Significance
Spinal cord injury (SCI) often results in severely impaired locomotor, sensory, and autonomic function. Although inflammation contributes to the physiopathology of SCI, several clinical trials of high doses of dexamethasone or methylprednisolone have not resulted in improved recovery of function. IL-37, a member of the IL-1 family, exerts broad antiinflammatory effects in several mouse models of inflammatory diseases. We report here that mice expressing human IL-37 exhibit reduced inflammation in the central nervous system and demonstrate significantly improved functional disabilities following SCI. The administration of recombinant forms of human IL-37 enhances motor skills after SCI, suggesting that IL-37 could provide a new therapeutic approach to limit the harmful effects of inflammation in neurologic conditions.
Keywords: IL-37, spinal cord injury, inflammation, neuroprotection, cytokines
Abstract
IL-37, a member of the IL-1 family, broadly reduces innate inflammation as well as acquired immunity. Whether the antiinflammatory properties of IL-37 extend to the central nervous system remains unknown, however. In the present study, we subjected mice transgenic for human IL-37 (hIL-37tg) and wild-type (WT) mice to spinal cord contusion injury and then treated them with recombinant human IL-37 (rIL-37). In the hIL-37tg mice, the expression of IL-37 was barely detectable in the uninjured cords, but was strongly induced at 24 h and 72 h after the spinal cord injury (SCI). Compared with WT mice, hIL-37tg mice exhibited increased myelin and neuronal sparing and protection against locomotor deficits, including 2.5-fold greater speed in a forced treadmill challenge. Reduced levels of cytokines (e.g., an 80% reduction in IL-6) were observed in the injured cords of hIL-37tg mice, along with lower numbers of blood-borne neutrophils, macrophages, and activated microglia. We treated WT mice with a single intraspinal injection of either full-length or processed rIL-37 after the injury and found that the IL-37–treated mice had significantly enhanced locomotor skills in an open field using the Basso Mouse Scale, as well as supported faster speed on a mechanical treadmill. Treatment with both forms of rIL-37 led to similar beneficial effects on locomotor recovery after SCI. This study presents novel data indicating that IL-37 suppresses inflammation in a clinically relevant model of SCI, and suggests that rIL-37 may have therapeutic potential for the treatment of acute SCI.
The inflammatory response plays an essential role in tissue protection after injury or invasion by microorganisms (1, 2). Regardless of the tissue, unless regulated, inflammation can become chronic and result in tissue damage and loss of function (1, 2). This is particularly the case in spinal cord injury (SCI). After spinal cord contusion or compression injury, there is a rapid initiation of inflammation in rodents and in humans (2). This response is orchestrated by endogenous microglial cells and by circulating leukocytes, especially monocytes and neutrophils, which invade the lesion site during the first hours and days after injury (2–4). Although these cells are required for the clearance of cellular and myelin debris, they also release cytokines and cytotoxic factors, which are harmful to neurons, glia, axons, and myelin, resulting in secondary damage to adjacent regions of the spinal cord that had been previously unaffected by the insult (2, 5, 6). Indeed, it is currently well accepted that inflammation is a major contributor to secondary cell death after SCI. The damaging effects of inflammation are more pronounced in the central nervous system (CNS) than in other tissues, because of the limited capacity for axon regeneration and replenishment of damaged neurons and glial cells, which leads to irreversible functional disabilities (7, 8). Therefore, targeting inflammation is a valuable approach to promoting neuroprotection and limiting functional deficits in SCI.
Cytokines are key players in the initiation, progression, and suppression of inflammation. Although several members of the IL-1 family are proinflammatory (9, 10), IL-37 has broad suppressive effects on innate inflammation and acquired immunity (11–14). Because a complete ORF for the mouse homolog of IL-37 has not yet been found, it was necessary to generate a strain of transgenic mice expressing human IL-37, designated hIL-37tg mice. These mice are protected against endotoxin shock, colitis, hepatitis, and myocardial infarction (9, 13, 15–18); however, a role for IL-37 after CNS trauma remains unexplored. In the present study, we subjected hIL-37tg mice to SCI and studied subsequent functional impairments in comparison with wild type (WT) mice. We also administered recombinant human IL-37 (rIL-37) to WT mice, to provide a rationale for clinical use of IL-37 as a therapeutic agent. We provide direct evidence for the first time, to our knowledge, that IL-37 exerts marked antiinflammatory properties on the contused spinal cord and confers protection from tissue damage and functional loss.
Results
hIL-37tg Mice Exhibit Reduced Functional Deficits and Tissue Damage After SCI.
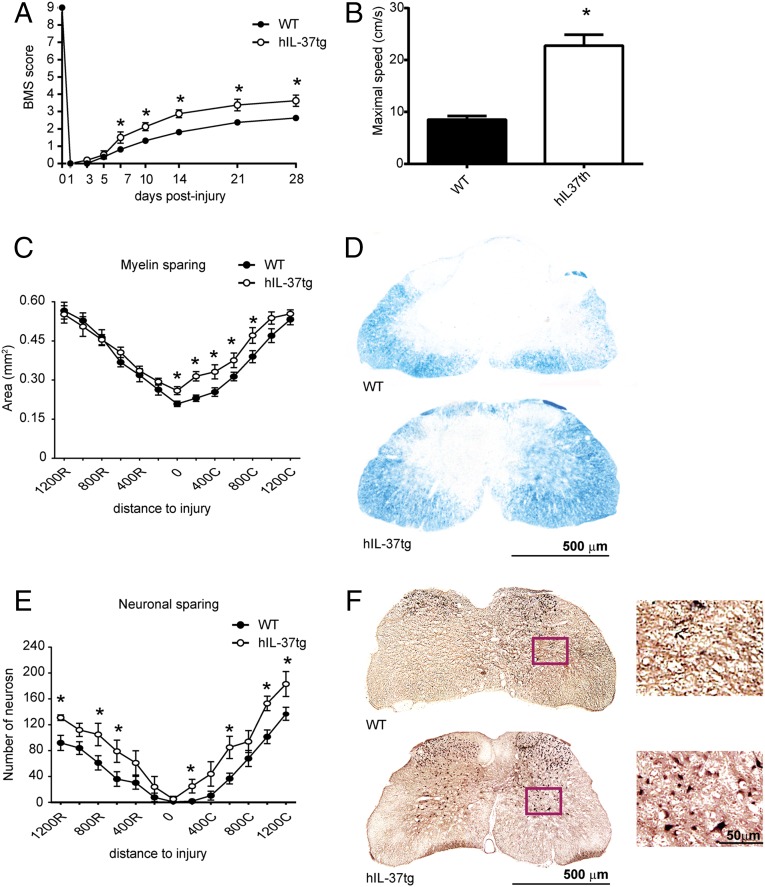
As shown in Fig. 1A, hIL-37tg mice displayed significant improvement in locomotor recovery after SCI. There were significant improvements in Basso Mouse Scale (BMS) scores starting at 7 days postinjury (dpi), and BMS scores remained significantly enhanced for the subsequent 3-wk period (Fig. 1A). At 28 dpi, all WT mice showed extensive ankle movement, but only 50% of these mice showed plantar paw placement but no weight-bearing stepping (BMS score 2.5). In contrast, all hIL-37tg mice showed extensive ankle movement and plantar paw placement with weight support, and the majority displayed occasional stepping (BMS score 3.8) (Fig. 1A). Moreover, the hIL-37tg mice performed ∼2.5-fold faster locomotion on the treadmill compared with the WT mice (Fig. 1B), further demonstrating the protective effect of IL-37 against functional loss in SCI.
