Abstract
Objectives
Although persons who inject drugs have high prevalence of hepatitis C virus (HCV) infection, few receive treatment mostly because of lack of knowledge about the infection and its treatment. We assessed the level of HCV-related knowledge and willingness to participate in HCV treatment among methadone-maintained patients.
Methods
A 30-item survey covering HCV-related knowledge and willingness to engage in HCV-related education and treatment was developed and completed by 320 methadone-maintained patients.
Results
Respondents’ mean age was 53 ± 8.7 years, 59.5% were male, 55.1% were African American, and 38.3% were Hispanic. The mean duration of methadone maintenance was 7 ± 6.7 years. In the preceding 6 months, 6.9% of patients reported injection drug use, whereas 37.3% used noninjection drugs. Hepatitis C virus seropositivity was self-reported by 46.3% of patients. The majority of patients (78%) expressed willingness to participate in HCV-related education and to receive HCV treatment. Most patients (54.7%) correctly answered 5 or more of 7 questions assessing HCV knowledge. Hepatitis C virus–seropositive individuals and prior attendees at HCV-related educational activities demonstrated a higher level of HCV-related knowledge (P < 0.001 and P = 0.002, respectively). Younger patients (P = 0.014), those willing to attend an HCV-related educational activity (P < 0.001), and those with higher–HCV-related knowledge (P = 0.029) were more accepting of HCV treatment. Fear of medication-related side effects was the most common reason for treatment avoidance.
Conclusions
The majority of patients reported willingness to receive HCV-related education and treatment. Treatment willingness was significantly associated with previous attendance at an HCV educational activity and a higher level of HCV-related knowledge.
Keywords: drug treatment, HCV education, knowledge, models of care for hepatitis C, persons who inject drugs
Hepatitis C virus (HCV) infection affects more than 150 million people worldwide (World Health Organization, 2013) and an estimated 3.2 million individuals in the United States (Armstrong et al., 2006; Chak et al., 2011). Acute HCV infection is usually asymptomatic and is rarely diagnosed; yet, 75% to 85% of acutely infected persons develop chronic infection that can eventually progress to liver cirrhosis and/or hepatocellular carcinoma (World Health Organization, 2013). Consequently, HCV-related disease is the most common indication for liver transplantation in the United States.
Because HCV is a blood-borne pathogen and injection drug use is a primary mode of its transmission in developed countries, HCV infection prevalence among persons who inject drugs (PWID) reaches as high as 80%, whereas the annual incidence ranges from 16% to 42% (Edlin and Carden, 2006; Amon et al., 2008; Nelson et al., 2011). Despite high prevalence, however, participation of PWID in HCV-related care has been extremely low (Mehta et al., 2008).
Until recently, the combination of pegylated-interferon and ribavirin was the standard treatment for HCV infection, and it resulted in viral eradication in approximately one-half of treated patients (Manns et al., 2001; Fried et al., 2002). Since 2011, several additional drugs belonging to the category of directly acting antivirals (DAAs) have been approved in combination with pegylated-interferon and ribavirin for treatment of HCV-infected patients. These medications have shown significantly increased treatment efficacy in clinical trials, but certain agents may result in an increase in both the frequency and severity of treatment-associated adverse effects. In addition, treatment with DAAs requires rigorous adherence to minimize the development of resistant viral variants with some of the agents (Kwo et al., 2010; Jacobson et al., 2011; Vermehren and Sarrazin, 2012). The increased complexity of new treatment regimens with choices from different DAA classes, their increased expense, and the necessity of strict adherence to therapy adds new challenges to HCV treatment of PWID.
Despite the fact that PWID, both former and current, represent the majority of HCV-infected people in the United States, only a small minority have been evaluated and treated for HCV infection (Schackman et al., 2007; Grebely et al., 2008; Mehta et al., 2008). There are several reasons for the suboptimal HCV treatment uptake among PWID, attributable to both patient and provider factors (Zeremski et al., 2013). Historically, limited knowledge about hepatitis C and concerns about treatment-related side effects among PWID have resulted in a low perceived need for and fear of treatment (Strauss et al., 2007; Mehta et al., 2008; Cohen-Moreno et al., 2010; Chen et al., 2013). Also, longstanding distrust between PWID and the medical community has contributed to feelings of stigmatization, which has been shown to negatively influence willingness of PWID to initiate and maintain HCV therapy (Mehta et al., 2008). Many medical practitioners are hesitant to prescribe HCV treatment to patients with a history of injection drug use largely because of concerns about poor adherence to therapy, psychiatric comorbidities, and the potential for re-infection after treatment cessation (Morrill et al, 2005; Myles et al., 2011). However, multiple studies have demonstrated that PWID achieve sustained virological responses similar to non-injectors in registration trials (Dimova et al., 2013).
Opiate agonist treatment (OAT) clinics and other drug treatment facilities may provide an infrastructure that can be used to circumvent many obstacles to evaluation and treatment for HCV of PWID. In addition to the stabilization of patients’ addictions, these venues may also provide a portal for PWID to enter into the health care system. In some cases, these facilities employ multidisciplinary teams that may be capable of addressing the medical comorbidities and the social service needs of PWID on-site. The majority of OAT facilities, however, do not have the capacity or the infrastructure necessary to offer this level of care (Bini et al., 2011, 2012); even among those venues where primary care medical services are available on-site, few programs seem to offer HCV-related care. Other barriers to the engagement of PWID in hepatitis C treatment, such as patients’ lack of knowledge, must also be addressed to obtain their full participation in care. New models that address these issues are urgently needed for successful HCV treatment among former and current PWID.
This study was conducted as the initial stage of the “Prevention, Evaluation, and Treatment of Hepatitis C in Opiate Agonist Treatment (PET-C)” project. The PET-C project has an overarching objective of testing the feasibility of a unique care model for HCV management and treatment for PWID enrolled in OAT. The objective of this study was to examine the willingness of PWID to receive HCV-related education and treatment and to better understand the relationship between HCV-related knowledge and willingness to receive treatment.
