Significance
Parkinson’s disease (PD) is symptomatically treated with levodopa (L-DOPA), but this treatment often results in disabling dyskinesias. Subchronic L-DOPA increases striatal levels of adaptor protein, p11. Using experimental mouse models of Parkinsonism, we report here that global p11 knockout mice show reduced therapeutic responses to L-DOPA on rotational motor sensitization, but also develop less L-DOPA–induced dyskinesias. Mice lacking p11 in dopamine D2R-containing neurons have reduced response to L-DOPA on the therapeutic parameters, but develop dyskinetic side effects. In contrast, mice lacking p11 in dopamine D1R-containing neurons exhibit rotational responses toward L-DOPA, but develop less dyskinesia. These results imply that reductions of p11 in D1R-containing cells may be a viable therapy against L-DOPA–induced dyskinesias.
Keywords: S100A10, dopamine, 6-hydroxydopamine, tremor, tacrine
Abstract
The reduced movement repertoire of Parkinson’s disease (PD) is mainly due to degeneration of nigrostriatal dopamine neurons. Restoration of dopamine transmission by levodopa (L-DOPA) relieves motor symptoms of PD but often causes disabling dyskinesias. Subchronic L-DOPA increases levels of adaptor protein p11 (S100A10) in dopaminoceptive neurons of the striatum. Using experimental mouse models of Parkinsonism, we report here that global p11 knockout (KO) mice develop fewer jaw tremors in response to tacrine. Following L-DOPA, global p11KO mice show reduced therapeutic responses on rotational motor sensitization, but also develop less dyskinetic side effects. Studies using conditional p11KO mice reveal that distinct cell populations mediate these therapeutic and side effects. Selective deletion of p11 in cholinergic acetyltransferase (ChAT) neurons reduces tacrine-induced tremor. Mice lacking p11 in dopamine D2R-containing neurons have a reduced response to L-DOPA on the therapeutic parameters, but develop dyskinetic side effects. In contrast, mice lacking p11 in dopamine D1R-containing neurons exhibit tremor and rotational responses toward L-DOPA, but develop less dyskinesia. Moreover, coadministration of rapamycin with L-DOPA counteracts L-DOPA–induced dyskinesias in wild-type mice, but not in mice lacking p11 in D1R-containing neurons. 6-OHDA lesioning causes an increase of evoked striatal glutamate release in wild type, but not in global p11KO mice, indicating that altered glutamate neurotransmission could contribute to the reduced L-DOPA responsivity. These data demonstrate that p11 located in ChAT or D2R-containing neurons is involved in regulating therapeutic actions in experimental PD, whereas p11 in D1R-containing neurons underlies the development of L-DOPA–induced dyskinesias.
Parkinson’s disease (PD) is characterized by a progressive degeneration of dopaminergic neurons projecting from substantia nigra pars compacta (SNc) to striatum, eventually resulting in bradykinesia, rigidity, resting tremor, and postural imbalance (1). Dopamine replacement strategies are effective for many motor symptoms. At early stages, MAO-B inhibitors or dopamine D2 receptor agonists may provide sufficient symptomatic relief, but as the disease progresses essentially all patients will require treatment with levodopa (L-DOPA) (1). Following decarboxylation, L-DOPA is converted to dopamine acting on both dopamine D1 and D2 receptors. D1 and D2 receptors are segregated in striatonigral and striatopallidal pathway neurons, respectively (2). Activation of D1 and D2 receptors causes synergistic stimulation of locomotion, explaining the stronger anti-Parkinsonian action of L-DOPA compared with selective D2 receptor agonists (1, 2). Whereas dopaminergic agents often successfully treat bradykinesia and rigidity in PD, additional therapy with anticholinergic agents is sometimes required for optimal treatment of resting tremor (1). Moreover, the therapeutic effect of L-DOPA is gradually shortened as disabling dyskinetic side effects emerge (3). There is no licensed treatment against L-DOPA–induced dyskinesias (LIDs).
Targeting the serotonergic, glutamatergic, and/or cholinergic systems have been reported to counteract LIDs (3). p11 (i.e., S100A10) is a member of the S100 EF-hand protein family, which increase the levels of distinct serotonin (5-HT1BR and 5-HT4R) and glutamate (mGluR5) receptors at the cell surface, resulting in enhanced effects on cell signaling via these receptors (4, 5). p11 is widely expressed in the brain with particularly high levels in cholinergic neurons (6–9). p11 is regulated by a variety of stimuli and therapies, most notably antidepressants (4). p11 is strongly up-regulated in the striatum following repeated treatment with L-DOPA in the 6-OHDA–lesioning model of experimental parkinsonism (10). p11 can therefore influence several pathways implicated in LIDs.
In the present study, we examined global and cell-specific conditional p11 knockout (KO) mice in experimental Parkinsonism models to identify the brain circuitries that mediate p11-dependent therapeutic effects of L-DOPA as well as LIDs.
Results
Conditional Deletion of p11 from Distinct Cell Populations.