Fig. 1.
hIL-37-tg mice show enhanced functional outcomes and reduced tissue damage after SCI. (A and B) Locomotor skill assessment using the nine-point BMS (A) and on a treadmill (B). (C) Myelin sparing at various distances rostral and caudal to the injury epicenter. (D) Representative micrographs showing myelin sparing at the injury epicenter in sections stained against LFB from WT and hIL-37tg mice. (E) Ventral horn neuron survival at various distances rostral and caudal to the injury epicenter. (F) Representative micrographs showing sparing of ventral horn neurons in WT mice (Upper) and hIL-37tg mice (Lower) in sections stained against NeuN 200 µm rostral to the injury epicenter. (Insets) Higher-magnification views of the areas outlined in the boxes. Data are expressed as mean ± SEM. n = 8 per group. *P < 0.05, two-way repeated-measures ANOVA with Bonferroni’s post hoc correction in A, C, and D and the t test in B.
We then assessed whether the improvement in motor skills of hIL-37tg mice was associated with reduced secondary tissue damage after SCI. Histological sections from the injury epicenter stained with Luxol fast blue (LFB) revealed enhanced myelin sparing in hIL-37tg mice compared with WT mice (Fig. 1 C and D). Prevention of myelin loss by transgenic expression of IL-37 was evident at the lesion epicenter and at distances up to 800 µm caudal to the injury (Fig. 1 C and D). Assessment of neuronal sparing in the ventral horns also demonstrated attenuated neuronal loss in hIL-37tg mice (Fig. 1 E and F). Spinal cord sections stained against NeuN revealed greater numbers of neurons at several regions rostral and caudal to the injury epicenter in hIL-37tg mice (Fig. 1 E and F).
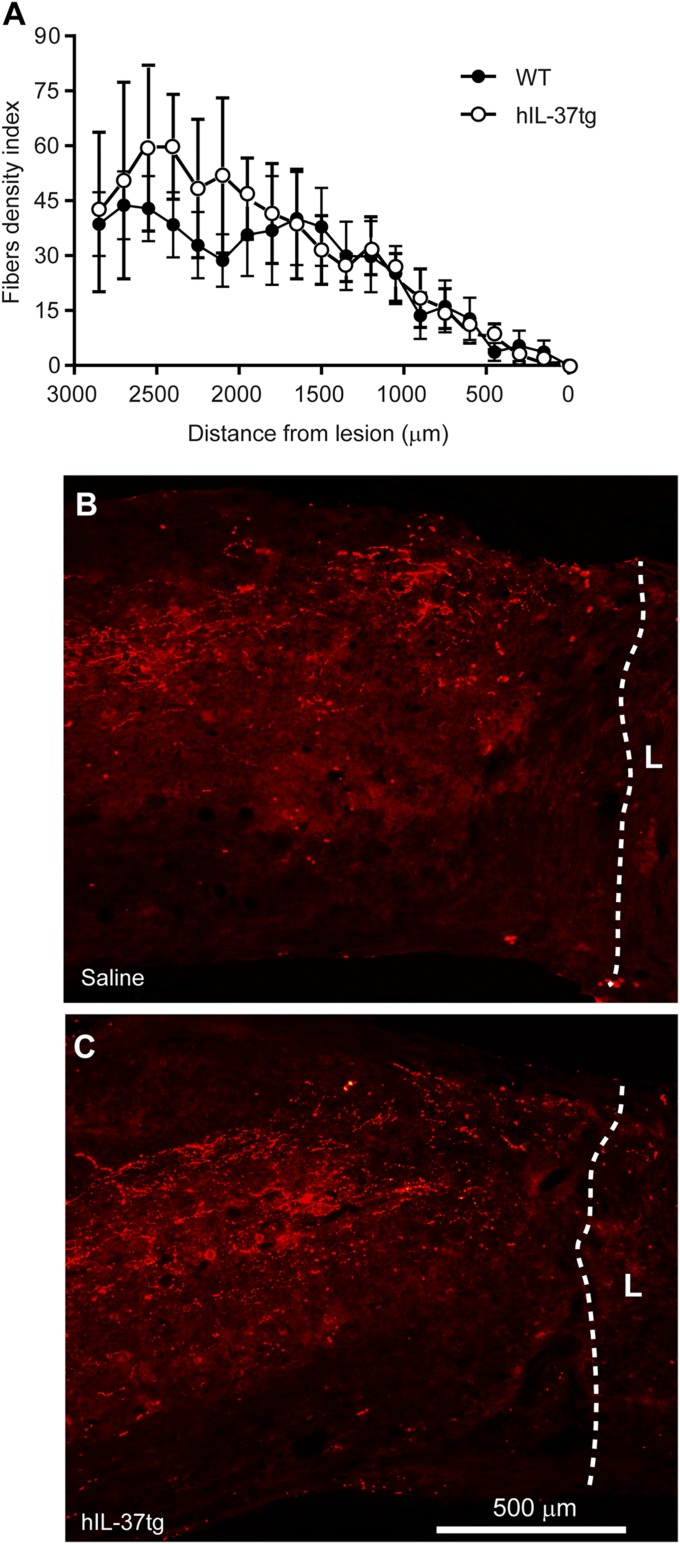
Because infiltrating macrophages inhibit axonal outgrowth by releasing soluble factors and by cell–cell interaction (19, 20), we sought to evaluate whether there was enhanced axonal regeneration in hIL-37tg mice after complete spinal cord transection. Both the hIL-37tg and WT mice presented complete hindlimb paralysis after the injury and lacked functional improvement at 10 wk postinjury (BMS score 0). Histological assessment of sagittal spinal cord tissue sections revealed that transgenic expression of IL-37 did not lead to regeneration of axons beyond the transection site. Similarly, axonal counts at rostral distances to the injury did not reveal any difference between the hIL-37tg and WT mice (Fig. 2), suggesting that IL-37 does not promote regeneration/sprouting of corticospinal axons (Fig. 2). Overall, these data provide evidence that IL-37 confers protection from functional disabilities and secondary tissue damage after spinal cord contusion injury, but does not promote axonal outgrowth of the corticospinal tract.
Fig. 2.
hIL-37tg expression does not promote axonal regeneration. (A) BDA-labeled corticospinal axons at different distances to the transection site. (B and C) Low-magnification images of complete transected spinal cords showing BDA-labeled corticospinal fibers in WT mice (B) and hIL-37tg mice (C). Lines define the transection sites. L, lesion. Data are expressed as mean ± SEM. n = 8 per group. *P < 0.05, one-way ANOVA with Bonferroni’s post hoc correction.
IL-37 Is Induced in hIL-37tg Mice After SCI.
