Abstract
The foundation of modern neuroscience and psychology about intention for action was laid by Libet and his colleagues (Libet, B., et al, Brain 106: 623-42, 1983). They reported the time of awareness of wanting to move to be about 0.2 s before voluntary movement onset. However, despite repeated confirmation of the result, their method has been criticized for its dependence on self-reported timing and subjective memory and the interpretation has been widely debated without general consensus. Here, we show that the mean time of the conscious intention to move was 1.42 seconds before movement, estimated based on subjects’ real-time decision of whether or not there was a thought to move when a tone occurred. This event is after the onset of the Bereitschaftspotential, an electroencephalographic activity preceding voluntary movement, but about one second earlier than the timing of intention reported previously based on subject’s recall. Our result solves some problems of the conventional method, thus giving clearer answer to the controversies. The difference between the conventional result and our result suggests that the perception of intention rises through multiple levels of awareness, starting just after the brain initiates movement.
Keywords: Neurophysiology, Electroencephalography, Readiness potential, free will, point of no return
Introduction
In order to understand the cortical mechanism underlying voluntary movement, it is essential to know how and when we consciously think and plan the forthcoming movement and then execute it (Hallett, 2007). In this report, we operationally define the terms “intention” as the specific thought that you will be making the movement, and “movement genesis” as the brain process of making movement. The most renowned work on this topic was done by Libet et al (1983). They asked subjects to recall when they first felt they wanted to move their hands, based on the position of an observed fast-rotating clock. They found the time of intention (W time) to be about 0.2 s before the movement onset. This was a substantial time after the beginning of simultaneously recorded Bereitschaftspotential (BP), the slow negative shift preceding voluntary movement by as long as 2 s (Shibasaki & Hallett, 2006) observed in electroencephalogram (EEG). This result has been confirmed by several reports (Haggard & Eimer, 1999; Lau et al., 2004; Sirigu et al., 2004) showing similar W estimates (0.20--0.35 s), but the interpretation of the discrepancy has been controversial. In addition, a recent fMRI study showed that human intention can be traced back as long as 5 seconds before the action onset (Soon et al., 2008), making the discrepancy even larger. There has been no clear explanation why there is such a long gap between BP and fMRI onset and W.
There are three main concerns with Libet et al’s conventional paradigm, which make the interpretation of their result complicated:
-
i)
The method completely relies on subjective recall after the events, and therefore is susceptive to various biases such as retrospective construction (Dennett & Kinsbourne, 1992) and backward referral (Libet, 1985). It is also vulnerable to turbulence after the events, such as magnetic cortical stimulation (Lau et al., 2007).
-
ii)
The introduction of the clock causes additional preparation and latency times for the subject to read the clock position and memorize it (Rollman, 1985; Gomes, 2002).
-
iii)
It was not clear whether the experimental paradigm might have altered the mental process of voluntary behavior into a reaction triggered by the feeling of intention (Keller & Heckhausen, 1990).
Based on the above reasons, our study aimed specifically to present a new method to measure the timing of intention to move with minimal dependence on subjective recall, and to explore the relationship between intention and movement genesis. The subjects performed self-paced finger movement with explicit direction to react to their feeling of intention as quick as possible. They were not interrupted after each movement to avoid external start cues. Intermittent tones were applied randomly throughout the task and the subjects decided real-time, instead of post-hoc recall, whether or not there was an intention to move when a tone occurred. If there was already an intention at the time of tones, the subjects simply canceled the intended motion (see Figure 1A). The timing of tones and movements were all recorded, and the distribution of relative times between movements and tones was constructed. If the subject completely ignored the randomly presented tone, the distribution should be uniform before the movement onset. However, in this study design, tones that happened in a certain period before the movement onset would cause cancellation of the following action and would not contribute to the constructed tone distribution, making a dip in this otherwise uniform distribution. We tried to estimate the timing of intention from the shape of this distribution by two different approaches to ensure the reliability of the method. The hypothesis was that the recall-independent time of intention to move is earlier than the recall-dependent W time and close to but still later than the onset of BP recorded simultaneously. In order to answer the issue (iii) above, we also measured simple reaction times and calculated the correlation to the time of intention.
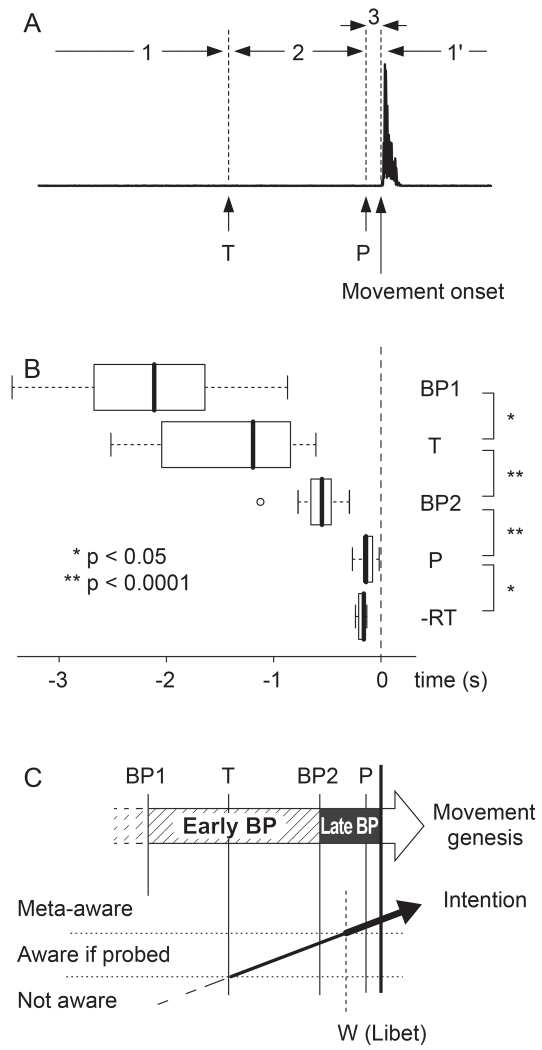
Figure 1.
