Summary
While cellular GTP concentration dramatically changes in response to an organism’s cellular status, whether it serves as a metabolic cue for biological signaling remains elusive due to the lack of molecular identification of GTP sensors. Here we report that PI5P4Kβ, a phosphoinositide kinase that regulates PI(5)P levels, detects GTP concentration and converts them into lipid second messenger signaling. Biochemical analyses show that PI5P4Kβ preferentially utilizes GTP, rather than ATP, for PI(5)P phosphorylation and its activity reflects changes in direct proportion to the physiological GTP concentration. Structural and biological analyses reveal that the GTP-sensing activity of PI5P4Kβ is critical for metabolic adaptation and tumorigenesis. These results demonstrate that PI5P4Kβ is the missing GTP sensor and that GTP concentration functions as a metabolic cue via PI5P4Kβ. The critical role of the GTP-sensing activity of PI5P4Kβ in cancer signifies this lipid kinase as a cancer therapeutic target.
Introduction
Energy molecules such as adenosine triphosphate (ATP) and guanosine triphosphate (GTP) are evolutionarily conserved metabolites and drive numerous enzymatic reactions in cells. Concentrations of the energy molecules vary by tissue type, environment, and pathological condition. The changes in the concentrations of energy molecules affect cell status not only in a passive manner but also in an active manner, in which these concentration changes are detected by specific molecular sensors and converted into signaling for metabolic adaptations. For example, AMP-activated protein kinase (AMPK) integrates the relative ATP concentration against AMP and/or ADP concentrations for signaling (Oakhill et al., 2011; Xiao et al., 2011). Mammalian target of rapamycin (mTOR) has been suggested as another type of ATP sensor because its Michaelis constant (Km) value for ATP falls within the range of physiological cellular concentration and its kinase activity changes in direct proportion to the cellular ATP concentration (Chen et al., 2013; Dennis et al., 2001). AMPK and mTOR modulate cellular metabolism by direct phosphorylation of effector proteins (Hardie, 2008; Laplante and Sabatini, 2012; Mihaylova and Shaw, 2011). The levels of other metabolites essential for cell viability are also monitored by respective sensors. Cellular oxygen levels are detected by hypoxia inducible factor (HIF) prolyl-hydroxylases, whose Km for oxygen is in the range of the atmospheric oxygen concentration (Schofield and Ratcliffe, 2004; Stiehl et al., 2006). Recently an amino acid sensor, SLC38A9, has been reported to have a Km value for arginine corresponding to its physiological concentration and transmits the amino acid concentration to cellular signaling (Wang et al., 2015). These studies reveal that molecular sensors for metabolites must have three fundamental features: (i) ability to bind directly to the metabolite; (ii) appropriate Km value so that its activity is regulated by physiological changes of the concentration of the target metabolite; and (iii) ability to evoke a signal for cellular functions.
GTP is particularly important for protein synthesis where two GTP molecules are consumed per every one amino acid incorporation into a polypeptide (Ertel et al., 1968; Green and Noller, 1997; Haselkorn and Rothman-Denes, 1973). Therefore, a large amount of GTP is required in rapidly dividing cells, such as tumor cells, and tissues producing serum proteins, such as the liver, pancreas, and adipose tissue (Caro and Palade, 1964; Havel, 2002; Ma and Blenis, 2009; Miller et al., 1951; Weiss, 1953). Cellular GTP concentration changes independently of the concentrations of ATP, ADP, and AMP and the ratios of ATP/AMP and GTP/GDP in bacterial, yeast, and mammalian cells (Lopez, 1982; Matsushika et al., 2013; Neidhardt et al., 1990; Traut, 1994). Regulatory links between GTP concentration and metabolic adaptation have been found: GTP concentration actively controls ribosomal RNA synthesis rates in bacterial and mammalian cells (Gaal, 1997; Grummt and Grummt, 1976; Krásný and Gourse, 2004). In gram-positive bacteria, a transcriptional factor, CodY, controls the expression of genes involved in branched-chain amino acid biosynthesis in response to changes in GTP concentration (Ratnayake-Lecamwasam, 2001). These studies indicated a fundamental requirement for cells to sense the GTP concentration in an active manner and alter their metabolisms with a GTP sensor acting as a gauge. G-proteins have some aspects of being a GTP sensor. However, G-proteins are unlikely to respond to the changes in GTP concentration because their Km values for GTP and GDP are, in most cases, in the pM to nM range, which is far below cellular GTP concentration (∼0.1 mM to 1 mM) (Traut, 1994). Until now, no molecular sensor for cellular GTP concentration has been identified in higher organisms. For this reason, molecular mechanisms of GTP-responsive signaling remain unclear. In the present study, we set out to identify a protein that would meet the requirements for the GTP sensor, and discovered a GTP sensor, Phosphatidylinositol 5-phosphate 4-kinase β (PI5P4Kβ), which converts GTP-concentration cues into phosphatidylinositol 5-phosphate (PI(5)P) second messenger signaling and tumorigenesis.
Results
Proteomic screen identifies PI5P4Ks as GTP-binding proteins
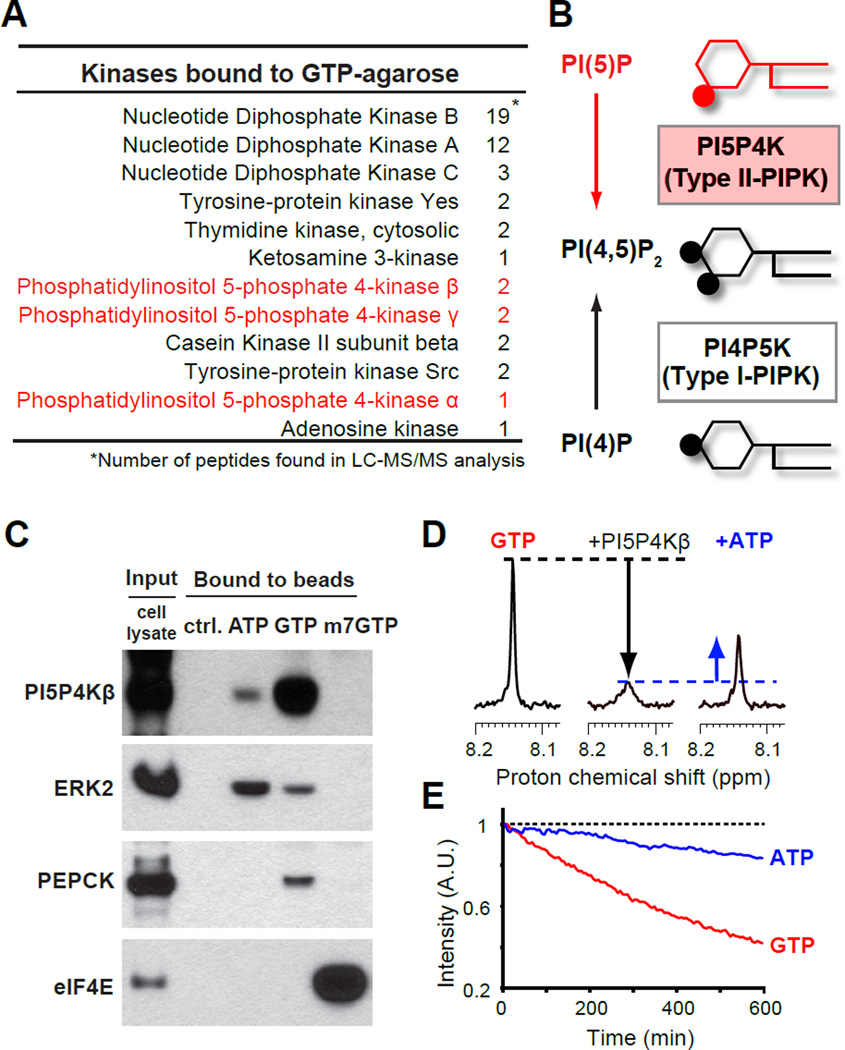
To identify a candidate for the GTP sensor, we performed a proteomic screening for signaling proteins that have an ability to bind to GTP. The GTP-conjugated agarose beads were incubated with dialyzed cell lysates, and proteins bound to the agarose beads were eluted with GTP. The eluted proteins were precipitated, digested with trypsin, and identified by mass spectrometry. As expected, the GTP-eluted fraction contained several G-proteins and two types of protein kinases, Src-family tyrosine-protein kinases and Casein kinase II (CKII), which have been shown to utilize GTP with less or equal efficiency as compared to ATP (Feder and Bishop, 1990; Niefind et al., 1999). Notably, all three isoforms of PI5P4K/Type II PIPK were also found in the GTP-eluted fraction (Fig. 1 A and B), while other families of phosphoinositide kinases (PI4P5K/Type I PIPK and PI3P5K/Type III PIPK/PIKfyve) were not detected. PI5P4Ks, an emerging target for cancer therapy, control the levels of lipid second messenger, PI(5)P (Clarke and Irvine, 2013; Emerling et al., 2013; Jude et al., 2015; Keune et al., 2013). These results raised the possibility that PI5P4Ks have an intrinsic ability to bind to GTP.
Figure 1. PI5P4Kβ is a GTP-binding phosphatidylinositol kinase.
(A) A list for kinases that were identified in a proteome screening with GTP-conjugated agarose beads.
(B) Enzymatic functions of PI5P4K/Type II PIPK and PI4P5K/Type I PIPK.
(C) Cellular PI5P4Kβ binds to GTP-conjugated agarose beads more than ATP- and m7GTP-conjugated agarose beads. After incubated with HEK 293T cell lysates, PI5P4Kβ as well as control proteins that bound to the indicated control or nucleotide conjugated beads were immunoblotted.
(D) Direct binding of GTP to PI5P4Kβ assessed by solution-state NMR. The signal intensity of GTP (200 µM, H8 position) was reduced by addition of PI5P4Kβ (10 µM, middle), which was partially recovered by addition of physiological amount of ATP (1 mM, right). See also Figure S1A.
