Abstract
Skeletal muscle loss is associated with aging as well as pathological conditions. Satellite cells (SCs) play an important role in muscle regeneration. Omega-3 fatty acids are widely studied in a variety of muscle wasting diseases; however, little is known about their impact on skeletal muscle regeneration. The aim of this review is to evaluate studies examining the effect of omega-3 fatty acids, α-linolenic acid, eicosapentaenoic acid, and docosahexaenoic acid on the regulation of SC proliferation and differentiation. This review highlights mechanisms by which omega-3 fatty acids may modulate the myogenic program of the stem cell population within skeletal muscles and identifies considerations for future studies. It is proposed that minimally three myogenic transcriptional regulatory factors, paired box 7 (Pax7), myogenic differentiation 1 protein, and myogenin, should be measured to confirm the stage of SCs within the myogenic program affected by omega-3 fatty acids.
Keywords: muscle stem cells, fish oil, muscle loss
Introduction
Skeletal muscles comprise 40%–50% of the total body mass in an adult human being and include a broad range of muscle types. Skeletal muscle mass and muscle fiber size change in response to physiological and pathological conditions. Muscle loss occurs as a normal process due to aging, and is also a characteristic of catabolic hormonal stimulation or diseases, such as cancer cachexia, diabetes, renal failure, denervation, motor neuron disease, and heart failure. Skeletal muscle depletion is associated with reduced muscle function and performance,1 decreased quality of life, and shorter length of survival in older adults and in people with cancer.2–4 Poor clinical outcomes associated with muscle loss present compelling reasons to develop strategies to reverse or slow down muscle loss. The development of effective therapies requires an understanding of contributing mechanisms and factors required to reverse or prevent muscle loss. An emerging area of research is the potential dysregulation of myogenic stem cells as contributors to muscle loss.
The importance of nutritional status in regulating muscle protein synthesis and muscle mass is well accepted, but limited literature exists regarding the role of nutrients, specifically on satellite cells (SCs).5 Dietary proteins and amino acids of sufficient quality and quantity are key nutrients required for muscle health. Recent studies have provided evidence that protein supplementation combined with exercise may accelerate SC proliferation.6–8 In the last decade, nutritional compounds, such as resveratrol, epigallocatechin gallate (catechins in green tea), β-hydroxy-β-methylbutyrate (leucine metabolite) have been reported to improve SC proliferation, especially in fast muscles in experimental models.9–11 It is suggested that resveratrol and epigallocatechin gallate buffer high levels of reactive oxygen species and reduce oxidative stress in aging muscles, thus favoring SC differentiation and proliferation.12 HMB reduces protein catabolism and muscle loss during disease and/or disuse.13
Polyunsaturated omega-3 fatty acids are a family of essential fatty acids with many biological activities. There are three major dietary n-3 fatty acids: α-linolenic acid (ALA; 18:3n-3), eicosapentaenoic acid (EPA; 20:5n-3), and docosahexaenoic acid (DHA; 22:6n-3). Omega-3 fatty acids comprise cell membrane phospholipids, contributing to structural and functional characteristics by altering signaling platforms for membrane proteins and lipids.14–17 In obese adolescents, supplementation with omega-3 fatty acids increased proportions of EPA and DHA in the muscle, while improving glucose tolerance and insulin sensitivity.18 Providing EPA and DHA in the form of fish oils in human diets has been reported to enhance anabolic potential and reduce muscle loss (reviewed by Ewaschuk et al19). While several mechanisms may be contributing to disease-associated muscle atrophy, little is known about the impact of omega-3 fatty acids on the differentiation and proliferation of SCs. This article reviews studies examining the effect of ALA, EPA, and DHA on myogenic regulation of SC proliferation and differentiation. Possible mechanisms by which omega-3 fatty acids modulate the myogenic lineage of SCs are also explored. Currently, there are no studies exploring these questions in humans, and therefore, emphasis is on studies performed in experimental systems.
Role of SCs in Myogenesis
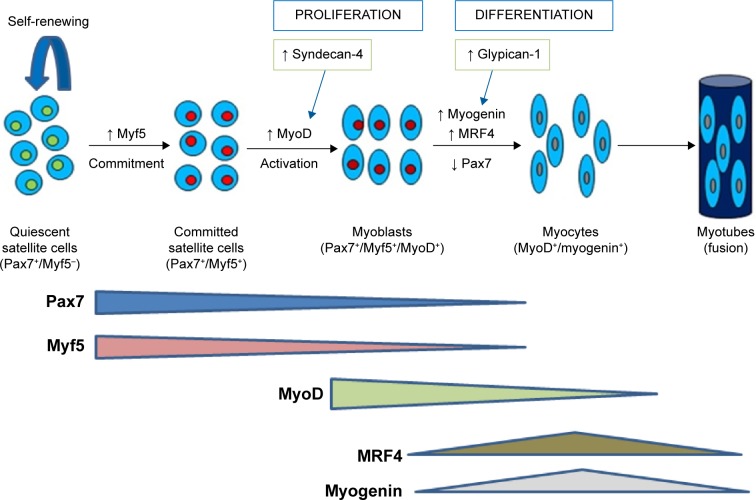
SCs are a heterogeneous collection of quiescent muscle stem cells that reside within adult myofibers, between the basement membrane and sarcolemma.20 Normally, in adult muscles, SCs are in a quiescent state and express Pax7.9,21,22 These mesodermally derived multipotent stem cells are capable of self-renewing proliferation, and are responsible for muscle growth and regeneration. In response to muscle damage, SCs are activated to proliferate, differentiate, and fuse with the existing muscle fibers. Pax7-positive daughter cells either differentiate by migrating through the sarcolemma and fusing with existing muscle fibers during the growth of the existing muscle fiber or have the capacity to fully regenerate new myotubes (Fig. 1).23,24 Commitment of SCs to a myogenic lineage is indicated by the expression of specific myogenic transcriptional regulatory factors (MRFs), which include, but are not limited to, myogenic differentiation 1 protein (MyoD), myogenic factor 5 (Myf5), and myogenic regulatory factor 4 (MRF4).25 On activation, committed SCs upregulate MyoD and enter the cell cycle to proliferate as myoblasts and differentiate by downregulating Pax7 and upregulating myogenin and MRF4 (Fig. 1).26 Thus, the expression of Pax7, MyoD, and myogenin identifies whether SCs are in a quiescent or committed state (Pax7+/MyoD−/myogenin−), proliferation state (Pax7+/MyoD+/myogenin−), or differentiation state (Pax7−/MyoD+/myogenin+).27 Measuring three MRFs concurrently enables the status of SCs to be determined as activated, proliferating, or differentiating.
Figure 1.
Model for changes in expression of myogenic regulatory factors through the myogenic program. In quiescence, satellite cells reside between basal lamina and sarcolemma as satellite stem cells (Pax7+/Myf5−) and/or as committed satellite cells (Pax7+/Myf5+). On activation, committed satellite cells upregulate myoblast determination protein (MyoD). Satellite progeny then follow one of two fates. They either enter the cell cycle to proliferate as myoblast and differentiate by down regulating Pax7 and up regulating myogenin and MRF4 or down regulate MyoD and self renew to give rise to Pax7+ satellite cells. Syndecan-4 and glypican-1 are regulators of expression of myogenic regulatory factors during satellite cell proliferation and differentiation.