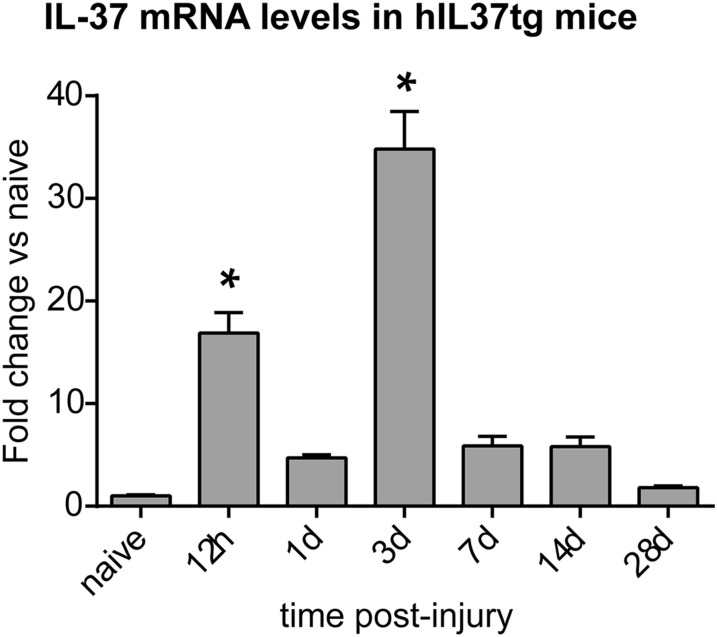
Because hIL-37tg mice were markedly protected against functional disabilities and tissue loss after SCI, we studied the inflammatory response in these mice. We first assessed the expression profile of IL-37 in the spinal cord of hIL-37tg mice as determined by PCR. As expected, IL-37 was not present in the intact or damaged spinal cord obtained from WT mice. The hIL-37tg mice exhibited low levels of constitutive expression of IL-37 in the spinal cord. Low levels of constitutive IL-37 expression in transgenic mice also has been observed in the colon, skin, circulating leukocytes, and cell lines transfected with IL-37 (11, 13, 16). The low levels are due to the instability sequence in human IL-37 (14). However, after contusion injury, induction of IL-37 in the spinal cord parenchyma was observed (Fig. 3). The IL-37 expression profile showed two peaks of expression, after 12 h postinjury (hpi) and at 3 dpi, when IL-37 levels increased by ∼17- and ∼35- fold, respectively (Fig. 3). The early peak of IL-37 coincides with the induction of cytokines in the injured spinal cord at 6–24 hpi (Figs. S1 and S2), whereas the latter peak (at 3 dpi) correlates with the infiltration of blood monocytes (2).
Fig. 3.
Expression of IL-37 after SCI. Shown is the time course of IL-37 transcripts in the spinal cords of hIL-37tg mice after contusion injury. n = 4 per time point. *P < 0.05, one-way ANOVA with Bonferroni’s post hoc test.
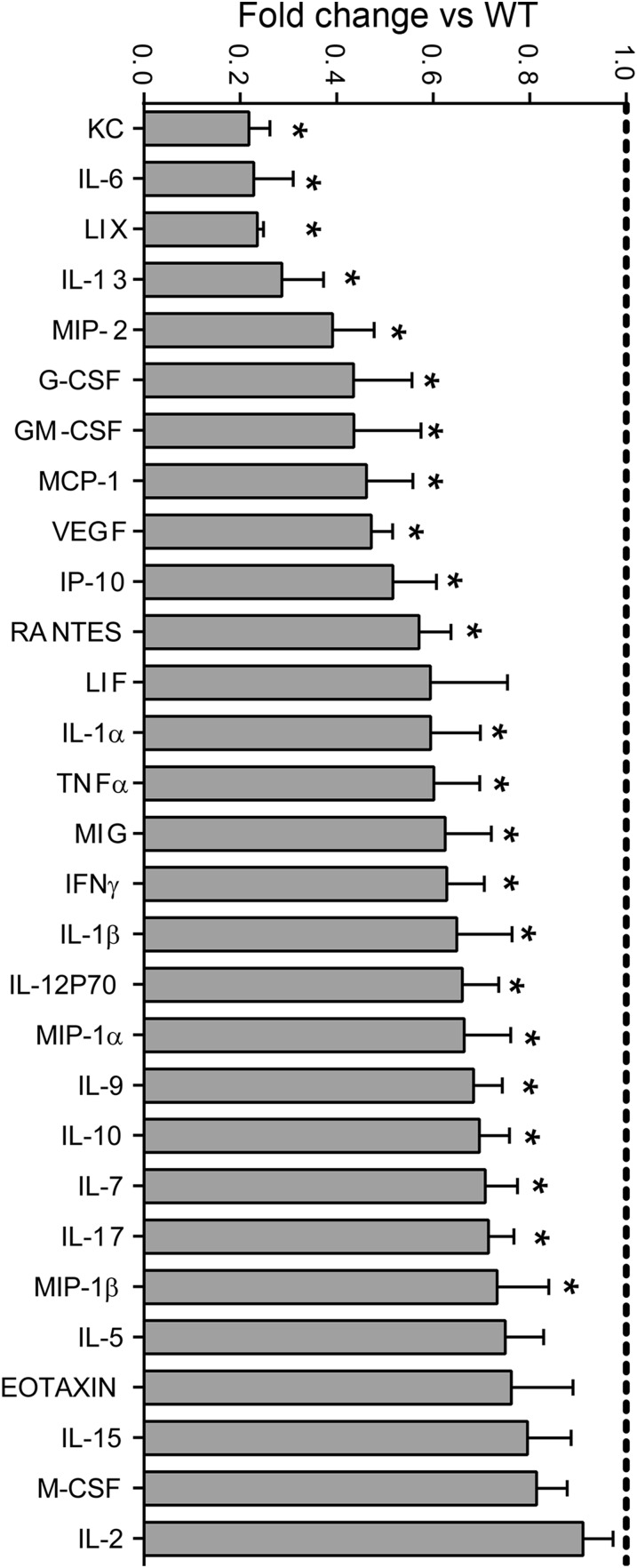
Fig. S1.
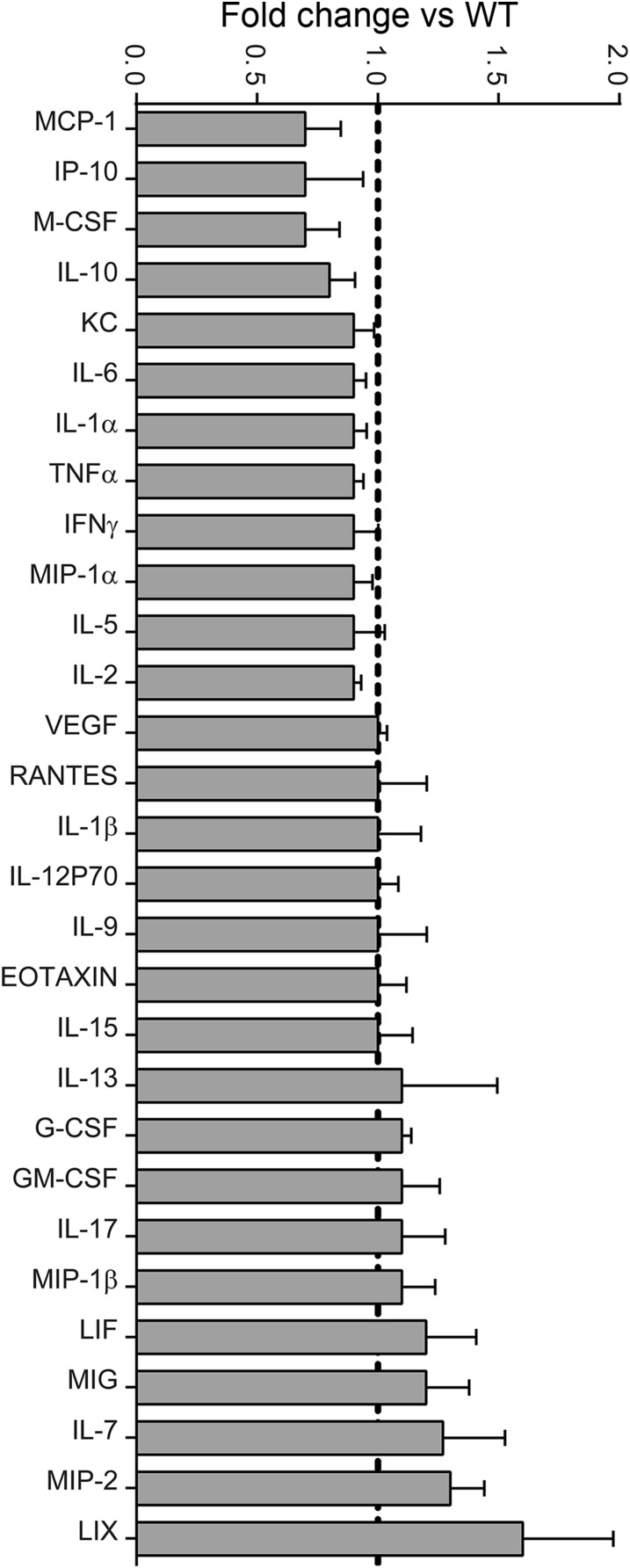
Multiplex analysis of cytokine protein profiles from spinal cords of WT and hIL-37tg mice at 24 hpi. Data are expressed as fold change vs. WT mice (mean ± SEM).