(E) PI5P4Kβ possesses superior GTP hydrolysis activity in vitro. The real-time reduction of GTP and ATP specific signals was monitored by NMR. See also Figure S1B.
PI5P4Kβ directly binds to GTP
To validate the binding of PI5P4Ks to GTP, dialyzed lysates from HEK 293T cells were incubated with ATP-, GTP-, or 7-methyl-GTP (m7GTP)-conjugated agarose beads, and proteins bound to those beads were analyzed by SDS-polyacrylamide gel electrophoresis followed by western blotting (Fig. 1C). Immunoblotting of extracellular signal-regulated kinase-2 (ERK2), phosphoenolpyruvate carboxykinase (PEPCK), and eukaryotic initiation factor-4E (eIF4E) indicated selective binding of these proteins to ATP-, GTP-, and m7GTP-conjugated agarose beads, respectively. We found that PI5P4Kβ in cell extracts bound to the GTP-conjugated agarose beads more strongly than to the ATP-conjugated agarose beads, which verified the result of the proteomic screening. Thus, we purified recombinant PI5P4Kβ and assessed its direct binding to GTP in vitro by solution-state nuclear magnetic resonance (NMR) spectroscopy (Fig. 1D). The NMR signal of GTP was reduced by the addition of PI5P4Kβ (Fig. 1D middle). The addition of excess ATP competed with the GTP binding to PI5P4Kβ (Fig. 1D right). These results indicated that PI5P4Kβ directly binds to GTP and that GTP and ATP shared the binding site in PI5P4Kβ. Given that PI5P4Kβ has only one nucleotide-binding site, we hypothesized that PI5P4Kβ binds to GTP through its catalytic site for its kinase reaction.
PI5P4Ks can hydrolyze GTP in vitro
Recently, it has been shown that PI5P4Kβ hydrolyzes ATP in vitro (Clarke and Irvine, 2013). ATP-dependent kinases often possess intrinsic ATP hydrolysis activity (phosphoryl transfer to water), because of their resemblance to kinase reactions (Kashem et al., 2006). To test if PI5P4Kβ can utilize GTP as a phosphodonor, we performed a real-time GTP hydrolysis assay using NMR (Fig. 1E and Fig. S1A). PI5P4Kβ hydrolyzed not only ATP but also GTP, and the rate of GTP hydrolysis was 5-times faster than its ATP hydrolysis. In contrast, PI5P4Kα showed 0.6-fold slower GTP hydrolysis than ATP hydrolysis (Fig. S1B). The PI5P4Kγ isoform displayed marginal ATP and GTP hydrolysis activities (Fig. S1A bottom). These results indicated that each PI5P4K possesses a different degrees of intrinsic activity toward GTP, and raised the possibility that PI5P4Ks use GTP for their kinase reactions.
PI5P4Kβ is a GTP-dependent kinase and its Km value is within physiological variation of GTP concentration
To examine whether the PI5P4K isoforms use GTP to phosphorylate their substrate PI(5)P, we performed in vitro kinase assays. We found that all PI5P4K isoforms possess GTP-dependent kinase activities (Fig. 2A and Fig. S2A). Their ATP-dependent kinase activities were also confirmed, as previously reported (Demian et al., 2009; Kunz et al., 2002). Interestingly, the Michaelis-Menten kinetics analysis showed that PI5P4K isoforms displayed distinct activities and Km values. PI5P4Kα showed the highest activity and the lowest Km values, which are 5 µM and 3 µM for ATP and GTP, respectively (Fig. S2B upper). This suggests that both the ATP- and GTP-dependent kinase activities of PI5P4Kα are always near their maximal levels at cellular ATP (1 −5 mM) and GTP (∼ 0.1 – 1 mM) concentrations (Traut, 1994). Thus, PI5P4Kα is not suitable for the GTP sensor. PI5P4Kγ displayed weak ATP- and GTP-dependent kinase activities (Fig. S2 A and B bottom): two orders of magnitude lower than PI5P4Kα and PI5P4Kβ. PI5P4Kγ could be a GTP sensor, because of its higher GTP-dependent kinase activity as compared to the ATP-dependent kinase activity and the Km value within the physiological variation of GTP concentration. However, it was difficult to analyze the functions of PI5P4Kγ as the first candidate GTP-sensor due to its low enzyme activity.
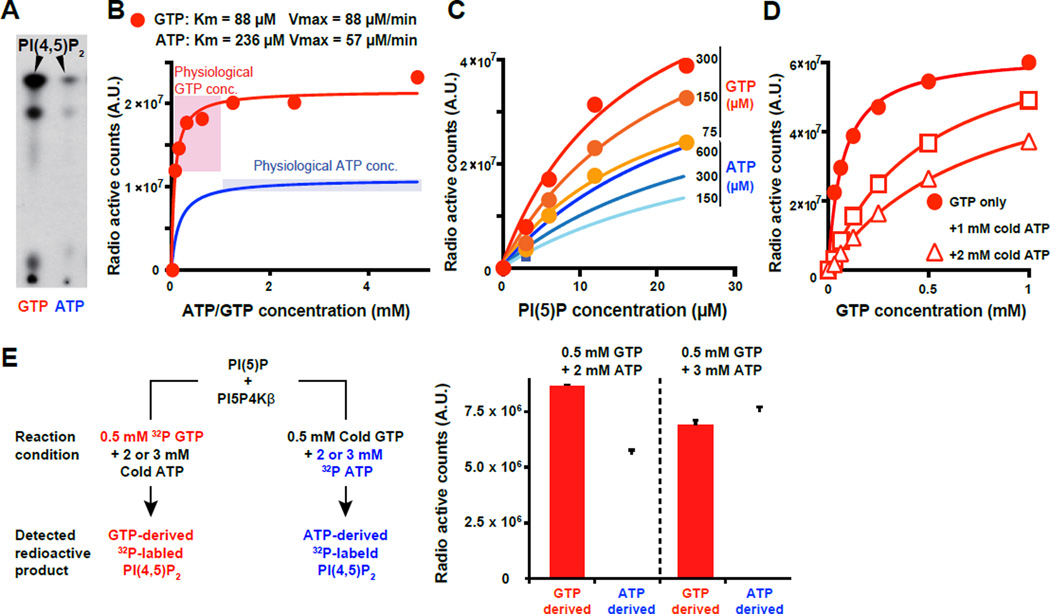
Figure 2. PI5P4Kβ has ability to sense GTP concentration.
(A) PI5P4Kβ utilizes GTP as a preferred phosphodonor to produce PI(4,5)P2 from PI(5)P. The bacterially produced recombinant PI5P4Kβ was incubated with PI(5)P in the presence of 0.2 µM γ-32P radiolabeled GTP or ATP. The phospholipids were extracted and analyzed by thin layer chromatography and autoradiography.
(B) PI5P4Kβ activity changes within the physiological variations of GTP concentration. PI5P4Kβ kinase activity was assessed with the indicated levels of γ-32P radiolabeled GTP or ATP and 20 µM of PI(5)P.
(C) PI5P4Kβ retains its preference to GTP at any PI(5)P concentration.
(D) PI5P4Kβ retains GTP responsiveness in the presence of physiological amount of ATP. PI5P4Kβ kinase activity with the indicated amount of γ-32P radiolabeled GTP was assessed in the presence of 1 mM and 2 mM of cold (non-radiolabeled) ATP.
(E) PI5P4Kβ efficiently utilizes GTP in the presence of physiological amount of ATP. GTP- and ATP-dependent kinase activities of PI5P4Kβ were assessed with mixtures of cold and γ-32P radiolabeled ATP and GTP as shown in the left panel. 0.2 µg PI5P4Kβ and 1.0 µg PI(5)P was used for each reaction and only radiolabeled PI(4,5)P2 was quantified (right panel). Data are displayed as means ± SD, n=3.
See also Figure S2.
Based on its kinetic property, PI5P4Kβ is a good candidate for a functional GTP sensor. PI5P4Kβ displayed a higher GTP-dependent kinase activity than ATP-dependent kinase activity (Fig. 2B), regardless of PI(5)P levels and the types of divalent metal ions (Fig. 2C and Fig. S2C). The GTP-dependent kinase activity can change in the range of physiological GTP concentration due to its relatively high Km value for GTP (Fig. 2B red). On the other hand, the ATP-dependent kinase activity is always near saturated level at physiological ATP concentration (Fig. 2B blue). Even in the presence of physiological concentration of ATP (1 mM and 2 mM), PI5P4Kβ still responded to the changes in GTP concentration (Fig. 2D). In addition, relative contribution of ATP and GTP for PI(4,5)P2 production was assessed under physiologically relevant conditions (Fig. 2E), which revealed that nearly or over 50% of PI(4,5)P2 was produced by using GTP at 0.5 mM concentration. These results suggest that concentration of cellular GTP (∼ 0.1 – 1 mM) has substantial impact on total PI5P4Kβ activity. The results also revealed that PI5P4Kβ has the appropriate characteristics of a GTP sensor.