METHODS
Study participants were recruited from an OAT clinic with a patient population of approximately 550 to 600. The agency is a private not-for-profit 501(c)(3) corporation with 7 treatment programs located in inner-city neighborhoods in New York City, serving predominantly minority populations that experience disparities in health care–related access and outcomes. The medical personnel at these programs also provide primary care, including management of human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS).
The survey was conducted between November 2012 and February 2013. All methadone-maintained patients currently attending the OAT clinic and willing to participate in the survey were included in the study. To recruit patients, flyers were posted at the patient sign-in counter located near the common entrance to the clinic beginning several days before the start of the survey. Participation in the study was voluntary, and all participants provided written informed consent before initiation of study activities. The survey and informed consent were offered in English and Spanish. Patients were compensated with a 5-ride transportation card (a value of $11.25) for their participation. The study was approved by institutional review boards of the Weill Cornell Medical College, University at Buffalo, and the study site and was performed in accordance with the Declaration of Helsinki.
Recruitment for the survey was conducted by 2 members of the research team who were present on-site to offer bilingual assistance (English and Spanish) during a 6-hour period, 2 days per week. Patients were instructed to answer the HCV-related knowledge questions to the best of their ability and to skip questions to which they did not know the answer. Patients were also reminded that the disclosure of their HCV infection status was voluntary and that they could decline to answer any questions they wished. The questionnaire was administered to groups of 6 patients and was supervised by 1 researcher who was available to answer any questions that arose. Several patients who had difficulties with reading or writing completed the questionnaire in a face-to-face interview with a researcher.
Data Collection
The survey consisted of 2 parts. In the first part, composed of 23 questions, participants were asked about their demographics (age, sex, and race/ethnicity), education level, and employment/disability status. We inquired about their former and current (within last 6 months) drug use and their HCV infection status. We assessed willingness to participate in HCV-related educational and treatment activities. For individuals who indicated that they were unwilling to participate in HCV treatment and educational activities, we inquired about the specific reasons for the lack of willingness to participate and whether their willingness could be affected by the offer of an incentive. The second part of the survey was composed of 7 questions designed to assess general knowledge regarding hepatitis C pathogenesis and treatment. The survey instrument is provided as Supplemental Digital Content 1, available at http://links.lww.com/JAM/A18.
Statistical Analysis
Statistical analysis was performed with SAS (SAS Institute Inc, Cary, North Carolina) and R (http://www.r-project.org/). Associations between categorical variables were assessed through the Fisher exact test and logistic regression. For continuous variables, comparisons between groups were performed using the Wilcoxon rank sum or Kruskal-Wallis tests. We assessed the effect of independent variables (eg, demographics) on willingness to participate in the HCV-related education and treatment via logistic regression. We used a stepwise selection strategy on the basis of the Wald test for the individual parameters. The goodness of fit of the final model was tested through the Hosmer-Lemeshow goodness-of-fit test. The HCV-related knowledge level is reported as the number of correctly answered questions, and we assessed the influence of potential covariates on patient's knowledge level through ordinal logistic regression in which the outcome was the cumulative log-odds of achieving a higher score on the HCV-related knowledge assessment test. The proportional odds assumption was verified through the score chi-square test, and in the case when it was not satisfied, the generalized logit model was used. The significance level in all tests (2-sided) was set to 0.05.
RESULTS
Study Participants
The mean age of the 320 respondents was 53 ± 8.7 years, 59.5% were male, 55.1% were African American, and 38.3% were Hispanic (Table 1). Respondents were largely stable on methadone with a mean duration of methadone maintenance of 7 ± 6.7 years. Five patients had attended the OAT clinic for more than 28 years. The majority of the patients had completed at least secondary education: 48.1% had a high school diploma or the equivalent (GED), and 12.2% had at least an associate degree. The vast majority of patients (93.4%) were unemployed, and 62.3% were on disability.
TABLE 1.
Patient Characteristics
| Variable | n Respondents | n (%) or Mean (SD) |
|---|---|---|
| Age, yr | 319 | 53.15 (8.72) |
| Sex | 316 | |
| Male | 188 (59.49) | |
| Female | 128 (40.51) | |
| Race | 312 | |
| White | 28 (8.97) | |
| Hispanic | 7 (2.32) | |
| Non-Hispanic | 21 (6.95) | |
| African American | 172 (55.13) | |
| Hispanic | 7 (2.32) | |
| Non-Hispanic | 157 (51.99) | |
| Mixed | 21 (6.73) | |
| Hispanic | 14 (4.64) | |
| Non-Hispanic | 6 (1.99) | |
| Other | 91 (29.17) | |
| Hispanic | 84 (27.81) | |
| Non-Hispanic | 6 (1.99) | |
| Ethnicity | 308 | |
| Hispanic | 118 (38.31) | |
| Non-Hispanic | 190 (61.69) | |
| Duration of OAT program attendance, yrs | 314 | 7.03 (6.70) |
| Education | 320 | |
| No GED/high school diploma | 127 (39.69) | |
| GED/high school | 154 (48.13) | |
| Associates degree | 22 (6.88) | |
| College degree | 14 (4.38) | |
| Masters or doctorate degree | 3 (0.94) | |
| Employed | 320 | 21 (6.56) |
| On disability | 318 | 198 (62.26) |
GED, general equivalency degree; OAT, opiate agonist therapy.
Drug Use and HCV Infection Status
History of injection and noninjection drug use was reported by 56.9% and 93.8% of respondents, respectively. Participants who admitted a history of injection drug use were older (55.1 ± 9.1 vs 50.6 ± 7.5 years, P < 0.001) and had been on methadone substitution for a longer period of time (7.8 ± 7 vs 6 ± 6.2 years, P = 0.015) compared with those who denied injection drug use.=Recent (preceding 6 months) injection and noninjection drug use occurred among 6.9% (22/320) and 37.3% (119/319) of respondents, respectively, with heroin, cocaine, and crack being the preferred drugs in both patient groups. Use of benzodiazepines, marijuana, prescription opioids, and amphetamines was much less common (Table 1).
