Abstract
The aging of the human immunodeficiency virus type 1 (HIV-1)–infected population obligates a focus on the interaction between aging, comorbid conditions, and HIV-1. We recruited a cohort of HIV-1–infected men aged ≤35 years or ≥50 years who were receiving fully suppressive antiretroviral therapy (ART). We analyzed plasma markers of inflammation; T-cell activation, exhaustion, proliferation; and innate cellular subsets and functional capacity. Levels of lipopolysaccharide and the plasma marker of chemokine (C-C motif) ligand 2 were significantly elevated in older HIV-infected men despite comparable cellular phenotypes. Compared with similarly age-stratified uninfected subjects, older HIV-1–infected adults were also more frequently in the upper quartile of soluble CD14 expression.
Keywords: HIV-1, inflammation, monocytes, chemokine
Human immunodeficiency virus type 1 (HIV-1) infection has been associated with increased risk of cardiovascular disease, non–AIDS-related cancers, frailty, osteoporosis, liver and kidney disease, and neurologic dysfunction [1]. The similarity of this clinical phenotype to that among older individuals without HIV-1 infection raises questions about the epidemiologic and biologic factors that contribute to earlier onset of these clinical conditions in the HIV-infected population. Age-associated noncommunicable comorbidities are more prevalent in the HIV-1–infected population, compared with uninfected controls. In persons with cardiovascular disease, HIV-1 infection has been associated with an increase in surrogate markers of disease and in clinical events across multiple cohorts [2]. Although traditional cardiovascular risk factors such as smoking are generally more prevalent in HIV-1–infected individuals, these factors alone do not account for the excess risk of cardiovascular events [3]. A broad consensus supports the association of immune activation with morbidity and mortality among people with HIV-1 infection [4], as well as a critical interaction between inflammation and traditional cardiac risk factors [5]. The parallels between phenotypes of cardiovascular and other comorbid diseases during aging and associated phenotypes during chronic HIV-1 infection suggest that inflammation and immune dysfunction/senescence is a common pathway to these outcomes.
To identify etiologic pathways and potential therapeutic targets responsible for excess morbidity and mortality in treated HIV-1 infection, we evaluated early signs of immune senescence and aging. We recruited a cohort of HIV-1–infected individuals in 2 age-stratified groups and performed detailed phenotypic and functional analyses to identify signatures of aberrant immune activation, to suggest targets for therapeutic intervention.
METHODS
Study Subjects
Male HIV-positive subjects with ≥1 year of combination antiretroviral therapy (ART)–associated viral suppression were enrolled prospectively in groups aged ≤35 years (n = 22) or ≥50 years (n = 23) from infectious diseases clinics at 3 Boston hospitals. Demographically similar HIV-1–uninfected men in both age groups (n = 45) were enrolled from a cohort of men at high risk for HIV infection, as previously described (Supplementary Methods). Study protocols were approved by the institutional review boards at each hospital.
Plasma Inflammatory Profile
Plasma samples were analyzed for interferon α (IFN-α), IFN-γ–induced protein 10, CCL2 (also known as monocyte chemotactic protein 1), macrophage inflammatory protein 1β, tumor necrosis factor α (TNF-α), IFN-γ, interleukin 1β (IL-1β), interleukin 6, and interleukin 7 (Milliplex) on the Luminex 3D instrument (Bioplex 3D, BioRad). Lipopolysaccharide (LPS) levels were measured with the LAL assay (Pierce), and soluble CD163 (sCD163; Trillium Diagnostics), sCD14 (R&D), intestinal fatty acid–binding protein (Hycult), and CCL2 (Millipore) were measured by enzyme-linked immunosorbent assay (ELISA).
