) was grown overnight to stationary phase, and then 3 µL were mixed with 27 µL of CVL or control buffer (20 mmol/L potassium phosphate, 60 mmol/L sodium chloride, and 0.2 mg/mL albumin) and incubated at 37°C for 2 hours. The mixtures were further diluted in buffer to yield 800–1000 colony-forming units (CFU) on control plates, plated in duplicate on agar enriched with trypticase soy broth, and incubated overnight at 37°C. Colonies were counted using ImageQuant TL v2005 software (GE Healthcare Bio-Sciences, Pittsburgh, Pennsylvania), and the percentage inhibition was determined relative to the number of colonies on control plates [5].
The impact of seminal plasma on E. coli growth was assessed by mixing E. coli with serial dilutions of pooled human seminal plasma (diluted in normal saline containing 40 mg/mL BSA) or with control buffer. Seminal plasma was isolated by centrifugation of pooled human semen (Lee Biosolutions, St. Louis, Missouri) at 1811g for 5 minutes. CVL and semen mixing experiments were conducted using CVL specimens with >90% E. coli inhibitory activity that were obtained from sexually abstinent women. CVL specimens were mixed with serial dilutions of seminal plasma or control buffer (final pH range, 6.6–7.8) and assayed for E. coli inhibitory activity.
Statistical Analyses
Continuous variables that were not normally distributed were log transformed to reduce skew. Continuous variables were compared by paired t tests or by Wilcoxon signed rank tests. Categorical variables were analyzed by the Fisher exact or χ2 tests. Spearman correlation coefficients were calculated to assess potential associations between continuous variables. No adjustment for multiple comparisons was used, given the small sample size and the hypothesis-generating nature of the study [15]. Simple linear regression was used to assess the relationship between Yc DNA copies and hours after unprotected sex. Two-sided P values of <.05 were considered significant. Based on a previous study [14], we calculated that a sample size of 15 women would yield >85% power to detect a 1–standard deviation (SD) difference in mean HIV inhibition between a baseline and postcoital visit. All analyses were performed using Stata, version 12.1 (College Station, Texas), and GraphPad Prism, version 6.0 (La Jolla, California).
RESULTS
Subject Characteristics
Seventeen couples were enrolled into the study (Table 1). Fourteen of 17 women completed all 5 study visits; 78 study visits were included in the analysis. Nine couples completed the condom-associated visits first, and 8 completed the non–condom-associated visits first. Study subjects reported 100% adherence with the study protocol. A negative PSA test result was detected in 16 of 17 women (94%) at the baseline visit and in 13 of 17 (76%) and 12 of 14 women (86%) 2–6 hours and 10–14 hours, respectively, after condom-protected sex. Conversely, 14 of 15 women (93%) and 11 of 14 (78%) had positive PSA test results 2–6 hours and 10–14 hours, respectively, after unprotected sexual intercourse.
Table 1.
Characteristics of Study Participants at Study Entry
| Characteristic | Value |
|---|---|
| Women | |
| Age, y | 30 (24–44) |
| Race | |
| White | 8 (47) |
| Black | 5 (29) |
| Other | 4 (24) |
| Ethnicity | |
| Hispanic | 4 (24) |
| Non-Hispanic | 13 (76) |
| Nulliparous | 8 (47) |
| Birth control method | |
| OCP | 9 (53) |
| IUD | 5 (29) |
| Tubal ligation | 2 (12) |
| NuvaRing | 1 (6) |
| Cervical ectopy | |
| None noted | 11 (65) |
| <50% cervix | 3 (18) |
| >50% cervix | 3 (18) |
| Signs or symptoms of UTI | 0 (0) |
| Nugent score | |
| 0–3 | 10 (59) |
| 4–6 | 3 (18) |
| 7–10 | 4 (23) |
| Men | |
| Age, y | 30 (24–46) |
| Race | |
| White | 9 (53) |
| Black | 5 (29) |
| Other | 3 (18) |
| Ethnicity | |
| Hispanic | 7 (41) |
| Non-Hispanic | 10 (59) |
| Couples | |
| Length of monogamous relationship, mo | 36 (9–240) |
| Reported no. of sex acts per mo | 9 (2–28) |
Data are median value (range) or no. (%) of participants.
Abbreviations: IUD, intrauterine device; OCP, oral contraceptive pill; UTI, urinary tract infection.
