Abstract
Aim
In this study, we investigated γH2AX foci as markers of DSBs in normal brain and brain tumor tissue in mouse after BNCT.
Background
Boron neutron capture therapy (BNCT) is a particle radiation therapy in combination of thermal neutron irradiation and boron compound that specifically accumulates in the tumor. 10B captures neutrons and produces an alpha (4He) particle and a recoiled lithium nucleus (7Li). These particles have the characteristics of extremely high linear energy transfer (LET) radiation and therefore have marked biological effects. High LET radiation causes severe DNA damage, DNA DSBs. As the high LET radiation induces complex DNA double strand breaks (DSBs), large proportions of DSBs are considered to remain unrepaired in comparison with exposure to sparsely ionizing radiation.
Materials and methods
We analyzed the number of γH2AX foci by immunohistochemistry 30 min or 24 h after neutron irradiation.
Results
In both normal brain and brain tumor, γH2AX foci induced by 10B(n,α)7Li reaction remained 24 h after neutron beam irradiation. In contrast, γH2AX foci produced by γ-ray irradiation at contaminated dose in BNCT disappeared 24 h after irradiation in these tissues.
Conclusion
DSBs produced by 10B(n,α)7Li reaction are supposed to be too complex to repair for cells in normal brain and brain tumor tissue within 24 h. These DSBs would be more difficult to repair than those by γ-ray. Excellent anti-tumor effect of BNCT may result from these unrepaired DSBs induced by 10B(n,α)7Li reaction.
Keywords: Boron neutron capture therapy, High LET radiation, DSBs, γH2AX foci
1. Background
Boron neutron capture therapy (BNCT) is a modality that involves the selective uptake of boron-10 (10B) compounds by tumor cells and subsequent irradiation using thermal or epithermal neutrons. 10B absorbs a thermal neutron with an extremely higher probability than 14N, the element with the largest cross section in the human body. The subsequent nuclear fission reaction yields an α (4He) particle and a lithium (7Li) nucleus, with high linear energy transfer (LET) values of 163 and 210 keV/μm, respectively. Another important characteristic of these particles is their extremely short track ranges, which are generally shorter than the diameters of tumor cells. In Kyoto University Research Reactor Institute (KUR), more than 400 cancer patients have been treated with BNCT since 1990, obtaining good results even after other conventional radiation and chemotherapies. Especially, malignant glioma patients have had great benefit from BNCT.1, 2, 3 The neutron beam used for BNCT is generated by a nuclear reactor and is inevitably a mixed beam that contains thermal, epithermal and fast neutrons, as well as γ-rays. Understanding the biological effects of the mixed beam will help improve the efficiency of this therapy and reduce the side effects in normal tissue. The biological effects of neutrons have been studied with respect to DNA damage,4, 5 but little is known about the effects induced by the mixed beam in BNCT. Among biological endpoints, DNA double-strand breaks (DNA-DSBs) are important and have been frequently used in many recent studies. The numbers of foci that immunochemically stain for γH2AX and other DNA repair proteins have previously been shown to be related to the number of DNA-DSBs and to be efficient markers for monitoring DNA-DSB induction and repair.6, 7 As the high LET radiation induces complex DNA DSBs, large proportions of DSBs are considered to remain unrepaired in comparison with exposure to low LET radiation.8, 9
2. Aim
In this study, we investigate γH2AX foci as markers of DSBs in normal brain and brain tumor in mouse after neutron irradiation by immunohistochemistry.
3. Materials and methods
3.1. Cell line and culture conditions
The human glioblastoma U251 cells was cultured in Dulbecco's modified Eagle's medium (Sigma–Aldrich Co. LLC, St. Louis, MO, USA) supplemented with 10% fetal bovine serum, and maintained at 37 °C in an atmosphere of 95% air and 5% CO2.
3.2. Animals and brain tumor model
Female BALB/C nu-nu mice, aged 8 weeks, were purchased from Japan Animal Co., Ltd, Osaka, Japan. The mice were anesthetized with an intraperitoneal injection of Nembutal (50 mg/kg) and placed in a stereotactic frame (Narishige, Japan). A midline scalp incision was made and the bregma was identified. A 1 mm burr hole was made in the right frontal region of the skull and a Hamilton syringe was inserted 4 mm into the dura. An injection of 106 human glioblastoma U251 cells in 5 μl of serum free medium was administered at a rate of 1 μl/min. After the infusion, the needle was left in place for 2 min and the burr hole was then covered with bone wax.
