Abstract
Background
Injectable botulinum neurotoxin (BoNT) is the principal effective treatment for blepharospasm (BSP). This trial explores the safety and efficacy of topical Acetyl Hexapeptide-8 (AH8), a competitive SNAP25 inhibitor, as a potential new therapy in BSP.
Methods
Double-blind, placebo-controlled, randomized trial of daily topical application of AH8 in 24 patients with BSP. The primary outcome was time to return to baseline Jankovic Rating Scale (JBRS) after a BoNT injection simultaneously with initiation of AH8. Patients displaying a strictly regular pattern of response to 3-monthly injections of BoNT were included.
Results
There were no significant adverse events. There was a trend for longer time until return to baseline JBRS after injection in the active group compared to placebo (3.7 vs 3.0 months), and for better scores in the active group. One third (4/12) of the patients in the active group had a significant extension of symptom control after BoNT (range: 3.3-7.1 months).
Conclusions
Topical AH8 is safe and promising for extending the duration of action of BoNT therapy for BSP.
Keywords: Blepharospasm, therapy, topical, dystonia, botulinum
Introduction
Blepharospasm (BSP) is a focal dystonia affecting primarily the orbicularis oculi (OO) muscles [1]. Botulinum neurotoxin (BoNT) injection therapy is the principal effective therapy [2]. The mechanism of action involves interference with the formation of the SNARE complex, which mediates neurotransmitter exocytosis at the neuromuscular junction [3].
Acetyl hexapeptide-8 (AH8) is a topically applied hexapeptide that competitively inhibits the SNAP-25 component of SNARE, a mechanism analogous to BoNT type A [4]. It has been used successfully in cosmetic applications for wrinkles, where it works by relaxing superficial dermal muscles [4]. Given that the musculature implicated in BSP is superficial and within reach of the topical agent, we have studied AH8 in its first medical application for the treatment of BSP.
Methods
This is a double-blind, placebo-controlled, randomized trial of daily topical application of 0.005% AH8 in patients with BSP. Ninety-six patients were pre-screened, and only patients receiving BoNT therapy at regular 3-month intervals, with no change in the injection pattern for at least three previous treatments, were selected for inclusion.
The study was approved by the NIH Neuroscience IRB. All patients signed informed consent at the time of the screening visit. The study was FDA monitored, under IND registration number 105,646, clinicaltrials.gov identifier NCT00942851.
Emulsions containing 0.005% AH8 and a placebo preparation, consisting of an identically appearing cream of the same formulation without AH8 content, were prepared by BCN peptides and delivered in automated dispensing containers. The initial visit occurred at the time of a scheduled BoNT treatment. The Jankovic Blepharospasm Rating Scale (JBRS) [5] and the Blepharospasm Disability Scale (BDS) [6] were measured before the injection (nadir of the response cycle to BoNT).
After the evaluation and baseline scores recording, patients received BoNT injections in the same pattern as previously successfully employed. Twenty-three patients received onabotulinumtoxin A at doses ranging from 25 to 100 units (median 51.25), and one patient received rimabotulinumtoxin B at a total dose of 3000 units. All injections targeted the palpebral portion of the OO muscle, and approximately ¾ of the patients also received injections of the orbital portion, the procerus, or corrugator muscles. No changes were made to the pattern, technique, or dose of the injection with the treatment at study initiation, compared to the previous regular treatments.
Starting the day after the injection treatment, the patients applied the topical agent twice daily to the eyelids, following written instructions, including a graphic representation of the targeted periocular area with the areas of application clearly marked. The application was standardized and targeted only the eyelids, independent of involvement of the orbital OO or surrounding muscles. The amount needed for each application was dispensed automatically with one manual press of the container. The correct technique for application of the study substance was reviewed at each visit, and the patients demonstrated the correct procedure before proceeding. JBRS and BDS were followed at least monthly, with expected improvement after BoNT therapy followed by decline as the BoNT effect wore off. In addition to scheduled monthly visits, the subjects were asked to report whether they subjectively appreciated a return to baseline severity, at which time a visit was immediately arranged for evaluation (within 48 h). Compliance with the therapy was verified by interview and verification of residual study substance at each visit. A full neurologic evaluation was performed at each visit, and side effects were screened for. Four patients who reported mild local discomfort had a same-day ophthalmology consultation at the time of the event. All visits took place at the NIH Clinical Center. The primary outcome was time to return to baseline JBRS, and secondary outcomes were JBRS and BDS score changes from baseline at 3 months.
Survival data analysis was used for the primary outcome, with logrank testing of the difference between groups. The return to baseline JBRS was used as the defined event. Wilcoxon signed rank test was used for the secondary measure comparisons. Once the patients returned to baseline pre-treatment JBRS, BoNT injection therapy was resumed and the study ended.