MRF expression is also affected by extracellular matrix molecules, such as heparan sulfate proteoglycans (HSPGs). Syndecan-4 and glypican-1 are the most studied HSPGs in relation to myogenesis. Syndecan-4 is required for activation and proliferation of SCs and regulates MyoD and MRF4 expressions.28 Syndecan-4 also serves as a marker for quiescent and activated SCs and subsets of SCs.29 In contrast, knockdown of glypican-1 inhibited mouse muscle cell differentiation and delayed or decreased myogenin expression.30 Glypican-1 plays a primary role in SC differentiation by sequestering fibroblast growth factor 2, a potent inhibitor of differentiation.30,31 These data suggest a regulatory role of syndecan-4 and glypican-1 in SC function and expression of transcriptional regulatory factors during SC myogenesis.
Effect of Omega-3 Fatty Acids on SC Function
Low plasma levels of EPA and DHA have been associated with skeletal muscle depletion and impaired muscle function in older adults and in people with type-2 diabetes, obesity, and cancer.32–34 A cross-sectional and retrospective cohort study reported an increase in the grip strength of men and women with each additional portion of fatty fish per week, independent of their height and age.32 The low level of plasma omega-3 fatty acids was related to a decline in physical performance in middle-aged and older adults in the large InCHI-ANTI cohort study.35 Supplementation with EPA has been reported to enhance muscle protein synthesis, increase muscle mass, and improve function in elderly individuals.36 Human studies have reported a relationship between EPA and DHA supplementation and the preservation or gain of muscle mass in patients with cancer, who would otherwise experience muscle loss.37,38 EPA and DHA can reduce contributing factors, such as acute-phase response, proinflammatory cytokines, and insulin resistance, which mediate muscle loss.39 Few studies have examined the effect of EPA and DHA on the myogenic potential of SCs. The studies that do exist have been performed in a variety of experimental models (Table 1).
Table 1.
Summary of studies exploring effects of omega-3 fatty acids on proliferation and differentiation of satellite cells.
| REFERENCES | OBJECTIVE | EXPERIMENTAL MODEL | MEDIUM OR DIET USED | PARAMETERS MEASURED | MUSCLE FATTY ACID COMP. | TREATMENT (DOSE) | OUTCOMES |
|---|---|---|---|---|---|---|---|
| Cell culture studies | |||||||
| Magee et al,52 2004 | To evaluate differentiation in response to TNF-α and EPA treatments. | C2C12 cell line | DMEM with fetal bovine serum | Expression of MyHC, myotube size and myoblast fusion index. Caspase-8 activity | – | EPA (50 μM) | EPA blocked TNF-α induced reduction of MyHC expression and caspase-8 mediated apoptosis. Increased myotube size and myoblast fusion index |
| Peng et al,68 2012 | To determine the effects of omega-3 fatty acids on proliferation. | C2C12 cell line | DMEM with fetal bovine serum | Cell morphology, Cyclin-E, cyclin-D1 and CDK2 (progression of cell cycle) | – | ALA, DHA and EPA (50 μM and 100 μM) | DHA and EPA ↓ C2C12 myoblasts proliferation, and the effect was concentration dependent whereas ALA did not show any inhibitory effect |
| Bryner et al,66 2012 | To determine if DHA treatment is protective against palmitate-associated muscle cell atrophy and reducing intramyocellular lipid content. | C2C12 cell line | DMEM with fetal bovine serum | Myotube morphology, PGC1α | – | DHA (100 μM) | DHA maintained myotube morphology, diameter and intramyocellular lipid content. ↑ AMPK levels. Maintained PGC1α and ↑ in oxidative metabolism |
| McFarland et al,74 2011 | To examine the proliferation and differentiation responses of cultured satellite cells when administered different types of fatty acids in the media. | Turkey and broiler chicken (isolated satellite cells) | DMEM with chicken serum or horse serum | Syndecan-4, glypican-1, morphology, differentiation, proliferation | No | LA, ALA, EPA, DHA and AA (5 μM) | ↓ Proliferation and differentiation in turkey cells and no modifcation in chicken cells, ↑ in syndecan-4 expression during proliferation and differentiation and ↑ glypican-1 expression during satellite cell differentiation |
| Castillero et al,71 2009 | To examine whether EPA is able to prevent an arthritis-induced decrease in body weight and muscle wasting. | Arthritic rats | Standard chow | MuRF-1, atrogin-1, MyoD, myogenin and myostatin | No | EPA (1 g/kg) | EPA ↓ gene expression of TNF-α, atrogin-1 and MuRF-1. No effect on MyoD and myogenin |
| Animal studies | |||||||
| Fiaccavento et al,77 2010 | To determine if n-3 PUFAs alleviate the dystrophic skeletal muscle damage differently modulating the myocyte membrane composition and conformation and, hence, intracellular signaling. | Hamster with muscular dystrophy | Chow pellet/ALA enriched-flaxseeds | Pax7 and myogenin expression of satellite cells, molar percentage of EPA, DHA, AA and ALA. Membrane proteins (caveolin-3 and β-catenin) | EPA, DHA | – | ↓ Satellite cells expressing Pax7, ↑ in myogenin expression, ↑ molar percentage of ALA and EPA. Normal sarcolemmal pattern of caveolin-3 and β-catenin |
| Penna et al,72 2011 | To verify the ability of EPA to prevent muscle depletion in lung carcinoma-bearing mice and to test the ability of endurance exercise training to increase the EPA effect. | Lung carcinoma-bearing mice | Not given | Pax7, atrogin-1 | No | EPA(0.5 g/kg) and exercise | EPA alone did not prevent muscle loss induced by tumor growth while the combination with exercise induced a partial rescue of muscle strength and mass. Association of EPA and exercise ↓ PAX-7 accumulation |
| Fappi et al,70 2014 | To evaluate whether n-3 supplementation could mitigate the development of dexamethasone-induced muscle atrophy. | Rats (dexamethasone induced muscle atrophy) | a standard commercial diet (Nuvilab CR1) | MuRF-1, atrogin-1, MyoD and myogenin | AA, ALA | EPA and DHA (0.1 g/kg) | EPA and DHA did not prevent the decreased expression of MyoD and myogenin. ↑ expression of Atrogin-1 and MuRF-1 |
| Apolinario et al,56 2015 | To evaluate the long term effect of n-3 PUFA on muscle regeneration and inflammation. | Mdx mice | Not given | MyoD, NF-κB, TNF-α | No | EPA (0.04 g/kg) and DHA (0.02 g/kg) | ↑ in MyoD, ↓ NF-κB and ↓ TNF-α protein expression |
Abbreviations: ALA, alpha linolenic acid; EPA, eicosapentanoic acid; LA, Linoleic acid; AA, Arachidonic acid; MyHC, Myosin Heavy Chain; DHA, docosahexanoic acid; DM EM, Dulbecco’s Modified Eagle’s medium.