Schematic diagrams of mental and physiological procedures proposed in this study. The three diagrams are shown in approximately the same time scale.
A: Illustration showing the T (onset of thought to move) and P (point of no return) time relationship in a single movement. The subjects were told to perform self-paced finger extension while tones were administered randomly. The solid horizontal line with a burst represents sample surface EMG waveform. Period 1: The subject was waiting. Period 2: The subject was thinking of and initiating the forthcoming movement. If there was a tone in this period the process was vetoed and no movement followed. Period 3: The subject could not stop his/her movement if he/she heard the tone in this period. Period 1’: The subject was again waiting and ignored any tones heard in this period.
B: Box-and-whisker plot of estimated times in all subjects. RT (simple reaction time) sign is shown as its negative. The boxes are aligned to the first and third quartile, and whiskers extend out to the most extreme data which is no more than 1.5 times the interquartile range from the box. The thick vertical line in the box denotes the median. An outlier is shown by an empty circle. BP1: Early BP onset. BP2: Late BP onset.
C: Proposed summary showing the relationship of the physiologically-determined movement genesis and the behaviorally-identified development of intention. The latter was described in the framework by Smallwood and Schooler (2006) with approximate W time by Libet et al (1983) added.
Materials and Methods
Subjects
This study was approved by the Institutional Review Board of the National Institute of Neurological Disorders and Stroke as part of protocol 02-N-0109 and conforms to The Code of Ethics of the World Medical Association (Declaration of Helsinki.) The experiments were undertaken with the understanding and written consent of each subject. 16 right-handed healthy volunteers (10 men and 6 women, age 42.2±13.0 (mean ± s.d.)) participated in the study. Handedness was screened by Edinburgh inventory (Oldfield, 1971) and described in Table 1.
Table 1.
Estimated results of all 15 subjects analyzed.
The first column denotes subject number. The second column shows handedness score. All other values are in seconds (s) and rounded to 2 digits below decimal point (also in the following tables). Subjects 5, 7 and 10 did not show identifiable LRP.
| T | P | RT | BP1 | BP2 | LRP | ||
|---|---|---|---|---|---|---|---|
| 1 | 100 | −2.52 | −0.22 | 0.21 | −0.87 | −0.56 | −0.70 |
| 2 | 90 | −2.42 | −0.15 | 0.16 | −1.62 | −0.77 | −0.57 |
| 3 | 80 | −2.21 | −0.06 | 0.14 | −1.34 | −0.29 | −0.22 |
| 4 | 80 | −2.17 | −0.11 | 0.14 | −1.65 | −0.53 | −0.75 |
| 5 | 100 | −1.92 | −0.16 | 0.24 | −1.91 | −0.34 | |
| 6 | 70 | −1.86 | −0.12 | 0.22 | −2.89 | −0.65 | −0.69 |
| 7 | 100 | −1.46 | −0.16 | 0.20 | −2.91 | −0.60 | |
| 8 | 100 | −1.19 | −0.16 | 0.14 | −2.11 | −0.52 | −0.95 |
| 9 | 100 | −0.98 | −0.06 | 0.19 | −2.37 | −0.66 | −0.62 |
| 10 | 100 | −0.97 | −0.02 | 0.13 | −1.93 | −0.44 | |
| 11 | 54 | −0.91 | −0.05 | 0.15 | −3.44 | −0.69 | −0.53 |
| 12 | 90 | −0.78 | −0.14 | 0.15 | −1.63 | −0.49 | −1.04 |
| 13 | 56 | −0.73 | −0.15 | 0.21 | −2.64 | −1.12 | −0.08 |
| 14 | 90 | −0.62 | −0.27 | 0.16 | −2.71 | −0.39 | −0.62 |
| 15 | 100 | −0.61 | −0.10 | 0.15 | −2.45 | −0.55 | −0.34 |
Recording
Subjects sat on a chair in a quiet room. Two loudspeakers 1 m in front of the subjects were used to apply 1 kHz tone bursts of 50 ms, with 5 ms rise and fall time, generated by a Grass S10 Click-Tone Control Module (Astro-Med. Inc., West Warwick, RI, USA). Nineteen tin EEG electrodes were placed over the scalp according to international 10-20 system using a cap (Electro-Cap International, Inc., Eaton, OH, USA) and two electrodes were placed on the left and right earlobes. Left earlobe electrode was used as the system reference and the data were later converted to digitally-linked earlobe reference. Surface electromyogram (EMG) was recorded from a pair of tin electrodes placed in the subject’s right forearm over the index finger extensor muscle (extensor digitorum communis). Electrooculogram was recorded to detect and reject blinks and large eye movements.
EEG, EMG, Electrooculogram and tone timing data were collected with Scan 4.3 system with SynAmps (Compumedics Limited, Abbotsford, VIC, Australia) as continuous data. Sampling rate was set to 1 kHz, with DC-200 Hz low-pass filter.