Of the 320 respondents, 148 (46.3%) reported positive HCV infection status, 155 (48.4%) reported negative status, and 17 (5.4%) were unsure of their status. Self-reported HCV-positive respondents were significantly older (P = 0.009) than other patients. HCV positivity was associated with a history of injection drug use (P < 0.001) and recent injection drug use (P = 0.049); HCV infection was reported by 70.9% (129 of the 182) of persons who reported ever injecting and among only 13.8% (19 of the 138) of persons who never injected (P < 0.001).
Willingness to Engage in HCV Education
Half of respondents (58.3%) were aware of the periodically available on-site provision of HCV-related education, and one-third (35.5%) had attended such activities. An additional one-quarter (25.5%) reported attending such activities elsewhere. The majority of respondents (78.3%) stated that they would be willing to participate in future on-site educational activities. Whites and participants without a high school diploma or the equivalent were significantly more likely to indicate willingness to participate in future educational activities than non-whites (96.3% vs 76.7%; odds ratio [OR] = 7.90; 95% confidence interval [CI], 1.05-59.33; P = 0.044) or patients with at least an associate degree (81% vs 64.1%; OR = 2.38; 95% CI, 1.08-5.25; P = 0.032), respectively (Table 2). There was no significant difference in willingness to engage in HCV education between those who reported HCV positivity and those who did not (P = 0.326). Participants who had previously attended an HCV educational activity were also more willing to attend one in the future (85.6% vs 66.9%; OR = 2.93; 95% CI, 1.69-5.07; P < 0.001). Males and employed participants were less willing to attend future educational activities compared with females (73.8% vs 84.3%; OR = 0.53; 95% CI, 0.30-0.94; P = 0.030) and with those who were unemployed (52.4% vs 80.1%; OR = 0.27; 95% CI, 0.11-0.67; P = 0.005), respectively. Finally, patients who were willing to receive HCV treatment were more likely to attend a future educational activity than those who were unwilling to be treated (85% vs 54.3%; OR = 4.76; 95% CI, 2.65-8.55; P < 0.001). On multivariable analysis, willingness to participate in an HCV educational program was associated with female (P = 0.040), unemployment (P = 0.010), previous participation in an educational program (P = 0.014), and willingness to receive HCV treatment (P < 0.001) (Table 2).
TABLE 2.
Factors Associated With Willingness to Participate in HCV Education
| Variable | n | Willing n (%) or Mean (SD) | Not Willing n (%) or Mean (SD) | Univariable Model |
Multivariable Model |
||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | ||||
| Age, yr | 317 | 53.24 (8.91) | 53.19 (7.83) | 1 | 0.97-1.03 | 0.964 | |||
| Sex, n (%) | 314 | ||||||||
| Male | 138 (73.8) | 49 (26.2) | 0.53 | 0.30-0.94 | 0.030* | 0.52 | 0.27;0.97 | 0.040* | |
| Female† | 107 (84.25) | 20 (15.75) | |||||||
| Race, n (%) | 310 | ||||||||
| White | 26 (96.3) | 1 (3.7) | 7.9 | 1.05-59.33 | 0.044* | ||||
| Non-White† | 217 (76.68) | 66 (23.32) | |||||||
| Ethnicity, n (%) | 306 | ||||||||
| Latin | 92 (78.63) | 25 (21.37) | 1.02 | 0.58-1.79 | 0.946 | ||||
| Non-Latin† | 148 (78.31) | 41 (21.69) | |||||||
| Duration of OAT attendance, yrs | 312 | 6.87 (6.36) | 7.71 (7.86) | 0.98 | 0.95-1.02 | 0.362 | |||
| Education, n (%) | 318 | 0.077 | |||||||
| No GED/high school diploma† | 102 (80.95) | 24 (19.05) | |||||||
| GED/high school | 122 (79.74) | 31 (20.26) | 0.93 | 0.51-1.68 | |||||
| Associates degree or higher | 25 (64.10) | 14 (35.90) | 0.42 | 0.19-0.93 | |||||
| Employed, n (%) | 318 | 0.27 | 0.11-0.67 | 0.005* | 0.010*‡ | ||||
| Yes | 11 (52.38) | 10 (47.62) | |||||||
| No† | 238 (80.13) | 59 (19.87) | |||||||
| Disability, n (%) | 316 | 0.57 | 0.32-1.02 | 0.059 | |||||
| Yes | 148 (74.75) | 50 (25.25) | |||||||
| No† | 99 (83.90) | 19 (16.10) | |||||||
| History of injecting drugs | 318 | 1.10 | 0.64-1.88 | 0.727 | |||||
| Yes | 143 (79.01) | 38 (20.99) | |||||||
| No† | 106 (77.37) | 31 (22.63) | |||||||
| History of noninjection drugs | 318 | 3.25 | 1.29-8.19 | 0.013* | |||||
| Yes | 238 (79.87) | 60 (20.13) | |||||||
| No† | 11 (55) | 9 (45) | |||||||
| Injected drugs during the last 6 months | 318 | 1.27 | 0.41-3.87 | 0.679 | |||||
| Yes | 18 (81.82) | 4 (18.18) | |||||||
| No† | 231 (78.04) | 65 (21.96) | |||||||
| Used noninjection drugs in the last 6 months | 317 | 1.47 | 0.83-2.60 | 0.189 | |||||
| Yes | 97 (82.20) | 21 (17.80) | |||||||
| No† | 151 (75.88) | 48 (24.12) | |||||||
| Tested for HCV | 318 | 2.38 | 0.94-6.00 | 0.066 | |||||
| Yes | 236 (79.46) | 61 (20.54) | |||||||
| No† | 13 (61.90) | 8 (38.10) | |||||||
| Do you have hepatitis C? | 318 | ||||||||
| Yes† | 113 (76.87) | 34 (23.13) | 0.326 | ||||||
| No | 120 (77.92) | 34 (22.08) | 1.06 | 0.62-1.82 | |||||
| I do not know | 16 (94.12) | 1 (5.88) | 4.81 | 0.62-37.63 | |||||
| Have you ever attended an educational program about hepatitis C? | 318 | ||||||||
| Yes | 166 (85.57) | 28 (14.43) | 2.93 | 1.69-5.07 | <0.001* | 0.014*‡ | |||
| No† | 83 (66.94) | 41 (33.06) | |||||||
| If you have ever been diagnosed with hepatitis C virus infection, would you be willing to be treated? | 316 | ||||||||
| Yes | 209 (84.96) | 37 (15.04) | 4.76 | 2.65-8.55 | <0.001* | 4.75 | 2.53;8.92 | <0.001* | |
| No or not sure† | 38 (54.29) | 32 (45.71) | |||||||
P < 0.05.
