Abstract
Currently there are no disease-modifying alternatives for the treatment of most neurodegenerative disorders. The available therapies for diseases such as Parkinson’s disease (PD), PD dementia (PDD), Dementia with Lewy bodies (DLB) and Multiple system atrophy (MSA), in which the protein alpha-synuclein (α-syn) accumulates within neurons and glial cells with toxic consequences, are focused on managing the disease symptoms. However, utilizing strategic drug combinations and/or multi-target drugs might increase the treatment efficiency when compared to monotherapies. Synucleinopathies are complex disorders that progress through several stages, and toxic α-syn aggregates exhibit prion-like behavior spreading from cell to cell. Therefore, it follows that these neurodegenerative disorders might require equally complex therapeutic approaches in order to obtain significant and long-lasting results. Hypothetically, therapies aimed at reducing α-syn accumulation and cell-to-cell transfer, such as immunotherapy against α-syn, could be combined with agents that reduce neuroinflammation with potential synergistic outcomes. Here we review the current evidence supporting this type of approach, suggesting that such rational therapy combinations, together with the use of multi-target drugs, may hold promise as the next logical step for the treatment of synucleinopathies.
Keywords: Alpha-synuclein, Parkinson’s disease, Synucleinopathies, Therapeutics, Combination therapy
Synucleinopathies1 are a group of neurodegenerative disorders characterized by the abnormal deposition of the protein alpha-synuclein (α-syn) in the form of oligomeric and fibrillary aggregates within neuronal and glial cell populations, this accumulation leading to cell death and subsequent behavioral and motor deficits. Α-syn is a protein found in presynaptic terminals that is involved in synaptic transmission2, 3, and its abnormal accumulation in the form of oligomers and fibrils is toxic4–6. Synucleinopathies include Parkinson’s disease (PD), PD dementia (PDD), Dementia with Lewy bodies (DLB) and Multiple system atrophy (MSA). Specific neuronal populations degenerate in each of these disorders7: dopaminergic neurons in the substantia nigra, midbrain, nucleus basalis of Meynert and brainstem are affected in PD; in DLB, both dopaminergic and cholinergic neurons in the nucleus basalis of Meynert and limbic system degenerate; and finally, the pattern of neurodegeneration in MSA (putamen, middle cerebellar peduncle, pons, and/or cerebellum) is characteristic for each subtype (MSA-P or MSA-C)8. Moreover, in MSA α-syn accumulates not only within neurons but also within oligodendroglial cells9, 10, leading to demyelination and the loss of trophic support to neurons, which translates into secondary neurodegeneration. Symptoms of synucleinopathies include a chronic and progressive decline in motor (ataxia, parkinsonism), cognitive (memory loss) and behavioral functions (depression, anxiety, REM sleep disorder)11, and dysautonomia, depending on the distribution of the lesions. The incidence rate of synucleinopathies is 21 per 100,000 persons per year, and increases with age12. PD is the most prevalent synucleinopathy and the second most common neurodegenerative disorder after Alzheimer’s disease (AD), affecting 1.5 million people in the US and 1% of people over 60 years old. The prevalence of Lewy body dementias in the US is estimated to be 1.3 million13. Finally, MSA affects approximately 14,000 people in the US.
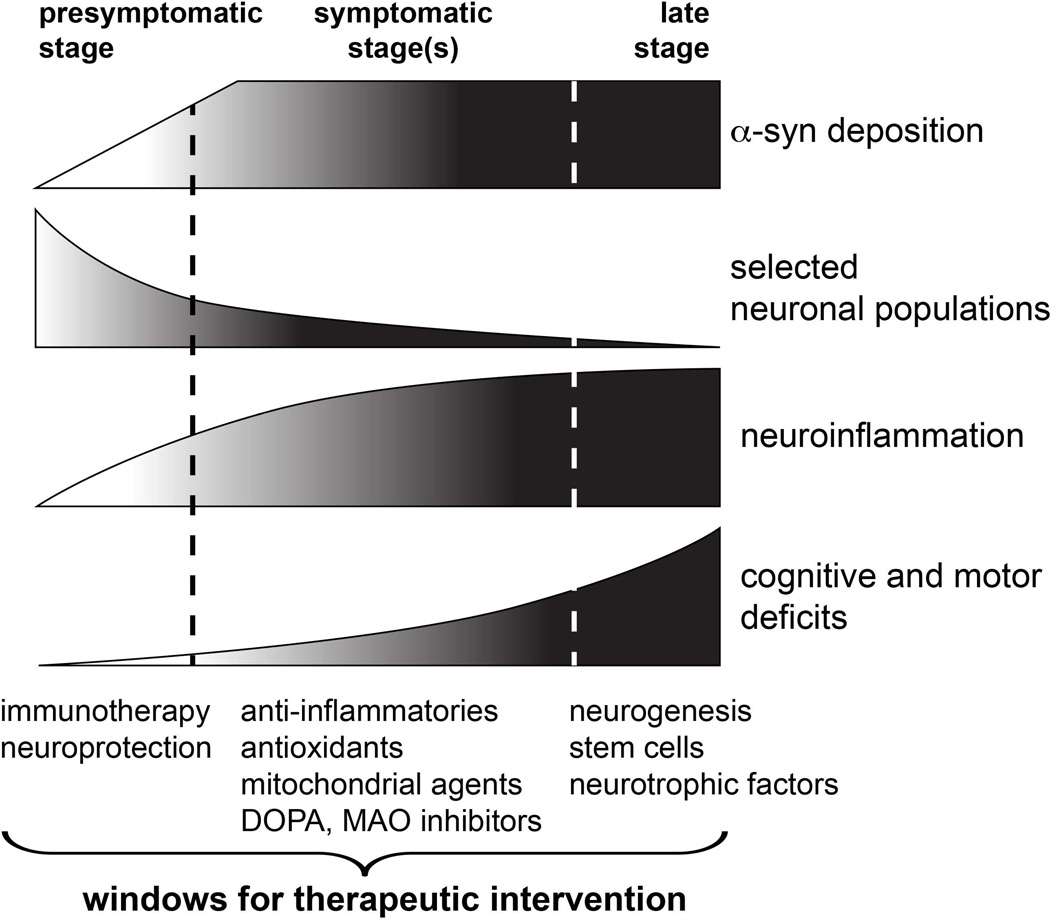
Synucleinopathies are complex diseases that progress through several stages14–16 (Figure 1), and increasing evidence suggest that an early diagnosis would increase the efficacy of the therapeutic intervention. A rational approach for the treatment of synucleinopathies dictates that preventive efforts aimed at protecting from neurodegeneration and stopping the early deposition of α-syn would be more effective if administered before or during the pre-symptomatic stage (Figure 1). However, the diagnosis usually occurs during the symptomatic stage, once α-syn deposition is already established, neuroinflammation is widespread, and approximately 75% of dopaminergic neurons have died17. At this point, restoring neurotransmitter signaling (dopamine, acetylcholine) and reducing inflammation may help alleviating symptoms and preventing further neuronal loss. Finally, late disease stages are characterized by extended neurodegeneration and loss of the neurotrophic support, and in this period the use of regenerative therapies and neurotrophic factors18, 19 can help delaying neurodegeneration and palliating its behavioral and motor correlates (Figure 1). From the analysis of these disease stages it can be concluded that developing therapies that make use of the natural windows of therapeutic intervention by targeting the most relevant events in each disease period may stop degenerative and inflammatory cascades from progressing further. For example, early stage interventions should be focused on prevention by reducing α-syn synthesis, propagation and accumulation; during the symptomatic stages, when the majority of α-syn deposition has already occurred and behavioral and motor symptoms are significant, treatments should be aimed at reducing neuroinflammation and preventing further neuronal death; and finally, slowing down neurodegeneration and restoring trophic support should be the main therapeutic goal at late stages.
Figure 1. Windows for therapeutic intervention in synucleinopathies.
Hypothetical disease progression in stages postulated for PD and other synucleinopathies, including α-syn deposition, death of selected neuronal populations, neuroinflammation and cognitive and motor deficits. Suggested treatments that could be suitable for each therapeutic window of opportunity are depicted below.
Therapeutic alternatives for synucleinopathies
Currently there are no disease-modifying treatments for synucleinopathies, and existing therapies are directed at managing the symptoms of the disease. Drugs that restore dopamine signaling such as L-DOPA20–22 (L-3,4-dihydroxyphenylalanine, levodopa), dopamine agonists (ropinirole23, pramipexole24, 25) and dopamine reuptake inhibitors (amantadine26) are widely used to treat parkinsonian symptoms in PD patients. Moreover, drugs used to treat PD symptoms may also be prescribed for other synucleinopathies such as MSA; however, these treatments may show reduced efficiency when used for other disorders, and for example approximately one third of MSA patients do not respond to levodopa therapy27. Apart from dopamine signaling, cholinesterase inhibitors such as donepezil28, 29, rivastigmine30 and galantamine31 are used to slow or prevent the decline of cognitive function in DLB and PDD. Low blood pressure, urinary incontinence, REM sleep disorder, dystonia and impotence are also targets for pharmacological intervention in synucleinopathies32. Nevertheless, none of these therapies is able to slow down the neurodegeneration cascades that affect the diseased brain.
