Abstract
Face recognition is fundamental to successful social interaction. Individuals with deficits in face recognition are likely to have social functioning impairments that may lead to heightened risk for social anxiety. A critical component of social interaction is how quickly a face is learned during initial exposure to a new individual. Here, we used a novel Repeated Faces task to assess how quickly memory for faces is established. Face recognition was measured over multiple exposures in 52 young adults ranging from low to high in social inhibition, a core dimension of social anxiety. High social inhibition was associated with a smaller slope of change in recognition memory over repeated face exposure, indicating participants with higher social inhibition showed smaller improvements in recognition memory after seeing faces multiple times. We propose that impaired face learning is an important mechanism underlying social inhibition and may contribute to, or maintain, social anxiety.
Keywords: social anxiety, novel faces, habituation, individual differences, temperament
1. Introduction
Humans are highly social beings. We are dependent on social groups to provide a sense of belonging (Hagerty et al., 1996), provide feelings of security (Sherman, 1977), and aid in partner selection and parenting (Hamilton, 1964). Thus, the ability to form and maintain strong social relationships is both rewarding and evolutionarily advantageous (Hamilton, 1964; Alexander, 1974). Individuals who are able to form large social circles are better protected against harm and adversity, while those who have difficulty developing new relationships experience fewer social benefits and are more susceptible to negative life events. For example, social anxiety is associated with myriad negative consequences, including reduced educational attainment (Schneier et al., 1994), occupational status (Schneier et al., 1994), and quality of life (Wittchen and Fehm, 2003).
Social anxiety may be associated with impairments in face recognition. The basic abilities to detect and process facial information form the basis of successful social interactions. Faces convey a wealth of information about an individual, and the ability to quickly recognize faces is a vital skill necessary to build and maintain social relationships. Accurate and rapid recognition of a face is critical for social functioning because it allows one to gauge behavior, intent, and appropriate social response, based on previous experiences. The importance of face recognition is evidenced by three distinct features: 1) humans have a preference at birth for processing and recognizing faces (Pascalis and Slater, 2003) and already show processing patterns during infancy similar to adults (Farzin et al., 2012); 2) face recognition is highly specific and dissociable from both general intelligence and from other types of recognition memory, like object recognition (Wilmer et al., 2010; Zhu et al., 2010); and 3) face processing relies on a dedicated neural substrate (Tsao et al., 2006; McKone et al., 2007; Tsao and Livingstone, 2008; Wilmer et al., 2010).
However, the ability to recognize faces is also surprisingly variable. Studies in healthy individuals reveal a dramatic range of ability on standardized face recognition assessments (Wilmer et al., 2010; Zhu et al., 2010; Skuse et al., 2014). This suggests that face recognition may vary along a dimension, with clinical recognition deficits as seen in prosopagnosia at the extreme end. Interestingly, individuals with prosopagnosia also show evidence of chronic and severe social anxiety (Yardley et al., 2008). Given the importance of face recognition in human social interaction, the notion that individual variability in the ability to recognize faces may affect social functioning is cogent. Face memory deficits have been observed in children with autism spectrum disorder (Corbett et al., 2014) and in patients with schizophrenia (Martin et al., 2005), two disorders characterized by social functioning impairments. However, the association between social functioning and face recognition ability remains largely untested outside of clinical populations. Given the importance of face recognition and the variability in face recognition ability across healthy people, we propose that this ability is coupled with social function at a fundamental level; that is, we propose that face recognition ability varies dimensionally with social inhibition. Social inhibition—defined as the tendency to withdrawal from new people and avoidance of social situations—is strongly related to social functioning (Bohlin et al., 2000; Rothbart et al., 2000) as it forms the foundation for developing effective social skills. Furthermore, social inhibition is one of the best established risk factors for the development of social anxiety disorder (Clauss and Blackford, 2012).