PI5P4Kβ recognizes guanine nucleotides via a “Tetris-spin”
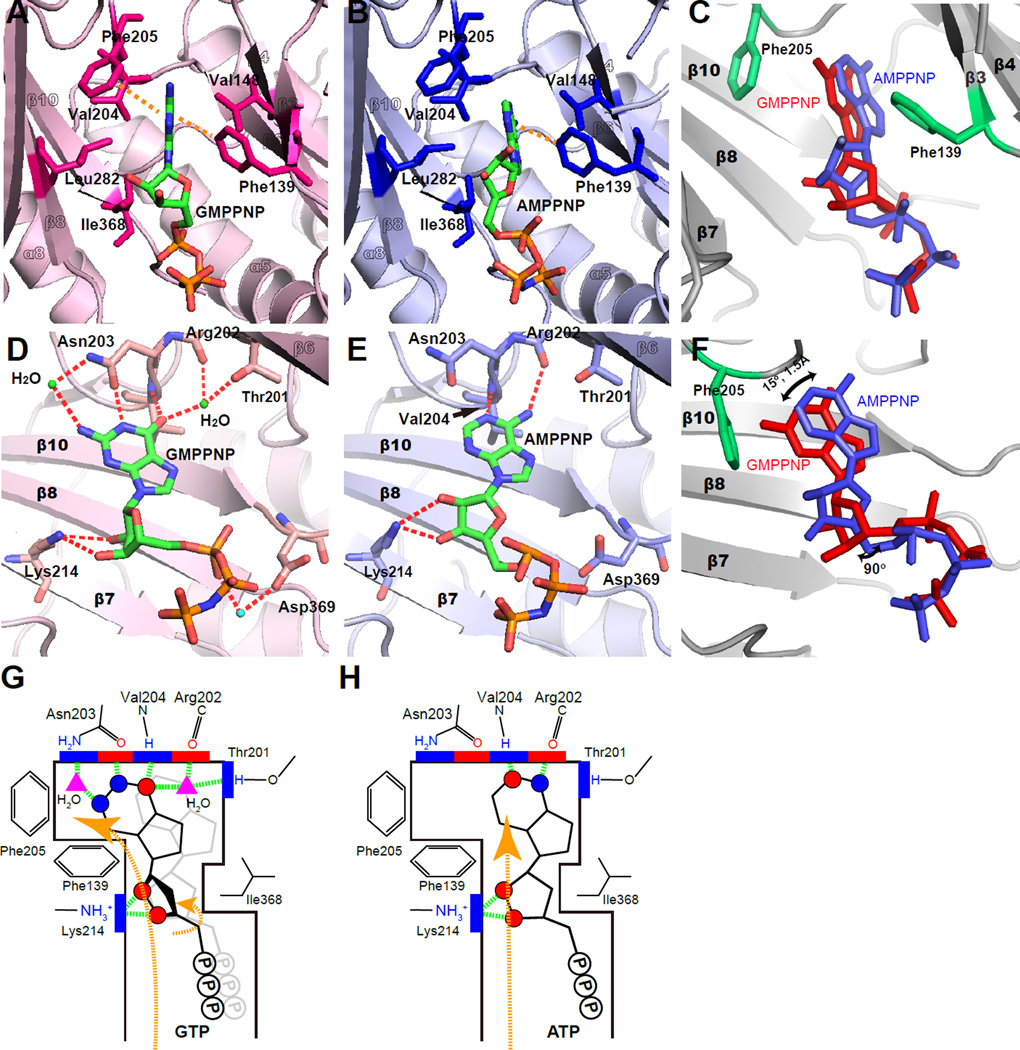
To further analyze the GTP-dependent activity of PI5P4Kβ at the atomic level, we carried out the structural analyses by X-ray crystallography. Crystal structures of human PI5P4Kβ in complex with non-hydrolysable GTP and ATP analogues (GMPPNP and AMPPNP, respectively) were determined at 2.6 Å and 2.7 Å resolutions, respectively, under physiological pH and Mg2+ concentration (Table 1 and Fig. S3). The bases of both the ATP and GTP analogues are placed in a hydrophobic groove formed by Phe-139, Val-148, Val-204, Phe-205, Leu-282, and Ile-368 (Fig. 3 A and B). The positions of the nucleotide bases are restrained by the sidechain of Ile-368 that protrudes out into the hydrophobic groove and the I368A mutation altered the position of guanine and adenine nucleotides in the binding pocket (Fig. S3I–L and Table 2).
Table 1.
Crystallographic summary of WT-PI5P4Kβ
| Apo | WT- AMP |
WT- GMP |
WT-AMPPNP | WT-GMPPNP | |
|---|---|---|---|---|---|
| Data collection | |||||
| Space group | C2221 | C2221 | C2221 | C2221 | C2221 |
| Cell dimensions | |||||
| a, b, c (Å) | 107.1, 182.4, 105.4 90.0, |
108.4, 181.8, 107.6 90.0, |
108.8, 183.2, 106.8 90.0, |
108.6, 183.0, 106.0 90.0, |
109.8, 185.2, 106.5 90.0, |
| α, β,γ (°) | 90.0, 90.0 |
90.0, 90.0 |
90.0, 90.0 |
90.0, 90.0 |
90.0, 90.0 |
| Resolution (Å)* | 69.47– 2.60 (2.74– 2.60) |
70.41– 2.15 (2.27– 2.15) |
46.76– 2.45 (2.58– 2.45) |
91.49–2.70 (2.85–2.70) |
94.45–2.60 (2.74–2.60) |
| Rmerge(%) | 3.8 (41.4) | 3.1 (45.0) | 4.2 (59.5) | 2.5 (39.6) | 3.1 (48.5) |
| I /σI | 27.7 (3.7) | 27.9 (3.4) | 21.4 (2.6) | 37.5 (3.8) | 29.3 (3.2) |
| Completeness (%) | 99.6 (100.0) |
97.5 (95.7) |
99.2 (99.9) |
98.6 (99.5) | 97.9 (99.8) |
| Redundancy | 3.8 (3.8) | 3.8 (3.7) | 3.8 (3.8) | 3.8 (3.8) | 3.7 (3.6) |
| Refinement | |||||
| Resolution (Å) | 52.70– 2.60 |
48.40– 2.15 |
46.76– 2.45 |
53.18–2.70 | 94.45–2.60 |
| No. reflections | 32017 | 56883 | 39252 | 29012 | 33027 |
| Rwork / Rfree (%) | 21.1/26.0 | 24.6/27.9 | 22.4/25.5 | 23.4/28.0 | 22.9/27.7 |
| No. atoms | |||||
| Protein | 5162 | 5063 | 5111 | 5284 | 5035 |
| Ligand/ion | 0 | 140 | 120 | 141 | 128 |
| Water | 51 | 106 | 42 | 25 | 17 |
| B-factors | |||||
| Protein | 71.9 | 69.6 | 77 | 97.9 | 83.7 |
| Ligand/ion | - | 74.5 | 74.1 | 125 | 114.8 |
| Water | 45.6 | 47.2 | 50.3 | 50.2 | 54 |
| R.m.s deviations | |||||
| Bond lengths (Å) | 0.009 | 0.012 | 0.009 | 0.019 | 0.01 |
| Bond angles (°) | 1.149 | 1.182 | 1.223 | 2.381 | 1.236 |
| PDB ID | 3WZZ | 3X01 | 3X02 | 3X03 | 3X04 |
Each structure was determined from single-crystal diffraction.
Highest resolution shell is shown in parenthesis.
Figure 3. Recognition of GTP and ATP by PI5P4Kβ.
(A–F) Hydrophobic and hydrogen-bond interactions in the human PI5P4Kβ-nucleotide complexes. (A) Guanine and (B) adenine nucleotides are tightly fitted into the slit formed by the hydrophobic residues shown in the stick representation. Aromatic interactions between the protein and nucleotide bases are highlighted by orange dotted lines. In panels D and E, the observed hydrogen-bonding network between the protein and nucleotides are highlighted by red dotted lines. Panels C and F show the displacement of GMPPNP relative to AMPPNP. The GMPPNP (red) in PI5P4Kβ complex is superimposed against AMPPNP (blue). The side chains of Phe-139 and Phe-205, which form aromatic interactions against the guanine base, are shown in green.
(G and H) Schematic representation of (G) GTP and (H) ATP recognition by PI5P4Kβ. The red and blue circles indicate the positions of proton donors and acceptors in the nucleotides, respectively. The red and blue squares indicate those in PI5P4Kβ. Hydrogen bonds are depicted as dashed green lines. For comparison, the superimposed ATP structure is represented as light gray in the GTP scheme.
Table 2.
Crystallographic summary of PI5P4Kβ mutants in complex with nucleotides.
| T201M- AMP |
T201M- GMP |
F205L- AMP |
F205L- GMP |
I368A- AMP |
I368A- GMP |
|
|---|---|---|---|---|---|---|
| Data collection | ||||||
| Space group | C2221 | C2221 | C2221 | C2221 | C2221 | C2221 |
| Cell dimensions | ||||||
| a, b, c (Å) | 108.7, 181.5, 107.4 |
108.8, 182.9, 106.7 |
108.3, 183.4, 107.3 |
107.9, 183.0, 107.0 |
108.6, 182.1, 107.2 |
109.1, 183.4, 106.5 |
| α, β, γ (°) | 90.0, 90.0, 90.0 93.26- |
90.0, 90.0, 90.0 91.44- |
90.0, 90.0, 90.0 70.37- |
90.0, 90.0, 90.0 48.18- |
90.0, 90.0, 90.0 93.27- |
90.0, 90.0, 90.0 93.76- |
| Resolution (Å)* | 2.50 (2.64– 2.50) |
2.65 (2.79– 2.65) |
2.70 (2.85– 2.70) |
2.60 (2.74– 2.60) |
2.60 (2.74– 2.60) |
2.55 (2.69– 2.55) |
| Rmerge (%) | 4.0 (52.4) | 3.3 (45.3) | 3.4 (42.5) | 2.7 (43.7) | 2.8 (41.7) | 2.6 (47.0) |
| I /σI | 32.7 (4.5) | 29.5 (3.6) | 33.5 (3.7) | 35.0 (3.1) | 33.8 (3.8) | 36.9 (3.1) |
| Completeness (%) |
98.8 (99.2) |
99.0 (99.5) |
99.5 (100.0) |
98.2 (99.1) |
98.0 (98.3) |
98.3 (99.0) |
| Redundancy | 7.4 (7.5) | 3.8 (3.8) | 3.8 (3.8) | 3.8 (3.6) | 3.8 (3.9) | 3.8 (3.8) |
| Refinement | ||||||
| Resolution (Å) | 54.35– 2.50 |
54.41– 2.65 |
53.64– 2.70 |
48.18– 2.60 |
52.99– 2.60 |
54.55– 2.55 |
| No. reflections | 36672 | 30962 | 29554 | 32433 | 32395 | 34600 |
| Rwork / Rfree (%) | 22.6/27.3 | 21.2/26.1 | 20.3/26.0 | 22.8/27.2 | 25.3/28.2 | 22.3/26.8 |
| No. atoms | ||||||
| Protein | 5121 | 5113 | 5100 | 5111 | 5099 | 5127 |
| Ligand/ion | 140 | 180 | 138 | 173 | 140 | 142 |
| Water | 40 | 31 | 36 | 40 | 34 | 42 |
| B-factors | ||||||
| Protein | 76 | 80 | 79 | 82.9 | 78.9 | 80.1 |
| Ligand/ion | 79.2 | 114.5 | 106.05 | 101.2 | 104.2 | 102.1 |
| Water | 49.5 | 51.8 | 52.5 | 56.4 | 53.8 | 49.9 |
| R.m.s deviations | ||||||
| Bond lengths (Å) | 0.01 | 0.011 | 0.01 | 0.013 | 0.011 | 0.011 |
| Bond angles (°) | 1.114 | 1.112 | 1.3 | 1.293 | 1.253 | 1.146 |
| PDB ID | 3X05 | 3X06 | 3X09 | 3X0A | 3X0B | 3X0C |
Each structure was determined from single-crystal diffraction.