Reference group for the reported odds ratios.
There is an interaction between employed and previous attendance of an educational program, P = 0.046:
Employed vs unemployed at ever attended HCV education: OR = 0.91; 95% CI, 0.18-4.70.
Employed vs unemployed atneverattended HCV education: OR= 0.06; 95% CI, 0.01-0.50.
Ever vs never attended HCV education at employed: OR = 35.08; 95% CI, 2.43-506.13.
Ever vs never attended HCV education at unemployed: OR = 2.17; 95% CI, 1.17-4.00.
CI, confidence interval; GED, general equivalency degree; HCV, hepatitis C virus; OAT, opiate agonist therapy; OR, odds ratio.
Of the 68 patients who reported an unwillingness to attend a future HCV-related educational activity, 26 (38%) indicated that an incentive could positively affect their decision with 17 (65%) preferring money and 9 (35%) preferring a transportation voucher. The majority, 42 patients, indicated that an incentive would not affect their decision to attend an HCV-related educational activity.
Willingness to Engage in HCV Treatment
When asked whether participants would be willing to be treated if they were ever diagnosed with HCV, 78% (248 of the 318) indicated willingness to be treated, 16.7% (53 of the 318) were unwilling, and 5.4% (17 of the 318) were unsure. Participants who expressed willingness to receive HCV treatment were significantly younger (52.5 ± 9 vs 55.4 ± 7.3 years, P < 0.013), a finding that persisted on multivariable analysis (OR = 0.96; 95% CI, 0.92-0.99; P = 0.014) (Table 3). There was a trend for lower willingness=among those who reported HCV positivity (P = 0.076), but it was not significant on multivariable analysis. We also found that participation in HCV-related education, through either previous attendance (P = 0.038) or willingness for future participation (P < 0.001), predicted willingness to receive HCV treatment. In addition, patients who scored higher on HCV-related knowledge questions (responding correctly to 5 or more questions) were more likely to be willing to receive HCV treatment (P = 0.029). Reasons for unwillingness to be treated included fear of side effects (n = 9), a prior unsuccessful treatment course (n = 8), a desire for further discussion with a health care provider (n = 6), patient request for additional information (n = 4), questionable diagnosis of HCV infection (n = 4), and competing medical priorities (n = 3). Fifteen participants did not provide a clear explanation for their lack of willingness to receive HCV treatment; answers typical of these individuals were “I just do not want to” and “I will leave it to the Lord.”
TABLE 3.
Predictors of Willingness to Be Treated
| Variable | n | Willing to Be Treated, n (%) or Mean (SD) | Not Willing or Not Sure, n (%) or Mean (SD) | Univariable Logistic Regression |
Multivariable Regression |
||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | ||||
| Age, yr | 317 | 52.47 (9.01) | 55.45 (7.28) | 0.96 | 0.93-0.99 | 0.013* | 0.96 | 0.92-0.99 | 0.014* |
| Sex, n (%) | 314 | ||||||||
| Male | 146 (78.49) | 40 (21.51) | 1.07 | 0.62-1.84 | 0.809 | ||||
| Female† | 99 (77.34) | 29 (22.66) | |||||||
| Race, n (%) | 310 | ||||||||
| White | 27 (96.43) | 1 (3.57) | 8.85 | 1.17-67.10 | |||||
| African American† | 128 (75.29) | 42 (24.71) | 0.216 | ||||||
| Mixed | 16 (76.19) | 5 (23.81) | 1.05 | 0.36-3.04 | |||||
| Other | 70 (76.92) | 21 (23.08) | 1.09 | 0.60-1.99 | |||||
| Ethnicity, n (%) | 306 | ||||||||
| Latin | 94 (79.66) | 24 (20.34) | 1.18 | 0.68-2.10 | 0.530 | ||||
| Non-Latin† | 144 (76.60) | 44 (23.40) | |||||||
| Duration of OAT attendance, yrs | 312 | 6.633 (6.45) | 8.59 (7.45) | 0.96 | 0.93-1.00 | 0.037* | |||