Task
Reaction time (RT) task
The tones were applied at pseudo-random intervals of 2 to 10 s. The subjects performed brisk right index finger extension as quick as possible every time they heard the tone. 60 responses with clear EMG onset were recorded in two sessions.
Veto task
The subjects performed self-paced index finger extension at intervals of 5 to 10 s. They were instructed to make brisk extensions as soon as the thought of the movement came to their mind, and not to count, keep time or think about the movement during the waiting interval. Tones were applied pseudo-randomly at intervals of 3 to 20 s, controlled by one of the investigators in a way not predictable by the subjects. They were instructed to ignore the tone when they heard it while they were not thinking about the next movement (period 1 in Figure 1A.) During the next period, they continued the self-paced movement. However, if they heard the tone after started thinking about the finger movement (period 2 in Figure 1A), they had to cancel (therefore, ‘veto’) the movement in progress and wait another 5 to 10 s interval before the next movement. They did not have to report each time if they vetoed the movement, since we did not want to prolong the experiment or complicate the self-paced movements by periodic oral reporting. When the tone was too close to the movement as in period 3, the subjects might not be able to stop the movement (“point of no return”). The tones that came after the movement (period 1’) were ignored. The actual written instruction given to the subjects also included instructions for blink control and relaxation as shown below. Each subject was asked to read the written instruction, and then verbal explanation was given as necessary. A brief practice session without and with tones was performed to help the subjects to understand the task and to enable brisk finger movements. During the practice session, the subjects were sometimes interrupted and asked if they were able to follow the instructions correctly.
Please quickly extend your index finger, following the instructions below:
-
a.)
The interval between your movements should be about 5-10 seconds. However, do not count or keep time. Just wait for a while. We will let you know if your intervals are too long or short. As soon as you think about the next movement, immediately extend your index finger as briskly as possible.
-
b.)
You will hear tones throughout the experiment. If you hear the tone while you are waiting and not thinking about the next movement, just ignore the tone and do your movement at your own will.
-
c.)
If you hear the tone after you have started thinking about the next movement or making the movement, stop the movement and relax. Wait for another 5-10 seconds without counting and make the next movement.
-
d.)
If you hear the tone after you have extended your finger, the tone should be ignored.
-
e.)
Refrain from blinking as long as possible. When you feel you must blink, stop the task and blink as many times as you want. Continue the task 5-10 seconds after blinking.
-
f.)
REMEMBER: Arm and hand muscles should be completely relaxed during the interval between movements.
Subjects performed the task in four separate sessions with short breaks in-between. At least 200 movements―characterized by brisk and clear EMG onset, sufficient muscle relaxation before the movement onset, and EEG without blink or muscle artifacts―were recorded. After each session and occasionally during the first session, the subjects were asked if they understood and followed the instructions correctly. Based on their report, those trials or sessions that the subject admitted they misunderstood or did not follow the instructions correctly were excluded from the analysis. The typical behaviors excluded were counting during intervals and failure to veto. Two (Subject 7 and 15 in Table 1) of them also reported that they moved their fingers in response to the tones, immediately afterwards or shortly after the tone, and we excluded such trials. One of the subjects could not perform the brisk and discrete finger movement in the practice session without tones and it was impossible to determine the precise timing of the movement onset. Therefore, this subject was excluded from further recording and analysis.
Analysis
All off-line analyses were performed using Matlab (Mathworks, Natick, MA, USA). Movement onsets were marked manually at the beginning of EMG burst.
Behavioral analysis
With the reaction time task data, the timing of the movement onset relative to each corresponding tone onset was accumulated and its mean value was calculated for each subject. With the veto task data, the timing of the tone onset relative to movement onset was accumulated across all marked movements. T time and P time were estimated from this distribution of relative tone timing using two methods below.
Sigmoid curve fitting
We assumed mental process timing jittered according to normal distribution across trials. A cumulative normal distribution function F(x) with scaling coefficients (p1, p2 and p3) was used to fit the distribution of the cue timing:
| (1) |
where erf(x) is an error function defined as
| (2) |
and x is the timing of tones relative to the movement onset. The operator at the left of erf is minus to estimate T and plus to estimate P. The parameters p1, p2 and p3 were estimated with least-square fitting by a Matlab function and p2was adopted for the estimated T and P (we call them T-s and P-s, respectively).
Density estimation
Smoothed tone distribution function was calculated with variable bandwidth kernel density estimation (Hall et al., 1995; Simonoff, 1996) using Gaussian kernel K(t) as follows:
| (3) |
where
| (4) |
and n is total number of tones, x is the time relative to the movement onset measured at 2.5 ms interval, Xi is the timing of the i-th tone, h0 is a global fixed bandwidth, and fp is a pilot distribution function. Density estimation was performed using fp equal to the average density of the whole analysis period and repeated twice with estimated used as the pilot function of the next iteration. Variability of estimation was obtained from 1000 bootstrap resamples. A sample size of n was drawn with replacement from the set of tone timing, and a density estimate was determined in the same way for each sample. The estimated density was recorded at each x and upper and lower 0.5% points were obtained as 99% variability interval (Simonoff, 1996). For T estimation, tone density distribution between −5.0 and +0.5 s relative to the movement onset was smoothed with h0 of 1.0 s. Baseline was determined as the average frequency of tones in the period of at least 1 s, in which the tone distribution reached plateau in each subject. This is supposed to be the tone frequency when the subject ignored all tones. T-d was estimated as the latest point earlier than −0.3 s, where the upper 0.5% of the variability exceeded the baseline (see second panels of Figure2A,B). For P time estimation, smoothed density and variability interval was estimated from the data between −1.0 and +2.0 s with h0 of 0.3 s. Baseline density was evaluated in 0.0 to 0.2 s range. P-d was estimated as the earliest point after −0.5 s, where the upper 0.5% of the variability exceeded the baseline frequency.