Results
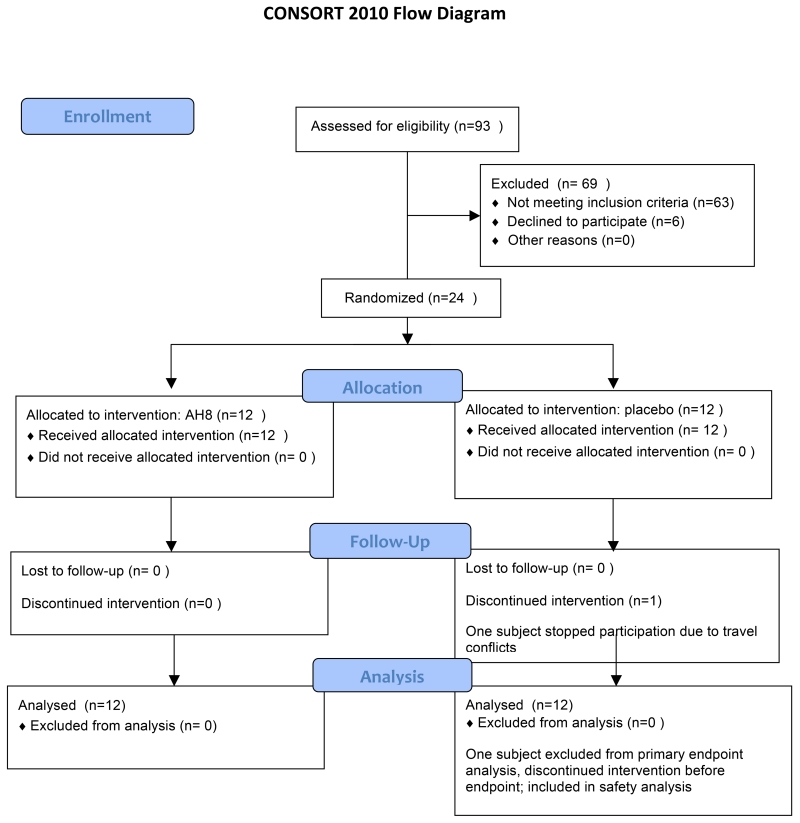
24 patients, average age 57 (range 42-73), were randomized to AH8 vs placebo. Fig.1 shows the CONSORT flow diagram for the trial. One patient discontinued the intervention before the endpoint due to unrelated family difficulties, which prevented travel to appointments.
Figure 1.
CONSORT flow diagram for the trial. 12 patients were randomized in each group. 1 patient (in the placebo arm) discontinued the intervention due to unrelated factors. This subject was included in the safety analysis but not in the primary efficacy analysis.
No severe adverse events were recorded. Four subjects (two on active substance and two on placebo) experienced minor, self-limiting eyelid irritation, for which no modification of the study procedure was needed.
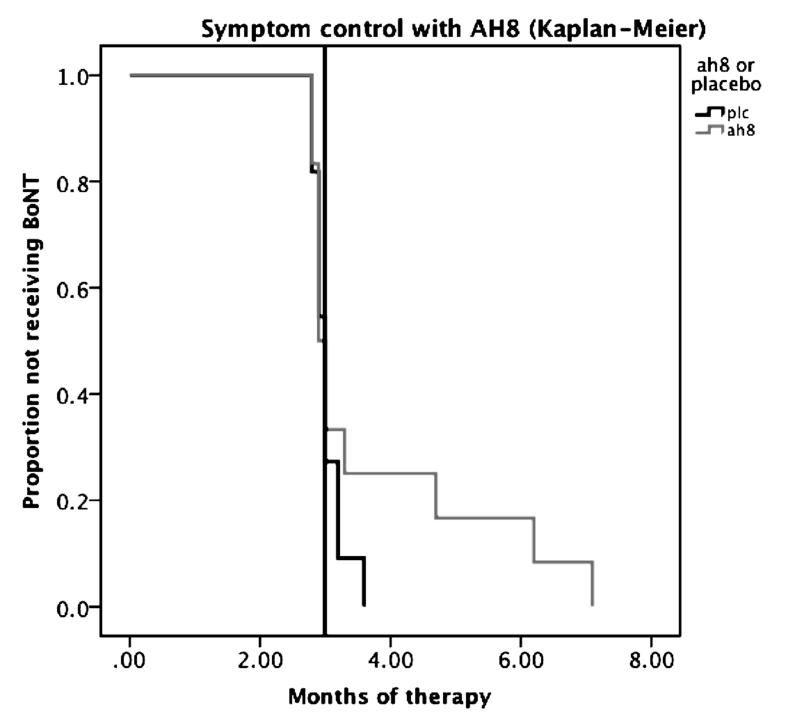
There was a clear trend for longer time until return to baseline JBRS after an injection in the active group compared to placebo (average 3.7 months vs. 3.0 months) (Fig. 2). This did not reach statistical significance in our analysis, which considered the entire duration from one BoNT treatment to the next. As can be seen in the figure though, considering the period from 3 months (the usual time of return to baseline severity in this group of patients) forward, the two groups separate out with the active group continuing to have symptom control for a longer period. One-third (4/12) of the patients in the active group had a significantly longer interval between two BoNT injections than their usual 3 months (range: 3.3–7.1 months). The study was underpowered to show significance with this amount of benefit. There were no significant differences in the BDS and JBRS scores between the two groups at 3 months.
