Significance
Medications for Parkinson’s disease (PD) are designed to replace lost dopamine. Although effective, they often cause abnormal involuntary movements (AIMs), also called dyskinesias, which can be difficult to resolve without worsening PD symptoms. We report that p11, a small protein necessary for neurotransmitter receptor function, is critical to dopamine responses in a mouse PD model. Blocking p11 production in the dorsal striatum, a brain region that responds to dopamine and regulates movement, can improve forced movements and normalize spontaneous movements in parkinsonian mice while dramatically reducing AIMs after dopamine replacement therapy. Our data identify a new target for therapeutic development to both improve symptoms and reduce drug-related side effects in human PD.
Keywords: gene therapy, p11, Parkinson's disease, dyskinesia, striatum
Abstract
Complications of dopamine replacement for Parkinson’s disease (PD) can limit therapeutic options, leading to interest in identifying novel pathways that can be exploited to improve treatment. p11 (S100A10) is a cellular scaffold protein that binds to and potentiates the activity of various ion channels and neurotransmitter receptors. We have previously reported that p11 can influence ventral striatal function in models of depression and drug addiction, and thus we hypothesized that dorsal striatal p11 might mediate motor function and drug responses in parkinsonian mice. To focally inhibit p11 expression in the dorsal striatum, we injected an adeno-associated virus (AAV) vector producing a short hairpin RNA (AAV.sh.p11). This intervention reduced the impairment in motor function on forced tasks, such as rotarod and treadmill tests, caused by substantia nigra lesioning in mice. Measures of spontaneous movement and gait in an open-field test declined as expected in control lesioned mice, whereas AAV.sh.p11 mice remained at or near normal baseline. Mice with unilateral lesions were then challenged with l-dopa (levodopa) and various dopamine receptor agonists, and resulting rotational behaviors were significantly reduced after ipsilateral inhibition of dorsal striatal p11 expression. Finally, p11 knockdown in the dorsal striatum dramatically reduced l-dopa–induced abnormal involuntary movements compared with control mice. These data indicate that focal inhibition of p11 action in the dorsal striatum could be a promising PD therapeutic target to improve motor function while reducing l-dopa–induced dyskinesias.
Pharmacologic replacement of depleted dopamine is the primary therapeutic approach to treating Parkinson’s disease (PD). Although this usually improves the major motor problems of this disorder, complications of medical therapy can often limit both dosing and effectiveness. Among the most common adverse effects limiting dopamine replacement therapy for PD is the development of abnormal involuntary movements (AIMs), also known as levodopa-induced dyskinesia (LID) (1). Treatment of LID usually requires reducing the dosage of dopaminergic medications to below the threshold for major complications, although certain pharmacotherapies or surgeries can improve LID as well (1). Understanding both the anatomic location and molecular pathways underlying dyskinesia responses to dopamine replacement therapy is necessary to develop improved therapies, which can reduce motor symptoms without this debilitating problem.
Previous studies have identified certain signaling pathways that may influence the development of dyskinesia. The primary site of action of l-dopa (levodopa) on PD motor symptoms after conversion to dopamine is the dorsal striatum, owing to the loss of the normal dopaminergic inputs from the substantia nigra pars compacta (2). This same region has also been shown to be responsible for motor complications of l-dopa therapy, including LID. Specifically, neurons harboring the D1 dopamine receptor appear to be primarily involved in these responses (3–5). Furthermore, other signaling pathways, including the serotonin 5-HT1B receptor, seem to modulate the response of these neurons to dopamine replacement therapy (6, 7). Nonetheless, it has been difficult to identify potential therapeutic targets that both improve motor function and reduce dyskinesia.
Here we demonstrate that dorsal striatal p11 is a key regulator of dopamine responses in PD. We previously reported that p11, a small adaptor protein also known as S100A10, binds to specific serotonin receptor subtypes, including 5-HT1B (8–10). Because activation of the 5-HT1B serotonin receptor (5-HT1BR) reduces dyskinesia, and p11 binds to 5-HT1BR and potentiates 5-HT1B activity, we hypothesized that dorsal striatal p11 may influence the response to dopamine replacement therapy. We found that inhibition of p11 expression in the dorsal striatum improved motor function in parkinsonian mice. Surprisingly, blockade of dorsal striatal p11 expression profoundly inhibited dyskinesias in response to chronic l-dopa treatment, to a greater extent than pharmacologic activation of 5-HT1B in controls. This indicates that inhibition of striatal p11 is a promising potential target to block dyskinesias while improving motor function in PD, and that these effects likely occur through a mechanism other than 5-HT1B.
Results
Reduction of p11 in the Dorsal Striatum Significantly Improves Rotarod and Treadmill Performance in Parkinsonian Mice.