Omega-3 fatty acids can affect inflammatory pathways
Inflammation plays a significant role in muscle damage and loss. Inflammatory processes that disrupt muscle homeostasis and promote injury have been an area of intense research. Cellular mediators, such as interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and transforming growth factor-β (TGF-β) are key regulators of skeletal muscle cell responses to injury. Disruption of TGF-β by introducing the dominant-negative mutant receptor (ie, truncated type II TGF-β receptor transfected into C2C12 cells) results in inhibition of myogenic differentiation and suppression of MyoD expression.40 Low physiological concentrations of TNF-α appear to activate myogenesis, whereas sustained high levels of TNF-α in chronic inflammatory disease have been associated with impaired myogenic differentiation.41 TNF-α induces muscle loss by inhibiting myogenic differentiation and by stimulating apoptosis of differentiated myotubes.42,43 Also, TNF-α is associated with increased activation of nuclear factor-kappaB (NF-κB), proinflammatory signaling pathway, and high expression of atrogenes, such as atrogin-1 and muscle RING-finger protein-1 (MuRF-1), leading to protein degradation.44 In response to various stimuli, immune cells secrete lipid mediators, prostaglandins (PGs) and leukotrienes, that may influence myogenesis and are potent mediators of pain and inflammation.45 Resolvins, maresins, and protectins are eicosanoids derived from EPA and DHA that act as anti-inflammatory agents; however, their impact on myogenic progenitors requires further investigation.46
C2C12 is a well-established murine skeletal muscle cell line extensively used in SC research. It is a subclone of the murine myoblast cell line established by Yaffe and Saxel and produced by Blau et al.47,48 C2C12 myoblasts exhibit the majority of features and proteins as SC-derived myoblasts.49,50 C2C12 has been extensively used as a model for skeletal muscle proliferation, differentiation, cell-based therapies, and other research related to muscle development. The effects of TNF-α and EPA treatment on skeletal muscle cells during their differentiation from myoblasts to myotubes have been studied in C2C12 cells (Table 1).51 C2C12 cells were differentiated in the presence or absence of TNF-α (20 ng/mL) with or without EPA (50 μM). EPA was added concurrently with TNF-α either as a co-treatment or as a pretreatment (two hours) after which EPA was withdrawn and replaced by TNF-α alone in Dulbecco’s modified eagle medium. The inhibitory effect of TNF-α on myotube differentiation and the modified pattern of myosin heavy chain expression was prevented by both pre- and co-treatment with EPA. TNF-α treatment significantly reduced myotube size and myoblast fusion index and induced cellular necrosis compared to their respective untreated controls. These deleterious effects of TNF-α were inhibited by EPA treatment.51 In addition, the activation of apoptosis by caspase-8 induced by TNF-α was completely blocked by EPA co-treatment.51 Pax7 and MyoD levels were not measured in the study, limiting the understanding of how EPA affects the myogenic program. Other studies have reported TNF-α to enhance proliferation and aggregation of myoblast cells, while inhibiting chief regulatory molecules of myogenic differentiation, MyoD and myogenin in C2C12 myoblasts (Fig. 2).52,53 A subsequent study evaluated the effects of EPA treatment on TNF-α-induced NF-κB activation in the C2C12 myoblast line (Table 1).54 It set out to determine whether EPA anti-inflammatory activity was dependent on the altered expression and activation of peroxisome proliferator-activated receptors (PPARs).54 Impairment of skeletal muscle cell differentiation induced by TNF-α was associated with increased NF-kB transcriptional activity as well as inhibition of PPAR-γ expression. On the other hand, EPA co-treatment or pretreatment was associated with the inhibition of NF-kB and upregulation of PPAR-γ expression. IL-6 expression was also significantly inhibited when EPA was administered either as a co-treatment or pretreatment with TNF-α. The inhibitory effect on NF-kB and IL-6 was specific to EPA and DHA as disrupted morphology was observed in control cell lines with omega-6 and omega-9 fatty acids. Experimental and human studies have suggested that fish oil-derived omega-3 fatty acids exhibit an anti-inflammatory effect by inhibiting the activation of NK-κB, either by decreasing phosphorylation of its inhibitory subunit, IκB, or by binding to PPAR-γ, which in turn interacts with NF-κB to prevent translocation to the nucleus (Fig. 2).17 A recent study explored the long-term (five months) effect of omega-3 fatty acid supplementation on muscle regeneration and inflammation in the mdx mouse model of Duchenne muscular dystrophy (Table 1).55 Mdx mice receiving omega-3 (60 mg/kg) capsules exhibited increase in muscle regeneration and higher expression of MyoD as compared to the control group. Long-term omega-3 supplementation resulted in lower expression of inflammatory markers, NF-κB and TNF-α. Measures of Pax7, MyoD, and myogenin protein expression in this model would determine which phase of the myogenic program of SCs is most affected by omega-3 fatty acids.
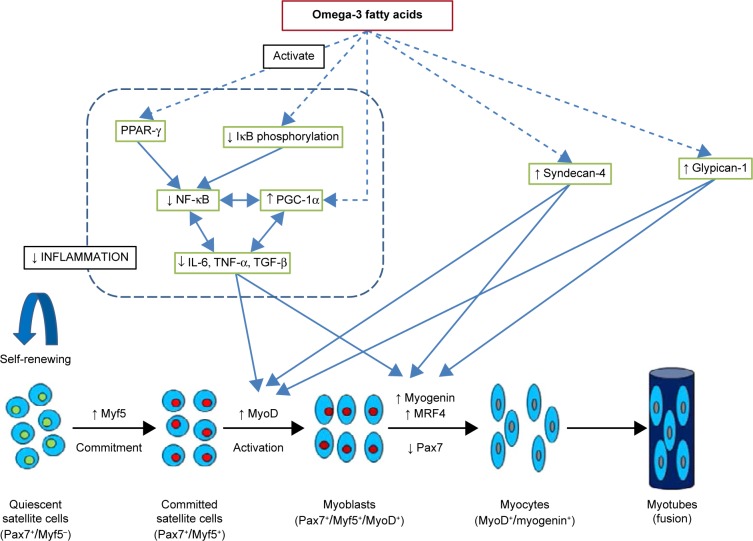
Figure 2.
Possible mechanisms by which omega-3 fatty acids promote myogenesis. Omega-3 fatty acids inhibit NF-κB activation and decrease release of inflammatory cytokines and proteins, including IL-6, TNF-α and TGF-β, by activating PPAR-γ, decreasing IκB phosphorylation or/and increasing PGC-1α expression in skeletal muscle. PGC-1 co activators and inflammatory processes in skeletal muscle are linked in reciprocal manner. Decrease in inflammatory proteins promotes proliferation and differentiation of satellite cells. Omega-3 fatty acids may increase syndecan-4 and glypican-1 expression which in turn increase myoD and myogenin expression. Dashed arrows indicate that effects may be indirect with involvement of other metabolites and signaling molecules.
