Abstract
Interleukin 19 (IL-19) and interleukin 24 (IL-24) are cytokines that are highly expressed in filarial infections. To study the role of IL-19 and IL-24 in regulating T-cell responses, we examined the frequency of T-helper type 1 (Th1)/Tc1, Th2/Tc2, Th9/Tc9, Th17/Tc17, Th22/Tc22, and Tr1 cells in 26 filariae-infected individuals stimulated with filarial antigen following IL-19 or IL-24 neutralization. IL-19 or IL-24 neutralization resulted in significantly enhanced frequencies of Th1/Tc1 and/or Th17/Tc17 cells and significantly reduced frequencies of Th2/Tc2, Tr1, and/or Th9/Tc9 cells. Thus, we demonstrate that IL-19 and IL-24 are associated with the modulation of T-cell responses in filarial infections.
Keywords: filarial infections, T cells, cytokines, IL-19, IL-24, IL-10
The interleukin 20 (IL-20) cytokine subfamily comprises of interleukin 19 (IL-19), IL-20, interleukin 22 (IL-22), interleukin 24 (IL-24), and interleukin 26 (IL-26). Although members of the IL-20 subfamily are part of the larger interleukin 10 (IL-10) family, they are grouped together because of their use of common receptor subunits and similarities in their biological functions [1]. These cytokines signal through heterodimeric receptors, composed of IL-20 receptor 2 (IL-20R2) or IL-10R2 paired with either IL-20R1 or IL-22R1 [1, 2]. T cells and myeloid cells are the primary sources of IL-19, IL-20, IL-22, IL-24, and IL-26 [3]. Although epithelial cells are the main target of these cytokines, previous reports suggest that IL-19, IL-20, and IL-24 can also modulate the function of T cells [3–5]. Thus, IL-19, IL-20, and IL-24 have been shown to downregulate T-helper type 1 (Th1) responses and upregulate the Th2 response in murine models [4, 5].
Among the disease-causing filarial parasites, those that cause lymphatic filariasis afflict >120 million people worldwide and, similar to most helminth infections, are characterized by a down-modulation of antigen-specific CD4+ and CD8+ Th1/Tc1, Th17/Tc17, and Th22/Tc22 responses and an expansion of Th2/Tc2, Th9/Tc9, and Tr1 responses [6]. The modulation of Th1, Th17, and Th22 responses have been previously shown to be mediated by 2 regulatory cytokines, IL-10 and transforming growth factor β [6]. We have previously reported that IL-19 and IL-24 are highly expressed in chronic filarial infections and that these cytokines are associated with protection against lymphatic pathology associated with lymphatic filariasis [7]. While IL-10, with its primary effects on CD4+ and CD8+ T cells, is a known regulator of T-cell function in lymphatic filariasis [6], the effect of IL-19 and IL-24 in this chronic infection remains unexplored. Hence, we sought to explore the impact of these cytokines on T cells in filarial infections and compare this to the effect of IL-10. We demonstrate that, while IL-10 functions as a global modulator of CD4+ and CD8+ T-cell responses, IL-19 and/or IL-24 modulate primarily Th1/Tc1 and Th17/Tc17 responses while enhancing Th2/Tc2, Th9/Tc9, and Tr1 responses in filarial infection.
MATERIALS AND METHODS
Study Population
We studied a group of 26 clinically asymptomatic, filariae-infected individuals and 10 uninfected control individuals in an area of Tamil Nadu, South India, where Wuchereria bancrofti is endemic. All infected individuals were positive for circulating filarial antigen by both an immunochromatographic test (Alere, Portland, Maine) and the TropBio Og4C3 enzyme-linked immunosorbent assay (ELISA; Cellabs, Australia) and had not received any antifilarial treatment prior to enrollment in this study. Uninfected control individuals had negative results of both tests. All individuals were examined as part of a natural history study with the protocol approved by the institutional review boards of both the National Institutes of Allergy and Infectious Diseases and the National Institute for Research in Tuberculosis (clinical trials registration NCT00001230), and informed written consent was obtained from all participants.
Parasite Antigen
Saline extracts of Brugia malayi adult worms (BmA) at 10 µg/mL were used for stimulation. Endotoxin levels in the BmA was determined to be <0.1 EU/mL, using the QCL-1000 Chromogenic LAL test kit (BioWhittaker, Radnor, Pennsylvania).