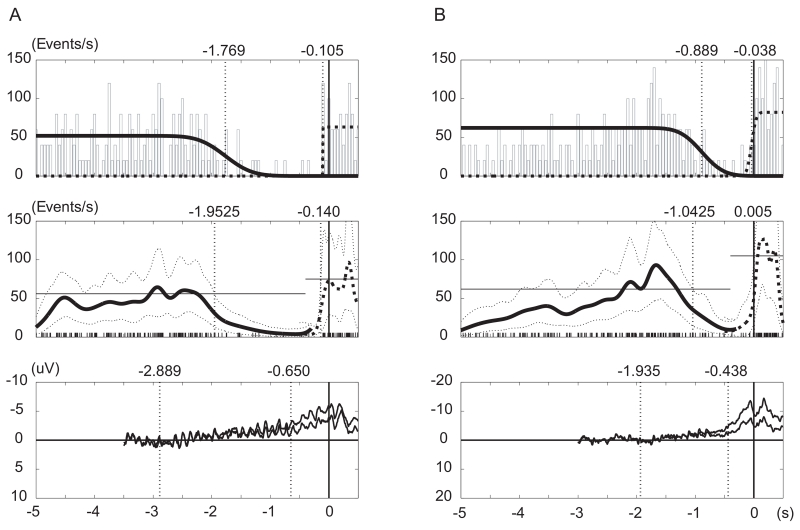
Figure 2.
The tone distribution, estimated density curves and BP waveforms of subject 6 (A) and subject 10 (B) in the Table 1. Upper panels: thin squares represent histogram of tone distribution relative to the movement onset. Vertical axis is scaled to number of tone events per second. Thick solid and dotted lines are fitted sigmoid curves for T and P time estimation, respectively. The estimates are shown by thin dotted vertical lines and numbers on the top. Middle panels: Short vertical lines at the bottom represent tone distribution. Thick solid and dotted lines are smoothed tone distribution functions for T and P time, respectively, calculated by variable bandwidth kernel density estimation. Thin dotted curves are upper and lower limit of 99% variability distribution. T and P are defined as the time upper 99% line crosses baseline level (thin horizontal line) and are shown by thin dotted vertical lines and numbers on the top. Lower panels: averaged BP waveforms recorded at C3 and CZ electrodes are superimposed. BP1 and BP2 are obtained as intersections among baseline and liner regression lines for early BP and late BP periods. Values at C3 and CZ electrodes are averaged and shown by thin dotted vertical lines and numbers on the top.
T-s and T-d were averaged to give an estimate of T time, and in the same way P-s and P-d to P time estimate.
BP analysis
EEG data during the veto task was first linearly detrended for the entire recording period or for the periods delimited by DC corrections, whichever was shorter. Then the data was averaged with an analysis window 2.5 s before the movement onset to 0.5 s after the movement onset. The first 0.5 s of the analysis window was used as the baseline. The analysis window could be extended up to 5 s before the movement onset so that the measured response onset was not included in the baseline. Epochs that fulfilled one or more of the following criteria were excluded from the analysis:
-
-
Eye blinks within the analysis window
-
-
EEG amplitude more than 200 μV peak to peak within the analysis window
-
-
EMG amplitude more than 30 μV peak to peak within the analysis window. Some subjects could not maintain complete relaxation of hand muscles and therefore 50 or 100 μV threshold was used instead.
-
-
When there were one or more tones in the period between −0.5 s before the beginning of analysis window and the movement onset.
We adopted relatively lenient criteria for artifact rejection, but due to the last criterion the effective number of epochs that fulfilled all the criteria above is significantly smaller than the number of trials recorded, ranging from 35 to 145 per subject, on average 79 epochs.
BP1 was measured as the time when the linear regression line of the early slow negative shift crossed zero. BP2 was measured as the time when the regression line of the late negative shift crossed the regression line of the preceding slope. These onset latencies were measured at C3 and Cz electrodes separately and then averaged.
Lateralized Readiness Potential (LRP) was measured by subtracting the averaged waveform at C4 electrode from that at C3 electrode during right index finger movement. The subtracted waveform was linearly detrended using the early BP period as baseline to compensate for possible asymmetry of electrode location and then the zero-crossing point of the regression line to the pre-movement negative slope was calculated as LRP onset. It has to be noted that our task includes right hand movement only, and therefore is not comparable to the authentic LRP, which used both left hand movement and right hand movement data to account for anatomical asymmetry.
Statistical evaluation
All statistical analyses are performed with R software package (R Development Core Team, 2005).
Temporal order of the physiological and behavioral measures
By definition, BP1 always precedes BP2 and T always precedes P. Therefore, 2-tailed Wilcoxon signed rank test was performed for the following pairs: BP1 and T, T and BP2, BP2 and P, and P and RT expressed as its negative. Multiple measurement correction was performed based on Holm (Holm, 1979).