CCL2 Genotype and Messenger RNA (mRNA) Expression
DNA was extracted from peripheral blood mononuclear cells (PBMCs; AllPrep kit, Qiagen), and the CCL2 -2518A→G single-nucleotide polymorphism was assigned on the basis of restriction digestion findings [6]. Total RNA was extracted (RNeasy Plus, Qiagen), and quantitative real-time PCR (QuantiFast SYBR Green real-time PCR kit), using previously validated primers for CCL2 and Quantitect primers for β-actin, was performed with amplified complementary DNA detection on the LightCycler 480 instrument (Roche; Supplementary Methods).
Proviral DNA and Cell-Associated RNA Levels
Total HIV-1 proviral DNA and cell-associated RNA (ca-RNA) were quantified from isolated PBMCs by using quantitative PCR and quantitative real-time PCR assays as previously described on the Applied Biosystems 7300 real-time PCR system (Life Technologies; Supplementary Methods).
Statistical Analysis
Flow cytometry data were analyzed using nonparametric Mann–Whitney testing. For plasma levels of sCD14, sCD163, and CCL2 in the full cohort of infected and uninfected individuals, quartiles were determined, and odds ratios (ORs) for the highest quartile were calculated using contingency tables and χ2 testing. Quantitative real-time PCR data for CCL2 were analyzed as CCL2 expression relative to β-actin expression. A 2-log10 statistical outlier was excluded from the final analysis, identified as an outlier by ROUT with a Q value of 0.1% (Prism, GraphPad), and comparison between groups was determined by Mann–Whitney testing.
RESULTS
Clinical Characteristics of Subjects With HIV-1 Infection
HIV-1–infected men were recruited into one of 2 age groups, ≤35 years and ≥50 years (Supplementary Table 1), matched by duration of ART (within 5 years) and clinical characteristics, including CD4+ T-cell count at ART initiation, pretreatment log viral load, and current smoking status. Both groups were predominantly cytomegalovirus (CMV) seropositive (≥90%). The HIV-1–negative comparators were healthy male subjects with a self-reported elevated risk for HIV-1 acquisition.
Plasma Profile of Inflammatory Cytokines
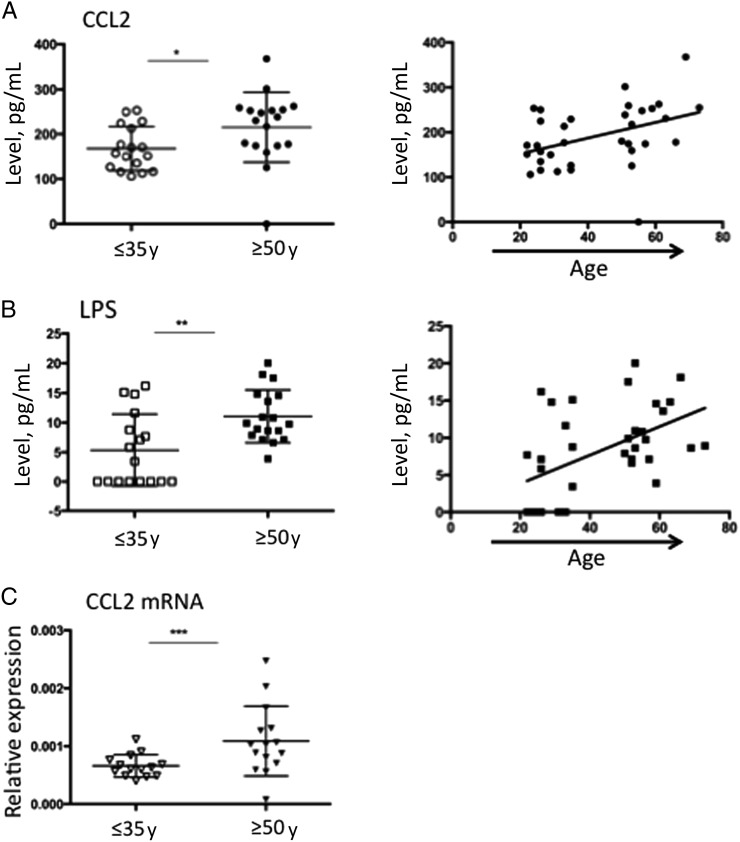
Multiplex analysis of plasma specimens for inflammatory cytokines identified significant elevations in CCL2 levels (Figure 1A). LPS levels were likewise elevated in the older age stratum (Figure 1B). Both of these measures also showed a correlation with age as a continuous variable (Figure 1A and 1B). We further verified the elevation in CCL2 plasma levels in the older group by demonstrating elevated CCL2 mRNA expression in PBMCs matched to the plasma specimens (Figure 1C). We assessed for the -2518A→G polymorphism, which has been associated with higher CCL2 expression and a higher risk for inflammatory disease and atherosclerosis [6]. The G allele was present in 16 of 22 older subjects and 12 of 21 younger subjects and was not significantly associated with age (OR, 2.00; 95% confidence interval [CI], .56–7.2; P = .28, by the χ2 test) or with plasma CCL2 levels in the upper quartile (OR, 3.6; 95% CI, .7–19.3; P = .12, by the χ2 test), suggesting that the differences observed in this study are not due to an imbalance in genotypes between the groups. No other inflammatory marker showed a significant association with age.