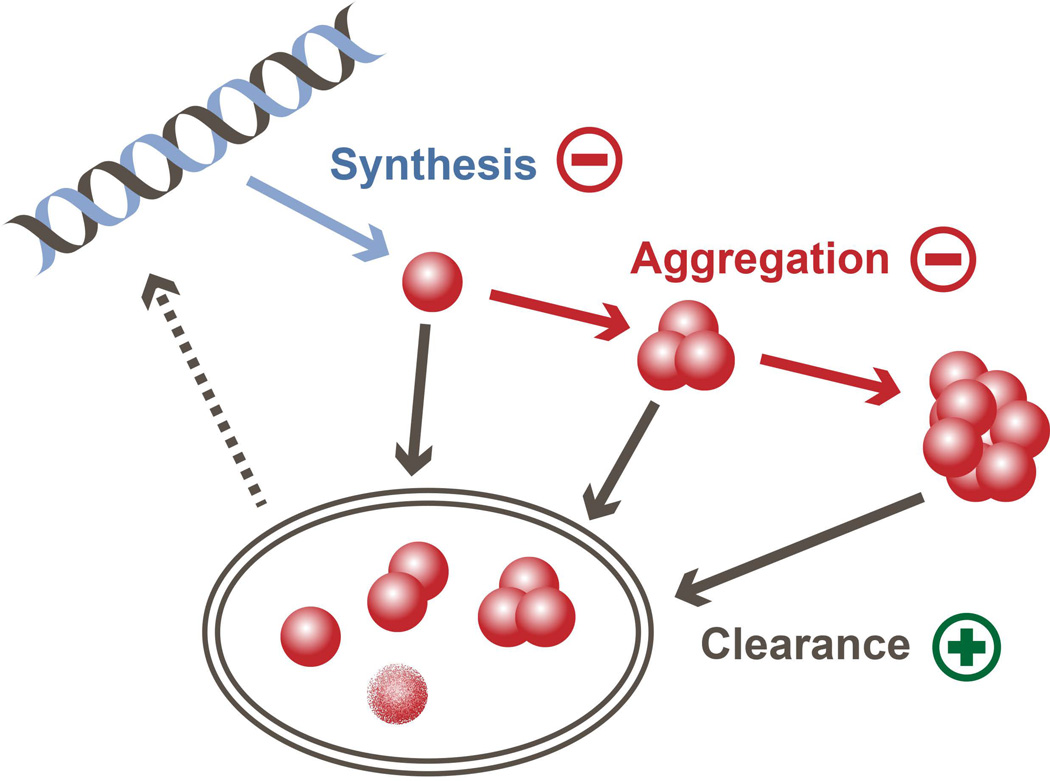
Therefore, in order to effectively delay or halt the progression of the disease it is necessary to develop effective disease-modifying alternatives. The vast majority of research efforts towards the development of disease-modifying therapies are focused on targeting α-syn deposition in the brain, since it seems to be a critical step in the development of synucleinopathies. Abnormal α-syn accumulation occurs early in the disease progression and spreads following a quite defined pattern through the brain14, suggesting that it is the driving force in PD pathogenesis5, 33. And, as it occurs with the accumulation of other toxic proteins34, the intracellular build-up of α-syn can be reduced by either 1) inhibiting α-syn synthesis, 2) blocking its aggregation, and/or 3) increasing its degradation and clearance (Figure 2). Postmortem brain samples of sporadic PD patients have higher levels of α-syn mRNA, which is relatively specific to neurons35. In the special case of MSA, the mRNA levels of α-syn are 1.5 higher in oligodendrocytes that in neurons36, suggesting that increased expression rates may be involved in the oligodendroglial α-syn accumulation observed in this synucleinopathy. Furthermore, duplications and triplications of the α-syn gene (SNCA) are associated with familial PD37–39, and genome-wide association studies (GWAS) show that SNCA single-nucleotide polymorphisms are risk factors for sporadic PD40, indicating a mechanistic role of increased α-syn in the origin of the pathology. Levels of α-syn expression can be therapeutically reduced using siRNAs41 or miRNAs42, 43. However, given that this protein is involved in normal synaptic transmission, successfully modulating α-syn synthesis in sporadic cases can be challenging. High intracellular levels of α-syn may increase its tendency to aggregate, together with other factors such as protein misfolding44, 45, limited proteolysis46, mutations47–49, and post-transcriptional modifications such as phosphorylation50, 51 and truncation52, 53. The processes of α-syn aggregation and fibrillation can be used as therapeutic targets for the development of drugs that act as conformational stabilizers and anti-aggregation agents (e.g., small molecules54, rifampicin55). Interestingly, the recent discovery that different α-syn conformations (“strains”) are specific of different synucleinopathies56 suggests that conformation-specific anti-aggregation agents may be more effective at reducing aggregation than more generic alternatives. Finally, the increased tendency of α-syn to propagate from cell to cell and to accumulate within neurons and glial cells might be due to deficits in protein clearance mechanisms in donor and/or acceptor cells. Autophagy impairments have been associated to PD and other synucleinopathies57, 58, and dysfunctions in other clearance mechanisms such as proteolysis and unfolded protein response have also been reported59, 60. Increasing α-syn degradation using autophagy inducers, unfolded protein response inducers61 and enzymes such as kallikrein-6 (neurosin)62, 63, MMP964 or cathepsin D65, 66 could help reduce both α-syn propagation and accumulation.
Figure 2. Disease-modifying therapeutic strategies focused on intracellular α-syn accumulation.
Intracellular α-syn levels are regulated by the balance between α-syn synthesis, aggregation and clearance. Strategies to reduce α-syn accumulation include decreasing its synthesis (−) with siRNA or miRNAs, reducing its aggregation (−) using anti-aggregation agents or post-translational modifications, and/or activating clearance mechanisms (+) such as autophagy.
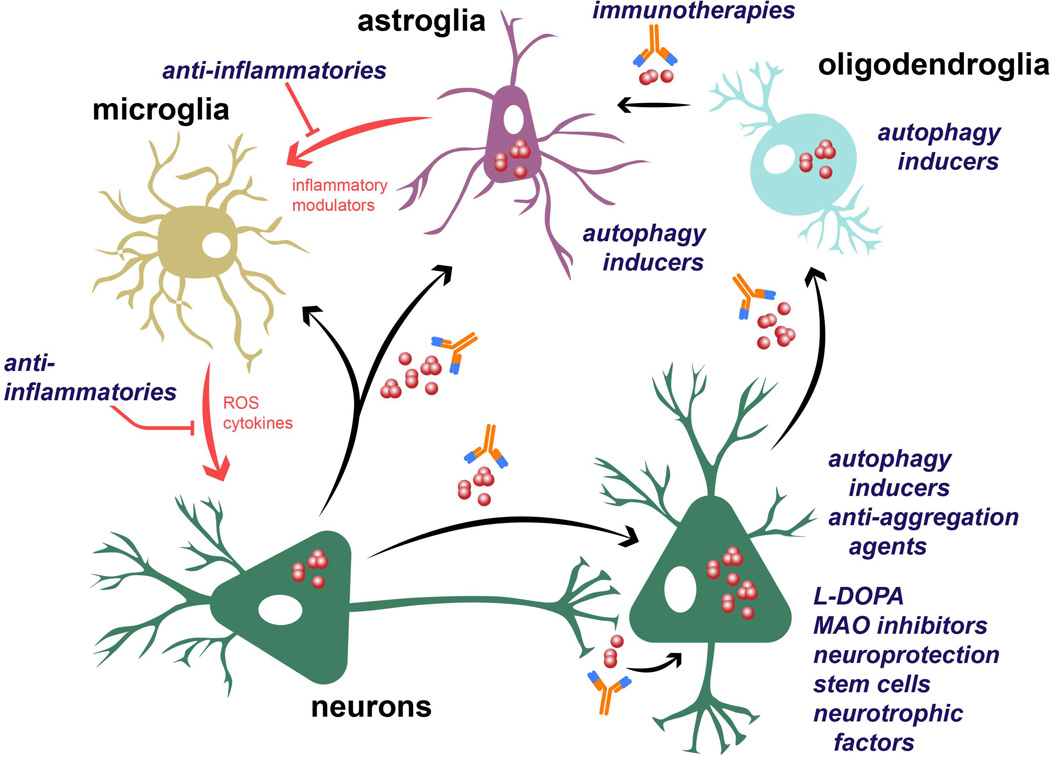
Importantly, it has been suggested that cell-to-cell propagation of α-syn aggregates plays a pivotal role in the mechanism of α-syn toxicity (Figure 3), and it is responsible for the prion-like spreading of the disease through the brain. Propagating α-syn aggregates, once taken up by acceptor cells, may act as seeds for further α-syn deposition within recipient cells, thus explaining the neurodegeneration pattern observed at different stages14, 67. It follows that α-syn-reducing agents that target extracellular α-syn or inhibit its endocytosis may inhibit propagation and therefore stop or delay the progression of the disease. One of such approaches is the use immunotherapy against toxic conformations of α-syn. Active and passive immunotherapeutic approaches have been effective at reducing toxic α-syn aggregates (dimers, oligomers) and improving behavioral deficits in transgenic (tg) mouse models of PD and MSA68–73. Active immunization with full human α-syn68 or with peptides (AFFITOPEs®) that mimic the C-terminus region of α-syn70, 71 results in the production of high relative affinity antibodies, decreased accumulation of α-syn aggregates and reduced neurodegeneration. Antibodies produced by immunized mice recognize abnormal α-syn and promote its degradation, probably via microglial lysosomal pathways. In this sense, clinical trials using the AFFITOPE® PD03A for PD and MSA are currently undergoing. Likewise, passive immunization with antibodies against the C-terminus of α-syn are able to cross into the CNS, attenuate synaptic and axonal pathology and reduce the accumulation and propagation of C-terminus-truncated α-syn69, 72, thus improving behavioral and motor functions in a mouse model of PD. Finally, the clearance of extracellular α-syn can also be achieved by targeting extracellular chaperones such as Hsp7074 and enzymes such as neurosin63.