Linear correlation between the measures
Spearman’s correlation coefficient was calculated with all possible pairs between two groups: (RT, BP2 LRP and BP1) and (RT, P, and T). 95% confidence intervals (CI) were estimated using Fisher’s Z transformation.
Reliability Estimation
Equivalence of two estimation methods
The mean difference of T-d and T-s, two estimates of T time by density estimation and that by sigmoid fitting, was −0.062 s. This means T-d tended to be slightly earlier than T-s. T-d and T-s were not normally or log-normally distributed, and the 95% CI of the difference evaluated by 10,000 bootstrapping was −0.115 to −0.004 s, corresponding to 8.1% to 0.3% of the average T time. The 95% CI of P time mean difference (P-d – P-s) was −0.033 to −0.002 s (average −0.015 s, 25.3% to 1.6% of average P time.)
Reproducibility by repeated measurements
To evaluate reliability of this new method, six subjects agreed to visit our laboratory again at least 1 month after the first recording and performed the same task under the same instruction. Data acquisition and analysis were performed in the same way in the two recordings. Intraclass correlation coefficient (ICC) with one-way random effects model was calculated as follows for RT, T, P, LRP, BP1 and BP2 (Shrout & Fleiss, 1979; Fleiss, 1999).
BMS: between-subject mean square
WMS: within-subject mean square
k: number of observations (2 in this study)
ICC close to one means good reliability, whereas ICC close to or below zero indicates poor reliability.
Results
The time of intention to move, estimated as T time as in the Figure 1A, was −1.42 ± 0.69 s (mean ± s.d., n=15) relative to movement onset. We estimated the point of no return for self-paced movement, which was −0.13 ± 0.07 s (P time in Figure 1A). The onsets of the early and late components of BP (BP1 and BP2) were −2.17 ± 0.69 s and −0.57 ± 0.20 s. The RT for random interval tone stimuli was 0.17 ± 0.04 s. These results are displayed in a box plot in Figure 1B. The distribution of tone timing relative to movement onset, estimated and fitted density curves, and BP waveforms of two representative subjects analyzed are presented in Figure 2. Estimated results from all subjects are summarized in Table 1. To compare the order of these events, we performed pair-wise comparisons (Figure 1B). T time significantly preceded BP2 (p= 3.5×10−5, corrected for multiple comparisons) and BP2 significantly preceded P (p = 5.2×10−8, corrected). The comparison of BP1 and T was minimally significant (p = 0.023, corrected), with four of the 15 subjects having a T earlier than BP1. P vs. RT (in negative sign) was also minimally significant (p = 0.045, corrected). We also measured the LRP (Eimer, 1998) using right-hand movement only and found its onset to be −0.59 ± 0.28 s (Table 1).
Correlation analyses among these estimated times across subjects revealed the strongest correlation to be a negative one of −0.60 (95% CI: −0.85 -- −0.13, uncorrected) between T and BP1 (Spearman’s rank correlation coefficient, Table 2). P and RT also showed negative correlation of −0.56 (95% CI: −0.84 -- −0.07, uncorrected). All other combinations had correlation coefficients between −0.4 and +0.4.
Table 2.
Spearman’s rank correlation coefficients and their 95% confidence intervals (not corrected for multiple comparisons).
| T | P | RT | |
|---|---|---|---|
| BP1 | −0.60 (−0.85~−0.13) |
−0.07 (−0.56~0.46) |
−0.21 (−0.65~0.33) |
| BP2 | −0.04 (−0.54~0.49) |
−0.11 (−0.59~0.42) |
−0.40 (−0.76~0.15) |
| LRP | 0.24 (−0.38~0.72) |
0.29 (−0.34~0.74) |
0.04 (−0.55~0.60) |
| RT | −0.14 (−0.61~0.40) |
−0.56 (−0.84~−0.07) |
- |
The reproducibility of the times T and P was estimated by repeated measurements in six subjects. The average T and P times of the first visit were −1.40 s and −0.13 s and those of the second visit were −1.44 s and −0.13 s, respectively (Table 3). Despite these good agreements of average estimates, ICC of the estimated timings are all below 0.4, except for RT which showed ICC of 0.92 (Table 3).
Table 3.
Estimated results of 6 subjects from their second recordings. The subject numbers in the first column correspond to those in the Table 1. The bottom row shows ICC calculated from the first and second recordings of these 6 subjects.
| T | P | RT | BP1 | BP2 | LRP | |
|---|---|---|---|---|---|---|
| 4 | −2.50 | −0.09 | 0.14 | −3.47 | −0.53 | −0.18 |
| 6 | −0.98 | −0.13 | 0.22 | −1.76 | −0.54 | −0.14 |
| 7 | −1.08 | −0.05 | 0.20 | −2.34 | −0.19 | −0.45 |
| 8 | −1.28 | −0.18 | 0.14 | −1.10 | −0.27 | −0.12 |
| 9 | −0.80 | −0.17 | 0.16 | −2.42 | −0.34 | −1.26 |
| 13 | −2.01 | −0.17 | 0.22 | −4.16 | −0.52 | −0.32 |
|
| ||||||
| ICC | 0.35 | −0.11 | 0.92 | −0.01 | −0.07 | 0.02 |
Discussion
The relationship between components of the BP and the time of intention, estimated as T time, are of central interest. We have shown that, statistically, the onset of BP1 is earlier than T; however, some of the subjects had a time T that preceded BP1 onset, suggesting that BP onset does not relate directly to the thought of movement initiation. The lack of positive correlation between T and BP1 also puts the causal relationship into doubt. Haggard and Eimer reported that the LRP onset correlated with and preceded Libet’s W time (Haggard & Eimer, 1999). Their LRP onset was −0.9 to −0.7 s, slightly earlier than our LRP measurement, but still later than the T time and therefore the physiology underlying it cannot be causal for T.