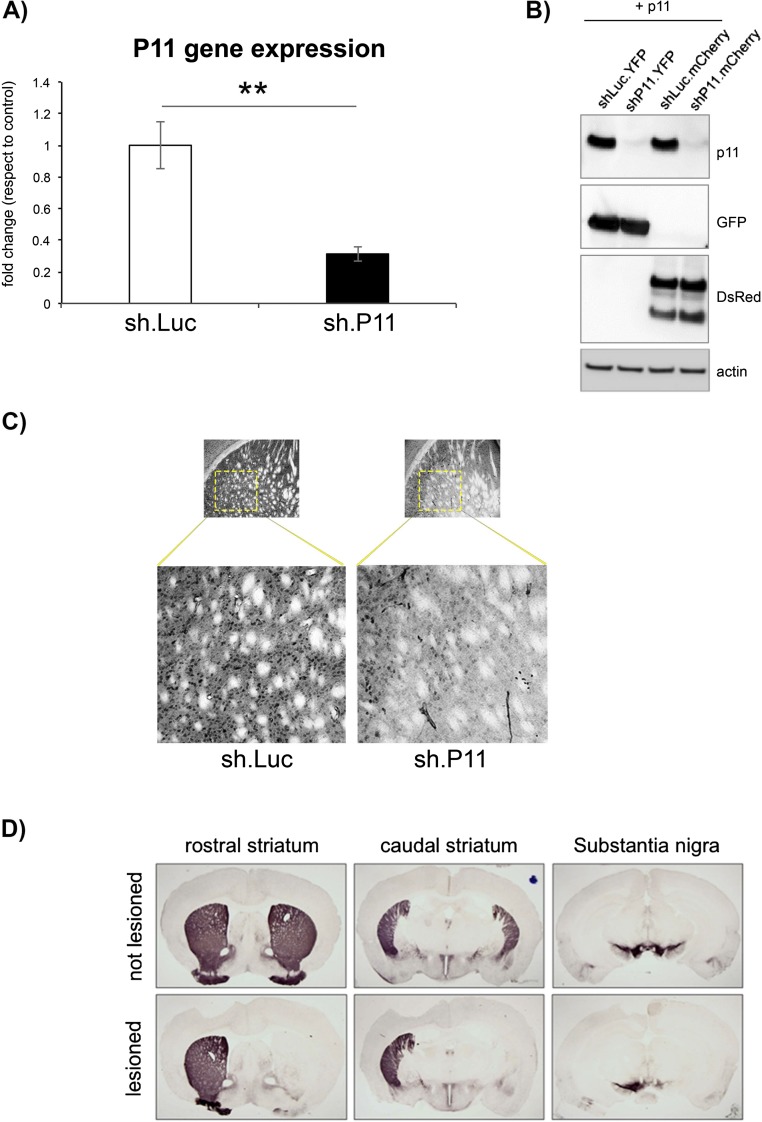
To address the influence of striatal p11 on motor function, we generated serotype 2 adeno-associated virus (AAV) vectors encoding for either a short hairpin RNA (shRNA) to block murine p11 expression (AAV.sh.p11) or an shRNA against firefly luciferase (AAV.sh.Luc) as a negative control (Fig. S1 A and B) (8). At 12 wk after bilateral intrastriatal injection of the AAV vectors, the mice demonstrated a significant increase in the time spent on an accelerating rotarod compared with controls (P < 0.05), suggesting that inhibition of striatal p11 expression could modestly improve motor function even in normal mice (Fig. 1C). Then, 6 wk later, these same animals were subjected to unilateral 6-hydroxydopamine (6-OHDA) lesions, and after another 6 wk, overall rotarod performance declined in all groups, as expected. The better performance of the p11 knockdown group compared with controls was maintained after lesioning, however (P < 0.05) (Fig. 1C).
Fig. S1.
(A) qPCR analysis of total mRNA from cultured 293 cells, transfected with either p11 or control luciferase (Luc) shRNA constructs, demonstrates substantial inhibition of p11 expression from the shRNA. Analyses were performed in triplicate. **P < 0.01, two-tailed t test. (B) Western blot of transfected cells demonstrating expression of p11 in two control samples, expressing an shRNA against luciferase and coexpressing either YFP (lane 1) or mCherry (lane 3), with no p11 detected in two p11 shRNA lanes coexpressing YFP (lane 2) or mCherry (lane 4). (C) Immunostaining of representative striatal sections from mice receiving dorsal striatal injections of AAV vectors expressing either control shRNA (Left) or p11 shRNA (Right), demonstrating reduced p11 staining in the p11 shRNA section. (D) Representative sections from a lesioned animal at the level of the rostral striatum, caudal striatum, and substantia nigra, immunostained for TH expression. Striatal and nigral TH expression is full and symmetrical bilaterally in the control sections (Upper), whereas expression is lost in the striatum and nigra of the lesioned hemisphere compared with the normal unlesioned hemisphere, in sections from a well-lesioned 6-OHDA mouse (Lower). No difference was observed in the extent of nigral or striatal loss of TH staining in animals receiving striatal AAV.shp11 compared with controls.
Fig. 1.
Reduction of p11 in the dorsal striatum improves evoked motor behavior in adult mice. (A) Diagram of the experimental design. (B) Immunostaining demonstrating equivalent striatal AAV vector transduction based on YFP expression, with similar overall cell numbers based on staining for DARPP-32 and DAPI. (C) Knockdown of p11 in the dorsal striatum of adult mice increased endurance on the rotarod test. A significant increase in rotarod time was observed in AAV.sh.p11 mice just before the 6-OHDA lesion, and this increase was maintained after the lesion. *P < 0.05, sh.p11 relative to sh.Luc controls, two-tailed t test. (D) Striatal p11 reduction improved performance on the treadmill speed challenge after the 6-OHDA lesion compared with controls. *P < 0.05, **P < 0.01, sh.p11 relative to sh.Luc controls, two-tailed Fisher’s exact test. The numbers of mice per experimental group were as follows: no virus, n = 12; sh.Luc, n = 10; sh.P11, n = 11.
Mice were also tested before and after lesioning on an accelerating treadmill, and were assayed for their ability to maintain ambulation at a given speed. Before lesioning, nearly all animals were able to ambulate effectively at on a forced treadmill, with no difference between groups. After lesioning, there was a progressive decline in the ability of mice with either no virus or intrastriatal infusion of control virus to ambulate on the treadmill, with no animal able to walk at a rate of 3 cm/s at 7 wk after lesioning (Fig. 1D). In contrast, 60% of the AAV.sh.p11 mice were still able to ambulate effectively on the treadmill at that time point (P = 0.01, two-tailed Fisher’s exact test). These data indicate that reduction of striatal p11 could improve motor performance after 6-OHDA lesioning and might even improve the ability of normal mice to respond to motor challenges.
Histological assessment on completion of the motor studies indicated comparable expression throughout most of the striatum in both groups, using immunostaining for yellow fluorescent protein (YFP) expressed from a second cassette in both vectors (Fig. 1B). There was no evidence of loss of medium spiny neurons, as measured by DARPP-32 staining (Fig. 1B), and no evidence of any difference in nigral dopaminergic neuronal loss between groups, as measured by tyrosine hydroxylase (TH) staining (Fig. S1D). This indicates that the motor effects after inhibition of dorsal striatal p11 expression were due to altered striatal function rather than to differences in neuronal survival compared with controls.
Reduction of p11 in the Dorsal Striatum Normalizes Spontaneous Motor Behaviors in Parkinsonian Mice.