Figure 3. Potential therapeutic interventions for the treatment of synucleinopathies.
α-syn aggregation can take place either in the cytoplasm or in association with the cellular membrane of neuronal cells. Interestingly, α-syn oligomers and fibrils, as well as the monomers, can be transferred between cells and induce disease spreading to other brain regions. Propagation of α-syn to astroglial and microglial cells can induce glial activation and neuroinflammation. In MSA, α-syn also accumulates within oligodendrocytes and propagate from these cells to astroglia. Potential therapeutic interventions include immunotherapy against extracellular α-syn; the use of anti-inflammatories to reduce glial neuroinflammation; autophagy inducers to stimulate the clearance of intracellular α-syn; and mechanisms to reduce or compensate neurodegeneration such as L-DOPA, MAO inhibitors, neuroprotective compounds, neurotrophic factors (BDNF, GDNF) or regenerative therapy with stem cells.
The late stages of most neurodegenerative disorders are characterized by significant neuronal loss, which drastically reduces the effectiveness of therapies prescribed for earlier stages. Once neurodegeneration is widespread, behavioral and motor impairments can only be reverted by either restoring neuronal populations or compensating for their loss. In this case, disease-modifying efforts are focused on the use of neurotrophic factors (BDNF, GDNF)75–78 or neurotrophic factor inducers79, drugs that enhance neurogenesis80, or regenerative cell therapy with stem cells81. However, it is important to consider that α-syn propagation still occurs at late stages, and regenerative efforts may be hindered if the propagation of α-syn aggregates from surrounding diseased tissues is not inhibited82, 83.
Finally, mutations in genes other than SNCA, namely LRRK2, PARK2 (parkin), PINK1, PARK7 (DJ-1), ATP13A2, VPS35, EIF4G1, GBA (β-Glucocerebrosidase) and UCHL184–94, have been considered as potential therapeutic targets, as mutations on those genes are associated with an increased risk of developing PD. Other genes of susceptibility for synucleinopathies are COQ2 for MSA95, 96, and PARK1197 and GBA98 for DLB. Interestingly, most of the proteins encoded by these genes are involved in lysosomal and mitochondrial functions, highlighting the crucial role of mitochondria and cell metabolism in the origin and progression of these disorders. For example, mutations that induce a gain on function on LRRK2 could be managed using LRRK2 kinase inhibitors such as sorafenib99, GW507499 and staurosporine100–102. However, as most of these mutations lead to a loss of function, gene therapy has been suggested for PARK2103, 104 and GBA105. The potential use of the DJ-1 products glycolate and D-lactate, that are neuroprotective, has been explored as well106. In the case of MSA, mutations in the coenzyme Q10 encoding gene COQ2 have been associated with an increase risk of suffering this disease95, 96; however treatment with coenzyme Q10 did not slow down the progression of PD in a Phase III study107, suggesting that its efficacy may be limited by other factors. Although therapies focused on these proteins might prove useful for synucleinopathies, potential treatment are still on the validation stage. Importantly, mutations in these genes can be used as biomarkers for prevention in pre-symptomatic stages, in which the use mitochondrial agents and neuroprotective compounds could prevent or delay the onset of the disease.
Neuroinflammation as therapeutic target in synucleinopathies
Synucleinopathies are complex disorders and, as mentioned before, using the opportunity of available windows for therapeutic intervention, although not without its challenges, may drastically affect the outcome of the pharmacological intervention (Figure 1). While early pre-symptomatic stages are characterized by an increased production, propagation and accumulation of α-syn, more advanced, symptomatic stages are characterized by the onset of non-autoimmune neuroinflammation. Neuroinflammation in synucleinopathies is characterized by microglial and astroglial activation with α-syn accumulation, cytokine dysregulation and immune cell infiltration into the brain108. Neuroinflammation is linked to memory retrieval dysfunction109, 110, and it is responsible for some of the cognitive impairments observed during the symptomatic stages of the disease111, 112. Importantly, only removing the initial neuroinflammatory stimulus (e.g., α-syn) would not be enough to put a stop to neuroinflammatory cascades once initiated113. Inflammatory mediators released by activated glial cells self-perpetuate their activation in a positive feedback loop manner, leading to over-production of pro-inflammatory cytokines and reactive oxygen species (ROS), oxidative stress and secondary neurodegeneration. Pro-inflammatory cytokines such as TNFα, IL-1β and IL-6 are elevated in the brain of PD patients114, 115, and are important mediators of inflammatory cascades and glial activation (Figure 3). Importantly, the presence of unaddressed neuroinflammation may be one of the reasons why effective anti-aggregation and α-syn-reducing agents have shown suboptimal results at reducing the pathology when used autonomously, and other disorders that accumulate toxic proteins, such as AD, have yielded similar results. In the case of AD, it has been shown that reducing amyloid beta levels using immunotherapy does not significantly improve memory deficits in humans116, 117, suggesting that factors other than toxic protein accumulation, such as neuroinflammation, may be responsible for behavioral outcomes in these neurodegenerative disorders.
Consequently, recent research efforts have been devoted to targeting neuroinflammation as a palliative treatment for synucleinopathies. Preclinical and clinical anti-inflammatory efforts have included nonsteroidal anti-inflammatory drugs (ibuprofen, indomethacin)118, 119, TNFα inhibitors (XPro1595, immunomodulatory drugs)120, 121, antidepressants (fluoxetine)80, 122, 123, antioxidants (coenzyme Q10, quercetin, curcumin)124–128, polyphenols (apigenin, luteolin)129, 130 and others. However, several clinical trials have shown that anti-inflammatories do not significantly reduce behavioral and motor deficits. In this sense it is important to note that most anti-inflammatories do not reduce extracellular α-syn levels, which constitutes a significant pro-inflammatory stimulus for glial cells in synucleinopathies131–133. However, treatment with some of these anti-inflammatory compounds can have more than one beneficial outcome and even disease-modifying properties, thus increasing their potential as therapeutic alternatives. For example, the antidepressant fluoxetine reduces neuroinflammation, increases neurogenesis, and inhibits α-syn propagation and accumulation in a tg model of MSA122, 123. It follows that approaches using similar multi-target drugs might be viable choices for the treatment of neurodegenerative disorders.
Combination therapies – a rational approach for the treatment of synucleinopathies
However, despite the considerable effort devoted to developing therapies that stop or delay the progression of synucleinopathies, substantially effective treatments have not been identified yet. This lack of success raises the question of whether conventional drug development approaches are appropriate for neurodegenerative diseases. Monotherapies, although effective for numerous diseases and with fewer side effects, might not be enough to induce significant improvements in patients with neurodegenerative disorders such as PD. Because of the complexity of these disorders, it may take a combination of therapies and/or the use of multi-target drugs to help stop the progression of the disease. Moreover, the need for combination therapies is being increasingly recognized due to the failure, or marginally protective effects, observed when using single-target agents134.
Among the reasons supporting the use of a logical combination of therapies for the treatment of neurodegenerative disorders is that utilizing drugs that target different signaling pathways that complement each other, or the same pathway at different levels, might have synergistic effects. For example, anti-inflammatories inhibit pro-inflammatory cascades while immunotherapy against α-syn reduces the levels of aggregated α-syn, which in turn may act as a pro-inflammatory stimulus for glial cells131, 132. Likewise, some anti-inflammatories are also able to reduce α-syn accumulation123, probably by stimulating the clearance of extracellular α-syn by physiologically-activated microglial cells (M2 polarized microglia)135. However, among the potential weaknesses of combining drugs is that it might lead to negative interactions among metabolites, resulting in a reduction in the positive outcome of the treatment, or even to increased side effects and/or hepatic toxicity. In this sense, some chronic diseases known to have competing therapies include diabetes, heart failure, high blood pressure, high cholesterol, and osteoarthritis136, 137. In synucleinopathies, some anti-TNFα and anti-inflammatory therapies might hypothetically interfere with responses to active immunization, as they modulate immune cell activation138, 139. Therefore, it is important to perform an in-depth analysis of each drug combination in order to prevent potential undesired effects.