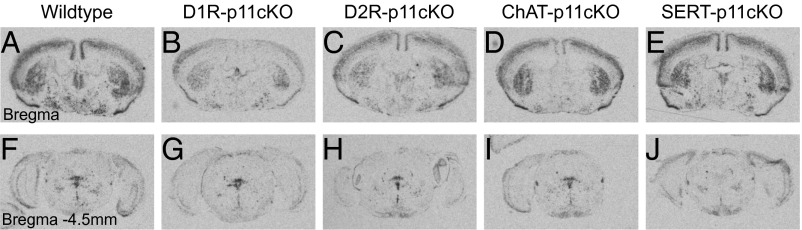
As illustrated in Fig. 1 from the bregma levels 0 and −4.5 mm, p11 mRNA is widely expressed in the brain (6). Conditional knockout of p11 in D1R- or D2R-containing neurons led to significant (P < 0.001) reductions (−64 and −58%, respectively) of p11 mRNA in the striatum (Fig. 1 B and C). No significant changes of striatal p11 mRNA were found in mice with conditional knockout of p11 in choline acetyltransferase (ChAT) or serotonin transporter (SERT)-expressing neurons (Fig. 1 D and E). Conditional knockout of p11 in D1R-containing neurons also led to a significant (P < 0.01) reduction (−60%) of p11mRNA in the cortex (Fig. 1B). Mice with conditional knockout of p11 in ChAT-expressing neurons showed lower expression of p11 mRNA in basal forebrain structures including substantia innominata (Fig. 1D). At the pontine level (bregma −4.5 mm), no significant changes of p11 mRNA were found in mice with conditional knockout of p11 in D1R-, D2R-, or ChAT-expressing neurons (Fig. 1 G, H, and I). p11 plays an important role in modulating serotonin neurotransmission (4), and conditional knockout of p11 in SERT-expressing neurons abolishes (−100%; P < 0.001) p11 mRNA expression in the raphe nuclei (Fig. 1J). In the following experiments, the phenotype of global p11KO mice was compared with that of mice with conditional knockout of p11 in the aforementioned cellular populations, hereafter referred to as D1R-p11cKO, D2R-p11cKO, ChAT-p11cKO, and SERT-p11cKO mice, respectively.
Fig. 1.
p11 mRNA expression in wild-type, D1R-p11cKO, D2R-p11cKO, ChAT-p11cKO, and SERT-p11cKO mice. In situ hybridization to detect p11 mRNA from sections at (A–E) bregma level 0 and at (F–J) bregma level −4.5 mm. (A and F) Wild-type mice show a widespread distribution of p11 mRNA. Reductions of p11 mRNA are found in striatum in (B) D1R-p11cKO and (C) D2R-p11cKO mice, cortex in (B) D1R-p11cKO mice, basal forebrain in (D) ChAT-p11cKO mice, and the raphe nuclei in (J) SERT-p11cKO mice. The number of mice per group was three. The experiment was repeated twice.
Tacrine-Induced Jaw Tremor Movements Involve p11 in ChAT-Containing Neurons.
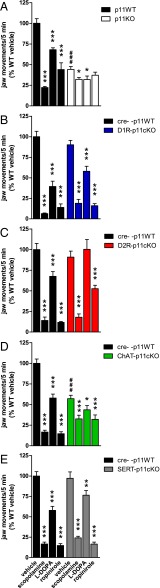
Treatment with the cholinesterase inhibitor tacrine induces jaw movements with a frequency between 3 and 7 Hz, i.e., in the same range as human parkinsonian resting tremor (1, 11). Because tacrine-induced jaw movements are diminished by several anti-Parkinsonian treatments, it is used to model aspects of Parkinsonian tremor (11). As shown in Fig. 2, both baseline alterations and responsivity to antitremor treatments were compared between WT cohorts and global p11KO as well as conditional p11KO mouse lines. There were significantly (P < 0.001) reduced tremorgenic effects of tacrine in global p11KO mice (Fig. 2A) and in ChAT-p11cKO (Fig. 2D) compared with WT littermates. The responses to tacrine per se were not altered in D1R-p11cKO, D2R-p11cKO, or SERT-p11cKO mice (P = 0.14, P = 0.31, P = 0.65, respectively) (Fig. 2 B, C, and E).
Fig. 2.
Jaw movement tremors in response to tacrine along with vehicle, scopolamine, L-DOPA, and ropinirole in wild-type, global, and conditional p11KO mice. (A–E) Jaw tremor was induced by tacrine (2.5 mg/kg, i.p.) in all wild-type (WT) cohorts, and this effect was potently inhibited by scopolamine (1 mg/kg, i.p.), L-DOPA/benserazide (10/7.5 mg/kg, i.p.), and ropinirole (3 mg/kg, i.p.). (A and D) Tacrine per se induced fewer jaw movements in p11KO mice and ChAT-p11cKO mice. (C) L-DOPA did not reduce tacrine-induced tremor in D2R-p11cKO mice. (B and E) D1-p11cKO and SERT-p11cKO mice showed similar tremor responses as WT mice toward tacrine and antitremor drugs. Values are expressed as percentage of jaw movements in tacrine+vehicle-treated WT mice. The number of mice per group was 8–13. Two-way ANOVA followed by Fisher’s LSD post hoc test: *P < 0.05, **P < 0.01, ***P < 0.001 versus corresponding vehicle; ###P < 0.001 versus corresponding WT.
As expected, scopolamine (a muscarine receptor antagonist), L-DOPA, and ropinirole (a D2/3R agonist) significantly (P < 0.001) reduced tremors in all WT cohorts (Fig. 2 A–E). Global p11KO mice did not respond to ropinirole (P = 0.25), but had small, yet significant, responses to scopolamine (P = 0.046) and L-DOPA (P = 0.03) (Fig. 2A). L-DOPA did not (P = 0.30) counteract tremor in D2R-p11cKO mice (Fig. 2C). There was also a comparably smaller response to ropinirole, but not scopolamine, in these mice (Fig. 2C). D1R-p11cKO, ChAT-p11cKO, and SERT-p11cKO mice responded significantly to scopolamine (P < 0.001 in all lines), L-DOPA (P < 0.001, P = 0.04, P = 0.003, respectively) and ropinirole (P < 0.001 in all lines) (Fig. 2 B, D, and E).
Contralateral Rotations in Response to L-DOPA Involve p11 Expressed in D2R-Containing Neurons.