Sexual Intercourse Is Associated With Decreased E. coli Inhibitory Activity
CVL inhibitory activity at baseline varied between women, with a median reduction in E. coli CFU of 63% (range, −34% to 99%), a median reduction in the number of HSV-2 plaques of 34% (range, −38% to 59%), and a median change in HIV RLU of 27% (range, −249% to 100%; Table 2). Median E. coli inhibitory activity was reduced following unprotected sex at both the 2–6-hour visit (5% [range, −51% to 100%]; P = .02) and 10–14-hour visit (5.5% [range, −19% to 92%]; P = .02; Figure 1). Although 9 of 17 women had >50% E. coli inhibitory activity at baseline, only 2 had >50% activity 2–6 hours after unprotected sex, and only 1 had >50% activity 10–14 hours after unprotected sex (P = .013). We also observed a modest but statistically significant reduction in median E. coli inhibitory activity after condom-protected sex at the 2–6 hour visit (27% [range, −37% to 96%]; P = .006). We did not observe significant differences in E. coli inhibitory activity at any visit between women who completed the condom-protected sex visits first, compared with those who completed the unprotected sex visits first (data not shown). There were no significant changes in the anti-HIV or anti-HSV-2 activity of CVL specimens obtained following unprotected or condom-protected intercourse.
Table 2.
Comparison of Vaginal pH, Immune Mediators, and Antimicrobial Activity in Cervicovaginal Lavage Specimens Between Postcoital and Baseline Visits
| Variable | Baseline Value | 2–6 h, No Condom |
10–14 h, No Condom |
2–6 h, Condom |
10–14 h, Condom |
||||
|---|---|---|---|---|---|---|---|---|---|
| Value | P Valuea | Value | P Valuea | Value | P Valuea | Value | P Valuea | ||
| E. coli, % inhibition | 63 (−34 to 99) | 5 (−51 to 100) | .02 | 5.5 (−19 to 92) | .02 | 27 (−37 to 96) | .006 | 39 (−49 to 99) | |
| HIV, % inhibition | 27 (−249 to 100) | 25 (−125 to 93) | 34 (−123 to 98) | 24.5 (−90 to 99) | −5 (−103 to 90) | .09 | |||
| HSV, % inhibition | 34 (−38 to 59) | 17 (−25 to 76) | 21 (−111 to 71) | 24 (−4 to 69) | 32 (−7 to 52) | ||||
| Vaginal pH | 4.6 (4.2–6.0) | 5.5 (4.4–7.5) | .002 | 4.9 (4.3–6.8) | .04 | 4.6 (4.2–6.5) | 4.5 (4.2–6.1) | ||
| Protein level, µg/mL | 2.7 ± 0.2 | 2.7 ± 0.3 | 2.7 ± 0.4 | 2.6 ± 0.2 | 2.5 ± 0.3 | .03 | |||
| HBD-1 level, pg/mL | 3.5 ± 0.5 | 3.6 ± 0.5 | 3.8 ± 0.4 | .08 | 3.5 ± 0.4 | 3.5 ± 0.4 | |||
| HBD-2 level, pg/mL | 8.3 ± 1.7 | 7.5 ± 1.4 | .04 | 8.1 ± 1.7 | .19 | 8.2 ± 1.6 | 8.0 ± 1.8 | .11 | |
| HBD-3 level, pg/mL | 3.3 ± 0.5 | 2.8 ± 0.5 | .003 | 3.1 ± 0.8 | .15 | 3.2 ± 0.6 | 3.1 ± 0.8 | .18 | |
| HNP-1–3 level, pg/mL | 5.2 ± 0.8 | 5.0 ± 0.9 | 5.2 ± 0.9 | 5.2 ± 0.7 | 5.0 ± 0.8 | ||||
| SLPI level, pg/mL | 5.2 ± 0.7 | 5.5 ± 0.6 | .11 | 5.3 ± 1.3 | 5.3 ± 0.5 | 5.3 ± 0.8 | |||