Although the time estimates were reproducible across repeated measurements as a whole, the low ICC suggests large day-to-day fluctuations in subject performance. This may be due to inherent physiological and psychological variability that is not yet well understood physiologically, such as variable degree of attention to the task and sleepiness.
When compared to the values in previously reported studies, our T time is more than one second earlier than the reported W time in Libet-style clock studies (Haggard & Eimer, 1999; Lau et al., 2004; Sirigu et al., 2004). Other parameters are consistent with previous results: P time was similar or slightly shorter than stop signal RT (reported 0.15 to 0.25 s) (Logan et al., 1984; De Jong et al., 1990; De Jong et al., 1995). Simple RT to auditory stimuli was reported to be around 0.2 to 0.25 s (Gordon, 1967; Wagner et al., 2004) and consistent with our result taking into account the electromechanical delay. BP1 and BP2 are similar but slightly earlier than those reported with simple self-paced movement as about 2 and 0.4 s, respectively (Shibasaki & Hallett, 2006).
The large difference between T and W times is not just a matter of different number. In the quest for the physiological correlate of intention, it requires further consideration. First, there are well established biases that may affect the estimated timing in general, such as flash-lag effect, prior entry bias and difference of modality specific sensory processing (Nijhawan, 1994; Eagleman & Sejnowski, 2000; Spence et al., 2001). They can each account for up to 100 ms and therefore may explain a small proportion of the difference. There are other proposed mechanisms that may add larger bias: backward referral and retrospective construction (Libet, 1982; Dennett & Kinsbourne, 1992). However, these mechanisms shift the perceived time of event to an earlier time than the true time of the event. As our estimated timings do not depend on the subject’s memory or self-estimation, such mechanisms do not explain why our T time, based on on-line processing, is so much earlier than W time, based on recall.
Second, there may have been an attentional refractory period after the subjects heard the tone, causing decreased attention as a whole including that for the attempt to move. There has not been a study precisely targeted to this type of refractory period in our task, but similar phenomena are reported and investigated intensively. In the case of two successive stimulus-response tasks with short stimulus onset asynchrony, the response to the second stimulus is delayed as compared with the same stimulus when presented alone. This is known as the psychological refractory period effect and the delay can be up to a few hundred ms (Pashler, 1994). When the first response is a no-go response, the delay is shorter (De Jong, 1993). Similarly, when two visual targets are presented successively at an interval of less than 0.5 s, the identification of the second target is impaired, a phenomenon called attentional blink. The mechanism of attentional blink is thought to be modality specific, at least partially, and cross-modal attentional blink has not been constantly observed (Marois & Ivanoff, 2005; Hein et al., 2006). The process responsible for psychological refractory period and AB may be partly involved in the process during our task of self-paced movement. The delay by these effects can be long because there is no second stimulus or target to facilitate attention in our task. Future studies might investigate such delays and explore this possible mechanism.
Third, the instruction of our paradigm was not the traditional “self-paced” movement. Rather, we asked the subject explicitly to react to their internal intention. This was designed to clarify criticism (iii) against the conventional method (Libet et al., 1983) as stated previously. If the task was transformed into a simple reaction task to the internal cue (the intention), then the T time should have been as late as or later than the W time and as close as RT to the movement onset. However, the result was the opposite, showing that the process in our paradigm is not a simple reaction to intention. The similarity of the recorded BP waveform to that of conventional self-paced movement paradigm suggests that the physiological process in the current paradigm may be comparable. Another deviation from classical self-paced movement is that the subjects may have had negative emotional reactions as their intentions were being frequently interrupted by the tones. Such subjects may have entered a ‘race game’ trying to complete the movement before the tone comes. Since this attitude is not a violation to our instruction, and such behavior would work to shorten our measurement and to diminish the difference, we did not discourage the subjects from doing so. However, the interpretation of BP should be made with caution. In the Libet et al. experiments, movements made “quickly” had a shorter duration of the BP.
Fourth, it is suggestive that the W time is close to our P time, the point of no return, rather than the T time, athough this may be just a coincidence of the two different methods. Instead of the intention onset, the W may indicate maturation of a complex process consisting of thought, decision and initiation of a movement ready for execution. This remains a point to be investigated more.
This discrepancy as discussed above can be explained by the similarity of the current task and a study of mind-wandering (Smallwood & Schooler, 2006). In the mind-wandering study, while subjects were continuously performing a simple task (such as reading a book), they either reported spontaneously when they realized their mind was wandering, or were intermittently interrupted and questioned by the investigator if their mind was wandering at that moment. The latter (probe-caught mind-wandering) was found more frequently than the former (self-monitored mind-wandering). The study implies that there were times when subjects were often not aware that their mind was wandering and they only became aware of it when they were alerted by the probes. Libet et al’s W is analogous to the self-monitored mind-wandering because the subjects spontaneously became aware of their own conscious experience (“meta-awareness”). On the other hand, the period between T and W may correspond to the probe-caught awareness, and before T time the probe failed to bring the awareness of one’s own movement genesis. According to this analogy, the process of voluntary movement in the current paradigm goes as follows (Figure 1C):
The first detected event in most subjects was the onset of BP. They were not aware of the movement genesis at this time, even if they were alerted by tones.