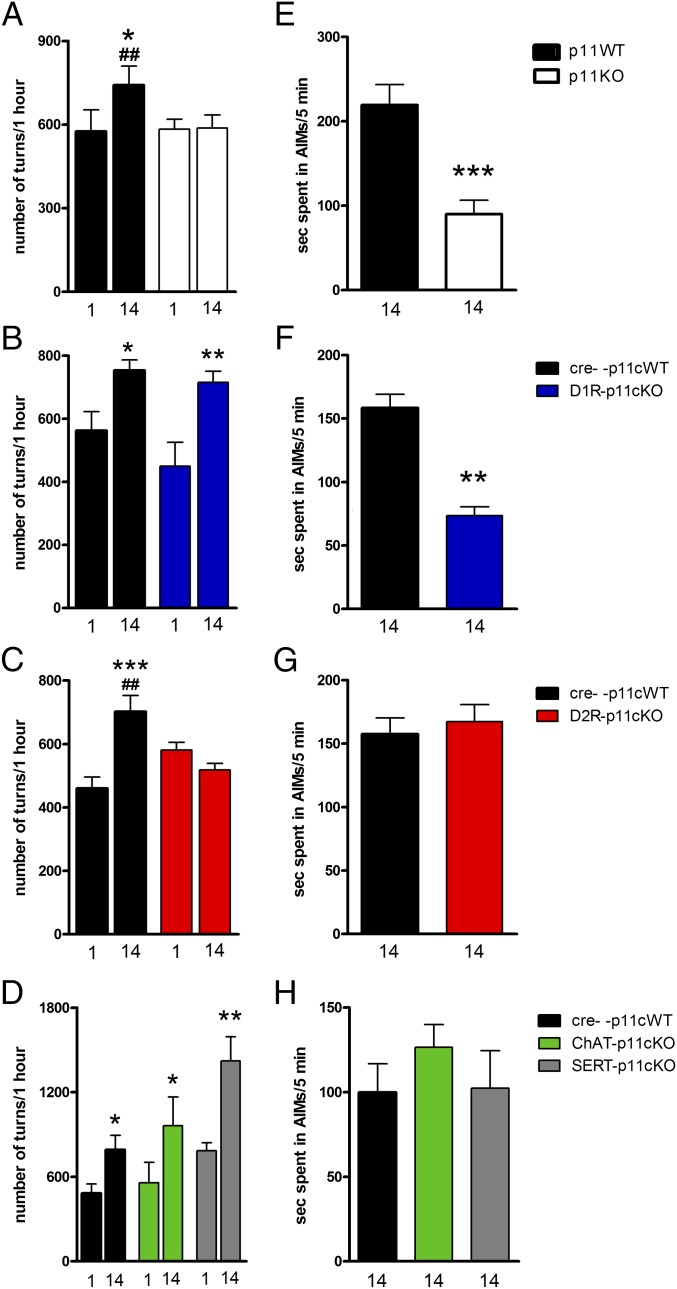
Contralateral rotations in unilaterally 6-OHDA–lesioned mice are commonly used to assess the anti-parkinsonian potential of compounds (12, 13). The contralateral turning response to L-DOPA sensitizes upon repeated administration (13). Two weeks of treatment with L-DOPA (10 mg/kg) caused a significant (F2,12 = 9.71; P = 0.0003) sensitization of contralateral rotation in WT mice (P = 0.01), but not in global p11KO mice (Fig. 3A). Accordingly, a single injection of L-DOPA caused a similar rotational response in WT mice and global p11KO mice, but following repeated L-DOPA (10 mg/kg) treatment, WT mice rotated significantly (P = 0.003) more than p11KO mice (Fig. 3A). Likewise, subsequent treatment with an escalated dose of L-DOPA (50 mg/kg) for another week significantly (F2,12 = 17.1; P < 0.0001) increased contralateral rotations in WT mice (P = 0.04) but not in p11KO mice (P = 0.96), and there was a significant (P = 0.0008) difference between WT and p11KO mice (Fig. S1A).
Fig. 3.
L-DOPA–induced contralateral turning and AIMs in unilaterally 6-OHDA–lesioned wild-type, global, and conditional p11KO mice. Mice were treated once daily with L-DOPA/benserazide (10/7.5 mg/kg, i.p.) for 2 wk. Contralateral rotations were assessed at days 1 and 14, and AIMs at day 14. (A–D) L-DOPA induced significant sensitization of rotations in WT cohorts, (B) D1R-p11cKO and (D) ChAT-p11cKO and SERT-p11cKO mice, but not in (A) global p11KO or (C) D2R-p11cKO mice. (E–H) L-DOPA induced AIMs in all WT cohorts. There were significantly fewer AIMs in (E) global p11KO and (F) D1R-p11cKO mice, but not in (G) D2R-p11cKO or (H) ChAT-p11cKO or SERT-p11cKO mice. (A–D) Values are presented as the number of contralateral rotations in 1 h. Two-way ANOVA followed by Fisher’s LSD post hoc test: *P < 0.05, **P < 0.01, ***P < 0.001 versus L-DOPA day 1; ##P < 0.01 versus corresponding KO. (E–H) Values indicate seconds in AIMs during a 5-min period. The number of mice per group was three to eight. One-way ANOVA or two-tailed Student t test: **P < 0.01, ***P < 0.001 versus WT.
Fig. S1.