Highest resolution shell is shown in parenthesis.
In the deep end of the nucleotide-binding pocket, the guanine nucleotide base is secured by four hydrogen bonds with Thr-201, Arg-202, Asn-203, and Val-204 (Fig. 3D). In contrast, the adenine nucleotide base forms two hydrogen bonds with Arg-202 and Val-204 (Fig. 3E). These include newly formed indirect hydrogen bonds via water between the O6 position of the guanine nucleotide base and Thr-201 sidechain, as well as direct and indirect hydrogen bonding from the N1 and N2 positions of the guanine nucleotide base, respectively, to the side chain of Asn-203. In addition, the main chain HN of Val-204 coordinates to the O6 position of the guanine nucleotide base instead of the N1 nitrogen in the adenine nucleotide base. These characteristic interactions for the GTP analogue are enabled by ∼15° rotation of the base moiety and ∼90° rotation of the ribose group relative to the ATP-analogue within the hydrophobic groove (Fig. 3 C and F). These rotations induce a shift of the guanine nucleotide base by ∼1.5 Å toward Phe-205, promoting the formation of aromatic-aromatic interactions with Phe-139 and Phe-205 (Fig. 3C). In contrast, the aromatic-aromatic interaction of the adenine nucleotide base to Phe-205 is less significant due to its separation from the phenyl ring compared to the guanine nucleotide base. The present crystallographic analysis revealed that GTP binds to its designated position in PI5P4Kβ by rotating and shifting within the hydrophobic groove relative to ATP, which could be analogous to the “Tetris spin” in the tile matching video game, Tetris (Fig. 3 G and H).
Structure-based development of the GTP-insensitive mutant of PI5P4Kβ
To analyze the GTP-sensing function of PI5P4K in vivo, generation of a PI5P4Kβ mutant that specifically lacks the GTP-dependent kinase activity without perturbing the ATP-dependent kinase activity is critical. Since methods such as PI5P4Kβ knockout, gene silencing, and production of a kinase-dead mutations would disrupt both GTP- and ATP-dependent PI5P4Kβ activities. Therefore, we engineered PI5P4Kβ based on structural data to specifically reduce its GTP preference, so that the role of PI5P4Kβ in GTP sensing can be directly tested.
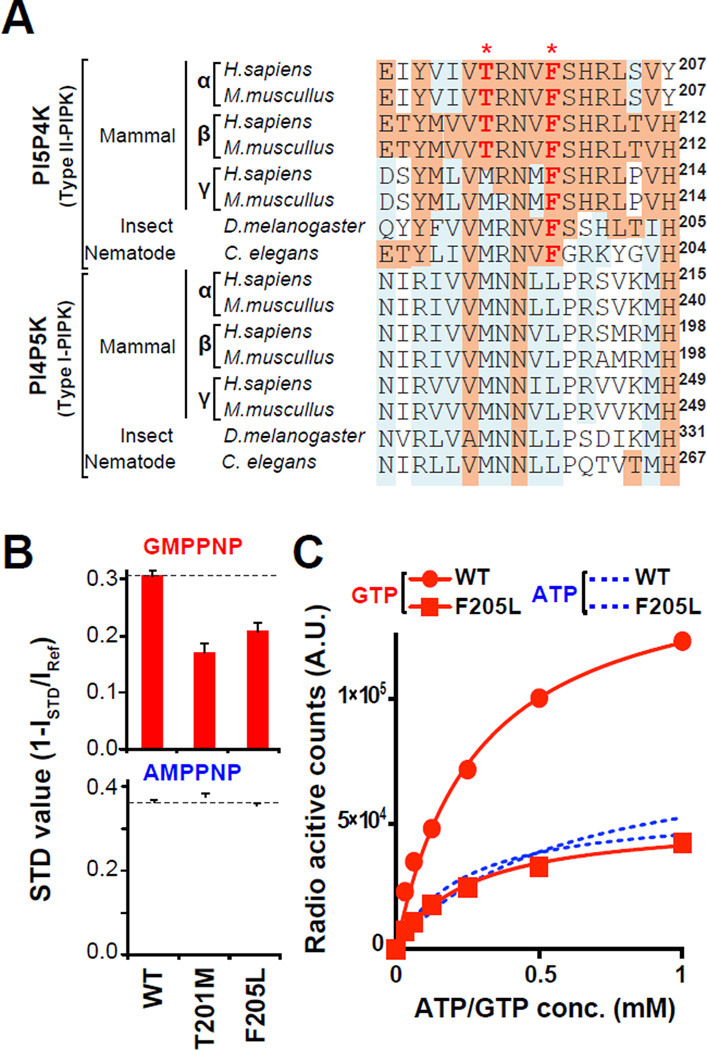
The crystal structures indicated that Thr-201 and Phe-205 are critical for guanine base recognition in PI5P4Kβ (Fig. 3). Thr-201 is conserved in PI5P4Kα and PI5P4Kβ while it is substituted to methionine in PI5P4Kγ and PI4P5K/Type-I PIPKs. Phe-205 is conserved among PI5P4Ks while it is leucine in PI4P5K/Type-I PIPKs (Fig. 4A). To analyze the effect of these residues on nucleotide recognition, Thr-201 and Phe-205 in PI5P4Kβ were mutated to methionine and leucine, respectively, and the direct interactions between nucleotides and PI5P4Kβs were analyzed. A saturation transfer difference (STD) NMR experiment revealed that PI5P4KβT201M and PI5P4KβF205L specifically reduced binding to the GTP analogue (Fig. 4B). The real-time GTP hydrolysis and in vitro kinase assays verify that PI5P4KβT201M and PI5P4KβF205L mutants showed a decrease in both GTP hydrolysis and GTP-dependent kinase activity (Fig. 4C and Fig. S4A–C). While PI5P4KβF205L retained ATP-dependent activity comparable to the WT, PI5P4KβT201M showed higher ATP-dependent activity in physiological nucleotide concentrations (Fig. S4 A – B). The nucleotide specificities of the two mutants were analyzed by crystal structures (Fig. S4D and Table 2). The decreased GTP-dependent kinase activity of PI5P4KβT201M could be explained by the loss of indirect hydrogen bonding by the T201M mutation and its higher ATP-dependent activity could arise from an additional contact between the substituted methionine and ATP (Fig. S4D). The reduction of the GTP-dependent activity of PI5P4KβF205L could be explained by the loss of the aromatic-aromatic interaction between guanine nucleotide base and Phe-205. The reduced binding affinity to the guanine nucleotide is supported by weak electron density for GMP in PI5P4KβF205L (Fig. S4D). In contrast, no significant differences were observed in AMP-binding mode in PI5P4KβF205L (Fig. S4D). Taken together, we selected PI5P4KβF205L to analyze the in vivo significance of the GTP-sensing activity.
Figure 4. Development of the GTP-insensitive PI5P4Kβ mutant.
(A) A sequence alignment of PI5P4K and PI4P5K family proteins. The positions Thr-201 and Phe-205 in human PI5P4Kβ are indicated by asterisk (*).
(B) STD-NMR experiments using PI5P4Kβ mutants. The smaller STD values of GMPPNP relative to WT indicate that PI5P4KβF205L and PI5P4KβT201M mutants specifically decreased the affinity to the GTP analogue. The STD values are not proportional to the reduction in affinity to nucleotides but reflect the population of nucleotides in complex with PI5P4Ks under experimental condition. Decrease in the STD values for GMPPNP from 0.3 to 0.2 observed here for mutants would correspond to 3-fold decrease in affinity. Data are displayed as means ± SD, n=3. See supplemental for calculation of STD value.
(C) PI5P4KβF205L specifically decreases GTP-dependent kinase activity, yet retains full ATP-dependent kinase activity.