Figure 1.
Analysis of plasma specimens to determine levels of inflammatory cytokines and markers of microbial translocation. Plasma samples from individuals aged ≤35 years or ≥50 years were analyzed for the expression of inflammatory cytokines, using the Luminex 3D instrument. Significant differences were identified for CCL2 (A), with higher levels of CCL2 in older individuals detected by dichotomous comparison (*P = .007, by the Mann–Whitney test) and a correlation with age as a continuous variable (Spearman r = 0.45; P = .007). Soluble CD163 (sCD163), sCD14, intestinal fatty acid–binding protein (FABP), and lipopolysaccharide (LPS) levels were also measured in plasma specimens. LPS (B) showed a significant relationship to age, with a significant difference between groups (**P = .005, by the Mann–Whitney test) and a correlation with age as a continuous variable (Spearman r = 0.53; P = .001). Bulk RNA from peripheral blood mononuclear cells (PBMCs; C) matched to the plasma samples was extracted, and the number of CCL2 messenger RNA (mRNA) copies, relative to that for β-actin was assessed, demonstrating significantly higher expression in the group aged ≥50 year (***P = .010, by the Mann–Whitney test).
Associated HIV-1 Virological Factors and Cellular Immune Markers
We investigated the virologic status of the cohort, determining levels of proviral DNA as a measure of reservoir size and levels of ca-RNA as an indicator of residual virus production and potential source of cellular stimulation. There were no significant differences in the proviral DNA level. However, there was a borderline increase in the ca-RNA level in the older stratum (OR, 5.1; 95% CI, .94–28.2; P = .045, by the χ2 test). We stratified by nadir CD4+ T-cell count of <350 cells/mm3 to assess the impact of advanced disease to CCL2 levels; there was still an increase in CCL2 protein and mRNA levels in older individuals within this subgroup (Supplementary Figure 1).
We sought to determine whether the differences in CCL2 levels were driven by different levels of cellular immune activation. There were no differences in CD4+ or CD8+ T-cell activation, proliferation, or exhaustion or in dendritic cell (both myeloid and plasmacytoid dendritic cells) or monocyte subset distribution (Supplementary Figure 2). Likewise, intracellular cytokine staining for innate cellular responses to a panel of stimulants did not identify any differences in functional capacity between the 2 age groups (Supplementary Figure 3).