There are three members of the PPAR-γ coactivator 1 (PGC-1) family, namely PGC-1α, PGC-1β, and PGC-1-related coactivator. PGC-1α is a transcriptional coactivator that is a central inducer of mitochondrial biogenesis in cells.56 The results from several studies suggest that PGC-1α targets the NF-κB pathway and favors an anti-inflammatory environment in skeletal muscles (Fig. 2).56 Muscle loss under pathological conditions, such as diabetes, cancer, obesity, uremia, and denervation-induced atrophy, has been associated with low levels of PGC-1α and/or PGC-1β and high levels of TNF-α and IL-1β.57–61 High PGC-1α levels could prevent the progression of muscle wasting by suppressing atrogenes in vitro and in vivo.59,60,62 A recent study revealed a positive relationship between PGC-1α expression and myogenic differentiation. Transcriptional factors critical for differentiation of skeletal muscle cells, such as MyoD and myogenin, were enhanced by PGC-1α in C2C12 cells.63 DHA and EPA treatment for 24 and 48 hours was reported to induce PGC-1α expression in human rhabdomyosarcoma cells.64 In another study, DHA treatment maintained the myotube morphology, diameter, and intramyocellular lipid content similar to control levels and retained PGC-1α near control levels.65 Also, DHA treatment caused myotube hypertrophy and showed a protective effect against palmitate-induced muscle atrophy.65 A high concentration of palmitate has been associated with insulin resistance and contributes to muscle atrophy.66 Peng et al reported that C2C12 myoblast proliferation was significantly reduced by EPA and DHA in a concentration-dependent manner, whereas ALA did not show any inhibitory effect (Table 1).67
Omega-3 fatty acids affect glucocorticoid-induced muscle degradation
Glucocorticoids are released as a component of the stress response and evoke catabolism by increasing protein degradation and decreasing protein synthesis.68 Synthetic glucocorticoids, such as dexamethasone, are used to model muscle atrophy. The effects of dietary EPA and DHA on dexamethasone-induced muscle atrophy were evaluated by measuring expression of genes involved in protein synthesis and degradation and myogenic proliferation and differentiation (MyoD, myogenin, atrogin-1, and MuRF-1), which was concurrent with histological analysis in rat muscles (Table 1).69 Four experimental groups consisted of control, dexamethasone alone, omega-3 supplement alone, and dexamethasone groups with an omega-3 supplement (EPA 180 mg and DHA 120 mg capsules). The group supplemented with omega-3 showed higher levels of MyoD compared to controls who were not sustained when dexamethasone was added. In contrast, myogenin levels were lower in both omega-3-supplemented and dexamethasone plus omega-3-supplemented groups compared to controls. The dexamethasone group with omega-3 supplementation showed higher activation of atrogenes, such as atrogin-1 and MuRF-1, indicating increased protein degradation and decreased protein synthesis. Ex vivo fatty acid analysis of muscles showed incorporation of omega-3 fatty acids; however, the DHA and EPA contents of muscles were not reported for any experimental group. Diets provided in this study were not matched for calories, and no data regarding food intake were presented, limiting the ability to interpret the results from this study.69 In a model of arthritis-induced muscle atrophy, dietary EPA decreased proteolysis as observed by lower gene expressions of TNF-α and atrogin-1 (Table 1).70 Arthritis increased gene and protein expressions of MyoD and myogenin, and EPA administration did not modify this effect. No change in the expression of these proteins was seen in the gastrocnemius muscle of pair-fed and control rats (Fig. 2).
Omega-3 fatty acids induces myogenesis in cancer-induced muscle atrophy
Muscle wasting is an important component of pathophysiology of cancer. A recent study evaluated the effect of EPA and endurance exercise training on muscle depletion in lung carcinoma-bearing mice (Table 1).71 Control (n = 15) and tumor-bearing (n = 24) mice were randomized into three groups (n = 5 for controls and n = 8 for tumor-bearing mice): untreated, treated with EPA, or treated with EPA and subjected to treadmill running. All groups had free access to food and water during the experimental period. The EPA-treated groups received daily administration of 0.5 g/kg EPA in corn oil starting from the fourth day of tumor growth, and the untreated groups received corn oil alone. Tumor-bearing mice were killed 14 days after tumor injection. EPA did not prevent muscle wasting when administered alone but was able to improve both muscle mass and strength when coupled with exercise. Untreated tumor-bearing mice showed higher Pax7 protein expression, whereas endurance exercise combined with EPA had lower levels of Pax7. A single measure of Pax7 does not enable interpretation with regard to alterations in myogenic proliferation or differentiation potential, as Pax7 is expressed by SCs at different stages within the myogenic program. Measuring other MRFs, MyoD and myogenin, would determine the step of SC-derived myogenesis affected by treatment. Food intake was partially corrected in the group receiving EPA plus exercise, and no pair-fed group was available for comparison. No effect of EPA alone was observed, potentially because the dose of EPA (0.5 g/kg) administered might not be sufficient to see desired effects. When a 1 g/kg dose was applied, a five-fold increase in the ratio of protein synthesis to protein degradation was reported in the gastrocnemius muscle of cachectic mice bearing the MAC16 tumor.72
Effect of omega-3 fatty acids on proteoglycans needed for myogenesis
A spectrum of polyunsaturated fatty acids on proliferation and differentiation, as well as on the expression of syndecan-4 and glypican-1 in avian myogenic SCs, has been recently studied (Table 1).73 SCs were isolated from the pectoralis major and biceps femoris muscles of 13-week old turkeys and 5-week old chickens and cultivated with linoleic acid, α-linolenic acid, arachidonic acid, DHA, or EPA. Decreases in proliferation and differentiation were reported in turkey cells treated with EPA and DHA. Evaluation of cell morphology suggested a toxic effect of EPA and DHA on turkey SCs. However, the proliferation of chicken SCs was not depressed by EPA and DHA, and a microscopic examination revealed that chicken cells receiving these treatments maintained normal morphology. The differences in SCs from turkeys and chickens in response to EPA and DHA may be attributed to differences in the composition of muscle membrane lipid in turkeys and chickens.73 After treating with spectrum of fatty acids, glypican-1 and syndecan-4 gene expressions were measured in proliferating and differentiating SCs from turkey and chicken muscles. Expressions of glypican-1 and syndecan-4 were increased during proliferation in all treatments compared to control serum in turkey and chicken SCs. Both glypican-1 and syndecan-4 differentially regulate expressions of myogenic regulatory transcriptional factors during proliferation and differentiation of SCs. The knockdown of glypican-1 in turkey SCs has been associated with decreased gene expression of MyoD and myogenin, whereas the knockdown of syndecan-4 has been associated primarily with increased MyoD and MRF4 expressions during proliferation and differentiation (Fig. 2).74 Single fatty acids as opposed to physiological fatty acid mixtures are likely to evoke different metabolic effects.75
Incorporation of omega-3 fatty acids into membrane lipids can affect muscle atrophy in dystrophic muscles
In dystrophic hamsters, the effect of flaxseed-enriched diet on myocyte membrane composition, conformation, and intracellular signaling has been investigated (Table 1).76 The hamster model of dystrophy (UM-X7.1 Syrian hamster) used for this study is a model for human limb-girdle muscular dystrophy. The experiment tested four groups in total, the first and second groups (n = 50 each) were fed either a chow diet or a flaxseed-enriched diet from weaning to death. To determine if ALA reverses a well-established muscular dystrophy, a third group of dystrophic hamsters (n = 30) were fed standard chow from weaning to 100 days (until development of dystrophy) followed by a flaxseed-rich diet for the next 50 days before death. Healthy animals were fed only chow and were used as a control. The histological analysis of muscles from dystrophic hamsters fed a flaxseed-enriched diet showed preservation of the typical skeletal muscle morphology compared to those fed the control diet. A significant reduction of Pax7 expression was evident, whereas myogenin was highly expressed in ALA-fed hamsters versus control, suggesting the occurrence of SC differentiation. However, no effect was seen in those fed a chow diet for 100 days followed by ALA for 50 days, suggesting an inability of ALA to reverse the established injury. Biochemical analysis revealed that ALA and EPA levels in muscles from ALA-fed hamsters were almost 2.5-fold higher than controls. Cytoplasmic accumulation of membrane proteins, caveolin-3 and β-catenin, involved in cell adhesion, membrane repair, and plasma membrane integrity was observed in the muscles of chow-fed dystrophic hamsters, whereas the normal morphological sarcolemmal pattern of these proteins was maintained in the muscles of dystrophic hamsters fed ALA. The diets (chow and ALA-rich) used in this study were not isocaloric, and their macronutrient composition was not reported. Also, the amounts of omega-6 and omega-3 fatty acids in the diets were not reported. The study may have produced different results if a higher amount of ALA was provided in the treatment arm. Providing longer chain fatty acids (ie, DHA, EPA, and DPA) derived from ALA may have produced different effects.77 An animal study showed that it is the provision of DHA and not the ratio of n-3/n-6 that is critical for skeletal muscle membrane change.78 Also, supplementation with EPA, but not DHA, partially improves skeletal muscle oxidative capacity.79
Limitations of the Studies Reviewed
The findings based on cell lines using omega-3 fatty acid treatment or supplementation may not necessarily correspond with physiologic responses in vivo. Although C2C12 cells express proteins necessary for myogenic differentiation and display the morphology of individual fiber units, there are striking differences between these cells and human adult muscles, particularly in their degree of maturation and mode of glucose transport.80,81 Nevertheless, this in vitro model is widely used to study the role of ALA, EPA, and DHA in activation, proliferation, and differentiation of SCs. In reviewed studies using C2C12 cell lines, the effect of omega-3 fatty acids on myogenic regulatory factors (ie, Pax7, MyoD, Myf5, and myogenin) has not been reported. These transcriptional factors are expressed in distinct expression patterns in fusing cells and influence skeletal muscle development.82 Also, studies demonstrate an inconsistent effect of ALA, EPA, and DHA on myoblast proliferation and myotube morphology.51,65,67 Bovine serum, commonly used in cell culture experiments, is an ill-defined mixture of components in culture media of varied fatty acid compositions from batch to batch.83,84 The fatty acid composition of the medium can significantly influence the fatty acid composition of cell lines and potentially alter actions of SCs.85 Chemically designed, serum-free media can be used to ensure the consistency of experimental conditions and results.