The GTP-sensing activity of PI5P4Kβ is required for metabolic adaptation and PI(5)P accumulation under a GTP-energy crisis
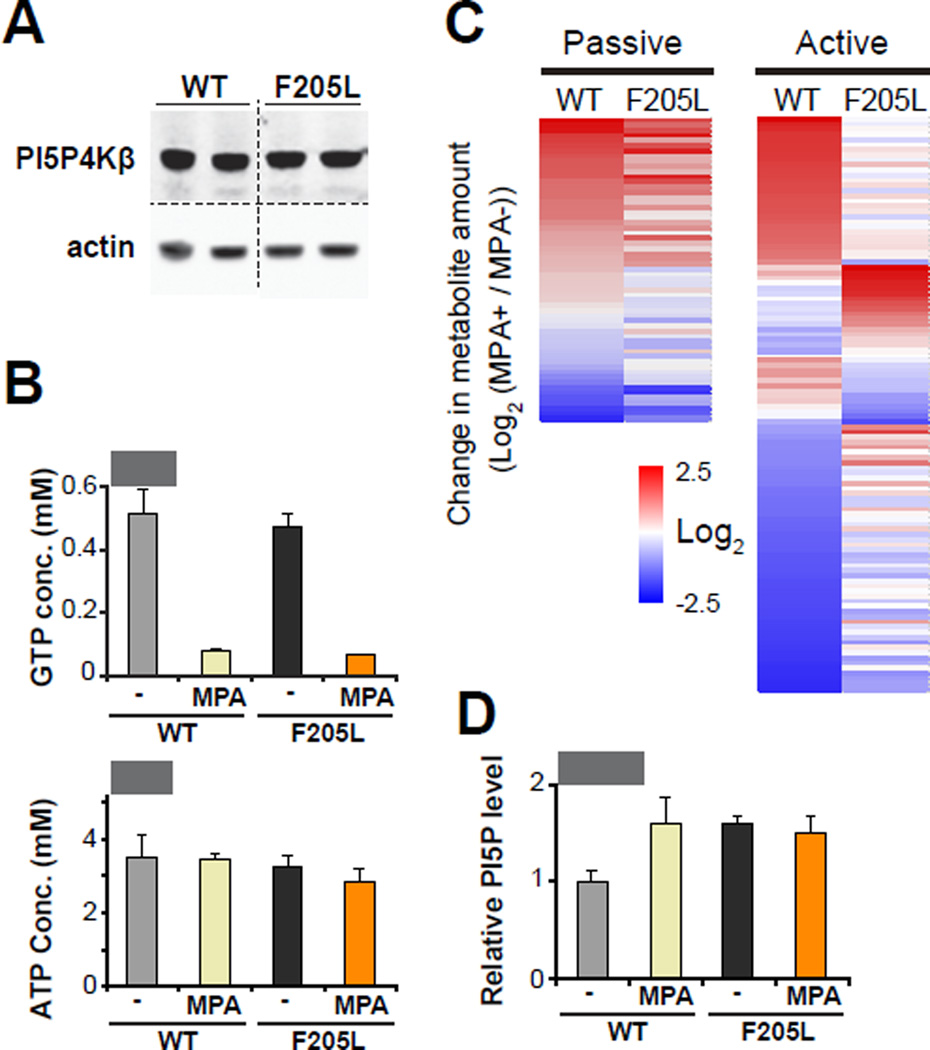
Previously identified molecular sensors, such as AMPK, mTOR, and HIF prolyl-hydroxylases, alter metabolism via cellular signaling in response to changes in their respective metabolite concentrations. We therefore investigated whether the GTP-sensing activity of PI5P4Kβ is involved in metabolic regulation. WT-PI5P4Kβ or PI5P4KβF205L was stably expressed in PI5P4Kβ-deficient SV40-transformed mouse embryonic fibroblasts (MEFs) (Fig. 5A). The metabolic states of PI5P4KβF205L cells are compared with those of WT-PI5P4Kβ cells in the presence and absence of mycophenolic acid (MPA), an inhibitor for inosine monophosphate dehydrogenase (Hedstrom, 2009; Weigel et al., 2001). Treatment with MPA decreased cellular GTP concentration within four hours without significantly altering ATP concentration (Fig. 5B). LC-MS/MS analysis revealed that metabolites can be categorized into two classes, passive and active classes (Fig. 5C and Table S1). The passive metabolites showed only a marginal difference between WT-PI5P4Kβ and PI5P4KβF205L cells in response to the change in GTP concentration (changes in PI5P4KβF205L cells are in between 0.5 ∼ 2-fold compared to that in WT-PI5P4Kβ cells). This class of metabolites would not be directly regulated by the GTP-sensing activity of PI5P4Kβ, yet they appear to be affected by changes in GTP concentration in a passive manner. On the other hand, there are metabolites showing distinct responses to GTP concentration changes in PI5P4KβF205L cells as compared to WT-PI5P4Kβ cells (changes in PI5P4KβF205L cells are <0.5-fold or >2-fold compared to that in WT-PI5P4Kβ cells). It appears reasonable to consider that these differences in “active-class metabolites” arise from the GTP-sensing activity of PI5P4Kβ. It has been suggested that PI5P4Ks control the levels of PI(5)P, a second lipid messenger, for downstream signaling. Thus, we assessed the levels of PI(5)P in WT-PI5P4Kβ and PI5P4KβF205L cells under normal and the MPA-induced GTP-depleted conditions. The MPA treatment increases the levels of PI(5)P by 50% in WT-PI5P4Kβ cells. In contrast, PI5P4KβF205L cells showed higher basal levels of PI(5)P under normal conditions, and the PI(5)P level did not change upon treatment with MPA (Fig. 5D). These results suggest that cellular GTP concentration is reflected in the levels of PI(5)P lipid second messenger via the GTP-sensing activity of PI5P4Kβ.
Figure 5. GTP-sensing activity of PI5P4Kβ regulates metabolism and PI(5)P level.
(A) The comparable expression levels of WT-PI5P4Kβ or PI5P4KβF205L in the isogenic PI5P4Kβ null MEFs.
(B) Cellular GTP and ATP concentrations of WT-PI5P4Kβ and PI5P4KβF205L cells with or without MPA treatment for 4hr. The average and deviation of two independent samples per group is presented. The data are representative of two separate experiments.
(C) Heat map of the metabolic responses to the GTP concentration changes. Each metabolite is color coded by the changes in amount by the MPA treatment.
(D) Cellular PI(5)P levels of WT-PI5P4Kβ and PI5P4KβF205L cells with or without MPA treatment for 4 hr. The average and deviation of two independent samples per group is presented. The data are representative of two separate experiments.
See also Table S1.
Taken together, PI5P4Kβ can be considered to be a GTP-sensor in mammalian cells; PI5P4Kβ binds to GTP, changes its activity depending on the cellular GTP concentration, and transfers biological signals presumably through PI(5)P to alter the metabolism of cells.
GTP sensing by PI5P4Kβ is critical for tumorigenesis
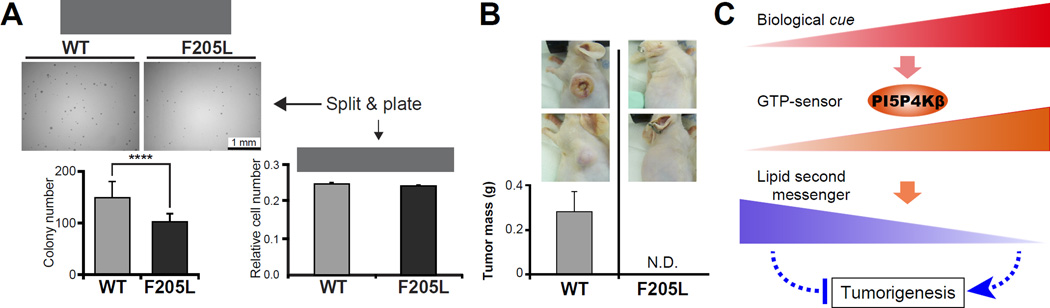
We next asked the biological significance of the GTP-sensing activity. It has been shown that solid tumor cells need to adapt their metabolism in order to cope with nutrient and energy stresses during tumorigenesis (Cantor and Sabatini, 2012; Jones and Thompson, 2009; Laderoute et al., 2006; Schafer et al., 2009). PI5P4Ks have been shown to promote tumorigenesis in several types of cancers (Emerling et al., 2013; Jude et al., 2015; Keune et al., 2013; Luoh et al., 2003), raising the possibility that the GTP-sensing activity of PI5P4Kβ is involved in its tumorigenic activity. Thus, we compared anchorage-independent soft-agar colony formation of WT-PI5P4Kβ cells to that of PI5P4KβF205L cells, where cells are exposed to similar nutrient depleted conditions as cancer cells in tissues (Paoli et al., 2013; Schafer et al., 2009). In the soft-agar assay, PI5P4KβF205L cells produced significantly fewer colonies than WT-PI5P4Kβ cells (Fig. 6A), while the cell proliferation rates of WT-PI5P4Kβ and PI5P4KβF205L cells were indistinguishable on plastic plates (Fig. 6A). These results suggest the possibility that the GTP-sensing activity of PI5P4Kβ is important for withstanding metabolic stress during tumorigenesis. To test this possibility in vivo, we performed an allograft study using WT-PI5P4Kβ and PI5P4KβF205L cells. Immunocompromised mice were injected with WT-PI5P4Kβ and PI5P4KβF205L cells, and tumor growth was monitored over an 11-week period. As shown in Fig. 6B, 50% of mice injected with the WT-PI5P4Kβ cells developed tumors, however no tumor formation was observed in mice injected with PI5P4KβF205L cells. These results indicate that the GTP-sensing activity of PI5P4Kβ provides an advantage in tumorigenesis in vivo.
Figure 6. GTP-sensing activity of PI5P4Kβ regulates cell proliferation.
(A) GTP-sensing activity of PI5P4Kβ is critical for anchorage-independent soft-agar colony formation of MEFs. The isogenic PI5P4Kβ null SV40-transformed MEFs that stably expressing WT-PI5P4Kβ or PI5P4KβF205L were split and plated. Data are displayed as means ± SE, n=10 for soft-agar assay and n=3 for cell proliferation assay. ****: p < 0.0001.
(B) GTP-sensing activity of PI5P4Kβ is critical for tumorigenesis of MEFs. The average of two independent samples per group is presented. The data are representative of two separate experiments.