The combination of therapeutic approaches has been successfully explored for other ailments such as autoimmune diseases and cancer140, 141. In the case of synucleinopathies some combinations have been recently suggested, including lithium plus levodopa/carbidopa142, rasagiline and levodopa143, and creatine plus coenzyme Q10, that produce additive neuroprotective effects in models of PD128. However, due to the inherent complexity of neurodegenerative disorders, patients are usually prescribed with more than one medication and, for example, it is not uncommon to combine the use of antidepressants with treatments aimed at managing the disease symptoms, such as parkinsonism and ataxia. Such unintended combinations have yielded some interesting results. For example, simultaneous treatment with rasagiline (monoamine oxidase (MAO) inhibitor) and an antidepressant has been associated with a reduced worsening of non-motor symptoms in PD patients144, encouraging further studies in this direction. Yet, the combination of pharmacological therapies has not been as extensively explored as monotherapeutic approaches. More frequent is the mix of pharmacological treatments with non-pharmacological approaches such as deep brain stimulation145–147 or physical exercise148 for the treatment of motor and cognitive symptoms in synucleinopathies.
As mentioned before, more research is needed for elucidating how drugs interact, and how we could use their synergy to our advantage. Following a rational approach focused on complementary targets and mechanisms of action, researchers are able to design specific therapeutic combinations to be explored for the treatment of PD and related disorders. For example, one of such combinations might be the use antidepressants with anti-inflammatory activity (e.g., fluoxetine) together with immunotherapy against toxic α-syn conformations. The rationale for this combination is that it would achieve a reduction in α-syn propagation and accumulation (immunotherapy) together with a reduction in neuroinflammation and secondary neurodegeneration (antidepressant). Both active and passive immunotherapies reduce α-syn levels, most likely by stimulating microglia to clear out extracellular antibody-α-syn complexes70–72. Additionally, antidepressants not only stabilize mood changes and anxiety, but also reduce microglial and astroglial pro-inflammatory cytokine responses123, 149. Furthermore, additional α-syn reduction and increased neurogenesis can be achieved using specific antidepressants (e.g., fluoxetine)150. Together, these results suggest that this or similar combinations would yield better results than using each drug separately, specially if administered at pre-symptomatic or early symptomatic stages, when α-syn is actively propagating (Figure 1). Another example would be combining the use of TNFα inhibitors such as lenalidomide, together with passive immunization against the C-terminal end of α-syn. Lenalidomide reduces microgliosis and inhibits the expression of pro-inflammatory cytokines in a tg mouse model of PD120, while antibodies against the C-terminal site of α-syn reduce its propagation, ameliorate the pathology and improve behavioral and motor functions in the same mouse model-term72. Such complementary modes of action suggest that combining both drugs may lead to synergistic results. However, at late disease stages, once neuroinflammation and neurodegeneration are widespread, α-syn-reducing agents would be less effective. In this situation, mixing anti-inflammatory approaches with regenerative therapy might lead to improved cognitive and motor functions. Other specific examples of rational therapy combinations are: anti-inflammatory and anti-oxidant molecules such as curcumin or derivatives, together with L-DOPA for PD; or minocycline151, an antibiotic that modulates microglial activation, combined with an α-syn endocytosis inhibitor for MSA. However, although the possibilities are multiple, a rational approach must be followed for designing such combination therapies. Logical pairings would include a) drugs that have different targets that complement each other (eg. TNFα inhibitors plus α-syn-reducing agents); b) drugs that act on the same pathway but targeting different mechanisms (eg. antidepressants that reduce α-syn propagation plus immunotherapy against α-syn); or c) multi-target drugs that inhibit several pathways, or one pathway at different levels (e.g., lenalidomide actions on several members of the NF-κB pathway and on TNFα mRNA stability).
Closing remarks
In conclusion, there is an urgent need for the development of new, effective therapeutic alternatives for the treatment of neurodegenerative disorders. This includes not only the development of reliable predictive and early diagnostic tools for synucleinopathies, but also of novel therapeutic alternatives that significantly down the progression of the disease at later stages. Available therapies to date are focused on managing disease symptoms, and a therapeutic breakthrough is sorely needed for PD and related disorders. In this sense, recent clinical trials have yielded suboptimal results, suggesting that a combination of two or more therapies may be needed in order to achieve significant improvements and disease modification. Taking advantage of the potential synergistic effects derived from combining therapies could be the next logical step for the treatment of synucleinopathies.
ACKNOWLEDGEMENTS
This work was funded by the National Institutes of Health (NIH) grants NS044233, AG18440, NS047303, AG022074 and NS057096.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest, support or financial issues.
BIBLIOGRAPHY
- 1.Marti MJ, Tolosa E, Campdelacreu J. Clinical overview of the synucleinopathies. Mov Disord. 2003;18(Suppl 6):S21–S27. doi: 10.1002/mds.10559. [DOI] [PubMed] [Google Scholar]
- 2.Fortin DL, Nemani VM, Voglmaier SM, Anthony MD, Ryan TA, Edwards RH. Neural activity controls the synaptic accumulation of alpha-synuclein. J Neurosci. 2005;25(47):10913–10921. doi: 10.1523/JNEUROSCI.2922-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.George JM, Jin H, Woods WS, Clayton DF. Characterization of a novel protein regulated during the critical period for song learning in the zebra finch. Neuron. 1995;15(2):361–372. doi: 10.1016/0896-6273(95)90040-3. [DOI] [PubMed] [Google Scholar]
- 4.Danzer KM, Haasen D, Karow AR, et al. Different species of alpha-synuclein oligomers induce calcium influx and seeding. J Neurosci. 2007;27(34):9220–9232. doi: 10.1523/JNEUROSCI.2617-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lashuel HA, Petre BM, Wall J, et al. Alpha-synuclein, especially the Parkinson's disease-associated mutants, forms pore-like annular and tubular protofibrils. J Mol Biol. 2002;322(5):1089–1102. doi: 10.1016/s0022-2836(02)00735-0. [DOI] [PubMed] [Google Scholar]
- 6.Winner B, Jappelli R, Maji SK, et al. In vivo demonstration that alpha-synuclein oligomers are toxic. Proc Natl Acad Sci USA. 2011;108(10):4194–4199. doi: 10.1073/pnas.1100976108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jellinger KA. Neuropathological spectrum of synucleinopathies. Mov Disord. 2003;18(Suppl 6):S2–S12. doi: 10.1002/mds.10557. [DOI] [PubMed] [Google Scholar]
- 8.Gilman S, Wenning GK, Low PA, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71(9):670–676. doi: 10.1212/01.wnl.0000324625.00404.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Papp MI, Kahn JE, Lantos PL. Glial cytoplasmic inclusions in the CNS of patients with multiple system atrophy (striatonigral degeneration, olivopontocerebellar atrophy and Shy-Drager syndrome) J Neurol Sci. 1989;94(1–3):79–100. doi: 10.1016/0022-510x(89)90219-0. [DOI] [PubMed] [Google Scholar]