Fig. S2.
Multiplex analysis of cytokine protein profiles from spinal cords of WT and hIL-37tg mice at 12 hpi. Data are expressed as fold change vs. WT mice (mean ± SEM).
Inhibition of Cytokine and Chemokine Expression After SCI.
We next sought to examine whether the early increase in IL-37 modulates gene expression of cytokines in the contused spinal cord. We evaluated the protein levels of 32 cytokines in injured spinal cords harvested at 12 hpi and 24 hpi, periods when the protein levels of most cytokines and chemokines reach maximal concentrations after SCI (2). At 12 hpi, cytokine levels were unchanged in the hIL-37tg mice relative to WT mice (Fig. S2); however, at 24 hpi, significant reductions in the expression of 23 of the 32 cytokines in were observed in the hIL-37tg mice (Fig. S1). Moreover, the expression of IL-3, which was detected at low levels in the spinal cord homogenates of WT mice (0.56 ± 0.03 pg/mg protein), was undetectable in hIL-37tg mice (<0.45 pg/mg protein). The levels of six cytokines (LIF, M-CSF, IL-2, IL-5, IL-15, and eotaxin) were unchanged in the hIL-37tg mice, whereas expression of IL-4 and IL-12p40 was not detected in the contused spinal cords of both experimental groups (Fig. S1).
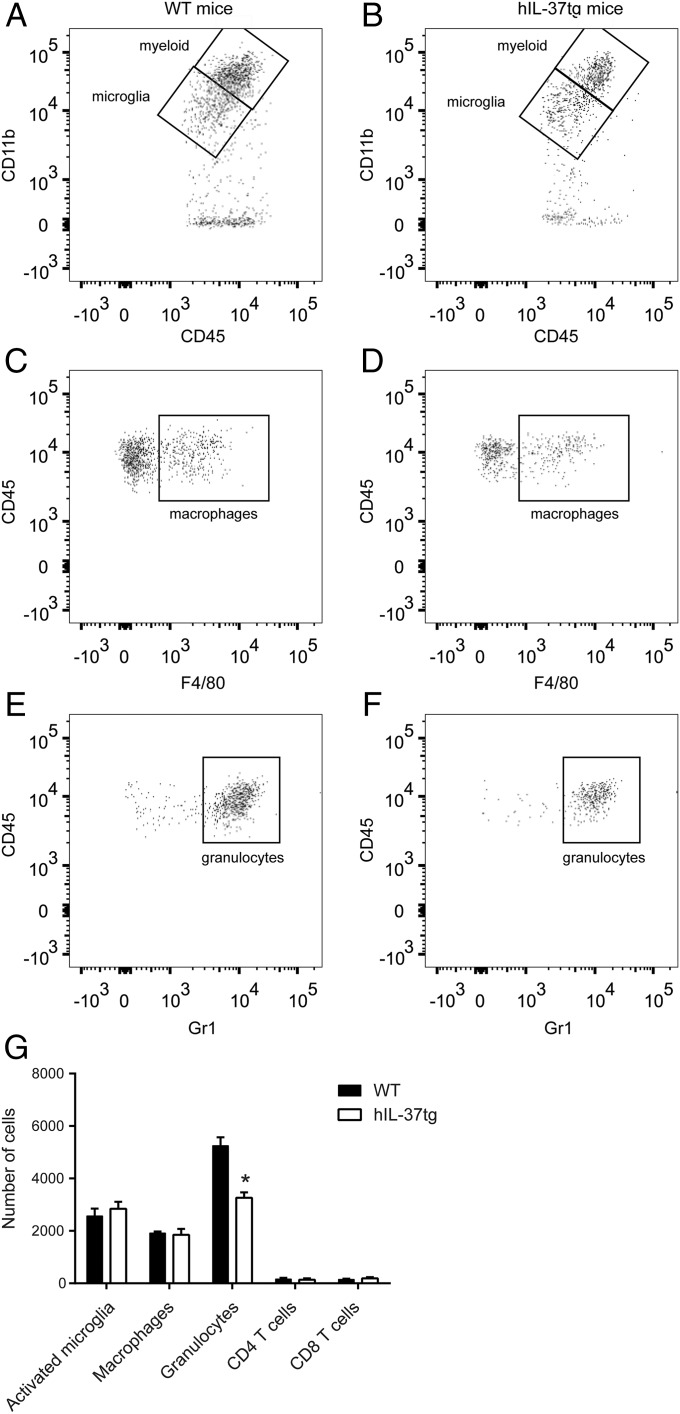
We next evaluated the accumulation of inflammatory cells in WT mice and hIL-37tg mice following SCI. At 1 dpi, when granulocyte infiltration reaches peak levels, the spinal cords of hIL-37tg mice showed an ∼40% reduction in the number of granulocytes (Fig. 4). There were no between-group differences in cell counts of activated microglia, blood-borne macrophages, CD4 and CD8 T cells at this time point (Fig. 4).
Fig. 4.
Spinal cord immune cell counts at 1 dpi. (A–F) Representative density plots of FACS analysis showing myeloid cells and microglia (A and B), macrophages (CD45high, CD11b+, F4/80+) (C and D), and granulocytes (CD45high, CD11b+, Gr1high) (E and F) in the spinal cords of WT and hIL-37tg mice. (G) Quantification of the different immune cell populations in the injured spinal cords. Data are expressed as mean ± SEM. n = 4 per group. *P < 0.05, t test.