In Vitro Culture
Whole-blood specimens were cultured in the presence of anti–IL-10, anti–IL-19, anti–IL-24, or isotype control antibody (all 5 µg/mL; R & D Systems, Minneapolis, Minnesota) for 18 hours, after which BmA was added and cultured for a further 6 hours. Briefly, whole-blood specimens were diluted 1:1 with Roswell Park Memorial Institute 1640 medium supplemented with penicillin/streptomycin (100 U/100 mg/mL), L-glutamine (2 mM), and HEPES (10 mM; all from Invitrogen, San Diego, California) and placed in 12-well tissue culture plates (Costar, Corning, New York). FastImmune Brefeldin A Solution (10 µg/mL; BD Biosciences, San Diego, California) was added 2 hours before culturing ended. Whole-blood specimens were then centrifuged and washed with cold phosphate-buffered saline (PBS), and 1× fluorescence-activated cell-sorting (FACS) lysing solution (BD Biosciences) was added. The cells were fixed using cytofix/cytoperm buffer (BD Biosciences), cryopreserved, and stored at −80°C until use.
Intracellular Cytokine Staining
The cells were thawed and washed with PBS first and PBS/1% bovine serum albumin later and then stained with surface antibodies for 30–60 minutes. Surface antibodies used were CD3-AmCyan, CD4–allophycocyanin–Hilite 7, and CD8–phycoerythrin–cyanine 7 (all from BD Biosciences). The cells were washed and permeabilized with BD Perm/Wash buffer (BD Biosciences) and stained with intracellular cytokines for an additional 30 minutes before washing and acquisition. Cytokine antibodies used were anti–interferon γ (IFN-γ), anti–tumor necrosis factor α (TNF-α), anti–interleukin 2 (IL-2), anti–interleukin 4 (IL-4), anti–interleukin 5 (IL-5), anti–interleukin 9 (IL-9), IL-10, anti–interleukin 17 (IL-17), and anti–interleukin 22 (IL-22) (BD Biosciences). Flow cytometry was performed on a FACS Canto II flow cytometer with FACSDiva software v.6 (Becton Dickinson, Franklin Lakes, New Jersey). The lymphocyte gating was set by forward and side scatter, and 100 000 gated lymphocyte events were acquired. Data were collected and analyzed using FlowJo software (FlowJo, Ashland, Oregon). All data are depicted as frequencies, with frequencies denoting the percentage of CD4+ and CD8+ T cells expressing the respective cytokine(s).
Statistical Analysis
Data analyses were performed using GraphPad PRISM (GraphPad Software, San Diego). Geometric means were used for measurements of central tendency. Statistically significant differences were analyzed using the Wilcoxon signed rank test, followed by the Holm correction for multiple comparisons.
RESULTS
Regulation of CD4+ T-Cell Subsets by IL-10, IL-19, and IL-24
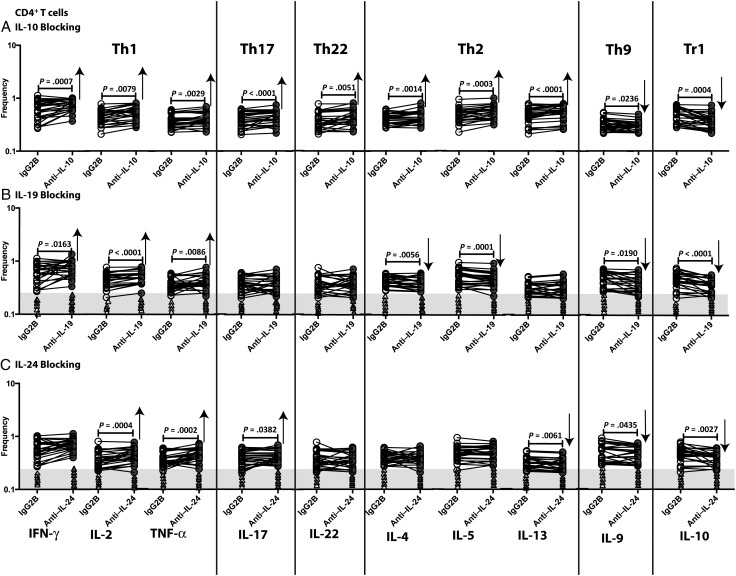
To examine the effect of IL-19 and IL-24 (as well as IL-10) on CD4+ T cells in filarial infections, we measured the frequencies of Th1 (IFN-γ–, TNF-α–, or IL-2–expressing), Th17 (IL-17–expressing), Th22 (IL-22–expressing), Th2 (IL-4–, IL-5–, or interleukin 13 [IL-13]–expressing), Th9 (IL-9–expressing), and Tr1 (IL-10–expressing but not IFN-γ– and IL-4–expressing) cells following neutralization of IL-10, IL-19, or IL-24 and stimulation with BmA in 26 filariae-infected individuals. As shown in Figure 1A, IL-10 neutralization resulted in significantly increased frequencies of CD4+ Th1, Th17, Th22, and Th2 cells and significantly decreased frequencies of CD4+ Th9 and Tr1 cells. As shown in Figure 1B, IL-19 neutralization resulted in significantly increased frequencies of Th1 cells and decreased frequencies of Th2, Th9, and Tr1 cells. As shown in Figure 1C, IL-24 neutralization resulted in significantly increased frequencies of Th1 and Th17 cells and significantly decreased frequencies of Th9 and Tr1 cells. In addition, IL-19 or IL-24 neutralization had no significant effect on the CD4+ T-cell frequencies in response to BmA in filariae-uninfected individuals. Also, neutralization of IL-19 or IL-24 in unstimulated samples had no effect on CD4+ T-cell frequencies (data not shown). Thus, IL-19 and/or IL-24 appear to act on CD4+ T cells by downregulating antigen-specific Th1 and Th17 responses and upregulating Th2, Th9, and Tr1 responses in filarial infection.