We posit that a critical component of face recognition is how quickly memory for faces is established. In a normal social environment (e.g., interaction with coworkers, mingling at a party) face exposures are repeated many times over a prolonged duration, and memory for a specific face increases with repeated exposures. However, whether there are individual differences in acquisition of face memory over repeated exposures remains unknown. Standardized clinical memory assessments, which typically provide a single exposure prior to an explicit recognition test, are not well-positioned to capture individual differences in memory acquisition over time. To address this limitation, we developed a novel task (Repeated Faces task) to explicitly assess face memory at different degrees of exposure to a novel face. Because we expect differences in memory acquisition for faces to be associated with social functioning, we investigated the relationship between face recognition over repeated exposures and social inhibition.
2. Methods
2.1. Participants
Study participants were 52 young adults (18 - 30 years of age; mean age = 23 years, SD = 2.56) with social inhibition scores ranging from low social inhibition to high social inhibition. Participants were recruited using recruitment databases, flyers, and mass distribution email. To ensure adequate sampling at the extreme ends of the social inhibition spectrum, we used additional advertisements seeking young adults who were “especially shy or outgoing as children”. Social inhibition was assessed using well-established self-report measures: the Adult Self-Report of Inhibition (ASRI) and the companion Retrospective Self-Report of Inhibition (RSRI) (Reznick, Hegeman, Kaufman, Woods, & Jacobs, 1992). The ASRI and RSRI both use a 1-5 likert scale and show good reliability and validity (Reznick et al., 1992; Rohrbacher et al., 2008). In this sample, internal consistency was excellent for both the ASRI (Cronbach's α = 0.92) and RSRI (Cronbach's α = 0.93). The childhood and adult social inhibition scores were averaged to create a combined social inhibition score for each participant. Participants ranged across the full continuum, from very low social inhibition (minimum = 1.32) to very high social inhibition (max = 4.21) with a mean score of 2.58 (SD = 0.83).
Participants were excluded for past or current psychiatric illness, except anxiety disorders based on self-report; head injury resulting in loss of consciousness; significant medical illness; or current use of psychoactive medications (previous six months). Fifty-four individuals participated in this study; however, two participants had missing data due to technical issues, resulting in the final analysis sample size of 52 participants. The study cohort was 65% female (n = 34) and reflected the racial composition of the surrounding Nashville community (73% Caucasian, 15% African-American, 8% Asian, and 4% other). There were no associations between social inhibition and age, sex, or race (all ps > 0.13).
This research was conducted in accordance with the Vanderbilt Human Research Protection Program and all participants provided written informed consent. Participants received financial compensation.
2.2. Repeated Faces Task
The Repeated Faces task was developed to test memory accuracy for faces with differing numbers of exposure, also known as depth of encoding. The task was comprised of an initial exposure phase followed by a testing phase. During the exposure phase, participants viewed a total of 128 face presentations in pseudo-random order. To provide a range of face exposures, we presented four sets of eight faces (n = 32 face identities) one, three, five, or seven times. Repeated face images were identical in expression and viewing perspective across presentations. Participants were told “In this study a face will appear in the middle of the screen. Your job is to stay focused on the screen and look at each face. The faces will flash quickly”. Faces were presented for 1 s each with a 250 ms fixation cross image presented between each face presentation. Prior to the testing phase, participants were told “Now you will see some more faces. Your job is to determine whether you have seen the face before or not.” During the testing phase, participants were shown each of the 32 previously-seen faces (1, 3, 5 or 7 previous exposures) and 32 novel faces (0 previous exposures) in random order and asked to indicate by button press whether each face was new or was previously-seen. Faces were presented until the button press was made, for up to 5 seconds. E-prime software (Version 1.1, Psychology Software Tools, Pittsburgh, PA, USA) was used to present stimuli and record button press responses. Both accuracy and reaction time were collected.
Stimuli were black and white images of human faces with neutral expressions, selected from the Gur (Gur et al., 2002) and Karolinska (Lundqvist, D, Flykt, A, Ohman et al., 1998; Goeleven et al., 2008) datasets, two standard sets of emotional expression. For each participant, an equal number of male and female faces were used for each exposure level; model-code numbers are included in Appendix A. We used neutral faces for several reasons: relatively mild stimuli, like non-emotional faces, may be ideal for eliciting individual differences (Lissek et al., 2006); and recent studies demonstrate that patients with social anxiety respond differently to neutral faces (Cooney et al., 2006; Yoon and Zinbarg, 2008). All stimuli were edited to ensure uniform face size, eye position and nose position, and all extraneous features (e.g. shirt collars, hair) were removed.