Comparison of Age-Associated Differences From HIV-Uninfected Controls
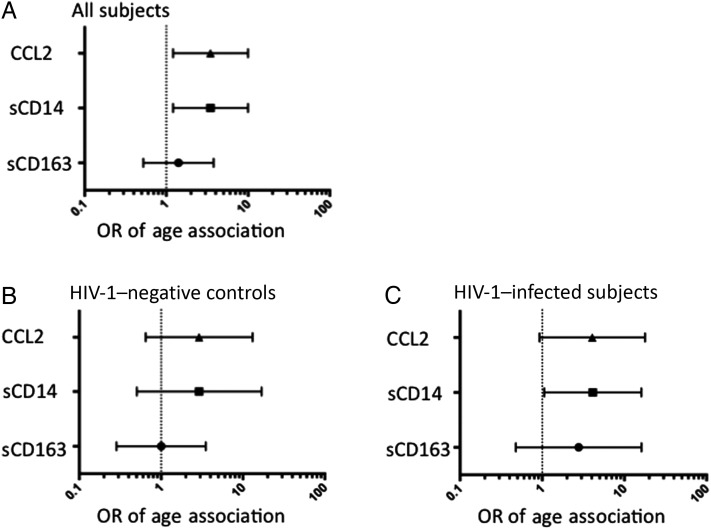
To determine whether the increase in levels of CCL2 and markers of microbial translocation is related to age alone or represents a feature of aging that is enhanced by HIV-1 infection, we measured levels of sCD14, sCD163, and CCL2 (by ELISA) in a group of demographically similar uninfected control men. All values were used to determine median values and quartiles for each analyte, and ORs for values in the upper quartile were calculated. Taken together, older individuals had an elevated OR for upper quartile values of CCL2 and sCD14 (Figure 2A). To assess whether this association was driven primarily by age or HIV-1 infection, we then analyzed the data for age associations separately for the infected and uninfected groups. Uninfected individuals did not show a significant age association for any of the analytes (Figure 2B), whereas older HIV-1–infected individuals had an increased OR for sCD14 values in the upper quartile (Figure 2C). It is notable that the small sample size may have limited detection of age-associated relationships in both the HIV-infected and HIV-uninfected populations.
Figure 2.
Relationship of age and human immunodeficiency virus type 1 (HIV-1) infection to plasma markers of inflammation and microbial translocation. Plasma samples from the 2 groups of HIV-1–infected individuals (n = 44) and 2 groups of uninfected control subjects aged ≤35 years or ≥50 years (n = 45), were assessed for levels of CCL2, soluble CD14 (sCD14), and sCD163 (all by enzyme-linked immunosorbent assay). Median values and quartiles were determined for each analyte across the entire cohort. Data were analyzed to determine the odds ratio (OR) for values of each analyte in the upper quartile by contingency table for age. Overall, regardless of HIV status, individuals ≥50 years old had increased ORs for upper quartile values of CCL2 and sCD14 (OR for both analytes, 3.467; 95% confidence interval, 1.210–9.930; P = .0169, by the χ2 test; A). Analysis of uninfected subjects separately demonstrated no significant association of age with upper quartile values of any of the 3 analyte (B). HIV-1–infected individuals ≥50 years old maintained a higher OR for upper quartile values of sCD14 (OR, 4.125; 95% CI, 1.061–16.04; P = .0350, by the χ2 test; C).
DISCUSSION
We recruited a cohort of 2 groups of individuals who had suppressed HIV-1 levels and were separated by a minimum of 15 years to identify markers of HIV-related premature/accelerated aging at a potentially reversible stage. Confounders were minimized by excluding patients with hepatitis, recent infection, frequent viral blips, autoimmune disease, or current receipt of immunomodulatory therapy and by matching on smoking status, ART duration, and CMV serostatus. With this cohort, we uncovered an association between aging and increased levels of CCL2 and microbial translocation markers despite similar adaptive and innate immune cellular subsets and functional competence.
Significantly, CCL2 levels were elevated in the older age group despite comparable levels of traditional markers of immune senescence associated with aging in the uninfected population, including T-cell markers of differentiation and exhaustion and innate immune functional capacity. This suggests that CCL2 may be a more sensitive or earlier indicator of immune dysfunction and is consistent with prior work linking increased CCL2 expression with subclinical atherosclerosis, particularly when elevated in younger patients [7]. We verified this elevated plasma CCL2 level among older HIV-infected men with significantly greater CCL2 mRNA expression in bulk PBMCs and did not identify any association with the G polymorphism or smoking status. Taken together, these observations suggest that HIV-1 infection is driving increased expression of CCL2.