(C) A model of regulation of PI5P4Kβ activity by GTP concentration. Changes of GTP concentration acts as cue to evoke lipid second messenger signaling for metabolic adaptation and tumorigenesis via PI5P4Kβ.
Discussion
PI5P4Kβ is a GTP sensor that couples GTP concentration to phosphoinositide signaling
Detecting and responding to changes in the levels of high energy metabolites are of fundamental importance for survival. Failure to respond to such change is detrimental to cells and predisposes organisms for systemic dysfunction or metabolic disease. While regulatory links between GTP concentration and metabolism have been found in unicellular organisms (Gaal, 1997; Grummt and Grummt, 1976; Krásný and Gourse, 2004; Ratnayake-Lecamwasam, 2001), the molecular identity of a GTP sensor has been missing in higher eukaryotes. Our data show that (i) PI5P4Kβ directly binds to GTP; (ii) PI5P4Kβ activity is regulated by the GTP concentration because its Km value is within the range of physiological variations of GTP concentration; (iii) PI5P4Kβ changes the levels of a lipid second messenger, PI(5)P, in response to changes in GTP concentration. In addition, the specific disruption of GTP-dependent kinase activity of PI5P4Kβ showed that GTP-sensing activity of PI5P4Kβ is required for metabolic adaptation and tumorigenesis. These results demonstrate that PI5P4Kβ is a molecular sensor for GTP concentration (Fig. 6C).
Among the three criteria for the GTP sensor, GTP preference is a critical biochemical characteristic. Since most kinases utilize ATP as a phosphor donor, GTP-dependent kinase activity of PI5P4Kβ seems to be evolved from an ATP-dependent kinase. Acquisition of the GTP preference, however, seems to be not simple. Our crystal structures demonstrate that Thr-201 and Phe-205 are responsible for the GTP-dependent kinase activity of PI5P4Kβ. While sequence alignment of PI5P4K/Type II PIPKs shows two patterns in combination of residues 201 and 205 (T-F and M-F types) (Fig. 4A), it is difficult to deduce nucleotide preference only from the two residues. For example, PI5P4Kγ has methionine (Met-203) in the position corresponding to Thr-201 in PI5P4Kβ, yet possesses a strong GTP-preference. Since there are additional structural differences in the nucleotide binding residues in PI5P4Kα and PI5P4Kγ compared to PI5P4Kβ, different set of residues seem to contribute to nucleotide selectivity of individual PI5P4Ks. Indeed, Clarke and Irvine (2013) indicate that the amino-acid sequence difference in G-loop, from Pro-126 to Arg-138 in human PI5P4Kβ, is critical for determining the ATP-dependent activity of PI5P4Ks. Therefore, structures of PI5P4Kα and PI5P4Kγ in complex with ATP or GTP are needed to clarify the mechanism for nucleotide preference of PI5P4Ks in the future, which will be a key to decipher the evolutional mechanism of the GTP-dependent kinase activity.
Given the higher GTP-dependent activity over ATP-dependent activity in PI5P4Kγ and existence of its Km value within the range of physiological variation in GTP concentration, it is certainly a possibility that PI5P4Kγ plays a role in GTP sensing. There is a recent report that PI5P4Kγ is involved in the development and maintenance of epithelial cell functional polarity (Clarke et al., 2015). The role of the PI5P4Kγ isoform in GTP sensing would be an important next step to explore.
The GTP-sensing activity of PI5P4Kβ fills a gap in the PI5P4K paradox
Mammalian genes encode three isoforms of PI5P4Ks (α, β, and γ). Among the isoforms, PI5P4Kα has the highest kinase activity. The kinase activity of PI5P4Kα is reported to be 100-fold higher than that of PI5P4Kβ and 2,000-fold higher than that of PI5P4Kγ, and therefore it has been considered that PI5P4Kα has a dominant role in cells and PI5P4Kβ and PI5P4Kγ may act as scaffolds to recruit PI5P4Kα by heterodimerization (Bultsma et al., 2010; Clarke and Irvine, 2013). Paradoxically, PI5P4Kα knockout mice do not exhibit any obvious phenotypes under normal conditions (Emerling et al., 2013), while the knock out mice of the less active PI5P4Kβ display dramatic phenotypes: reduced body weight, resistance to obesity induced by high-fat diets, and increased insulin sensitivity (Lamia et al., 2004). In the present study, we established that the GTP-dependent activity of PI5P4Kβ is of the same order of magnitude as PI5P4Kα, indicating that the PI5P4Kβ activity is strong enough to vary the PI5P4K activity. In addition, PI5P4Kβ has the additional feature of its kinase activity being regulated by the GTP concentration. The GTP-sensing activity of PI5P4Kβ could affect a PI(5)P population and might play a role in the different signal pathway from PI5P4Kα with distinct cellular localization. These observations would fill the gap in the PI5P4K paradox and explain why the PI5P4Kβ knockout shows a more severe phenotype than the PI5P4Kα knockout.
PI5P4Kβ converts metabolic cues from GTP into PI(5)P signaling
PI5P4Kβ regulates two lipid second messengers, PI(5)P and PI(4,5)P2. It has been suggested that a major role of PI5P4K is to regulate the levels of PI(5)P, since the majority of PI(4,5)P2 is produced by another pathway by PI4P5K/Type-I PIPKs from PI(4)P (Clarke and Irvine, 2012; 2013). Thus far, a series of effector molecules of PI(5)P have been reported. Transcriptional regulators, ING2, UHRF1, IRF3, and TAF3, have been shown to bind to PI(5)P to change their subcellular localization and/or activity (Bua et al., 2013; Gelato et al., 2014; Kawasaki et al., 2013; Stijf-Bultsma et al., 2015). Binding of PI(5)P to WIPI2, an autophagy effector, has been shown to induce Vps34-independent non-canonical autophagy (Vicinanza et al., 2015). PI(5)P’s role in regulating membrane trafficking and cytoskeletal rearrangement, via TOM1, TIAM1, and the Doc family, has been reported (Boal et al., 2015; Guittard et al., 2012; Viaud et al., 2014). Our data suggest that there may be unidentified effectors of PI(5)P that play a role in regulating metabolism, and that the changes of GTP concentration would induce systemic responses in cells through these PI(5)P effectors.
One critical question is by how much the amount of GTP-concentration changes could be sensed by this signaling system. The results of the present study using MPA suggest that an approximately 85% reduction of GTP concentration, which leads to a 1.5-fold increase in PI(5)P level, is enough to evoke the GTP-dependent PI5P4Kβ signaling (Fig. 5) Indeed, with the 85% reduction of GTP concentration, cells harboring the GTP-insensitive PI5P4Kβ mutant (PI5P4KβF205L) showed no variation of the PI(5)P concentration and significant differences in metabolic responses as compared to WT cells (Fig. 5). In addition, PI5P4KβF205L cells decrease the tumorigenic activities in vitro and in vivo (Fig. 6). Thus, it is reasonable to suggest that the reduction of GTP-dependent kinase activities of PI5P4Kβ and the resulting changes in PI(5)P level is sufficient for trigger functional signaling. Presumably, there may be a mechanism that amplifies the changes in PI5P4Kβ activity. In support of this notion, many biological systems are not linear and signaling activities are dynamically propagated in a spatio-temporal manner through feedback amplification and site specific localization (Kholodenko, 2006). It would also be possible to consider contribution of another GTP sensor.
The GTP-sensing activity of PI5P4Kβ may be the Achilles' heel for human diseases
Receiving biological cues from GTP concentration may be important in pathological and metabolic conditions that lead to GTP depletion, because rapidly dividing tumor cells as well as metabolic tissues, including the liver, pancreas, and adipose tissue, consume massive amounts of GTP to maintain tumorigenesis and/or to produce serum proteins (Caro and Palade, 1964; Havel, 2002; Ma and Blenis, 2009; Miller et al., 1951; Weiss, 1953). In line with this notion, it is intriguing that the PI5P4Kβ knockout mice display the phenotypic link to tumorigenesis as well as whole body metabolism (Emerling et al., 2013; Lamia et al., 2004). Taken into consideration with the present work, these observations argue that biological cues from GTP concentration need to be integrated into the current energy model as an independent and crucial benchmark for tumorigenesis and metabolic diseases. In addition, increased dependence of those pathological states on GTP makes the GTP-sensing activity of PI5P4Kβ an Achilles' heel for those human diseases. Our crystal structures reveal the unpredicted recognition mode of GTP by PI5P4Kβ, and importantly, we were able to modulate GTP-dependent activity by introducing rational mutations. We believe that the structural data presented in this work will benefit pharmaceutical targeting of the GTP-sensing activity of PI5P4Kβ that provides an opportunity to develop unique cancer therapeutics.
Experimental Procedures
A proteomic screening for GTP-binding proteins
A proteomic screening for GTP-binding proteins was carried out by pulling down by GTP-conjugated agarose beads and the successive LC-MS/MS analysis. The detail is described in the supplemental materials and methods.
The PI5P4K kinase assay
The in vitro kinase assay was carried out using γ-32P radiolabeled ATP or GTP (Perkin Elmer), synthetic PI(5)P diC16 (Echelon Biosciences), and recombinant PI5P4Ks. The radiolabeled PI(4,5)P2 was quantified with a phosphorimager (Molecular Dynamics, STORM840, GE Healthcare). Details of in vitro kinase assay are described in the supplemental materials and methods. Non-tagged recombinant PI5P4Ks were used otherwise mentioned.
Crystallization, data collection, and structure determination
Diffraction data were collected at PF in KEK (Tsukuba, Japan). Crystallographic calculations were performed by PHENIX (Tables 1 and 2) (Adams et al., 2010). Details of the crystallographic work are described in the supplemental materials and methods.