- 10.Tsuboi K, Grzesiak JJ, Bouvet M, Hashimoto M, Masliah E, Shults CW. Alpha-synuclein overexpression in oligodendrocytic cells results in impaired adhesion to fibronectin and cell death. Mol Cell Neurosci. 2005;29(2):259–268. doi: 10.1016/j.mcn.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Kao AW, Racine CA, Quitania LC, Kramer JH, Christine CW, Miller BL. Cognitive and neuropsychiatric profile of the synucleinopathies: Parkinson disease, dementia with Lewy bodies, and multiple system atrophy. Alzheimer disease and associated disorders. 2009;23(4):365–370. doi: 10.1097/WAD.0b013e3181b5065d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Savica R, Grossardt BR, Bower JH, Ahlskog JE, Rocca WA. Incidence and Pathology of Synucleinopathies and Tauopathies Related to Parkinsonism. JAMA neurology. 2013:1–7. doi: 10.1001/jamaneurol.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Savica R, Grossardt BR, Bower JH, Boeve BF, Ahlskog JE, Rocca WA. Incidence of dementia with Lewy bodies and Parkinson disease dementia. JAMA neurology. 2013;70(11):1396–1402. doi: 10.1001/jamaneurol.2013.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24(2):197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 15.Jellinger KA. A critical evaluation of current staging of alpha-synuclein pathology in Lewy body disorders. Biochim Biophys Acta. 2009;1792(7):730–740. doi: 10.1016/j.bbadis.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Goedert M, Spillantini MG, Del Tredici K, Braak H. 100 years of Lewy pathology. Nat Rev Neurol. 2013;9(1):13–24. doi: 10.1038/nrneurol.2012.242. [DOI] [PubMed] [Google Scholar]
- 17.Lloyd KG. CNS compensation to dopamine neuron loss in Parkinson's disease. Adv Exp Med Biol. 1977;90:255–266. doi: 10.1007/978-1-4684-2511-6_16. [DOI] [PubMed] [Google Scholar]
- 18.Chiocco MJ, Harvey BK, Wang Y, Hoffer BJ. Neurotrophic factors for the treatment of Parkinson's disease. Parkinsonism Relat Disord. 2007;13(Suppl 3):S321–S328. doi: 10.1016/S1353-8020(08)70024-5. [DOI] [PubMed] [Google Scholar]
- 19.Taylor H, Minger SL. Regenerative medicine in Parkinson's disease: generation of mesencephalic dopaminergic cells from embryonic stem cells. Current opinion in biotechnology. 2005;16(5):487–492. doi: 10.1016/j.copbio.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Cotzias GC, Papavasiliou PS, Gellene R. L-dopa in parkinson's syndrome. The New England journal of medicine. 1969;281(5):272. doi: 10.1056/NEJM196907312810518. [DOI] [PubMed] [Google Scholar]
- 21.Yahr MD, Duvoisin RC, Schear MJ, Barrett RE, Hoehn MM. Treatment of parkinsonism with levodopa. Archives of neurology. 1969;21(4):343–354. doi: 10.1001/archneur.1969.00480160015001. [DOI] [PubMed] [Google Scholar]
- 22.Carlsson A, Lindqvist M, Magnusson T. 3,4-Dihydroxyphenylalanine and 5-hydroxytryptophan as reserpine antagonists. Nature. 1957;180(4596):1200. doi: 10.1038/1801200a0. [DOI] [PubMed] [Google Scholar]
- 23.Rascol O, Brooks DJ, Korczyn AD, De Deyn PP, Clarke CE, Lang AE. A five-year study of the incidence of dyskinesia in patients with early Parkinson's disease who were treated with ropinirole or levodopa. The New England journal of medicine. 2000;342(20):1484–1491. doi: 10.1056/NEJM200005183422004. [DOI] [PubMed] [Google Scholar]
- 24.Holloway RG, Shoulson I, Fahn S, et al. Pramipexole vs levodopa as initial treatment for Parkinson disease: a 4-year randomized controlled trial. Archives of neurology. 2004;61(7):1044–1053. doi: 10.1001/archneur.61.7.1044. [DOI] [PubMed] [Google Scholar]
- 25.Parkinson Study Group CCI. Long-term effect of initiating pramipexole vs levodopa in early Parkinson disease. Archives of neurology. 2009;66(5):563–570. doi: 10.1001/archneur.66.1.nct90001. [DOI] [PubMed] [Google Scholar]
- 26.Mizoguchi K, Yokoo H, Yoshida M, Tanaka T, Tanaka M. Amantadine increases the extracellular dopamine levels in the striatum by re-uptake inhibition and by N-methyl-D-aspartate antagonism. Brain Res. 1994;662(1–2):255–258. doi: 10.1016/0006-8993(94)90821-4. [DOI] [PubMed] [Google Scholar]
- 27.Hughes AJ, Colosimo C, Kleedorfer B, Daniel SE, Lees AJ. The dopaminergic response in multiple system atrophy. J Neurol Neurosurg Psychiatry. 1992;55(11):1009–1013. doi: 10.1136/jnnp.55.11.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ikeda M, Mori E, Matsuo K, Nakagawa M, Kosaka K. Donepezil for dementia with Lewy bodies: a randomized, placebo-controlled, confirmatory phase III trial. Alzheimer's research & therapy. 2015;7(1):4. doi: 10.1186/s13195-014-0083-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dubois B, Tolosa E, Katzenschlager R, et al. Donepezil in Parkinson's disease dementia: a randomized, double-blind efficacy and safety study. Mov Disord. 2012;27(10):1230–1238. doi: 10.1002/mds.25098. [DOI] [PubMed] [Google Scholar]
- 30.Reingold JL, Morgan JC, Sethi KD. Rivastigmine for the treatment of dementia associated with Parkinson's disease. Neuropsychiatric disease and treatment. 2007;3(6):775–783. doi: 10.2147/ndt.s1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edwards K, Royall D, Hershey L, et al. Efficacy and safety of galantamine in patients with dementia with Lewy bodies: a 24-week open-label study. Dement Geriatr Cogn Disord. 2007;23(6):401–405. doi: 10.1159/000101512. [DOI] [PubMed] [Google Scholar]
- 32.Poewe W. Parkinson disease: treatment of the nonmotor symptoms of Parkinson disease. Nat Rev Neurol. 2010;6(8):417–418. doi: 10.1038/nrneurol.2010.87. [DOI] [PubMed] [Google Scholar]
- 33.Stefanis L. α-Synuclein in Parkinson's disease. Cold Spring Harb Perspect Med. 2012;2(2):a009399. doi: 10.1101/cshperspect.a009399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor JP, Hardy J, Fischbeck KH. Toxic proteins in neurodegenerative disease. Science. 2002;296(5575):1991–1995. doi: 10.1126/science.1067122. [DOI] [PubMed] [Google Scholar]
- 35.Chiba-Falek O, Lopez GJ, Nussbaum RL. Levels of alpha-synuclein mRNA in sporadic Parkinson disease patients. Mov Disord. 2006;21(10):1703–1708. doi: 10.1002/mds.21007. [DOI] [PubMed] [Google Scholar]
- 36.Asi YT, Simpson JE, Heath PR, et al. Alpha-synuclein mRNA expression in oligodendrocytes in MSA. Glia. 2014;62(6):964–970. doi: 10.1002/glia.22653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singleton AB, Farrer M, Johnson J, et al. alpha-Synuclein locus triplication causes Parkinson's disease. Science. 2003;302(5646):841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 38.Ibanez P, Bonnet AM, Debarges B, et al. Causal relation between alpha-synuclein gene duplication and familial Parkinson's disease. Lancet. 2004;364(9440):1169–1171. doi: 10.1016/S0140-6736(04)17104-3. [DOI] [PubMed] [Google Scholar]
- 39.Ahn TB, Kim SY, Kim JY, et al. alpha-Synuclein gene duplication is present in sporadic Parkinson disease. Neurology. 2008;70(1):43–49. doi: 10.1212/01.wnl.0000271080.53272.c7. [DOI] [PubMed] [Google Scholar]
- 40.Edwards TL, Scott WK, Almonte C, et al. Genome-wide association study confirms SNPs in SNCA and the MAPT region as common risk factors for Parkinson disease. Annals of human genetics. 2010;74(2):97–109. doi: 10.1111/j.1469-1809.2009.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takahashi M, Suzuki M, Fukuoka M, et al. Normalization of Overexpressed alpha-Synuclein Causing Parkinson's Disease By a Moderate Gene Silencing With RNA Interference. Molecular therapy Nucleic acids. 2015;4:e241. doi: 10.1038/mtna.2015.14. [DOI] [PubMed] [Google Scholar]
- 42.Junn E, Lee KW, Jeong BS, Chan TW, Im JY, Mouradian MM. Repression of alpha-synuclein expression and toxicity by microRNA-7. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(31):13052–13057. doi: 10.1073/pnas.0906277106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Doxakis E. Post-transcriptional regulation of alpha-synuclein expression by mir-7 and mir-153. J Biol Chem. 2010;285(17):12726–12734. doi: 10.1074/jbc.M109.086827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Breydo L, Wu JW, Uversky VN. Alpha-synuclein misfolding and Parkinson's disease. Biochim Biophys Acta. 2012;1822(2):261–285. doi: 10.1016/j.bbadis.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 45.Berrocal R, Vasquez V, Krs SR, Gadad BS, Rao KS. alpha-Synuclein Misfolding Versus Aggregation Relevance to Parkinson's Disease: Critical Assessment and Modeling. Mol Neurobiol. 2015;51(3):1417–1431. doi: 10.1007/s12035-014-8818-2. [DOI] [PubMed] [Google Scholar]
- 46.Levin J, Giese A, Boetzel K, et al. Increased alpha-synuclein aggregation following limited cleavage by certain matrix metalloproteinases. Exp Neurol. 2009;215(1):201–208. doi: 10.1016/j.expneurol.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 47.Conway KA, Harper JD, Lansbury PT. Accelerated in vitro fibril formation by a mutant alpha-synuclein linked to early-onset Parkinson disease. Nat Med. 1998;4(11):1318–1320. doi: 10.1038/3311. [DOI] [PubMed] [Google Scholar]
- 48.Ostrerova-Golts N, Petrucelli L, Hardy J, Lee JM, Farer M, Wolozin B. The A53T alpha-synuclein mutation increases iron-dependent aggregation and toxicity. J Neurosci. 2000;20(16):6048–6054. doi: 10.1523/JNEUROSCI.20-16-06048.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lazaro DF, Rodrigues EF, Langohr R, et al. Systematic comparison of the effects of alpha-synuclein mutations on its oligomerization and aggregation. PLoS genetics. 2014;10(11):e1004741. doi: 10.1371/journal.pgen.1004741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oueslati A, Fournier M, Lashuel HA. Role of post-translational modifications in modulating the structure, function and toxicity of alpha-synuclein: implications for Parkinson's disease pathogenesis and therapies. Prog Brain Res. 2010;183:115–145. doi: 10.1016/S0079-6123(10)83007-9. [DOI] [PubMed] [Google Scholar]