Animal models are an invaluable component of biomedical research, and many factors, such as genetic background and environmental factors, that can introduce variability need to be considered.86 However, most studies overlook the factors of dietary design, diet composition, and intake.9,86–88 Velleman et al reported an association between feed restriction and MyoD/myogenin expression of broiler pectoralis muscles, and decrease in SC proliferation and differentiation have been observed in methionine- and cysteine-restricted cell culture medium.89,90 Of the studies reviewed, two studies used standard chow diet and another used standard commercial diet (Nuvilab CR1), each of which has undefined macronutrient composition.69,70,76 The treatment groups were not exposed to isocaloric and isonitrogenous diets, which introduces variation in results as measures of muscle anabolism are highly responsive to anabolic effects of protein and insulin response. Individual omega-3 fatty acids have different therapeutic potentials, and so it is important to know the fat composition of diets used in the study, as dietary fat alters the fatty acid composition of the membrane, which in turn can affect the membrane and cell function.14–16,91,92 Of the studies reviewed, only two studies determined the fatty acid composition of muscles to confirm incorporation of omega-3 fatty acids in cell membranes after treatment or supplementation; however, in one study, DHA and EPA fatty acid levels were not presented.69,76 In addition to the fat composition of the diet, dosages of EPA and DHA should also be considered to determine if they are dietary achievable or therapeutic. Most studies that showed a systemic effect of EPA and DHA used intakes >0.03 g/kg.93 All animal studies reviewed employed higher dosages of EPA and DHA (0.06–1 g/kg); a wide variation in dosages makes it difficult to compare between studies. Studies should employ pair-fed controls to determine the extent to which the effect of a treatment on muscle wasting occurred independently of changes of energy intake and differentiate reduced feeding from other causes of muscle loss. The use of undefined or ill-matched dietary designs limits comparison between studies of results within the same study, translation across different study designs, as well as application to clinical studies.
While our understanding of murine SC biology is rapidly expanding, little is known about alterations in activation, proliferation, and differentiation of SCs in relation to various nutrient interventions. There are a few studies in C2C12 cells and five animal studies evaluating the effect of omega-3 fatty acids on the myogenic program. In contrast, there is no study exploring effects of omega-3 fatty acids on human SCs. This is primarily because of difficulty in obtaining human muscle biopsy, particularly from vulnerable populations like older adults and those with existing musculoskeletal disorders. Additionally, there are challenges to isolate SCs, such as scarcity, location under the basal lamina of muscle fibers, and separation from other cells within skeletal muscles. However, studies can be designed using commercially available human cell lines. There is need to conduct experiments using human SCs to explore the effects of omega-3 fatty acids, as it is difficult to determine if the phenotypes and functions of human and murine SCs are equivalent.
Future Perspectives
Omega-3 fatty acids show an inconsistent effect on SC proliferation, differentiation, and muscle regeneration in the experimental systems. Future studies should attend to dietary design (ie, isocaloric, isonitrogenous, and similar fatty acid composition), and food intake in animal studies needs to be recorded to control dietary intake as a variable in these studies.86 There are several inherent limitations of the experimental studies exploring this question to date, and a few studies have shown mechanisms by which omega-3 fatty acids induce their anabolic effect. There is a need to clarify the nature and mechanisms by which omega-3 fatty acids affect the myogenic potential of SCs. In C2C12 cell line studies, the effect of omega-3 fatty acids on myogenic regulatory factors (eg, Pax7, MyoD, myogenin, and Myf5) needs to be explored and at least three factors need to be measured to determine which part of the myogenic program is being affected. Also, it is important to consider differences between human SCs and experimental models. The effect of omega-3 fatty acids on human SCs has not been studied directly because of practical and methodological issues. However, to use omega-3 fatty acids as a potential therapeutic agent to prevent or treat muscle loss in chronic disease population, it is important to design clinical studies and investigate effects of these essential fatty acids on the myogenic program of human SCs.
Footnotes
ACADEMIC EDITOR: Joseph Zhou, Editor in Chief
PEER REVIEW: Four peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1861 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no external funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: VCM, ASB, CTP. Analyzed the data: VCM, ASB. Wrote the first draft of the manuscript: ASB. Contributed to the writing of the manuscript: ASB, CTP, VCM. Agree with manuscript results and conclusions: ASB, CTP, VCM. Jointly developed the structure and arguments for the paper: ASB, CTP, VCM. Made critical revisions and approved final version: ASB, CTP, VCM. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Evans W. Functional and metabolic consequences of sarcopenia. J Nutr. 1997;127(5 suppl):998s–1003s. doi: 10.1093/jn/127.5.998S. [DOI] [PubMed] [Google Scholar]
- 2.Martin L, Birdsell L, Macdonald N, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31(12):1539–1547. doi: 10.1200/JCO.2012.45.2722. [DOI] [PubMed] [Google Scholar]
- 3.Visser M, Schaap LA. Consequences of sarcopenia. Clin Geriatr Med. 2011;27(3):387–399. doi: 10.1016/j.cger.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Prado CM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9(7):629–635. doi: 10.1016/S1470-2045(08)70153-0. [DOI] [PubMed] [Google Scholar]
- 5.Phillips BE, Hill DS, Atherton PJ. Regulation of muscle protein synthesis in humans. Curr Opin Clin Nutr Metab Care. 2012;15(1):58–63. doi: 10.1097/MCO.0b013e32834d19bc. [DOI] [PubMed] [Google Scholar]
- 6.Farup J, Rahbek SK, Knudsen IS, et al. Whey protein supplementation accelerates satellite cell proliferation during recovery from eccentric exercise. Amino Acids. 2014;46(11):2503–2516. doi: 10.1007/s00726-014-1810-3. [DOI] [PubMed] [Google Scholar]