As the movement genesis progressed, the awareness state rose higher and after the T time, if the subjects were alerted, they could consciously access awareness of their movement genesis as intention. The late BP began within this period.
The awareness state rose even higher as the process went on, and at the W time it reached the level of meta-awareness without being probed. In Libet et al’s clock task, subjects could memorize the clock position at this time.
Shortly after that, the movement genesis reached its final point, after which the subjects could not veto the movement any more (P time).
Since we failed to show a positive correlation between physiological and behavioral indices, the relationship between them is not clear. Our result showed that the onset of BP appears to be independent of the time when the intention to move becomes detectable. The temporal order suggests that the late BP starts after T time and is not responsible for the formation of intention. It is thought to be closely related to movement genesis (Shibasaki & Hallett, 2006), but it is not the final stage of execution as one can still cancel the forthcoming movement at the time of BP2.
Although we tried to remove subjectivity from the estimate of intention, we still depend on the subject’s conscious behavior. For an extreme example, our estimates can be completely meaningless if the subjects told us lies and there are many strategies that may mimic our result. It is also possible that the subjects might have had some unconscious response or behavior to the tone signal, in a way we did not specify. The subjects may have unconsciously employed strategies simply not to move within certain periods after the tone, even though we explicitly told the subjects not to do so when they noticed such behavior themselves. However, the compatibility of our result with the previous studies and the reproducibility of the result across subjects with large variability within subject make us believe that our result is reliable.
Intention as examined here is not a thought that is planned or scheduled long before the action (i.e., intention generated when the subject agreed to participate in the study). We studied the immediate intention directly preceding the action. We think it best to understand movement genesis and intention as separate phenomena, both measurable. Movement genesis begins at a level beyond awareness and over time gradually becomes accessible to consciousness as the perception of intention. Determining the true onset of movement genesis is difficult. A recent fMRI study reported that the decision of when to move could be traced back to 5 seconds before the action in the supplementary motor area, and the decision of which side to move was detected up to 10 seconds before the movement in the prefrontal and medial parietal cortex (Soon et al., 2008). This is such a long time before one perceives his intention, that it is likely that the unconscious process of movement genesis fluctuates and may even often fade away without coming out as actual movements. The task in our study is not directed to such premature “intention” as mentioned before. Therefore, a complete physiological explanation of movement genesis still remains to be determined. EEG has the potential to give additional physiological information about it with excellent time resolution. The averaged BP, however, is just a measure of premotor and motor cortical activity (Shibasaki & Hallett, 2006) and scalp recording has limitations in its sensitivity. Hence better information about the mechanisms of movement genesis and brain correlates of the perception of intention is anticipated with improved tasks and other techniques of measuring brain activity.
Acknowledgements
We thank D. M. Eagleman (Baylor College of Medicine), A. R. Mele (Florida State University), P. Strick (University of Pittsburgh), and D. M. Wegner (Harvard University) for discussion and advice for the study; S. Pirio Richardson for discussion and critically reviewing the manuscript; N. Jeffries for advice and verification in statistical analysis; and S. Vorbach for technical assistance. This research was supported by the Intramural Research Program of the NIH, National Institute of Neurological Disorders and Stroke.
Abbreviations
- EEG
Electroencephalogram
- BP
Bereitschaftspotential
- BP1
Early Bereitschaftspotential onset
- BP2
Late Bereitschaftspotential onset
- EMG
Electromyogram
- RT
Reaction time
- LRP
Lateralized readiness potential
- CI
Confidence interval
- ICC
Intraclass correlation coefficient
- P time
Point of no return
- P-d
Point of no return determined by density estimation
- P-s
Point of no return estimated by sigmoid curve fitting
- T time
Time of thought to act
- T-d
Time of though to act determined by density estimation
- T-s
Time of though to act estimated by sigmoid curve fitting
- W time
Time of will to act by Libet’s method
References
- De Jong R. Multiple bottlenecks in overlapping task performance. J Exp Psychol Hum Percept Perform. 1993;19:965–980. doi: 10.1037//0096-1523.19.5.965. [DOI] [PubMed] [Google Scholar]
- De Jong R, Coles MG, Logan GD. Strategies and mechanisms in nonselective and selective inhibitory motor control. J Exp Psychol Hum Percept Perform. 1995;21:498–511. doi: 10.1037//0096-1523.21.3.498. [DOI] [PubMed] [Google Scholar]