- 51.Lee KW, Chen W, Junn E, et al. Enhanced phosphatase activity attenuates α-synucleinopathy in a mouse model. J Neurosci. 2011;31(19):6963–6971. doi: 10.1523/JNEUROSCI.6513-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoyer W, Cherny D, Subramaniam V, Jovin TM. Impact of the acidic C-terminal region comprising amino acids 109–140 on alpha-synuclein aggregation in vitro. Biochemistry. 2004;43(51):16233–16242. doi: 10.1021/bi048453u. [DOI] [PubMed] [Google Scholar]
- 53.Li W, West N, Colla E, et al. Aggregation promoting C-terminal truncation of alpha-synuclein is a normal cellular process and is enhanced by the familial Parkinson's disease-linked mutations. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(6):2162–2167. doi: 10.1073/pnas.0406976102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fonseca-Ornelas L, Eisbach SE, Paulat M, et al. Small molecule-mediated stabilization of vesicle-associated helical alpha-synuclein inhibits pathogenic misfolding and aggregation. Nature communications. 2014;5:5857. doi: 10.1038/ncomms6857. [DOI] [PubMed] [Google Scholar]
- 55.Li J, Zhu M, Rajamani S, Uversky VN, Fink AL. Rifampicin inhibits alpha-synuclein fibrillation and disaggregates fibrils. Chem Biol. 2004;11(11):1513–1521. doi: 10.1016/j.chembiol.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 56.Bousset L, Pieri L, Ruiz-Arlandis G, et al. Structural and functional characterization of two alpha-synuclein strains. Nature communications. 2013;4:2575. doi: 10.1038/ncomms3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Crews L, Spencer B, Desplats P, et al. Selective molecular alterations in the autophagy pathway in patients with Lewy body disease and in models of alpha-synucleinopathy. PloS one. 2010;5(2):e9313. doi: 10.1371/journal.pone.0009313. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 58.Lynch-Day MA, Mao K, Wang K, Zhao M, Klionsky DJ. The role of autophagy in Parkinson's disease. Cold Spring Harb Perspect Med. 2012;2(4):a009357. doi: 10.1101/cshperspect.a009357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McNaught KS, Belizaire R, Isacson O, Jenner P, Olanow CW. Altered proteasomal function in sporadic Parkinson's disease. Exp Neurol. 2003;179(1):38–46. doi: 10.1006/exnr.2002.8050. [DOI] [PubMed] [Google Scholar]
- 60.Maor G, Rencus-Lazar S, Filocamo M, Steller H, Segal D, Horowitz M. Unfolded protein response in Gaucher disease: from human to Drosophila. Orphanet journal of rare diseases. 2013;8:140. doi: 10.1186/1750-1172-8-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Valera E, Dargusch R, Maher PA, Schubert D. Modulation of 5-lipoxygenase in proteotoxicity and Alzheimer's disease. J Neurosci: Society for Neuroscience. 2013:10512–10525. doi: 10.1523/JNEUROSCI.5183-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tatebe H, Watanabe Y, Kasai T, et al. Extracellular neurosin degrades alpha-synuclein in cultured cells. Neurosci Res. 2010;67(4):341–346. doi: 10.1016/j.neures.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 63.Spencer B, Michael S, Shen J, et al. Lentivirus mediated delivery of neurosin promotes clearance of wild-type alpha-synuclein and reduces the pathology in an alpha-synuclein model of LBD. Mol Ther. 2013;21(1):31–41. doi: 10.1038/mt.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 64.Sung JY, Park SM, Lee CH, et al. Proteolytic cleavage of extracellular secreted {alpha}-synuclein via matrix metalloproteinases. J Biol Chem. 2005;280(26):25216–25224. doi: 10.1074/jbc.M503341200. [DOI] [PubMed] [Google Scholar]
- 65.Sevlever D, Jiang P, Yen SH. Cathepsin D is the main lysosomal enzyme involved in the degradation of alpha-synuclein and generation of its carboxy-terminally truncated species. Biochemistry. 2008;47(36):9678–9687. doi: 10.1021/bi800699v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bae EJ, Lee HJ, Rockenstein E, et al. Antibody-aided clearance of extracellular alpha-synuclein prevents cell-to-cell aggregate transmission. J Neurosci. 2012;32(39):13454–13469. doi: 10.1523/JNEUROSCI.1292-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Braak H, Sastre M, Del Tredici K. Development of alpha-synuclein immunoreactive astrocytes in the forebrain parallels stages of intraneuronal pathology in sporadic Parkinson's disease. Acta neuropathologica. 2007;114(3):231–241. doi: 10.1007/s00401-007-0244-3. [DOI] [PubMed] [Google Scholar]
- 68.Masliah E, Rockenstein E, Adame A, et al. Effects of alpha-synuclein immunization in a mouse model of Parkinson's disease. Neuron. 2005;46(6):857–868. doi: 10.1016/j.neuron.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 69.Masliah E, Rockenstein E, Mante M, et al. Passive immunization reduces behavioral and neuropathological deficits in an alpha-synuclein transgenic model of Lewy body disease. PloS one. 2011;6(4):e19338. doi: 10.1371/journal.pone.0019338. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 70.Mandler M, Valera E, Rockenstein E, et al. Next-generation active immunization approach for synucleinopathies: implications for Parkinson's disease clinical trials. Acta neuropathologica. 2014;127(6):861–879. doi: 10.1007/s00401-014-1256-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mandler M, Valera E, Rockenstein E, et al. Active immunization against alpha-synuclein ameliorates the degenerative pathology and prevents demyelination in a model of multiple system atrophy. Mol Neurodegener. 2015;10(1):10. doi: 10.1186/s13024-015-0008-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Games D, Valera E, Spencer B, et al. Reducing C-terminal-truncated alpha-synuclein by immunotherapy attenuates neurodegeneration and propagation in Parkinson's disease-like models. J Neurosci. 2014;34(28):9441–9454. doi: 10.1523/JNEUROSCI.5314-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Valera E, Masliah E. Immunotherapy for neurodegenerative diseases: Focus on α-synucleinopathies. Pharmacol Ther. 2013 doi: 10.1016/j.pharmthera.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Danzer KM, Ruf WP, Putcha P, et al. Heat-shock protein 70 modulates toxic extracellular alpha-synuclein oligomers and rescues trans-synaptic toxicity. Faseb J. 2011;25(1):326–336. doi: 10.1096/fj.10-164624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kordower JH, Emborg ME, Bloch J, et al. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson's disease. Science. 2000;290(5492):767–773. doi: 10.1126/science.290.5492.767. [DOI] [PubMed] [Google Scholar]
- 76.Rangasamy SB, Soderstrom K, Bakay RA, Kordower JH. Neurotrophic factor therapy for Parkinson's disease. Prog Brain Res. 2010;184:237–264. doi: 10.1016/S0079-6123(10)84013-0. [DOI] [PubMed] [Google Scholar]
- 77.Allen SJ, Watson JJ, Shoemark DK, Barua NU, Patel NK. GDNF, NGF and BDNF as therapeutic options for neurodegeneration. Pharmacol Ther. 2013;138(2):155–175. doi: 10.1016/j.pharmthera.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 78.Drinkut A, Tereshchenko Y, Schulz JB, Bahr M, Kugler S. Efficient gene therapy for Parkinson's disease using astrocytes as hosts for localized neurotrophic factor delivery. Mol Ther. 2012;20(3):534–543. doi: 10.1038/mt.2011.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Visanji NP, Orsi A, Johnston TH, et al. PYM50028, a novel, orally active, nonpeptide neurotrophic factor inducer, prevents and reverses neuronal damage induced by MPP+ in mesencephalic neurons and by MPTP in a mouse model of Parkinson's disease. FASEB J. 2008;22(7):2488–2497. doi: 10.1096/fj.07-095398. [DOI] [PubMed] [Google Scholar]
- 80.Kohl Z, Winner B, Ubhi K, et al. Fluoxetine rescues impaired hippocampal neurogenesis in a transgenic A53T synuclein mouse model. The European journal of neuroscience. 2012;35(1):10–19. doi: 10.1111/j.1460-9568.2011.07933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim JH, Auerbach JM, Rodriguez-Gomez JA, et al. Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson's disease. Nature. 2002;418(6893):50–56. doi: 10.1038/nature00900. [DOI] [PubMed] [Google Scholar]