2.3. Data Analysis
To validate our novel task, we first examined percent accuracy and reaction times by exposure category (0, 1, 3, 5, 7). We expected percent accuracy to increase and reaction times to decrease with increased exposure to faces; t-tests compared accuracy and reaction times for faces previously seen once with faces previously seen seven times (1 vs. 7) across participants. To test our primary outcome measure, rate of change in memory acquisition, we computed slope of change (b′) in face recognition. For each participant, percent accuracy values were natural log-transformed and b′ was calculated using linear regression (Appendix B; Montagu, 1963; Plichta et al., 2014). Because slope of change over repeated faces is highly dependent on initial accuracy to faces seen once, slopes were normalized to recognition of faces with for one exposure (Appendix B) (Montagu, 1963; Plichta et al., 2014). To test our primary hypothesis, correlations between social inhibition and slope of change (b′) in face recognition (1 to 7 previous exposures) were performed. Significant differences were followed by post-hoc analyses of change in memory acquisition for adjacent levels (1 to 3 exposures, 3 to 5 exposures, 5 to 7 exposures). Slope analyses were performed in SAS (SAS Institute Inc., Cary, NC, USA) with α = .05.
3. Results
3.1. Task Validation
We first examined percent accuracy across participants. As expected, participants were more accurate in recognizing faces that had been seen seven times relative to faces seen once (Table 1). Percent accuracy was lowest for faces with one previous exposure, at 45%, but improved to 86% for faces with seven previous exposures. Novel face discrimination accuracy (73%) was similar to other face recognition tasks (Pérez-López and Woody, 2001). Participants were also faster at identifying faces seen seven times relative to faces seen once (Table 1).
Table 1.
Percent accuracy and reaction time by exposure category
| Previous exposures | ||||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 3 | 5 | 7 | T-test (1 vs. 7) | |||
| mean (sd) | mean (sd) | mean (sd) | mean (sd) | mean (sd) | t | d | p | |
| Percent accuracy | 0.73 (0.15) | 0.45 (0.23) | 0.67 (0.21) | 0.77 (0.21) | 0.86 (0.16) | −12.31 | −2.07 | <0.001 |
| Reaction time (ms) | 1317 (342) | 1312 (417) | 1266 (503) | 1161 (344) | 1087 (294) | 4.44 | 0.62 | <0.001 |
Note: standard deviation (sd); reaction time (ms).
3.2. Social Inhibition
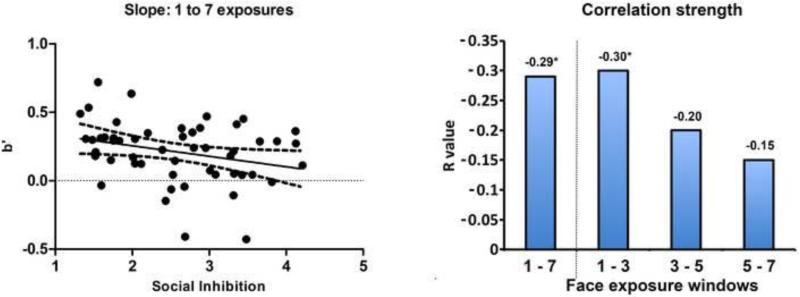
We investigated the association between social inhibition and the ability to benefit from repeated exposure to a face by examining change in face recognition accuracy over repeated exposures using slope (b′) analysis. Social inhibition scores were negatively correlated with face recognition slope across the experiment (change from 1 to 7 previous exposures, r = −0.29, p = 0.04; Figure 1). The direction of the correlation suggests that higher social inhibition scores are associated with smaller increases in memory accuracy with repeated exposures.
Figure 1. Rate of improvement in face recognition memory correlates with social inhibition.