Figure 1.
Altered frequencies of CD4+ T-helper type 1 (Th1), Th17, Th22, Th2, Th9, and Tr1 cells following neutralization of interleukin 10 (IL-10), interleukin 19 (IL-19), and interleukin 24 (IL-24). Frequencies of CD4+ Th1, Th2, Th9, Th17, Th22, and Tr1 cells stimulated by saline extracts of Brugia malayi adult worms were measured by flow cytometry following neutralization of IL-10 (A), IL-19 (B), IL-24 (C), or isotype control antibody in 26 filariae-infected individuals. The data are represented as line diagrams, with each line representing a single individual. The shaded portion represents the upper threshold for the frequencies in uninfected subjects, and each triangle represents a single individual. P values were calculated by the Wilcoxon signed rank test, followed by the Holm correction. Abbreviations: IFN-γ, interferon γ; IgG2B, immunoglobulin G2B; IL-2, interleukin 2; IL-4, interleukin 4; IL-5, interleukin 5; IL-9, interleukin 9; IL-10, interleukin 10; IL-13, interleukin 13; IL-17, interleukin 17; IL-22, interleukin 22; TNF-α, tumor necrosis factor α.
Regulation of CD8+ T-Cell Subsets by IL-10, IL-19, and IL-24
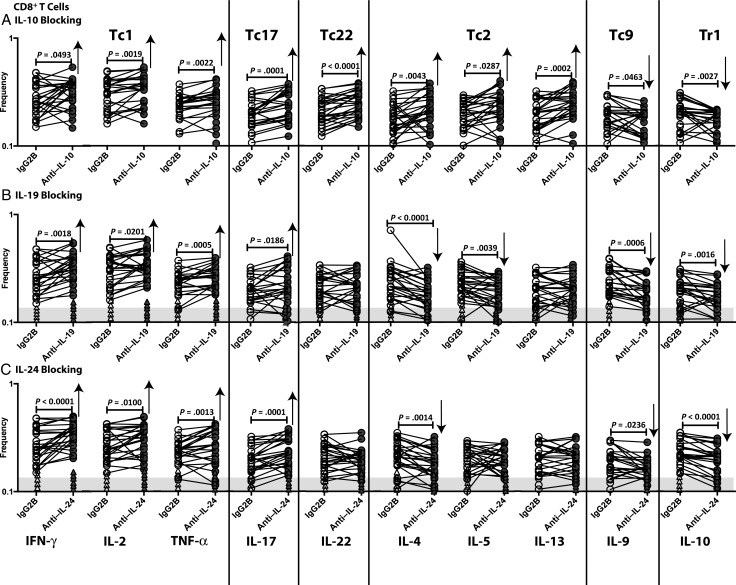
To examine the effect of IL-19 and IL-24 (as well as IL-10) on CD8+ T cells in filarial infections, we measured the frequencies of Tc1 (IFN-γ–, TNF-α–, or IL-2–expressing), Tc17 (IL-17–expressing), Tc22 (IL-22–expressing), Tc2 (IL-4–, IL-5–, or IL-13–expressing), Tc9 (IL-9–expressing), and Tr1 (IL-10–expressing but not IFN-γ– and IL-4–expressing) CD8+ T cells following neutralization of IL-10, IL-19, or IL-24 and stimulation with BmA in 26 filariae-infected individuals. As shown in Figure 2A, IL-10 neutralization resulted in significantly increased frequencies of Tc1, Tc17, Tc22, and Tc2 cells and significantly decreased frequencies of Tc9 and Tr1 cells. As shown in Figure 2B, IL-19 neutralization resulted in significantly increased frequencies of Tc1 and Tc17 cells and decreased frequencies of Tc2 and Tr1 cells. As shown in Figure 2C, IL-24 neutralization resulted in significantly increased frequencies of Tc1 and Tc17 cells and significantly decreased frequencies of Tc2, Tc9, and Tr1 cells. In addition, IL-19 or IL-24 neutralization had no significant effect on CD8+ T-cell frequencies in response to BmA in filariae-uninfected individuals. Also, neutralization of IL-19 or IL-24 in unstimulated samples had no effect on CD8+ T-cell frequencies (data not shown). Thus, IL-19 and IL-24 both appear to act on CD8+ T cells by downregulating Tc1 and Tc17 responses and upregulating Tc2, Tc9, and Tr1 responses in filarial infection.