| MIP-3α level, pg/mL | 1.7 ± 0.9 | 1.9 ± 1.1 | 1.8 ± 1.1 | 1.6 ± 0.9 | 1.6 ± 0.9 | .18 | |||
| TGF-β1 level, pg/mL | 1.4 ± 0.3 | 2.1 ± 1.1 | .02 | 1.8 ± 0.8 | .03 | 1.5 ± 0.5 | 1.4 ± 0.4 | ||
| GM-CSF level, pg/mL | UD | UD | UD | UD | UD | ||||
| IFN-γ level, pg/mL | 0.2 ± 0.5 | 0.3 ± 0.5 | 0.2 ± 0.4 | 0.1 ± 0.4 | 0.2 ± 0.5 | ||||
| IL-17 level, pg/mL | 0.1 ± 0.4 | 0.1 ± 0.4 | 0.2 ± 0.3 | 0.1 ± 0.4 | 0.2 ± 0.7 | ||||
| IL-1α level, pg/mL | 2.2 ± 0.5 | 2.2 ± 0.4 | 2.2 ± 0.5 | 2.2 ± 0.6 | 2.1 ± 0.4 | ||||
| IL-1β level, pg/mL | 0.8 ± 1.1 | 0.6 ± 1.0 | .11 | 0.9 ± 0.8 | 0.9 ± 1.0 | 0.7 ± 1.1 | |||
| IL-6 level, pg/mL | 1.0 ± 0.6 | 1.2 ± 0.9 | 1.4 ± 0.7 | .08 | 1.1 ± 0.6 | 0.9 ± 0.7 | |||
| IL-8 level, pg/mL | 2.9 ± 0.7 | 2.6 ± 0.8 | .003 | 2.9 ± 0.7 | 2.8 ± 0.7 | 2.7 ± 0.7 | .11 | ||
| MCP-1 level, pg/mL | 1.9 ± 0.8 | 2.1 ± 0.6 | .13 | 2.1 ± 0.7 | .17 | 1.9 ± 0.7 | 1.9 ± 0.8 | ||
| MIP-1α level, pg/mL | 1.0 ± 0.7 | 1.0 ± 0.7 | 0.9 ± 0.6 | 1.0 ± 0.6 | 1.0 ± 0.7 | ||||
| MIP-1β level, pg/mL | 0.9 ± 0.5 | 1.1 ± 0.7 | 1.2 ± 0.4 | .10 | 1.0 ± 0.5 | .06 | 0.9 ± 0.8 | ||
| RANTES level, pg/mL | 0.6 ± 0.4 | 0.8 ± 0.8 | .11 | 0.7 ± 0.6 | 0.5 ± 0.3 | 0.6 ± 0.6 | |||
| TNF-α level, pg/mL | 0 ± 0.3 | 0.2 ± 0.5 | 0.1 ± 0.4 | 0 ± 0.2 | 0.2 ± 0.4 | .16 | |||
Data are median value (range) or mean value ± SD. All values were log transformed, except those for antimicrobial activity and vaginal pH.
Abbreviations: E. coli, Escherichia coli; GM-CSF, granulocyte macrophage colony-stimulating factor; HBD, human beta defensin; HIV, human immunodeficiency virus; HNP-1–3, human neutrophil peptides 1–3; HSV, herpes simplex virus; IFN-γ, interferon γ; IL, interleukin; MCP-1, monocyte chemotactic protein 1; MIP, macrophage inflammatory protein; RANTES, regulated on activation, normal T-cell expressed; SLPI, secretory leukocyte protease inhibitor; TGF-β1, transforming growth factor β1; TNF-α, tumor necrosis factor α; UD, undetectable.
a Data denote comparisons with baseline by paired t tests or Wilcoxon signed rank tests (for nonparametric data). P values of <.2 are specified.
Figure 1.
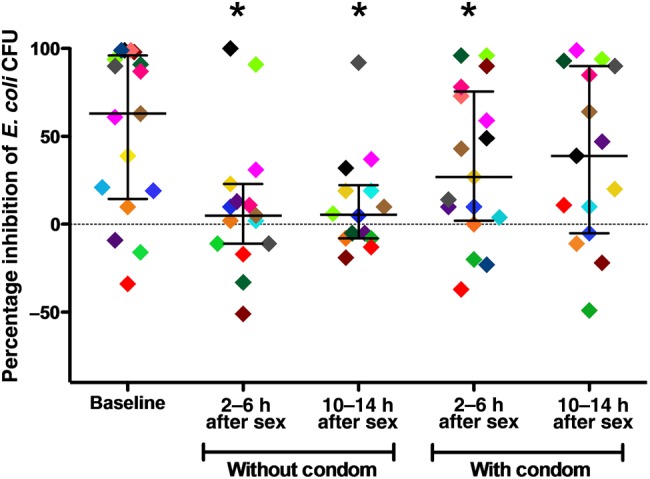
Escherichia coli inhibitory activity in cervicovaginal lavage (CVL) specimens obtained at postcoital visits, compared with baseline. E. coli (approximately 109 colony-forming units [CFU]/mL) were mixed with each CVL specimen or control buffer for 2 hours, diluted in buffer to yield approximately 1000 CFU on control plates, and incubated overnight. Each point represents the percentage inhibition relative to the control buffer. Horizontal and vertical lines indicate median values and interquartile ranges, respectively. Each participant is color-coded to highlight individual changes over time. *P < .05, by the Wilcoxon signed rank test, compared with baseline.