3.3. Drug treatment
A stock solution of boronophenylalanine (BPA) was used for all experiments. The 10B concentrations were measured by means of prompt γ-ray spectrometry using a thermal neutron guide tube installed at the KUR and the value was about 1000 ± 4.55 ppm. This stock solution consisted of sterilized water, glucose and BPA. BPA (500 mg/kg) or saline was administered subcutaneously 1 h before neutron irradiation.
3.4. Radiation sources and neutron fluences
The Heavy Water Neutron Irradiation Facility of the KUR was used for 1 MW neutron irradiation (1 h irradiation). The thermal neutron fluences were measured by gold foil (3 mm in diameter, 0.05 mm thick) activation analysis. The epi-thermal and fast neuron fluences were estimated by the normalization of the nominal values using the measured thermal neutron fluences. Contaminating gamma rays, including secondary gamma rays, were measured with thermoluminescence dosimeter (TLD). The TLD used was beryllium oxide (BeO) enclosed in a quartz glass capsule. BeO itself is sensitive to thermal neutrons. In terms of TLD sensitivity, the thermal neutron fluence of 8 × 1012 cm−2 is equal to approximately 1 cGy gamma-ray dose. For the neutron-sensitivity correction, gold activation foil was placed together with TLD. The average neutron fluxes were 1.0 × 109 cm−2 s−1 for the thermal neutron range (less than 0.5 eV), 1.6 × 108 cm−2 s−1 for the epithermal neutron range (0.5 eV–10 keV), and 9.4 × 106 cm−2 s−1 for the fast neutron range (more than 10 keV). The total absorbed doses were calculated as the sum of the absorbed doses attributed primarily to 1H(n,n)1H, 14N(n,p)14C, 10B(n,α)7Li and contaminating γ-rays. The dose-converting coefficients and details of the calculation method were described previously.10 The percentage of 14N was assumed to be 2%. The cobalt-60 γ-ray source at our institute was used for the γ-ray irradiations at the dose rate of 2.7 Gy/h.
3.5. Analysis of 10B concentrations in tissue
10B concentration of normal brain was analyzed by prompt γ-ray analysis using a thermal neutron guide tube installed at KUR.
3.6. Detection of γH2AX foci by immunohistochemistry (IHC)
After 30 min and 24 h, the mice were sacrificed and the brains were flash-frozen. Sections of 7-μm thickness were cut on a microtome (CM 1850; Leica Microsystems, Wetzlar, Germany). Briefly, frozen tissue sections were fixed with 4% paraformaldehyde buffer solution for 30 min. The slices were then permeabilized with 0.25% Triton X-100 for 15 min, blocked with 1% BSA in PBS for 60 min at 37 °C, and then incubated with a 1:300 dilution of mouse anti-phospho-histone H2AX (Ser139) monoclonal antibody (Millipore, USA) overnight at 4 °C. The slides were then washed in PBS and incubated with 1:100 dilution, Alexa Fluor 594 or Alexa Fluor 488-labeled goat anti-mouse IgG secondary antibodies (Molecular Probes, Eugene, OR, USA). The slices were then mounted on slides using 50% glycerol with 4,6-diamino-2-phenylindole (DAPI) (1:500). Immunofluorescence staining was observed using a fluorescence microscope (BZ-9000, KEYENCE, Japan). When we counted the number of gamma H2AX foci, we regarded them as positive whose sizes were bigger than 0.01 μm2.
4. Results
Table 1 shows the physical dose of neutron irradiation. These neutron beams consisted of fast, epithermal, and thermal neutrons and contaminated γ-rays. The calculated physical dose to saline treated mouse was sum of neutron dose and γ-ray dose, i.e. 2.045 Gy. The value of 10B concentration in normal brain was 9 ppm. Therefore the calculated physical dose to BPA treated mouse was 4.655 Gy.
Table 1.
Physical dose of mixed neutron beam.
| Thermal neutrons (<0.5 eV) | Epithermal neutron (<0.5 eV–10 keV) | Fast neutron (>10 keV) | γ-Ray | 10B (1 ppm) | |
|---|---|---|---|---|---|
| Physical dose (Gy) | 0.51 | 0.055 | 0.38 | 1.1 | 0.29 |
The physical dose of thermal neutron is almost due to the high-LET proton produced by nitrogen capture reaction (14N(n,p)14C). The physical dose of fast neutron is almost due to the proton recoiled by elastic scattering (1H(n,n)1H). Fast neutron also causes the reactions producing alpha particle, but the contribution of these reactions is small in the neutron irradiation field used in this study. The contribution of epi-thermal neutron in the neutron physical dose is very small, so the protons produced by the nitrogen-reaction and elastic scattering with epi-thermal neutron are almost negligible.