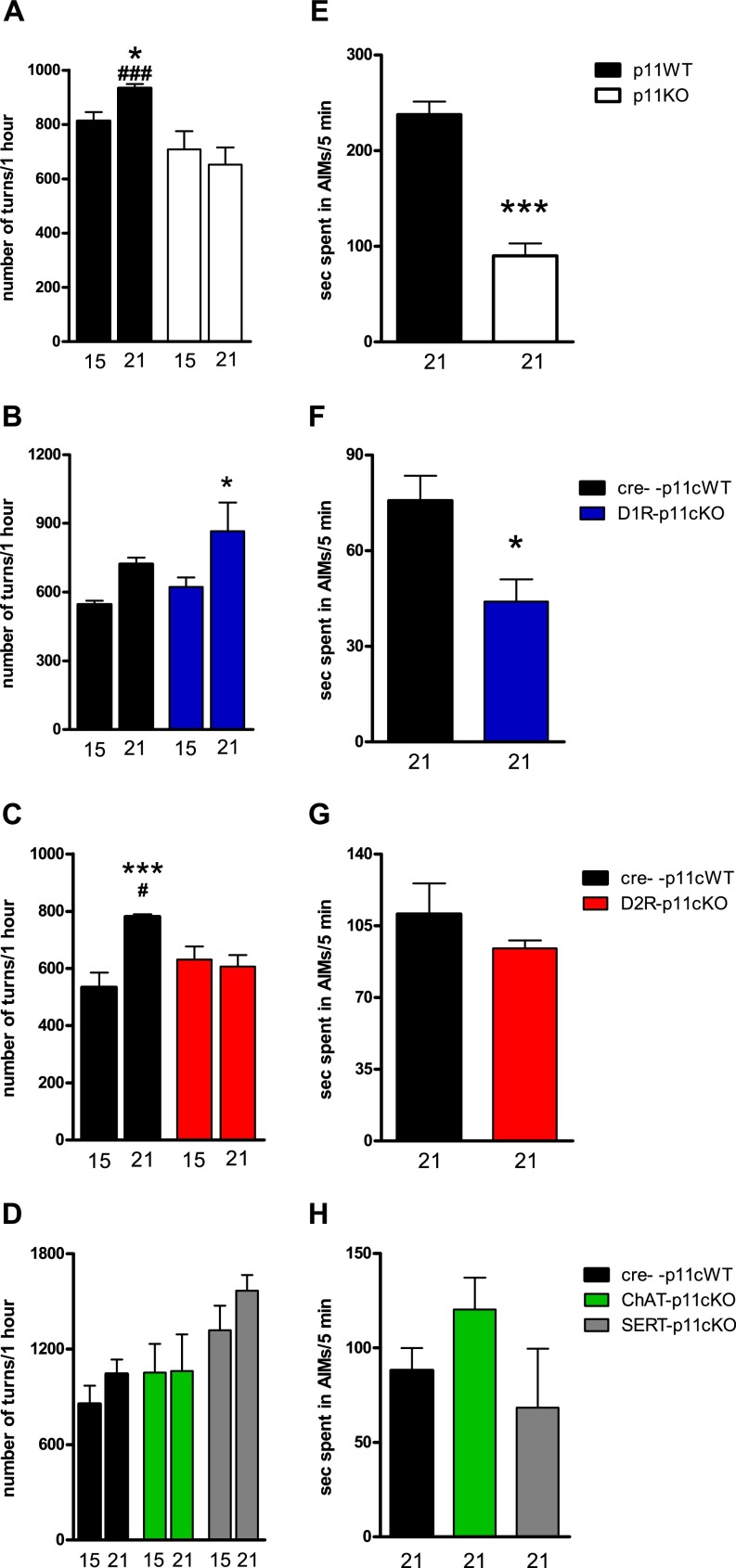
L-DOPA (50 mg/kg)-induced contralateral turning and AIMs in unilaterally 6-OHDA–lesioned wild-type, global, and conditional p11KO mice. Subsequent to the 2-wk treatment with 10/7.5 mg/kg of L-DOPA/benserazide, mice received an escalated dose (50/12.5 mg/kg i.p., once daily) for another week. Contralateral rotations were assessed at days 15 and 21, and AIMs at day 21. (A–D) High-dose L-DOPA continued to induce significant sensitization of rotations in some (A and C) WT cohorts and (B) D1R-p11cKO mice, but not in other (B and D) WT cohorts, (A) global p11KO mice, (C) D2R-p11cKO mice, or (D) ChAT-p11cKO or SERT-p11cKO mice. (E–H) High-dose L-DOPA–induced AIMs were significantly lower in (E) global p11KO and (F) D1R-p11cKO mice, but not in (G) D2R-p11cKO or (H) ChAT-p11cKO or SERT-p11cKO mice. (A–D) Values are presented as the number of contralateral rotations in 1 h. The number of mice per group was three to eight. Two-way ANOVA followed by LSD post hoc test: *P < 0.05, ***P < 0.001 versus L-DOPA day 1; ###P < 0.001 versus corresponding KO. (E–H) Values indicate seconds in AIMs during a 5-min period. One-way ANOVA or two-tailed Student t test; *P < 0.05, ***P < 0.001 versus WT.
Similar to global p11KO mice, D2R-p11cKO mice did not (P = 0.31) develop sensitization of contralateral rotations to repeated L-DOPA (10 mg/kg) (Fig. 3C). They differed significantly (P = 0.008) from WT littermates that develop sensitization toward L-DOPA (Fig. 3C). Even at 50 mg/kg of L-DOPA, no (P = 0.69) sensitization in D2R-p11cKO mice occurred, and they continued to differ significantly (P = 0.01) from WT littermates (Fig. S1C). The fact that global p11KO and D2R-p11cKO exhibit significantly less sensitization of contralateral rotations compared with WT at both L-DOPA doses demonstrates that this phenotype cannot be explained only by a shifted dose–response to L-DOPA.
In contrast to global p11KO and D2R-p11cKO mice, D1R-p11cKO, ChAT-p11cKO, and SERT-p11cKO developed sensitization of contralateral rotations to repeated L-DOPA (10 mg/kg) (P = 0.04, P = 0.04, and P = 0.002) (Fig. 3 B and D). Upon repeated treatment with 50 mg/kg of L-DOPA, D1R-p11cKO mice continued to develop sensitization (P = 0.04) (Fig. S1B). No further sensitization toward 50 mg/kg of L-DOPA occurred in the WT cohort matched with ChAT-p11cKO and SERT-p11cKO (Fig. S1D), making it difficult to interpret the lack of continued sensitization found in ChAT-p11cKO and SERT-p11cKO mice following the higher dose of L-DOPA (Fig. S1D).
L-DOPA–Induced Abnormal Involuntary Movements Involve p11 Expressed in D1R-Containing Neurons.
Abnormal involuntary movements (AIMs) in rodents have been used as a model of LIDs in human PD (14). Here, we used 6-OHDA–lesioned mice and a modified protocol starting with a clinically meaningful dose, 10 mg/kg, of L-DOPA for 14 d followed by an escalation to 50 mg/kg for 1 wk. AIMs were assessed at days 14 and 21. Repeated treatment with L-DOPA, both at 10 and 50 mg/kg, resulted in AIMs in WT mice, which were significantly (P = 0.0006 and P = 0.0001, respectively) lowered in global p11KO mice (Fig. 3A and Fig. S1A). This was reproduced by D1R-p11cKO mice, which developed significantly (P = 0.003 and P = 0.04, respectively) fewer AIMs in response to L-DOPA (10 and 50 mg/kg) compared with their WT littermates (Fig. 3B and Fig. S1B).