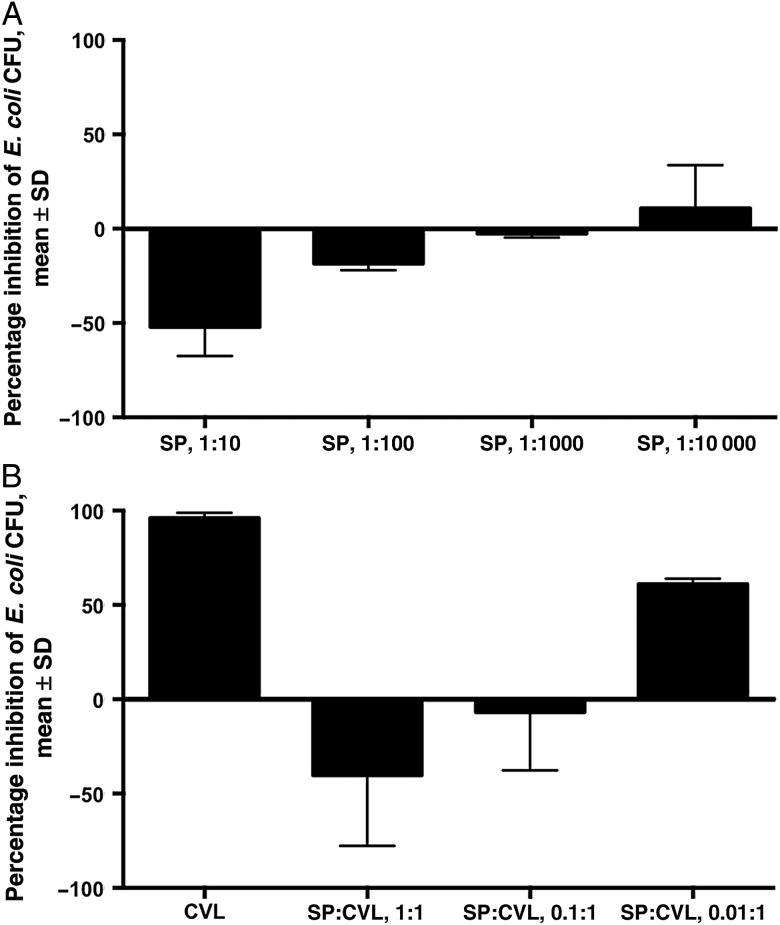
We then examined the impact of pooled human seminal plasma on E. coli growth in vitro. Seminal plasma significantly enhanced the number of E. coli CFU in a dose-dependent manner (Figure 2A). When diluted 1:10 in pH buffers 4.0–9.5, seminal plasma still enhanced E. coli growth in vitro relative to pH-matched buffer controls (data not shown). The addition of seminal plasma to CVL specimens from sexually abstinent women with >90% inhibitory activity resulted in a dose-dependent loss in inhibition that persisted over a pH range of 6.6 to 7.8 (Figure 2B).
Figure 2.
Effect of human seminal plasma (SP) on Escherichia coli growth in vitro. A, E. coli were mixed with control buffer (40 mg/mL bovine serum albumin in normal saline) or pooled human SP diluted 1:10, 1:100, 1:1000, or 1:10 000 in control buffer and assayed for inhibitory activity. B, Human SP or control buffer was mixed at a ratio of 0 (no SP), 1:1, 0.1:1, or 0.01:1 with cervicovaginal lavage (CVL) known to have high inhibitory activity against E. coli and then assayed for E. coli inhibitory activity. Results are presented as percentage inhibition (or enhancement) of colony-forming units (CFU), relative to the respective control, from 2 independent experiments each performed in duplicate. Abbreviation: SD, standard deviation.
Changes in Mucosal Immune Molecules, Vaginal pH, and Nugent Scores Following Unprotected Vaginal Intercourse
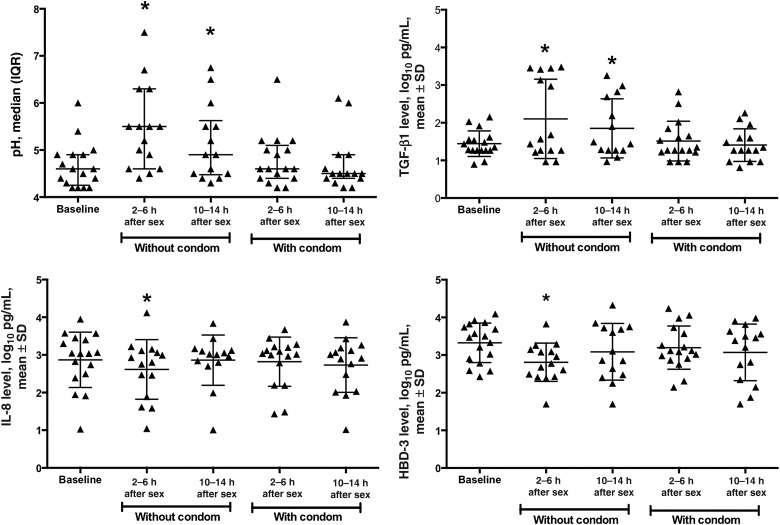
Median vaginal pH was significantly higher 2–6 hours (5.5 [range, 4.4–7.5]; P = .002) and 10–14 hours (4.9 [median, 4.3–6.8]; P = .04) after unprotected sex, compared with baseline (4.6 [range, 4.2–6.0]; Table 2 and Figure 3). There was no significant difference in pH following condom-protected sex.