The left panel shows the results of the main analysis. Higher social inhibition scores were correlated with smaller b′ slope values across the experiment (change from 1 to 7 exposures, p=0.04), indicating that higher social inhibition was associated with smaller improvement in recognition memory for faces seen seven times compared to faces seen once. The right panel shows the results of 1–7 face exposures exploratory post hoc analyses. Significant correlations are denoted with an asterisk (*). Post-hoc analyses showed a moderate association between higher social inhibition and smaller memory improvements during early face exposures (change from 1 to 3 exposures , p=0.03 uncorrected) and modest, nonsignificant (p>0.05) correlations across later exposure windows; however, the strength of correlations across adjacent windows were not significantly different, suggesting a similar overall impairment across discreet repetition windows.
As an exploratory post-hoc analysis, we also examined the correlations between social inhibition and slopes for adjacent exposure levels (i.e., change from 1–3, 3–5, 5–7 exposures). This analysis showed modest to moderate correlations between social inhibition and slope of memory change for each adjacent level (Figure 1). The strongest correlation between slope and social inhibition was observed for the early face exposures (change from 1 to 3 exposures, r=−0.30, p=0.03 uncorrected; Fig. 1). The correlations for the other adjacent levels were: change from 3 to 5 exposures, r = −0.20, p = −0.16; and change from 5 to 7 exposures, r = −0.15, p = 0.28. It should be noted that the strength of correlations across adjacent windows were not significantly different from one another using a formal statistical test comparing the difference between correlations. Social inhibition was not correlated with false alarm rate to novel faces (p = 0.61) or reaction times (all ps > 0.50).
4. Discussion
The major finding from the current study is that social inhibition is associated with impairments in face recognition memory. The ability to recognize previously-seen faces is a critical component of social interaction; in a typical social situation exposure to a face builds over time, with increasing exposure promoting better subsequent recognition of an individual. Here, we used a unique Repeated Faces task to build tiered levels of exposure to individual faces and test subsequent recognition ability. Overall, face recognition improved across all subjects with repeated exposures. However, subjects with higher social inhibition scores had smaller slopes, indicating smaller increases in recognition memory improvement with repeated face exposures. These findings demonstrate that people with higher social inhibition show less benefit from repeated face exposures and suggest a difference in face learning. Because social inhibition confers substantial risk for the development of social anxiety disorder, differences in face learning may be a cognitive mechanism contributing to vulnerability to social anxiety.
Although the relationship between face memory and social inhibition is largely understudied, there are several previous findings of interest. Davis and colleagues (2011) examined the association between a clinical measure of face recognition—where three different perspectives of a single face identity were shown once prior to testing—and a dimensional measure of social anxiety in an unselected sample. In that study, higher levels of social anxiety correlated with worse face recognition, although the effect sizes were relatively small (R2 = 0.02-0.04)—potentially due to a restricted range of anxiety scores. Studies of people with social anxiety disorder may also be relevant. To date, the majority of studies in social anxiety disorder have examined recognition memory for threatening compared to accepting faces. Two studies have shown enhanced recognition for faces that had previously been rated as critical, but not for faces rated as accepting (Lundh and Ost, 1996; Coles and Heimberg, 2005). However, another study showed that under conditions of stress, patients with social anxiety had lower recognition memory for both threatening and reassuring faces (Pérez-López and Woody, 2001). Because recognition memory may be modulated by emotional expressions in people with high levels of social anxiety (Button et al., 2013); future research should investigate the time course of memory acquisition for emotional faces.
Face recognition impairments may result from differences in the processing or interpretation of facial information by socially inhibited individuals. Studies have shown that shy children spend a longer time viewing the eyes of novel neutral faces (Brunet et al., 2009) and show reduced sensitivity to variations in the spacing of facial features (Brunet et al., 2010), suggesting they may not use global face viewing strategies when processing novel faces—in healthy adults, a global face viewing strategy, relative to a feature-based strategy, is associated with better recognition memory (Richler et al., 2011). Also, people with higher levels of social anxiety interpret neutral faces as more negative or threatening (Yoon and Zinbarg, 2008; Perlman et al., 2009; Jun et al., 2013) and patients with social anxiety disorder avoid looking at the eye regions of angry faces (Horley et al., 2004). These findings suggest that sub-optimal face viewing strategies may be associated with differences in learning in socially inhibited individuals, although this should be tested in future studies.