In contrast, the development of AIMs in D2R-p11cKO, ChAT-p11cKO, and SERT-p11cKO mice did not differ from WT mice following L-DOPA at 10 mg/kg (P = 0.62 and F2,15 = 0.62; P = 0.55, respectively) or 50 mg/kg (P = 0.39 and F2,15 = 1.90; P = 0.19, respectively) (Fig. 3 C and D; Fig. S1 C and D).
p11 Is Required for the Inhibitory Influence of Rapamycin on L-DOPA–Induced AIMs.
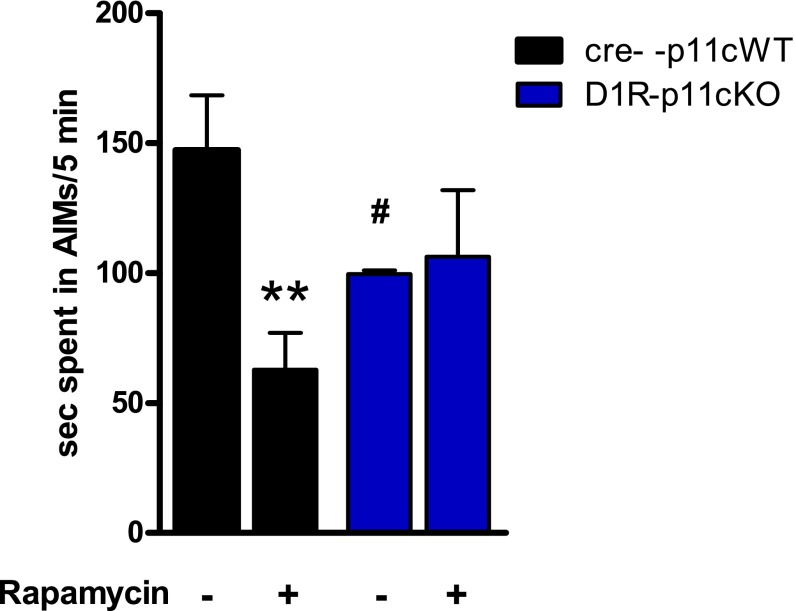
To explore one potential mechanism whereby p11 may modulate L-DOPA–induced AIMs in D1R-expressing neurons, we studied the role of p11 in a pharmacological intervention known to occur through striatonigral neurons, namely rapamycin inhibition of AIMs (15). In agreement with our previous experiments, we found that D1R-p11cKO mice developed significantly (P = 0.04) fewer AIMs toward L-DOPA than the p11 WT mice (Fig. 4). Rapamycin significantly (P = 0.001) counteracted L-DOPA–induced AIMs in WT mice, but not in D1R-p11cKO mice (P = 0.80) (Fig. 4).
Fig. 4.
Effects of rapamycin on L-DOPA–induced AIMs in unilaterally 6-OHDA–lesioned WT and D1R-p11cKO mice. Mice were treated once daily with L-DOPA/benserazide (10/7.5 mg/kg, i.p.) together with vehicle or rapamycin (5 mg/kg, i.p.) for 10 d. D1R-p11cKO mice developed fewer AIMs than WT mice. Rapamycin reduced AIMs in WT mice, but not in D1R-p11cKO mice. Values indicate seconds in AIMs during a 5-min period. The number of mice per group was three to six. Two-way ANOVA followed by Fisher’s LSD post hoc test: **P < 0.01 versus L-DOPA alone; #P < 0.05 versus WT and L-DOPA alone.
Evoked Glutamate Release Induced by 6-OHDA Lesioning Is Counteracted in p11KO Mice.
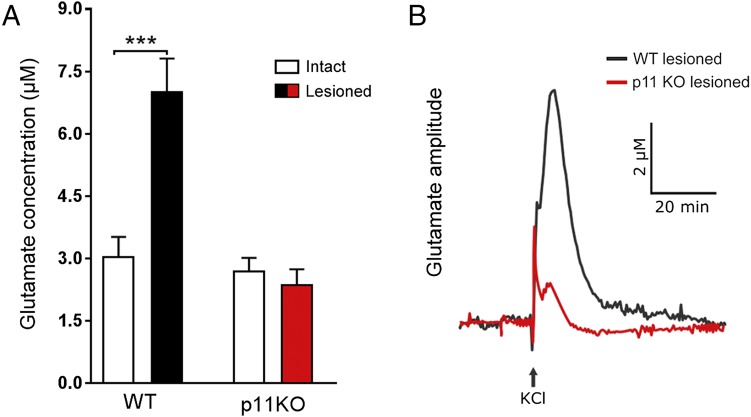
Because striatal glutamate influences responses to dopamine replacement therapies (2, 16), we examined interactions between p11 and the glutamate system. Electrophysiological (16) and neurochemical (17) studies have demonstrated that degeneration of nigrostriatal dopaminergic neurons leads to excess of corticostriatal glutamatergic activity in the striatum. In agreement with these data, we found that 6-OHDA lesioning significantly (P < 0.0001) increased evoked glutamate release in striatum in WT mice (Fig. 5). However, this induction of evoked glutamate release was not found (P = 0.76) in p11KO mice (Fig. 5). It is therefore possible that a reduced ability to evoke glutamate release in p11KO mice underlies some of the lowered responses to L-DOPA in these mice.
Fig. 5.
Evoked glutamate release in intact and 6-OHDA–lesioned hemispheres of wild-type and p11KO mice. Recordings of potassium chloride (KCl; 70 mM)-evoked glutamate release amplitudes in the dorsal striatum of anesthetized unilaterally 6-OHDA–lesioned wild-type (WT) and p11KO mice. (A) The glutamate concentration is increased in the 6-OHDA–lesioned hemisphere in WT, but not p11KO, mice. (B) Representative evoked glutamate peaks from 6-OHDA–lesioned striata of WT and p11KO mice. The number of mice per group was 7–12. Two-way ANOVA followed by Fisher’s LSD post hoc test: ***P < 0.001 versus intact hemisphere.