Figure 3.
Changes in vaginal pH and concentrations of immune mediators following sexual intercourse. Vaginal pH and the concentrations of transforming growth factor β1 (TGF-β1), interleukin 8 (IL-8), and human beta-defensin 3 (HBD-3) in cervicovaginal lavage (CVL) specimens at each visit. Lines indicate mean and standard deviation (SD) (or median and interquartile range [IQR] for pH) and asterisks indicate significant change from baseline *P < .05, by the paired t test or the nonparametric equivalent, compared with baseline.
At baseline, 58.8% of women had a Nugent score of 0–3, 17.6% had a score of 4–6, and 23.5% had a score of 7–10. The proportions of women with a Nugent score of 0–3 or 7–10 did not differ significantly from baseline at 2–6 hours after unprotected sex (0–3, 53.3%; 7–10, 40%) or 10–14 hours after unprotected sex (0–3, 40%; 7–10, 26.7%; P = .34). The proportions of women with Nugent scores of 0–3 or 7–10 also did not differ significantly from baseline at 2–6 hours following condom-protected sex (0–3, 52.9%; 7–10, 23.5%) or 10–14 hours after unprotected sex (0–3, 60%; 7–10, 26.7%; P = .95).
Although the total protein concentration in seminal plasma is approximately 100-fold higher (approximately 53 mg/mL in the pooled seminal plasma tested in this study) than that of CVL specimen (200–800 μg/mL) and approximately 10-fold higher than that of undiluted cervical secretions collected by cervical cup (2.5–5 mg/mL), we did not observe an increase in total protein recovered from CVL specimens obtained following unprotected sex. However, TGF-β1, which is present in high concentrations in semen [11, 16], was significantly higher in CVL specimens obtained 2–6 hours (P = .02) and 10–14 hours (P = .03) after unprotected coitus, relative to baseline (Table 2 and Figure 3). There was a modest but statistically significant decrease in mean CVL protein levels (±SD) 10–14 hours after condom-protected sex, relative to baseline (2.5 ± 0.3 vs 2.7 ± 0.2 log10 µg/mL; P = .03).
The IL-8 level was significantly decreased at 2–6 hours following unprotected sex (P = .003). HBD-2 and HBD-3 levels were significantly lower in CVL specimens obtained 2–6 hours after unprotected sex (P = .04 and P = .003, respectively) but began to recover by 10–14 hours (Table 2 and Figure 3). There were no statistically significant changes in levels of other antimicrobial proteins or cytokines measured following unprotected sex.
Correlation of Yc DNA Copy Number With Time Since Sex
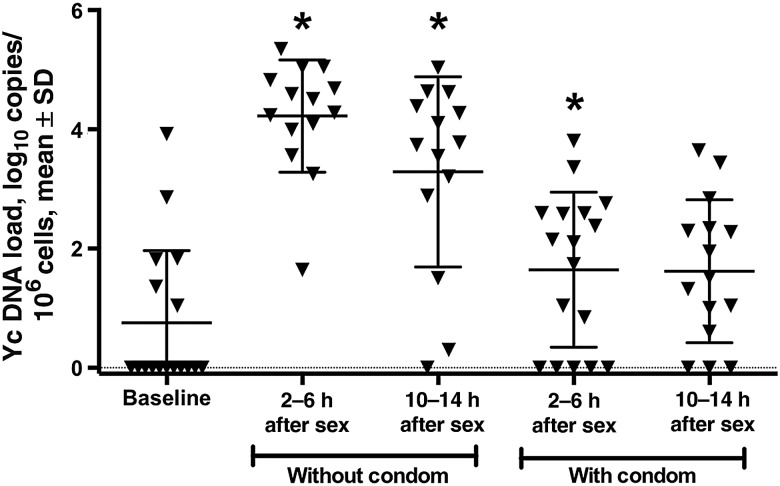
The mean number of Yc DNA copies (±SD) in the CVL cell pellet at the baseline visit was 0.8 ± 1.2 log10 copies/106 cells, with 6 of 17 women having detectable Yc DNA (range, 0–3.9 log10 copies/106 cells). This low number is consistent with prior studies indicating that Yc DNA can be detected up to 15 days after coitus [17]. The mean Yc DNA copy number (±SD) increased to 4.2±0.9 log10 copies/106 cells 2–6 hours after unprotected sex and to 3.3 ± 1.6 log10 copies/106 10–14 hours after unprotected sex (P = <.001 for both comparisons; Figure 4). Mean Yc DNA copies (±SD) after condom-protected sex were higher than those at baseline, but the difference reached statistical significance only at 2–6 hours (1.6 ± 1.3 log10 copies/106 cells at 2–6 hours and 1.6 ± 1.2 log10 copies/106 cells at 10–14 hours; P = .03 and P = .12, respectively).