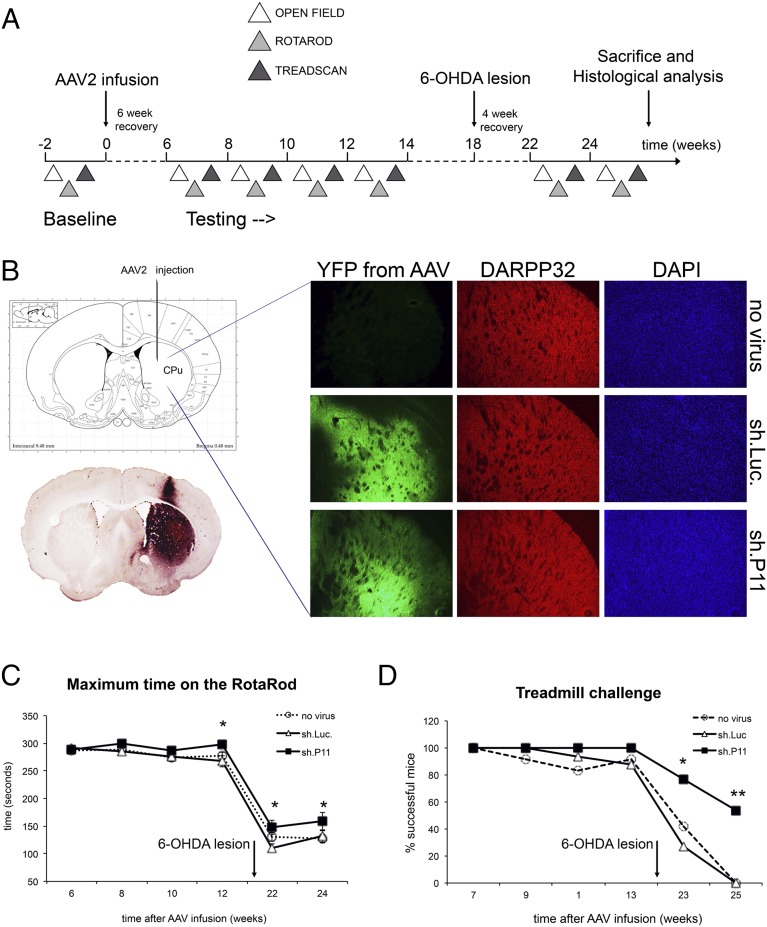
To determine whether blockade of striatal p11 expression influences spontaneous motor function, we analyzed open-field activity in mice both before and after 6-OHDA lesioning. Before the lesion, there was no difference in any parameter between control and AAV.sh.p11 mice. After lesioning, control mice exhibited a substantial decline in several parameters, including the number of ambulatory episodes and average velocity of gait, with a concomitant increase in time spent resting (Fig. 2 A–C). As with the rotarod and treadmill tests, dorsal striatal p11 knockdown significantly improved these parameters. Unlike the modest improvements in treadmill and rotarod performance, however, the open-field parameters in AAV.sh.p11 mice after lesioning were indistinguishable from the prelesioning baseline behaviors (Fig. 2 A–C). There was no evidence of pathological hyperactivity in these mice; they were no different from either control group before lesioning, and motor parameters were maintained but not increased after lesioning. This finding further supports the conclusion that inhibition of dorsal striatal p11 expression improves motor function in parkinsonian mice, and suggests that spontaneous motor activity may be affected to a greater degree than movement in response to a forced challenge.
Fig. 2.
Reduction of p11 in the dorsal striatum improved spontaneous open-field motor behavior in 6-OHDA lesioned mice. After an initial 5-min acclimation period, open-field activity was measured over 25 min using a photocell-based tracking system in a Plexiglas arena. Compared with control mice receiving AAV.sh.luc, 6-OHDA lesioned mice with intrastriatal AAV.sh.p11 ipsilateral to the lesion showed normalization of ambulatory episodes (A), resting time (B), and average gait velocity (C). *P < 0.05, **P < 0.01, ***P < 0.001, sh.p11 relative to sh.Luc controls, two-tailed t test. The numbers of mice per group were as follows: no virus, n = 12; sh.Luc, n = 10; sh.P11, n = 11.
Blockade of Striatal p11 Expression Influences the Response to Dopamine in a Mouse Model of PD.
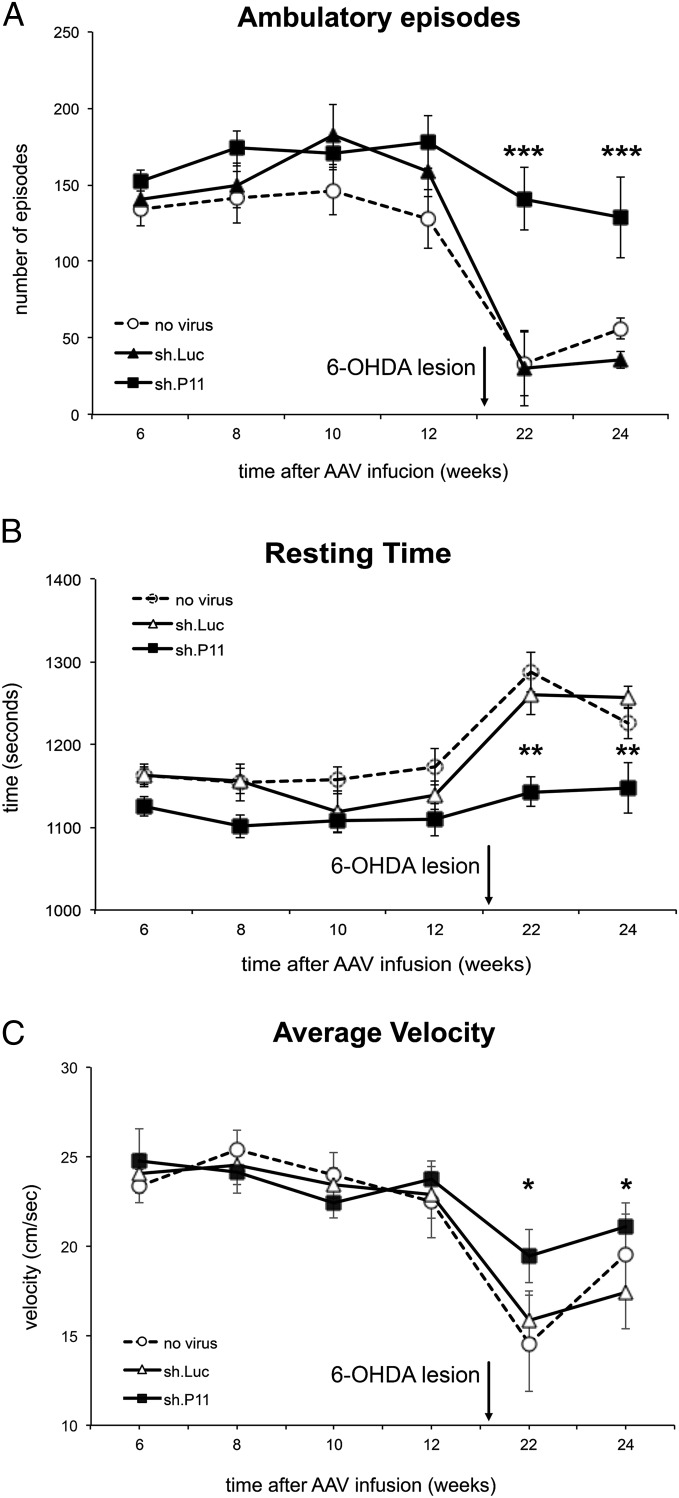
Given the effect of p11 on evoked and spontaneous motor behaviors, we next determined whether altered p11 expression after 6-OHDA lesioning could influence motor responses to dopamine. A loss of striatal dopamine after the creation of unilateral 6-OHDA nigral lesions leads to rotational behaviors following administration of dopamine agonists, owing to hypersensitivity of striatal dopamine receptors only in the lesioned hemisphere. To test the effect of p11 loss on striatal responses to dopamine, we randomized mice into equal groups based on their rotational counts in response to the dopamine receptor agonist apomorphine and then administered injections of either AAV.sh.p11 or control AAV.sh.Luc in the ipsilateral striatum. A third group of mice received no viral injection. After a 6-wk period to optimize AAV expression, the mice were challenged again with apomorphine. There was a significant reduction in apomorphine-induced rotations in the AAV.sh.p11 group compared with both the control AAV.sh.Luc and no virus groups (P < 0.0001) (Fig. 3B).