The physical dose for γ-ray in Table 1 is the average value of the measured data by TLD. The measured γ-ray consists of the primary γ-ray, and the secondary γ-ray such as the prompt γ-rays from the neutron-capture reactions of hydrogen and boron-10. The values for the γ-ray dose were almost the same, with or without BPA. It is because the contribution of the prompt γ-rays from boron-reaction is much smaller than that from hydrogen-reaction, calculated to be almost 1% even for the boron-10 concentration of 10 ppm. The contribution for the primary γ-ray in the neutron irradiation field used in this study is much larger than that for the prompt γ-ray from hydrogen-reaction. Then, the contribution of the prompt γ-ray from boron-reaction is almost negligible for the total γ-ray dose.
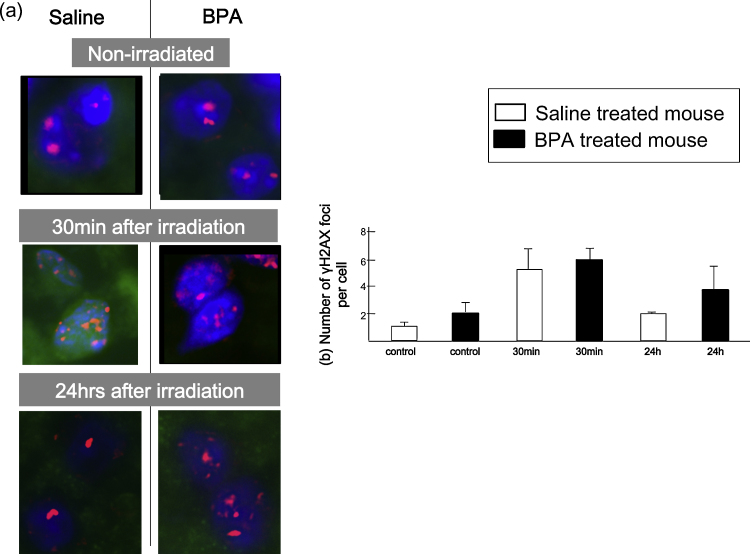
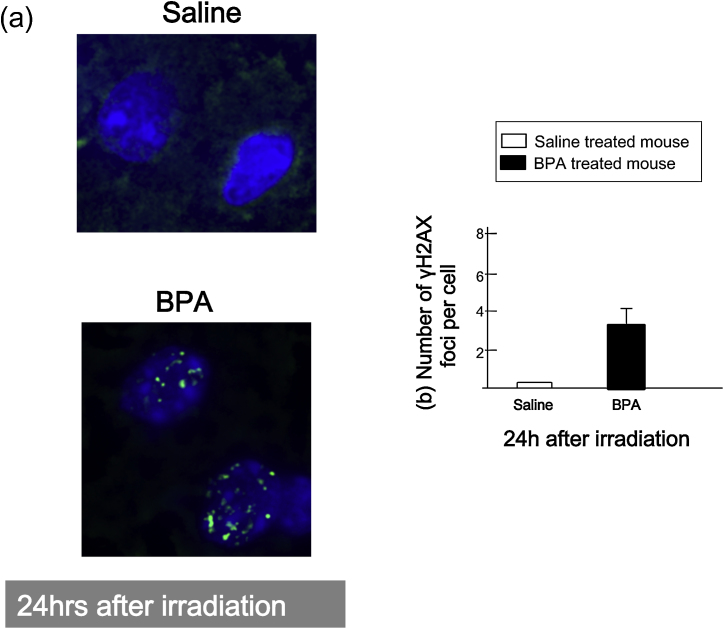
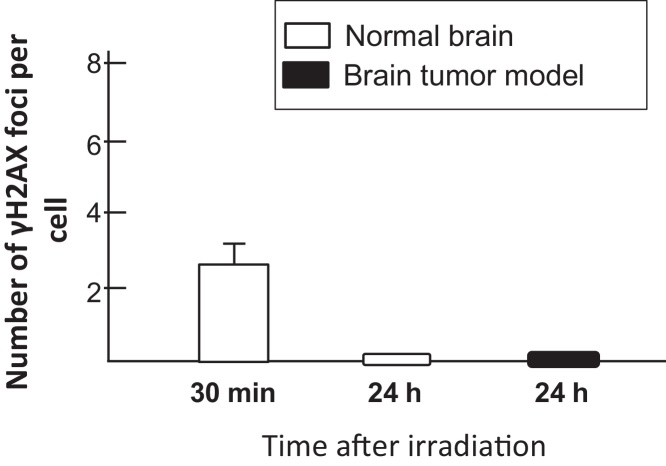
Fig. 1a shows representative image of normal brain before and after neutron beam irradiation. In case of normal brain, the number of γH2AX foci in both saline and BPA treated mice increased up to 5–6 per cell 30 min after neutron beam irradiation and decreased 24 h after neutron beam irradiation. However in BPA treated mouse, there were more number of γH2AX foci (4/cell) as compared to that of saline treated mouse (2/cell) 24 h after neutron beam irradiation (Fig. 1b). Next, Fig. 2a shows representative image of brain tumor 24 h after neutron beam irradiation. In case of brain tumor model 24 h following neutron beam irradiation, there were 3 γH2AX foci remaining in BPA treated mouse, on the other hand, there were no γH2AX foci in saline treated mouse (Fig. 2b). To know the impact of DSBs induced by contaminated γ-ray, γ-ray irradiation to normal brain and brain tumor in mouse was conducted (Fig. 3).