Figure 4.
Y chromosome (Yc) DNA copies in cervicovaginal lavage (CVL) cell pellets. Yc DNA was quantified by DNA polymerase chain reaction analysis. *P < .05, by the paired t test, compared with baseline.
The number of Yc DNA copies in the CVL pellet was inversely and significantly associated with the reported hours since unprotected sex (β coefficient = −0.13; P = .03). In addition, the concentration of TGF-β1 in CVL specimens correlated with the number of Yc DNA copies (r = 0.42; P < .001). E. coli inhibitory activity did not correlate with the number of Yc DNA copies or with the TGF-β1 level, although it correlated positively with protein concentration in CVL specimens (r = 0.33; P = .003) and negatively with vaginal pH (r = −0.27; P = .02).
DISCUSSION
In this hypothesis-generating study, we identified a significant decline in innate anti–E. coli but not anti-HIV or anti–HSV-2 activity following unprotected sex. The lack of seminal plasma–mediated impact on anti-HIV or anti–HSV-2 activity is consistent with results from our prior human and in vitro studies [14, 18]. The reduction in E. coli inhibitory activity may be partially attributed to seminal plasma components that specifically enhance bacterial replication, as evidenced by the findings that seminal plasma directly enhanced E. coli growth independent of pH and that combining seminal plasma with CVL reduced E. coli inhibitory activity. However, E. coli inhibitory activity did not correlate with other markers of semen exposure, including Yc DNA and TGF-β1. These findings, coupled with the observation that there was also a modest but transient decrease in the E. coli inhibitory activity 2–6 hours following condom-protected sex, suggest that factors other than seminal plasma may also contribute to the reduction in E. coli inhibitory activity following sex.
Prior work has demonstrated an increased risk of vaginal E. coli colonization following intercourse [19, 20], and we hypothesize that this may be secondary to the observed decline in E. coli inhibitory activity. Conversely, high E. coli inhibitory activity has been associated with a healthy, Lactobacillus-dominant vaginal microbiome [2, 5, 21, 22], and proteomic studies identified Lactobacillus surface proteins as contributing to E. coli inhibitory activity in CVL [5]. Consistent with these observations, women with E. coli colonization are more likely to have a paucity of vaginal lactobacilli [23]. Although we did not observe significant differences in Nugent scores following unprotected vaginal intercourse, it is possible that a transient decrease in vaginal lactobacilli following vaginal intercourse contributed to the postcoital reduction in E. coli inhibitory activity [20]. Quantification of vaginal lactobacilli by molecular detection methods, such as RT-PCR, could yield more informative data.
Vaginal pH significantly increased following unprotected coitus and remained elevated even after 10–14 hours. The vaginal pH is naturally acidic, in part because of commensal H2O2-producing lactobacilli, and is an important component of the female genital tract host defense. Lower pH has been associated with increased E. coli inhibitory activity [2, 24]. We did not observe a change in pH following condom-protected sex, indicating that the pH increase can be attributed to the buffering effects of semen, which has a pH of approximately 8.0 [25].
Despite the high protein concentration of seminal plasma, we did not detect the significant increase in CVL protein levels following unprotected intercourse that we observed in a prior study [14]. This may reflect leakage and/or redistribution of the semen prior to sampling, as the couples had sex at home and then traveled to our facility for sample collection. However, the TGF-β1 level, which is found in high concentration in semen, was significantly higher at both time points after unprotected sexual intercourse. TGF-β1 has been proposed as a primary mediator of immunologic changes in the female genital tract following coitus [26] and has been shown to induce an inflammatory response in the female genital tract [11], although we did not detect a significant increase in levels of the proinflammatory cytokines or chemokines we measured.
We observed a decrease in levels of the neutrophil chemokine IL-8 following unprotected sex. This differs from a prior study that demonstrated an increase in cervical expression of IL8 and other proinflammatory cytokines after unprotected coitus [10]. These conflicting results could reflect the method of sample collection. The prior study evaluated gene expression in cervical biopsy specimens, but we quantified mediators in CVL specimens. Cervical biopsy specimens allow access to deeper tissues, whereas CVL specimens reflect contributions from superficial epithelial cells.