Fig. 3.
Reduction of p11 in the dorsal striatum decreases rotational turns in response to dopamine agonists in adult mice. (A) Diagram of the experimental design. (B–E) Contralateral rotations were decreased in animals with inhibition of striatal p11 ipsilateral to the 6-OHDA lesions compared with lesioned mice receiving a control AAV after administration of apomorphine (B), l-dopa (C), D1R agonist SKF81297 (D), and D2R agonist quinpirole (E). ***P < 0.001, ****P < 0.0001, sh.p11 relative to sh.Luc controls, two-tailed t test. The numbers of mice per group were as follows: no virus, n = 19; sh.Luc, n = 18; sh.P11, n = 18. The experiment was repeated three times.
To evaluate a more clinically relevant condition, we then tested the animals with l-dopa, which is the gold standard therapy for human PD. Again, the AAV.sh.p11 group showed a significant reduction in rotational behaviors compared with controls (P < 0.001) (Fig. 3C). To determine whether this effect was specific to a single dopamine receptor subtype, we challenged mice with the D1 receptor agonist SKF81297 and the D2 receptor agonist quinpirole. Again, there was a significant reduction in rotations in response to either agonist compared with controls (P < 0.001 for the SKF81297 group; P < 0.0001 for the quinpirole group), indicating that the effect was not specific to one dopamine receptor subtype (Fig. 3 D and E). These data are similar to the results seen with focal striatal dopamine replacement therapies, such as gene therapy and cell transplantation (11, 12). Therefore, our results suggest that p11 knockdown normalizes dorsal striatal function ipsilateral to the lesion, with the resultant decrease in up-regulation of dopamine receptor activity leading to reduced striatal asymmetry and decreased rotations.
Down-Regulation of p11 Expression Significantly Reduces l-Dopa–Induced AIMs.
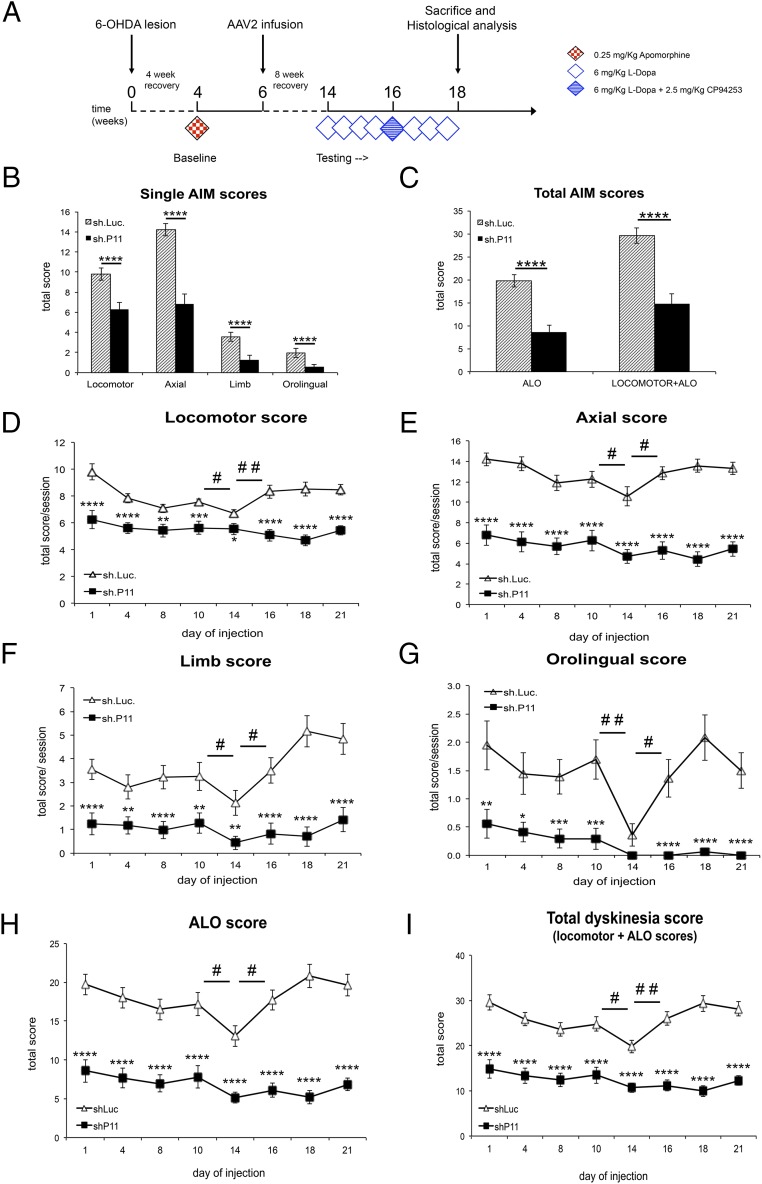
Dyskinesia is one of the major complications of dopamine replacement therapy, and, based on our foregoing findings, we hypothesized that p11 might influence the development of AIMs in parkinsonian mice. To test this, mice received unilateral 6-OHDA lesions and 1 mo later were randomized to equal groups based on rotational turns after systemic apomorphine administration. AAV vectors were then injected into the ipsilateral striatum, and starting 8 wk later, the mice received daily therapy with high-dose l-dopa for 3 wk to induce AIMs (Fig. 4A). The AIMs induced in mice by chronic l-dopa administration can be classified into four phenotypes: limb, orolingual, axial, and locomotor dyskinesias (13). On the first testing day after l-dopa administration, compared with the mice receiving control AAV.sh.Luc, those with intrastriatal AAV.sh.p11 ipsilateral to the 6-OHDA lesion demonstrated significant reductions in all AIM subscores and in the composite scores of the axial, limb, and orolingual (ALO) and the total composite of ALO plus locomotor scores (Fig. 4 B and C).