| Education | 318 | ||||||||
| No GED/high school diploma† | 102 (80.95) | 24 (19.05) | 0.496 | ||||||
| GED/high school | 115 (75.16) | 38 (24.84) | 0.71 | 0.40-1.27 | |||||
| Associates degree or higher | 31 (79.49) | 8 (20.51) | 0.91 | 0.37-2.23 | |||||
| Employed | 318 | ||||||||
| Yes | 14 (66.67) | 7 (33.33) | 0.54 | 0.21-1.39 | 0.201 | ||||
| No† | 234 (78.79) | 63 (21.21) | |||||||
| Disability | 316 | ||||||||
| Yes | 146 (74.11) | 51 (25.89) | 0.51 | 0.28-0.92 | 0.026* | ||||
| No† | 101 (84.87) | 18 (15.13) | |||||||
| History of injecting drugs | 318 | ||||||||
| Yes | 138 (76.67) | 42 (23.33) | 0.84 | 0.49-1.44 | 0.517 | ||||
| No† | 110 (79.71) | 28 (20.29) | |||||||
| History of noninjecting drugs | 318 | ||||||||
| Yes | 237 (79.53) | 61 (20.47) | 3.18 | 1.26-8.02 | 0.014* | ||||
| No† | 11 (55.00) | 9 (45.00) | |||||||
| Injected drugs during the last 6 months | 318 | ||||||||
| Yes | 19 (86.36) | 3 (13.64) | 1.85 | 0.53-6.45 | 0.333 | ||||
| No† | 229 (77.36) | 67 (22.64) | |||||||
| Used noninjection drugs in the last 6 months | 317 | ||||||||
| Yes | 95 (79.83) | 24 (20.17) | 1.20 | 0.69-2.09 | 0.525 | ||||
| No† | 152 (76.77) | 46 (23.23) | |||||||
| Tested for HCV | 318 | ||||||||
| Yes | 234 (78.79) | 63 (21.21) | 1.86 | 0.72-4.80 | 0.201 | ||||
| No† | 14 (66.67) | 7 (33.33) | |||||||
| Do you have hepatitis C? | 318 | ||||||||
| Yes† | 107 (72.30) | 41 (27.70) | 0.076 | ||||||
| No | 127 (83.01) | 26 (16.99) | 1.87 | 1.08-3.26 | |||||
| I do not know | 14 (82.35) | 3 (17.65) | 1.79 | 0.49-6.55 | |||||
| Have you ever attended an educational program about hepatitis C? | 316 | ||||||||
| Yes | 157 (81.77) | 35 (18.23) | 1.76 | 1.03-3.02 | 0.038* | ||||
| No† | 89 (71.77) | 35 (28.23) | |||||||
| Would you be willing to attend an educational activity about hepatitis C? | 316 | ||||||||
| Yes | 209 (84.62) | 38 (15.38) | 4.76 | 2.65-8.55 | <0.001* | 4.26 | 2.30-7.88 | <0.001* | |
| No† | 37 (53.62) | 32 (46.38) | |||||||
| 5 or more correct answers on the HCV knowledge assessment test | 318 | ||||||||
| Yes | 148 (85.06) | 26 (14.94) | 2.51 | 1.45-4.33 | 0.001* | 1.91 | 1.07-3.43 | 0.029* | |
| No† | 100 (69.44) | 44 (30.56) | |||||||
P < 0.05.
Reference group for the reported odds ratios.
CI, confidence interval; GED, general equivalency degree; HCV, hepatitis C virus; OAT, opiate agonist therapy; OR, odds ratio.
HCV-Related Knowledge
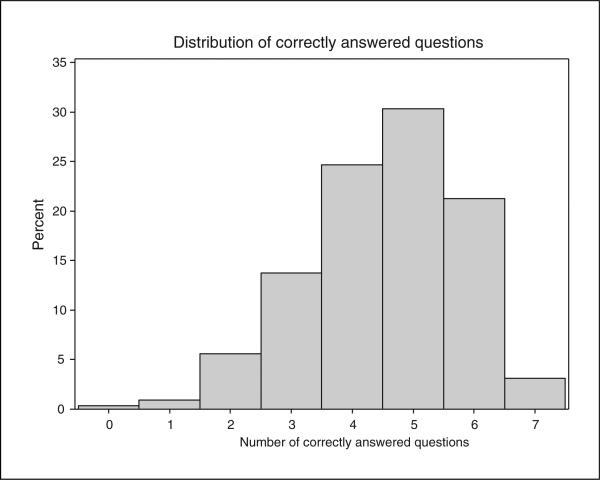
Hepatitis C virus–related knowledge was assessed by the last 7 questions of the survey, and the median number of correctly answered questions was 5 (interquartile range: 4-5) (Fig. 1). Most patients were well aware of basic facts about the infection that injection drug use is the primary route of HCV transmission (90.3%), that HCV treatment exists (87.9%), and that spontaneous resolution of the infection or clearance upon treatment does not provide protection against future infections (78.4%). The most frequently incorrectly answered question assessed patient knowledge of a hepatitis C vaccine; only 32.7% were aware that a vaccine is unavailable.
FIGURE 1.
Distribution of correct responses to knowledge survey.
We then investigated the effect of the different covariates on the number of correct answers (Table 4). Patients who reported positive HCV infection status, those who had previously attended an educational activity about hepatitis C, those who were willing to attend an educational activity in the future, and those who were willing to be treated for HCV scored higher on the hepatitis C knowledge assessment.
TABLE 4.