Figure 2.
Altered frequencies of CD8+ Tc1, Tc17, Tc22, Tc2, Tc9, and Tr1 cells following neutralization of interleukin 10 (IL-10), interleukin 19 (IL-19), and interleukin 24 (IL-24). Frequencies of CD8+ Tc1, Tc17, Tc22, Tc2, Tc9, and Tr1 cells stimulated by saline extracts of Brugia malayi adult worms were measured by flow cytometry following neutralization of IL-10 (A), IL-19 (B), IL-24 (C), or isotype control antibody in 26 filariae-infected individuals. The data are represented as line diagrams, with each line representing a single individual. The shaded portion represents the upper threshold for the frequencies in uninfected subjects, and each triangle represents a single individual. P values were calculated by the Wilcoxon signed rank test, followed by the Holm correction. Abbreviations: IFN-γ, interferon γ; IgG2B, immunoglobulin G2B; IL-2, interleukin 2; IL-4, interleukin 4; IL-5, interleukin 5; IL-9, interleukin 9; IL-10, interleukin 10; IL-13, interleukin 13; IL-17, interleukin 17; IL-22, interleukin 22; TNF-α, tumor necrosis factor α.
DISCUSSION
Although the role of IL-22 in host defense during infection with extracellular pathogens is well established [1], very little is known about the contribution of the other IL-20 subfamily cytokines to host resistance during infection. We have previously reported that the frequencies of CD4+ and CD8+ T cells expressing IL-19, IL-24, and IL-26 are expanded in filarial infections [7], and others have shown increased IL-19 production by dendritic cells in Klebsiella pneumoniae infection [8]. Moreover, IL-19, IL-20, and IL-24 were shown to be induced in response to Staphylococcus aureus infection [9], in which these cytokines potently inhibit the production of interleukin 1β and IL-17, resulting in increased susceptibility and a greater severity of infection [9]. In terms of the mode of action, signaling through IL-20R2 has been shown to downregulate antigen-specific T-cell responses in mice [5], and IL-19, IL-20, and IL-24 have been shown to depress IFN-γ expression and enhance IL-4 and IL-13 expression in human T cells in vitro [4]. Thus, it has been assumed that the IL-20 subfamily of cytokines could potentially play an antiinflammatory role similar to that of the parent cytokine, IL-10 [2].
In this study, we provide definitive evidence for a modulatory role for 2 of the IL-20 subfamily members: IL-19 and IL-24. Our findings confirm the potent role played by IL-10 in filarial infection by demonstrating its effect on all the subsets of both CD4+ and CD8+ T cells responding to parasite antigen. Thus, IL-10 clearly down-modulates the Th1/Tc1, Th2/Tc2, Th17/Tc17, and Th22/Tc22 arms of T-cell subsets responding to parasite antigen, while facilitating the expansion of Th9/Tc9 and Tr1 cells. Although the influence of IL-10 in T-cell responses in helminth infections have been examined previously [6], this study, to our knowledge, is the most comprehensive examination of all currently known subsets of CD4+ and CD8+ T cells in a helminth infection.
In addition, our data also reveal a novel role for both IL-19 and IL-24 in human helminth infections. Examination of antigen-specific responses clearly reveals a major effect of both IL-19 and IL-24 in modulating T-cell responses in filarial infections. Both IL-19 and IL-24 downregulate Th1/Tc1 responses in filariae-infected individuals but not those in uninfected individuals. Similarly, both cytokines appear to also inhibit Tc1 responses and, to a lesser extent, Th1 responses. IL-19 is known to be upregulated in macrophages and T cells and lessens inflammation by suppressing the production of TNF-α, IL-6, and IL-12 [10]. Moreover, IL-19–deficient mice are susceptible to experimental colitis induced by dextran sodium sulfate, a disease characterized by excessive inflammatory responses [11]. Our data expand on these antiinflammatory effects of IL-19 by demonstrating that IL-19 can directly inhibit Th1/Tc1 and Th17/Tc17 responses and thereby promote antiinflammatory host responses. The role of IL-24 in modulating T-cell responses has not been explored in detail. Our study suggests that IL-24 behaves similarly to IL-19 in terms of downregulating both Th1/Tc1 and Th17/Tc17 responses. Interestingly, neither IL-19 nor IL-24 had any effect on Th22/Tc22 responses.