Fig. 4.
Reduction of striatal p11 expression significantly reduces l-dopa-induced AIMs. (A) Diagram of the experimental design. (B) Individual AIM subscores per group in a single testing session. (C) Total AIMs composite score in a single testing session. (D–G) Individual AIM subscores per group across the entire 3 wk of daily l-dopa treatment. On day 14, the mice also received the 5-HT1B agonist CP94253. (H) Composite AIM scores of ALO subscores across the entire study. (I) Composite total AIM scores (ALO plus locomotor subscores) across the entire study. Administration of CP94253 to control mice modestly reduced AIMs by roughly 20% in all subscores and in total composite AIM scores compared with l-dopa alone, but this effect was not observed in AAV.sh.p11 mice. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, sh.p11 relative to sh.Luc controls; #P < 0.05, ##P < 0.01 for day 14 sh.Luc compared with either day 10 or day 16 for the same group. n = 18 sh.Luc mice; n = 17 sh.p11 mice. The experiment was repeated three times.
To determine the stability of this effect, animals were scored periodically over 3 wk of daily l-dopa administration, and this profound effect was observed on each testing day (Fig. 4 D–I). Because p11 has been shown to potentiate 5-HT1B receptor activity, we examined the effect of inhibiting striatal p11 on AIM scores after coadministration of the 5-HT1B agonist CP94253 with l-dopa. Although modest reductions in AIM subscores and total composite score were observed in control mice, as expected, this effect was not seen in the mice receiving intrastriatal AAV.sh.p11 (Fig. 4 D–I). After CP94253 administration, the mice returned to baseline, and the effect of l-dopa continued for another 1 wk. Immunostaining for YFP confirmed equivalent AAV transduction in control and AAV.sh.p11 mice (Fig. S2). These data demonstrate that inhibition of dorsal striatal p11 expression is profoundly antidyskinetic, to a greater degree than pharmacologic 5-HT1B receptor activation.
Fig. S2.
Representative sections of the whole mouse brain at the level of the dorsal striatum, immunostained for YFP expression, from animals used for dyskinesia testing and infused with either AAV.shp11 or AAV.shLuc negative control. Both vectors contain the same YFP expression cassette.
Discussion
Dyskinesia represents one of the most disabling consequences of dopamine replacement therapy for PD. The small adaptor protein p11 has been identified as critical for the function of ventral striatum in models of depression and addiction (8, 14, 15). Here we identify p11 as a protein that profoundly influences the development of abnormal involuntary movements in parkinsonian mice exposed to dopamine replacement therapy. We examined p11 in the dorsal striatum, and found that inhibition of p11 expression resulted in a reduction in AIMs after l-dopa administration. Furthermore, significant improvements in motor behavior were seen, indicating that therapies directed at blocking the action of p11 in the dorsal striatum could improve motor function while reducing abnormal movements. The effect on rotarod and treadmill testing was significant but small, whereas complete normalization of spontaneous motor activity was seen. This suggests that there might be an effect on motivation as well as on movement. Histological analyses confirmed that our transduction was limited to the dorsal striatum, and thus any motivational or behavioral consequences were unlikely to be related to off-target effects of p11. Our data are also consistent with the observation in our companion report (16), which demonstrated improvement in tacrine-induced tremor in transgenic global p11 knockout (KO) mice, an effect replicated in cell type-specific cholinergic p11 KO mice. Taken together, our data suggest that the loss of p11 within cholinergic neurons of the dorsal striatum can improve a variety of motor functions, both evoked and spontaneous. This will be explored more fully in future studies.
Our data raise intriguing questions regarding p11’s mechanism of action. The original description of p11 as a potential mediator of neuronal activity identified the 5-HT1B receptor as a key binding partner (17). p11 was found to bind to and increase surface presentation of 5-HT1B receptors, thereby potentiating their activity, and this was confirmed in subsequent studies. Because 5-HT1B agonists are known to reduce dyskinesias (6), we had originally hypothesized that inhibition of p11 expression would block the effect of 5-HT1B agonists, and possibly worsen baseline AIMs. In fact, we found the opposite result. Although focal knockdown of p11 expression in the dorsal striatum did reduce the effect of a 5-HT1B agonist, the overall effect of reduced p11 was profoundly antidyskinetic. Given the dramatic reduction in AIMs, it is difficult to determine whether the effect of reducing p11 in the dorsal striatum on 5-HT1B agonist function is due to decreased 5-HT1B activity or to a floor effect in which a further reduction in AIMs cannot be readily appreciated. Regardless, our data indicate that inhibition of p11 in the dorsal striatum is antidyskinetic, and that this action is not mediated through previously reported potentiation of 5-HT1B activity.
This action also represents, to our knowledge, the first reported potential therapeutic benefit of inhibiting rather than increasing p11 activity, which is again consistent with the finding of reduced AIMs in total p11 KO mice reported in our companion paper (16). The companion study also reported reduced sensitization to rotational behaviors following l-dopa administration in lesioned p11 KO mice, but no difference compared with control mice at baseline. We did observe reduced rotations without sensitization, and we hypothesize that this reflects improved functioning of neurons in the denervated striatum, leading to reduced receptor hypersensitivity and reduced rotations. This would explain the reduced rotations, reduced AIMs, and improved motor function, which were also reported in the companion paper. This isolated difference between studies likely reflects either variations due to p11 loss during development in KO mice compared with later loss in normal adult mice or potential effects elsewhere in the brain or body in KO mice compared with focal knockdown after viral infusion in otherwise normal mice.
Several other p11 actions that could mediate the effect described here have been reported. In many cases, p11 forms a heterotetramer with annexin A2, and this appears to be necessary for interaction with a variety of receptors and channels, such as the TASK-1 potassium channel (18). p11 also has been reported to interact with SMARCA3, a chromatin remodeling factor, and this interaction appears to be important for some antidepressant effects of p11 (19). Most relevant to our current findings, however, is a recent report of p11 binding to the mGluR5 metabotropic glutamate receptor (20). In prefrontal cortex, p11 was found to potentiate the effects of mGluR5 to regulate depression-like behaviors. mGluR5 also has been shown to play a role in dyskinesia, and unlike 5-HT1B, mGluR5 appears to potentiate abnormal involuntary movements after dopamine replacement therapy (21, 22). Striatal medium spiny projection neurons and cholinergic interneurons express mGluR5 receptors, and pharmacologic inhibition of mGluR5 is antidyskinetic (23, 24); thus, our finding that genetic inhibition of p11 is antidyskinetic could be consistent with this mechanism.