One important question is whether differences in face learning contribute to, or result from, social inhibition. Although our study is correlational, and therefore cannot be used to determine directionality, several other studies have attempted to address this question. One hypothesis is that early developmental differences in face viewing contribute to long-term developmental differences in face memory ability—in other words, that high social inhibition results in avoidance of viewing faces, which leads to lack of expertise in face processing (Brunet et al., 2010). However, the existing literature currently does not support the lack of expertise hypothesis; instead, a recent review suggests that face processing abilities are present very early in life and are not the result of lack of expertise (McKone et al., 2007). These findings support a domain-specific impairment in face recognition, suggesting that early face recognition differences drive the later development of social function and possibly contribute to development of social inhibition. Future longitudinal studies will be necessary to tease apart the relationships among face viewing patterns, recognition memory, and social inhibition.
Of interest, recent studies have shown differences in habituation of neural activity to repeated exposures to faces in socially inhibited people. Blackford and colleagues (2013) found that the young adults with high social inhibition fail to show the typical pattern of habituation to repeated presentations of neutral faces in both the amygdala and hippocampus, two brain regions implicated in both novelty detection and social behavior. Schwartz and colleagues (2012) also found a habituation failure to neutral faces in the amygdala in young adults who were highly reactive infants—a developmental precursor to inhibited temperament and social inhibition—although the finding was limited to males. Habituation is one of the most basic learning processes and serves a critical function of allocating attentional resources (Rankin et al., 2009). Based on the findings of the current study and previous neuroimaging studies, it is possible that memory impairments are associated with the habituation failure observed in the amygdala and hippocampus.
Individual variability in face recognition ability may also have important implications for understanding psychiatric illness more broadly. Social anxiety is a leading cause of disability in psychiatric disorders. Face recognition impairments have been demonstrated in disorders associated with social anxiety, including social anxiety disorder (Davis et al., 2011), autism (Weigelt et al., 2012), and schizophrenia (Martin et al., 2005). Face recognition is also highly heritable (Wilmer et al., 2010), suggesting that it may represent a valuable intermediate phenotype, or endophenotype, to explain genetic influence on social behavior across a spectrum of psychiatric illnesses. This approach is in line with the aims of the Research Domain Criteria initiative (RDoC) from the National Institute of Mental Health (Cuthbert and Insel, 2013), which aims to develop a research classification for mental disorders based on dimensional constructs.
One limitation of the present study is that we tested for memory for social, but not non-social, stimuli. For example, a previous study (Davis et al., 2011) found that social anxiety was associated with impairment in face, but not car, recognition. Another limitation is that we are unable to determine whether recognition memory is deficient or simply delayed in socially inhibited individuals. Social inhibition was associated with a smaller recognition memory benefit across repeated face exposures; however, it remains unclear whether memory accuracy converges across social inhibition with additional presentations (> 7), or remains deficient. While we expect that socially inhibited individuals will reach a ceiling level with further repetitions, indicating a delay rather than deficit, future studies are needed.
In summary, social inhibition confers substantial risk for the development of anxiety disorders and is a contributor to disability within numerous psychiatric illnesses, including social anxiety disorder, schizophrenia, and autism; however, the mechanisms that underlie social inhibition remain largely unknown. The current study provides novel evidence that adults with high social inhibition show impaired memory acquisition for faces. We propose that the inability to successfully benefit from repeated exposures to a face may contribute to vulnerability for, or maintenance of, social anxiety.
We explore face recognition ability across the social inhibition continuum.
Faces are presented 1, 3, 5, or 7 times to build tiered levels of exposure.
Repeated exposure improves face recognition across all individuals.
Socially inhibited individuals show smaller improvements with repeated exposure.
Face recognition deficits may contribute to social dysfunction and impairment.
Acknowledgements
Research reported in this publication was supported in part by funding from the National Institute of Mental Health (K01-MH083052, JUB; F31-MH102008-01, SNA).