Fig. 1.
γH2AX foci in normal brain after neutron irradiation. (a) Representative images of nuclear γH2AX foci of normal brain. Images on the left show saline-treated mouse, and those on the right show BPA treated mouse. Upper: non-irradiated control, middle: 30 min after irradiation; lower: 24 h after irradiation. DAPI = staining of nuclear DNA. (b) Changes in the number of γH2AX foci at the times indicated post-irradiation. Bars represent the standard errors.
Fig. 2.
γH2AX foci in brain tumor model after neutron irradiation. (a) Representative images of nuclear γH2AX foci of brain tumor cells. Upper image shows saline-treated mouse, and lower shows BPA treated mouse. DAPI = staining of nuclear DNA. (b) The number of γH2AX foci 24 h after irradiation. Bars represent the standard errors.
Fig. 3.
γH2AX foci in normal brain and brain tumor model after γ-irradiation. The number of γH2AX foci 24 h after irradiation at the times indicated post-irradiation.
In the γ-ray (1.1 Gy) irradiated mouse, the number of γH2AX foci was detected in normal brain 30 min after irradiation; however, no γH2AX foci were detected 24 h after irradiation. Also, γH2AX foci were not detected in tumor 24 h after γ-ray irradiation.
5. Discussion
From a previous report, the persistence of γH2AX foci 24 h after treatments could be judged as unrepairable DSB.11 From our data, DSBs caused by γ-ray could be repaired 24 h after irradiation in both normal and tumor model. This γ-ray dose was equal dose to contaminated γ-ray dose (1.1 Gy) of neutron beam irradiation. Therefore, DSBs which were still retained 24 h after neutron beam irradiation in the normal brain of saline treated mouse may be consisted of DSBs mainly caused by elastic scattering (1H(n,n)1H) or nitrogen capture reaction (14N(n,p)14C). These particles exhibited high-LET radiation. The LET of proton particles produced by the former reaction was about 50 keV/μm, and that produced by the latter reaction was about 35 keV/μm, whereas the γ rays exhibited low-LET radiation. On the other hand, in the saline treated tumor model, DSBs caused by elastic scattering (1H(n,n)1H) or nitrogen capture reaction (14N(n,p)14C) were almost repaired. This may suggests higher capacity to repair DSBs as compared as normal brain. However, capability of repair to DSBs may differs between each tumor cell lines because in C6 rat glioma model, C6 rat glioma cells could not completely repair the DSBs produced by elastic scattering (1H(n,n)1H) or nitrogen capture reaction (14N(n,p)14C)(date not shown). In this study, even in the BPA treated tumor model, DSBs were unrepaired 24 h after neutron beam irradiation. In both normal brain and tumor model, the differences of γH2AX foci between BPA treated mouse and saline treated mouse 24 h after neutron beam irradiation were supposed to be caused by 10B(n,α)7Li reaction. These differences were not detected 30 min after neutron beam irradiation. The DSBs caused by 10B(n,α)7Li reaction could be more difficult to repair even for the tumor model than those by elastic scattering (1H(n,n)1H) or nitrogen capture reaction (14N(n,p)14C). The LET of α particle and Li particle produced by 10B(n,α)7Li reaction are about 163 keV/μm and 210 keV/μm respectively, which are higher than those of proton particles produced by elastic scattering (1H(n,n)1H) or nitrogen capture reaction (14N(n,p)14C). High-LET radiation induces DNA-DSBs that are not repaired or are more difficult to repair compared to low-LET radiation9 and theoretical analysis and experimental evidence have shown an increased complexity and severity of complex DNA damage with increasing LET.12 From the clinical point of view, it is a great advantage that the DSBs produced by 10B(n,α)7Li reaction may be unrepairable even in tumor tissue and BNCT will be more effective than treatment with low-LET radiation, such as γ-rays and X-rays. Also we have to consider carefully that DSBs produced by neutrons except γ-rays could remain in normal brain tissue 24 h after irradiation. To investigate the real capacity of tumor tissue to repair DSBs caused by 10B(n,α)7Li reaction, we will measure the concentration of 10B in tumor tissue although we have not yet.