- 7.Olsen S, Aagaard P, Kadi F, et al. Creatine supplementation augments the increase in satellite cell and myonuclei number in human skeletal muscle induced by strength training. J Physiol. 2006;573(2):525–534. doi: 10.1113/jphysiol.2006.107359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farup J, Rahbek SK, Riis S, et al. Influence of exercise contraction mode and protein supplementation on human skeletal muscle satellite cell content and muscle fiber growth. J Appl Physiol. 2014;117(8):898–909. doi: 10.1152/japplphysiol.00261.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alway SE, Bennett BT, Wilson JC, et al. Epigallocatechin-3-gallate improves plantaris muscle recovery after disuse in aged rats. Exp Gerontol. 2014;50:82–94. doi: 10.1016/j.exger.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alway SE, Bennett BT, Wilson JC, et al. Green tea extract attenuates muscle loss and improves muscle function during disuse, but fails to improve muscle recovery following unloading in aged rats. J Appl Physiol. 2014;118(3):319–330. doi: 10.1152/japplphysiol.00674.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alway SE, Pereira SL, Edens NK, et al. beta-Hydroxy-beta-methylbutyrate (HMB) enhances the proliferation of satellite cells in fast muscles of aged rats during recovery from disuse atrophy. Exp Gerontol. 2013;48(9):973–984. doi: 10.1016/j.exger.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Alway SE, Myers MJ, Mohamed JS. Regulation of satellite cell function in sarcopenia. Front Aging Neurosci. 2014;6:246. doi: 10.3389/fnagi.2014.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson GJ, Wilson JM, Manninen AH. Effects of beta-hydroxy-beta-methylbutyrate (HMB) on exercise performance and body composition across varying levels of age, sex, and training experience: a review. Nutr Metab. 2008;5:1–1. doi: 10.1186/1743-7075-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clandinin MT, Cheema S, Field CJ, et al. Dietary fat: exogenous determination of membrane structure and cell function. FASEB J. 1991;5(13):2761–2769. doi: 10.1096/fasebj.5.13.1916101. [DOI] [PubMed] [Google Scholar]
- 15.Hulbert AJ, Turner N, Storlien LH, et al. Dietary fats and membrane function: implications for metabolism and disease. Biol Rev Camb Philos Soc. 2005;80(1):155–169. doi: 10.1017/s1464793104006578. [DOI] [PubMed] [Google Scholar]
- 16.Endo J, Arita M. Cardioprotective mechanism of omega-3 polyunsaturated fatty acids [published online ahead of print September 7, 2015] Journal of cardiology. doi: 10.1016/j.jjcc.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Calder PC. Mechanisms of action of (n-3) fatty acids. J Nutr. 2012;142(3):592s–599s. doi: 10.3945/jn.111.155259. [DOI] [PubMed] [Google Scholar]
- 18.Dangardt F, Chen Y, Gronowitz E, et al. High physiological omega-3 fatty acid supplementation affects muscle fatty acid composition and glucose and insulin homeostasis in obese adolescents. J Nutrit Metabol. 2012;2012:395757. doi: 10.1155/2012/395757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ewaschuk JB, Almasud A, Mazurak VC. Role of n-3 fatty acids in muscle loss and myosteatosis. Appl Physiol Nutr Metab. 2014;39(6):654–662. doi: 10.1139/apnm-2013-0423. [DOI] [PubMed] [Google Scholar]
- 20.Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seale P, Ishibashi J, Scime A, et al. Pax7 is necessary and sufficient for the myogenic specification of CD45+:Sca1+ stem cells from injured muscle. PLoS Biol. 2004;2(5):E130. doi: 10.1371/journal.pbio.0020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seale P, Sabourin LA, Girgis-Gabardo A, et al. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102(6):777–786. doi: 10.1016/s0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- 23.Collins-Hooper H, Woolley TE, Dyson L, et al. Age-related changes in speed and mechanism of adult skeletal muscle stem cell migration. Stem Cells. 2012;30(6):1182–1195. doi: 10.1002/stem.1088. [DOI] [PubMed] [Google Scholar]
- 24.von Maltzahn J, Jones AE, Parks RJ, et al. Pax7 is critical for the normal function of satellite cells in adult skeletal muscle. Proc Natl Acad Sci U S A. 2013;110(41):16474–16479. doi: 10.1073/pnas.1307680110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cornelison DD, Wold BJ. Single-cell analysis of regulatory gene expression in quiescent and activated mouse skeletal muscle satellite cells. Dev Biol. 1997;191(2):270–283. doi: 10.1006/dbio.1997.8721. [DOI] [PubMed] [Google Scholar]
- 26.Olguin HC, Yang Z, Tapscott SJ, et al. Reciprocal inhibition between Pax7 and muscle regulatory factors modulates myogenic cell fate determination. J Cell Biol. 2007;177(5):769–779. doi: 10.1083/jcb.200608122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dumont NA, Bentzinger CF, Sincennes MC, et al. Satellite cells and skeletal muscle regeneration. Compr Physiol. 2015;5(3):1027–1059. doi: 10.1002/cphy.c140068. [DOI] [PubMed] [Google Scholar]
- 28.Cornelison DD, Wilcox-Adelman SA, Goetinck PF, et al. Essential and separable roles for Syndecan-3 and Syndecan-4 in skeletal muscle development and regeneration. Genes Dev. 2004;18(18):2231–2236. doi: 10.1101/gad.1214204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cornelison DD, Filla MS, Stanley HM, et al. Syndecan-3 and syndecan-4 specifically mark skeletal muscle satellite cells and are implicated in satellite cell maintenance and muscle regeneration. Dev Biol. 2001;239(1):79–94. doi: 10.1006/dbio.2001.0416. [DOI] [PubMed] [Google Scholar]
- 30.Gutierrez J, Brandan E. A novel mechanism of sequestering fibroblast growth factor 2 by glypican in lipid rafts, allowing skeletal muscle differentiation. Mol Cell Biol. 2010;30(7):1634–1649. doi: 10.1128/MCB.01164-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dollenmeier P, Turner DC, Eppenberger HM. Proliferation and differentiation of chick skeletal muscle cells cultured in a chemically defined medium. Exp Cell Res. 1981;135(1):47–61. doi: 10.1016/0014-4827(81)90298-6. [DOI] [PubMed] [Google Scholar]
- 32.Robinson SM, Jameson KA, Batelaan SF, et al. Diet and its relationship with grip strength in community-dwelling older men and women: the Hertfordshire Cohort Study. J Am Geriatr Soc. 2008;56(1):84–90. doi: 10.1111/j.1532-5415.2007.01478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delarue J, LeFoll C, Corporeau C, et al. N-3 long chain polyunsaturated fatty acids: a nutritional tool to prevent insulin resistance associated to type 2 diabetes and obesity? Reprod Nutr Dev. 2004;44(3):289–299. doi: 10.1051/rnd:2004033. [DOI] [PubMed] [Google Scholar]
- 34.Murphy RA, Mourtzakis M, Chu QS, et al. Skeletal muscle depletion is associated with reduced plasma (n-3) fatty acids in non-small cell lung cancer patients. J Nutr. 2010;140(9):1602–1606. doi: 10.3945/jn.110.123521. [DOI] [PubMed] [Google Scholar]
- 35.Abbatecola AM, Cherubini A, Guralnik JM, et al. Plasma polyunsaturated fatty acids and age-related physical performance decline. Rejuvenation Res. 2009;12(1):25–32. doi: 10.1089/rej.2008.0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith GI, Atherton P, Reeds DN, et al. Dietary omega-3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: a randomized controlled trial. Am J Clin Nutr. 2011;93(2):402–412. doi: 10.3945/ajcn.110.005611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murphy RA, Mourtzakis M, Mazurak VC. n-3 polyunsaturated fatty acids: the potential role for supplementation in cancer. Curr Opin Clin Nutr Metab Care. 2012;15(3):246–251. doi: 10.1097/MCO.0b013e328351c32f. [DOI] [PubMed] [Google Scholar]