Predictors of Higher Level of HCV Knowledge
| Univariable Model |
Multivariable Model |
||||||
|---|---|---|---|---|---|---|---|
| Variable | n | OR* | 95% CI | P | OR* | 95% CI | P |
| Age, yr | 319 | 0.99 | 0.97-1.02 | 0.574 | |||
| Sex, n (%) | 316 | ||||||
| Male | 0.81 | 0.54-1.21 | 0.305 | ||||
| Female† | |||||||
| Race, n (%) | 312 | ||||||
| White | 2.14 | 1.03-4.43 | |||||
| African American† | 0.228 | ||||||
| Mixed | 1.15 | 0.51-2.59 | |||||
| Other | 1.21 | 0.77-1.90 | |||||
| Ethnicity, n (%) | 308 | ||||||
| Latin | 1.02 | 0.68-1.54 | 0.924 | ||||
| Non-Latin† | |||||||
| Duration of OAT attendance, yrs | 314 | 0.99 | 0.96-1.02 | 0.328 | |||
| Education, n (%) | 320 | ||||||
| No GED/high school diploma† | 0.542 | ||||||
| GED/high school | 1.26 | 0.83-1.92 | |||||
| Associates degree or higher | 1.22 | 0.64-2.32 | |||||
| Employed, n (%) | 320 | ||||||
| Yes | 1.07 | 0.49-2.37 | 0.859 | ||||
| No† | |||||||
| Disability, n (%) | 318 | ||||||
| Yes | 0.78 | 0.52-1.17 | 0.233 | ||||
| No† | |||||||
| History of injecting drugs | 320 | ||||||
| Yes | 2.01 | 1.35-3.00 | <0.001‡ | ||||
| No† | |||||||
| History of noninjection drugs | 320 | ||||||
| Yes | 2.27 | 1.01-5.10 | 0.047‡ | ||||
| No† | |||||||
| Injected drugs during the last 6 months | 318 | ||||||
| Yes | 00.735§ | ||||||
| No† | |||||||
| Used noninjection drugs in the last 6 months | 319 | ||||||
| Yes | 0.84 | 0.56-1.25 | 0.383 | ||||
| No† | |||||||
| Tested for HCV | 320 | ||||||
| Yes | 2.61 | 1.18-5.78 | 0.018‡ | ||||
| No† | |||||||
| Do you have hepatitis C? | 320 | ||||||
| Yes | 2.10 | 1.39-3.16 | 0.002‡ | 2.27 | 1.49; 3.45 | ||
| No† | <0.001‡ | ||||||
| I do not know | 1.65 | 0.67-4.04 | 1.63 | 0.66; 4.04 | |||
| Have you ever attended an educational program about hepatitis C? | 318 | ||||||
| Yes | 2.37 | 1.57-3.58 | <0.001‡ | 1.99 | 1.30; 3.03 | 0.002‡ | |
| No† | |||||||
| Would you be willing to attend an educational activity about hepatitis C? | 318 | ||||||
| Yes | 2.52 | 1.56-4.10 | <0.001‡ | 1.84 | 1.10; 3.09 | 0.021‡ | |
| No† | |||||||
| If you have ever been diagnosed with hepatitis C virus infection, would you be willing to be treated? | 318 | ||||||
| Yes | 2.14 | 1.32-3.45 | 0.002‡ | 1.76 | 1.06; 2.92 | 0.030‡ | |
| No or not sure† | |||||||
OR of having higher score.
Reference group for the reported odds ratios.
P < 0.05.
From generalized logit model.
CI, confidence interval; GED, general equivalency degree; HCV, hepatitis C virus; OAT, opiate agonist therapy; OR, odds ratio.
DISCUSSION
In this study, we assessed the level of HCV-related knowledge and willingness to participate in HCV-related education and treatment among 320 patients enrolled in an OAT clinic in New York City. We found that the majority of patients (78%) indicated a willingness to be treated for HCV; more than half (61%) had previously participated in an HCV-related educational activity, and an even higher percentage (78%) expressed willingness to participate in a future educational activity. In general, respondents demonstrated moderate HCV-related knowledge with more than half (54.7%) correctly responding to at least 5 of the 7 HCV-related knowledge questions. Higher levels of knowledge were associated with self-reported HCV-positive status and with prior attendance at an HCV-related educational activity. Participants who scored higher on HCV-related knowledge questions were also more willing to receive HCV treatment.
Multiple studies have analyzed HCV-related knowledge among PWID (Stein et al., 2001; Carey et al., 2005; Doab et al., 2005; Walley et al., 2005; Strauss et al., 2007; Cohen-Moreno et al., 2010; Treloar et al., 2012) and the general public (Balfour et al., 2009; Krauskopf et al., 2011; Denniston et al., 2012) and found many knowledge gaps. In general, PWID exhibited either poor or moderate knowledge about HCV depending on the particular study. Comparisons between these studies are difficult considering that they were performed in different countries and used different instruments. However, it does seem that older studies recorded somewhat lower levels of HCV-related knowledge, with smaller patient percentages recalling accurate information about HCV treatment (Carey et al., 2005; Doab et al., 2005; Walley et al., 2005) or even routes of HCV transmission (Carey et al., 2005). Similar to our findings, in more recent studies (Cohen-Moreno et al., 2010; Treloar et al., 2012), the majority of PWID were found to be aware that HCV is a treatable disease, and they knew that injection drug use represents the major route of HCV transmission. The increased HCV awareness among PWID might be linked with the recent development of new antiviral therapies, increased media coverage of HCV related to new Centers for Disease Control and Prevention screening recommendations, and tendencies in many countries toward increased HCV screening and treatment. Other studies have also recorded low levels of knowledge about the availability of HCV vaccine (Strauss et al., 2007; Cohen-Moreno et al., 2010).
The majority of study participants expressed willingness to receive HCV treatment, a finding similar to previously published data (Stein et al., 2001; Doab et al., 2005; Strathdee et al., 2005; Grebely et al., 2008; Treloar et al., 2012). Most respondents were well stabilized on methadone, with an average treatment duration of 7 years, similar to other studies that found that OAT-stabilized patients (Treloar et al., 2012) and PWID who are not currently injecting drugs (Grebely et al., 2008) were more willing to accept HCV treatment. In addition, men stabilized on methadone for at least 3 years were found to be significantly more likely to receive an HCV clinical evaluation than men on methadone for a shorter time interval, although among women, duration of methadone use was not associated with the likelihood of receiving an HCV evaluation (Martinez et al., 2012). Taken together, these findings indicate that PWID stabilized on OAT might be a population to preferentially target for HCV evaluation and treatment.