Discussion
We report here that p11 affects experimental PD and L-DOPA responses via different cell types. In accordance with the high levels of p11 in cholinergic neurons, we found that global p11KO mice develop less jaw tremors in response to tacrine. This beneficial response was mimicked by selective deletion of p11 in ChAT neurons. These data indicate that lowered levels of p11 per se may result in some anti-Parkinsonian effects. Interestingly, in the PNAS paper by Marongiu et al. (18), it is shown that viral-mediated reduction of p11 from dorsal striatum has anti-parkinsonian effects on gait and locomotion. Taken together, the data indicate that p11 in cholinergic neurons from the dorsal striatum may produce symptoms of PD. Moreover, these behavioral responses provide further evidence of an important role of p11 in cholinergic neurons and add to the previous findings that a depressive-like behavior is generated when p11 is deleted from cholinergic neurons of the ventral striatum (i.e., nucleus accumbens) (7, 8).
L-DOPA is very potent in alleviating parkinsonian bradykinesia and fine-movement dysfunctions along with a modest action against resting tremor (1). The reduced L-DOPA–induced sensitization of contralateral rotations in unilaterally 6-OHDA–lesioned mice found in global p11KO mice was reproduced in D2R-p11cKO mice. There was also a reduced tremor response to L-DOPA in D2R-p11cKO mice. However, the initial anti-akinetic response to L-DOPA was not significantly affected in global p11KO or D2R-p11cKO mice. The first exposure of single housing and measurement of rotational response to L-DOPA involve significant novelty and stress factors. p11KO mice may be more reactive than wild-type mice to such factors overshadowing a reduced L-DOPA responsivity. Nonetheless, our data indicate that D2R-containing cells are critical in mediating certain p11-dependent therapeutic responses to L-DOPA. Interestingly, the L-DOPA–induced jaw tremor and rotational sensitization were intact in D1R-p11cKO mice. In this context, it is noteworthy that there are several selective D2R agonists used to treat PD in the clinics (1), but no selective D1R agonists have proven beneficial in this regard.
AIMs developed in D2R-p11cKO mice, but not in global p11KO or D1R-p11 cKO mice. Thus, whereas no therapeutic effects of L-DOPA involve p11 in D1R-containing cells, disabling dyskinesias are generated from the D1R-containing cell population. There is a lack of positive correlations between sensitization of contralateral rotations and AIMs in global p11KO, D2R-p11cKO, and D1R-p11 cKO mice. In a previous study (10), where the primary objective was to examine the antidyskinetic effect of a 5-HT1BR agonist in wild-type and p11KO mice, we administered a high dose of L-DOPA (50 mg/kg) and studied dyskinesias after 1 wk. Using this protocol, we could demonstrate a reduced antidyskinetic effect of the 5-HT1BR agonist in p11 KO mice, but we found no obvious difference in the occurrence of L-DOPA–induced AIMs between wild-type and p11KO mice. Here, we used an improved protocol with escalating L-DOPA dosing, starting with a clinically meaningful dose, and prolonged treatment durations. These improvements in experimental design may explain the differences in AIM scores between our current and previous data. To further examine the role of D1R-containing cells in the development of AIMs and to obtain some mechanistic insight, we coadministered L-DOPA with vehicle or rapamycin, an mTOR inhibitor. Rapamycin blocks L-DOPA–induced mTOR/Akt/Rhes signaling in D1R-containing cells and thereby counteracts AIMs (15, 19). The fact that rapamycin was inefficient in counteracting AIMs in D1R-p11cKO mice indicates that interference with mTOR/Akt/Rhes signaling may underlie p11-dependent modulation of AIMs.
It is possible that p11 modulates L-DOPA responses by altering serotonin and glutamate neurotransmission. p11 facilitates signal transduction via serotonin 5-HT1BR and 5-HT4R and glutamate mGluR5 (4, 5). Preclinical studies (10, 20) have shown that 5-HT1BR agonism counteracts L-DOPA responses via inhibition of release of “false dopamine” from serotonin terminals (20) and by counteracting D1-mediated signaling in striatonigral neurons (10). In a previous (10) and an accompanying paper by Marongiuet al. (18), we have shown that 5-HT1BR agonist-mediated reduction of L-DOPA–induced AIMs involves p11. However, lowered signaling via 5-HT1BR receptors cannot explain the reduced AIMs in p11KO mice, because it would be expected to result in an increase, rather than the observed decrease, of AIMs. Instead, our combined results indicate that reduced glutamatergic neurotransmission via mGluR5 may underlie the lowered development of AIMs in p11KO and D1R-p11cKO mice. This is consistent with preclinical studies showing that antagonism of mGluR5 counteracts AIMs in both rodent and monkey models of parkinsonism (21). Our observation that evoked glutamate release is reduced in p11KO mice provides additional support for a blunted glutamatergic neurotransmission in these mice. Because both 5-HT1A/BR agonists and mGluR5 antagonists have shown antidyskinetic properties in PD patients with LIDs (22, 23), it will be important to further delineate the pathways whereby p11 influences serotonergic and glutamatergic mechanisms underlying LIDs.
It has been suggested that similar mechanisms underlie both L-DOPA–induced locomotor sensitization and LIDs (e.g., 24). However, here we demonstrate that the p11-dependent sensitization of L-DOPA–induced contralateral rotations involves a D2R-containing cell population that is distinct from a D1R-containing population that generates LIDs. These results imply that reductions of p11 in D1R-containing cells, for example, by viral-mediated gene transfer (18), may be a viable therapy against LIDs.
Materials and Methods
Wild-Type, Global, and Conditional p11KO Mice.