- 82.Li JY, Englund E, Holton JL, et al. Lewy bodies in grafted neurons in subjects with Parkinson's disease suggest host-to-graft disease propagation. Nat Med. 2008;14(5):501–503. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- 83.Stefanova N, Hainzer M, Stemberger S, et al. Striatal transplantation for multiple system atrophy--are grafts affected by alpha-synucleinopathy? Exp Neurol. 2009;219(1):368–371. doi: 10.1016/j.expneurol.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 84.Chartier-Harlin MC, Dachsel JC, Vilariño-Güell C, et al. Translation initiator EIF4G1 mutations in familial Parkinson disease. Am J Hum Genet. 2011;89(3):398–406. doi: 10.1016/j.ajhg.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lesage S, Brice A. Role of mendelian genes in "sporadic" Parkinson's disease. Parkinsonism Relat Disord. 2012;18(Suppl 1):S66–S70. doi: 10.1016/S1353-8020(11)70022-0. [DOI] [PubMed] [Google Scholar]
- 86.Matsumine H, Yamamura Y, Hattori N, et al. A microdeletion of D6S305 in a family of autosomal recessive juvenile parkinsonism (PARK2) Genomics. 1998;49(1):143–146. doi: 10.1006/geno.1997.5196. [DOI] [PubMed] [Google Scholar]
- 87.Ramirez A, Heimbach A, Gründemann J, et al. Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase. Nat Genet. 2006;38(10):1184–1191. doi: 10.1038/ng1884. [DOI] [PubMed] [Google Scholar]
- 88.Valente EM, Abou-Sleiman PM, Caputo V, et al. Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science. 2004;304(5674):1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- 89.van Duijn CM, Dekker MC, Bonifati V, et al. Park7, a novel locus for autosomal recessive early-onset parkinsonism, on chromosome 1p36. Am J Hum Genet. 2001;69(3):629–634. doi: 10.1086/322996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zimprich A, Biskup S, Leitner P, et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44(4):601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 91.Zimprich A, Benet-Pagès A, Struhal W, et al. A mutation in VPS35, encoding a subunit of the retromer complex, causes late-onset Parkinson disease. Am J Hum Genet. 2011;89(1):168–175. doi: 10.1016/j.ajhg.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lincoln S, Vaughan J, Wood N, et al. Low frequency of pathogenic mutations in the ubiquitin carboxy-terminal hydrolase gene in familial Parkinson's disease. Neuroreport. 1999;10(2):427–429. doi: 10.1097/00001756-199902050-00040. [DOI] [PubMed] [Google Scholar]
- 93.Lwin A, Orvisky E, Goker-Alpan O, LaMarca ME, Sidransky E. Glucocerebrosidase mutations in subjects with parkinsonism. Mol Genet Metab. 2004;81(1):70–73. doi: 10.1016/j.ymgme.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 94.Sutherland GT, Halliday GM, Silburn PA, et al. Do polymorphisms in the familial Parkinsonism genes contribute to risk for sporadic Parkinson's disease? Mov Disord. 2009;24(6):833–838. doi: 10.1002/mds.22214. [DOI] [PubMed] [Google Scholar]
- 95.Collaboration M-SAR. Mutations in COQ2 in familial and sporadic multiple-system atrophy. The New England journal of medicine. 2013:233–244. doi: 10.1056/NEJMoa1212115. [DOI] [PubMed] [Google Scholar]
- 96.Fujioka S, Ogaki K, Tacik PM, Uitti RJ, Ross OA, Wszolek ZK. Update on novel familial forms of Parkinson's disease and multiple system atrophy. Parkinsonism Relat Disord. 2014:S29–S34. doi: 10.1016/S1353-8020(13)70010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bogaerts V, Engelborghs S, Kumar-Singh S, et al. A novel locus for dementia with Lewy bodies: a clinically and genetically heterogeneous disorder. Brain : a journal of neurology. 2007;130(Pt 9):2277–2291. doi: 10.1093/brain/awm167. [DOI] [PubMed] [Google Scholar]
- 98.Tsuang D, Leverenz JB, Lopez OL, et al. GBA mutations increase risk for Lewy body disease with and without Alzheimer disease pathology. Neurology. 2012;79(19):1944–1950. doi: 10.1212/WNL.0b013e3182735e9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu Z, Hamamichi S, Lee BD, et al. Inhibitors of LRRK2 kinase attenuate neurodegeneration and Parkinson-like phenotypes in Caenorhabditis elegans and Drosophila Parkinson's disease models. Hum Mol Genet. 2011;20(20):3933–3942. doi: 10.1093/hmg/ddr312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Covy JP, Giasson BI. Identification of compounds that inhibit the kinase activity of leucine-rich repeat kinase 2. Biochem Biophys Res Commun. 2009;378(3):473–477. doi: 10.1016/j.bbrc.2008.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee BD, Shin JH, VanKampen J, et al. Inhibitors of leucine-rich repeat kinase-2 protect against models of Parkinson's disease. Nat Med. 2010;16(9):998–1000. doi: 10.1038/nm.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee BD, Dawson VL, Dawson TM. Leucine-rich repeat kinase 2 (LRRK2) as a potential therapeutic target in Parkinson's disease. Trends Pharmacol Sci. 2012;33(7):365–373. doi: 10.1016/j.tips.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Winklhofer KF. The parkin protein as a therapeutic target in Parkinson's disease. Expert Opin Ther Targets. 2007;11(12):1543–1552. doi: 10.1517/14728222.11.12.1543. [DOI] [PubMed] [Google Scholar]
- 104.Kubo S, Hatano T, Takanashi M, Hattori N. Can parkin be a target for future treatment of Parkinson's disease? Expert Opin Ther Targets. 2013;17(10):1133–1144. doi: 10.1517/14728222.2013.827173. [DOI] [PubMed] [Google Scholar]
- 105.Sardi SP, Clarke J, Viel C, et al. Augmenting CNS glucocerebrosidase activity as a therapeutic strategy for parkinsonism and other Gaucher-related synucleinopathies. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(9):3537–3542. doi: 10.1073/pnas.1220464110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Toyoda Y, Erkut C, Pan-Montojo F, et al. Products of the Parkinson's disease-related glyoxalase DJ-1, D-lactate and glycolate, support mitochondrial membrane potential and neuronal survival. Biology open. 2014;3(8):777–784. doi: 10.1242/bio.20149399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Parkinson Study Group QEI. Beal MF, Oakes D, et al. A randomized clinical trial of high-dosage coenzyme Q10 in early Parkinson disease: no evidence of benefit. JAMA neurology. 2014;71(5):543–552. doi: 10.1001/jamaneurol.2014.131. [DOI] [PubMed] [Google Scholar]
- 108.Sekiyama K, Sugama S, Fujita M, et al. Neuroinflammation in Parkinson's Disease and Related Disorders: A Lesson from Genetically Manipulated Mouse Models of α-Synucleinopathies. Parkinsons Dis. 2012;2012:271732. doi: 10.1155/2012/271732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bettcher BM, Wilheim R, Rigby T, et al. C-reactive protein is related to memory and medial temporal brain volume in older adults. Brain, behavior, and immunity. 2012;26(1):103–108. doi: 10.1016/j.bbi.2011.07.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Czerniawski J, Guzowski JF. Acute neuroinflammation impairs context discrimination memory and disrupts pattern separation processes in hippocampus. J Neurosci. 2014;34(37):12470–12480. doi: 10.1523/JNEUROSCI.0542-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lindqvist D, Hall S, Surova Y, et al. Cerebrospinal fluid inflammatory markers in Parkinson's disease--associations with depression, fatigue, and cognitive impairment. Brain, behavior, and immunity. 2013:183–189. doi: 10.1016/j.bbi.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 112.Barnum CJ, Tansey MG. Neuroinflammation and non-motor symptoms: the dark passenger of Parkinson's disease? Current neurology and neuroscience reports. 2012;12(4):350–358. doi: 10.1007/s11910-012-0283-6. [DOI] [PubMed] [Google Scholar]
- 113.Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140(6):918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hernández-Romero MC, Delgado-Cortés MJ, Sarmiento M, et al. Peripheral inflammation increases the deleterious effect of CNS inflammation on the nigrostriatal dopaminergic system. Neurotoxicology. 2012:347–360. doi: 10.1016/j.neuro.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 115.Nagatsu T, Mogi M, Ichinose H, Togari A. Changes in cytokines and neurotrophins in Parkinson's disease. J Neural Transm Suppl. 2000;(60):277–290. doi: 10.1007/978-3-7091-6301-6_19. [DOI] [PubMed] [Google Scholar]
- 116.Gilman S, Koller M, Black RS, et al. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005;64(9):1553–1562. doi: 10.1212/01.WNL.0000159740.16984.3C. [DOI] [PubMed] [Google Scholar]