HBD-2 and HBD-3 concentrations were also decreased following unprotected sexual activity. HBD-2 and HBD-3 are antimicrobial peptides that mediate mucosal host defense through disruption of target membranes and are induced by inflammation [1]. Although defensins have bactericidal activity against E. coli in vitro [27], the concentrations of these proteins did not correlate with anti–E coli activity, and the levels detected in CVL specimens of individual mediators are probably not sufficient to inhibit E. coli growth. For example, the mean 90% lethal dose (±SD) for HBD-3 against E. coli is 6.6 ± 1.9 μg/mL [28], but concentrations in CVL specimens ranged from 50 pg/mL to 21 ng/mL in this study. Presuming that a CVL specimen is a 50-fold dilute sample of genital tract secretions [29], the concentration is still orders of magnitude less than what is needed for bacterial inhibition. Thus, it is more likely that multiple antimicrobial peptides act in concert to contribute to innate antimicrobial defense.
We observed substantial variability in Yc levels, which could represent differences in the quantity of semen and sperm cells deposited during ejaculation and/or variation in the kinetics of elimination [13]. The modest but statistically significant increase in Yc DNA copies after condom-protected sex could reflect delayed condom application.
There are several limitations to this study, including small sample size and limited formal testing for STIs. Additionally, there was variability in type of contraception used by female participants and in the phase of the menstrual cycle during which they completed study visits [30]. However, this study is the first to demonstrate that unprotected sexual intercourse leads to a decrease in innate antimicrobial activity and that seminal plasma directly enhances E. coli growth. This previously unrecognized effect of semen could contribute to increased risk of vaginal colonization and urinary tract infections with E. coli after sexual intercourse [31]. This finding may also be important in the setting of pregnancy, E. coli–related chorioamnionitis, and neonatal sepsis. Identification of the seminal plasma factors and mechanisms that contribute to the enhancement of E. coli growth could provide a new strategy for protection against E. coli colonization.
Notes
Acknowledgments. We thank Nicole Marshall, for her assistance in performing subject visits; and Yungtai Lo, for his assistance with the statistical analysis.
Disclaimer. The contents are solely the responsibility of the authors and do not necessarily represent the official view of the National Institutes of Health.
Financial support. This work was supported by the National Institutes of Health (NIH; grants T32 AI070117 to N. A. N.; K23 A1089271 to R. P. M.; and U19 AI103461, AI079763, and AI065309 to B. C. H.), the NIH/National Center for Advancing Translational Science Einstein-Montefiore CTSA (grant UL1TR001073), and the Center for AIDS Research at the Albert Einstein College of Medicine and Montefiore Medical Center (grant AI51519).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Cole AM. Innate host defense of human vaginal and cervical mucosae. Curr Top Microbiol Immunol 2006; 306:199–230. [PubMed] [Google Scholar]
- 2.Valore EV, Park CH, Igreti SL, Ganz T. Antimicrobial components of vaginal fluid. Am J Obstet Gynecol 2002; 187:561–8. [DOI] [PubMed] [Google Scholar]
- 3.John M, Keller MJ, Fam EH et al. Cervicovaginal secretions contribute to innate resistance to herpes simplex virus infection. J Infect Dis 2005; 192:1731–40. [DOI] [PubMed] [Google Scholar]
- 4.Ghosh M, Fahey JV, Shen Z et al. Anti-HIV activity in cervical-vaginal secretions from HIV-positive and -negative women correlate with innate antimicrobial levels and IgG antibodies. PLoS One 2010; 5:e11366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalyoussef S, Nieves E, Dinerman E et al. Lactobacillus proteins are associated with the bactericidal activity against E. coli of female genital tract secretions . PLoS One 2012; 7:e49506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharkey DJ, Macpherson AM, Tremellen KP, Robertson SA. Seminal plasma differentially regulates inflammatory cytokine gene expression in human cervical and vaginal epithelial cells. Mol Hum Reprod 2007; 13:491–501. [DOI] [PubMed] [Google Scholar]
- 7.Berlier W, Cremel M, Hamzeh H et al. Seminal plasma promotes the attraction of Langerhans cells via the secretion of CCL20 by vaginal epithelial cells: involvement in the sexual transmission of HIV. Hum Reprod 2006; 21:1135–42. [DOI] [PubMed] [Google Scholar]
- 8.Pandya IJ, Cohen J. The leukocytic reaction of the human uterine cervix to spermatozoa. Fertil Steril 1985; 43:417–21. [DOI] [PubMed] [Google Scholar]