Although education alone is not enough to change behavior, it is a prerequisite to implement behavior changes. It has been shown that even simple educational interventions, such as informational presentations, can lead to significant improvements in knowledge (Shah and Abu-Amara, 2013). Similarly, in our study, patients who previously participated in an HCV-related educational activity knew more about hepatitis C. Furthermore, treatment willingness was significantly associated with both previous attendance at an HCV educational activity and a higher level of HCV-related knowledge. These findings support the notion that the more patients know about their disease, the more they will be willing to receive treatment.
Incentives have been shown to be effective in motivating PWID to participate in medical interventions (Perlman et al., 2003) and experimental studies (Park et al., 2012). Interestingly, we found that the majority of respondents who were unwilling to participate in HCV-related educational activities would not be swayed to do so by provision of incentives. These findings suggest that other factors might increase willingness of PWID to engage in educational interventions. Additional investigation is required to identify what these other factors could be.
This study has several limitations. Most notably, it relied on patient self-report of drug use and HCV infection status, which were not confirmed by serology. Although the survey instrument was developed with expert opinion from individuals with a variety of professional backgrounds, the instrument itself was not validated before implementation. Other limitations include self-administration of the instrument, although assistance from the research staff was available when requested, and the fact that only a limited number of questions assessing HCV-related knowledge were included.
CONCLUSIONS
On the basis of these findings, we conclude that OAT and other drug treatment facilities with an infrastructure capable of supporting patients’ basic medical and educational needs are venues that can enroll PWID into HCV-related care. Furthermore, multiple activities supported by these venues, including participation in HCV-related education sessions, can promote hepatitis C awareness among OAT patients. Lack of knowledge combined with mistrust of the health care system has been a major obstacle to PWID receiving HCV-related care. Long-term participation in OAT might increase the level of HCV-related knowledge and increase patients’ receptivity to treatment. In addition, if HCV treatment is offered in OAT facilities, either on-site or remotely by telemedicine, a major obstacle to PWID receipt of HCV-related care could be mitigated through treatment delivery in a familiar and trusted environment. Opiate agonist treatment facilities also have the advantage of linking HCV-related care to drug treatment, thereby facilitating close patient evaluation potentially increasing adherence to the treatment regimen. Therefore, care models that permit specialty care to be delivered on-site in OAT facilities should be pursued to increase the number of PWID who receive care for hepatitis C.
Supplementary Material
Acknowledgments
Supported by the CDC Foundation through the Viral Hepatitis Action Coalition with individual sponsorships from Gilead Sciences, Vertex Pharmaceuticals, Abbvie and Abbott Molecular Inc. Andrew H. Talal has served on the advisory board for Abbott Molecular and has received research support from Gilead Sciences.
Footnotes
Supplemental digital content is available for this article. Direct URL citation appears in the printed text and is provided in the HTML and PDF versions of this article on the journal's Web site (http://www.journaladdictionmedicine.com).
The remaining authors declare no conflicts of interest.
REFERENCES
- Amon JJ, Garfein RS, Ahdieh-Grant L, et al. Prevalence of hepatitis C virus infection among injection drug users in the United States, 1994–2004. Clin Infect Dis. 2008;46(12):1852–1858. doi: 10.1086/588297. [DOI] [PubMed] [Google Scholar]
- Armstrong GL, Wasley A, Simard EP, et al. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144(10):705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- Balfour L, Kowal J, Corace KM, et al. Increasing public awareness about hepatitis C: development and validation of the brief hepatitis C knowledge scale. Scand J Caring Sci. 2009;23(4):801–808. doi: 10.1111/j.1471-6712.2008.00668.x. [DOI] [PubMed] [Google Scholar]
- Bini EJ, Kritz S, Brown LS, Jr, et al. Barriers to providing health services for HIV/AIDS, hepatitis C virus infection and sexually transmitted infections in substance abuse treatment programs in the United States. J Addict Dis. 2011;30(2):98–109. doi: 10.1080/10550887.2011.554780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bini EJ, Kritz S, Brown LS, Jr, et al. Hepatitis B virus and hepatitis C virus services offered by substance abuse treatment programs in the United States. J Subst Abuse Treat. 2012;42(4):438–445. doi: 10.1016/j.jsat.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey J, Perlman DC, Friedmann P, et al. Knowledge of hepatitis among active drug injectors at a syringe exchange program. J Subst Abuse Treat. 2005;29(1):47–53. doi: 10.1016/j.jsat.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Chak E, Talal AH, Sherman KE, et al. Hepatitis C virus infection in USA: an estimate of true prevalence. Liver Int. 2011;31(8):1090–1101. doi: 10.1111/j.1478-3231.2011.02494.x. [DOI] [PubMed] [Google Scholar]
- Chen EY, North CS, Fatunde O, et al. Knowledge and attitudes about hepatitis C virus (HCV) infection and its treatment in HCV mono-infected and HCV/HIV co-infected adults. J Viral Hepat. 2013;20(10):708–714. doi: 10.1111/jvh.12095. [DOI] [PubMed] [Google Scholar]
- Cohen-Moreno R, Schiff M, Levitt S, et al. Knowledge about hepatitis-C among methadone maintenance treatment patients in Israel. Subst Use Misuse. 2010;45(1/2):58–76. doi: 10.3109/10826080902864894. [DOI] [PubMed] [Google Scholar]