Identification of p11 inhibition as a previously unidentified antidyskinesia pathway raises the potential of exploiting this for therapeutic development. Dyskinesia is a common complication of dopamine replacement therapy, is often dose-limiting, and can be sufficiently difficult to manage such that many patients opt for deep brain stimulation surgery in an attempt to obtain better control without symptomatic decline (1). The complex actions of p11 in different brain regions highlight the difficulties in developing systemic pharmacotherapies for neurologic disease. Although our data suggest that inhibition of p11 throughout the brain might reduce dyskinesia, our previous study in the ventral striatum indicates that this also could cause or worsen depression, which is a major comorbidity of PD (8, 25). Our data suggest that systemic p11 inhibition might be effective in reducing dyskinesia in patients without major depression, whereas for more complex patients, focal therapies, such as gene therapy to specifically inhibit striatal p11, could be optimal for improving dyskinesia and motor function while limiting potential unintended consequences of extrastriatal inhibition.
Materials and Methods
Generation of AAV Vectors Expressing shRNAs.
To silence p11 expression, we generated an shRNA against mouse p11 shRNA (sh.p11) and a luciferase shRNA (sh.Luc) as a negative control. These were packaged into AAV vectors using a two-plasmid system in HEK 293 cells. The production and purification procedures are described in detail in SI Materials and Methods.
Animals.
Wild type C57BL/6 mice, obtained from Charles River Laboratory, were housed two to five per cage and kept at 22 °C on a reverse 12-h light/12-h dark cycle, with standard mouse chow and water provided ad libitum throughout the duration of the study. All animal procedures were approved by the Institutional Animal Care and Use Committee of Weill Cornell Medical College and were in accordance with National Institutes of Health guidelines.
Stereotactic Surgery and Behavioral Assessments.
Viral vectors were infused stereotactically into the striata of 8- to 12-wk-old mice, followed by behavioral assessments 6–8 wk later. Details of the surgical infusion and behavioral assessments are provided in SI Materials and Methods.
Immunohistochemistry.
On completion of all behavioral assessments, mice were deeply anesthetized with sodium pentobarbital (150 mg/kg) and transcardially perfused with 4% paraformaldehyde (PFA). Brains were extracted and postfixed overnight in 4% PFA, cryoprotected in 30% sucrose, and cut into 40-μm sections using a microtome.
Free-floating sections were treated with various antibodies to visualize proteins of interest, including TH, DARPP-32, and YFP, using immunofluorescence or immunoperoxidase labeling. Detailed information is provided in SI Materials and Methods.
Statistical Analysis.
The two-tailed t test was used for statistical comparisons of all paired animal group data with the exception of the treadmill gait system. All data are expressed as mean ± SEM. The gait system behavior data were evaluated using the two-tailed Fisher’s exact test. A P value < 0.05 was considered to indicate statistical significance.
SI Materials and Methods
Creation of shRNA Constructs.
For shRNA construction, the human H1 promoter was amplified from genomic DNA as described previously (26). The PCR product was cloned into an AAV cis-plasmid to generate pAAV.H1. This vector also contains a YFP expression cassette under the control of a hybrid chicken b-actin/CMV promoter. The shRNAs were cloned immediately downstream from the H1 promoter of pAAV.H1 between BglII and SpeI restriction sites. The following ShRNA sequences were used in this study: sh.Luc: 5′-CGCTGGAGAGCAACTGCATcttcctgtcaATGCAGTTGCTCTCCAGCGttttt-3′; sh.p11: 5′-GGATCCTCTGGCTGTGGACActtcctgtcaTGTCCACAGCCAGAGGATCCttttt-3′.
The integrity of the constructs was verified by sequencing. The efficiency of the shRNA constructs was further confirmed by Western blot analysis of extracted protein and quantitative real-time PCR (qPCR) using Syber Green chemistry and an Applied Biosystems ABI Prism Fast-7500 Sequence Detection System of extracted mRNA from cultured 293 cells transfected with p11 shRNA or control constructs (Fig. S1 A and B).
Cell Culture and Adeno-Associated Virus Preparation.
Human embryonic kidney (HEK293) cells were cultured in DMEM (Gibco), supplemented with 10% (vol/vol) FBS (Sigma-Aldrich) and 1% (vol/vol) penicillin-streptomycin (Gibco), at 37 °C in 95% humidified air and 5% CO2.
The viral vectors used in this study were AAV2.sh.Luc.YFP and AAV2.sh.p11.YFP. Vector stocks were prepared by packaging the plasmids into AAV2 particles using a calcium phosphate transfection system as described previously (27). In brief, cells were harvested and lysed at 72 h after transfection. The vectors were purified using heparin- affinity chromatography and dialyzed against PBS. AAV titers were determined by qPCR using Syber Green chemistry and with primers to the CMV-enhancer fragment of the AAV backbone.
Stereotactic Surgery.
All stereotactic surgical procedures were performed on 8–12-wk-old mice under a mixture of ketamine-xylazine anesthesia. Ketamine (Butler Animal Health Supply) and xylazine (Lloyd Laboratories) were administered at concentrations of 110 and 4.4 mg/kg body weight, respectively.
After the induction of anesthesia, the animals were placed into a stereotactic frame (David Kopf Instruments). All infusions were performed using a 10-μL Hamilton syringe with a 33 G needle attached to a microinfusion pump (World Precision Instruments) at a rate of 0.1–0.4 μL/min. To prevent reflux, after each infusion, the injection needle was left in place for 5 min, withdrawn a short distance (0.3–0.5 mm), and then left in the new position for an additional 2–3 min before removal.
To generate 6-OHDA lesioned mice, normal C57BL/6 mice were injected with a total volume of 0.5 μL of 6-OHDA hydrobromide (Sigma-Aldrich) unilaterally into the medial forebrain bundle (AP −1.1 mm, ML ±1.1 mm, DV −4.8 mm from bregma) at a concentration of 3.0 mg/mL and an infusion rate of 0.1 μL/min. Before lesion surgery, the norepinephrine reuptake inhibitor desipramine (25 mg/kg) was administered via i.p. injection at least 30 min before 6-OHDA injection, to protect neostriatal and cerebellar noradrenergic neurons from the toxin-induced damage. AAV2 vectors were infused into striatum (AP +0.5 mm, ML ±2.3 mm, DV −3.5 mm from bregma) of either normal or lesioned mice using a microinfusion pump (World Precision Instruments) at a rate of 400 nL/min. A total of 2 × 109 (2 μL in PBS) genomic particles of AAV2 vectors were injected over 5 min.