In our age-stratified cohort, we also observed increased levels of markers of microbial translocation (LPS and sCD14) in the older age stratum, suggesting that copathogen burden may be a critical factor in driving the elevated levels of CCL2. Microbial translocation is a driver of persistent inflammation in HIV infection [8], and it is notable that increased microbial translocation has also been identified in animal models of aging [9]. These findings raise the possibility of synergism between HIV-1–mediated gut injury and aging as a common inflammatory pathway to increase exposure to microbial products and stimulate CCL2 production, leading to morbidity.
The role of CCL2 in disease is complex: it is produced by a variety of cell types, predominantly monocytes/macrophages; it is a potent regulator of migration and local infiltration of monocytes, memory T cells, and natural killer cells; and its activity is balanced by expression of decoy receptors at sites of inflammation. Recruitment of inflammatory cells is critical to pathogen responses but can also be detrimental in the setting of pathologic inflammation. CCL2 production can be stimulated by a variety of signals, including cytokines (TNF-α and IL-1β), oxidative stress, growth factors, and Toll-like receptor agonists. Elevated plasma levels and genetic polymorphisms influencing expression have been associated with several disease processes [10], including cardiovascular disease, in which CCL2 recruits monocytes that become lipid-laden foam cells at sites of atherosclerotic plaque [6]. CCL2 levels have also been associated with neuronal degeneration, inflammatory bowel disease, asthma, and nephropathy [10].
In HIV-1 infection, the dual roles of CCL2 as both critical in pathogen responses [11] and detrimental through promoting inflammatory disease [12] are recapitulated. Genotypic variants that increase expression of CCL2 are associated with resistance to HIV-1 infection. However, once HIV-1 infection is established, an elevated CCL2 level may contribute to inflammatory sequelae and has been linked to elevated coronary artery calcium scores [13]. The increased levels of CCL2 described in this cohort suggest that elevated levels may be contributing to morbidity and inflammatory pathology in aging patients.
The data from this cohort also suggest that the stimulus for CCL2 production may be an ongoing inflammatory response to microbial translocation. In contrast to previous studies with healthy populations with a more divergent age range [14], the innate functional capacity was comparable between our 2 age strata. Thus, the difference in CCL2 level appears to be most likely related to responses to an ongoing inflammatory stimulus, rather than baseline cellular dysfunction. These inflammatory stimuli may include residual HIV-1, coinfecting pathogens (eg, CMV or herpes simplex virus), or products of microbial translocation (eg, LPS), the latter of which shows significant differences even with the relatively narrow age spread in this cohort and offers a potential intervention point [15]. The weak association noted in this cohort between age and upper quartile values of ca-RNA, despite the lack of association with proviral DNA, raises the possibility that, in older individuals, there is less efficient control of integrated HIV-1 DNA and will be explored in future studies.
In summary, we identified higher plasma CCL2 levels in HIV-1–infected individuals aged ≥50 years, compared with levels in those ≤35 years of age. These increases were greater than those expected due to aging alone and were accompanied by increased levels of LPS and sCD14. This occurred despite similar immune cellular phenotypes and function. Given the multiple pathways leading to CCL2 production, further work is necessary to dissect which stimuli are the primary mediators of increased expression. The identification of early measurable changes in plasma markers prior to detectable cellular signs of functional impairment has important clinical implications. Identifying individuals with this early signature could potentially direct interventions aimed at disrupting the pathways leading to non–AIDS-related morbidity.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank all of the participants enrolled in the study; all of the human immunodeficiency virus physicians and nurses at Brigham and Women's Hospital, Massachusetts General Hospital, Beth Israel Deaconess Hospital, and Fenway Health, for referral of eligible participants for this cohort; Andrea Kershaw, NP, for her assistance in screening and enrolling of participants for this study from the infectious diseases outpatient clinic at the Beth Israel Deaconess Medical Center; and Martin Hirsch and Manish Sagar, for their critical review of the draft manuscript.