- Denniston MM, Klevens RM, Mcquillan GM, et al. Awareness of infection, knowledge of hepatitis C, and medical follow-up among individuals testing positive for hepatitis C: National Health and Nutrition Examination Survey 2001–2008. Hepatology. 2012;55(6):1652–1661. doi: 10.1002/hep.25556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimova RB, Zeremski M, Jacobson IM, et al. Determinants of hepatitis C virus treatment completion and efficacy in drug users assessed by meta-analysis. Clin Infect Dis. 2013;56(6):806–816. doi: 10.1093/cid/cis1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doab A, Treloar C, Dore GJ. Knowledge and attitudes about treatment for hepatitis C virus infection and barriers to treatment among current injection drug users in Australia. Clin Infect Dis. 2005;40(suppl 5):S313–S320. doi: 10.1086/427446. [DOI] [PubMed] [Google Scholar]
- Edlin BR, Carden MR. Injection drug users: the overlooked core of the hepatitis C epidemic. Clin Infect Dis. 2006;42(5):673–676. doi: 10.1086/499960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347(13):975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- Grebely J, Genoway KA, Raffa JD, et al. Barriers associated with the treatment of hepatitis C virus infection among illicit drug users. Drug Alcohol Depend. 2008;93(1/2):141–147. doi: 10.1016/j.drugalcdep.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Jacobson IM, Mchutchison JG, Dusheiko G, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364(25):2405–2416. doi: 10.1056/NEJMoa1012912. [DOI] [PubMed] [Google Scholar]
- Krauskopf K, McGinn TG, Federman AD, et al. HIV and HCV health beliefs in an inner-city community. J Viral Hepat. 2011;18(11):785–791. doi: 10.1111/j.1365-2893.2010.01383.x. [DOI] [PubMed] [Google Scholar]
- Kwo PY, Lawitz EJ, Mccone J, et al. Efficacy of boceprevir, an NS3 protease inhibitor, in combination with peginterferon alfa-2b and ribavirin in treatment-naive patients with genotype 1 hepatitis C infection (SPRINT-1): an open-label, randomised, multicentre phase 2 trial. Lancet. 2010;376(9742):705–716. doi: 10.1016/S0140-6736(10)60934-8. [DOI] [PubMed] [Google Scholar]
- Manns MP, Mchutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358(9286):958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- Martinez AD, Dimova R, Marks KM, et al. Integrated internist—addiction medicine—hepatology model for hepatitis C management for individuals on methadone maintenance. J Viral Hepat. 2012;19(1):47–54. doi: 10.1111/j.1365-2893.2010.01411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta SH, Genberg BL, Astemborski J, et al. Limited uptake of hepatitis C treatment among injection drug users. J Community Health. 2008;33(3):126–133. doi: 10.1007/s10900-007-9083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrill JA, Shrestha M, Grant RW. Barriers to the treatment of hepatitis C. Patient, provider, and system factors. J Gen Intern Med. 2005;20(8):754–758. doi: 10.1111/j.1525-1497.2005.0161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myles A, Mugford GJ, Zhao J, et al. Physicians’ attitudes and practice toward treating injection drug users with hepatitis C: results from a national specialist survey in Canada. Can J Gastroenterol. 2011;25(3):135–139. doi: 10.1155/2011/810108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PK, Mathers BM, Cowie B, et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet. 2011;378(9791):571–583. doi: 10.1016/S0140-6736(11)61097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JN, White B, Bates A, et al. Motivators and barriers influencing willingness to participate in candidate HCV vaccine trials: perspectives of people who inject drugs. Drug Alcohol Depend. 2012;123(1–3):35–40. doi: 10.1016/j.drugalcdep.2011.10.009. [DOI] [PubMed] [Google Scholar]
- Perlman DC, Friedmann P, Horn L, et al. Impact of monetary incentives on adherence to referral for screening chest x-rays after syringe exchange-based tuberculin skin testing. J Urban Health. 2003;80(3):428–437. doi: 10.1093/jurban/jtg044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schackman BR, Teixeira PA, Beeder AB. Offers of hepatitis C care do not lead to treatment. J Urban Health. 2007;84(3):455–458. doi: 10.1007/s11524-007-9180-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah HA, Abu-Amara M. Education provides significant benefits to patients with hepatitis B virus or hepatitis C virus infection: a systematic review. Clin Gastroenterol Hepatol. 2013;11(8):922–933. doi: 10.1016/j.cgh.2013.04.024. [DOI] [PubMed] [Google Scholar]
- Stein MD, Maksad J, Clarke J. Hepatitis C disease among injection drug users: knowledge, perceived risk and willingness to receive treatment. Drug Alcohol Depend. 2001;61(3):211–215. doi: 10.1016/s0376-8716(00)00144-7. [DOI] [PubMed] [Google Scholar]
- Strathdee SA, Latka M, Campbell J, et al. Factors associated with interest in initiating treatment for hepatitis C virus (HCV) infection among young HCV-infected injection drug users. Clin Infect Dis. 2005;40(suppl 5):S304–S312. doi: 10.1086/427445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss SM, Astone-Twerell J, Munoz-Plaza CE, et al. Drug treatment program patients’ hepatitis C virus (HCV) education needs and their use of available HCV education services. BMC Health Serv Res. 2007;7:39. doi: 10.1186/1472-6963-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treloar C, Hull P, Dore GJ, et al. Knowledge and barriers associated with assessment and treatment for hepatitis C virus infection among people who inject drugs. Drug Alcohol Rev. 2012;31(7):918–924. doi: 10.1111/j.1465-3362.2012.00468.x. [DOI] [PubMed] [Google Scholar]
- Vermehren J, Sarrazin C. The role of resistance in HCV treatment. Best Pract Res Clin Gastroenterol. 2012;26(4):487–503. doi: 10.1016/j.bpg.2012.09.011. [DOI] [PubMed] [Google Scholar]
- Walley AY, White MC, Kushel MB, et al. Knowledge of and interest in hepatitis C treatment at a methadone clinic. J Subst Abuse Treat. 2005;28(2):181–187. doi: 10.1016/j.jsat.2004.12.004. [DOI] [PubMed] [Google Scholar]
- World Health Organization [August 21, 2013];Hepatitis C. Fact sheet no. 164. Available at http://www.who.int/mediacentre/factsheets/fs164/en/.
- Zeremski M, Zibbell JE, Martinez AD, et al. Hepatitis C virus control among persons who inject drugs requires overcoming barriers to care. World J Gastroenterol. 2013;19(44):7846–7851. doi: 10.3748/wjg.v19.i44.7846. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.