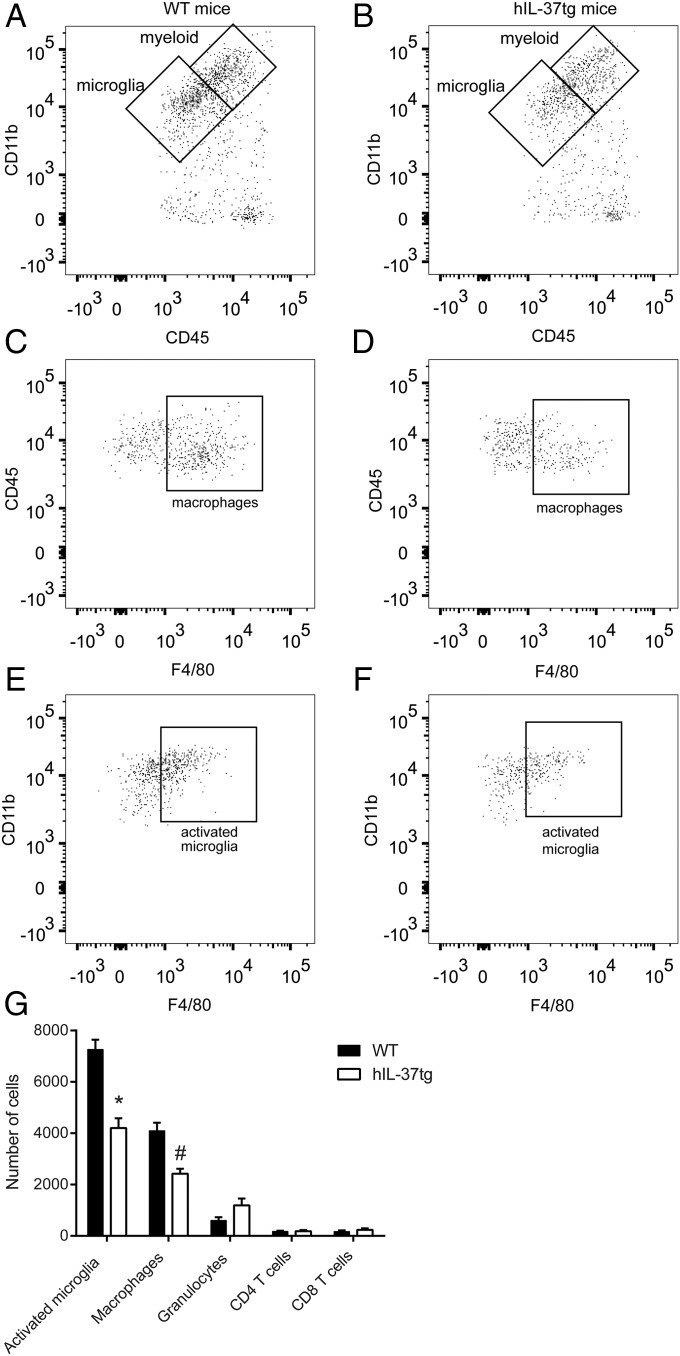
At 7 dpi, when the accumulation of activated microglia and macrophages reaches peak levels in the injured spinal cord and accounts for ∼80% of total immune cells, spinal cords from the hIL-37tg mice exhibited significantly lower numbers of these two cell subsets (Fig. 5). The number of granulocytes, which was reduced by 90% compared with that at 1 dpi, was slightly increased in the hIL-37tg mice, but the difference did not reach statistical significance, whereas CD4 and CD8 lymphocyte counts were unchanged (Fig. 3).
Fig. 5.
Recruitment of immune cells within the spinal cord at 7 dpi. (A–F) Representative density plots of FACS analysis showing myeloid cells and microglia (A and B), macrophages (CD45high, CD11b+, F4/80+) (C and D), and activated microglia (CD45low, CD11b+, F4/80+) (E and F) in the spinal cords of WT and hIL-37tg mice. (G) Quantification of the different immune cell populations in the injured spinal cords. Data are expressed as mean ± SEM. n = 4 per group. *P < 0.05; *P < 0.001; #P = 0.001, t test.
We also assessed whether IL-37 modulates macrophage and microglia polarization after SCI. FACS analysis of contused spinal cords revealed no between-group differences in the expression of CD16/32 and CD206, known markers of M1 and M2 activation, respectively, on microglia (CD16/32: 76.2 ± 4.2 in WT vs. 74.6 ± 3.8 in hIL-37tg; CD206: 14.1 ± 3.1 in WT vs. 15.2 ± 3.1 in hIL-37tg) or macrophages (CD16/32: 82.2 ± 6.7 in WT vs. 79.4 ± 5.3 in hIL-37tg; CD206: 14.5 ± 1.9 in WT vs. 16.6 ± 3.2 in hIL-37tg).
Intralesional Administration of rIL-37 Improves Functional Outcomes After SCI.
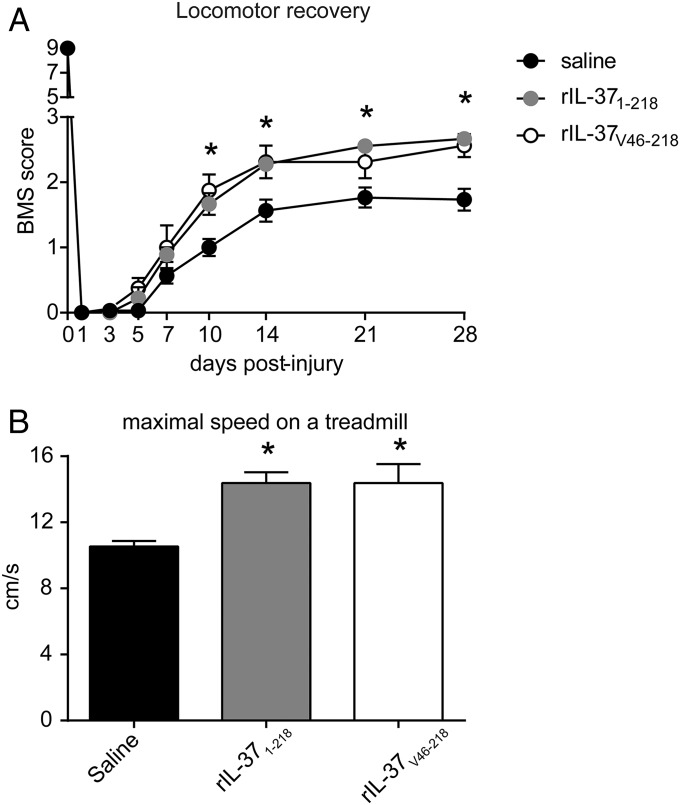
To assess a therapeutic use of IL-37 in SCI, we administered rIL-37 to determine possible beneficial effects in SCI. We first tested the IL-37 precursor (full-length IL-37 isoform b), because previous studies have shown efficacy in vivo (21) and in vitro (22). We also administered a processed form of IL-37 with the N terminus at valine 46 (22, 23). Because the blood-brain barrier prevents the entry of most molecules into the CNS, we infused rIL-37 into the lesion site at 5 min after the contusion injury, using a glass capillary (30-µm diameter). As shown in Fig. 6A, intraspinal injection of either full-length or processed rIL-37 significantly enhanced locomotor recovery as demonstrated by the BMS score, starting at 10 dpi. At 28 dpi, mice injected with saline had a BMS score of 1.6, which corresponds to slight or extensive movement of the ankle; however, treatment with full-length rIL-37 improved the BMS score by 1 point (Fig. 6A). The rIL-37–treated mice showed extensive movement of the ankle, and most also displayed plantar paw placement in at least one paw, but no stepping. Moreover, treatment with full-length rIL-37 increased the speed that mice were able to achieve on a treadmill by 50% (Fig. 6B), further supporting the beneficial effects of rIL-37 in preventing functional deficits after SCI. Overall, both forms of rIL-37 led to similar beneficial effects on motor skills (Fig. 6).
Fig. 6.
Infusion of rIL-37 promotes functional recovery after SCI. Locomotor performance of C57BL/6 mice treated with intraspinal injections of saline, full-length IL-37b (IL-371–218), or the processed form of IL-37b (IL-37V46–218) after SCI using the BMS (A) and treadmill (B). Data are shown as mean ± SEM. n = 14 for saline; n = 9 for rIL-371–218 and rIL-37V46–218. *P < 0.05, two-way repeated-measures ANOVA with Bonferroni’s post hoc correction in A and the t test in B.
Discussion
Although IL-37 is lacking in mice, hIL-37tg mice are protected against several inflammatory challenges. Previous studies using hIL-37tg mice have consistently demonstrated the suppressive properties of IL-37 on inflammation (11, 13, 16); however, whether IL-37 exerts a similar antiinflammatory effect in the CNS trauma has remained unknown. Here we demonstrate for the first time, to our knowledge, that IL-37 suppresses inflammation and limits locomotor deficits and tissue damage in spinal cord contusion injury in mice. This conclusion is based on observations in hIL-37tg mice as well as in WT mice treated with either rIL-37 precursor or processed IL-37.