6. Conclusions
In conclusion, DSBs produced by 10B(n,α)7Li reaction are supposed to be too complex to repair for cells in normal brain and even in brain tumor tissue within 24 h. These DSBs would be more difficult to repair than those produced by γ-ray, low LET radiation. Excellent anti-tumor effect of BNCT may result from these unrepaired DSBs induced by 10B(n,α)7Li reaction.
Conflict of interest
None declared.
Financial disclosure
This work was supported by a Grant-in-Aid for Young Scientists (25870382) from the Japan Ministry of Education, Science, Sports and Culture to N. Kondo.
Acknowledgements
The authors are grateful to Ms. Kaori Fukuda and Ms. Masami Fukui for their technical assistance during the study.
References
- 1.Miyatake S., Kawabata S., Yokoyama K. Survival benefit of Boron neutron capture therapy for recurrent malignant gliomas. J Neurooncol. 2009;91(2):199–206. doi: 10.1007/s11060-008-9699-x. [DOI] [PubMed] [Google Scholar]
- 2.Kawabata S., Miyatake S., Kuroiwa T. Boron neutron capture therapy for newly diagnosed glioblastoma. J Radiat Res. 2009;50(1):51–60. doi: 10.1269/jrr.08043. [DOI] [PubMed] [Google Scholar]
- 3.Kawabata S., Miyatake S., Hiramatsu R. Phase II clinical study of boron neutron capture therapy combined with X-ray radiotherapy/temozolomide in patients with newly diagnosed glioblastoma multiforme-study design and current status report. Appl Radiat Isot. 2011;69(12):1796–1799. doi: 10.1016/j.apradiso.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 4.Peak M.J., Wang L., Hill C.K. Comparison of repair of DNA double-strand breaks caused by neutron or γ-radiation in cultured human cells. Int J Radiat Biol. 1991;60(6):891–898. [PubMed] [Google Scholar]
- 5.Tanaka K., Gajendiran N., Endo S. Neutron energy-dependent initial DNA damage and chromosomal exchange. J Radiat Res. 1999;40(Suppl):36–44. doi: 10.1269/jrr.40.s36. [DOI] [PubMed] [Google Scholar]
- 6.Kinner A., Wu W., Staudt C. γ-H2AX in recognition and signaling of DNA double-strand breaks in the context of chromatin. Nucleic Acids Res. 2008;36(17):5678–5694. doi: 10.1093/nar/gkn550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan D.W., Chen B.P.C., Prithivirajsingh S. Autophosphorylation of the DNA-dependent protein kinase catalytic subunit is required for rejoining of DNA double-strand breaks. Genes Dev. 2002;16(18):2333–2338. doi: 10.1101/gad.1015202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nikjoo H., O’Neil P., Wilson W.E. Computational approach for determining the spectrum of DNA damage induced by ionizing radiation. Radiat Res. 2001;156(5 Pt 2):577–583. doi: 10.1667/0033-7587(2001)156[0577:cafdts]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 9.Okayasu R. Repair of DNA damage induced by accelerated heavy ions – a mini review. Int J Cancer. 2012;130(5):991–1000. doi: 10.1002/ijc.26445. [DOI] [PubMed] [Google Scholar]
- 10.Sakurai Y., Kobayashi T. Characteristics of the KUR Heavy Water Neutron Irradiation Facility as a neutron field with variable energy spectra. Nucl Instrum Methods Phys Res Sect A. 2000;453:569–596. [Google Scholar]
- 11.Schmid T.E., Dollinger G., Beisker W. Differences in the kinetics of γ-H2AX fluorescence decay after exposure to low and high LET radiation. Int J Radiat Biol. 2010;86(8):682–691. doi: 10.3109/09553001003734543. [DOI] [PubMed] [Google Scholar]
- 12.Hada M., Georgakilas A.G. Formation of clustered DNA damage after high-LET irradiation: a review. J Radiat Res. 2008;49(3):203–210. doi: 10.1269/jrr.07123. [DOI] [PubMed] [Google Scholar]