N. H. L. and E. S. served as the chief investigators. N. H. L. designed and implemented the clinical study, identified and enrolled the patient subgroups, and, together with E. S., designed, developed, and coordinated the experimental studies and interpreted the data. D. R. K. and M. A. provided scientific guidance, assistance with the experimental approaches, and clinical infrastructure for the clinical study. C. B., L. H., M. A. A., and Y. R. assisted with regulatory issues for the clinical cohort, patient recruitment, specimen collection, specimen processing, and analyses. A. L., M. R.-T., C. D. P., and L. H. performed the flow and immunological assays. E. S. performed the statistical analysis, with assistance and input from R. J. B. and N. H. L. N. H. L. and E. S. wrote and edited the manuscript.
Disclaimer. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (grant 5 K08 AI081545 to N. H. L.); the Harvard University Center for AIDS Research, a National Institutes of Health (NIH)–funded program (grant P30 A1060354; supplemental award to N. H. L.); and the Ragon Institute Immune Monitoring Core and NIH (grant P01 AI074415).
Potential conflicts of interest. N. H. L. has received grant support from Gilead Sciences and Merck for other studies. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med 2011; 62:141–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Currier JS, Lundgren JD, Carr A et al. Epidemiological evidence for cardiovascular disease in HIV-infected patients and relationship to highly active antiretroviral therapy. Circulation 2008; 118:e29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freiberg MS, Chang CC, Kuller LH et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med 2013; 173:614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lederman MM, Funderburg NT, Sekaly RP, Klatt NR, Hunt PW. Residual immune dysregulation syndrome in treated HIV infection. Adv Immunol 2013; 119:51–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsue PY, Deeks SG, Hunt PW. Immunologic basis of cardiovascular disease in HIV-infected adults. J Infect Dis 2012; 205(suppl 3):S375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joven J, Coll B, Tous M et al. The influence of HIV infection on the correlation between plasma concentrations of monocyte chemoattractant protein-1 and carotid atherosclerosis. Clin Chim Acta 2006; 368:114–9. [DOI] [PubMed] [Google Scholar]
- 7.Alonso-Villaverde C, Coll B, Parra S et al. Atherosclerosis in patients infected with HIV is influenced by a mutant monocyte chemoattractant protein-1 allele. Circulation 2004; 110:2204–9. [DOI] [PubMed] [Google Scholar]
- 8.Sandler NG, Wand H, Roque A et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis 2011; 203:780–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tran L, Greenwood-Van Meerveld B. Age-associated remodeling of the intestinal epithelial barrier. J Gerontol A Biol Sci Med Sci 2013; 68:1045–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res 2009; 29:313–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oliver BG, Elliott JH, Price P et al. Mediators of innate and adaptive immune responses differentially affect immune restoration disease associated with Mycobacterium tuberculosis in HIV patients beginning antiretroviral therapy. J Infect Dis 2010; 202:1728–37. [DOI] [PubMed] [Google Scholar]
- 12.O'Brien SJ, Moore JP. The effect of genetic variation in chemokines and their receptors on HIV transmission and progression to AIDS. Immunol Rev 2000; 177:99–111. [DOI] [PubMed] [Google Scholar]
- 13.Shikuma CM, Barbour JD, Ndhlovu LC et al. Plasma monocyte chemoattractant protein-1 and tumor necrosis factor-alpha levels predict the presence of coronary artery calcium in HIV-infected individuals independent of traditional cardiovascular risk factors. AIDS Res Hum Retroviruses 2014; 30:142–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Panda A, Qian F, Mohanty S et al. Age-associated decrease in TLR function in primary human dendritic cells predicts influenza vaccine response. J Immunol 2010; 184:2518–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sandler NG, Douek DC. Microbial translocation in HIV infection: causes, consequences and treatment opportunities. Nat Rev Microbiol 2012; 10:655–66. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.