Appendix A
Karolinska faces: AF02, AF06, AF07, AF12, AF16, AF20, AF22, AF28, AF35, AM06, AM07, AM08, AM13, AM15, AM17, AM18, AM23, AM27, AM33, AM34, AM35, BF04, BF13, BF14, BF15, BF21, BF25, BF27, BF32, BF33, BM01, BM03, BM09, BM21, BM25, BM32
Gur faces: FN_003, FN_054, FN_057, FN_058, FN_064, FN_065, FN_066, FN_078, FN_079, FN_083, FN_086, FN_088, FN_089, FN_128, FN_138, FN_141, FN_165, MN_026, MN_031, MN_041, MN_092, MN_095, MN_098, MN_104, MN_105, MN_108, MN_112, MN_121, MN_124, MN_145, MN_150, MN_153, MN_155
Appendix B
b′ = b – c(a – mean(a))
where:
b = participant's regression slope
c = mean regression parameter estimate (exposures) of the sample
a = recognition for faces with one exposure
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no competing financial interests.
References Cited
- Alexander R. The evolution of social behavior. Annual review of ecology and systematics. 1974;5:325–383. [Google Scholar]
- Blackford JU, Allen AH, Cowan RL, Avery SN. Amygdala and hippocampus fail to habituate to faces in individuals with an inhibited temperament. Social cognitive and affective neuroscience. 2013;8:143–50. doi: 10.1093/scan/nsr078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlin G, Bengtsgård K, Andersson K. Social inhibition and overfriendliness as related to socioemotional functioning in 7- and 8-year-old children. Journal of clinical child psychology. 2000;29:414–23. doi: 10.1207/S15374424JCCP2903_12. [DOI] [PubMed] [Google Scholar]
- Brunet PM, Heisz JJ, Mondloch CJ, Shore DI, Schmidt LA. Shyness and face scanning in children. Journal of anxiety disorders. 2009;23:909–14. doi: 10.1016/j.janxdis.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Brunet PM, Mondloch CJ, Schmidt LA. Shy children are less sensitive to some cues to facial recognition. Child psychiatry and human development. 2010;41:1–14. doi: 10.1007/s10578-009-0150-0. [DOI] [PubMed] [Google Scholar]
- Button K, Lewis G, Penton-Voak I, Munafò M. Social anxiety is associated with general but not specific biases in emotion recognition. Psychiatry Research. 2013;210:199–207. doi: 10.1016/j.psychres.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Clauss JA, Blackford JU. Behavioral inhibition and risk for developing social anxiety disorder: a meta-analytic study. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51:1066–1075. e1. doi: 10.1016/j.jaac.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles ME, Heimberg RG. Recognition bias for critical faces in social phobia: a replication and extension. Behaviour research and therapy. 2005;43:109–20. doi: 10.1016/j.brat.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Cooney RE, Atlas LY, Joormann J, Eugene F, Gotlib IH. Amygdala activation in the processing of neutral faces in social anxiety disorder: Is neutral really neutral? Psychiatry Research: Neuroimaging. 2006;148:55–59. doi: 10.1016/j.pscychresns.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Corbett BA, Newsom C, Key AP, Qualls LR, Edmiston EK. Examining the relationship between face processing and social interaction behavior in children with and without autism spectrum disorder. Journal of neurodevelopmental disorders. 2014;6:35. doi: 10.1186/1866-1955-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Medicine. 2013;11:126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JM, McKone E, Dennett H, O'Connor KB, O'Kearney R, Palermo R. Individual differences in the ability to recognise facial identity are associated with social anxiety. PloS one. 2011;6:e28800. doi: 10.1371/journal.pone.0028800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzin F, Hou C, Norcia AM. Piecing it together: infants' neural responses to face and object structure. Journal of Vision. 2012;12:1–14. doi: 10.1167/12.13.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeleven E, De Raedt R, Leyman L, Verschuere B. The Karolinska Directed Emotional Faces: A validation study. Cognition & Emotion. 2008;22:1094–1118. [Google Scholar]