NMR spectroscopy
STD experiment and real time GTP- and ATP-hydrolysis assays were performed on Bruker Avance 700 MHz spectrometer equipped with triple resonance probe at 25 °C. Details of the NMR experiments are described in the supplemental materials and methods.
Analysis of metabolites by targeted mass spectrometry
The metabolomics study was carried out by the targeted mass spectrometry using the SV40-transformed PI5P4Kβ-deficient MEFs stably expressing WT-PI5P4Kβ or PI5P4KβF205L. Details of the sample preparation, data acquisition, and processing are described in supplemental materials and methods.
Colony formation assays
Anchorage-independent colony formation assay for assessing the transforming activity and cell proliferation assays on plastic plate were carried out. The details are described in supplemental materials and methods.
Allograft assay
SV40-transformed PI5P4Kβ -deficient MEFs stably expressing WT-PI5P4Kβ or PI5P4KβF205L were subcutaneously injected into the flank of 8–10 week old four athymic NCr nu/nu mice. Tumors were harvested at the 76 days. Details of allograft assay are described in supplemental materials and methods.
Supplementary Material
Acknowledgments
First and foremost, we thank Dr. Lewis C. Cantley for his supports and comments. We thank Drs. George Thomas, David Plas, Sarah Kozma, and Carol Mercer for their support. We also thank Susanne Breitkopf and Min Yuan for help with the mass spectrometry experiments, Mr. Toshiaki Takano and Ms. Imai Misaki for helping protein expression and purification, and Drs. Mindy Davis, Janice Connelly, Shingo Kajimura, Takanari Inoue, Koichi Okumura, Keiko Kono, Satoshi Yoshida, Yoji Minamishima, Maria Czyzyk-Krzeska, and Jun-Lin Guan for critical reading and comments, Ms. Kaori Kofuji for manuscript preparation. We acknowledge the members of the UC-Cancer Institute, UC-Brain Tumor Center, and the Izayoi Meeting for stimulating discussions. K.S. was supported, in part, by the American Association of Neurological Surgeons. S.K was supported, in part, by Kanae Foundation. H.Y. was supported, in part, by the Uehara Memorial Foundation. The work was partially supported by NIH grants 2P01CA120964 (J.M.A.), 5P30CA006516 (J.M.A.), 1R00CA168997 (J.W.L) and MTP UC-Brain Tumor Center grant, UC Internal Medicine grant, 1R03MH096575 & 1R01NS089815 (A.T.S.). Funding was also provided by Grants-in-Aid for Scientific Research (KAKENHI; grant numbers 24370048 and 25121743 to K. T.; 22121005 to T.S.) and Platform for Drug Discovery, Informatics, and Structural Life Science from the Ministry of Education, Culture, Sports, Science and Technology and Japan Society for the Promotion of Science. Work in the D.A. lab is supported by MRC grant MC_UP_1202/1. Author Contributions: K.S., S.K., Y.I., performed in vitro assays. K.T., Y.H.L., M.S. and T.Y. performed X-ray crystallography. K.T. performed NMR experiments. J.T. and T.D. performed xenograft assay. M.S., H.Y., S.K., N.M., Y.Z., E.R.K. and B.M.E. performed biochemical analysis and support analysis. J.M.A. performed LC/MS/MS analysis. T.I. supported kinetics analysis of the kinase. D.A., J.W.L., assisted the initiation of screening and consulted the project. K.T., T.S. and A.T.S. conceived the study and wrote the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Accession Numbers:
All atomic coordinates used in this manuscript have been deposited in the Protein Data Bank under following accession codes: 3WZZ, 3X01, 3X02, 3X03, 3X04, 3X05, 3X06, 3X09, 3X0A, 3X0B, 3X0C
Authors declare no conflicts of interests on this paper.
Supplemental Materials:
Figure S1, related to Figure 1. GTP and ATP hydrolysis activity of human PI5P4K isoforms in vitro.
Figure S2, related to Figure 2. In vitro kinase activity of human PI5P4K isoforms.
Figure S3, related to Figure 3. Overall structure of PI5P4Kβ in complex with nucleotide.
Figure S4, related to Figure 4. Enzymatic and structural characterizations of GTP-insensitive PI5P4Kβ mutants.
Table S1, related to Figure 5. Polar metabolites measured by LC-MS/MS. Supplemental Experimental Procedures
References
- Adams PD, Afonine PV, Bunkóczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boal F, Mansour R, Gayral M, Saland E, Chicanne G, Xuereb JM, Marcellin M, Burlet-Schiltz O, Sansonetti PJ, Payrastre B, et al. TOM1 is a PI5P effector involved in the regulation of endosomal maturation. J Cell Sci. 2015;128:815–827. doi: 10.1242/jcs.166314. [DOI] [PubMed] [Google Scholar]
- Bua DJ, Martin GM, Binda O, Gozani O. Nuclear phosphatidylinositol-5-phosphate regulates ING2 stability at discrete chromatin targets in response to DNA damage. Sci. Rep. 2013;3:2137. doi: 10.1038/srep02137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bultsma Y, Keune W-J, Divecha N. PIP4Kbeta interacts with and modulates nuclear localization of the high-activity PtdIns5P-4-kinase isoform PIP4Kalpha. Biochem J. 2010;430:223–235. doi: 10.1042/BJ20100341. [DOI] [PubMed] [Google Scholar]
- Cantor JR, Sabatini DM. Cancer Cell Metabolism: One Hallmark, Many Faces. Cancer Disc. 2012;2:881–898. doi: 10.1158/2159-8290.CD-12-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro Palade GE. Protein synthesis, storage, and discharge in the pancreatic exocrine cell. An autoradiographic study. J Cell Biol. 1964;20:473–495. doi: 10.1083/jcb.20.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Kiyan V, Zhylkibayev AA, Kazyken D, Bulgakova O, Page KE, Bersimbaev RI, Spooner E, Sarbassov DD. Autoregulation of the Mechanistic Target of Rapamycin (mTOR) Complex 2 Integrity Is Controlled by an ATP-dependent Mechanism. J Biol Chem. 2013;288:27019–27030. doi: 10.1074/jbc.M113.498055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke JH, Irvine RF. The activity, evolution and association of phosphatidylinositol 5-phosphate 4-kinases. Adv Biol Reg. 2012;52:40–45. doi: 10.1016/j.advenzreg.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Clarke JH, Irvine RF. Evolutionarily conserved structural changes in phosphatidylinositol 5-phosphate 4-kinase (PI5P4K) isoforms are responsible for differences in enzyme activity and localization. Biochem J. 2013;454:49–57. doi: 10.1042/BJ20130488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke JH, Giudici ML, Burke JE, Williams RL, Maloney DJ, Marugan J, Irvine RF. The function of phosphatidylinositol 5-phosphate 4-kinase γ (PI5P4Kγ) explored using a specific inhibitor that targets the PI5P–binding site. Biochem J. 2015;466:359–367. doi: 10.1042/BJ20141333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demian DJ, Clugston SL, Foster MM, Rameh L, Sarkes D, Townson SA, Yang L, Zhang M, Charlton ME. High-throughput, cell-free, liposome-based approach for assessing in vitro activity of lipid kinases. J Biomol Screen. 2009;14:838–844. doi: 10.1177/1087057109339205. [DOI] [PubMed] [Google Scholar]
- Dennis PB, Jaeschke A, Saitoh M, Fowler B, Kozma SC, Thomas G. Mammalian TOR: a homeostatic ATP sensor. Science. 2001;294:1102–1105. doi: 10.1126/science.1063518. [DOI] [PubMed] [Google Scholar]
- Emerling BM, Hurov JB, Poulogiannis G, Tsukazawa KS, Choo-Wing R, Wulf GM, Bell EL, Shim H-S, Lamia KA, Rameh LE, et al. Depletion of a Putatively Druggable Class of Phosphatidylinositol Kinases Inhibits Growth of p53-Null Tumors. Cell. 2013;155:844–857. doi: 10.1016/j.cell.2013.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertel R, Redfield B, Brot N, Weissbach H. Role of GTP in protein synthesis: interaction of GTP with soluble transfer factors from E. coli. Arch Biochem Biophys. 1968;128:331–338. doi: 10.1016/0003-9861(68)90039-8. [DOI] [PubMed] [Google Scholar]
- Feder D, Bishop JM. Purification and enzymatic characterization of pp60c–src from human platelets. J Biol Chem. 1990;265:8205–8211. [PubMed] [Google Scholar]
- Gaal T. Transcription Regulation by Initiating NTP Concentration: rRNA Synthesis in Bacteria. Science. 1997;278:2092–2097. doi: 10.1126/science.278.5346.2092. [DOI] [PubMed] [Google Scholar]
- Gelato KA, Tauber M, Ong MS, Winter S, Hiragami-Hamada K, Sindlinger J, Lemak A, Bultsma Y, Houliston S, Schwarzer D, et al. Accessibility of different histone H3-binding domains of UHRF1 is allosterically regulated by phosphatidylinositol 5-phosphate. Mol Cell. 2014;54:905–919. doi: 10.1016/j.molcel.2014.04.004. [DOI] [PubMed] [Google Scholar]
- Green R, Noller HF. Ribosomes and translation. Annu Rev Biochem. 1997;66:679–716. doi: 10.1146/annurev.biochem.66.1.679. [DOI] [PubMed] [Google Scholar]
- Grummt I, Grummt F. Control of nucleolar RNA synthesis by the intracellular pool sizes of ATP and GTP. Cell. 1976;7:447–453. doi: 10.1016/0092-8674(76)90175-6. [DOI] [PubMed] [Google Scholar]
- Guittard G, Mortier E, Tronchère H, Firaguay G, Gérard A, Zimmermann P, Payrastre B, Nunès JA. Evidence for a positive role of PtdIns5P in T-cell signal transduction pathways. FEBS Letters. 2012;584:2455–2460. doi: 10.1016/j.febslet.2010.04.051. [DOI] [PubMed] [Google Scholar]