- 9.Thompson LA, Barratt CL, Bolton AE, Cooke ID. The leukocytic reaction of the human uterine cervix. Am J Reprod Immunol 1992; 28:85–9. [DOI] [PubMed] [Google Scholar]
- 10.Sharkey DJ, Tremellen KP, Jasper MJ, Gemzell-Danielsson K, Robertson SA. Seminal fluid induces leukocyte recruitment and cytokine and chemokine mRNA expression in the human cervix after coitus. J Immunol 2012; 188:2445–54. [DOI] [PubMed] [Google Scholar]
- 11.Sharkey DJ, Macpherson AM, Tremellen KP, Mottershead DG, Gilchrist RB, Robertson SA. TGF-beta mediates proinflammatory seminal fluid signaling in human cervical epithelial cells. J Immunol 2012; 189:1024–35. [DOI] [PubMed] [Google Scholar]
- 12.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol 1991; 29:297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Penrose KJ, Richardson BA, Besson G et al. Y chromosome and HIV DNA detection in vaginal swabs as biomarkers of semen and HIV exposure in women. Sex Transm Dis 2014; 41:674–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keller MJ, Mesquita PM, Torres NM et al. Postcoital bioavailability and antiviral activity of 0.5% PRO 2000 gel: implications for future microbicide clinical trials. PLoS One 2010; 5:e8781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology 1990; 1:43–6. [PubMed] [Google Scholar]
- 16.Robertson SA. Seminal plasma and male factor signalling in the female reproductive tract. Cell Tissue Res 2005; 322:43–52. [DOI] [PubMed] [Google Scholar]
- 17.Zenilman JM, Yuenger J, Galai N, Turner CF, Rogers SM. Polymerase chain reaction detection of Y chromosome sequences in vaginal fluid: preliminary studies of a potential biomarker for sexual behavior. Sex Transm Dis 2005; 32:90–4. [DOI] [PubMed] [Google Scholar]
- 18.Patel S, Hazrati E, Cheshenko N et al. Seminal plasma reduces the effectiveness of topical polyanionic microbicides. J Infect Dis 2007; 196:1394–402. [DOI] [PubMed] [Google Scholar]
- 19.Eschenbach DA, Patton DL, Hooton TM et al. Effects of vaginal intercourse with and without a condom on vaginal flora and vaginal epithelium. J Infect Dis 2001; 183:913–8. [DOI] [PubMed] [Google Scholar]
- 20.Hooton TM, Roberts PL, Stamm WE. Effects of recent sexual activity and use of a diaphragm on the vaginal microflora. Clin Infect Dis 1994; 19:274–8. [DOI] [PubMed] [Google Scholar]
- 21.Ghartey JP, Carpenter C, GialanellaP et al. Association of bactericidal activity of genital tract secretions with Escherichia coli colonization in pregnancy. Am J Obstet Gynecol 2012; 207:297.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghartey JP, Smith BC, Chen Z et al. Lactobacillus crispatus dominant vaginal microbiome is associated with inhibitory activity of female genital tract secretions against Escherichia coli. PLoS One 2014; 9:e96659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta K, Hillier SL, Hooton TM, Roberts PL, Stamm WE. Effects of contraceptive method on the vaginal microbial flora: a prospective evaluation. J Infect Dis 2000; 181:595–601. [DOI] [PubMed] [Google Scholar]
- 24.Keller MJ, Carpenter CA, Lo Y et al. Phase I randomized safety study of twice daily dosing of acidform vaginal gel: candidate antimicrobial contraceptive. PLoS One 2012; 7:e46901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolters-Everhardt E, Dony JM, Lemmens WA, Doesburg WH, De Pont JJ. Buffering capacity of human semen. Fertil Steril 1986; 46:114–9. [DOI] [PubMed] [Google Scholar]
- 26.Robertson SA, Ingman WV, O'Leary S, Sharkey DJ, Tremellen KP. Transforming growth factor beta--a mediator of immune deviation in seminal plasma. J Reprod Immunol 2002; 57:109–28. [DOI] [PubMed] [Google Scholar]
- 27.Valore EV, Park CH, Quayle AJ, Wiles KR, McCray PB Jr, Ganz T. Human beta-defensin-1: an antimicrobial peptide of urogenital tissues. J Clin Invest 1998; 101:1633–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei G, de Leeuw E, Pazgier M et al. Through the looking glass, mechanistic insights from enantiomeric human defensins. J Biol Chem 2009; 284:29180–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dezzutti CS, Hendrix CW, Marrazzo JM et al. Performance of swabs, lavage, and diluents to quantify biomarkers of female genital tract soluble mucosal mediators. PLoS One 2011; 6:e23136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shust GF, Cho S, Kim M et al. Female genital tract secretions inhibit herpes simplex virus infection: correlation with soluble mucosal immune mediators and impact of hormonal contraception. Am J Reprod Immunol 2010; 63:110–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hooton TM. Recurrent urinary tract infection in women. Int J Antimicrob Agents 2001; 17:259–68. [DOI] [PubMed] [Google Scholar]