- 38.Pappalardo G, Almeida A, Ravasco P. Eicosapentaenoic acid in cancer improves body composition and modulates metabolism. Nutrition. 2015;31(4):549–555. doi: 10.1016/j.nut.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 39.Wigmore SJ, Fearon KC, Maingay JP, et al. Down-regulation of the acute-phase response in patients with pancreatic cancer cachexia receiving oral eicosapentaenoic acid is mediated via suppression of interleukin-6. Clin Sci. 1997;92(2):215–221. doi: 10.1042/cs0920215. [DOI] [PubMed] [Google Scholar]
- 40.Filvaroff EH, Ebner R, Derynck R. Inhibition of myogenic differentiation in myoblasts expressing a truncated type II TGF-beta receptor. Development. 1994;120(5):1085–1095. doi: 10.1242/dev.120.5.1085. [DOI] [PubMed] [Google Scholar]
- 41.Langen RC, Schols AM, Kelders MC, et al. Tumor necrosis factor-alpha inhibits myogenesis through redox-dependent and -independent pathways. Am J Physiol Cell Physiol. 2002;283(3):C714–C721. doi: 10.1152/ajpcell.00418.2001. [DOI] [PubMed] [Google Scholar]
- 42.Langen RC, Schols AM, Kelders MC, et al. Inflammatory cytokines inhibit myogenic differentiation through activation of nuclear factor-kappaB. FASEB J. 2001;15(7):1169–1180. doi: 10.1096/fj.00-0463. [DOI] [PubMed] [Google Scholar]
- 43.Tolosa L, Morla M, Iglesias A, et al. IFN-gamma prevents TNF-alpha-induced apoptosis in C2C12 myotubes through down-regulation of TNF-R2 and increased NF-kappaB activity. Cell Signal. 2005;17(11):1333–1342. doi: 10.1016/j.cellsig.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 44.Lecker SH, Goldberg AL, Mitch WE. Protein degradation by the ubiquitin-proteasome pathway in normal and disease states. J Am Soc Nephrol. 2006;17(7):1807–1819. doi: 10.1681/ASN.2006010083. [DOI] [PubMed] [Google Scholar]
- 45.Loell I, Lundberg IE. Can muscle regeneration fail in chronic inflammation: a weakness in inflammatory myopathies? J Intern Med. 2011;269(3):243–257. doi: 10.1111/j.1365-2796.2010.02334.x. [DOI] [PubMed] [Google Scholar]
- 46.Calder PC. Marine omega-3 fatty acids and inflammatory processes: effects, mechanisms and clinical relevance. Biochim Biophys Acta. 2015;1851(4):469–484. doi: 10.1016/j.bbalip.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 47.Yaffe D, Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 1977;270(5639):725–727. doi: 10.1038/270725a0. [DOI] [PubMed] [Google Scholar]
- 48.Blau H, Pavlath G, Hardeman E, et al. Plasticity of the differentiated state. Science. 1985;230(4727):758–766. doi: 10.1126/science.2414846. [DOI] [PubMed] [Google Scholar]
- 49.Burattini S, Ferri P, Battistelli M, et al. C2C12 murine myoblasts as a model of skeletal muscle development: morpho-functional characterization. Eur J Histochem. 2004;48(3):223–233. [PubMed] [Google Scholar]
- 50.Diel P, Baadners D, Schlupmann K, et al. C2C12 myoblastoma cell differentiation and proliferation is stimulated by androgens and associated with a modulation of myostatin and Pax7 expression. J Mol Endocrinol. 2008;40(5):231–241. doi: 10.1677/JME-07-0175. [DOI] [PubMed] [Google Scholar]
- 51.Magee P, Pearson S, Allen J. The omega-3 fatty acid, eicosapentaenoic acid (EPA), prevents the damaging effects of tumour necrosis factor (TNF)-alpha during murine skeletal muscle cell differentiation. Lipids Health Dis. 2008;7:24. doi: 10.1186/1476-511X-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Langen RC, Van Der Velden JL, Schols AM, et al. Tumor necrosis factor-alpha inhibits myogenic differentiation through MyoD protein destabilization. FASEB J. 2004;18(2):227–237. doi: 10.1096/fj.03-0251com. [DOI] [PubMed] [Google Scholar]
- 53.Layne MD, Farmer SR. Tumor necrosis factor-alpha and basic fibroblast growth factor differentially inhibit the insulin-like growth factor-I induced expression of myogenin in C2C12 myoblasts. Exp Cell Res. 1999;249(1):177–187. doi: 10.1006/excr.1999.4465. [DOI] [PubMed] [Google Scholar]
- 54.Magee P, Pearson S, Whittingham-Dowd J, et al. PPARgamma as a molecular target of EPA anti-inflammatory activity during TNF-alpha-impaired skeletal muscle cell differentiation. J Nutr Biochem. 2012;23(11):1440–1448. doi: 10.1016/j.jnutbio.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 55.Apolinario LM, De Carvalho SC, Santo Neto H, et al. Long-term therapy with omega-3 ameliorates myonecrosis and benefits skeletal muscle regeneration in Mdx mice. Anat Rec (Hoboken) 2015;298(9):1589–1596. doi: 10.1002/ar.23177. [DOI] [PubMed] [Google Scholar]
- 56.Eisele PS, Handschin C. Functional crosstalk of PGC-1 coactivators and inflammation in skeletal muscle pathophysiology. Semin Immunopathol. 2014;36(1):27–53. doi: 10.1007/s00281-013-0406-4. [DOI] [PubMed] [Google Scholar]
- 57.Sacheck JM, Hyatt JP, Raffaello A, et al. Rapid disuse and denervation atrophy involve transcriptional changes similar to those of muscle wasting during systemic diseases. FASEB J. 2007;21(1):140–155. doi: 10.1096/fj.06-6604com. [DOI] [PubMed] [Google Scholar]
- 58.Adhihetty PJ, O’Leary MF, Chabi B, et al. Effect of denervation on mitochondrially mediated apoptosis in skeletal muscle. J Appl Physiol. 2007;102(3):1143–1151. doi: 10.1152/japplphysiol.00768.2006. [DOI] [PubMed] [Google Scholar]
- 59.Hanai J, Cao P, Tanksale P, et al. The muscle-specific ubiquitin ligase atrogin-1/MAFbx mediates statin-induced muscle toxicity. J Clin Invest. 2007;117(12):3940–3951. doi: 10.1172/JCI32741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sandri M, Lin J, Handschin C, et al. PGC-1alpha protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc Natl Acad Sci U S A. 2006;103(44):16260–16265. doi: 10.1073/pnas.0607795103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Handschin C, Choi CS, Chin S, et al. Abnormal glucose homeostasis in skeletal muscle-specific PGC-1alpha knockout mice reveals skeletal muscle-pancreatic beta cell crosstalk. J Clin Invest. 2007;117(11):3463–3474. doi: 10.1172/JCI31785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chaturvedi RK, Adhihetty P, Shukla S, et al. Impaired PGC-1alpha function in muscle in Huntington’s disease. Hum Mol Genet. 2009;18(16):3048–3065. doi: 10.1093/hmg/ddp243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lin Y, Zhao Y, Li R, et al. PGC-1α is associated with C2C12 Myoblast differentiation. Cent Eur J Biol. 2014;9(11):1030–1036. [Google Scholar]