The mice were allowed 6–8 wk of recovery before being subjected to the behavioral tests. After all behavioral tests, injection site accuracy was determined by immunohistochemistry for YFP, and mice with mistargeted injections were excluded from analysis before their behavioral data were unblinded.
Behavioral Assessments.
All behavioral experiments except dyskinesia scoring were run during the dark phase of the daily cycle, which corresponds to the period of maximal activity in rodents. The experiments were performed by an examiner blinded to treatment group.
Locomotor Quantification with the Rotarod Treadmill.
The rotarod test examines balance and coordination by recording the ability of mice to stay on a rotating rod (Med Associates), which gradually increases the rotational rate from 4 to 40 rpm over a 5-min period. The time spent on the accelerating rotarod was averaged over a five-trial testing session. The latency to fall off the rotarod was determined automatically by a photobeam-based system.
Locomotor Quantification with the Gait Analysis System.
The apparatus consisted of a transparent treadmill (Clever Sys) fitted with a mirror and camera that recorded the underside of the animal. Two fluorescent lamps were used to illuminate the testing chamber. Each mouse was trained at 5 cm/s for 180 s on the day before testing. Testing consisted of 30 s to allow the mouse to acclimate to the chamber with the treadmill off, followed by 20 s of recorded running at 7.5 cm/s on the moving treadmill. Gait analysis was performed using Bcamcap for data collection and Treadscan 2.0 for data analysis (Clever Sys).
Locomotor Quantification in an Open-Field Arena.
Locomotor behavior in an open-field arena was tracked automatically via a photocell-based system in a Plexiglas arena (43.2 × 43.2 cm2), and spontaneous horizontal and vertical behaviors were recorded during 30-min sessions. Parameters including distance traveled, speed, jumps, and rotational and exploratory activity were analyzed offline.
Locomotor Quantification Using a Rotometer.
Rotational behaviors were performed as described previously (28) with minor modifications. In brief, mice were placed in a body harness connected to a transducer/swivel in a bowl-shaped testing arena (rotometer). Full 360° clockwise and counter-clockwise rotations were measured over a 45-min period after drug injection. All drugs were dissolved in saline solution, 0.9% NaCl, and 0.1% ascorbic acid, and administered via s.c. injection. Testing with apomorphine (0.25 mg/kg; Sigma-Aldrich) was initiated at 4 wk postlesion, and an average score of at least five turns/min was thought to reflect an adequate lesion. Animals that failed to reach this threshold were not used for further study. The D1 receptor agonist SKF81297 (Sigma-Aldrich) was injected at a concentration of 0.5 mg/kg, and the D2 receptor agonist quinpirole was injected at a concentration of 1 mg/kg. l-dopa (6 mg/kg; Tocris) was administered in combination with 12 mg/kg benserazide (Tocris), to peripherally inhibit l-dopa degradation by the l-amino acid decarboxylase enzyme.
Quantification of LID or AIMs.
The AIMs induced in mice by chronic l-dopa administration can be classified into four different phenotypes: limb, orolingual, axial, and locomotor dyskinesias. The dyskinesia scoring quantifies the degree of dystonic or hyperactive abnormal involuntary movements on a scale of 0–4 (29). Chronic l-dopa treatment was maintained for a period of 3–4 wk, which resulted in a stable dyskinetic phenotype and allowed the observer to score the mice multiple times. On the day of scoring, mice were placed individually in clear Plexiglas boxes at a distance of at least 10 cm from one another. Each mouse was scored for LID presentation for 1 min every 20 min starting at minute 20 from l-dopa injection and continuing until the l-dopa dyskinetic effect wore off.
Scores were analyzed by comparing the scores between the two groups of mice per monitoring session or same day of injection, and also by analyzing the scores for the two groups of mice per testing session, which allowed a comparison of how the two groups behaved with respect to each other and with respect to the other monitoring sessions.
Immunohistochemistry.
The following primary antibodies were used: anti-GFP to detect YFP (1:5,000; Abcam), anti-TH (1:4,000; Chemicon), and anti-DARPP32 (1:2,000; Millipore). Immunoperoxidase labeling was done using secondary biotinylated antibodies, the Vectastain Elite ABC Kit, the Mouse on Mouse Kit, and avidin-streptavidin blocking reagents (Vector Laboratories). The blue 3,3′-diaminobenzidine (DAB) reagent contained 0.05% DAB, 0.05% cobalt chloride, 0.05% nickel ammonium sulfate, and 0.015% hydrogen peroxide (all from Sigma-Aldrich). The brown DAB reagent contained 0.03% DAB and 0.015% hydrogen peroxide. Fluorescent secondary antibodies (Alexa Fluor-conjugated donkey anti-rabbit) were obtained from Life Technologies. Fluorescent images were acquired with an Olympus BX61 microscope fluorescent microscope fitted with an Olympus DP71 digital camera. Images were processed with Microsuite Basic Edition version 2.5 (Olympus) and ImageJ (rsbweb.nih.gov/ij/index.html).
Acknowledgments
The authors thank Sophie Ewald, Cara Berkowitz, and Eric Stewart for their excellent technical assistance. This work was supported by the JPB Foundation (P.G. and M.G.K.), the US Army Medical Research and Material Command (Awards W81XWH-14-1-0390, to P.G., and W81XWH-09-1-0381, to M.G.K.), the Fisher Center for Alzheimer’s Disease Research Foundation (P.G. and P.S.), the Swedish Research Council (P.S. and M.A.C.), and the Swedish Foundation for International Cooperation in Research and Higher Education (M.A.C.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1524387113/-/DCSupplemental.