- De Jong R, Coles MG, Logan GD, Gratton G. In search of the point of no return: the control of response processes. J Exp Psychol Hum Percept Perform. 1990;16:164–182. doi: 10.1037/0096-1523.16.1.164. [DOI] [PubMed] [Google Scholar]
- Dennett DC, Kinsbourne M. Time and the Observer - the Where and When of Consciousness in the Brain. Behav Brain Sci. 1992;15:183–201. [Google Scholar]
- Eagleman DM, Sejnowski TJ. Motion integration and postdiction in visual awareness. Science. 2000;287:2036–2038. doi: 10.1126/science.287.5460.2036. [DOI] [PubMed] [Google Scholar]
- Eimer M. The lateralized readiness potential as an on-line measure of central response activation processes. Behav Res Methods Instrum Comput. 1998;30:146–156. [Google Scholar]
- Fleiss JL. Reliability of Measurement Design and Analysis of Clinical Experiments. Wiley; New York: 1999. pp. 1–32. [Google Scholar]
- Gomes G. The interpretation of Libet’s results on the timing of conscious events: a commentary. Conscious Cogn. 2002;11:221–230. doi: 10.1006/ccog.2002.0556. discussion 308-213, 314-225. [DOI] [PubMed] [Google Scholar]
- Gordon IE. Stimulus probability and simple reaction time. Nature. 1967;215:895–896. doi: 10.1038/215895a0. [DOI] [PubMed] [Google Scholar]
- Haggard P, Eimer M. On the relation between brain potentials and the awareness of voluntary movements. Exp Brain Res. 1999;126:128–133. doi: 10.1007/s002210050722. [DOI] [PubMed] [Google Scholar]
- Hall P, Hu TC, Marron JS. Improved Variable Window Kernel Estimates of Probability Densities. Ann Statist. 1995;23:1–10. [Google Scholar]
- Hallett M. Volitional control of movement: the physiology of free will. Clin Neurophysiol. 2007;118:1179–1192. doi: 10.1016/j.clinph.2007.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein G, Parr A, Duncan J. Within-modality and cross-modality attentional blinks in a simple discrimination task. Percept Psychophys. 2006;68:54–61. doi: 10.3758/bf03193655. [DOI] [PubMed] [Google Scholar]
- Holm S. A Simple Sequentially Rejective Multiple Test Procedure. Scand J Statist. 1979;6:65–70. [Google Scholar]
- Keller I, Heckhausen H. Readiness potentials preceding spontaneous motor acts: voluntary vs. involuntary control. Electroencephalogr Clin Neurophysiol. 1990;76:351–361. doi: 10.1016/0013-4694(90)90036-j. [DOI] [PubMed] [Google Scholar]
- Lau HC, Rogers RD, Haggard P, Passingham RE. Attention to intention. Science. 2004;303:1208–1210. doi: 10.1126/science.1090973. [DOI] [PubMed] [Google Scholar]
- Lau HC, rogers RD, Passingham RE. Manipulating the Experienced Onset of Intention after Action Execution. J Cogn Neurosci. 2007;19:81–90. doi: 10.1162/jocn.2007.19.1.81. [DOI] [PubMed] [Google Scholar]
- Libet B. Brain stimulation in the study of neuronal functions for conscious sensory experiences. Hum Neurobiol. 1982;1:235–242. [PubMed] [Google Scholar]
- Libet B. Unconscious Cerebral Initiative and the Role of Conscious Will in Voluntary Action. Behav Brain Sci. 1985;8:529–566. [Google Scholar]
- Libet B, Gleason CA, Wright EW, Pearl DK. Time of conscious intention to act in relation to onset of cerebral activity (readiness-potential). The unconscious initiation of a freely voluntary act. Brain. 1983;106(Pt 3):623–642. doi: 10.1093/brain/106.3.623. [DOI] [PubMed] [Google Scholar]
- Logan GD, Cowan WB, Davis KA. On the ability to inhibit simple and choice reaction time responses: a model and a method. J Exp Psychol Hum Percept Perform. 1984;10:276–291. doi: 10.1037//0096-1523.10.2.276. [DOI] [PubMed] [Google Scholar]
- Marois R, Ivanoff J. Capacity limits of information processing in the brain. Trends Cogn Sci. 2005;9:296–305. doi: 10.1016/j.tics.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Nijhawan R. Motion extrapolation in catching. Nature. 1994;370:256–257. doi: 10.1038/370256b0. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pashler H. Dual-task interference in simple tasks: data and theory. Psychol Bull. 1994;116:220–244. doi: 10.1037/0033-2909.116.2.220. [DOI] [PubMed] [Google Scholar]
- R Development Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; 2005. [Google Scholar]
- Rollman GB. Sensory events with variable central latencies provide inaccurate clocks. Behav Brain Sci. 1985;8:551–552. [Google Scholar]
- Shibasaki H, Hallett M. What is the Bereitschaftspotential? Clin Neurophysiol. 2006 doi: 10.1016/j.clinph.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Shrout PE, Fleiss JL. Intraclass Correlations: Uses in Assessing Rater Reliability. Psychol Bull. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Simonoff JS. Smoothing Methods in Statistics. Springer; New York: 1996. [Google Scholar]
- Sirigu A, Daprati E, Ciancia S, Giraux P, Nighoghossian N, Posada A, Haggard P. Altered awareness of voluntary action after damage to the parietal cortex. Nat Neurosci. 2004;7:80–84. doi: 10.1038/nn1160. [DOI] [PubMed] [Google Scholar]
- Smallwood J, Schooler JW. The restless mind. Psychol Bull. 2006;132:946–958. doi: 10.1037/0033-2909.132.6.946. [DOI] [PubMed] [Google Scholar]
- Soon CS, Brass M, Heinze HJ, Haynes JD. Unconscious determinants of free decisions in the human brain. Nat Neurosci. 2008;11:543–545. doi: 10.1038/nn.2112. [DOI] [PubMed] [Google Scholar]
- Spence C, Shore DI, Klein RM. Multisensory prior entry. J Exp Psychol Gen. 2001;130:799–832. doi: 10.1037//0096-3445.130.4.799. [DOI] [PubMed] [Google Scholar]
- Wagner E, Florentine M, Buus S, McCormack J. Spectral loudness summation and simple reaction time. J Acoust Soc Am. 2004;116:1681–1686. doi: 10.1121/1.1780573. [DOI] [PubMed] [Google Scholar]