Our data also show that IL-19 has an important effect on Th2/Tc2 induction, since IL-19 blockade resulted in significantly diminished Th2/Tc2 responses. IL-19 has been shown to induce Th2 responses and to be present in elevated levels in Th2-dominant conditions, such as asthma [12]. Our study expands on these findings and reveals an important role for IL-19 in regulating Th2/Tc2 responses in filarial infections, as well. Finally, our findings also reveal a novel but important role for IL-19 and IL-24 in promoting Th9/Tc9 and Tr1 responses. Since both these responses appear to modulate resistance to filarial infections [13, 14], our data define IL-19 and IL-24 as 2 additional important regulatory cytokines modulating the frequency of T-cell subsets in this infection. The future exploration of the mechanism by which these cytokines modulate T-cell responses could provide major insight into the role of this relatively understudied family of cytokines in infectious diseases.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank the staff of the Department of Epidemiology, NIRT, for valuable assistance in recruiting the patients for this study; and M. Satiswaran, J. Jeevan, Prabbu Balakrishnan, Pavan Kumar, Yukti Bhootra, and Jovvian George of the NIH-ICER, for technical assistance.
Financial support. This work was supported by the Intramural Research Program of the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol 2011; 29:71–109. [DOI] [PubMed] [Google Scholar]
- 2.Sabat R. IL-10 family of cytokines. Cytokine Growth Factor Rev 2010; 21:315–24. [DOI] [PubMed] [Google Scholar]
- 3.Wolk K, Kunz S, Asadullah K, Sabat R. Cutting edge: immune cells as sources and targets of the IL-10 family members? J Immunol 2002; 168:5397–402. [DOI] [PubMed] [Google Scholar]
- 4.Oral HB, Kotenko SV, Yilmaz M et al. . Regulation of T cells and cytokines by the interleukin-10 (IL-10)-family cytokines IL-19, IL-20, IL-22, IL-24 and IL-26. Eur J Immunol 2006; 36:380–8. [DOI] [PubMed] [Google Scholar]
- 5.Wahl C, Muller W, Leithauser F et al. . IL-20 receptor 2 signaling down-regulates antigen-specific T cell responses. J Immunol 2009; 182:802–10. [DOI] [PubMed] [Google Scholar]
- 6.Babu S, Nutman TB. Immunology of lymphatic filariasis. Parasite Immunol 2014; 36:338–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anuradha R, George PJ, Hanna LE et al. . Expansion of parasite-specific CD4+ and CD8+ T cells expressing IL-10 superfamily cytokine members and their regulation in human lymphatic filariasis. PLoS Negl Trop Dis 2014; 8:e2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hackstein H, Kranz S, Lippitsch A et al. . Modulation of respiratory dendritic cells during Klebsiella pneumonia infection. Respir Res 2013; 14:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Myles IA, Fontecilla NM, Valdez PA et al. . Signaling via the IL-20 receptor inhibits cutaneous production of IL-1beta and IL-17A to promote infection with methicillin-resistant Staphylococcus aureus. Nat Immunol 2013; 14:804–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azuma YT, Nakajima H, Takeuchi T. IL-19 as a potential therapeutic in autoimmune and inflammatory diseases. Curr Pharm Des 2011; 17:3776–80. [DOI] [PubMed] [Google Scholar]
- 11.Azuma YT, Matsuo Y, Kuwamura M et al. . Interleukin-19 protects mice from innate-mediated colonic inflammation. Inflamm Bowel Dis 2010; 16:1017–28. [DOI] [PubMed] [Google Scholar]
- 12.Liao SC, Cheng YC, Wang YC et al. . IL-19 induced Th2 cytokines and was up-regulated in asthma patients. J Immunol 2004; 173:6712–8. [DOI] [PubMed] [Google Scholar]
- 13.Anuradha R, George PJ, Hanna LE et al. . IL-4-, TGF-beta-, and IL-1-dependent expansion of parasite antigen-specific Th9 cells is associated with clinical pathology in human lymphatic filariasis. J Immunol 2013; 191:2466–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Metenou S, Dembele B, Konate S et al. . At homeostasis filarial infections have expanded adaptive T regulatory but not classical Th2 cells. J Immunol 2010; 184:5375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.