In hIL-37tg mice, IL-37 expression is regulated by a constitutive cytomegalovirus promoter (13), and thus expression should be induced in all cells. However, constitutive expression of IL-37 in the spinal cord is low, owing to the presence of an instability sequence that limits the half-life of the IL-37 transcript (14), as has been shown in other models using these mice (11, 13, 16). However, in inflammatory conditions, such as tissue trauma, activation of inflammatory components increases the half-life of IL-37 mRNA and allows for translation of the protein IL-37. IL-37 mRNA stability is apparently regulated by instability elements present in exon 5, given that deletion of exon 5 can significantly increase the mRNA stability of both IL-37b and IL-37c. Similar mRNA stabilization on LPS stimulation has been reported for IL-18 (14). We observed that mRNA levels were increased at 12 hpi, followed by a secondary increase at 3 dpi. Whether endogenous IL-37 is expressed in human SCI remains unknown, however. Cytokines such as IL-1β, TLR agonist, and TGF-β are known to induce IL-37 in vitro (13). Cytokine expression reaches maximal levels in the spinal cord within 6–24 hpi (2), which may account for the first peak of IL-37 expression. Although IL-37 is expressed mainly in macrophages in hIL-37tg mice after several inflammatory challenges (13, 16, 24), endogenous glial cells (astrocytes and microglia) are likely the early source of IL-37, owing to the low amount of infiltrated leukocytes at this time point. However, the later expression peak of IL-37 at 3 dpi coincides with the entrance of blood-borne monocytes into the spinal cord, suggesting that macrophages are the main source of this second peak (2).
IL-37 reduces the expression of several proinflammatory cytokines in cell cultures and in various inflammatory disorders (13, 15, 16, 18, 25). We also found that IL-37 attenuates the protein levels of most proinflammatory cytokines evaluated in the contused spinal cord, with IL-6 the most markedly effected. The capacity of IL-37 to reduce cytokine production after SCI affects the infiltration and activation of immune cells. Indeed, we found that IL-37 reduced the recruitment of neutrophils and macrophages into the injured spinal cord, as well as the activation state of the microglia; however, it did not affect the infiltration of T cells.
Regardless of the species or type of SCI, antiinflammatories or myeloid cell depletion consistently reveal reduced tissue damage and greater functional outcomes (2, 3). Here we show that hIL-37tg mice exhibit enhanced locomotor function associated with attenuated tissue damage after SCI. Blood-borne monocytes infiltrating into the injured spinal cord mediate axonal retraction (19, 20), and this deleterious effect is due to products released by macrophages or by integral macrophage membrane proteins, which inhibit the growth and guidance of axons (19, 20). Other inhibitory molecules include chondroitin sulfate proteoglycans, Nogo, ephrins, and semaphorins, which are expressed at the lesion site by astrocytes, oligodendrocytes, and some precursor cells (7). In addition, adult CNS neurons have a limited ability to switch on the intrinsic regeneration machinery after axotomy (26). However, despite reduced macrophage numbers in the spinal cords of hIL-37tg mice, axonal outgrowth was not observed, because these other factors, which hamper the ability of axons to regenerate in the injured CNS, might not be altered in these mice. Therefore, our data indicate that attenuation of inflammation in the hIL-37tg mice did not overcome the extrinsic and intrinsic factors that curtail the relatively limited regenerative potential of injured corticospinal axons. It is possible that increasing the dosage of rIL-37 could enhance the axonal outgrowth of other motor or sensory tracts with better regeneration capabilities.
Once synthesized after activation, up to 30% of the IL-37 precursor is cleaved intracellularly by activated caspase-1 and translocates to the nucleus, most likely in association with Smad3 (13, 17). Indeed, the antiinflammatory effects of IL-37 are abolished in LPS-stimulated macrophages when incubated with caspase-1 inhibitors, or when the caspase-1 site (D20) is mutated and cannot translocate to the nucleus (27). Similarly, in vivo studies also have revealed that the antiinflammatory actions of IL-37 are lost when Smad3 is silenced after LPS challenge (13).
On the other hand, the precursor form of IL-37 is released from human monocytes, or cell lines transfected with IL-37 after LPS stimulation (13). The release of IL-37 is independent of caspase-1 cleavage at D20, however (27). The administration of neutralizing antibodies against IL-37 enhances cytokine production in in vitro and in vivo, highlighting the extracellular antiinflammatory function of IL-37 (22, 27). These observations are further supported by studies showing the ability of rIL-37 protein to reduce cytokine production (22). Extracellular IL-37 carries out its antiinflammatory actions by recruiting the decoy IL-1R8 to the IL-18Rα/IL-37 complex (28–30). Administration of rIL-37 improves functional recovery after SCI, indicating that extracellular IL-37 is sufficient for its beneficial effects in the CNS. Nevertheless, we cannot rule out the possibility that the translocation of IL-37 to the nucleus also plays a role in the transgenic mice, as well as in humans.
Processing of the IL-37 precursor appears to occur extracellularly. Edman degradation of supernatants from cells lines transfected with IL-37b revealed a processed form starting at valine 46 (IL-37V46–218) (23). Both full-length and processed IL-37 exhibit antiinflammatory effects (22). We found that both IL-37 forms exerted similar beneficial effects on functional outcomes after SCI, suggesting that the IL-37 precursor may be processed when administered in the injured spinal cord.
In summary, rIL-37 prevents functional deficits and secondary tissue damage in SCI, suggesting a potential clinical application. To our knowledge, this is the first report suggesting that IL-37 may be an effective therapy during the acute phase after SCI, for which there is currently no effective treatment.
Materials and Methods
Animals.
All experimental procedures were approved by the Universitat Autònoma de Barcelona Animal Experimentation Ethical Committee (CEEAH 1188R3-DMAH 6131) and followed the European Communities Council Directive 2010/63/EU, and the methods for each procedure were carried out in accordance with the approved guidelines. Experiments were performed in adult (8–10 wk) female mice expressing the human form of IL-37 (hIL-37tg) and C57BL/6 (WT) mice (Charles River Laboratory). After SCI, motor skills were assessed in hIL-37tg mice, WT mice, and WT mice treated with rIL-37. Detailed information on SCI models, rIL-37 delivery, and functional assessment is provided in SI Materials and Methods.
Tissue Processing.
Spinal cords were cut on a cryostat and stained for assessment of neuron survival, myelin sparing, and axonal regeneration. Spinal cords were also processed for RNA, cytokine protein level, and FACS analyses. Details are provided in SI Materials and Methods.
SI Materials and Methods
Surgical Procedure.
All surgical procedures were approved by the Universitat Autònoma de Barcelona Animal Care Committee were performed in accordance with the guidelines of the European Commission on Animal Care. Adult (8–10 wk old) female C57BL/6 mice (Charles River Laboratory) and hIL-37tg mice (13) were anesthetized with ketamine (90 mg/kg, i.m.) and xylazine (10 mg/kg, i.m.). After laminectomy at the 11th thoracic vertebrae, the exposed spinal cord was contused with a force of 60 kdyn using the Infinite Horizon Impactor device (Precision Scientific Instrumentation) (31) or was completely transected using a microscalpel. At 56 d after complete spinal cord transection, the corticospinal tract was labeled by injecting a 10% (wt/vol) solution of the axonal tracer biotinylated dextran amine (BDA) into the sensorimotor cortex (32).