References
- 1.Manson A, Stirpe P, Schrag A. Levodopa-induced-dyskinesias: Clinical features, incidence, risk factors, management, and impact on quality of life. J Parkinsons Dis. 2012;2(3):189–198. doi: 10.3233/JPD-2012-120103. [DOI] [PubMed] [Google Scholar]
- 2.Nelson AB, Kreitzer AC. Reassessing models of basal ganglia function and dysfunction. Annu Rev Neurosci. 2014;37:117–135. doi: 10.1146/annurev-neuro-071013-013916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Francardo V, Cenci MA. Investigating the molecular mechanisms of L-DOPA–induced dyskinesia in the mouse. Parkinsonism Relat Disord. 2014;20(Suppl 1):S20–S22. doi: 10.1016/S1353-8020(13)70008-7. [DOI] [PubMed] [Google Scholar]
- 4.Feyder M, Bonito-Oliva A, Fisone G. L-dopa–induced dyskinesia and abnormal signaling in striatal medium spiny neurons: Focus on dopamine D1 receptor-mediated transmission. Front Behav Neurosci. 2011;5:71. doi: 10.3389/fnbeh.2011.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guigoni C, et al. Pathogenesis of levodopa-induced dyskinesia: Focus on D1 and D3 dopamine receptors. Parkinsonism Relat Disord. 2005;11(Suppl 1):S25–S29. doi: 10.1016/j.parkreldis.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Bézard E, et al. Anti-dyskinetic effect of anpirtoline in animal models of L-dopa–induced dyskinesia. Neurosci Res. 2013;77(4):242–246. doi: 10.1016/j.neures.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Svenningsson P, et al. Eltoprazine counteracts L-dopa–induced dyskinesias in Parkinson's disease: A dose-finding study. Brain. 2015;138(Pt 4):963–973. doi: 10.1093/brain/awu409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alexander B, et al. Reversal of depressed behaviors in mice by p11 gene therapy in the nucleus accumbens. Sci Transl Med. 2010;2(54):54ra76. doi: 10.1126/scitranslmed.3001079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Svenningsson P, Greengard P. p11 (S100A10): An inducible adaptor protein that modulates neuronal functions. Curr Opin Pharmacol. 2007;7(1):27–32. doi: 10.1016/j.coph.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Warner-Schmidt JL, et al. Role of p11 in cellular and behavioral effects of 5-HT4 receptor stimulation. J Neurosci. 2009;29(6):1937–1946. doi: 10.1523/JNEUROSCI.5343-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leone P, et al. Multi-site partitioned delivery of human tyrosine hydroxylase gene with phenotypic recovery in Parkinsonian rats. Neuroreport. 2000;11(6):1145–1151. doi: 10.1097/00001756-200004270-00002. [DOI] [PubMed] [Google Scholar]
- 12.Park S, et al. Genetically modified human embryonic stem cells relieve symptomatic motor behavior in a rat model of Parkinson’s disease. Neurosci Lett. 2003;353(2):91–94. doi: 10.1016/j.neulet.2003.08.082. [DOI] [PubMed] [Google Scholar]
- 13.Lundblad M, et al. Pharmacological validation of a mouse model of L-dopa–induced dyskinesia. Exp Neurol. 2005;194(1):66–75. doi: 10.1016/j.expneurol.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Arango-Lievano M, et al. Cell type-specific expression of p11 controls cocaine reward. Biol Psychiatry. 2014;76(10):794–801. doi: 10.1016/j.biopsych.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Warner-Schmidt JL, et al. Cholinergic interneurons in the nucleus accumbens regulate depression-like behavior. Proc Natl Acad Sci USA. 2012;109(28):11360–11365. doi: 10.1073/pnas.1209293109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schintu N, et al. p11 modulates L-DOPA therapeutic effects and dyskinesia via distinct cell types in experimental Parkinsonism. Proc Natl Acad Sci USA. 2016;113:1429–1434. doi: 10.1073/pnas.1524303113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Svenningsson P, et al. Alterations in 5-HT1B receptor function by p11 in depression-like states. Science. 2006;311(5757):77–80. doi: 10.1126/science.1117571. [DOI] [PubMed] [Google Scholar]
- 18.Foulkes T, et al. Deletion of annexin 2 light chain p11 in nociceptors causes deficits in somatosensory coding and pain behavior. J Neurosci. 2006;26(41):10499–10507. doi: 10.1523/JNEUROSCI.1997-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oh YS, et al. SMARCA3, a chromatin-remodeling factor, is required for p11-dependent antidepressant action. Cell. 2013;152(4):831–843. doi: 10.1016/j.cell.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee KW, et al. Alteration by p11 of mGluR5 localization regulates depression-like behaviors. Mol Psychiatry. 2015;20(12):1546–1556. doi: 10.1038/mp.2015.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cenci MA. Glutamatergic pathways as a target for the treatment of dyskinesias in Parkinson’s disease. Biochem Soc Trans. 2014;42(2):600–604. doi: 10.1042/BST20140006. [DOI] [PubMed] [Google Scholar]
- 22.Fieblinger T, et al. Mechanisms of dopamine D1 receptor-mediated ERK1/2 activation in the parkinsonian striatum and their modulation by metabotropic glutamate receptor type 5. J Neurosci. 2014;34(13):4728–4740. doi: 10.1523/JNEUROSCI.2702-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bezard E, et al. The mGluR5 negative allosteric modulator dipraglurant reduces dyskinesia in the MPTP macaque model. Mov Disord. 2014;29(8):1074–1079. doi: 10.1002/mds.25920. [DOI] [PubMed] [Google Scholar]
- 24.Vallano A, et al. Targeting striatal metabotropic glutamate receptor type 5 in Parkinson’s disease: Bridging molecular studies and clinical trials. CNS Neurol Disord Drug Targets. 2013;12(8):1128–1142. [PubMed] [Google Scholar]
- 25.Vriend C, et al. Depression and impulse control disorders in Parkinson’s disease: Two sides of the same coin? Neurosci Biobehav Rev. 2014;38:60–71. doi: 10.1016/j.neubiorev.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 26.Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296(5567):550–553. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- 27.Morgenstern PF, Marongiu R, Musatov SA, Kaplitt MG. Adeno-associated viral gene delivery in neurodegenerative disease. Methods in Molecular Biology. 2011;793:443–455. doi: 10.1007/978-1-61779-328-8_29. [DOI] [PubMed] [Google Scholar]
- 28.Herrera-Marschitz M, Arbuthnott G, Ungerstedt U. The rotational model and microdialysis: Significance for dopamine signaling, clinical studies, and beyond. Prog Neurobiol. 2010;90(2):176–189. doi: 10.1016/j.pneurobio.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 29.Cenci MA, Lundblad M. Ratings of L-DOPA–induced dyskinesia in the unilateral 6-OHDA lesion model of PD in rats and mice. Curr Protoc Neurosci. 2007;Chapter 9:Unit 9.25. doi: 10.1002/0471142301.ns0925s41. [DOI] [PubMed] [Google Scholar]