Figure 2.
Kaplan-Meier curve illustrating the return to baseline JBRS after BoNT treatment. The interpolation line is at 3 months, the usual duration of action of the regularly scheduled BoNT injections in this group of patients. A clear separation of the active and placebo groups can be seen beyond this point in terms of return to baseline severity.
Discussion
In its most severe forms, BSP can result in functional blindness or severe impairment, at significant cost in disability, loss of productivity, or overall health [5]. The only reliably effective treatment, BoNT injection therapy, has the disadvantages of significant cost, discomfort, and risks of side effects [7] and needs to be repeated on average every 3 months. Oral medications are generally not useful, and DBS surgery has given mixed results with significant risks of adverse effects [8]. A practical topical alternative would be a welcome addition to the therapeutic armamentarium.
Acetyl hexapeptide-8 has a mechanism of action similar to BoNT A, acting by competitive inhibition of the SNAP25 component of the SNARE complex (BoNT type A acts by cleaving SNAP25 [9]). It has been used safely and effectively in cosmetic applications under the brand name Argireline®, patent WO00/64932/Lipotec S.A [4, 10] (BCN Peptides and Lipotec SA are part of the Lipotec Group). Extensive toxicology and safety studies in animals and humans showed no topical or systemic toxicity at concentrations of 0.05% and above (BCN Peptides Toxicology Report, 2006, data on file).
In this initial study, we have used the study substance in addition to BoNT therapy. As the vast majority of patients are being treated with BoNT with some success, and BoNT is a proven effective therapy [2], we elected to enroll patients already in therapy and focus on the duration of action, to avoid the recruitment and ethical challenges otherwise posed by interrupting an effective therapy. We have included only patients who had been receiving stable benefit from BoNT injections strictly at 3-month intervals, excluding all patients who displayed any variation in the pattern of response or injection treatment over the previous 3 BoNT injection cycles. As these subjects had been returning to the severity requiring re-injection 3 months after each treatment (for at least three cycles prior to enrollment), any extended benefit beyond the 3-month point (expressed as a delay in returning to the BSP severity previously recorded as the worst in the patients’ therapeutic cycles) was attributed to the added AH8 treatment. By enrolling only the patients with very stable recent pattern of symptoms, we have also attempted to avoid large natural variations in the severity of BSP, which can occur in some patients.
The primary outcome of the study was negative, as the extension of duration of symptom control in patients receiving BoNT therapy did not reach statistical significance. However, we did observe a trend for longer intertreatment interval in the active group. In several patients, the duration of effective symptom control extended considerably (more than 7 months in one case). Post hoc review of the subject characteristics did not suggest that certain subgroups of patients may be more susceptible to the treatment.
No severe adverse events were recorded, and all subjects completed the intervention without significant problems. Four subjects reported mild discomfort with a feeling of ‘heaviness’ affecting the eyelids. Prompt ophthalmologic evaluation concluded this to be mild non-septic blepharitis associated with the application of a thick cream, a known risk independent of the active ingredients (and seen in our patients with both active and placebo formulations). The symptom resolved spontaneously in all subjects, with at most simple hygiene measures, such as daily washing of the eyelids with a warm moist towel [11].
The concentration chosen for this study was low, as previously used in cosmetic applications. The toxicology data showed no evidence of significant risks for human use at concentrations 10 times higher (0.05%). It is possible that the limited efficacy seen in this study is related to the concentration employed. The targeted OO is situated deeper to the skin and is much larger than the intradermal muscles targeted in cosmetic application [12]. The topical application targeted only the palpebral portion of the OO, as the orbital portions are deeper and likely out of reach of the active substance.
One patient was in treatment with BoNT type B after developing resistance to BoNT type A. This subject was in the active group and returned to baseline at 3 months, with no change. It is unlikely but not impossible that resistance to BoNT A would translate to resistance to the topical substance, but we cannot draw any conclusions on this point. This problem would be avoided in future trials targeting BoNT-naïve patients.