Administration of rIL-37 protein (b isoform) was performed intraspinally by means of a glass capillary (30 µm internal diameter; Eppendorf) coupled to a 10-mL syringe (Hamilton #701). Then, at 5 min postinjury, 1 μL of saline, saline containing 100 ng of full-length (rIL-371–218), or rIL-37V46–218 was injected into the injured spinal cord at the lesion site. Injections were made at a perfusion speed of 2 µL/min, controlled by an automatic injector (KDS 310 Plus; KD Scientific), and the tip of the glass capillary was left in the cord tissue for 3 min after each injection to avoid liquid reflux.
RNA Isolation, Reverse Transcription, and Real-Time PCR.
The mice were perfused with sterile saline, and a 5-mm-long section of uninjured spinal cord was removed. Tissue was homogenized with QIAzol lysis reagent (Qiagen), and RNA was extracted using the RNeasy Lipid Tissue Kit (Qiagen), according to the manufacturer’s protocol. RNA thus obtained was treated with DNase I (Qiagen) to eliminate genomic DNA contamination. Then 1 μg of RNA was primed with random hexamers (Promega) and reverse-transcribed using the Omniscript RT Kit (Qiagen). RNase inhibitor (Roche) was added (1 U/μL final concentration) to avoid RNA degradation. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) served as a housekeeping gene.
Cytokine Protein Expression.
The mice were perfused with sterile saline, and 5-mm lengths of spinal cord centered on the lesions were collected at 12 hpi and 24 hpi and then snap-frozen. The spinal cords were then homogenized, and protein concentration was determined using the DC Protein Assay (Bio-Rad). Samples were concentrated to 4 µg/µL using MicroCon centrifugation filters (Millipore) to ensure equal amounts of protein. The protein levels of 32 cytokines and chemokines were then analyzed using the Milliplex MAP Mouse Cytokine/Chemokine Magnetic Bead Panel on a Luminex system (EMD Millipore) in accordance with the manufacturer’s protocol.
Flow Cytometry.
Immune cells from the injured spinal cords were analyzed by flow cytometry at 1 dpi and 7 dpi as described previously (33). In brief, spinal cords were cut into small pieces and passed through a 70-µm cell strainer (BD Falcon) using saline. The cell suspension was centrifuged at 300 × g for 10 min at 4 °C. The pellet was then resuspended and centrifuged a second time. The samples were divided, and cells alone and isotype-matched control samples were generated to control for nonspecific binding of antibodies and for autofluorescence. The following antibodies were purchased from eBioscience: CD45-PerCP, CD11b-PE-Cy7, Gr1-FITC, F4/80-APC, F4/80-PE, CD3-FITC, CD4-APC, CD8-APC, and CD19-PE. After 30 min of incubation with combinations of antibodies at 4 °C, the samples were washed and fixed in 1% paraformaldehyde. Various combinations of markers were used to identify activated microglia (CD45low, CD11b+, F4/80+), granulocytes (CD45high, CD11b+, F4/80−, Gr-1high), macrophages (CD45high, CD11b+, F4/80+), CD4 T cells (CD45+, CD11b−, CD3+, CD4+), and CD8 T cells (CD45+, CD11b, CD3+, CD8+) (31). In addition, microglia and macrophages were further differentiated based on CD16/32 and CD206 and to assess M1 and M2 polarization, respectively. Cells were analyzed using FlowJo software on a FACSCanto flow cytometer (BD Biosciences).
Functional Assessment.
Locomotor recovery was evaluated at 1, 3, 5, 7, 10, 14, 21, and 28 dpi in an open-field test using the nine-point BMS (34), which was developed specifically for locomotor testing after contusion injuries in mice. The BMS analysis of hindlimb movements and coordination was performed by two independent and blinded assessors, and a consensus score was derived. In addition, at the end of the follow-up period (28 dpi), the highest locomotion speed of the mice was evaluated on a belt of a motorized treadmill. In brief, each mouse was allowed to explore the treadmill compartment for 5 min, with the motor speed set to zero. Then the speed was increased by 2 cm/s every 5 s until the animal was not able to maintain the speed. The maximum speed at which each mouse was able to achieve for 5 s was recorded.
Histology.
Mice were perfused with 4% paraformaldehyde in 0.1 M phosphate buffer (PB) at 12 hpi, 3 dpi, and 28 dpi. A 5-mm length of spinal cord containing the lesion site was removed, cryoprotected with 30% sucrose in 0.1 M PB at 4 °C, and six series of 10-µm-thick sections were picked up on glass slides. Thus, adjacent sections on the same slide were 100 µm apart. For demyelination analyses, sections were stained with LFB (Sigma-Aldrich). After graded dehydration, sections were placed in a 1 mg/mL LFB solution in 95% ethanol and 0.05% acetic acid overnight at 37 °C. Sections were then washed in 95% ethanol and distilled water before being placed into a solution of 0.5 mg/mL Li2CO3 in distilled water for 1 min at room temperature. After washing in distilled water, sections were dehydrated and mounted in DPX mounting medium (Sigma-Aldrich). For neuronal assessment, sections were incubated overnight at 4 °C with biotinylated antibodies against NeuN. After several washes in PBS, sections were stained using the ABC kit (Vector Laboratories), and a coverslip was applied in DPX mounting medium (Sigma-Aldrich).
The epicenter of the injection or contusion injury impact was determined for each mouse spinal cord by localizing the tissue section with the greatest damage using LFB-stained sections. Myelin sparing after SCI was calculated by delineating the spared LFB- stained tissue area from images taken at 40× magnification at the injury epicenter and every 200 µm rostral and caudal to the lesion site. Neuronal survival was assessed by manually counting the number of NeuN+ cells in high-magnification (400×) images obtained at both ventral horns at the injury epicenter and every 200 µm rostral and caudal to the lesion site. ImageJ software was used to quantify all of the histological parameters.
At 10 d after the BDA tracer injections (66 dpi), mice were perfused, and the transected spinal cords were harvested and cut into 20-µm-thick sagittal sections as described above. Visualization of corticospinal fibers was done by incubating the spinal cord sections with Alexa Fluor 594-conjugated streptavidin. Pictures from the spinal cord covering the transection lesion and up 3,000 µm rostral were obtained at 200× magnification and then merged into collages using Adobe Photoshop software. Only sections that contained BDA-labeled fibers were acquired. The number of pixels of BDA-labeled fibers with a dorsoventral line was counted every 150 µm over the rostrocaudal axis using ImageJ.
Statistical Analysis.
All analyses were conducted with SPSS version 19. The two-tailed Student’s t test was used for single comparisons between two groups. Maximal speed on a treadmill was analyzed using the Mantel–Cox test. Functional follow-up for BMS scores and subscores, along with histological analyses of myelin and neuronal sparing as well as BDA+ fibers, were done using two-way repeated-measures ANOVA. Post hoc comparisons were carried out only when a main effect showed statistical significance. P values for multiple comparisons were adjusted using Bonferroni’s post hoc correction. Results are expressed as mean ± SEM. Differences were considered significant at P < 0.05.
Acknowledgments
This work was supported by grants from the Fundació La Marató, Spanish Ministry of Economy and Competitiveness (SAF2013-48431-R), International Foundation for Research in Paraplegia, and Fondo de Investigación Sanitaria of Spain [Red de Terapia Celular (TERCEL) and Centro de Investigación Biomédica en Red, Enfermedades Neurodegenerativas (CIBERNED)], all to R.L.-V.; the National Institutes of Health (Grant AI-15614, to C.A.D.); and the Deutsche Forschungsgemeinschaft (Grant BU 1222/3-3, to P.B.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1523212113/-/DCSupplemental.