- 64.Vaughan RA, Garcia-Smith R, Bisoffi M, et al. Conjugated linoleic acid or omega 3 fatty acids increase mitochondrial biosynthesis and metabolism in skeletal muscle cells. Lipids Health Dis. 2012;11:142. doi: 10.1186/1476-511X-11-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bryner RW, Woodworth-Hobbs ME, Williamson DL, et al. Docosahexaenoic acid protects muscle cells from palmitate-induced atrophy. ISRN Obes. 2012;2012:14. doi: 10.5402/2012/647348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang M, Wei D, Mo C, et al. Saturated fatty acid palmitate-induced insulin resistance is accompanied with myotube loss and the impaired expression of health benefit myokine genes in C2C12 myotubes. Lipids Health Dis. 2013;12:104. doi: 10.1186/1476-511X-12-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Peng Y, Zheng Y, Zhang Y, et al. Different effects of omega-3 fatty acids on the cell cycle in C2C12 myoblast proliferation. Mol Cell Biochem. 2012;367(1–2):165–173. doi: 10.1007/s11010-012-1329-4. [DOI] [PubMed] [Google Scholar]
- 68.Dardevet D, Sornet C, Savary I, et al. Glucocorticoid effects on insulin- and IGF-I-regulated muscle protein metabolism during aging. J Endocrinol. 1998;156(1):83–89. doi: 10.1677/joe.0.1560083. [DOI] [PubMed] [Google Scholar]
- 69.Fappi A, Godoy TS, Maximino JR, et al. The effects of omega-3 fatty acid supplementation on dexamethasone-induced muscle atrophy. Biomed Res Int. 2014;2014:13. doi: 10.1155/2014/961438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Castillero E, Martin AI, Lopez-Menduina M, et al. Eicosapentaenoic acid attenuates arthritis-induced muscle wasting acting on atrogin-1 and on myogenic regulatory factors. Am J Physiol Regul Integr Comp Physiol. 2009;297(5):R1322–R1331. doi: 10.1152/ajpregu.00388.2009. [DOI] [PubMed] [Google Scholar]
- 71.Penna F, Busquets S, Pin F, et al. Combined approach to counteract experimental cancer cachexia: eicosapentaenoic acid and training exercise. J Cachexia Sarcopenia Muscle. 2011;2(2):95–104. doi: 10.1007/s13539-011-0028-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smith HJ, Greenberg NA, Tisdale MJ. Effect of eicosapentaenoic acid, protein and amino acids on protein synthesis and degradation in skeletal muscle of cachectic mice. Br J Cancer. 2004;91(2):408–412. doi: 10.1038/sj.bjc.6601981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McFarland DC, Velleman SG, Pesall JE, et al. Effect of lipids on avian satellite cell proliferation, differentiation and heparan sulfate proteoglycan expression. Comp Biochem Physiol A Mol Integr Physiol. 2011;159(2):188–195. doi: 10.1016/j.cbpa.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 74.Shin J, McFarland DC, Velleman SG. Heparan sulfate proteoglycans, syndecan-4 and glypican-1, differentially regulate myogenic regulatory transcription factors and paired box 7 expression during turkey satellite cell myogenesis: implications for muscle growth. Poult Sci. 2012;91(1):201–207. doi: 10.3382/ps.2011-01695. [DOI] [PubMed] [Google Scholar]
- 75.Libran-Perez M, Figueiredo-Silva AC, Panserat S, et al. Response of hepatic lipid and glucose metabolism to a mixture or single fatty acids: possible presence of fatty acid-sensing mechanisms. Comp Biochem Physiol A Mol Integr Physiol. 2013;164(1):241–248. doi: 10.1016/j.cbpa.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 76.Fiaccavento R, Carotenuto F, Vecchini A, et al. An omega-3 fatty acid-enriched diet prevents skeletal muscle lesions in a hamster model of dystrophy. Am J Pathol. 2010;177(5):2176–2184. doi: 10.2353/ajpath.2010.100174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Burdge GC, Calder PC. Conversion of alpha-linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reprod Nutr Dev. 2005;45(5):581–597. doi: 10.1051/rnd:2005047. [DOI] [PubMed] [Google Scholar]
- 78.Slee EL, McLennan PL, Owen AJ, et al. Low dietary fish-oil threshold for myocardial membrane n-3 PUFA enrichment independent of n-6 PUFA intake in rats. J Lipid Res. 2010;51(7):1841–1848. doi: 10.1194/jlr.M004069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Johnson ML, Lalia AZ, Dasari S, et al. Eicosapentaenoic acid but not docosahexaenoic acid restores skeletal muscle mitochondrial oxidative capacity in old mice. Aging Cell. 2015;14(5):734–743. doi: 10.1111/acel.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Langelaan ML, Boonen KJ, Rosaria-Chak KY, et al. Advanced maturation by electrical stimulation: Differences in response between C2C12 and primary muscle progenitor cells. J Tissue Eng Regen Med. 2011;5(7):529–539. doi: 10.1002/term.345. [DOI] [PubMed] [Google Scholar]
- 81.Kotliar N, Pilch PF. Expression of the glucose transporter isoform GLUT 4 is insufficient to confer insulin-regulatable hexose uptake to cultured muscle cells. Mol Endocrinol. 1992;6(3):337–345. doi: 10.1210/mend.6.3.1584210. [DOI] [PubMed] [Google Scholar]
- 82.Dedieu S, Mazeres G, Cottin P, et al. Involvement of myogenic regulator factors during fusion in the cell line C2C12. Int J Dev Biol. 2002;46(2):235–241. [PubMed] [Google Scholar]
- 83.Shah G. Why do we still use serum in the production of biopharmaceuticals? Dev Biol Stand. 1999;99:17–22. [PubMed] [Google Scholar]
- 84.Gstraunthaler G, Lindl T, van der Valk J. A plea to reduce or replace fetal bovine serum in cell culture media. Cytotechnology. 2013;65(5):791–793. doi: 10.1007/s10616-013-9633-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stoll L, Spector A. Changes in serum influence the fatty acid composition of established cell lines. In Vitro. 1984;20(9):732–738. doi: 10.1007/BF02618879. [DOI] [PubMed] [Google Scholar]
- 86.Giles K, Guan C, Jagoe TR, Mazurak V. Diet composition as a source of variation in experimental animal models of cancer cachexia. Journal of Cachexia, Sarcopenia and Muscle. doi: 10.1002/jcsm.12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hashimoto Y, Yamada K, Tsushima H, et al. Three dissimilar high fat diets differentially regulate lipid and glucose metabolism in obesity-resistant Slc:Wistar/ST rats. Lipids. 2013;48(8):803–815. doi: 10.1007/s11745-013-3805-3. [DOI] [PubMed] [Google Scholar]
- 88.Romsos DR, Leveille GA. Effect of diet on activity of enzymes involved in fatty acid and cholesterol synthesis. Adv Lipid Res. 1974;12(0):97–146. [PubMed] [Google Scholar]
- 89.Velleman SG, Nestor KE, Coy CS, et al. Effect of posthatch feed restriction on broiler breast muscle development and muscle transcriptional regulatory factor gene and heparan sulfate proteoglycan expression. Int J Poult Sci. 2010;9(5):417–425. [Google Scholar]
- 90.Powell DJ, McFarland DC, Cowieson AJ, et al. The effect of nutritional status on myogenic satellite cell proliferation and differentiation. Poult Sci. 2013;92(8):2163–2173. doi: 10.3382/ps.2013-03107. [DOI] [PubMed] [Google Scholar]
- 91.Field CJ, Ryan EA, Thomson AB, et al. Diet fat composition alters membrane phospholipid composition, insulin binding, and glucose metabolism in adipocytes from control and diabetic animals. J Biol Chem. 1990;265(19):11143–11150. [PubMed] [Google Scholar]
- 92.Fetterman JW, Jr, Zdanowicz MM. Therapeutic potential of n-3 polyunsaturated fatty acids in disease. Am J Health Syst Pharm. 2009;66(13):1169–1179. doi: 10.2146/ajhp080411. [DOI] [PubMed] [Google Scholar]
- 93.Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83(6 suppl):1505s–1519s. doi: 10.1093/ajcn/83.6.1505S. [DOI] [PubMed] [Google Scholar]