- Gur RC, Schroeder L, Turner T, McGrath C, Chan RM, Turetsky BI, Alsop D, Maldjian J, Gur RE. Brain activation during facial emotion processing. NeuroImage. 2002;16:651–62. doi: 10.1006/nimg.2002.1097. [DOI] [PubMed] [Google Scholar]
- Hagerty BM, Williams RA, Coyne JC, Early MR. Sense of belonging and indicators of social and psychological functioning. Archives of psychiatric nursing. 1996;10:235–44. doi: 10.1016/s0883-9417(96)80029-x. [DOI] [PubMed] [Google Scholar]
- Hamilton WD. The genetical evolution of social behaviour. I. Journal of theoretical biology. 1964;7:1–16. doi: 10.1016/0022-5193(64)90038-4. [DOI] [PubMed] [Google Scholar]
- Horley K, Williams LM, Gonsalvez C, Gordon E. Face to face: visual scanpath evidence for abnormal processing of facial expressions in social phobia. Psychiatry research. 2004;127:43–53. doi: 10.1016/j.psychres.2004.02.016. [DOI] [PubMed] [Google Scholar]
- Jun YY, Mareschal I, Clifford CWG, Dadds MR. Cone of direct gaze as a marker of social anxiety in males. Psychiatry Research. 2013;210:193–198. doi: 10.1016/j.psychres.2013.05.020. [DOI] [PubMed] [Google Scholar]
- Lissek S, Pine DS, Grillon C. The strong situation: a potential impediment to studying the psychobiology and pharmacology of anxiety disorders. Biological psychology. 2006;72:265–70. doi: 10.1016/j.biopsycho.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Lundh LG, Ost LG. Recognition bias for critical faces in social phobics. Behaviour research and therapy. 1996;34:787–94. doi: 10.1016/0005-7967(96)00035-6. [DOI] [PubMed] [Google Scholar]
- Lundqvist D, Flykt A, Ohman A, Lundqvist D, Flykt A, Ohman A. The Karolinska Directed Emotional Faces - KDEF, CD ROM from Department of Clinical Neuroscience, Psychology section. Karolinska Institutet; 1998. ISBN 91-630-7164-9. [Google Scholar]
- Martin F, Baudouin J-Y, Tiberghien G, Franck N. Processing emotional expression and facial identity in schizophrenia. Psychiatry Research. 2005;134:43–53. doi: 10.1016/j.psychres.2003.12.031. [DOI] [PubMed] [Google Scholar]
- McKone E, Kanwisher N, Duchaine BC. Can generic expertise explain special processing for faces? Trends in cognitive sciences. 2007;11:8–15. doi: 10.1016/j.tics.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Montagu JD. Habituation of the psycho-galvanic reflex during serial tests. Journal of psychosomatic research. 1963;52:199–214. doi: 10.1016/0022-3999(63)90004-7. [DOI] [PubMed] [Google Scholar]
- Pascalis O, Slater A. The development of face processing in infancy and early childhood: Current perspectives, Infant and Child Development. Nova Science Publishers; Hauppauge, New York: 2003. [Google Scholar]
- Pérez-López JR, Woody SR. Memory for facial expressions in social phobia. Behaviour research and therapy. 2001;39:967–75. doi: 10.1016/s0005-7967(00)00103-0. [DOI] [PubMed] [Google Scholar]
- Perlman SB, Morris JP, Vander Wyk BC, Green SR, Doyle JL, Pelphrey KA. Individual differences in personality predict how people look at faces. PloS one. 2009;4:e5952. doi: 10.1371/journal.pone.0005952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plichta MM, Grimm O, Morgen K, Mier D, Sauer C, Haddad L, Tost H, Esslinger C, Kirsch P, Schwarz AJ, Meyer-Lindenberg A. Amygdala habituation: A reliable fMRI phenotype. NeuroImage. 2014 doi: 10.1016/j.neuroimage.2014.09.059. [DOI] [PubMed] [Google Scholar]
- Rankin CH, Abrams T, Barry RJ, Bhatnagar S, Clayton DF, Colombo J, Coppola G, Geyer MA, Glanzman DL, Marsland S, McSweeney FK, Wilson DA, Wu C-FF, Thompson RF. Habituation revisited: An updated and revised description of the behavioral characteristics of habituation. Neurobiology of Learning and Memory. 2009;92:135–138. doi: 10.1016/j.nlm.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznick JS, Hegeman IM, Kaufman ER, Woods SW, Jacobs M. Retrospective and concurrent self-report of behavioral inhibition and their relation to adult mental health. Development and. 1992;4:301–321. [Google Scholar]