Adult global p11KO mice and WT littermates were used (6). Conditional p11KO mice were generated by flanking exon 2 (containing ATG) of the p11 gene with LoxP sites. Floxed p11 mice were bred with BAC transgenic mice expressing CRE under the promotors of D1R, D2R, ChAT, or SERT. The generated D1R-p11cKO, D2R-p11cKO, ChAT-p11cKO, and SERT-p11cKO were used in the studies. All experiments were performed in agreement with the European Communities Council (86/609/ECC) and approved by the ethics committee at the Karolinska Institute.
Unilateral 6-OHDA Lesioning.
For 6-OHDA lesioning, mice were anesthetized with 80 mg/kg ketamine i.p. (Parke-Davis) and 5 mg/kg xylazine i.p. (Bayer), pretreated with 25 mg/kg desipramine i.p. (Sigma-Aldrich) and 5 mg/kg pargyline i.p. (Sigma-Aldrich), placed in a stereotaxic frame, and injected, over 2 min, with 3 μg of 6-OHDA in 0.01% ascorbate (Sigma-Aldrich) into the median forebrain bundle of the right hemisphere. The coordinates for injection were anteroposterior (AP), −1.1 mm; mediolateral (ML), −1.1 mm; and dorsoventral (DV), −4.75 mm relative to bregma and the dural surface. Two weeks after unilateral 6-OHDA lesioning, rodents were administered 1 mg/kg apomorphine i.p (Sigma-Aldrich). Only mice rotating >50 turns per 30 min were included in further experiments.
In Situ Hybridization.
To detect p11 mRNA, a radioactive riboprobe in situ hybridization experiment was carried out as described previously (6). Coronal sections at the bregma levels 0 and −4.5 mm were exposed on Kodak MR films. These films were digitized (EPSON), and the optical density values of p11 mRNA in striatum, cortex, and raphe nuclei were measured using Image J (NIH).
Tacrine-Induced Jaw Movements.
To assess jaw movement in 3–7 Hz tremors, mice were treated with tacrine (2.5 mg/kg i.p.; Sigma). After 20 min, mice were treated with vehicle (saline), scopolamine (1 mg/kg i.p.; Sigma-Aldrich), L-DOPA/benzerazide (10/7.5 mg/kg i.p.; Sigma-Aldrich), or ropinirole (3 mg/kg i.p.; Sigma-Aldrich). Mice were then placed in a clean cage situated in an elevated position to allow complete observation of the experimental animal. After 10 min of habituation, tremulous jaw movements—defined as vertical and purposeless deflections of the lower jaw (11)—were manually scored for 5 min. Yawns, tongue protrusions, and stereotypes, such as grooming, were not included in the evaluation.
Rotational Testing.
Contralateral rotational turns were examined in unilaterally 6-OHDA–lesioned mice treated with 10/7.5 mg/kg L-DOPA/benserazide (i.p., once daily) for 2 wk followed by 50/12.5 mg/kg L-DOPA/benserazide (i.p., once daily) for another week. At days 1, 14, 15, and 21 the mice were placed individually in clean cages, and their number of contralateral rotations were counted for 60 min.
AIMs.
AIMs were studied in unilaterally 6-OHDA–lesioned mice treated with 10/7.5 mg/kg L-DOPA/benserazide (i.p., once daily) for 2 wk followed by 50/12.5 mg/kg L-DOPA/benserazide (i.p., once daily) for another week. Immediately after the quantification of rotational behaviors on days 14 and 21, the incidence of AIMs, classified into forelimb, orofacial, axial, and locomotive behaviors, were quantified for 5 min (14). In a separate experiment, unilaterally 6-OHDA–lesioned WT and D1R-p11cKO mice were treated with 10/7.5 mg/kg L-DOPA/benserazide (i.p., once daily) together with vehicle or 5 mg/kg of rapamycin (i.p., once daily) for 10 d. The AIMs were then quantified as mentioned above.
Fast Analytical Sensing Technology Measurements of Evoked Glutamate Release.
Unilaterally 6-OHDA–lesioned mice were anesthetized with isoflurane (3% for induction, 0.7–1% for maintenance) and mounted in a stereotaxic frame (David Kopf Instruments) fitted with a Cunningham mouse adapter (Stoelting Co.). Small cranial windows were drilled over the striata (AP: +1.3; ML: ±1.5; DV: −2.5 versus bregma), and microelectrode arrays (MEAs) were inserted into dorsal striatum of the intact and dopamine-depleted hemispheres. As previously described (17, 25), glutamate dynamics were assessed on a subsecond timescale by MEA recordings with two of four electrode sites coated with l-glutamate oxidase enzyme, which breaks down l-glutamate into α-ketoglutarate and peroxide (H2O2). By using constant voltage amperometry with application of a fixed potential, the H2O2 was oxidized, with electron loss, and the resulting current was recorded using a Fast Analytical Sensing Technology-16 electrochemistry instrument (Quanteon). Depolarization-induced glutamate release was induced by an isotonic solution of 70 mM KCl ejected for 1 s through a glass micropipette positioned at a distance of 50–100 μm from the MEA recording sites. A MATLAB graphic user interface was used to calculate concentrations of glutamate from an average of 3–5 amplitudes per mouse.
Statistical Analyses.
When two or more groups were analyzed, one- or two-way ANOVAs followed by a Fisher’s Least Significant Difference (LSD) test for pairwise comparisons were used. When two groups were compared, Student’s t test was used. P values less than 0.05 were considered significant.
Acknowledgments
This work was supported by the Fisher Center for Alzheimer’s Disease Research Foundation (P.G. and P.S.), Vetenskapsrådet Grant 2015-02966_3 (to P.S.), JPB Foundation for Medical Research (M.G.K. and P.G.), and the US Army Medical Research and Material Command under Awards W81XWH-09-1-0402 (to P.G.) and W81XWH-09-1-0381 (to M.G.K.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1524303113/-/DCSupplemental.