- 117.Spencer B, Masliah E. Immunotherapy for Alzheimer's disease: past, present and future. Frontiers in aging neuroscience. 2014;6:114. doi: 10.3389/fnagi.2014.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hirohata M, Ono K, Morinaga A, Yamada M. Non-steroidal anti-inflammatory drugs have potent anti-fibrillogenic and fibril-destabilizing effects for alpha-synuclein fibrils in vitro. Neuropharmacology. 2008;54(3):620–627. doi: 10.1016/j.neuropharm.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 119.Swiatkiewicz M, Zaremba M, Joniec I, Czlonkowski A, Kurkowska-Jastrzebska I. Potential neuroprotective effect of ibuprofen, insights from the mice model of Parkinson's disease. Pharmacological reports : PR. 2013;65(5):1227–1236. doi: 10.1016/s1734-1140(13)71480-4. [DOI] [PubMed] [Google Scholar]
- 120.Valera E, Mante M, Anderson S, Rockenstein E, Masliah E. Lenalidomide reduces microglial activation and behavioral deficits in a transgenic model of Parkinson's disease. J Neuroinflammation. 2015;12:93. doi: 10.1186/s12974-015-0320-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Barnum CJ, Chen X, Chung J, et al. Peripheral administration of the selective inhibitor of soluble tumor necrosis factor (TNF) XPro(R)1595 attenuates nigral cell loss and glial activation in 6-OHDA hemiparkinsonian rats. Journal of Parkinson's disease. 2014;4(3):349–360. doi: 10.3233/JPD-140410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ubhi K, Inglis C, Mante M, et al. Fluoxetine ameliorates behavioral and neuropathological deficits in a transgenic model mouse of α-synucleinopathy. Exp Neurol. 2012;234(2):405–416. doi: 10.1016/j.expneurol.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Valera E, Ubhi K, Mante M, Rockenstein E, Masliah E. Antidepressants reduce neuroinflammatory responses and astroglial alpha-synuclein accumulation in a transgenic mouse model of multiple system atrophy. Glia. 2014;62(2):317–337. doi: 10.1002/glia.22610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ono K, Yamada M. Antioxidant compounds have potent anti-fibrillogenic and fibril-destabilizing effects for alpha-synuclein fibrils in vitro. J Neurochem. 2006;97(1):105–115. doi: 10.1111/j.1471-4159.2006.03707.x. [DOI] [PubMed] [Google Scholar]
- 125.Spinelli KJ, Osterberg VR, Meshul CK, Soumyanath A, Unni VK. Curcumin Treatment Improves Motor Behavior in alpha-Synuclein Transgenic Mice. PloS one. 2015;10(6):e0128510. doi: 10.1371/journal.pone.0128510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Takahashi R, Ono K, Takamura Y, et al. Phenolic compounds prevent the oligomerization of alpha-synuclein and reduce synaptic toxicity. J Neurochem. 2015 doi: 10.1111/jnc.13180. [DOI] [PubMed] [Google Scholar]
- 127.Magalingam KB, Radhakrishnan A, Ramdas P, Haleagrahara N. Quercetin glycosides induced neuroprotection by changes in the gene expression in a cellular model of Parkinson's disease. Journal of molecular neuroscience : MN. 2015;55(3):609–617. doi: 10.1007/s12031-014-0400-x. [DOI] [PubMed] [Google Scholar]
- 128.Li Z, Wang P, Yu Z, et al. The effect of creatine and coenzyme q10 combination therapy on mild cognitive impairment in Parkinson's disease. European neurology. 2015;73(3–4):205–211. doi: 10.1159/000377676. [DOI] [PubMed] [Google Scholar]
- 129.Caruana M, Högen T, Levin J, Hillmer A, Giese A, Vassallo N. Inhibition and disaggregation of α-synuclein oligomers by natural polyphenolic compounds. FEBS Lett. 2011;585(8):1113–1120. doi: 10.1016/j.febslet.2011.03.046. [DOI] [PubMed] [Google Scholar]
- 130.Dajas F, Rivera-Megret F, Blasina F, et al. Neuroprotection by flavonoids. Braz J Med Biol Res. 2003;36(12):1613–1620. doi: 10.1590/s0100-879x2003001200002. [DOI] [PubMed] [Google Scholar]
- 131.Zhang W, Wang T, Pei Z, et al. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson's disease. FASEB J. 2005;19(6):533–542. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]
- 132.Lee HJ, Suk JE, Patrick C, et al. Direct transfer of alpha-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J Biol Chem. 2010;285(12):9262–9272. doi: 10.1074/jbc.M109.081125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lee HJ, Kim C, Lee SJ. Alpha-synuclein stimulation of astrocytes: Potential role for neuroinflammation and neuroprotection: Oxid Med Cell Longev. 2010;3(4):283–287. doi: 10.4161/oxim.3.4.12809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Klegeris A, McGeer PL. Non-steroidal anti-inflammatory drugs (NSAIDs) and other anti-inflammatory agents in the treatment of neurodegenerative disease. Curr Alzheimer Res. 2005;2(3):355–365. doi: 10.2174/1567205054367883. [DOI] [PubMed] [Google Scholar]
- 135.Tang Y, Le W. Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol Neurobiol. 2015 doi: 10.1007/s12035-014-9070-5. [DOI] [PubMed] [Google Scholar]
- 136.Lorgunpai SJ, Grammas M, Lee DS, McAvay G, Charpentier P, Tinetti ME. Potential therapeutic competition in community-living older adults in the U.S.: use of medications that may adversely affect a coexisting condition. PloS one. 2014;9(2):e89447. doi: 10.1371/journal.pone.0089447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tannenbaum C, Johnell K. Managing therapeutic competition in patients with heart failure, lower urinary tract symptoms and incontinence. Drugs & aging. 2014;31(2):93–101. doi: 10.1007/s40266-013-0145-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Carballido E, Veliz M, Komrokji R, Pinilla-Ibarz J. Immunomodulatory drugs and active immunotherapy for chronic lymphocytic leukemia. Cancer control : journal of the Moffitt Cancer Center. 2012;19(1):54–67. doi: 10.1177/107327481201900106. [DOI] [PubMed] [Google Scholar]
- 139.Bancos S, Bernard MP, Topham DJ, Phipps RP. Ibuprofen and other widely used non-steroidal anti-inflammatory drugs inhibit antibody production in human cells. Cellular immunology. 2009;258(1):18–28. doi: 10.1016/j.cellimm.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.De Cock D, Van der Elst K, Meyfroidt S, Verschueren P, Westhovens R. The optimal combination therapy for the treatment of early rheumatoid arthritis. Expert opinion on pharmacotherapy. 2015;16(11):1615–1625. doi: 10.1517/14656566.2015.1056735. [DOI] [PubMed] [Google Scholar]
- 141.Komarova NL, Boland CR. Cancer: calculated treatment. Nature. 2013;499(7458):291–292. doi: 10.1038/499291a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lazzara CA, Riley RR, Rane A, Andersen JK, Kim YH. The combination of lithium and l-Dopa/Carbidopa reduces MPTP-induced abnormal involuntary movements (AIMs) via calpain-1 inhibition in a mouse model: Relevance for Parkinsons disease therapy. Brain Res. 2015 doi: 10.1016/j.brainres.2015.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cai JP, Chen WJ, Lin Y, Cai B, Wang N. Safety and efficacy of rasagiline in addition to levodopa for the treatment of idiopathic Parkinson's disease: a meta-analysis of randomised controlled trials. European neurology. 2015;73(1–2):5–12. doi: 10.1159/000367892. [DOI] [PubMed] [Google Scholar]
- 144.Smith KM, Eyal E, Weintraub D Investigators A. Combined rasagiline and antidepressant use in Parkinson disease in the ADAGIO study: effects on nonmotor symptoms and tolerability. JAMA neurology. 2015;72(1):88–95. doi: 10.1001/jamaneurol.2014.2472. [DOI] [PubMed] [Google Scholar]
- 145.Rocchi L, Chiari L, Horak FB. Effects of deep brain stimulation and levodopa on postural sway in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2002;73(3):267–274. doi: 10.1136/jnnp.73.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Johnson L, Rodrigues J, Teo WP, et al. Interactive effects of GPI stimulation and levodopa on postural control in Parkinson's disease. Gait & posture. 2015;41(4):929–934. doi: 10.1016/j.gaitpost.2015.03.346. [DOI] [PubMed] [Google Scholar]
- 147.Rowland NC, Starr PA, Larson PS, Ostrem JL, Marks WJ, Jr, Lim DA. Combining cell transplants or gene therapy with deep brain stimulation for Parkinson's disease. Mov Disord. 2015;30(2):190–195. doi: 10.1002/mds.26083. [DOI] [PubMed] [Google Scholar]
- 148.David FJ, Robichaud JA, Leurgans SE, et al. Exercise Improves Cognition in Parkinson's Disease: The PRET-PD Randomized, Clinical Trial. Mov Disord. 2015 doi: 10.1002/mds.26291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hashioka S. Antidepressants and neuroinflammation: Can antidepressants calm glial rage down? Mini Rev Med Chem. 2011;11(7):555–564. doi: 10.2174/138955711795906888. [DOI] [PubMed] [Google Scholar]
- 150.Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20(24):9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Dodel R, Spottke A, Gerhard A, et al. Minocycline 1-year therapy in multiple-system-atrophy: effect on clinical symptoms and [(11)C] (R)-PK11195 PET (MEMSA-trial) Mov Disord. 2010;25(1):97–107. doi: 10.1002/mds.22732. [DOI] [PubMed] [Google Scholar]