- Hardie DG. AMPK: a key regulator of energy balance in the single cell and the whole organism. Int J Obes Relat Metab Disord. 2008;32:S7–S12. doi: 10.1038/ijo.2008.116. [DOI] [PubMed] [Google Scholar]
- Haselkorn R, Rothman-Denes LB. Protein synthesis. Ann Rev Biochem. 1973;42:397–438. doi: 10.1146/annurev.bi.42.070173.002145. [DOI] [PubMed] [Google Scholar]
- Havel PJ. Control of energy homeostasis and insulin action by adipocyte hormones: leptin, acylation stimulating protein, and adiponectin. Curr Opin Lipidol. 2002;13:51–59. doi: 10.1097/00041433-200202000-00008. [DOI] [PubMed] [Google Scholar]
- Hedstrom L. IMP dehydrogenase: structure, mechanism, and inhibition. Chem Rev. 2009;109:2903–2928. doi: 10.1021/cr900021w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RG, Thompson CB. Tumor suppressors and cell metabolism: a recipe for cancer growth. Gen Dev. 2009;23:537–548. doi: 10.1101/gad.1756509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jude JG, Spencer GJ, Huang X, Somerville TDD, Jones DR, Divecha N, Somervaille TCP. A targeted knockdown screen of genes coding for phosphoinositide modulators identifies PIP4K2A as required for acute myeloid leukemia cell proliferation and survival. Oncogene. 2015;34:1253–1262. doi: 10.1038/onc.2014.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashem MA, Nelson RM, Yingling JD, Pullen SS, Prokopowicz AS, Jones JW, Wolak JP, Rogers GR, Morelock MM, Snow RJ, et al. Three Mechanistically Distinct Kinase Assays Compared: Measurement of Intrinsic ATPase Activity Identified the Most Comprehensive Set of ITK Inhibitors. J Biomol Scr. 2006;12:70–83. doi: 10.1177/1087057106296047. [DOI] [PubMed] [Google Scholar]
- Kawasaki T, Takemura N, Standley DM, Akira S, Kawai T. The Second Messenger Phosphatidylinositol-5-Phosphate Facilitates Antiviral Innate Immune Signaling. Cell Host Microbe. 2013;14:148–158. doi: 10.1016/j.chom.2013.07.011. [DOI] [PubMed] [Google Scholar]
- Keune W-J, Sims AH, Jones DR, Bultsma Y, Lynch JT, Jirström K, Landberg G, Divecha N. Low PIP4K2B expression in human breast tumors correlates with reduced patient survival: A role for PIP4K2B in the regulation of E-cadherin expression. Cancer Res. 2013;73:6913–6925. doi: 10.1158/0008-5472.CAN-13-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kholodenko BN. Cell-signalling dynamics in time and space. Nat Rev Mol Cell Biol. 2006;7:165–176. doi: 10.1038/nrm1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krásný L, Gourse RL. An alternative strategy for bacterial ribosome synthesis: Bacillus subtilis rRNA transcription regulation. Embo J. 2004;23:4473–4483. doi: 10.1038/sj.emboj.7600423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz J, Fuelling A, Kolbe L, Anderson RA. Stereo-specific substrate recognition by phosphatidylinositol phosphate kinases is swapped by changing a single amino acid residue. J Biol Chem. 2002;277:5611–5619. doi: 10.1074/jbc.M110775200. [DOI] [PubMed] [Google Scholar]
- Laderoute KR, Amin K, Calaoagan JM, Knapp M, Le T, Orduna J, Foretz M, Viollet B. 5'-AMP-Activated Protein Kinase (AMPK) Is Induced by Low-Oxygen and Glucose Deprivation Conditions Found in Solid-Tumor Microenvironments. Mol Cell Biol. 2006;26:5336–5347. doi: 10.1128/MCB.00166-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamia KA, Peroni OD, Kim Y-B, Rameh LE, Kahn BB, Cantley LC. Increased insulin sensitivity and reduced adiposity in phosphatidylinositol 5-phosphate 4-kinase beta−/− mice. Mol Cell Biol. 2004;24:5080–5087. doi: 10.1128/MCB.24.11.5080-5087.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez JM. GTP pool expansion is necessary for the growth rate increase occurring in Bacillus subtilis after amino acids shift-up. Arch Microbiol. 1982;131:247–251. doi: 10.1007/BF00405887. [DOI] [PubMed] [Google Scholar]
- Luoh S-W, Venkatesan N, Tripathi R. Overexpression of the amplified Pip4k2β gene from 17q11–12 in breast cancer cells confers proliferation advantage. Oncogene. 2003;23:1354–1363. doi: 10.1038/sj.onc.1207251. [DOI] [PubMed] [Google Scholar]
- Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10:307–318. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- Matsushika A, Nagashima A, Goshima T, Hoshino T. Fermentation of xylose causes inefficient metabolic state due to carbon/energy starvation and reduced glycolytic flux in recombinant industrial Saccharomyces cerevisiae. PLoS ONE. 2013;8:e69005. doi: 10.1371/journal.pone.0069005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13:1016–1023. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LL, Bly CG, Watson ML, Bale WF. The dominant role of the liver in plasma protein synthesis; a direct study of the isolated perfused rat liver with the aid of lysine-epsilon-C14. J Exp Med. 1951;94:431–453. doi: 10.1084/jem.94.5.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt FC, Ingraham JL, Schaechter M. Physiology of the Bacterial Cell. Sunderland, MA: Sinauer Associates Incorporated; 1990. [Google Scholar]
- Niefind K, Pütter M, Guerra B, Issinger OG, Schomburg D. GTP plus water mimic ATP in the active site of protein kinase CK2. Nat Struct Biol. 1999;6:1100–1103. doi: 10.1038/70033. [DOI] [PubMed] [Google Scholar]
- Oakhill JS, Steel R, Chen Z-P, Scott JW, Ling N, Tam S, Kemp BE. AMPK is a direct adenylate charge-regulated protein kinase. Science. 2011;332:1433–1435. doi: 10.1126/science.1200094. [DOI] [PubMed] [Google Scholar]
- Paoli P, Giannoni E, Chiarugi P. Anoikis molecular pathways and its role in cancer progression. Biochim Biophys Acta. 2013;1833:3481–3498. doi: 10.1016/j.bbamcr.2013.06.026. [DOI] [PubMed] [Google Scholar]
- Ratnayake-Lecamwasam M. Bacillus subtilis CodY represses early-stationary-phase genes by sensing GTP levels. Gen Dev. 2001;15:1093–1103. doi: 10.1101/gad.874201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer ZT, Grassian AR, Song L, Jiang Z, Gerhart-Hines Z, Irie HY, Gao S, Puigserver P, Brugge JS. Antioxidant and oncogene rescue of metabolic defects caused by loss of matrix attachment. Nature. 2009;461:109–113. doi: 10.1038/nature08268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield CJ, Ratcliffe PJ. Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol. 2004;5:343–354. doi: 10.1038/nrm1366. [DOI] [PubMed] [Google Scholar]
- Stiehl DP, Wirthner R, Köditz J, Spielmann P, Camenisch G, Wenger RH. Increased prolyl 4-hydroxylase domain proteins compensate for decreased oxygen levels. Evidence for an autoregulatory oxygen-sensing system. J Biol Chem. 2006;281:23482–23491. doi: 10.1074/jbc.M601719200. [DOI] [PubMed] [Google Scholar]
- Stijf-Bultsma Y, Sommer L, Tauber M, Baalbaki M, Giardoglou P, Jones DR, Gelato KA, van Pelt J, Shah Z, Rahnamoun H, et al. The basal transcription complex component TAF3 transduces changes in nuclear phosphoinositides into transcriptional output. Mol Cell. 2015;58:453–467. doi: 10.1016/j.molcel.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traut T. Physiological concentrations of purines and pyrimidines. Mol Cell Biochem. 1994;140:1–22. doi: 10.1007/BF00928361. [DOI] [PubMed] [Google Scholar]
- Viaud J, Lagarrigue F, Ramel D, Allart S, Chicanne G, Ceccato L, Courilleau D, Xuereb J-M, Pertz O, Payrastre B, et al. Phosphatidylinositol 5-phosphate regulates invasion through binding and activation of Tiam1. Nat Commun. 2014;5:4080. doi: 10.1038/ncomms5080. [DOI] [PubMed] [Google Scholar]
- Vicinanza M, Korolchuk VI, Ashkenazi A, Puri C, Menzies FM, Clarke JH, Rubinsztein DC. PI(5)P Regulates Autophagosome Biogenesis. Mol Cell. 2015;57:219–234. doi: 10.1016/j.molcel.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Tsun Z-Y, Wolfson RL, Shen K, Wyant GA, Plovanich ME, Yuan ED, Jones TD, Chantranupong L, Comb W, et al. Metabolism. Lysosomal amino acid transporter SLC38A9 signals arginine sufficiency to mTORC1. Science. 2015;347:188–194. doi: 10.1126/science.1257132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel G, Griesmacher A, Zuckermann AO, Laufer G, Mueller MM. Effect of mycophenolate mofetil therapy on inosine monophosphate dehydrogenase induction in red blood cells of heart transplant recipients. Clin Pharm Ther. 2001;69:137–144. doi: 10.1067/mcp.2001.114166. [DOI] [PubMed] [Google Scholar]
- Weiss JM. The ergastoplasm; its fine structure and relation to protein synthesis as studied with the electron microscope in the pancreas of the Swiss albino mouse. J Exp Med. 1953;98:607–618. doi: 10.1084/jem.98.6.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao B, Sanders MJ, Underwood E, Heath R, Mayer FV, Carmena D, Jing C, Walker PA, Eccleston JF, Haire LF, et al. Structure of mammalian AMPK and its regulation by ADP. Nature. 2011;472:230–233. doi: 10.1038/nature09932. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.