- Richler JJ, Cheung OS, Gauthier I. Holistic processing predicts face recognition. Psychological science. 2011;22:464–71. doi: 10.1177/0956797611401753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrbacher H, Hoyer J, Beesdo K, Höfler M, Bittner A, Lieb R, Wittchen H. Psychometric properties of the Retrospective Self Report of Inhibition (RSRI) in a representative German sample. International journal of methods in psychiatric research. 2008;17:80–8. doi: 10.1002/mpr.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbart MK, Ahadi SA, Evans DE. Temperament and personality: origins and outcomes. Journal of personality and social psychology. 2000;78:122–35. doi: 10.1037//0022-3514.78.1.122. [DOI] [PubMed] [Google Scholar]
- Schneier FR, Heckelman LR, Garfinkel R, Campeas R, Fallon BA, Gitow A, Street L, Del Bene D, Liebowitz MR. Functional impairment in social phobia. The Journal of clinical psychiatry. 1994;55:322–31. [PubMed] [Google Scholar]
- Schwartz CE, Kunwar PS, Greve DN, Kagan J, Snidman NC, Bloch RB. A phenotype of early infancy predicts reactivity of the amygdala in male adults. Molecular Psychiatry. 2012;17:1042–50. doi: 10.1038/mp.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman PW. Nepotism and the evolution of alarm calls. Science (New York, N.Y.) 1977;197:1246–53. doi: 10.1126/science.197.4310.1246. [DOI] [PubMed] [Google Scholar]
- Skuse DH, Lori A, Cubells JF, Lee I, Conneely KN, Puura K, Lehtimäki T, Binder EB, Young LJ. Common polymorphism in the oxytocin receptor gene (OXTR) is associated with human social recognition skills. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:1987–92. doi: 10.1073/pnas.1302985111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao DY, Freiwald WA, Tootell RBH, Livingstone MS. A cortical region consisting entirely of face-selective cells. Science. 2006;311:670–4. doi: 10.1126/science.1119983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao DY, Livingstone MS. Mechanisms of face perception. Annual review of neuroscience. 2008;31:411–437. doi: 10.1146/annurev.neuro.30.051606.094238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigelt S, Koldewyn K, Kanwisher N. Face identity recognition in autism spectrum disorders: a review of behavioral studies. Neuroscience and Biobehavioral Reviews. 2012;36:1060–84. doi: 10.1016/j.neubiorev.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Wilmer JB, Germine L, Chabris CF, Chatterjee G, Williams M, Loken E, Nakayama K, Duchaine B. Human face recognition ability is specific and highly heritable. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:5238–41. doi: 10.1073/pnas.0913053107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittchen H-U, Fehm L. Epidemiology and natural course of social fears and social phobia. Acta psychiatrica Scandinavica. 2003;(Supplementum):4–18. doi: 10.1034/j.1600-0447.108.s417.1.x. [DOI] [PubMed] [Google Scholar]
- Yardley L, McDermott L, Pisarski S, Duchaine B, Nakayama K. Psychosocial consequences of developmental prosopagnosia: a problem of recognition. Journal of Psychosomatic Research. 2008;65:445–51. doi: 10.1016/j.jpsychores.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Yoon KL, Zinbarg RE. Interpreting neutral faces as threatening is a default mode for socially anxious individuals. Journal of abnormal psychology. 2008;117:680–5. doi: 10.1037/0021-843X.117.3.680. [DOI] [PubMed] [Google Scholar]
- Zhu Q, Song Y, Hu S, Li X, Tian M, Zhen Z, Dong Q, Kanwisher N, Liu J. Heritability of the specific cognitive ability of face perception. Current biology : CB. 2010;20:137–42. doi: 10.1016/j.cub.2009.11.067. [DOI] [PubMed] [Google Scholar]