References
- 1.Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015;386(9996):896–912. doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- 2.Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bastide MF, et al. Pathophysiology of L-DOPA-induced motor and non-motor complications in Parkinson’s disease. Prog Neurobiol. 2015;132:96–168. doi: 10.1016/j.pneurobio.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Svenningsson P, Kim Y, Warner-Schmidt J, Oh YS, Greengard P. p11 and its role in depression and therapeutic responses to antidepressants. Nat Rev Neurosci. 2013;14(10):673–680. doi: 10.1038/nrn3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee KW, et al. Alteration by p11 of mGluR5 localization regulates depression-like behaviors. Mol Psychiatry. 2015;20(12):1546–1556. doi: 10.1038/mp.2015.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Svenningsson P, et al. Alterations in 5-HT1B receptor function by p11 in depression-like states. Science. 2006;311(5757):77–80. doi: 10.1126/science.1117571. [DOI] [PubMed] [Google Scholar]
- 7.Alexander B, et al. Reversal of depressed behaviors in mice by p11 gene therapy in the nucleus accumbens. Sci Transl Med. 2010;2(54):54ra76. doi: 10.1126/scitranslmed.3001079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warner-Schmidt JL, et al. Cholinergic interneurons in the nucleus accumbens regulate depression-like behavior. Proc Natl Acad Sci USA. 2012;109(28):11360–11365. doi: 10.1073/pnas.1209293109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Egeland M, Warner-Schmidt J, Greengard P, Svenningsson P. Co-expression of serotonin 5-HT(1B) and 5-HT(4) receptors in p11 containing cells in cerebral cortex, hippocampus, caudate-putamen and cerebellum. Neuropharmacology. 2011;61(3):442–450. doi: 10.1016/j.neuropharm.2011.01.046. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X, Andren PE, Greengard P, Svenningsson P. Evidence for a role of the 5-HT1B receptor and its adaptor protein, p11, in L-DOPA treatment of an animal model of Parkinsonism. Proc Natl Acad Sci USA. 2008;105(6):2163–2168. doi: 10.1073/pnas.0711839105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salamone JD, et al. Neostriatal muscarinic receptor subtypes involved in the generation of tremulous jaw movements in rodents implications for cholinergic involvement in parkinsonism. Life Sci. 2001;68(22-23):2579–2584. doi: 10.1016/s0024-3205(01)01055-4. [DOI] [PubMed] [Google Scholar]
- 12.Ungerstedt U. 6-Hydroxy-dopamine induced degeneration of central monoamine neurons. Eur J Pharmacol. 1968;5(1):107–110. doi: 10.1016/0014-2999(68)90164-7. [DOI] [PubMed] [Google Scholar]
- 13.Herrera-Marschitz M, Arbuthnott G, Ungerstedt U. The rotational model and microdialysis: Significance for dopamine signalling, clinical studies, and beyond. Prog Neurobiol. 2010;90(2):176–189. doi: 10.1016/j.pneurobio.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Lundblad M, Picconi B, Lindgren H, Cenci MA. A model of L-DOPA-induced dyskinesia in 6-hydroxydopamine lesioned mice: Relation to motor and cellular parameters of nigrostriatal function. Neurobiol Dis. 2004;16(1):110–123. doi: 10.1016/j.nbd.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 15.Santini E, Heiman M, Greengard P, Valjent E, Fisone G. Inhibition of mTOR signaling in Parkinson’s disease prevents L-DOPA-induced dyskinesia. Sci Signal. 2009;2(80):ra36. doi: 10.1126/scisignal.2000308. [DOI] [PubMed] [Google Scholar]
- 16.Calabresi P, Picconi B, Tozzi A, Di Filippo M. Dopamine-mediated regulation of corticostriatal synaptic plasticity. Trends Neurosci. 2007;30(5):211–219. doi: 10.1016/j.tins.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Alvarsson A, et al. Modulation by trace amine-associated receptor 1 of experimental Parkinsonism, L-DOPA responsivity, and glutamatergic neurotransmission. J Neurosci. 2015;35(41):14057–14069. doi: 10.1523/JNEUROSCI.1312-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Marongiu R., et al. (2016) Gene therapy blockade of dorsal striatal p11 improves motor function and dyskinesia in parkinsonian mice. Proc Nat Acad Sci USA 113:1423–1428. [DOI] [PMC free article] [PubMed]
- 19.Subramaniam S, et al. Rhes, a striatal-enriched small G protein, mediates mTOR signaling and L-DOPA-induced dyskinesia. Nat Neurosci. 2012;15(2):191–193. doi: 10.1038/nn.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carta M, Carlsson T, Kirik D, Björklund A. Dopamine released from 5-HT terminals is the cause of L-DOPA-induced dyskinesia in parkinsonian rats. Brain. 2007;130(Pt 7):1819–1833. doi: 10.1093/brain/awm082. [DOI] [PubMed] [Google Scholar]
- 21.Rylander D, et al. A mGluR5 antagonist under clinical development improves L-DOPA-induced dyskinesia in parkinsonian rats and monkeys. Neurobiol Dis. 2010;39(3):352–361. doi: 10.1016/j.nbd.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Berg D, et al. AFQ056 treatment of levodopa-induced dyskinesias: Results of 2 randomized controlled trials. Mov Disord. 2011;26(7):1243–1250. doi: 10.1002/mds.23616. [DOI] [PubMed] [Google Scholar]
- 23.Svenningsson P, et al. Eltoprazine counteracts L-DOPA-induced dyskinesias in Parkinson’s disease: A dose-finding study. Brain. 2015;138(Pt 4):963–973. doi: 10.1093/brain/awu409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henry B, Crossman AR, Brotchie JM. Characterization of enhanced behavioral responses to L-DOPA following repeated administration in the 6-hydroxydopamine-lesioned rat model of Parkinson’s disease. Exp Neurol. 1998;151(2):334–342. doi: 10.1006/exnr.1998.6819. [DOI] [PubMed] [Google Scholar]
- 25.Hascup KN, Hascup ER, Pomerleau F, Huettl P, Gerhardt GA. Second-by-second measures of L-glutamate in the prefrontal cortex and striatum of freely moving mice. J Pharmacol Exp Ther. 2008;324(2):725–731. doi: 10.1124/jpet.107.131698. [DOI] [PMC free article] [PubMed] [Google Scholar]