Whilst the results did not reach efficacy significance, the safety profile and the trend for improvement in the active group suggest that AH8 could, potentially, prove effective with future studies, particularly targeting patients with predominantly palpebral OO involvement. This study is limited primarily by the inclusion of patients receiving BoNT therapy. Whilst we strived to include patients who had demonstrated a stable pattern of response to therapy, it is well known that response to BoNT is variable. The patients enrolled had a wide spectrum of BSP severity, which further complicated the analysis. Lastly, the concentration used was likely too low for this group, and a larger sample would have allowed more robust conclusions.
In this initial trial, AH8 treatment of BSP was safe and well tolerated. There was a trend for efficacy in extending the duration of action of concomitant BoNT therapy, without reaching significance. Further studies are in preparation, using higher concentrations and BoNT-naïve BSP patients, which would allow less variability and a more straightforward data analysis.
References
- 1.Jankovic J, Havins WE, Wilkins RB. Blinking and blepharospasm. Mechanism, diagnosis, and management. JAMA : the journal of the American Medical Association. 1982 Dec 17;248(23):3160–4. [PubMed] [Google Scholar]
- 2.Simpson DM, Blitzer A, Brashear A, Comella C, Dubinsky R, Hallett M, et al. Assessment: Botulinum neurotoxin for the treatment of movement disorders (an evidence-based review): report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. [Meta-Analysis] 2008 May 6;70(19):1699–706. doi: 10.1212/01.wnl.0000311389.26145.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Willis B, Eubanks LM, Dickerson TJ, Janda KD. The strange case of the botulinum neurotoxin: using chemistry and biology to modulate the most deadly poison. Angewandte Chemie. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Review] 2008;47(44):8360–79. doi: 10.1002/anie.200705531. [DOI] [PubMed] [Google Scholar]
- 4.Blanes-Mira C, Clemente J, Jodas G, Gil A, Fernandez-Ballester G, Ponsati B, et al. A synthetic hexapeptide (Argireline) with antiwrinkle activity. International journal of cosmetic science. 2002 Oct;24(5):303–10. doi: 10.1046/j.1467-2494.2002.00153.x. [DOI] [PubMed] [Google Scholar]
- 5.Jankovic J, Kenney C, Grafe S, Goertelmeyer R, Comes G. Relationship Between Various Clinical Outcome Assessments in Patients with Blepharospasm. Movement Disorders. 2009 Feb 15;24(3):407–13. doi: 10.1002/mds.22368. [DOI] [PubMed] [Google Scholar]
- 6.Lindeboom R, De Haan R, Aramideh M, Speelman JD. The blepharospasm disability scale: an instrument for the assessment of functional health in blepharospasm. Movement disorders : official journal of the Movement Disorder Society. 1995 Jul;10(4):444–9. doi: 10.1002/mds.870100407. [DOI] [PubMed] [Google Scholar]
- 7.Naumann M, Albanese A, Heinen F, Molenaers G, Relja M. Safety and efficacy of botulinum toxin type A following long-term use. European journal of neurology : the official journal of the European Federation of Neurological Societies. [Research Support, Non-U.S. Gov’t Review] 2006 Dec;13(Suppl 4):35–40. doi: 10.1111/j.1468-1331.2006.01652.x. [DOI] [PubMed] [Google Scholar]
- 8.Limotai N, Go C, Oyama G, Hwynn N, Zesiewicz T, Foote K, et al. Mixed results for GPi-DBS in the treatment of cranio-facial and cranio-cervical dystonia symptoms. J Neurol. Nov;258(11):2069–74. doi: 10.1007/s00415-011-6075-0. [DOI] [PubMed] [Google Scholar]
- 9.Grumelli C, Verderio C, Pozzi D, Rossetto O, Montecucco C, Matteoli M. Internalization and mechanism of action of clostridial toxins in neurons. Neurotoxicology. [Research Support, Non-U.S. Gov’t Review] 2005 Oct;26(5):761–7. doi: 10.1016/j.neuro.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Ruiz MA, Clares B, Morales ME, Cazalla S, Gallardo V. Preparation and stability of cosmetic formulations with an anti-aging peptide. Journal of cosmetic science. [Research Support, Non-U.S. Gov’t] 2007 Mar-Apr;58(2):157–71. [PubMed] [Google Scholar]
- 11.Eyelid hygiene for blepharitis. Insight. Jan-Mar;36(1):24. [PubMed] [Google Scholar]
- 12.Hallett M. Blepharospasm: recent advances. Neurology. [Review] 2002 Nov 12;59(9):1306–12. doi: 10.1212/01.wnl.0000027361.73814.0e. [DOI] [PubMed] [Google Scholar]