References
- 1.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510(7503):92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.David S, López-Vales R, Wee Yong V. Harmful and beneficial effects of inflammation after spinal cord injury: Potential therapeutic implications. Handb Clin Neurol. 2012;109:485–502. doi: 10.1016/B978-0-444-52137-8.00030-9. [DOI] [PubMed] [Google Scholar]
- 3.Popovich PG. Neuroimmunology of traumatic spinal cord injury: A brief history and overview. Exp Neurol. 2014;258:1–4. doi: 10.1016/j.expneurol.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 4.David S, Greenhalgh AD, López-Vales R. Role of phospholipase A2s and lipid mediators in secondary damage after spinal cord injury. Cell Tissue Res. 2012;349(1):249–267. doi: 10.1007/s00441-012-1430-8. [DOI] [PubMed] [Google Scholar]
- 5.López-Vales R, et al. Phospholipase A2 superfamily members play divergent roles after spinal cord injury. FASEB J. 2011;25(12):4240–4252. doi: 10.1096/fj.11-183186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alexander JK, Popovich PG. Neuroinflammation in spinal cord injury: Therapeutic targets for neuroprotection and regeneration. Prog Brain Res. 2009;175:125–137. doi: 10.1016/S0079-6123(09)17508-8. [DOI] [PubMed] [Google Scholar]
- 7.Silver J, Schwab ME, Popovich PG. Central nervous system regenerative failure: Role of oligodendrocytes, astrocytes, and microglia. Cold Spring Harb Perspect Biol. 2015;7(3):a020602. doi: 10.1101/cshperspect.a020602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rowland JW, Hawryluk GW, Kwon B, Fehlings MG. Current status of acute spinal cord injury pathophysiology and emerging therapies: Promise on the horizon. Neurosurg Focus. 2008;25(5):E2. doi: 10.3171/FOC.2008.25.11.E2. [DOI] [PubMed] [Google Scholar]
- 9.Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: Back to the future. Immunity. 2013;39(6):1003–1018. doi: 10.1016/j.immuni.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11(8):633–652. doi: 10.1038/nrd3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo Y, et al. Suppression of antigen-specific adaptive immunity by IL-37 via induction of tolerogenic dendritic cells. Proc Natl Acad Sci USA. 2014;111(42):15178–15183. doi: 10.1073/pnas.1416714111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dinarello CA, Bufler P. Interleukin-37. Semin Immunol. 2013;25(6):466–468. doi: 10.1016/j.smim.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Nold MF, et al. IL-37 is a fundamental inhibitor of innate immunity. Nat Immunol. 2010;11(11):1014–1022. doi: 10.1038/ni.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bufler P, Gamboni-Robertson F, Azam T, Kim SH, Dinarello CA. Interleukin-1 homologues IL-1F7b and IL-18 contain functional mRNA instability elements within the coding region responsive to lipopolysaccharide. Biochem J. 2004;381(Pt 2):503–510. doi: 10.1042/BJ20040217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ballak DB, et al. IL-37 protects against obesity-induced inflammation and insulin resistance. Nat Commun. 2014;5:4711. doi: 10.1038/ncomms5711. [DOI] [PubMed] [Google Scholar]
- 16.McNamee EN, et al. Interleukin 37 expression protects mice from colitis. Proc Natl Acad Sci USA. 2011;108(40):16711–16716. doi: 10.1073/pnas.1111982108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bulau AM, et al. In vivo expression of interleukin-37 reduces local and systemic inflammation in concanavalin A-induced hepatitis. ScientificWorldJournal. 2011;11:2480–2490. doi: 10.1100/2011/968479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teng X, et al. IL-37 ameliorates the inflammatory process in psoriasis by suppressing proinflammatory cytokine production. J Immunol. 2014;192(4):1815–1823. doi: 10.4049/jimmunol.1300047. [DOI] [PubMed] [Google Scholar]
- 19.Evans TA, et al. High-resolution intravital imaging reveals that blood-derived macrophages but not resident microglia facilitate secondary axonal dieback in traumatic spinal cord injury. Exp Neurol. 2014;254:109–120. doi: 10.1016/j.expneurol.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horn KP, Busch SA, Hawthorne AL, van Rooijen N, Silver J. Another barrier to regeneration in the CNS: Activated macrophages induce extensive retraction of dystrophic axons through direct physical interactions. J Neurosci. 2008;28(38):9330–9341. doi: 10.1523/JNEUROSCI.2488-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moretti S, et al. IL-37 inhibits inflammasome activation and disease severity in murine aspergillosis. PLoS Pathog. 2014;10(11):e1004462. doi: 10.1371/journal.ppat.1004462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li S, et al. Extracellular forms of IL-37 inhibit innate inflammation in vitro and in vivo but require the IL-1 family decoy receptor IL-1R8. Proc Natl Acad Sci USA. 2015;112(8):2497–2502. doi: 10.1073/pnas.1424626112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pan G, et al. IL-1H, an interleukin 1-related protein that binds IL-18 receptor/IL-1Rrp. Cytokine. 2001;13(1):1–7. doi: 10.1006/cyto.2000.0799. [DOI] [PubMed] [Google Scholar]
- 24.Quirk S, Agrawal DK. Immunobiology of IL-37: Mechanism of action and clinical perspectives. Expert Rev Clin Immunol. 2014;10(12):1703–1709. doi: 10.1586/1744666X.2014.971014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ye L, et al. IL-37 inhibits the production of inflammatory cytokines in peripheral blood mononuclear cells of patients with systemic lupus erythematosus: Its correlation with disease activity. J Transl Med. 2014;12:69. doi: 10.1186/1479-5876-12-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu K, et al. PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nat Neurosci. 2010;13(9):1075–1081. doi: 10.1038/nn.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bulau AM, et al. Role of caspase-1 in nuclear translocation of IL-37, release of the cytokine, and IL-37 inhibition of innate immune responses. Proc Natl Acad Sci USA. 2014;111(7):2650–2655. doi: 10.1073/pnas.1324140111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang Y, et al. IL-37 inhibits IL-18–induced tubular epithelial cell expression of pro-inflammatory cytokines and renal ischemia-reperfusion injury. Kidney Int. 2015;87(2):396–408. doi: 10.1038/ki.2014.295. [DOI] [PubMed] [Google Scholar]
- 29.Nold-Petry CA, et al. IL-37 requires the receptors IL-18Rα and IL-1R8 (SIGIRR) to carry out its multifaceted anti-inflammatory program upon innate signal transduction. Nat Immunol. 2015;16(4):354–365. doi: 10.1038/ni.3103. [DOI] [PubMed] [Google Scholar]
- 30.Lunding L, et al. IL-37 requires IL-18Rα and SIGIRR/IL-1R8 to diminish allergic airway inflammation in mice. Allergy. 2015;70(4):366–373. doi: 10.1111/all.12566. [DOI] [PubMed] [Google Scholar]
- 31.Klopstein A, et al. Beneficial effects of alphaB-crystallin in spinal cord contusion injury. J Neurosci. 2012;32(42):14478–14488. doi: 10.1523/JNEUROSCI.0923-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fry EJ, Chagnon MJ, Lopez-Vales R, Tremblay ML, David S. Corticospinal tract regeneration after spinal cord injury in receptor protein tyrosine phosphatase sigma deficient mice. Glia. 2010;58(4):423–433. doi: 10.1002/glia.20934. [DOI] [PubMed] [Google Scholar]
- 33.Stirling DP, Yong VW. Dynamics of the inflammatory response after murine spinal cord injury revealed by flow cytometry. J Neurosci Res. 2008;86(9):1944–1958. doi: 10.1002/jnr.21659. [DOI] [PubMed] [Google Scholar]
- 34.Basso DM, et al. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma. 2006;23(5):635–659. doi: 10.1089/neu.2006.23.635. [DOI] [PubMed] [Google Scholar]